Abstract
Background:
Studies have demonstrated the Timed Up and Go Test’s (TUGT) ability to forecast post-operative outcomes for several surgical specialties. Evaluations of TUGT for waitlist and post-transplant outcomes has yet to be examined in kidney transplantation.
Objective:
To assess the prognostic utility of TUGT and its associations with waitlist and post-transplant outcomes for kidney transplant candidates.
Design and Methods:
Single-center, prospective study of 518 patients who performed TUGT during their transplant evaluation between 9/1/2013–11/30/2014. TUGT times were evaluated as a continuous variable or 3-level discrete categorical variable with TUGT times categorized as long (>9s), average (8–9s), or short (5–8s).
Results:
Transplanted individuals had shorter TUGT times than those who remained on the waitlist (8.99 vs. 9.79 seconds, p<0.001). Bivariable and multivariable logistic regression showed that after adjusting for age, there was no association between TUGT times and probability of waitlist removal (OR 0.997[0.814–1.221]), prolonged length of stay post-transplant (OR 1.113[0.958–1.306] for deceased-donor, OR 0.983[0.757–1.277] for living-donor), and 30-day readmissions (OR 0.984[0.845–1.146] for deceased-donor, OR 1.254[0.976–1.613] for living-donor).
Conclusions:
TUGT was not associated with waitlist removal or prolonged hospitalization for kidney transplant candidates. Alternative assessments of global health, such as functional status or frailty, should be considered for evaluation of potential kidney transplant candidates.
Keywords: kidney transplantation, transplant evaluation, waitlisting, patient characteristics, risk stratification
Introduction
Kidney transplantation is the treatment of choice for patients with end-stage renal disease (ESRD) but is limited by the scarcity of organs available for transplantation. However, there an elevated mortality risk that seen immediately following a transplant that returns to and subsequently falls below that associated with remaining on dialysis fairly quickly.1 To ensure appropriate use of scarce deceased donor kidneys there are exhaustive processes in place at transplant centers that attempt to identify individuals who will benefit from transplantation in order to waitlist them while excluding those individuals who are either unlikely to benefit from transplantation or unlikely to survive long enough to receive a transplant. Transplant evaluations, although protocolized, can be very subjective at times and would benefit from the introduction of an objective measure in the evaluation process.2–4 Frailty, a biologic syndrome of decreased reserve and resistance to stressors, is reflective of global health status and is emerging as an important prognostic marker in surgical and transplant patient populations.5, 6 For example frailty appears to be associated with postoperative complications including length of stay and mortality.5, 7 In particular, frailty measured at the time of transplantation has been shown to be associated with a 94% increased risk of delayed graft function and a 61% increased risk of rehospitalization in kidney transplant recipients.8, 9 However, most of the studies that have measured frailty in transplant recipients have done so after the evaluation process precluding their potential use in that process.
While several groups have studied the impact of frailty and outcomes following kidney transplantation, this measure has yet to be evaluated as a screening tool for whether patients might be good candidates for transplantation. Consequently, there is currently no standardized, validated assessment of functional status or frailty or physical performance that has been shown to be useful in the evaluation of potential transplant candidates.
Given the recent concerns and focus on post-transplant metrics, the presence of a frailty measure to evaluate potential transplant recipients will reduce variation in the acceptance criteria across centers.
In September 2013, Columbia University Medical Center (CUMC) implemented the “Timed Up and Go Test” (TUGT) as a component of our evaluation process for all patients prior to potential waitlisting. The TUGT, a modified version of the “Get-Up and Go” test, is a previously validated measure used to evaluate a patient’s functional status.10, 11 The TUGT was also chosen in part because it could be incorporated in the clinical workflow of a busy evaluation day with minimal disruption.12–14 Recorded TUGT times were made available to the transplant selection committee for review during the waitlist selection process. TUGT times were considered as an additional clinical element in the overall evaluation of patients. Anecdotal data suggests that there was greater interest in the results of the TUGT for older individuals and those with complex medical histories; short TUGT times were considered favorable, whereas long TUGT times were concerning.
This study evaluates the associations of TUGT with waitlist and post-transplant outcomes in kidney transplantation. There is a high likelihood of frail waitlisted patients removed from the waitlist due to further deterioration of their global health status. Additionally, frailty has previously been shown to be associated with prolonged length of stay.7 As such, we assess the associations between the TUGT and the post-transplant outcomes of waitlist removal, length of stay, and hospital readmission for transplant candidates.
Methods
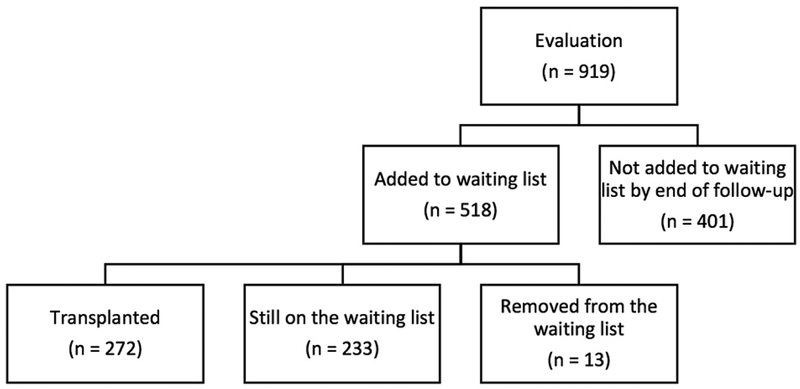
We conducted a single-center, retrospective cohort study of all patients undergoing kidney transplant evaluation at Columbia University Medical Center (CUMC) from September 01, 2013 through November 30, 2014. Patients were eligible for study inclusion if they were aged ≥18 years and were being evaluated for kidney transplant (n=919 patients). Patients unable to perform the TUGT due to physical limitations (0.4%) and those evaluated before the TUGT was incorporated as standard practice in clinic were excluded, resulting in a final cohort of 518 patients. All 518 patients in the final cohort who were evaluated for transplantation were subsequently waitlisted, and 272 patients went on to be transplanted (Table 1). All clinical and outcomes data were obtained through May 31, 2015. All procedures performed were approved by and conducted in accordance with the ethical standards of CUMC’s Institutional Review Board.
Table 1.
Characteristics of patients who performed the TUGT as part of their evaluation for kidney transplant at CUMC from 2013–2014, stratified by their transplant process status (Waitlisted Only or Transplanted) (n = 518)
| Total | Waitlisted Only | Transplanted | p-value | |
|---|---|---|---|---|
| N (row %) | 518 (100.0%) | 246 (47.5%) | 272 (52.5%) | |
| Age at the time of TUGT (years) | 52.9±13.5 | 54.2±12.9 | 51.7±13.9 | 0.033 |
| 18–39 | 90 (17.4) | 33 (13.4) | 57(21.0) | 0.024 |
| 40–59 | 244 (47.1) | 121(49.2) | 123(45.2) | 0.396 |
| 60≥ | 184 (35.5) | 92(37.4) | 92(33.8) | 0.396 |
| TUGT (seconds) | 9.70±2.87 | 9.79±2.80 | 8.99±2.22 | <0.001 |
| Min, Max | 5.00, 34.00 | 5.00, 34.00 | 5.00, 29.00 | |
| Short: 5-<8 | 61 (11.8) | 24 (9.8) | 37(13.6) | 0.175 |
| Average: 8–9 | 278 (53.7) | 113(24.9) | 165(60.7) | <0.001 |
| Long: >9 | 179 (34.6) | 109(44.3) | 70(34.56) | <0.001 |
| EPTS (%) | 34.9±25.3 | 37.2±24.2 | 32.8±26.0 | 0.046 |
| Male | 309 (59.7) | 143 (58.1) | 166 (61.0) | 0.502 |
| African-American/Black | 130 (25.0) | 78 (31.7) | 52 (19.1) | 0.001 |
| BMI (kg/m2) | 29.0±5.8 | 29.9±5.9 | 28.2±5.6 | 0.001 |
| Previous TX Recipients | 40 (7.7) | 19 (7.7) | 21 (7.7) | 0.999 |
| History of DM | 193 (37.3) | 100 (40.7) | 93 (34.2) | 0.129 |
| History of HTN | 448 (92.0) | 211 (90.1) | 237(92.9) | 0.418 |
| College Education | 180 (35.1) | 78 (32.1) | 102 (37.8) | 0.178 |
| Pre-emptive listing† | 238 (46.0) | 110 (44.7) | 128 (47.1) | 0.593 |
| Years on Dialysis at TUGT | 2.48±2.50 | 2.23±1.78 | 2.72±2.34 | 0.105 |
| Patient Mortality‡ | 26 (5.0) | 10 (4.1) | 16 (5.9) | 0.587 |
| 1 year* | 6 (23.1) | 0 (0.0) | 6 (37.5) | --- |
| 2 years* | 13 (50.0) | 3 (30.0) | 10 (62.5) | --- |
| Donor Type (% DDRT) | --- | --- | 142 (52.2) | --- |
| Use of Walking Aid | 5 (1.0) | 5 (2.0) | 0 | 0.018 |
Were not on chronic dialysis at the time of listing for a kidney transplant
Refers to patients who died while listed or following transplantation
*Patient died within given timeframe (1 or 2 years) of being waitlisted (for Waitlisted Only group) or transplanted (Transplanted group)
BMI, body mass index; DDRT, deceased donor renal transplant; DM, diabetes; EPTS, Estimated Post-Transplant Survival; HTN, hypertension; TUGT, Timed Up and Go Test
The TUGT protocol involves measuring how long it takes for a patient to perform the following sequence:10
Rise from a seated position in an arm chair
Walk 10 feet forward
Turn around
Walk back to the arm chair
Sit down again
Patients are allowed to use their customary walking aids during the task and patients walk through the test once to become familiar with the process before being timed. A faster TUGT time indicates better functional performance.10 TUGT times were recorded in seconds (s) and analyzed as both a continuous and 3-level discrete categorical variable: Long: >9s; Average: 8–9s; Short: <8s. A sensitivity analysis was performed to determine the threshold points at which to categorize TUGT times.
In addition to TUGT time, additional variables were examined that could serve to confound the relationship between TUGT time and short-term outcomes. Covariates such as age, presence of hypertension or diabetes, and years of dialysis at time of TUGT were included to assess whether adjustment was needed when examining the relationship between TUGT and short-term outcomes.
Study data were screened to detect erroneous data entries, missing data, and outliers to test normality. Pearson’s chi-square, Fischer’s exact tests, student t-tests, and ANOVA were performed for categorical and continuous variables, respectively, to compare characteristics between the TUGT groups. All continuous values are expressed as means and standard deviation (SD) and all categorical variables as counts and percentages. Univariate and multivariable logistic regression were constructed to evaluate the relationship between TUGT times and waitlist or post-transplant outcomes. Outcomes of interest included: (i) wait list removal for reasons other than receiving a transplant, (ii) length of stay (>7 days) during the index hospitalization for those individuals who subsequently received a transplant during the follow up period, and (iii) post-transplant hospital readmission within 30 days. All analyses were conducted using SAS 9.4 (SAS institute Inc., Cary, NC).
Results
All 518 patients in the final cohort who were evaluated for transplantation were subsequently waitlisted of which 272 patients (52.5%) went on to be transplanted. Among the 518 patients in our final cohort, 285 patients were removed from the waitlist, including 11 (2.1%) patients who died, 1 patient who changed status to kidney/pancreas, and 1 patient who transferred to a different transplant center. Of these transplanted patients, 130 (47.8%) received a living donor transplant while 142 (52.2%) received a deceased donor transplant (Table 1). The median time (IQR) between evaluation to listing was 27(13–109) days, and the median time (IQR) from evaluation to transplant was 83(8–267) days for living donors transplants and 332(141–633) days for deceased donor transplants.
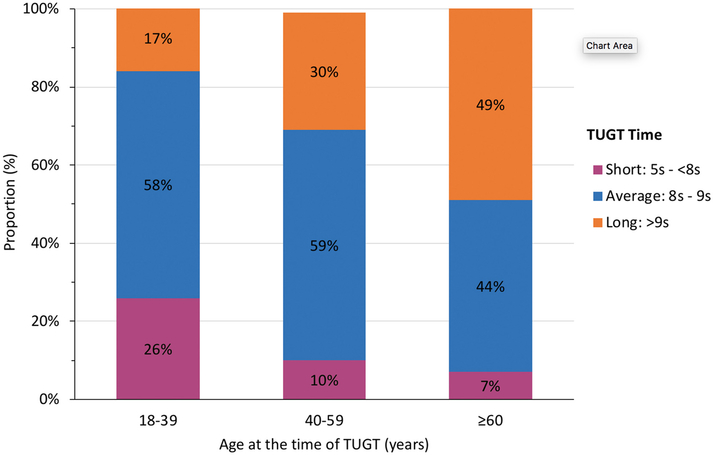
Only 3.3% of the 919 patients used a walking aid. When compared to patients who were waitlisted but not transplanted, transplanted patients were found to be younger at the time of TUGT evaluation (51.7 vs 54.2 years, p=0.033), had better expected post-transplant survival (EPTS) scores (32.8 vs 37.2%, p=0.046), lower mean BMI (28.2 vs 29.9 kg/m2, p=0.001), and were less likely to be Black (19.1 vs 31.7%, p=0.001). Transplanted patients also demonstrated significantly lower TUGT times compared to their counterparts who were not transplanted (8.99 vs 9.79 seconds, p<0.001). Distribution of TUGT performance varied by age group, with the oldest patients more likely to be categorized into the “long TUGT” group (TUGT >9s) and the youngest patients more likely to be categorized into the “short TUGT” group (TUGT <8s) (Table 2) (Figure 2). Additionally, when compared to patients in “short TUGT” group, patients categorized into the “long TUGT” group had a higher average BMI (29.7 vs. 27.4, p=0.023), higher incidence of diabetes (53.6 vs. 19.7%, p=0.001), and higher patient mortality (8.9 vs. 1.6%, p=0.011).
Table 2.
Characteristics of patients who performed the TUGT as part of their evaluation for kidney transplant at CUMC from 2013–2014, stratified by TUGT times (Short, Average, or Long) (n=518)
| Short (5-<8) | Average (8–9) | Long (>9) | p-value | |
|---|---|---|---|---|
| N (row %) | 61 (11.8%) | 278 (53.7%) | 179 (34.6%) | |
| Age at the time of TUGT (years) | 45.4±15.0 | 51.3±13.1 | 58.0±11.8 | <0.001abc |
| 18–39 | 23 (37.7) | 52 (18.7) | 15(8.4) | <0.001abc |
| 40–59 | 25 (41.0) | 145(52.2) | 74(41.3) | 0.046b |
| 60≥ | 13 (21.3) | 81 (29.1) | 90 (50.3) | <0.001bc |
| Male | 34 (55.7) | 170 (61.2) | 105 (58.7) | 0.697 |
| African-American/Black | 7 (11.5) | 66 (23.7) | 57 (31.8) | 0.005ac |
| BMI (kg/m2) | 27.4±5.1 | 28.9±5.7 | 29.7±6.1 | 0.023c |
| Previous TX Recipients | 6 (9.8) | 25 (9.0) | 9 (5.0) | 0.242 |
| History of DM | 12 (19.7) | 85 (30.6) | 96 (53.6) | <0.001bc |
| History of HTN | 56 (96.6) | 236 (89.7) | 156 (94.0) | 0.114 |
| College Education | 29 (47.5) | 103 (37.3) | 48 (27.3) | 0.009bc |
| Pre-emptive listing† | 33 (54.1) | 123 (44.2) | 82 (45.8) | 0.376 |
| Years on Dialysis at TUGT | 2.48±2.50 | 2.23±1.78 | 2.72±2.34 | 0.105 |
| Patient Mortality‡ | 1 (1.6) | 9 (3.2) | 16 (8.9) | 0.011bc |
| 1 year* | 0 (0) | 3 (33.3) | 3 (18.8) | --- |
| 2 years* | 1 (100) | 4 (44.4) | 8 (50.0) | --- |
| Transplanted | 37 (13.6) | 165 (60.7) | 70 (25.7) | <0.001bc |
| Donor Type (among transplanted) (% DDRT) | 16 (43.2) | 84 (50.9) | 28 (40.0) | 0.222 |
|
| ||||
Significant difference between short and long TUGT categories
Significant difference between average and long TUGT categories
Significant difference between short and average TUGT categoriess
Figure 2.
The association between a patient’s age at the time of evaluation at CUMC and their TUGT time (n = 518)
An assessment of short-term outcomes was performed to evaluate the associations between the TUGT and non-transplant waitlist removal and immediate post-transplant parameters (Table 3). There was no significant relationship found between TUGT time and probability of wait list removal. However, age at TUGT was found to be significantly associated with wait list removal for reasons other than transplant (OR 1.060, p=0.037). For both living and deceased donor transplants, TUGT time in seconds and TUGT time categorization were not significantly associated with the length of stay during the index transplant hospitalization and readmission post-transplant. In contrast, the EPTS was found to be significantly associated with prolonged length of stay for deceased donor transplants (OR 1.014, p=0.030), while BMI at listing was found to be significantly associated with prolonged length of stay for living donor transplants (OR 1.086, p=0.028). When examining odds of readmission for living donors, age at TUGT (OR 1.041, p=0.042), EPTS (OR 1.024, p = 0.018), and BMI at listing (OR 1.098, p=0.019) were found to be significant. After adjusting for age at TUGT and EPTS using multivariable logistic regression, TUGT time was not found to be significantly associated with any of the short-term outcomes (table 4). Additionally, age at TUGT and EPTS were also not significantly associated with the outcomes of interest.
Table 3.
Bivariable logistic regression models illustrating the relationship between TUGT times and short-term outcomes (i) waitlist removal, (ii) length of stay >7days, (iii) readmission within 30 days.
| Wait List Removal* | ||
|---|---|---|
| Parameters | Crude OR (95% CI) | p-value |
| TUGT (seconds) | 0.997 (0.814–1.221) | 0.977 |
| Short: 5-<8 | Ref | --- |
| Average: 8–9 | 0.627(0.062–6.303) | 0.692 |
| Long: >9 | 2.070 (0.250–17.162) | 0.500 |
| Age at TUGT (years) | 1.060 (1.004–1.120) | 0.037 |
| EPTS (%) | 1.020 (0.997–1.043) | 0.086 |
| BMI at listing | 0.994 (0.904–1.093) | 0.908 |
| African-American/Black | 0.376 (0.081–1.737) | 0.210 |
| DM | 0.908 (0.288–2.860) | 0.869 |
| HTN | --- | --- |
| Length of Stay >7 days† | ||||
|---|---|---|---|---|
| Deceased Donor Transplant | Living Donor Transplant | |||
| Parameters | Crude OR (95% CI) | p-value | Crude OR (95% CI) | p-value |
| TUGT (seconds) | 1.113(0.958–1.306) | 0.155 | 0.983(0.757–1.277) | 0.898 |
| Short: 5-<8 | Ref | --- | ref | --- |
| Average: 8–9 | 0.849(0.258–2.797) | 0.788 | 0.664(0.224–1.970) | 0.461 |
| Long: >9 | 2.223(0.627–7.890) | 0.216 | 0.417(0.101–1.724) | 0.227 |
| Age at TUGT (years) | 1.028(0.999–1.057) | 0.055 | 1.000(0.970–1.031) | 0.995 |
| EPTS (%) | 1.014(1.001–1.027) | 0.030 | 1.012(0.993–1.031) | 0.217 |
| BMI at listing | 0.992(0.930–1.058) | 0.804 | 1.086(1.009,1.168) | 0.028 |
| African-American/Black | 1.694(0.773–3.713) | 0.188 | 0.554(0.117,2.618) | 0.456 |
| DM | 1.461(0.709–3.012) | 0.304 | 1.742(0.709,4.279) | 0.226 |
| HTN | 1.045(0.237–4.600) | 0.954 | 0.766(0.145,4.036) | 0.753 |
| Readmission Within 30 Days† | ||||
|---|---|---|---|---|
| Deceased Donor Transplant | Living Donor Transplant | |||
| Parameters | Crude OR (95% CI) | p-value | Crude OR (95% CI) | p-value |
| TUGT (seconds) | 0.984(0.845–1.146) | 0.834 | 1.254(0.976–1.613) | 0.077 |
| Short: 5-<8 | Ref | --- | ref | --- |
| Average: 8–9 | 0.877(0.253, 3.035) | 0.836 | 0.739(0.212–2.579) | 0.636 |
| Long: >9 | 1.345(0.364, 4.970) | 0.657 | 1.159(0.282–4.770) | 0.838 |
| Age at TUGT (years) | 1.004(0.977–1.032) | 0.763 | 1.041(1.002–1.082) | 0.042 |
| EPTS (%) | 1.002(0.988–1.015) | 0.818 | 1.024(1.004–1.045) | 0.018 |
| BMI at listing | 0.931(0.861–1.006) | 0.072 | 1.098(1.016,1.188) | 0.019 |
| African-American/Black | 1.931(0.852–4.377) | 0.115 | 0.673(0.561,6.840) | 0.292 |
| DM | 1.088(0.505–2.347) | 0.829 | 0.975(0.349,2.727) | 0.962 |
| HTN | --- | --- | 1.343(0.156,11.552) | 0.788 |
Patient who were transplanted with a living or deceased donor kidney were excluded (n = 246)
Transplanted patients included (n = 272)
Table 4.
Multivariable logistic regression models illustrating the relationship between TUGT times and short-term outcomes of (i) waitlist removal, (ii) length of stay >7days, (iii) readmission within 30 days.
| Wait List Removal* | ||
|---|---|---|
| Parameters | Adjusted OR | p-value |
| TUGT (seconds) | 0.930(0.720–1.200) | 0.576 |
| Age at TUGT (years) | 1.063(0.980–1.153) | 0.138 |
| EPTS (%) | 1.000(0.965–1.037) | 0.982 |
| Length of Stay >7 days† | ||||
|---|---|---|---|---|
| Deceased Donor Transplant | Living Donor Transplant | |||
| Parameters | Adjusted OR | p-value | Adjusted OR (95% CI) | p-value |
| TUGT (seconds) | 1.091(0.941–1.264) | 0.248 | 0.940(0.707–1.249) | 0.669 |
| Age at TUGT (years) | 1.007(0.964–1.053) | 0.746 | 0.966(0.921–1.014) | 0.159 |
| EPTS (%) | 1.010(0.990–1.031) | 0.326 | 1.029(0.999–1.060) | 0.055 |
| Readmission Within 30 Days† | ||||
|---|---|---|---|---|
| Deceased Donor Transplant | Living Donor Transplant | |||
| Parameters | Adjusted OR | p-value | Adjusted OR (95% CI) | p-value |
| TUGT (seconds) | 0.980(0.838–1.146) | 0.800 | 1.172(0.900–1.526) | 0.239 |
| Age at TUGT (years) | 1.005(0.961–1.050) | 0.837 | 1.016(0.960–1.075) | 0.588 |
| EPTS (%) | 1.000(0.979–1.022) | 0.994 | 1.015(0.984–1.046) | 0.357 |
Patient who were transplanted with a living or deceased donor kidney were excluded (n = 246)
Transplanted patients included (n = 272)
Discussion
Chronic kidney disease is associated with decreased physical activity, low muscle mass, and frailty.15–21 Additionally, prior research has demonstrated a relationship between other surrogate markers for poorer overall health status, including higher comorbidity scores, lower serum albumin, and low recipient muscle mass (reflected by lower serum creatinine level prior to transplant) and inferior post-transplant outcomes.22–24 Recent studies have also shown that frailty in elderly surgical patients is associated with surgical complications, prolonged length of stay, as well as post-operative mortality for a variety of surgical specialties.25, 26 Finally, there is a strong association between frailty and 30-day patient mortality, particularly after major sugery.25, 26 These findings raise the possibility of using an objective assessment of patient frailty status prior to waitlisting for kidney transplantation to provide useful for risk stratification during pre-transplant evaluation.
Furthermore, there are currently more than 500,000 people on dialysis in the United States who are not on the kidney transplant waitlist. Transplant centers have variable waitlisting criteria due to concerns about post-transplant outcomes, resulting in some centers being much more selective than others, which in turn has the potential to exacerbate existing disparities in access to transplantation. Providing objective measures such as functional status or frailty assessments may help standardize the selection process and thus reduce these disparities.26
In our cohort the TUGT did not appear to provide any additional prognostic information for short-term outcomes after consideration of recipient age and EPTS score, variables already well-known to be associated with post-transplant outcomes.24, 27–30 Although prior research on the TUGT has found the test to be associated with post-operative complications in several non-transplant surgical populations,14 the failure of the TUGT to provide prognostic information here may be explained by differences between the nature of the surgical procedures (orthopedic, colorectal, and cardiac surgical recovery may have different physical demands than kidney transplantation), as well as by the extended time interval between the test and the actual surgical procedure.
Since the TUGT was not found to be associated with post-transplant outcomes, there is a need for a better tool to assess frailty that can be easily incorporated into a busy evaluation clinic day. The Fried Frailty Phenotype is a validated measure which proposes a standard of frailty among elderly patients but involves a multitude of steps that make its application outside the research setting a challenge. Prior studies have demonstrated the validity of this frailty measure at predicting poor health outcomes among geriatric patient populations. However, this frailty measure has not been studied in patients who are end-stage, a characteristic of all patients being evaluated for kidney transplantation.31 A further limitation of this frailty measure is that it involves extensive interviews, questionnaires, timed walking tests, and additional patient evaluations to assess a variety of health criteria used to determine frailty status. Despite being relatively inexpensive to implement, this frailty measure is time-intensive and may not be pragmatic for use in a clinical setting during transplant evaluation. Therefore, further research must be conducted to determine a practical, validated assessment of frailty in kidney transplant candidates.
It would also be of value to consider whether supplementing the TUGT with additional measures of global health status might improve the utility of the TUGT as a screening tool for transplant readiness. Prior studies have described measures of global health status, including functional status and physical performance, to be useful for determining which patients are good transplant candidates during waitlist selection.32–34 Lower physical function, as measured by the physical function scale of the Medical Outcomes Study 36-Item Short Form Health Survey (SF-36), was independently associated with an increased risk of becoming inactive on the waitlist and a decreased chance of receiving a kidney transplant while higher physical functioning scores on the SF-36 were associated with a reduced risk of hospitalization and mortality post-transplant.32, 33 Given that frailty is a promising measure, it is likely that a supplementation of frailty measures such as TUGT coupled with self-reported physical functioning data (SF-36) would provide a more complete picture of overall health status but this would need further study.
Limitations of this study include its single-center design and a focus on the immediate post-operative follow up period. In addition, we were unable to adjust for comorbidities other than diabetes and hypertension. A selection bias may also exist since all patients included were waitlisted, and outcomes for patients who were ultimately not waitlisted were unable to be assessed. Additionally, in this study we lacked an alternative measure of frailty to assess the performance of TUGT as a measure of patient frailty. Furthermore, the TUGT is a measure obtained months to years prior to transplantation when the patient is first evaluated for waitlisting. Given the time lag between when the TUGT is performed and the time of transplantation, patients may experience different frailty trajectories during this period. Consequently, a frailty assessment tool at the time of evaluation may not be particularly helpful in the decision to waitlist. Nevertheless, an alternative frailty measure assessed at the time of evaluation may be of value if patients are repeatedly tested throughout the duration of their time on the waitlist to determine whether they are deteriorating or whether they continue to be good transplant candidates. Implementing continual assessments in this manner would likely result in decreased disparities in access to transplantation.
In conclusion, after adjusting for age, the TUGT performed at the time of the evaluation was not associated with short-term outcomes including waitlist removal and length of stay following transplantation. Use of the TUGT to assess kidney transplant candidates therefore may bias against waitlisting older patients while not actually providing additional prognostic information. While measures of frailty may represent a useful means of risk stratification for patients being evaluated for or undergoing renal transplantation, the TUGT at the time of the initial evaluation does not appear to be able to identify individuals at an increased risk of adverse outcomes.
Figure 1.
Study population
Acknowledgements
SM is supported by the NIH/NIDDK (R01-DK114893 and U01-DK116066) as well as funding from the American Society of Transplantation and the Laura and John Arnold Foundation.
Footnotes
Conflicts of Interest
The authors declare that they have no financial conflicts of interest to disclose.
References:
- 1.Ojo AO, Hanson JA, Meier-Kriesche H, et al. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol. March 2001;12(3):589–597. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. December 2 1999;341(23):1725–1730. [DOI] [PubMed] [Google Scholar]
- 3.Rabbat CG, Thorpe KE, Russell JD, Churchill DN. Comparison of mortality risk for dialysis patients and cadaveric first renal transplant recipients in Ontario, Canada. J Am Soc Nephrol. May 2000;11(5):917–922. [DOI] [PubMed] [Google Scholar]
- 4.Oniscu GC, Brown H, Forsythe JL. Impact of cadaveric renal transplantation on survival in patients listed for transplantation. J Am Soc Nephrol. June 2005;16(6):1859–1865. [DOI] [PubMed] [Google Scholar]
- 5.McAdams-DeMarco MA, Law A, King E, et al. Frailty and mortality in kidney transplant recipients. Am J Transplant. January 2015;15(1):149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. February 2011;27(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAdams-DeMarco MA, King EA, Luo X, et al. Frailty, Length of Stay, and Mortality in Kidney Transplant Recipients: A National Registry and Prospective Cohort Study. Ann Surg. December 2017;266(6):1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garonzik-Wang JM, Govindan P, Grinnan JW, et al. Frailty and delayed graft function in kidney transplant recipients. Arch Surg. February 2012;147(2):190–193. [DOI] [PubMed] [Google Scholar]
- 9.McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty and early hospital readmission after kidney transplantation. Am J Transplant. August 2013;13(8):2091–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. February 1991;39(2):142–148. [DOI] [PubMed] [Google Scholar]
- 11.Mathias S, Nayak US, Isaacs B. Balance in elderly patients: the “get-up and go” test. Arch Phys Med Rehabil. June 1986;67(6):387–389. [PubMed] [Google Scholar]
- 12.Zeni JA Jr., Snyder-Mackler L. Early postoperative measures predict 1- and 2-year outcomes after unilateral total knee arthroplasty: importance of contralateral limb strength. Phys Ther. January 2010;90(1):43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizner RL, Petterson SC, Clements KE, Zeni JA Jr., JJ Irrgang, Snyder-Mackler L. Measuring functional improvement after total knee arthroplasty requires both performance-based and patient-report assessments: a longitudinal analysis of outcomes. J Arthroplasty. August 2011;26(5):728–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson TN, Wu DS, Sauaia A, et al. Slower walking speed forecasts increased postoperative morbidity and 1-year mortality across surgical specialties. Ann Surg. October 2013;258(4):582–588; discussion 588–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polinder-Bos HA, Nacak H, Dekker FW, Bakker SJL, Gaillard C, Gansevoort RT. Low Urinary Creatinine Excretion Is Associated With Self-Reported Frailty in Patients With Advanced Chronic Kidney Disease. Kidney Int Rep. July 2017;2(4):676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adey D, Kumar R, McCarthy JT, Nair KS. Reduced synthesis of muscle proteins in chronic renal failure. Am J Physiol Endocrinol Metab. February 2000;278(2):E219–225. [DOI] [PubMed] [Google Scholar]
- 17.Dalrymple LS, Katz R, Rifkin DE, et al. Kidney function and prevalent and incident frailty. Clin J Am Soc Nephrol. December 2013;8(12):2091–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fried LF, Boudreau R, Lee JS, et al. Kidney function as a predictor of loss of lean mass in older adults: health, aging and body composition study. J Am Geriatr Soc. October 2007;55(10):1578–1584. [DOI] [PubMed] [Google Scholar]
- 19.Johansen KL, Chertow GM, Kutner NG, Dalrymple LS, Grimes BA, Kaysen GA. Low level of self-reported physical activity in ambulatory patients new to dialysis. Kidney Int. December 2010;78(11):1164–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reese PP, Cappola AR, Shults J, et al. Physical performance and frailty in chronic kidney disease. Am J Nephrol. 2013;38(4):307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roshanravan B, Khatri M, Robinson-Cohen C, et al. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis. December 2012;60(6):912–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molnar MZ, Kovesdy CP, Bunnapradist S, et al. Associations of pretransplant serum albumin with post-transplant outcomes in kidney transplant recipients. Am J Transplant. May 2011;11(5):1006–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Streja E, Molnar MZ, Kovesdy CP, et al. Associations of pretransplant weight and muscle mass with mortality in renal transplant recipients. Clin J Am Soc Nephrol. June 2011;6(6):1463–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patzer RE, Basu M, Larsen CP, et al. iChoose Kidney: A Clinical Decision Aid for Kidney Transplantation Versus Dialysis Treatment. Transplantation. March 2016;100(3):630–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin HS, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr. August 31 2016;16(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. June 2010;210(6):901–908. [DOI] [PubMed] [Google Scholar]
- 27.Clayton PA, McDonald SP, Snyder JJ, Salkowski N, Chadban SJ. External validation of the estimated posttransplant survival score for allocation of deceased donor kidneys in the United States. Am J Transplant. August 2014;14(8):1922–1926. [DOI] [PubMed] [Google Scholar]
- 28.Dieplinger G, Everly MJ. Understanding trends and predictors of outcome in elderly renal transplant recipients: an analysis of the UNOS registry. Clin Transpl. 2013:1–11. [PubMed] [Google Scholar]
- 29.Laging M, Kal-van Gestel JA, van de Wetering J, et al. A High Comorbidity Score Should Not be a Contraindication for Kidney Transplantation. Transplantation. February 2016;100(2):400–406. [DOI] [PubMed] [Google Scholar]
- 30.Kasiske BL, Israni AK, Snyder JJ, Skeans MA, Peng Y, Weinhandl ED. A simple tool to predict outcomes after kidney transplant. Am J Kidney Dis. November 2010;56(5):947–960. [DOI] [PubMed] [Google Scholar]
- 31.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. March 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 32.Reese PP, Shults J, Bloom RD, et al. Functional status, time to transplantation, and survival benefit of kidney transplantation among wait-listed candidates. Am J Kidney Dis. November 2015;66(5):837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartmann EL, Kitzman D, Rocco M, et al. Physical function in older candidates for renal transplantation: an impaired population. Clin J Am Soc Nephrol. March 2009;4(3):588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kutner NG, Zhang R, Bowles T, Painter P. Pretransplant physical functioning and kidney patients’ risk for posttransplantation hospitalization/death: evidence from a national cohort. Clin J Am Soc Nephrol. July 2006;1(4):837–843. [DOI] [PubMed] [Google Scholar]