Abstract
The tremendous diversity of animal behaviors has inspired generations of scientists from an array of biological disciplines. To complement investigations of ecological and evolutionary factors contributing to behavioral evolution, modern computational, genetics, genomics, and neuroscience tools now provide a means to discover the proximate mechanisms upon which natural selection acts to generate behavioral diversity. Social behaviors are motivated behaviors that can differ tremendously between closely related species, suggesting phylogenetic plasticity in their underlying biological mechanisms. Additionally, convergent evolution has repeatedly given rise to similar forms of social behavior and mating systems in distantly related species. Social behavioral divergence and convergence provides an entry point for understanding the neurogenetic mechanisms contributing to behavioral diversity. We argue that the greatest strides in understanding social behavioral diversity will be achieved through integration of interdisciplinary comparative approaches with modern tools in diverse species systems. We review recent advances and future potential for discovering mechanisms underlying social behavioral variation, highlighting patterns of social behavioral evolution, oxytocin and vasopressin neuropeptide systems, genetic/transcriptional “toolkits,” modern techniques, and alternative species systems, with particular emphasis on Microtine rodents and Lake Malawi cichlid fishes.
Keywords: social behavior, oxytocin and vasopressin, toolkits, voles, cichlids
Introduction
Animal behavior has been of great interest to integrative zoologists and ethologists for many decades, and its scientific investigation led to the 1973 Nobel Prize in Physiology or Medicine being jointly awarded to Karl von Frisch, Konrad Lorenz and Nikolaas Tinbergen. This research tradition has not only aimed to discover biological mechanisms contributing to behavioral diversity, but also to understand the degree of variation and generalizability of these mechanisms within and across species. Interest in animal behavior has continued to grow as biomedical researchers have aimed to understand the biological basis of human behavior and cognition through animal models. In pursuit of this aim, behavioral genetics and neuroscience research has grown increasingly focused on a narrow range of model species. Here we argue that, with regards to social behavior in particular, the limitations of such strategies are amplified; and that such strategies will, by default, be unable to access a diverse array of gene regulatory and neural circuit mechanisms contributing to natural social behavioral variation. Instead we argue that a synthesis of comparative perspectives with modern neuroscience, genetics, genomics, and computational tools in alternative species systems will be necessary to discover the fundamental biological mechanisms and organizing principles underlying social behavioral diversity.
Social behavior as an integrative phenotype
Selective pressure to adaptively transmit, receive, and respond to signals with conspecifics has led to the evolution of complex social behaviors in diverse species spanning microbes and humans (Crespi; West et al, 2007). Social behaviors have long been recognized as fundamentally integrative phenotypes that emerge as functions of evolutionary history; external factors such as abiotic environment, resource distribution, population density, and behaviors or cues from other individuals; and internal biological factors including genetic composition and expression, developmental stage, experience, epigenetic modification, sex, hormonal state, and neurogenetic architecture (Nescent Working Group on Integrative Models of Vertebrate Sociality: Evolution et al, 2014). Interactions among these factors have profound effects on social behavioral variation across multiple levels of biological organization—spanning individuals, populations, and species—and a high degree of variation in any one or a combination of these factors frequently exists at these levels. The labile, variable, and combinatorial nature of these factors establish the potential for diverse gene regulatory and neurogenetic mechanisms to drive patterns of natural social behavioral variation in individuals, populations, and species.
Patterns of social behavioral variation in nature
Convergent evolution of social behaviors
Convergent evolution of integrative traits pervades the evolutionary history of life on Earth, including many examples of independently evolved and rare social behaviors. Several mating and parental systems—e.g. socially monogamous mating systems and biparental care—have repeatedly and independently evolved in invertebrates and every major vertebrate lineage (Bull, 2000; Burley & Johnson, 2002; French et al, 2017; Johnson & Young, 2015; Reynolds et al, 2002; Roland & O’Connell, 2015; Suzuki, 2013; Whiteman & Cote, 2004). Vocal learning, or the acquisition of learned vocalizations through imitation, has independently evolved in several avian and mammalian lineages, including humans (Jarvis, 2007). Mating systems in which males congregate into leks and construct elaborate courtship “bowers” have evolved in bowerbirds (Ptilonorhynchidae) and cichlid (Cichlidae) fishes (Kusmierski et al, 1997; York et al, 2015). The capacity for empathy-like consoling behavior has evolved in corvids (Corvidae), rodents (Rodentia), elephants (Elephantidae), dogs (Canis familiaris), and primates (Burkett et al, 2016; Clay & de Waal, 2013; de Waal & Preston, 2017; Fraser et al, 2008). And eusocial systems have evolved in termites (Isoptera); ants, bees, and wasps (Hymenoptera); Synalpheus shrimp; and African mole rats (Bathyergidae) (Chak et al, 2017; Duffy & Macdonald, 2010; Nowak et al, 2010; Thorne, 1997; Toth & Rehan, 2017).
The repeated evolution of nature’s rarest social behaviors in distant taxa—and in nervous systems of varying sizes, organizations, and complexities—suggests that in response to common selective forces, the underlying and diverse architectures of animal biology have frequently given rise to the same patterns of social behavior, either through distinct or shared mechanistic pathways. It has been proposed that evolutionary plasticity within common neurobehavioral substrates—including reward/reinforcement, feeding/foraging, sexual, aggression, and maternal behavior circuits; conserved neuromodulatory systems, including steroid hormone, oxytocin (OT), vasopressin (AVP), and dopamine systems; and conserved transcriptional “toolkits”—has repeatedly contributed to the evolution of convergent social behavioral phenotypes (Ament et al, 2010; Fischer & O’Connell, 2017; Johnson & Young, 2017; Newman, 1999; Numan & Young, 2016; Rittschof & Robinson, 2016; Toth et al, 2007). In vertebrates, these systems are thought to function and interact within a broader “social decision-making” neural network with a predominantly conserved core neuroanatomical architecture (O’Connell & Hofmann, 2011).
What is the degree of mechanistic overlap underlying independently evolved social behaviors? Evidence from butterflyfishes (Chaetodontidae), Microtine voles, Peromyscus mice, and primates suggest that evolutionary plasticity in conserved neuromodulatory systems—namely OT, AVP, and dopamine systems within ventral striatal reward and reinforcement circuits—has repeatedly contributed to the convergent evolution of socially monogamous mating systems across vertebrate lineages (Bendesky et al, 2017; Johnson & Young, 2015; 2017; Nowicki et al, 2017b). However, additional evidence from cichlid fishes and rodents suggests that the detailed gene regulatory and neural circuit mechanisms can differ between closely-related species (Fink et al, 2006; Johnson & Young, 2017; Renn et al, 2017; Turner et al, 2010). These findings are consistent with emerging evidence that common—but in some cases “loose”—genetic and/or transcriptional “toolkits” have repeatedly been selected upon in the evolution of vocal learning and eusociality (Berens et al, 2015; Pfenning et al, 2014; Rittschof & Robinson, 2016; Toth et al, 2010). To date, however, comparative neurogenetic investigations of social behavior are limited to a relatively small number of species systems, and therefore the degree of mechanistic overlap across species remains a major question.
Rapid evolutionary divergence of social behaviors
Social behaviors can rapidly diverge (and repeatedly evolve) in groups of closely-related species (York & Fernald, 2017). For example, divergent mating and parental care behaviors are exhibited between closely-related species of Hymenopteran insects (ants, bees, and wasps), butterflyfishes, cichlid fishes, poison frogs (Dendrobatidae), African mole rats, Microtine voles, Peromyscus mice, and primates (Emlen & Oring, 1977; Ferkin, 1990; French et al, 2017; Jasarevic et al, 2013; Kidd et al, 2012; Lukas & Clutton-Brock, 2013; Nowicki et al, 2017a; Opie et al, 2013; Roland & O’Connell, 2015; Toth & Rehan, 2017). Diverse patterns of flocking behavior are frequently exhibited between closely-related species of birds, such as Estrildidae finches and Emberizidae songbirds; and divergent group burrowing behaviors are exhibited between closely-related species of South American Ctenomys tuco-tuco rodents (Anacker & Beery, 2013; Beery et al, 2008; Goodson et al, 2009; Goodson et al, 2012).
Social behavior can also vary within species at the population level. For example, populations of threespine stickelback fish (Gasterosteus aculeatus) and cavefish (Astyanax mexicanus) exhibit differences in schooling behavior; and prairie voles (Microtus ochrogaster) exhibit population differences in socially monogamous behavior (Greenwood et al, 2015; Greenwood et al, 2016; Kowalko et al, 2013; McGraw & Young, 2010).
These and many other examples suggest that the genes and neurobiological systems modulating social behaviors are evolutionarily labile, frequently varying within and between closely-related species. Strategic comparisons of social behavioral divergence at the species and population levels have discovered causal genetic and neurobiological mechanisms contributing to specific social behaviors (Bendesky et al, 2017; Goodson et al, 2009; Greenwood et al, 2016; Haesler et al, 2007; Haesler et al, 2004; Insel & Shapiro, 1992; Insel et al, 1994; Kowalko et al, 2013; Lim et al, 2004). For example, investigations in alternative species systems spanning teleost fishes and mammals have begun to reveal the roles of brain-region specific OT and AVP receptor (OTR and V1aR, respectively) populations in modulating species-specific social behaviors (DeAngelis et al, 2017; Donaldson & Young, 2008; Goodson & Bass, 2000; Keebaugh et al, 2015; O’Connell et al, 2012; Oldfield & Hofmann, 2011; Song et al, 2014). Comparative investigations followed by causal manipulations and have demonstrated the role of region-specific OTR and V1aR populations in modulating flocking behavior in Estrildidae finches and pair bonding behavior in Microtine voles (Goodson et al, 2009; Insel & Shapiro, 1992; Insel et al, 1994; Lim et al, 2004; Young et al, 2001). However, much remains to be discovered about the detailed neuronal populations and neural circuits mediating these effects.
Individual variation and plasticity in social behaviors
Social behavioral divergence between closely-related species is sometimes paralleled by a high degree of social behavioral variation within species (Johnson & Young, 2017). For example, although prairie voles (Microtus ochrogaster) are classified as socially monogamous, individual males and females exhibit a high degree of variation in sociospatial and mating behaviors (McGraw & Young, 2010). This variation has been linked to single nucleotide polymorphisms in the OXTR/AVPR1A (the genes encoding OTR and V1aR, respectively) gene region, indicating that naturally-occurring genetic variation within species contributes to individual social behavioral phenotypes (King et al, 2016; Okhovat et al, 2015). In humans, genetic variation in OXTR and AVPR1A has also been linked to variation in brain region-specific OTR/V1aR expression; brain function during social contexts; and pair bonding, social recognition, and psychiatric phenotypes such as autism (Johnson & Young, 2017; LoParo & Waldman, 2015; Reuter et al, 2017; Skuse et al, 2014; Walum et al, 2012; Walum et al, 2008). Taken together, these findings suggest that naturally-occurring polymorphisms in homologous genes can contribute to individual variation in brain gene expression patterns and social behavior in humans and alternative species systems. Furthermore, the diversity of OTR/V1aR brain expression profiles within and between species suggests that diverse transcriptional mechanisms selectively recruit OTR/V1aR expression to specific neuronal populations and circuits to modulate behavior; and that understanding these mechanisms will require investigation in diverse species.
Individuals can also exhibit social behavioral plasticity at several timescales. For example, male cichlid fish rapidly transition to dominant physiological and behavioral phenotypes in response to social opportunities; Siberian hamsters (Phodopus sungorus) exhibit seasonal variation in aggression; and honeybees (Apis mellifera) transition through different social roles in the colony throughout the lifespan (Ben-Shahar, 2005; Maruska & Fernald, 2010; Maruska et al, 2013; Rendon et al, 2017; Robinson, 2002; Wen et al, 2004). These and many other examples suggest that the biological systems modulating social behavior exhibit functional plasticity across developmental, seasonal, and rapid neuromodulatory timescales in a species-specific fashion. Investigations in alternative species systems have discovered novel mechanisms regulating several forms of social behavioral plasticity at different timescales (Ament et al, 2008; Ben-Shahar, 2005; Carpenter et al, 2014; Juntti et al, 2016; Maruska et al, 2013). For example, Juntti et al. recently combined CRISPR/Cas9 and hormonal manipulations to demonstrate the causal role of prostaglandin F2α in modulating the rapid expression of female mating behaviors in Astatotilapia burtoni cichlid fish (Juntti et al, 2016).
Comparative investigations have also generated evidence that conserved gene regulatory networks contribute to similar forms of social behavioral plasticity across species. For example, studies in cichlid fishes have identified core transcriptional “modules” associated with rapid transitions to social dominance in both sexes and across species; and recent studies suggest that aggression and territorial behaviors are associated with conserved genetic “toolkits” across honeybees, stickelback fish, and house mice (Mus musculus) (Alaux et al, 2009; Liu et al, 2016; Renn et al, 2016; Rittschof et al, 2014).
Limitations of traditional laboratory models
For many decades Krogh’s principle (the notion that the most appropriate model system should be carefully selected for a given research question) led to numerous breakthrough discoveries in biology (Krogh, 1929). The electrochemical mechanisms of action potentials were discovered in giant axons of squid (Loligo pealii), and neuroplasticity mechanisms mediating learning and memory were elucidated through studying the accessible neurons of sea slugs (Aplysia) (Yartsev, 2017). In recent decades, however, behavioral genetics and neuroscience research has become predominantly focused on a narrow set of model species, most notably inbred laboratory strains of house mice. This general strategy aims to make biomedical discoveries by applying cutting-edge techniques to study conserved and translationally relevant biological functions in animal models. Concentrated effort in developing experimental resources for specific systems (e.g. transgenic mouse strains), combined with invasive approaches that cannot be employed in humans, allows such processes to be studied in unprecedented detail and has led to discoveries of novel mechanisms underlying human disease and other traits of interest.
Despite these strengths, strategies focusing on a small number of species are poorly suited for understanding unique functional specializations, complex integrative traits, and phenotypic diversity; and are therefore poorly positioned for understanding social behavioral diversity (Brenowitz & Zakon, 2015; Yartsev, 2017). In traditional and “knockout” laboratory strains, the vast majority of natural genetic variation present in wild populations has been eradicated through inbreeding, genetic drift, and artificial selection; eliminating naturally-occurring functional polymorphisms and reducing the genetic complexity underlying complex traits (Chalfin et al, 2014). Furthermore, inbreeding, adaptation to captivity, and artificial selection frequently impact reproductive, parental, aggressive, and other social behaviors; altering or eliminating wild-type behavioral phenotypes (Champagne et al, 2007; Connor, 1975; Margulis & Altmann, 1997; Wilson, 2000). Indeed, complete loss of natural social behaviors (and gain of “unnatural” social behaviors) has been demonstrated in common laboratory mouse strains (Chalfin et al, 2014). Therefore, traditional model systems have presumably lost many natural genetic polymorphisms and transcriptional mechanisms contributing to variation in brain gene expression patterns and social behavior at the individual, population, and species level.
Social behaviors are also specialized products of the unique evolutionary history, ecology, and biology of a given organism or lineage. For example, although sex steroids play a dominant role in regulating aggression in mice and rats, species with different life history strategies—some exhibiting stronger hormonal parallels to humans—regulate aggression through other hormonal mechanisms, including several species of hamsters and songbirds (Soma et al, 2008). Mechanistic diversity underlying conserved behaviors highlights an additional limitation of pursuing traditional laboratory systems as translational models for human social cognition and behavior. As another example, although investigations in laboratory mice have contributed to our understanding of parental behavior, laboratory mice exhibit differences in the onset, expression, and expiration of parental behaviors compared to wild house mice and other mammals; and in some instances exhibit complete loss of wild-type parental behaviors (Chalfin et al, 2014; Champagne et al, 2007; Olazabal et al, 2013a). Understanding how gene regulatory and neural circuit mechanisms shape natural variation in parental behavior will require investigation in alternative species systems exhibiting a range of parental behavioral phenotypes (Olazabal et al, 2013a; b). Other unique social behaviors reflect additional dimensions of human sociality and are completely absent in traditional laboratory species and their wild-type counterparts, such as pair bonding, vocal learning, and consoling behavior; the mechanistic basic of these behaviors can only be studied in alternative species systems.
Modern tools for investigating social behavior
Molecular genetics, neuroscience, genomic sequencing, and computational tools have rapidly advanced in recent decades, placing us in a unique position for studying the social brain. New and powerful technologies such as CRISPR/Cas9, single-cell RNA sequencing, computer vision, and optogenetics enable investigation of the biological mechanisms of social behavior in diverse species at unprecedented levels of precision. Decreasing costs of high-throughput and next-generation “omics” sequencing technologies and advances in bioinformatics have created opportunities for gene regulatory network analyses and large scale comparative genomic and transcriptomic investigations of social behavior across species (Baran et al, 2017). Taken together, these ongoing trends will continue to increase the cost-effectiveness and potential impact of prioritizing neurogenetic investigations in new and diverse species. In the following sections, we highlight discoveries in Microtine voles to illustrate the power of applying modern genetics and neuroscience tools to alternative species systems. We then highlight the social behavioral diversity of Lake Malawi cichlids as a promising system for future discoveries.
Microtine rodents and prairie voles
Background
Microtine rodents include more than 60 species of North American, European, and Asian voles exhibiting diverse mating strategies, parental care behaviors, and patterns of group living. For example, although socially monogamous mating systems are rare in mammals, these systems have repeatedly evolved in independent Microtine lineages. Comparative strategies focusing on Microtine social behaviors have provided mechanistic insights into convergent evolution, rapid divergence, and individual variation in complex social behaviors that are not expressed in laboratory mice; and have simultaneously provided translational insights relevant to human social cognition, behavior, and psychiatry
Comparative investigations in Microtine voles
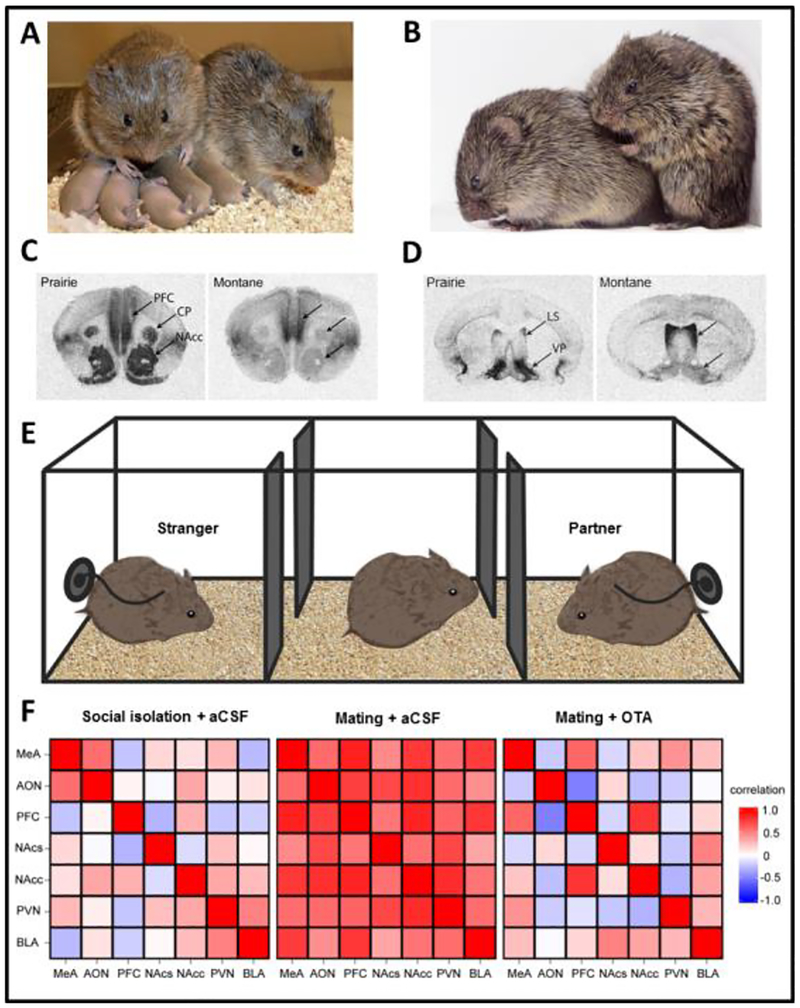
Pair bond formation in monogamous species such as the prairie vole (Microtus ochrogaster; Figure 1A, B) can be studied in the laboratory using a partner preference test (Fig. 1E), in which subjects can freely choose to spend time near a familiar mating partner, near an unfamiliar opposite-sex conspecific, or in a neutral chamber (Young & Wang, 2004). Socially monogamous and promiscuous vole species exhibit robust behavioral differences in the partner preference test. Behavioral pharmacological studies in socially monogamous prairie voles have demonstrated that the neuropeptides OT and AVP facilitate mating-induced partner preference formation (Winslow et al., 1993, Cho et al., 1999). Socially monogamous and promiscuous Microtine species exhibit strikingly different expression patterns of OTRs and V1aRs in the brain (e.g. Fig. 1C, D); with socially monogamous prairie and pine voles exhibiting higher expression levels of OTR and V1aR in a ventral striatal (nucleus accumbens-ventral pallidum) reward and reinforcement circuit compared to promiscuous meadow and montane voles (Insel & Shapiro, 1992; Insel et al, 1994).
Figure 1. Neurogenetic investigations of complex social behaviors in prairie voles (Microtus ochrogaster).
Prairie voles exhibit complex social behavioral phenotypes that are not exhibited in traditional laboratory organisms, including pair bonding and biparental care (A), and consoling behavior (B). Comparative neurogenetic investigations across Microtine species have revealed robust divergence in brain expression patterns of OTR and V1aR between closely related species. Socially monogamous prairie voles exhibit higher OTR expression selectively in the nucleus accumbens (NAcc) compared to promiscuous montane voles (C), meadow voles, mice, and rats (not depicted); and higher V1aR expression selectively in the ventral pallidum (VP) compared to promiscuous montane voles (D), meadow voles, mice, and rats (not depicted). The laboratory partner preference test (E), in which a male or female subject can freely spend time with a familiar mating partner, with a novel opposite-sex conspecific, or in a neutral chamber, has been used to discover detailed neurogenetic mechanisms regulating pair bonding behavior. Recent investigations in prairie voles suggest the OT/AVP systems can generate functional diversity in the brain and behavior by modulating conserved and widely distributed neural networks during social contexts (F) (Johnson et al, 2016; Johnson et al, 2017). Heatmaps represent pairwise correlation coefficients of Fos expression (an activity-dependent immediate early gene widely used to study brain function) between seven interconnected, OTR-expressing brain regions in three treatment groups. Following central administration of artificial cerebral spinal fluid (aCSF), social isolation is associated with weakly correlated Fos expression across brain regions. In contrast, aCSF-treatment followed by sociosexual interaction and mating with a female is associated with strongly and positively correlated Fos expression across the network. This effect is disrupted by central administration of a selective OTR antagonist (OTA) prior to sociosexual interaction and mating, which causes a significant decrease in correlated Fos expression across the network. Abbreviations (see above): AON=anterior olfactory nucleus; BLA=basolateral amygdala; CP=caudate putamen; LS=lateral septum; MeA=medial amygdala; NAcc=nucleus accumbens core; NAcs=nucleus accumbens shell; PFC=prefrontal cortex; PVN=paraventricular nucleus of the hypothalamus; VP=ventral pallidum. Autoradiograms (C-D) were adapted from (Young & Wang, 2004) with permission. Partner preference schematic (E) was adapted from (Barrett & Young) with permission. Heatmaps (F) were adapted from (Johnson et al, 2016) with permission. Images in A-B are courtesy of Todd Ahern, Quinnipiac University, USA; and Zachary Johnson, Georgia Institute of Technology, USA.
Comparative genetic investigations have revealed both individual and species level differences near the genes encoding OTR and V1aR, providing potential genetic mechanisms underlying species differences in the brain and social behavior (Hammock & Young, 2004; King et al, 2016; Young et al, 1996; Young et al, 1999). In one set of experiments, V1aR was selectively overexpressed in the same reward and reinforcement circuit in promiscuous meadow voles using viral vectors. This manipulation caused promiscuous meadow voles to exhibit prairie vole-like patterns of brain region-specific V1aR expression as well as monogamous-like behavior, demonstrating that variation in expression of a single gene in a conserved neural circuit can significantly contribute to variation in complex social behaviors (Lim et al, 2004). Unlike mice and rats, high expression levels of OTR and/or V1aR in this circuit have been noted in socially monogamous species of Peromyscus mice and primates, supporting the possibility of a convergent functional specialization involving expression patterns of conserved genes in a conserved neural circuit (Freeman & Young, 2016; Johnson & Young, 2015; Young, 1999). Investigations in human brain tissue have used ligands that bind promiscuously to both OTR and V1aR, and have also revealed high expression levels in this circuit, suggesting discoveries in alternative species systems can contribute unique discoveries relevant to human brain gene expression (Loup et al, 1991).
Prairie voles
Comparative investigations in Microtine voles also provided the foundation for laboratory investigation of additional complex social behaviors in prairie voles that are not exhibited in laboratory mice (Donaldson & Young, 2008; Lim et al, 2004; McGraw & Young, 2010). Like humans, prairie voles exhibit selective social attachments between mating partners, biparental and alloparental care (Fig. 1A), depressive-like behavior following partner loss, and consoling behavior towards partners following unobserved stress (Fig. 1B). Application of both traditional and cutting-edge genetic and neuroscience techniques have provided insights into the neurogenetic mechanisms underlying these behaviors. For example, pharmacological manipulations have shown that brain region-specific populations of OTR, V1aR, dopamine receptors, and other neuromodulatory receptors play causal roles in regulating pair bonding behavior (Aragona et al, 2003; Burkett et al, 2011; Curtis & Wang, 2005; Gingrich et al, 2000; Keebaugh et al, 2015; Lim et al, 2007; Lim & Young, 2004; Liu & Wang, 2003; Young et al, 2001). These techniques have also been used to investigate the neural basis of social attachments in socially monogamous Mandarin voles, and suggest similar neurogenetic circuit mechanisms may contribute to independently evolved mating systems across Microtine species (He et al, 2017; Yu et al, 2013). In addition to pair bonding, receptor autoradiography, selective antagonists, viral vector gene transfer, and RNA knockdown have been used to discover novel neuromodulatory mechanisms contributing to other complex social behaviors including selective aggression, alloparental care, and consoling behavior (Barrett et al, 2013; Burkett et al, 2016; Burkett et al, 2011; Gobrogge et al, 2009; Johnson et al, 2016; Keebaugh et al, 2015; Lim & Young, 2004; Liu et al, 2001; Young et al, 2001). These techniques have also been used in combination with patch-clamp electrophysiological recording and microdialysis to dissect neural circuits underlying depressive-like behavior following partner loss, which is thought to play a role in the maintenance of pair bonds (Bosch et al, 2016). Additional studies have revealed neuroepigenetic mechanisms contributing to pair bonding (Wang et al, 2013). Recently, we combined in vivo electrophysiological recordings in multiple brain regions, computer vision, and optogenetic manipulation to show that a corticostriatal circuit—a neural pathway that has been repeatedly implicated in human addiction and compulsive behavior—modulates pair bonding in prairie voles (Amadei et al, 2017; Horga et al, 2015; Marquand et al, 2017; Volkow et al, 2012). Taken together, these findings reinforce the potential for discovering neurogenetic and circuit mechanisms in alternative species that may be relevant to human cognition and behavior, and that may not be present in traditional laboratory models. The ability to use CRISPR/Cas9 to generate transgenic vole lines that express Cre-recombinase in specific neuronal populations will significantly enhance the usefulness of voles for elucidating the neural circuitry of social behaviors.
Investigations in prairie voles have also revealed neurogenetic mechanisms contributing to individual variation in social behavior. Recent investigations have identified single nucleotide polymorphisms in the OXTR and AVPR1A gene region that predict individual variation in brain region-specific OTR and V1aR expression and social behavior (King et al, 2016; Okhovat et al, 2015). Individual variation in OTR expression influences how early life social experience affects social bonding in adulthood, providing a potential mechanism for gene by environment interactions (Barrett et al, 2015). Additional experiments have demonstrated that central and region-specific OTR populations shift patterns of neural activation across widely distributed brain networks during sociosexual interactions, offering clues as to how individual and species variation in brain gene expression can generate functional diversity in the brain during social contexts (Fig. 1F) (Johnson et al, 2016; Johnson et al, 2017; Johnson & Young, 2017).
In humans, genetic variation in OXTR and AVPR1A predict individual variation in region-specific receptor expression, brain function during social contexts (including shifts in patterned activation across widely distributed brain networks), social behavior, social cognition, and psychiatric phenotypes, suggesting investigations of natural individual variation in alternative species systems can discover biological mechanisms that are relevant to human health (Johnson & Young, 2017; LoParo & Waldman, 2015; Reuter et al, 2017; Skuse et al, 2014; Walum et al, 2012; Walum et al, 2008). Indeed, clinical trials assessing the therapeutic potential of OT administration for autism spectrum disorders are currently underway (https://clinicaltrials.gov/), demonstrating that investigations of social behavior in alternative species systems has already helped stimulate lines of translational research (Johnson & Young, 2017).
Conclusions
The application of a wide array of experimental techniques to investigate social behavior in prairies voles has led to the discovery of novel neurogenetic mechanisms contributing to individual and species variation in brain function and behavior. Similar, but not identical, mechanisms have been linked to social behavior in other vertebrates and humans, contributing to our understanding of mechanistic variation and generalizability across species. Combining comparative approaches with modern tools in alternative species systems can thus accelerate discoveries of biological mechanisms driving ethologically relevant behaviors, and simultaneously advance biomedical research goals.
Cichlids and Lake Malawi bower builders
Background
Teleost fishes possess putative homologues for all core neuromodulatory and neuroanatomical components of the social decision making network, a conserved neural network that regulates social behaviors across vertebrates, including humans. Among teleosts, zebrafish have emerged as a “model” laboratory system for studying genes, brains, and behavior; and behavioral genetics and neuroscience studies in zebrafish have contributed to our understanding of genetic mechanisms underlying human health and psychiatric phenotypes (Norton, 2013). Despite their unique strengths, laboratory strains of zebrafish share many limitations of other laboratory model organisms (see above under “Limitations of traditional laboratory models“), notably the loss of natural genetic variation and the absence of many complex social behaviors that are expressed by other teleost species, such as cichlid fishes.
Cichlidae is the most species-rich family of vertebrates on Earth (Kocher, 2004). Cichlid evolution in the East African Rift Valley (Lakes Tanganyika, Malawi, and Victoria) has attracted the attention of evolutionary biologists due to the explosive speciation in recent evolutionary history, with more than 2,000 cichlid species estimated to populate these lakes today. East African cichlids exhibit tremendous phenotypic diversity in habitat preference, diet, morphology, neural organization, and behavior—including many rare and complex social behaviors that are not expressed in zebrafish or other traditional laboratory species (Brawand et al, 2014). Cichlids thus represent a premier system for discovering novel neurogenetic mechanisms and organizing principles underlying social behavioral diversity.
Comparative investigations in cichlids
To date, the majority of comparative genetic investigations in cichlids have focused on speciation, trophic morphology, and vision/color patterning (Albertson et al, 2005; Brawand et al, 2014; Gante et al, 2016; Hauser et al, 2017; Hulsey, 2009; Lee et al, 2005; Loh et al, 2008; Owens & Rennison, 2017; Parsons et al, 2014; Roberts et al, 2017; Spady et al, 2005; Streelman et al, 2003). Additional studies have investigated the diverse genetic mechanisms regulating sex determination, and differences in brain organization associated with ecology (Bohne et al, 2013; Sylvester et al, 2013). These efforts have discovered genes contributing to phenotypic variation, demonstrating proof of principle for behavioral studies. Despite this exciting progress, to date there have been relatively few comparative genetic and neural investigations of social behavior in cichlids.
Cichlids exhibit extraordinary social behavioral diversity including many examples of repeated behavioral evolution, rapid behavioral divergence, and robust behavioral plasticity within individuals (Balshine-Earn & Earn, 1998; Emlen & Oring, 1977; Kidd et al, 2012; Klett & Meyer, 2002; Maruska & Fernald, 2010; 2013). Cichlid species exhibit many social behaviors that cannot be studied in traditional laboratory species—including complex social hierarchies; cooperative breeding; biparental and exclusively paternal care systems; maternal care and mouthbrooding; monogamous and lek-like mating systems; and male bower building (Emlen & Oring, 1977; Fernald, 2017; Kidd et al, 2012; Kidd et al, 2006). Recent studies have also demonstrated OT and AVP modulation of social behavior in cichlids, including species-specific parental and mating behaviors (Huffman et al, 2015; O’Connell et al, 2012; Oldfield & Hofmann, 2011). Therefore, cichlids also represent an unparalleled opportunity to discover novel transcriptional mechanisms through which OTR/V1aR expression can be recruited to conserved neuronal populations and circuits to modulate behavior. Links between natural polymorphisms in the OXTR/AVPR1A gene region, brain region-specific OTR/V1aR expression, and social behavior and cognition in humans suggest that such discoveries may provide valuable translational insights (Johnson & Young, 2017). In the following section we highlight male bower-building behavior in Lake Malawi as a promising behavioral system for discovering neurogenetic mechanisms modulating complex social behavior.
Bower building behavior in Lake Malawi
Lake Malawi (Fig. 2A) is home to approximately 1,000 cichlid species, making it the most species-rich freshwater lake on Earth. Collectively, Lake Malawi cichlids are estimated to possess a total genetic diversity just 2.5 times greater than that of the human species; therefore, species exhibiting striking social behavioral differences are typically separated by a relatively small number of genetic polymorphisms, comparable to that between human individuals (Loh et al, 2013). Lake Malawi cichlids thus represent an unparalleled opportunity to discover causative natural polymorphisms contributing to social behavioral variation.
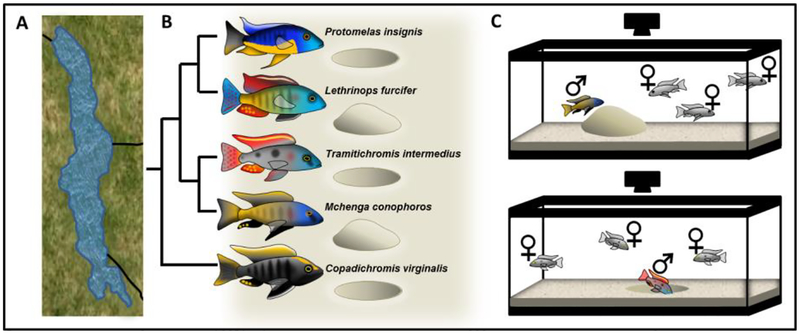
Figure 2. Bower building behavior in Lake Malawi cichlid (Cichlidae) fishes.
Lake Malawi (A) is the most species-rich freshwater lake on Earth, and is home to an estimated 1,000 species of cichlid fishes that have diverged within the past 5 million years, including dozens of species exhibiting bower building behavior during the breeding season. Different forms of bower-building behavior have repeatedly evolved, with many closely related species expressing divergent “pit” versus “castle” bower phenotypes. The phylogeny is based on York et al. 2015 (York et al, 2015) (B). Bower building behavior is reliably performed in aquarium tanks. Advances in computer vision, depth sensing, and neurogenetic approaches will facilitate rigorous investigation of bower building behavior in the laboratory (C).
Within Lake Malawi, dozens of sand-dwelling species exhibit “bower building” behaviors that parallel dimensions of bower building in Ptilonorhynchidae bowerbirds—an example of convergent evolution across long evolutionary scales (McKaye et al, 2001). During the breeding season, male bower-building cichlids congregate into leks—large communal breeding grounds—and devote enormous energy to building, maintaining, and defending species-typical structures, or bowers, in the sand that serve as temporary territories for courtship and spawning (Kidd et al, 2006; York et al, 2015). Species-typical bowers come in different shapes and sizes, and several forms have repeatedly evolved. For example, “pits,” or crater-like bowers that are dug into the sand, and “castles,” or volcano-like bowers that are built up from the sand, are independently expressed in dozens of Lake Malawi lineages (Fig. 2B) (Kidd et al, 2006; York et al, 2015).
Bowers are constructed through the same motor pattern: collecting or “scooping” a mouthful of sand and spitting it elsewhere. Species differences in bower form are thus mediated by differences in patterned spatial decision-making about where to scoop and spit sand over time. Field observations have revealed that courtship, spawning, and intermale aggression occur most frequently at the center of the bower, and that bower size, shape, and position interact within the social landscape of the lek to influence intermale aggression and female mate choice (Genner et al, 2008; Martin & Genner, 2009; McKaye et al, 1990; Young et al, 2009). Many closely related species express divergent bower forms (e.g. pit versus castle), and distantly related species express the same bower form (Fig. 2B). The evolution of bower building systems in Lake Malawi therefore represents rapid diversification as well as repeated evolution of unique and species-specific extended phenotypes and suites of sociospatial behaviors. Interestingly, some Lake Tanganyikan species also exhibit bower building behaviors, enabling comparisons across longer evolutionary timescales (Morita et al, 2014). As teleosts, bower building species possess putative homologues of all major components of the social-decision making network. Bower building behavior is thus a promising system for discovering how neurogenetic specializations within conserved neural circuits generate functional and behavioral variation across evolutionary, seasonal, and rapid timescales.
Interdisciplinary approaches to investigate bower building
Reproductively naive males of many bower-building species reliably construct species-typical bower forms in aquariums, facilitating investigation in laboratory environments. Many bower-building species are commercially available and can thus be easily introduced and maintained in the laboratory. Cichlid males can be socially housed with multiple reproductive females in aquarium tanks with sandy bottoms, enabling behavior in semi-naturalistic “home” environments to be investigated over extended periods of time (Fig. 2C). Advances in computer vision and depth sensing technologies also enable high-resolution and high-throughput behavioral phenotyping (as has recently been demonstrated to measure social behavior in mice) as well as structural analysis of bower form over time (Fig. 2C) (Hong et al, 2015).
High genetic similarity between Lake Malawi species enables powerful experimental strategies that are impossible or ineffective in other vertebrate systems. For example, divergent Malawi cichlids can be intercrossed in the laboratory, producing hybrid offspring that are genetic and phenotypic mosaics. First generation (F1) hybrids possess full copies of both parental genomes, enabling analyses of context-specific parental allele expression in the brain. Subsequent hybrid generations (F2, F3, F4, etc.) are genetic mosaics of the parent species, and can be used for quantitative trait loci (QTL) mapping of genes contributing to species-specific behaviors. QTL mapping has been used to identify genetic loci associated with many traits of interest in Malawi cichlids (Holzman & Hulsey, 2017; Husemann et al, 2017; Nandamuri et al, 2017; O’Quin et al, 2012; Parnell et al, 2008; 2012; Parsons et al, 2015; Selz et al, 2014; Svensson et al, 2011); and has recently been used to discover genetic loci contributing to extended phenotypes (burrow structures) and social behaviors in Peromyscus mice (Bendesky et al, 2017; Metz et al, 2017; Weber et al, 2013).
Rapid advances and decreasing costs of next-generation sequencing enable comparative genomic and brain transcriptomic investigations across bower building species. These approaches have already been integrated to reveal neurogenetic specializations associated with social behaviors in alternative species systems, including Lake Tanganyikan cichlids (Renn et al, 2016; Renn et al, 2017; Sanogo et al, 2011; Toth et al, 2010). New techniques such as single-cell RNA sequencing in the brain have the potential to reveal neuronal populations and neuromodulatory systems regulating social behavior in unprecedented detail. Additionally, in contrast to mammalian systems, eggs are fertilized externally in cichlids, facilitating CRISPR/Cas9 approaches that are more technically difficult in laboratory rodents. CRISPR/Cas9 has already been successfully applied in East African cichlids to link specific genes to social behavior; and can similarly be applied to investigate the roles of specific (and potentially translationally relevant) genes, single nucleotide polymorphisms, and neuronal populations modulate bower building (Juntti et al, 2016).
Conclusions
The explosive diversification of cichlids in the East African Rift Valley is perhaps the greatest opportunity on Earth for discovering causative genetic variants that shape brain gene expression and behavior. The clear success of comparative strategies in cichlids highlights the potential for discovering neurogenetic mechanisms contributing to complex social behaviors that are absent in traditional laboratory organisms. Several unique biological features position Lake Malawi cichlids as a particularly promising system for such discoveries. Integrating comparative approaches with modern tools to investigate bower building behavior has the potential to reveal novel mechanisms and organizing principles underlying neurogenetic and social behavioral diversity, and may simultaneously advance our understanding of conserved genes, transcriptional mechanisms, and neural circuits that are relevant to human behavior, cognition, and psychiatric phenotypes.
Summary
Social behavioral diversity has long been of interest to biologists, and is of growing interest to biomedical researchers seeking to understand human social cognition, behavior, and psychiatry. Traditional behavioral genetics and neuroscience strategies are poorly suited for understanding social behavioral diversity; in contrast, outbred and alternative species systems have already helped elucidate neurogenetic mechanisms and organizing principles underlying social behavioral diversity, while simultaneously advancing biomedical research goals. The greatest advances in the years to come will be rooted in a synthesis of comparative strategies with modern genetics, genomics, neuroscience, and computational tools in new and diverse species systems.
Acknowledgements:
Preparation of this manuscript was supported by NIH grants P50MH100023 to LJY and NIH OD P51OD11132 to YNPRC
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record.
References
- Alaux C, Sinha S, Hasadsri L, Hunt GJ, Guzman-Novoa E, DeGrandi-Hoffman G, Uribe-Rubio JL, Southey BR, Rodriguez-Zas S & Robinson GE (2009) Honey bee aggression supports a link between gene regulation and behavioral evolution. Proc Natl Acad Sci U S A, 106(36), 15400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertson RC, Streelman JT, Kocher TD & Yelick PC (2005) Integration and evolution of the cichlid mandible: the molecular basis of alternate feeding strategies. Proc Natl Acad Sci U S A, 102(45), 16287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadei EA, Johnson ZV, Jun Kwon Y, Shpiner AC, Saravanan V, Mays WD, Ryan SJ, Walum H, Rainnie DG, Young LJ & Liu RC (2017) Dynamic corticostriatal activity biases social bonding in monogamous female prairie voles. Nature, 546(7657), 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ament SA, Corona M, Pollock HS & Robinson GE (2008) Insulin signaling is involved in the regulation of worker division of labor in honey bee colonies. Proc Natl Acad Sci U S A, 105(11), 4226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ament SA, Wang Y & Robinson GE (2010) Nutritional regulation of division of labor in honey bees: toward a systems biology perspective. Wiley Interdiscip Rev Syst Biol Med, 2(5), 566–76. [DOI] [PubMed] [Google Scholar]
- Anacker AM & Beery AK (2013) Life in groups: the roles of oxytocin in mammalian sociality. Front Behav Neurosci, 7, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Curtis JT, Stephan FK & Wang Z (2003) A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J Neurosci, 23(8), 3483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balshine-Earn S & Earn DJD (1998) On the evolutionary pathway of parental care in mouth-brooding cichlid fishes. Proceedings of the Royal Society B: Biological Sciences, 265(1411), 2217–2217. [Google Scholar]
- Baran NM, McGrath PT & Streelman JT (2017) Applying gene regulatory network logic to the evolution of social behavior. Proc Natl Acad Sci U S A, 114(23), 5886–5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CE, Arambula SE & Young LJ (2015) The oxytocin system promotes resilience to the effects of neonatal isolation on adult social attachment in female prairie voles. Transl Psychiatry, 5, e606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CE, Keebaugh AC, Ahern TH, Bass CE, Terwilliger EF & Young LJ (2013) Variation in vasopressin receptor (Avpr1a) expression creates diversity in behaviors related to monogamy in prairie voles. Horm Behav, 63(3), 518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CE & Young LJ Molecular Neurobiology of Social Bonding, The Oxford Handbook of Molecular Psychology. [Google Scholar]
- Beery AK, Lacey EA & Francis DD (2008) Oxytocin and vasopressin receptor distributions in a solitary and a social species of tuco-tuco (Ctenomys haigi and Ctenomys sociabilis). J Comp Neurol, 507(6), 1847–59. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar Y (2005) The foraging gene, behavioral plasticity, and honeybee division of labor. J Comp Physiol A Neuroethol Sens Neural Behav Physiol, 191(11), 987–94. [DOI] [PubMed] [Google Scholar]
- Bendesky A, Kwon YM, Lassance JM, Lewarch CL, Yao S, Peterson BK, He MX, Dulac C & Hoekstra HE (2017) The genetic basis of parental care evolution in monogamous mice. Nature, 544(7651), 434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens AJ, Hunt JH & Toth AL (2015) Comparative transcriptomics of convergent evolution: different genes but conserved pathways underlie caste phenotypes across lineages of eusocial insects. Mol Biol Evol, 32(3), 690–703. [DOI] [PubMed] [Google Scholar]
- Bohne A, Heule C, Boileau N & Salzburger W (2013) Expression and sequence evolution of aromatase cyp19a1 and other sexual development genes in East African cichlid fishes. Mol Biol Evol, 30(10), 2268–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Dabrowska J, Modi ME, Johnson ZV, Keebaugh AC, Barrett CE, Ahern TH, Guo J, Grinevich V, Rainnie DG, Neumann ID & Young LJ (2016) Oxytocin in the nucleus accumbens shell reverses CRFR2-evoked passive stress-coping after partner loss in monogamous male prairie voles. Psychoneuroendocrinology, 64, 66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawand D, Wagner CE, Li YI, Malinsky M, Keller I, Fan S, Simakov O, Ng AY, Lim ZW, Bezault E, Turner-Maier J, Johnson J, Alcazar R, Noh HJ, Russell P, Aken B, Alfoldi J, Amemiya C, Azzouzi N, Baroiller JF, Barloy-Hubler F, Berlin A, Bloomquist R, Carleton KL, Conte MA, D’Cotta H, Eshel O, Gaffney L, Galibert F, Gante HF, Gnerre S, Greuter L, Guyon R, Haddad NS, Haerty W, Harris RM, Hofmann HA, Hourlier T, Hulata G, Jaffe DB, Lara M, Lee AP, MacCallum I, Mwaiko S, Nikaido M, Nishihara H, Ozouf-Costaz C, Penman DJ, Przybylski D, Rakotomanga M, Renn SCP, Ribeiro FJ, Ron M, Salzburger W, Sanchez-Pulido L, Santos ME, Searle S, Sharpe T, Swofford R, Tan FJ, Williams L, Young S, Yin S, Okada N, Kocher TD, Miska EA, Lander ES, Venkatesh B, Fernald RD, Meyer A, Ponting CP, Streelman JT, Lindblad-Toh K, Seehausen O & Di Palma F (2014) The genomic substrate for adaptive radiation in African cichlid fish. Nature, 513(7518), 375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz EA & Zakon HH (2015) Emerging from the bottleneck: benefits of the comparative approach to modern neuroscience. Trends Neurosci, 38(5), 273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull CM (2000) Monogamy in lizards. Behav Processes, 51(1–3), 7–20. [DOI] [PubMed] [Google Scholar]
- Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FB & Young LJ (2016) Oxytocin-dependent consolation behavior in rodents. Science, 351(6271), 375–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett JP, Spiegel LL, Inoue K, Murphy AZ & Young LJ (2011) Activation of mu-opioid receptors in the dorsal striatum is necessary for adult social attachment in monogamous prairie voles. Neuropsychopharmacology, 36(11), 2200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burley NT & Johnson K (2002) The evolution of avian parental care. Philos Trans R Soc Lond B Biol Sci, 357(1419), 241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RE, Maruska KP, Becker L & Fernald RD (2014) Social opportunity rapidly regulates expression of CRF and CRF receptors in the brain during social ascent of a teleost fish, Astatotilapia burtoni. PLoS One, 9(5), e96632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chak STC, Duffy JE, Hultgren KM & Rubenstein DR (2017) Evolutionary transitions towards eusociality in snapping shrimps. Nat Ecol Evol, 1(4), 96. [DOI] [PubMed] [Google Scholar]
- Chalfin L, Dayan M, Levy DR, Austad SN, Miller RA, Iraqi FA, Dulac C & Kimchi T (2014) Mapping ecologically relevant social behaviours by gene knockout in wild mice. Nat Commun, 5, 4569. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Curley JP, Keverne EB & Bateson PP (2007) Natural variations in postpartum maternal care in inbred and outbred mice. Physiol Behav, 91(2–3), 325–34. [DOI] [PubMed] [Google Scholar]
- Clay Z & de Waal FB (2013) Bonobos respond to distress in others: consolation across the age spectrum. PLoS One, 8(1), e55206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JL (1975) Genetic mechanisms controlling the domestication of a wild house mouse population (Mus musculus L.). J Comp Physiol Psychol, 89(2), 118–30. [DOI] [PubMed] [Google Scholar]
- Crespi BJ The evolution of social behavior in microorganisms. Trends in Ecology & Evolution, 16(4), 178–183. [DOI] [PubMed] [Google Scholar]
- Curtis JT & Wang Z (2005) Ventral tegmental area involvement in pair bonding in male prairie voles. Physiol Behav, 86(3), 338–46. [DOI] [PubMed] [Google Scholar]
- de Waal FBM & Preston SD (2017) Mammalian empathy: behavioural manifestations and neural basis. Nature Reviews Neuroscience, 18, 498. [DOI] [PubMed] [Google Scholar]
- DeAngelis R, Gogola J, Dodd L & Rhodes JS (2017) Opposite effects of nonapeptide antagonists on paternal behavior in the teleost fish Amphiprion ocellaris. Horm Behav, 90, 113–119. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR & Young LJ (2008) Oxytocin, vasopressin, and the neurogenetics of sociality. Science, 322(5903), 900–4. [DOI] [PubMed] [Google Scholar]
- Duffy JE & Macdonald KS (2010) Kin structure, ecology and the evolution of social organization in shrimp: a comparative analysis. Proc Biol Sci, 277(1681), 575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emlen ST & Oring LW (1977) Ecology, sexual selection, and the evolution of mating systems. Science, 197(4300), 215–23. [DOI] [PubMed] [Google Scholar]
- Ferkin MH (1990) Kin Recognition and Social Behavior in Microtine Rodents, in Tamarin RH, Ostfeld RS, Pugh SR & Bujalska G (eds), Social Systems and Population Cycles in Voles. Basel: Birkhäuser Basel, 11–24. [Google Scholar]
- Fernald RD (2017) Cognitive skills and the evolution of social systems. J Exp Biol, 220(Pt 1), 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink S, Excoffier L & Heckel G (2006) Mammalian monogamy is not controlled by a single gene. Proc Natl Acad Sci U S A, 103(29), 10956–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer EK & O’Connell LA (2017) Modification of feeding circuits in the evolution of social behavior. J Exp Biol, 220(Pt 1), 92–102. [DOI] [PubMed] [Google Scholar]
- Fraser ON, Stahl D & Aureli F (2008) Stress reduction through consolation in chimpanzees. Proc Natl Acad Sci U S A, 105(25), 8557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM & Young LJ (2016) Comparative Perspectives on Oxytocin and Vasopressin Receptor Research in Rodents and Primates: Translational Implications. J Neuroendocrinol, 28(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA, Cavanaugh J, Mustoe AC, Carp SB & Womack SL (2017) Social Monogamy in Nonhuman Primates: Phylogeny, Phenotype, and Physiology. J Sex Res, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gante HF, Matschiner M, Malmstrom M, Jakobsen KS, Jentoft S & Salzburger W (2016) Genomics of speciation and introgression in Princess cichlid fishes from Lake Tanganyika. Mol Ecol, 25(24), 6143–6161. [DOI] [PubMed] [Google Scholar]
- Genner MJ, Young KA, Haesler MP & Joyce DA (2008) Indirect mate choice, direct mate choice and species recognition in a bower-building cichlid fish lek. J Evol Biol, 21(5), 1387–96. [DOI] [PubMed] [Google Scholar]
- Gingrich B, Liu Y, Cascio C, Wang Z & Insel TR (2000) Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster). Behav Neurosci, 114(1), 173–83. [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Young LJ & Wang Z (2009) Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc Natl Acad Sci U S A, 106(45), 19144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL & Bass AH (2000) Vasotocin innervation and modulation of vocal-acoustic circuitry in the teleost Porichthys notatus. J Comp Neurol, 422(3), 363–79. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Schrock SE, Klatt JD, Kabelik D & Kingsbury MA (2009) Mesotocin and nonapeptide receptors promote estrildid flocking behavior. Science, 325(5942), 862–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Wilson LC & Schrock SE (2012) To flock or fight: neurochemical signatures of divergent life histories in sparrows. Proc Natl Acad Sci U S A, 109 Suppl 1, 10685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood AK, Ardekani R, McCann SR, Dubin ME, Sullivan A, Bensussen S, Tavare S & Peichel CL (2015) Genetic mapping of natural variation in schooling tendency in the threespine stickleback. G3 (Bethesda), 5(5), 761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood AK, Mills MG, Wark AR, Archambeault SL & Peichel CL (2016) Evolution of Schooling Behavior in Threespine Sticklebacks Is Shaped by the Eda Gene. Genetics, 203(2), 677–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haesler S, Rochefort C, Georgi B, Licznerski P, Osten P & Scharff C (2007) Incomplete and inaccurate vocal imitation after knockdown of FoxP2 in songbird basal ganglia nucleus Area X. PLoS Biol, 5(12), e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haesler S, Wada K, Nshdejan A, Morrisey EE, Lints T, Jarvis ED & Scharff C (2004) FoxP2 expression in avian vocal learners and non-learners. J Neurosci, 24(13), 3164–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock EA & Young LJ (2004) Functional microsatellite polymorphism associated with divergent social structure in vole species. Mol Biol Evol, 21(6), 1057–63. [DOI] [PubMed] [Google Scholar]
- Hauser FE, Ilves KL, Schott RK, Castiglione GM, Lopez-Fernandez H & Chang BSW (2017) Accelerated Evolution and Functional Divergence of the Dim Light Visual Pigment Accompanies Cichlid Colonization of Central America. Mol Biol Evol, 34(10), 2650–2664. [DOI] [PubMed] [Google Scholar]
- He Z, Hou W, Hao X, Dong N, Du P, Yuan W, Yang J, Jia R & Tai F (2017) Oxytocin receptor antagonist treatments alter levels of attachment to mothers and central dopamine activity in pre-weaning mandarin vole pups. Psychoneuroendocrinology, 84, 124–134. [DOI] [PubMed] [Google Scholar]
- Holzman R & Hulsey CD (2017) Mechanical Transgressive Segregation and the Rapid Origin of Trophic Novelty. Sci Rep, 7, 40306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W, Kennedy A, Burgos-Artizzu XP, Zelikowsky M, Navonne SG, Perona P & Anderson DJ (2015) Automated measurement of mouse social behaviors using depth sensing, video tracking, and machine learning. Proc Natl Acad Sci U S A, 112(38), E5351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horga G, Maia TV, Marsh R, Hao X, Xu D, Duan Y, Tau GZ, Graniello B, Wang Z, Kangarlu A, Martinez D, Packard MG & Peterson BS (2015) Changes in corticostriatal connectivity during reinforcement learning in humans. Hum Brain Mapp, 36(2), 793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman LS, Hinz FI, Wojcik S, Aubin-Horth N & Hofmann HA (2015) Arginine vasotocin regulates social ascent in the African cichlid fish Astatotilapia burtoni. Gen Comp Endocrinol, 212, 106–13. [DOI] [PubMed] [Google Scholar]
- Hulsey CD (2009) Cichlid genomics and phenotypic diversity in a comparative context. Integr Comp Biol, 49(6), 618–29. [DOI] [PubMed] [Google Scholar]
- Husemann M, Tobler M, McCauley C, Ding B & Danley PD (2017) Body shape differences in a pair of closely related Malawi cichlids and their hybrids: Effects of genetic variation, phenotypic plasticity, and transgressive segregation. Ecol Evol, 7(12), 4336–4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR & Shapiro LE (1992) Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci U S A, 89(13), 5981–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Wang ZX & Ferris CF (1994) Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J Neurosci, 14(9), 5381–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED (2007) Neural systems for vocal learning in birds and humans: a synopsis. J Ornithol, 148(1), 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasarevic E, Bailey DH, Crossland JP, Dawson WD, Szalai G, Ellersieck MR, Rosenfeld CS & Geary DC (2013) Evolution of monogamy, paternal investment, and female life history in Peromyscus. J Comp Psychol, 127(1), 91–102. [DOI] [PubMed] [Google Scholar]
- Johnson ZV, Walum H, Jamal YA, Xiao Y, Keebaugh AC, Inoue K & Young LJ (2016) Central oxytocin receptors mediate mating-induced partner preferences and enhance correlated activation across forebrain nuclei in male prairie voles. Horm Behav, 79, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Walum H, Xiao Y, Riefkohl PC & Young LJ (2017) Oxytocin receptors modulate a social salience neural network in male prairie voles. Horm Behav, 87, 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV & Young LJ (2015) Neurobiological mechanisms of social attachment and pair bonding. Curr Opin Behav Sci, 3, 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV & Young LJ (2017) Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neurosci Biobehav Rev, 76(Pt A), 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juntti SA, Hilliard AT, Kent KR, Kumar A, Nguyen A, Jimenez MA, Loveland JL, Mourrain P & Fernald RD (2016) A Neural Basis for Control of Cichlid Female Reproductive Behavior by Prostaglandin F2alpha. Curr Biol, 26(7), 943–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keebaugh AC, Barrett CE, Laprairie JL, Jenkins JJ & Young LJ (2015) RNAi knockdown of oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Soc Neurosci, 10(5), 561–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd MR, Duftner N, Koblmuller S, Sturmbauer C & Hofmann HA (2012) Repeated parallel evolution of parental care strategies within Xenotilapia, a genus of cichlid fishes from Lake Tanganyika. PLoS One, 7(2), e31236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd MR, Kidd CE & Kocher TD (2006) Axes of differentiation in the bower-building cichlids of Lake Malawi. Mol Ecol, 15(2), 459–78. [DOI] [PubMed] [Google Scholar]
- King LB, Walum H, Inoue K, Eyrich NW & Young LJ (2016) Variation in the Oxytocin Receptor Gene Predicts Brain Region-Specific Expression and Social Attachment. Biol Psychiatry, 80(2), 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klett V & Meyer A (2002) What, if anything, is a Tilapia?-mitochondrial ND2 phylogeny of tilapiines and the evolution of parental care systems in the African cichlid fishes. Mol Biol Evol, 19(6), 865–83. [DOI] [PubMed] [Google Scholar]
- Kocher TD (2004) Adaptive evolution and explosive speciation: the cichlid fish model. Nat Rev Genet, 5(4), 288–98. [DOI] [PubMed] [Google Scholar]
- Kowalko JE, Rohner N, Rompani SB, Peterson BK, Linden TA, Yoshizawa M, Kay EH, Weber J, Hoekstra HE, Jeffery WR, Borowsky R & Tabin CJ (2013) Loss of schooling behavior in cavefish through sight-dependent and sight-independent mechanisms. Curr Biol, 23(19), 1874–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A (1929) The Progress of Physiology. Science, 70(1809), 200–4. [DOI] [PubMed] [Google Scholar]
- Kusmierski R, Borgia G, Uy A & Crozier RH (1997) Labile evolution of display traits in bowerbirds indicates reduced effects of phylogenetic constraint. Proc Biol Sci, 264(1380), 307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B-Y, Lee W-J, Streelman JT, Carleton KL, Howe AE, Hulata G, Slettan A, Stern JE, Terai Y & Kocher TD (2005) A Second-Generation Genetic Linkage Map of Tilapia (Oreochromis spp.). Genetics, 170(1), 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Liu Y, Ryabinin AE, Bai Y, Wang Z & Young LJ (2007) CRF receptors in the nucleus accumbens modulate partner preference in prairie voles. Horm Behav, 51(4), 508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF & Young LJ (2004) Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature, 429(6993), 754–7. [DOI] [PubMed] [Google Scholar]
- Lim MM & Young LJ (2004) Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience, 125(1), 35–45. [DOI] [PubMed] [Google Scholar]
- Liu H, Robinson GE & Jakobsson E (2016) Conservation in Mammals of Genes Associated with Aggression-Related Behavioral Phenotypes in Honey Bees. PLoS Comput Biol, 12(6), e1004921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Curtis JT & Wang Z (2001) Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster). Behav Neurosci, 115(4), 910–9. [DOI] [PubMed] [Google Scholar]
- Liu Y & Wang ZX (2003) Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience, 121(3), 537–44. [DOI] [PubMed] [Google Scholar]
- Loh Y-HE, Katz LS, Mims MC, Kocher TD, Yi SV & Streelman JT (2008) Comparative analysis reveals signatures of differentiation amid genomic polymorphism in Lake Malawi cichlids. Genome Biology, 9(7), R113–R113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh YH, Bezault E, Muenzel FM, Roberts RB, Swofford R, Barluenga M, Kidd CE, Howe AE, Di Palma F, Lindblad-Toh K, Hey J, Seehausen O, Salzburger W, Kocher TD & Streelman JT (2013) Origins of shared genetic variation in African cichlids. Mol Biol Evol, 30(4), 906–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoParo D & Waldman ID (2015) The oxytocin receptor gene (OXTR) is associated with autism spectrum disorder: a meta-analysis. Mol Psychiatry, 20(5), 640–6. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M & Dreifuss JJ (1991) Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Res, 555(2), 220–32. [DOI] [PubMed] [Google Scholar]
- Lukas D & Clutton-Brock TH (2013) The evolution of social monogamy in mammals. Science, 341(6145), 526–30. [DOI] [PubMed] [Google Scholar]
- Margulis SW & Altmann J (1997) Behavioural risk factors in the reproduction of inbred and outbred oldfield mice. Anim Behav, 54(2), 397–408. [DOI] [PubMed] [Google Scholar]
- Marquand AF, Haak KV & Beckmann CF (2017) Functional corticostriatal connection topographies predict goal directed behaviour in humans. Nat Hum Behav, 1(8), 0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C & Genner M (2009) A role for male bower size as an intrasexual signal in a Lake Malawi cichlid fish. Behaviour, 146(7), 963–978. [Google Scholar]
- Maruska KP & Fernald RD (2010) Behavioral and physiological plasticity: rapid changes during social ascent in an African cichlid fish. Horm Behav, 58(2), 230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruska KP & Fernald RD (2013) Social regulation of male reproductive plasticity in an African cichlid fish. Integr Comp Biol, 53(6), 938–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruska KP, Zhang A, Neboori A & Fernald RD (2013) Social opportunity causes rapid transcriptional changes in the social behaviour network of the brain in an African cichlid fish. J Neuroendocrinol, 25(2), 145–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw LA & Young LJ (2010) The prairie vole: an emerging model organism for understanding the social brain. Trends Neurosci, 33(2), 103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKaye K, Stauffer J, Turner G, Konings A & Sato T (2001) Fishes, as well as birds, build bowers. Journal of Aquariculture and Aquatic Sciences, 9, 121–133. [Google Scholar]
- McKaye KR, Louda SM & Jay R Stauffer J (1990) Bower Size and Male Reproductive Success in a Cichlid Fish Lek. The American Naturalist, 135(5), 597–613. [Google Scholar]
- Metz HC, Bedford NL, Pan YL & Hoekstra HE (2017) Evolution and Genetics of Precocious Burrowing Behavior in Peromyscus Mice. Current Biology, 27(24), 3837–+. [DOI] [PubMed] [Google Scholar]
- Morita M, Awata S, Yorifuji M, Ota K, Kohda M & Ochi H (2014) Bower-building behaviour is associated with increased sperm longevity in Tanganyikan cichlids. J Evol Biol, 27(12), 2629–43. [DOI] [PubMed] [Google Scholar]
- Nandamuri SP, Dalton BE & Carleton KL (2017) Determination of the Genetic Architecture Underlying Short Wavelength Sensitivity in Lake Malawi Cichlids. J Hered, 108(4), 379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nescent Working Group on Integrative Models of Vertebrate Sociality: Evolution M, Emergent P, Hofmann HA, Beery AK, Blumstein DT, Couzin ID, Earley RL, Hayes LD, Hurd PL, Lacey EA, Phelps SM, Solomon NG, Taborsky M, Young LJ & Rubenstein DR (2014) An evolutionary framework for studying mechanisms of social behavior. Trends Ecol Evol, 29(10), 581–9. [DOI] [PubMed] [Google Scholar]
- Newman SW (1999) The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci, 877, 242–57. [DOI] [PubMed] [Google Scholar]
- Norton WHJ (2013) Toward developmental models of psychiatric disorders in zebrafish. Frontiers in Neural Circuits, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak MA, Tarnita CE & Wilson EO (2010) The evolution of eusociality. Nature, 466(7310), 1057–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki J, O’Connell L, Cowman PF, Walker S, Coker D & Pratchett M (2017a) Butterflyfishes as a System for Investigating Pair Bonding. bioRxiv. [Google Scholar]
- Nowicki J, Pratchett M, Walker S, Coker D & O’Connell LA (2017b) Neurobiology of pair bonding in fishes; convergence of neural mechanisms across distant vertebrate lineages. bioRxiv. [Google Scholar]
- Numan M & Young LJ (2016) Neural mechanisms of mother-infant bonding and pair bonding: Similarities, differences, and broader implications. Horm Behav, 77, 98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell LA & Hofmann HA (2011) The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol, 519(18), 3599–639. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Matthews BJ & Hofmann HA (2012) Isotocin regulates paternal care in a monogamous cichlid fish. Horm Behav, 61(5), 725–33. [DOI] [PubMed] [Google Scholar]
- O’Quin CT, Drilea AC, Roberts RB & Kocher TD (2012) A small number of genes underlie male pigmentation traits in Lake Malawi cichlid fishes. J Exp Zool B Mol Dev Evol, 318(3), 199–208. [DOI] [PubMed] [Google Scholar]
- Okhovat M, Berrio A, Wallace G, Ophir AG & Phelps SM (2015) Sexual fidelity trade-offs promote regulatory variation in the prairie vole brain. Science, 350(6266), 1371–4. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Pereira M, Agrati D, Ferreira A, Fleming AS, Gonzalez-Mariscal G, Levy F, Lucion AB, Morrell JI, Numan M & Uriarte N (2013a) Flexibility and adaptation of the neural substrate that supports maternal behavior in mammals. Neurosci Biobehav Rev, 37(8), 1875–92. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Pereira M, Agrati D, Ferreira A, Fleming AS, Gonzalez-Mariscal G, Levy F, Lucion AB, Morrell JI, Numan M & Uriarte N (2013b) New theoretical and experimental approaches on maternal motivation in mammals. Neurosci Biobehav Rev, 37(8), 1860–74. [DOI] [PubMed] [Google Scholar]
- Oldfield RG & Hofmann HA (2011) Neuropeptide regulation of social behavior in a monogamous cichlid fish. Physiol Behav, 102(3–4), 296–303. [DOI] [PubMed] [Google Scholar]
- Opie C, Atkinson QD, Dunbar RI & Shultz S (2013) Male infanticide leads to social monogamy in primates. Proc Natl Acad Sci U S A, 110(33), 13328–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens GL & Rennison DJ (2017) Evolutionary ecology of opsin gene sequence, expression and repertoire. Mol Ecol, 26(5), 1207–1210. [DOI] [PubMed] [Google Scholar]
- Parnell NF, Hulsey CD & Streelman JT (2008) Hybridization produces novelty when the mapping of form to function is many to one. BMC Evol Biol, 8, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell NF, Hulsey CD & Streelman JT (2012) The genetic basis of a complex functional system. Evolution, 66(11), 3352–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons KJ, Trent Taylor A, Powder KE & Albertson RC (2014) Wnt signalling underlies the evolution of new phenotypes and craniofacial variability in Lake Malawi cichlids. Nat Commun, 5, 3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons KJ, Wang J, Anderson G & Albertson RC (2015) Nested Levels of Adaptive Divergence: The Genetic Basis of Craniofacial Divergence and Ecological Sexual Dimorphism. G3 (Bethesda), 5(8), 1613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfenning AR, Hara E, Whitney O, Rivas MV, Wang R, Roulhac PL, Howard JT, Wirthlin M, Lovell PV, Ganapathy G, Mouncastle J, Moseley MA, Thompson JW, Soderblom EJ, Iriki A, Kato M, Gilbert MT, Zhang G, Bakken T, Bongaarts A, Bernard A, Lein E, Mello CV, Hartemink AJ & Jarvis ED (2014) Convergent transcriptional specializations in the brains of humans and song-learning birds. Science, 346(6215), 1256846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendon NM, Amez AC, Proffitt MR, Bauserman ER & Demas GE (2017) Aggressive behaviours track transitions in seasonal phenotypes of female Siberian hamsters. Funct Ecol, 31(5), 1071–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn SC, O’Rourke CF, Aubin-Horth N, Fraser EJ & Hofmann HA (2016) Dissecting the Transcriptional Patterns of Social Dominance across Teleosts. Integr Comp Biol, 56(6), 1250–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn SCP, Machado HE, Duftner N, Sessa AK, Harris RM & Hofmann HA (2017) Gene expression signatures of mating system evolution. Genome, 1–11. [DOI] [PubMed] [Google Scholar]
- Reuter M, Montag C, Altmann S, Bendlow F, Elger C, Kirsch P, Becker A, Schoch-McGovern S, Simon M, Weber B & Felten A (2017) Functional characterization of an oxytocin receptor gene variant (rs2268498) previously associated with social cognition by expression analysis in vitro and in human brain biopsy. Soc Neurosci, 12(5), 604–611. [DOI] [PubMed] [Google Scholar]
- Reynolds JD, Goodwin NB & Freckleton RP (2002) Evolutionary transitions in parental care and live bearing in vertebrates. Philos Trans R Soc Lond B Biol Sci, 357(1419), 269–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittschof CC, Bukhari SA, Sloofman LG, Troy JM, Caetano-Anolles D, Cash-Ahmed A, Kent M, Lu X, Sanogo YO, Weisner PA, Zhang H, Bell AM, Ma J, Sinha S, Robinson GE & Stubbs L (2014) Neuromolecular responses to social challenge: common mechanisms across mouse, stickleback fish, and honey bee. Proc Natl Acad Sci U S A, 111(50), 17929–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittschof CC & Robinson GE (2016) Behavioral Genetic Toolkits: Toward the Evolutionary Origins of Complex Phenotypes. Curr Top Dev Biol, 119, 157–204. [DOI] [PubMed] [Google Scholar]
- Roberts RB, Moore EC & Kocher TD (2017) An allelic series at pax7a is associated with colour polymorphism diversity in Lake Malawi cichlid fish. Mol Ecol, 26(10), 2625–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GE (2002) Genomics and integrative analyses of division of labor in honeybee colonies. Am Nat, 160 Suppl 6, S160–72. [DOI] [PubMed] [Google Scholar]
- Roland AB & O’Connell LA (2015) Poison frogs as a model system for studying the neurobiology of parental care. Current Opinion in Behavioral Sciences, 6(Supplement C), 76–81. [Google Scholar]
- Sanogo YO, Hankison S, Band M, Obregon A & Bell AM (2011) Brain transcriptomic response of threespine sticklebacks to cues of a predator. Brain Behav Evol, 77(4), 270–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selz OM, Lucek K, Young KA & Seehausen O (2014) Relaxed trait covariance in interspecific cichlid hybrids predicts morphological diversity in adaptive radiations. J Evol Biol, 27(1), 11–24. [DOI] [PubMed] [Google Scholar]
- Skuse DH, Lori A, Cubells JF, Lee I, Conneely KN, Puura K, Lehtimaki T, Binder EB & Young LJ (2014) Common polymorphism in the oxytocin receptor gene (OXTR) is associated with human social recognition skills. Proc Natl Acad Sci U S A, 111(5), 1987–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma KK, Scotti MA, Newman AE, Charlier TD & Demas GE (2008) Novel mechanisms for neuroendocrine regulation of aggression. Front Neuroendocrinol, 29(4), 476–89. [DOI] [PubMed] [Google Scholar]
- Song Z, McCann KE, McNeill J. K. t., Larkin TE 2nd, Huhman KL & Albers HE (2014) Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology, 50, 14–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady TC, Seehausen O, Loew ER, Jordan RC, Kocher TD & Carleton KL (2005) Adaptive Molecular Evolution in the Opsin Genes of Rapidly Speciating Cichlid Species. Molecular Biology and Evolution, 22(6), 1412–1422. [DOI] [PubMed] [Google Scholar]
- Streelman JT, Albertson RC & Kocher TD (2003) Genome mapping of the orange blotch colour pattern in cichlid fishes. Molecular Ecology, 12(9), 2465–2471. [DOI] [PubMed] [Google Scholar]
- Suzuki S (2013) Biparental care in insects: paternal care, life history, and the function of the nest. J Insect Sci, 13, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson O, Egger B, Gricar B, Woodhouse K, van Oosterhout C, Salzburger W, Seehausen O & Turner GF (2011) Segregation of species-specific male attractiveness in f(2) hybrid lake Malawi cichlid fish. Int J Evol Biol, 2011, 426179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester JB, Rich CA, Yi C, Peres JN, Houart C & Streelman JT (2013) Competing signals drive telencephalon diversity. Nat Commun, 4, 1745. [DOI] [PubMed] [Google Scholar]
- Thorne BL (1997) Evolution of Eusociality in Termites. Annual Review of Ecology and Systematics, 28(1), 27–54. [Google Scholar]
- Toth AL & Rehan SM (2017) Molecular Evolution of Insect Sociality: An Eco-Evo-Devo Perspective. Annual Review of Entomology, 62(1), 419–442. [DOI] [PubMed] [Google Scholar]
- Toth AL, Varala K, Henshaw MT, Rodriguez-Zas SL, Hudson ME & Robinson GE (2010) Brain transcriptomic analysis in paper wasps identifies genes associated with behaviour across social insect lineages. Proc Biol Sci, 277(1691), 2139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth AL, Varala K, Newman TC, Miguez FE, Hutchison SK, Willoughby DA, Simons JF, Egholm M, Hunt JH, Hudson ME & Robinson GE (2007) Wasp gene expression supports an evolutionary link between maternal behavior and eusociality. Science, 318(5849), 441–4. [DOI] [PubMed] [Google Scholar]
- Turner LM, Young AR, Rompler H, Schoneberg T, Phelps SM & Hoekstra HE (2010) Monogamy evolves through multiple mechanisms: evidence from V1aR in deer mice. Mol Biol Evol, 27(6), 1269–78. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS & Tomasi D (2012) Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol, 52, 321–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, Lichtenstein P, Neiderhiser JM, Reiss D, Ganiban JM, Spotts EL, Pedersen NL, Anckarsater H, Larsson H & Westberg L (2012) Variation in the oxytocin receptor gene is associated with pair-bonding and social behavior. Biol Psychiatry, 71(5), 419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, Westberg L, Henningsson S, Neiderhiser JM, Reiss D, Igl W, Ganiban JM, Spotts EL, Pedersen NL, Eriksson E & Lichtenstein P (2008) Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proc Natl Acad Sci U S A, 105(37), 14153–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Duclot F, Liu Y, Wang Z & Kabbaj M (2013) Histone deacetylase inhibitors facilitate partner preference formation in female prairie voles. Nat Neurosci, 16(7), 919–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JN, Peterson BK & Hoekstra HE (2013) Discrete genetic modules are responsible for complex burrow evolution in Peromyscus mice. Nature, 493(7432), 402–U145. [DOI] [PubMed] [Google Scholar]
- Wen JC, Hotchkiss AK, Demas GE & Nelson RJ (2004) Photoperiod affects neuronal nitric oxide synthase and aggressive behaviour in male Siberian hamsters (Phodopus sungorus). J Neuroendocrinol, 16(11), 916–21. [DOI] [PubMed] [Google Scholar]
- West SA, Diggle SP, Buckling A, Gardner A & Griffin AS (2007) The Social Lives of Microbes. Annual Review of Ecology, Evolution, and Systematics, 38(1), 53–77. [Google Scholar]
- Whiteman EA & Cote IM (2004) Monogamy in marine fishes. Biol Rev Camb Philos Soc, 79(2), 351–75. [DOI] [PubMed] [Google Scholar]
- Wilson EO (2000) Sociobiology Harvard University Press. [Google Scholar]
- Yartsev MM (2017) The emperor’s new wardrobe: Rebalancing diversity of animal models in neuroscience research. Science, 358(6362), 466–469. [DOI] [PubMed] [Google Scholar]
- York RA & Fernald RD (2017) The Repeated Evolution of Behavior. Frontiers in Ecology and Evolution, 4(143). [Google Scholar]
- York RA, Patil C, Hulsey CD, Anoruo O, Streelman JT & Fernald RD (2015) Evolution of bower building in Lake Malawi cichlid fish: phylogeny, morphology, and behavior. Frontiers in Ecology and Evolution, 3(18). [Google Scholar]
- Young KA, Genner MJ, Joyce DA & Haesler MP (2009) Hotshots, hot spots, and female preference: exploring lek formation models with a bower-building cichlid fish. Behavioral Ecology, 20(3), 609–615. [Google Scholar]
- Young LJ (1999) Frank A. Beach Award. Oxytocin and vasopressin receptors and species-typical social behaviors. Horm Behav, 36(3), 212–21. [DOI] [PubMed] [Google Scholar]
- Young LJ, Huot B, Nilsen R, Wang Z & Insel TR (1996) Species differences in central oxytocin receptor gene expression: comparative analysis of promoter sequences. J Neuroendocrinol, 8(10), 777–83. [DOI] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B & Insel TR (2001) Cellular mechanisms of social attachment. Horm Behav, 40(2), 133–8. [DOI] [PubMed] [Google Scholar]
- Young LJ, Nilsen R, Waymire KG, MacGregor GR & Insel TR (1999) Increased affiliative response to vasopressin in mice expressing the V1a receptor from a monogamous vole. Nature, 400(6746), 766–8. [DOI] [PubMed] [Google Scholar]
- Young LJ & Wang Z (2004) The neurobiology of pair bonding. Nat Neurosci, 7(10), 1048–54. [DOI] [PubMed] [Google Scholar]
- Yu P, An S, Tai F, Wang J, Wu R & Wang B (2013) Early social deprivation impairs pair bonding and alters serum corticosterone and the NAcc dopamine system in mandarin voles. Psychoneuroendocrinology, 38(12), 3128–38. [DOI] [PubMed] [Google Scholar]