Abstract
Objective
To evaluate the correlation of aging and upper airway collapse characteristics observed by drug-induced sleep endoscopy (DISE), and report the observed differences on obstructive sleep apnea (OSA) subjects, older and younger than 60 years-old.
Methods
Case series study that analyzed the data of 200 OSA patients who underwent DISE between January 1, 2013 and June 30, 2017. The variables sex, body mass index (BMI), Epworth Sleepiness Scale (ESS) score, tonsil size, modified Mallampati (MM) classification, apnea-hypopnea index (AHI), oxygen desaturation index (ODI), lowest oxygen saturation (LSAT) and VOTE classification were compared between two groups: <60 and ≥60yr.
Results
Older age had significant correlation with higher AHI, ODI, lower O2 nadir, multi-sites obstruction, combined upper (palatopharyngeal) + lower (hypopharyngeal) level obstructions, and complete, anterior-posterior (AP) velum collapse pattern. Lateral oropharyngeal wall collapse was significantly lower in the older group. Findings remained statistically significant when adjusted for sex, BMI, tonsil size and MM.
Conclusion
Aging was an independent factor that directly correlated with increased AHI and hypoxemia, multi-sites, combined levels of obstruction, complete AP velum collapse pattern. Being older than 60 years-old had higher incidence of complete AP velum collapse and lower of lateral oropharyngeal wall collapse, regardless of OSA severity and tonsil size.
Keywords: Age, drug-induced sedation endoscopy, sleep endoscopy, obstructive sleep apnea, upper airway
Introduction
Obstructive sleep apnea (OSA) is the most common sleep-related breathing disorder in general population and its prevalence has been suggested to be a mean of 22% in men and 17% in women based on review of epidemiological studies.1 Considering the aspect of a global aging population, the impact of OSA in older adults has become relevant.2 Increased OSA prevalence has been reported in subjects over 60 years old (yr.),3,4 and increased prevalence of 2.2x per 10 years of age has been described in subjects from 30 to 70 yr.5 Studies showed that the prevalence in community-dwelling population sub-groups aged 39–49, 50–59 and 60–70 increased by 30%, 40% and 51%, respectively.4 Increased severity of OSA was also correlated with aging in over 65 yr. group.6 Among hospitalized older patients, the prevalence increased from 1.47% in 2006 to 5.01% in 2012 in U.S.7 Older patients are more likely to have comorbid chronic conditions,8 such as cardiovascular disease9,10 and chronic obstructive pulmonary disease,11 that can potentially worsen OSA and heighten overall mortality. OSA has been also described as an independent risk factor for chronic kidney disease,12 stroke,13 and associated with impaired cognitive function14 and higher risk of dementia15 in older adults.
Previous studies demonstrated that older subjects were predisposed to severe OSA due to an increasing collapsibility of the upper airway, based on indirect assessment: continuous positive airway pressure (CPAP),16 nasal mask pressure measurements,17 acoustic reflection,18 electroneuromyography (EMG),19 and magnetic resonance imaging (MRI).20
To our knowledge, a detailed description about age-related changes in collapse pattern and/or degrees of obstruction in drug induced sleep endoscopy (DISE) is lacking. DISE is a direct, dynamic and low cost evaluation,21 useful for identifying obstruction sites and patterns in patients with OSA, and may be useful in directing surgical plan.22,23 It has increased in popularity and been utilized worldwide.24 The objective of this study was to evaluate the association between age and upper airway collapse characteristics during sedated endoscopy in patients with OSA.
Materials and Methods
Study Design
Retrospective review of 200 OSA patients who underwent DISE at Stanford Sleep Surgery Clinic between January 1, 2013 and June 30, 2017. Patients who underwent upper airway procedures for sleep disordered breathing including uvulopalatopharyngoplasty (UPPP), uvulo-palatal flap, genial tubercle advancement, tongue base reduction, maxillo-mandibular advancement or orthognathic surgeries were excluded. The following variables were extracted from Stanford Hospitals and Clinic REDCap25 (Research Electronic Data Capture) and EPIC databases: age, sex, body mass index (BMI), Epworth Sleepiness Scale (ESS) score, tonsil size, modified Mallampati (MM), apnea-hypopnea index (AHI), oxygen desaturation index (ODI), lowest oxygen saturation (LSAT), and DISE findings described by VOTE classification26. Patients were divided into two groups: younger than 60 years-old (< 60yr.) and 60 years-old or older (≥ 60yr.), baseline characteristics and DISE findings were compared. Meanwhile the associations between age and the above-mentioned variables were analyzed in all subjects. This project was approved by the Institutional Review Board and Hospital Research Ethics Committee of Stanford Hospital and Clinics (protocol 35054).
Sleep Studies
Only patients who underwent an overnight in-laboratory polysomnography (PSG) or a home sleep test (HST) that reported all assessed respiratory and oximetry variables were included. Respiratory variables were scored in accordance to the guidelines of the American Academy of Sleep Medicine 2012 edition.27 Apnea was identified when the amplitude of the airflow was decreased by at least 90% for longer than 10 seconds. Hypopnea was identified when there was a 30% reduction in the airflow amplitude for at least 10 seconds, associated with oxygen desaturation ≥ 3% or an arousal. OSA was classified based on the AHI as follows: mild (≥ 5 and < 15 events/hour), moderate (≥ 15 and < 30 events/hour) or severe (≥ 30 events/hour). The ODI represented the number of events of oxygen desaturation ≥ 3% per hour.
DISE Protocol
DISE was performed in the operating room in supine position. Topical nasal decongestion with the use of oxymetazoline and topical lidocaine gel was applied to the nasal valve area to decrease any discomfort caused by the endoscope introduction Dexmedetomidine was the sedative agent, administered with an IV bolus at 1.5 mcg/kg over 10 minutes, followed by a maintenance infusion rate of 1.5 mcg/kg/h. An Olympus® ear, nose, and throat flexible endoscope with a 3.2mm diameter was inserted into the nose, starting from nasopharynx to hypopharynx and larynx. The assessment of the upper airway obstruction during DISE was performed after the first cycle of snoring and obstruction had been completed. The cycle is here defined as a complete and stable sequence of snoring-obstructed breathing, or desaturations. At least 2 cycles of obstructed breathing were observed for each subsite, as recommended by the European position paper on DISE.24 Patients were evaluated in the drug-induced sleep state for approximately 15 to 20 minutes. The grade and patterns of upper airway collapse were recorded with the VOTE.26 All evaluations were performed and classified exclusively by a senior and experienced surgeon (R.C).
Statistical Analysis
Continuous variables were summarized with mean ± standard deviation as well as median (interquartile range) due to lack of normality in distribution in some variables (AHI, ODI, LSAT, ESS). Categorical variables were summarized with frequencies and percentages. Continuous variables were compared using Student t-test if normally distributed or Wilcoxon rank sum test otherwise. Categorical variables were compared using Fisher’s exact test or Chi-square test unless the categories were ordered, in which case Wilcoxon rank-sum test was used. Spearman correlation was analyzed between age and the outcome variables due to lack of normality in distribution. Linear regression model was used to analyze the association between age and AHI, ODI, LSAT, ESS. Various transformations of continuous outcome variables including log transformation were explored through Box-Cox transformation method. Logistical regression model was used to analyze the association between age and single/multiple obstructive sites; isolated /combined obstructive levels; non (0) and partial (1) / complete (2) collapse pattern in differential obstructive sites. All the models were presented with no adjustment and with adjustment for gender, BMI, tonsil size and MM. All the analyses were performed by SAS 9.4 (SAS Inc, Cary, NC, USA). P <0.05 was considered statistically significant.
Results
The average age was 48.1 yr. (standard deviation = ±15.3), range from 18 to 81yr. Further, there were 29 subjects in < 30 years-old subgroup, 33 in 30–39 yr., 42 in 40–49 yr., 44 in 50–59 yr., and 52 in ≥60yr. BMI, AHI, ODI and LSAT were, 27.9 kg/m2 (±4.6), 36.8 events/hr. (±21.2), 24.8 events/hr. (± 21.9) and 83.4% (± 8.2), respectively. There were significant differences between the <60 and ≥60 groups in baseline AHI, ODI, LSAT, tonsil size score and MM score (Table 1). No significant differences were found in subjects with severe OSA (AHI ≥ 30) and small tonsils (size= 0, 1 and 2).
Table 1.
Baseline Characteristics between groups
| Characteristics | OSA | P-value | ||
|---|---|---|---|---|
| < 60 yr. (n=148) |
≥ 60 yr. (n=52) |
|||
| Sex† | ||||
| Male | 122 (82.4%) | 41 (78.8%) | ||
| Female | 26 (17.6%) | 11 (21.2%) | 0.567* | |
| Age‡ | 41.2±11.1 (148) | 67.7±5.5 (52) | <.0001** | |
| BMI, kg/m2‡ | 28±4.8 (148) | 27.6±3.6 (52) | 0.657** | |
| ESS‡ | 9.8±5.2 (140) | 9.5±4.9 (51) | 0.676*** | |
| Tonsil size† | ||||
| 0 | 15 (10.1%) | 23 (44.2%) | ||
| 1 | 72 (48.6%) | 29 (55.8%) | ||
| 2 | 46 (31.1%) | 0 | ||
| 3 | 13 (8.8%) | 0 | ||
| 4 | 2 (1.4%) | 0 | <.0001**** | |
| M. Mallampati† | ||||
| 1 | 21 (14.2%) | 2 (3.8%) | ||
| 2 | 29 (19.6%) | 16 (30.8%) | ||
| 3 | 65 (43.9%) | 28 (53.8%) | ||
| 4 | 33 (22.3%) | 6 (11.5%) | 0.028**** | |
| AHI, events/hr.‡ | 34±20.9 (148) | 44.9±20 (52) | 0.001*** | |
| ODI, events/hr.‡ | 22±22.4 (99) | 36.2±15.7 (24) | <.0001*** | |
| LSAT, %‡ | 84.5±8.1 (147) | 80.4±8 (49) | <.0001*** | |
BMI, body mass index; ESS, Epwort Sleepiness Scale; AHI, apnea hypopnea index; ODI, oxygen desaturation index; LSAT, lowest oxygen saturation. Statistical analysis:
Pearson Chi-square test;
student t-test;
Wilcoxon rank-sum test;
Fisher exact test. Values are statistically significant at P < 0.05.
n (%);
Mean ± Standard Deviation (n).
Age had a significant positive correlation with AHI and ODI, negative correlation with LSAT and tonsil size (Figure 1), and no correlation with BMI, ESS, or MM (P >0.05). Increase in age had significant association with observed collapse in multiple sites as well, and combined obstruction levels in unadjusted and adjusted linear regression model and logistic regression models, respectively (Table 2). No significant association was found between age and ESS.
Figure 1. Correlation between age and AHI, ODI, LSAT and tonsil size.
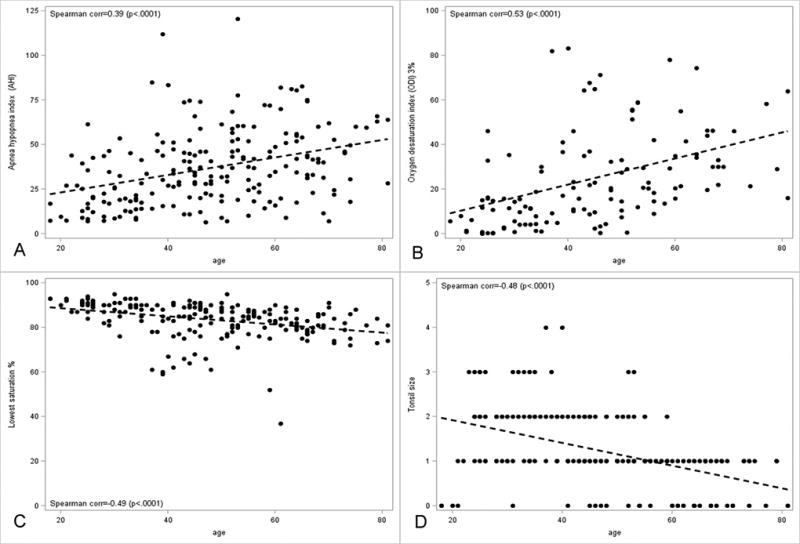
Age had a significant positive correlation with AHI (Figure 1A, Corr=0.39, P< 0.001 and ODI (Figure 1B, Corr=0.53, P< 0.001), negative correlation with lowest saturation (Figure 1C, Corr=∙0.49, P< 0.001) and tonsil size (Figure 1D, Corr=−0.48, P< 0.001). Statistically significant as P < 0.05.
Table 2.
Association between age (per year increase) and sleep study outcomes and obstruction levels
| Dependent Variables | Unadjusted % change | P-value | Adjusted % change | P-value |
|---|---|---|---|---|
| AHI | 1.7% (1.1%, 2.2%) | <.0001 | 1.7% (1.1%, 2.3%) | <.0001 |
| ODI | 4.6% (3.2%, 6.1%) | <.0001 | 4.1% (2.6%, 5.6%) | <.0001 |
| LSAT | −0.2% (−0.3%, −0.1%) | <.0001 | −0.2% (−0.3%, −0.1%) | <.0001 |
| ESS | −0.2% (−1.1%, 0.8%) | 0.723 | 0% (−1.1%, 1.1%) | 0.988 |
|
| ||||
| Unadjusted OR | P-value | Adjusted OR | P-value | |
| Multiple sites | 1.08 (1.03–1.13) | 0.002 | 1.07(1.01–1.12) | 0.013 |
| Combined levels | 1.04 (1.02–1.07) | 0.001 | 1.03 (1.00–1.06) | 0.025 |
AHI, apnea hypopnea index; ODI, oxygen desaturation index; LSAT, lowest oxygen saturation; ESS, Epworth sleepiness scale; Multiple sites, sum of obstructive sties ≥ 2; Combined levels, obstruction occurred in upper level (velum and/or oropharynx) and lower level (tongue base and/or epiglottis). OR adjusted for sex, body mass index, tonsil size and modified Mallampati. Statistically significant as P < 0.05.
Only 6.5% of the patients presented a single site of obstruction, and the remaining presented obstruction in multiple sites: 2 sites (29%), 3 (48.5%) and 4 (15.5%). Figure 2 shown no significant difference in number of obstruction sites between ≥ 60 yr. and < 60 yr. groups, however, combined upper and lower levels obstruction in the ≥ 60 yr. group were significantly higher than < 60 yr. group.
Figure 2. Obstructive sites and levels in groups.
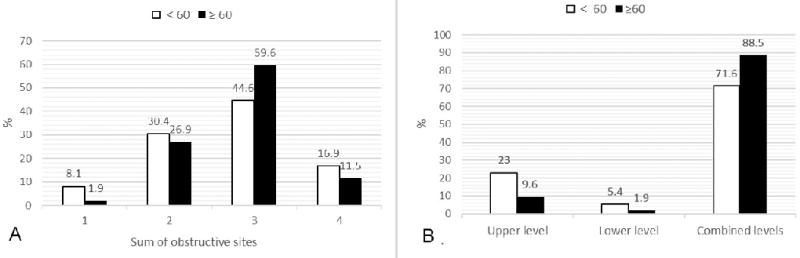
Figure 2A showed that there was no significant difference in single/multiple sites obstruction between groups (P= 0.468). Figure 2B showed combined upper and lower levels obstruction in the ≥ 60 yr. group were significantly higher than < 60 yr. group (P= 0.046). Upper level: obstruction in velum and/or oropharynx (V/O); Lower level: obstruction in tongue base and/or epiglottis (T/E); Combined levels: obstruction in both upper and lower levels (V/O + T/E). Statistically significant as P < 0.05.
As shown in Table 3, increase in age had a significant association with velum complete anterior-posterior collapse and tongue complete collapse. After adjusting for sex, BMI, tonsil size and MM, tongue complete collapse lost significance.
Table 3.
Association between age and complete collapse pattern in different sites
| Site | Pattern | OR unadjusted | P value | OR adjusted | P value |
|---|---|---|---|---|---|
| Velum | AP | 1.05(1.03–1.07) | 0.000 | 1.04(1.02–1.07) | 0.001 |
| L | 0.95(0.90–1.01) | 0.108 | 0.90(0.80–1.01) | 0.061 | |
| C | 1.00(0.98–1.02) | 0.999 | 1.01(0.98–1.03) | 0.634 | |
| Oropharynx | L | 0.99(0.97–1.01) | 0.265 | 0.99(0.97–1.02) | 0.581 |
| Tongue Base | AP | 1.02(1.00–1.04) | 0.037 | 1.01(0.99–1.03) | 0.352 |
| Epiglottis | AP | 1.01(0.98–1.03) | 0.630 | 1.01(0.98–1.03) | 0.682 |
| L | 1.01(0.99–1.03) | 0.789 | 1.00(0.99–1.02) | 0.999 |
AP, anterior-posterior; L, lateral; C, concentric. OR adjusted for sex, body mass index, tonsil size and modified Mallampati. Statistically significant as P < 0.05.
Collapse pattern and degree according to the VOTE classification in both groups was shown in Table 4. The proportion of velum complete AP collapse was higher (69.2%) and oropharynx complete lateral collapse was lower (23.1%) in the ≥ 60 yr. group (39.2% and 41.2%, respectively) (Table 5), despite significant difference in baseline characteristics between the two groups. Nevertheless, there was still significance (65.9% Vs 39.7%, P = 0.01 and 24.4% Vs 51.5%, P = 0.009, respectively) when analysis was limited to subjects with severe OSA and small or absent tonsils (0, 1 and 2) and without significance in baseline between groups.
Table 4.
Collapse pattern and obstruction degree between groups
| Site /Pattern/ Degree | OSA n (%) | P-value | ||
|---|---|---|---|---|
| < 60 yr. (n=148) |
≥ 60 yr. (n=52) |
|||
| Velum | ||||
| AP | 0 | 9 (6.1%) | 1 (1.9%) | |
| 1 | 16 (10.8%) | 0 (0%) | ||
| 2 | 58 (39.2%) | 36 (69.2%) | ||
| L | 0 | 0 (0%) | 0 (0%) | |
| 1 | 2 (1.4%) | 0 (0%) | ||
| 2 | 6 (4.1%) | 0 (0%) | ||
| C | 0 | 1 (0.7%) | 0 (0%) | |
| 1 | 9 (6.1%) | 0 (0%) | ||
| 2 | 47 (31.8%) | 15 (28.8%) | 0.002* | |
| Oropharynx | ||||
| L | 0 | 40(27%) | 17 (32.7%) | |
| 1 | 47(31.8%) | 23 (44.2%) | ||
| 2 | 61(41.2%) | 12 (23.1%) | 0.058** | |
| Tongue base | ||||
| AP | 0 | 42(28.4%) | 10 (19.2%) | |
| 1 | 63(42.6%) | 20 (38.5%) | ||
| 2 | 43(29%) | 22 (42.3%) | 0.066** | |
| Epiglottis | ||||
| AP | 0 | 98(66.2%) | 34(65.4%) | |
| 1 | 14(9.5%) | 4 (7.7%) | ||
| 2 | 32(21.6%) | 13 (25%) | ||
| L | 0 | 0(0%) | 0(0%) | |
| 1 | 2 (1.4%) | 0 (0%) | ||
| 2 | 2(1.4%) | 1 (1.9%) | 0.962* | |
AP, anterior-posterior; L, lateral; C, concentric;
statistical analysis by Fisher exact test;
Wilcoxon rank-sum test.
Statistically significant as P < 0.05.
Table 5.
Collapse pattern with complete obstruction between groups
| Site | Pattern/ Degree | OSA n (%) | P-value* | ||
|---|---|---|---|---|---|
| < 60 yr. (n=148) |
≥ 60 yr. (n=52) |
||||
| Velum | Anterior-posterior | ||||
| 0+1 | 90 (60.8%) | 16 (30.8%) | |||
| 2 | 58 (39.2%) | 36 (69.2%) | <.0001 | ||
| Lateral | |||||
| 0+1 | 142(95.9%) | 52 (100%) | |||
| 2 | 6(4.1%) | 0 (0%) | 0.342 | ||
| Concentric | |||||
| 0+1 | 101(68.2%) | 37 (71.2%) | |||
| 2 | 47 (31.8%) | 15 (28.8%) | 0.731 | ||
| Oropharynx | Lateral | ||||
| 0+1 | 87 (58.8%) | 40 (76.9%) | |||
| 2 | 61 (41.2%) | 12 (23.1%) | 0.02 | ||
0+1, none (0) and partial (1) collapse; 2, complete collapse;
statistical analysis by Fisher exact test.
Statistically significant as P < 0.05
Discussion
To our knowledge, this study was the first to use DISE to evaluate the association between age and upper airway collapse characteristics in patients with OSA. The main findings were that aging increases the probability of multiple obstruction sites, combined upper and lower obstruction levels, and velum complete AP collapse. All these factors are likely secondary to increased upper airway collapsibility and likely are at least partially responsible for a more severe OSA. In addition, more AP velum complete collapse and less lateral oropharynx complete collapse were the distinct characteristics in older (age ≥ 60) patients with OSA.
In general, most patients (93.47%) presented multiple sites of obstruction (≥ 2 sites). These results were higher than others already reported: 68.2%,28 73%29 and 84.06%.30 The sub-division on levels of obstruction (upper and lower), allowed an analysis from VOTE classification system adds some elements described in the traditional Fujita’s31 and the more recent nose, oropharynx, hypopharynx and larynx (NOHL) classifications.32 The proportion (76.38%) of combined upper and lower level obstruction was also higher than previous studies: 52.17%33 and 31.9%.34 There were many other differences among studies: demography, airway anatomy, baseline sleep study outcomes, and sedative medicine used during DISE. Therefore, multivariable regression analysis was used to assess the association between OSA severity, hypoxia, airway collapse characteristics, and age, by adjusted for some widely-accepted confounders such as sex, BMI, tonsil size, and MM.
Our main finding was that age was an independent predictor of multiple sites and combined levels of obstruction. Each additional year was associated with increased multiple sites of obstruction by 6.6%, combined levels obstruction by 3%, AHI by 1.7%, ODI by 4.1% and decreased LSAT by 0.2%. These results provided evidence on how aging affects upper airway collapsibility, leading to increased OSA severity. Carlisle et al. pointed that MRI studies of older adults had greater retropalatal and retroglossal airway length.18 By means of the EMG, studies found that age-related weakening in genioglossus muscle function induced susceptible tongue collapse in both human19 and mouse model study.35 Our study confirmed through DISE, which is a dynamic visual direct observation, that age predisposed older subjects to an increasing upper way multi-sites and multi-levels collapse.
The other main finding was that age affected the pattern and the degree of collapse. In our study, one year raise in age was associated with 8.0% increase in odds ratio of complete AP velum collapse and 2% of complete tongue base collapse. This can be due to upper airway anatomic changing and dysfunction of dilator muscles. Studies demonstrate that older adults have longer soft palate, more parapharyngeal fat deposit,36 longer hypopharyngeal airway, greater hypopharyngeal soft tissue volume,37 as well as obvious recruitment of genioglossus (GG) activity and higher upper airway resistance during sleep onset stage.38 The mechanism for tongue base collapse is complex. There is evidence that BMI was an independent predictor of tongue collapse,28 and that older patients with OSA showed lower BMI than young and middle-aged adults with equivalent AHI.39 The exact cause for increased upper airway collapsibility is likely multi-factorial. Sex hormones have been described to have an association with OSA. Decline in testosterone levels has been associated with decrease in ventilator response,40 and increased nocturnal hypoxemia in older men with OSA.41 In women, post menopause progesterone decline contributed to decreased activity of GG.42 While tonsil size and MM classification are well known factors that correlate with upper airway obstruction.31,43 In our study, once we adjusted for sex, BMI, tonsil size and MM, it was found that age was not an independent predictor to tongue base collapse. No significantly increased tongue base collapse was found in subjects with age ≥ 60yr.
In this study, the increase in age had an inverse correlation with complete lateral oropharynx collapse. Schwartz RN, et al.44 found that severe lateral pharyngeal wall collapse by Muller maneuver indicated increasing severity of OSA. Complete lateral pharyngeal wall collapse on preoperative DISE was reported to have an association with failure rate after surgery.45 Through sleep MRI, it was obviously observed that dynamic lateral wall collapse was attributed to lateral pharyngeal muscle median-direction movement,46 therefore the atrophy of pharyngeal muscle may be associated with age-related general muscle atrophy, thus contributing to the lateral wall collapse reduction. Also, aging was associated with increase in ratio of transverse/AP diameter of mandibular contour that provided a wider pharyngeal cavity,36 and with expansion in expiratory end lung volume that induced a decreasing inspiratory negative pressure to pharyngeal lateral wall.47,48 Clinically, it can be judged that older patients with OSA who are CPAP intolerant, may have upper airway stimulation considered early in a possible treatment strategy.
Although AHI and hypoxia increased with aging, ESS showed no significant changes. Other studies demonstrated that older people with OSA had lower ESS score than young and middle-aged subjects.49,39 Lower daytime activity intensity and more napping opportunity may be the reason of less subjective sleepiness symptoms in older people. Meanwhile, aging and decreased cognitive status also contributed to lower self-report ESS in older adults as compared with scores provided by their close relatives.50
Despite the novelty of the concepts present in our study, there are limitations that need to be acknowledged. First, subjects in this study were not from a general population sample, but from CPAP intolerant subjects referred to a tertiary center for possible surgical management of OSA, however, the research was carried out in a group of subjects with uniform age distribution and adequate age range. The patient percentage ≥ 60yr. was 26%, which fully met the needs of the study.
Conclusion
Age was an independent variable directly correlated with increased AHI and hypoxia in subjects with OSA. Aging has also contributed to increase in multiple sites, combined levels of collapse, and complete AP velum collapse. Tongue base complete collapse was positively associated with age, but depended on sex, BMI, tonsil size and MM. The older group of patients with OSA (≥ 60yr.) was associated with greater incidence of the complete collapse of velum AP and less oropharynx lateral, regardless of the OSA severity and tonsil size. Future studies will contribute to clarify the usefulness of these findings in defining treatments in older patients with OSA.
Acknowledgments
This work was supported by a National Institutes of Health National Center for Advancing Translational Science Clinical and Translational Science Award (UL1 TR001085). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Financial disclosure and Conflict of Interests: None
Previous presentation of work: None
References
- 1.Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311–1322. doi: 10.3978/j.issn.2072-1439.2015.06.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holtzman D, Anderson LA. Aging and health in America: a tale from two boomers. Am J Public Health. 2012;102:392. doi: 10.2105/AJPH.2011.300647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neikrug AB, Ancoli-Israel S. Sleep disorders in the older adult - a mini-review. Gerontology. 2010;56:181–189. doi: 10.1159/000236900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 5.Durán J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163:685–689. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 6.Hongyo K, Ito N, Yamamoto K, et al. Factors associated with the severity of obstructive sleep apnea in older adults. Geriatr Gerontol Int. 2017;17:614–621. doi: 10.1111/ggi.12768. [DOI] [PubMed] [Google Scholar]
- 7.Gamaldo AA, Beydoun MA, Beydoun HA, et al. Sleep Disturbances among older adults in the United States, 2002–2012: nationwide inpatient rates, predictors, and outcomes. Front Aging Neurosci. 2016;8:266. doi: 10.3389/fnagi.2016.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salive ME. Multimorbidity in older adults. Epidemiol Rev. 2013;35:75–83. doi: 10.1093/epirev/mxs009. [DOI] [PubMed] [Google Scholar]
- 9.Mokhlesi B, Ham SA, Gozal D. The effect of sex and age on the comorbidity burden of OSA: an observational analysis from a large nationwide US health claims database. Eur Respir J. 2016;47:1162–1169. doi: 10.1183/13993003.01618-2015. [DOI] [PubMed] [Google Scholar]
- 10.Johansson P, Svensson E, Alehagen U, Dahlström U, Jaarsma T, Broström A. Sleep disordered breathing, hypoxia and inflammation: associations with sickness behavior in community dwelling elderly with and without cardiovascular disease. Sleep Breath. 2015;19:263–271. doi: 10.1007/s11325-014-1006-9. [DOI] [PubMed] [Google Scholar]
- 11.Marrone O, Lo Bue A, Salvaggio A, Dardanoni G, Insalaco G. Comorbidities and survival in obstructive sleep apnea beyond the age of 50. Eur J Clin Invest. 2013;43:27–33. doi: 10.1111/eci.12011. [DOI] [PubMed] [Google Scholar]
- 12.Canales MT, Paudel ML, Taylor BC, et al. Sleep-disordered breathing and urinary albumin excretion in older men. Sleep Breath. 2011;15:137–144. doi: 10.1007/s11325-010-0339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stone KL, Blackwell TL, Ancoli-Israel S, et al. Sleep disordered breathing and risk of stroke in older community-dwelling men. Sleep. 2016;39:531–540. doi: 10.5665/sleep.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin MS, Sforza E, Roche F, Barthélémy JC, Thomas-Anterion C PROOF study group. Sleep breathing disorders and cognitive function in the elderly: an 8-year follow-up study. the proof-synapse cohort. Sleep. 2015;38:179–187. doi: 10.5665/sleep.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rostanski SK, Zimmerman ME, Schupf N, et al. Sleep disordered breathing and white matter hyperintensities in community-dwelling elders. Sleep. 2016;39:785–791. doi: 10.5665/sleep.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards BA, Wellman A, Sands SA, et al. Obstructive sleep apnea in older adults is a distinctly different physiological phenotype. Sleep. 2014;37:1227–1236. doi: 10.5665/sleep.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eikermann M, Jordan AS, Chamberlin NL, et al. The influence of aging on pharyngeal collapsibility during sleep. Chest. 2007;131:1702–1709. doi: 10.1378/chest.06-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlisle T, Carthy ER, Glasser M, et al. Upper airway factors that protect against obstructive sleep apnoea in healthy older males. Eur Respir J. 2014;44:685–693. doi: 10.1183/09031936.00177213. [DOI] [PubMed] [Google Scholar]
- 19.Saboisky JP, Stashuk DW, Hamilton-Wright A, Trinder J, Nandedkar S, Malhotra A. Effects of aging on genioglossus motor units in humans. PLoS ONE. 2014;9:e104572. doi: 10.1371/journal.pone.0104572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boutet C, Abdirahman Mohamed Moussa S, Celle S, et al. Supra-Epiglottic upper airway volume in elderly patients with obstructive sleep apnea hypopnea syndrome. PLoS One. 2016;11:e0157720. doi: 10.1371/journal.pone.0157720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vroegop AV, Vanderveken OM, Wouters K, et al. Observer variation in drug-induced sleep endoscopy: experienced versus nonexperienced, ear, nose, and throat surgeons. Sleep. 2013;36:947–953. doi: 10.5665/sleep.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez-Julian E, García-Pérez MÁ, García-Callejo J, Ferrer F, Martí F, Marco J. Surgical planning after sleep versus awake techniques in patients with obstructive sleep apnea. Laryngoscope. 2014;124:1970–1974. doi: 10.1002/lary.24577. [DOI] [PubMed] [Google Scholar]
- 23.Vanderveken OM, Maurer JT, Hohenhorst W, et al. Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J Clin Sleep Med. 2013;9:433–438. doi: 10.5664/jcsm.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Vito A, Carrasco Llatas M, Vanni A, et al. European position paper on drug-induced sedation endoscopy (DISE) Sleep Breath. 2014;18:453–465. doi: 10.1007/s11325-014-0989-6. [DOI] [PubMed] [Google Scholar]
- 25.Paul A Harris, Robert T, Robert T, Jonathon P, Nathaniel G, Jose G Conde. Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kezirian EJ, Hohenhorst W, de Vries N. Drug-induced sleep endoscopy: the VOTE classification. Eur Arch Otorhinolaryngol. 2011;268:1233–1236. doi: 10.1007/s00405-011-1633-8. [DOI] [PubMed] [Google Scholar]
- 27.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vroegop AV, Vanderveken OM, Boudewyns AN, et al. Drug-induced sleep endoscopy in sleep-disordered breathing: report on 1,249 cases. Laryngoscope. 2014;124:797–802. doi: 10.1002/lary.24479. [DOI] [PubMed] [Google Scholar]
- 29.Gillespie MB, Reddy RP, White DR, Discolo CM, Overdyk FJ, Nguyen SA. A trial of drug-induced sleep endoscopy in the surgical management of sleep-disordered breathing. Laryngoscope. 2013;123:277–282. doi: 10.1002/lary.23506. [DOI] [PubMed] [Google Scholar]
- 30.Koo SK, Choi JW, Myung NS, Lee HJ, Kim YJ, Kim YJ. Analysis of obstruction site in obstructive sleep apnea syndrome patients by drug induced sleep endoscopy. Am J Otolaryngol. 2013;34:626–630. doi: 10.1016/j.amjoto.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Fairbanks DN, Fujita S, Ikematsu T, et al. Snoring and Obstructive Sleep Apnea. New York: Raven Press; 1987. pp. 101–128. [Google Scholar]
- 32.Vicini C, De Vito A, Benazzo M, et al. The nose oropharynx hypopharynx and larynx (NOHL) classification: a new system of diagnostic standardized examination for OSAHS patients. Eur Arch Otorhinolaryngol. 2012;269:1297–1300. doi: 10.1007/s00405-012-1965-z. [DOI] [PubMed] [Google Scholar]
- 33.Rabelo FA, Küpper DS, Sander HH, et al. A comparison of the Fujita classification of awake and drug-induced sleep endoscopy patients. Braz J Otorhinolaryngol. 2013;79:100–105. doi: 10.5935/1808-8694.20130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamans E, Meeus O, Boudewyns A, Saldien V, Verbraecken J, Van de Heyning P. Outcome of sleep endoscopy in obstructive sleep apnea: the Antwerp experience. B-ENT. 2010;6:97–103. [PubMed] [Google Scholar]
- 35.Polotsky M, Elsayed-Ahmed AS, Pichard L, et al. Effect of age and weight on upper airway function in a mouse model. J Appl Physiol (1985) 2011;111:696–703. doi: 10.1152/japplphysiol.00123.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malhotra A, Huang Y, Fogel R, et al. Aging influences on pharyngeal anatomy and physiology: the predisposition to pharyngeal collapse. Am J Med. 2006;119:72.e9–72.14. doi: 10.1016/j.amjmed.2005.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shigeta Y, Ogawa T, Venturin J, et al. Gender- and age-based differences in computerized tomographic measurements of the orophaynx. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:563–570. doi: 10.1016/j.tripleo.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fogel RB, White DP, Pierce RJ, et al. Control of upper airway muscle activity in younger versus older men during sleep onset. J Physiol. 2003;553(Pt 2):533–544. doi: 10.1113/jphysiol.2003.045708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung S, Yoon IY, Lee CH, Kim JW. Effects of age on the clinical features of men with obstructive sleep apnea syndrome. Respiration. 2009;78:23–29. doi: 10.1159/000218143. [DOI] [PubMed] [Google Scholar]
- 40.Burschtin O, Wang J. Testosterone deficiency and sleep apnea. Sleep Med Clin. 2016;11:525–529. doi: 10.1016/j.jsmc.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Viana A, Jr, Daflon AC, Couto A, Neves D, de Araujo-Melo MH, Capasso R. Nocturnal hypoxemia is associated with low testosterone levels in overweight males and older men with normal weight. J Clin Sleep Med. 2017;13:1395–1401. doi: 10.5664/jcsm.6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Popovic RM, White DP. Upper airway muscle activity in normal women: influence of hormonal status. J Appl Physiol. 1998;84:1055–1062. doi: 10.1152/jappl.1998.84.3.1055. [DOI] [PubMed] [Google Scholar]
- 43.Friedman M, Tanyeri H, LaRosa M, et al. Clinical predictors of obstructive sleep apnea. Laryngoscope. 1999;109:1901–1907. doi: 10.1097/00005537-199912000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz RN, Payne RJ, Forest VI, Hier MP, Fanous A, Vallée-Gravel C. The relationship between upper airway collapse and the severity of obstructive sleep apnea syndrome: a chart review. J Otolaryngol Head Neck Surg. 2015;44:32. doi: 10.1186/s40463-015-0086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soares D, Sinawe H, Folbe AJ, et al. Lateral oropharyngeal wall and supraglottic airway collapse associated with failure in sleep apnea surgery. Laryngoscope. 2012;122:473–479. doi: 10.1002/lary.22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huon LK, Liu SY, Shih TT, Chen YJ, Lo MT, Wang PC. Dynamic upper airway collapse observed from sleep MRI: BMI-matched severe and mild OSA patients. Eur Arch Otorhinolaryngol. 2016;273:4021–4026. doi: 10.1007/s00405-016-4131-1. [DOI] [PubMed] [Google Scholar]
- 47.Kostikas K, Browne HA, Ghiassi R, Adams L, Simonds AK, Morrell MJ. The determinants of therapeutic levels of continuous positive airway pressure in elderly sleep apnea patients. Respir Med. 2006;100:1216–1225. doi: 10.1016/j.rmed.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 48.Heinzer RC, Stanchina ML, Malhotra A, et al. Effect of increased lung volume on sleep disordered breathing in sleep apnoea patients. Thorax. 2006;61:435–439. doi: 10.1136/thx.2005.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sforza E, Pichot V, Martin MS, Barthélémy JC, Roche F. Prevalence and determinants of subjective sleepiness in healthy elderly with unrecognized obstructive sleep apnea. Sleep Med. 2015;16:981–986. doi: 10.1016/j.sleep.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Onen F, Moreau T, Gooneratne NS, Petit C, Falissard B, Onen SH. Limits of the Epworth Sleepiness Scale in older adults. Sleep Breath. 2013;17:343–350. doi: 10.1007/s11325-012-0700-8. [DOI] [PubMed] [Google Scholar]


