Abstract
Background:
For 10 years, the Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet) conducted surveillance for Duchenne and Becker muscular dystrophy (DBMD). We piloted expanding surveillance to other MDs that vary in severity, onset, and sources of care.
Methods:
Our retrospective surveillance included individuals diagnosed with one of nine eligible MDs before or during the study period (January 2007–December 2011), one or more health encounters, and residence in one of four U.S. sites (Arizona, Colorado, Iowa, or western New York) at any time within the study period. We developed case definitions, surveillance protocols, and software applications for medical record abstraction, clinical review, and data pooling. Potential cases were identified by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 359.0, 359.1, and 359.21 and International Classification of Diseases, Tenth Revision (ICD-10) codes G71.0 and G71.1. Descriptive statistics were compared by MD type. Percentage of MD cases identified by each ICD-9-CM code was calculated.
Results:
Of 2,862 cases, 32.9% were myotonic, dystrophy 25.8% DBMD, 9.7% facioscapulohumeral MD, and 9.1% limb-girdle MD. Most cases were male (63.6%), non-Hispanic (59.8%), and White (80.2%). About, half of cases were genetically diagnosed in self (39.1%) or family (6.2%). About, half had a family history of MD (48.9%). The hereditary progressive MD code (359.1) was the most common code for identifying eligible cases. The myotonic code (359.21) identified 83.4% of eligible myotonic dystrophy cases (786/943).
Conclusions:
MD STARnet is the only multisite, population-based active surveillance system available for MD in the United States. Continuing our expanded surveillance will contribute important epidemiologic and health outcome information about several MDs.
Keywords: active surveillance, International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes, MD STARnet, medical record abstraction, muscular dystrophies, population-based
1 ∣. INTRODUCTION
Muscular dystrophies (MDs) are a set of rare genetic diseases resulting from a progressive, disabling loss of muscle function (Emery, 1998; Mah et al., 2014). The MDs differ from each other in the affected muscle groups, spectrum of causal genetic mutations, age of onset, severity, rate of disease progression, and prevalence. The progressive severity of MDs and their considerable impact on affected individuals, families, and society make them candidates for secondary and tertiary public health prevention. Because studies on the prevalence of MDs and the attributes of affected individuals in the United States are limited, an initial step in planning secondary and tertiary prevention activities is population-based surveillance (Miller et al., 2006; Turnock, 2009). Previously, administrative data have been used to conduct active and passive surveillance of rare conditions, such as MDs (Reichard et al., 2016; Smith, Royer, Mann, & McDermott, 2017; Smith, Royer, Mann, McDermott, & Valdez, 2017). Active surveillance uses data abstracted directly from medical records from medical facilities serving individuals with the condition under surveillance as a primary data source (Smith, Royer, Mann, & McDermott, 2017; Smith, Royer, Mann, McDermott, & Valdez, 2017). Active surveillance also often incorporates use of multiple administrative data sources. In passive surveillance, data are typically ascertained through multiple administrative data sources only.
From 2002 to 2011, the Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet) conducted active, population-based surveillance for Duchenne and Becker muscular dystrophy (DBMD), the most common childhood-onset MDs (Mathews et al., 2010; Miller et al., 2006). This surveillance included DBMD cases born on or after January 1, 1982 who were diagnosed on or before December 31, 2011 and resided at any time in one of the participating geographic sites (Arizona, Colorado, Georgia, Hawaii, Iowa, or western New York State). Details of the surveillance methods used by the MD STARnet have previously been published (Mathews et al., 2010; Miller et al., 2006). Using data collected from clinics and administrative data sources, MD STARnet investigators determined the prevalence of childhood-onset DBMD (Romitti et al., 2015) and characterized healthcare and health outcomes in this population (Andrews et al., 2016; Caspers Conway et al., 2015; Centers for Disease Control and Prevention, 2009; Ciafaloni et al., 2016; Fox et al., 2015; Kim, Campbell, Fox, Matthews, & Valdez, 2015; Lamb et al., 2016; Mathews et al., 2010; Miller et al., 2006; Pandya, Andrews, Campbell, & Meaney, 2016; Pandya, Campbell, Andrews, Meaney, & Ciafaloni, 2016; West et al., 2013). Given the ability of MD STARnet to enumerate DBMD cases and study this severe, childhood-onset condition, the purpose of our pilot was to determine whether MD STARnet methods could be applied to other major MDs that vary in severity, age of onset, and/or access to sources of clinical care. Specifically, we aimed to: (a) determine the feasibility of conducting surveillance on an expanded group of MDs; and (b) generate data to estimate period prevalence and describe selected attributes of affected individuals. Herein, we describe the methodology of collecting this information and the utility of using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and International Classification of Diseases, Tenth Revision (ICD-10) in identifying this expanded group of MDs.
2 ∣. METHODS
2.1 ∣. Study population and types of MD
To accomplish our objectives, we expanded on the methods used for DBMD surveillance to monitor seven additional MDs. Along with DBMD, we ascertained individuals diagnosed with congenital MD (CMD), distal MD, Emery– Dreifuss MD (EDMD), facioscapulohumeral MD (FSHD), limb-girdle MD (LGMD), myotonic dystrophy (DM), oculopharyngeal MD (OPMD), and MD not otherwise specified (MD-NOS). MD-NOS was selected when the MD type was not specified in the medical record.
This retrospective surveillance covered individuals of any age diagnosed with an eligible MD type before or during the study period (January 1, 2007–December 31, 2011) who:(a) resided for any length of time following diagnosis during the study period in one of the four MD STARnet sites (Arizona, Colorado, Iowa, or a 12-county area of western New York State [henceforth, termed New York]) and (b) had at least one health encounter in a healthcare setting (i.e., neuromuscular outpatient clinic, hospital, or emergency department visit from 2007 to 2011).
2.2 ∣. Surveillance authority
The Arizona MD STARnet site acted as an agent for the Arizona Department of Health Services to conduct MD surveillance. MD STARnet activities in Arizona were approved and monitored by an institutional review board at the University of Arizona, and as needed, from healthcare facilities where records were accessed. Colorado, Iowa, and New York operated through legal authority for public health surveillance from their respective state health department.
2.3 ∣. Case ascertainment and sources
The case-finding methodology for the pilot was similar to that used by the MD STARnet for DBMD surveillance (Mathews et al., 2010; Miller et al., 2006). Cases were identified using: (a) ICD-9-CM codes (359.0: congenital hereditary MD, 359.1: hereditary progressive MD, 359.21: DM) in healthcare data sources and administrative data; (b) ICD-10 in death certificate records (G71.0: MD, G71.1: DM); (c) DBMD cases from Arizona, Colorado, Iowa, and New York previously identified through the MD STARnet that met eligibility requirements. A clinical diagnosis of MD entered in the medical record was required for a case to be abstracted.
Case ascertainment was conducted using multiple sources. In general, sources were prioritized by volume of cases, data that the sources were expected to yield, and the anticipated workload to review the cases. The healthcare data sources included neuromuscular clinics, physical medicine and rehabilitation clinics, hospitals, and other outpatient clinics. Lists of patients with ICD-9-CM codes for MD were first obtained from neuromuscular and physical medicine and rehabilitation clinics. Abstractors screened cases from these lists to determine whether a case met the eligibility criteria, and if so, abstracted additional information. Sites had varying access to administrative data that included data from birth defects surveillance programs, healthcare administrative data (including accounting records), state hospital discharge summaries, Medicaid claims (Colorado only), and vital records (state birth and death certificates). Potential MD cases were also identified through searching these administrative data using ICD-9-CM and ICD-10 (death records only) MD codes. After identification of potential cases using hospital discharge and Medicaid data, hospital records of these cases were reviewed by abstractors to determine eligibility and to abstract information on eligible cases. With potential cases at hundreds of hospitals throughout each site, abstraction at these hospitals was prioritized by potential case volume and number of beds. Due to time and resource constraints, the four MD STARnet sites did not collect data from other outpatient facilities/providers for cases (i.e., physicians identified through hospital records). At each site, surveillance activities were conducted by one to three abstractors, a data manager, and a program manager, with oversight from a principal investigator, and case review and consultation from a neuromuscular specialist.
After a potential case was identified using the ICD-9-CM or ICD-10 code, a healthcare encounter from 2007 to 2011, residency, and diagnosis of MD were collected and used to determine eligibility. Source of information and time required to determine eligibility were also collected to evaluate the screening process. A full medical record abstraction was completed for all eligible cases. This abstraction included core variables (demographic information, visit information during the study period, clinical evidence supporting diagnosis, DNA testing, family history of MD, MD type, method of diagnosis as determined by the abstractor [i.e., clinical diagnosis, genetic diagnosis in self, genetic diagnosis in family], physical mobility, medications, medical interventions [e.g., tracheostomy], sources of healthcare for MD, and time to abstract a case [time from when the abstractor considered the case to be eligible to completion of data entry]), and supplemental variables (e.g., employment, height, weight). The collection of MD STARnet case data was standardized through detailed manual and protocol documentation, quality assurance activities, and training.
During abstraction, abstractors made an initial assessment of the MD type and the method of diagnosis. MD STARnet clinicians, whose practice included patients with pediatric and adult MDs, reviewed abstracted data on each eligible case at their site to determine MD type and method of diagnosis. Cases were flagged if the data to assign the MD type and/or method of diagnosis were unclear. Every month, the final determination for these cases was made by clinicians in the MD STARnet clinical review committee. This committee was comprised of one clinician from each site who had experience managing care of individuals with MD (pediatric neurologists or physiatrists). All screened cases that an abstractor excluded based on ineligible MD type were reviewed by the local MD STARnet clinician to validate the appropriateness of exclusion. If the clinician determined the case to be eligible, the abstractor abstracted the full record for the case.
2.4 ∣. Quality control
Before surveillance field activities began, sites collaboratively decided on the protocol, the anticipated analyses, and the data variables needed. Simultaneously, the MD STARnet Data Coordinating Center developed software for collection, review, quality control, and pooling of data. All abstractors were MD STARnet certified after receiving training, which included presentations on each MD and detailed instructions on the clinical information to be collected. Practice cases were abstracted, scored for required data variables, and discrepancies discussed. Abstractors received additional training for variables or conditions that demonstrated inconsistencies across sites. Equivalent terminology was developed for the lexicon of medical terms found in records describing these MDs. Data collection was piloted to test the abstraction instrument and manual of procedures and methods, and to address problems with the process flow from screening to abstraction, data cleaning, and clinical review. Lastly, abstractors repeated case abstraction exercises to evaluate reliability in the screening and abstraction of cases. Inter-rater reliability (IRR) prior to data collection showed high agreement (>90%) among all abstractors and the gold standard for all but one diagnostic variable (presence of variants of unknown significance found in DNA test results) and two clinical variables (height and number of medications documented in the medical record). Results were shared with abstractors and the MD STARnet, and adjustments to the abstractor manual and abstraction process were made. The completed IRR exercise following the data collection period demonstrated improvement across the variables observed to be below the established level (>90%) during the initial IRR. The MD STARnet Data Monitoring Committee conducted quarterly assessments of abstraction progress and data quality and completeness, and provided recommended changes for improving abstraction throughout the pilot. Monthly abstractor calls provided opportunities for targeted training and resolved issues identified by the MD STARnet Data Monitoring Committee.
2.5 ∣. Variables
The following variables were included in the current analysis: reporting source, gender (male/female), date of birth, race, ethnicity, vital status (deceased: yes/no and date of death), type of MD (DMD and BMD were combined as DBMD), method of diagnosis, family history of MD present (yes/no), and case identification by ICD-9-CM or ICD-10 code. Reporting sources were the sources that supplied data to the surveillance system, and were categorized as either administrative data or clinical record. A case may have had more than one reporting source.
Date of birth was collected as a continuous variable and calculated as the age (in years) of the case at the beginning of the study period on January 1, 2007 or age of death if the case was deceased. Race was collected as White/Caucasian, Black/African-American, American-Indian/Alaska Native, Asian, Native Hawaiian/Pacific Islander, multiple races, other, and unknown. Race was collapsed to White/Caucasian, Black/African-American, multiple/other races (American-Indian/Alaska Native, Asian, Native Hawaiian/Pacific Islander, multiple race, and other), and unknown. Ethnicity was categorized as Hispanic, non-Hispanic, or unknown. Method of diagnosis was categorized as clinical diagnosis, genetic diagnosis in self, or genetic diagnosis in family. A clinical diagnosis was defined as having signs and symptoms of MD without genetic confirmation. For cases who had genetic testing that identified a pathogenic variant, the method of diagnosis was genetic diagnosis in self. If the pathogenic variant was identified in a family member but not tested for in the index case, the method of diagnosis was genetic diagnosis in family.
2.6 ∣. Data analysis
All statistical analyses were conducted using SAS 9.4© (SAS Institute Inc., Cary, NC). Descriptive statistics (frequency and percentages) were used to compare the demographic and diagnostic characteristics of the eligible MD STARnet cases. Mean, median, and standard deviation (SD) were calculated for age at beginning of study period and age at death. Small cell sizes were not reported for demographic variables. To assess the utility of the ICD-9-CM codes in identifying a particular type of MD, the percentage of eligible MD cases identified by each ICD-9-CM code was calculated.
3 ∣. RESULTS
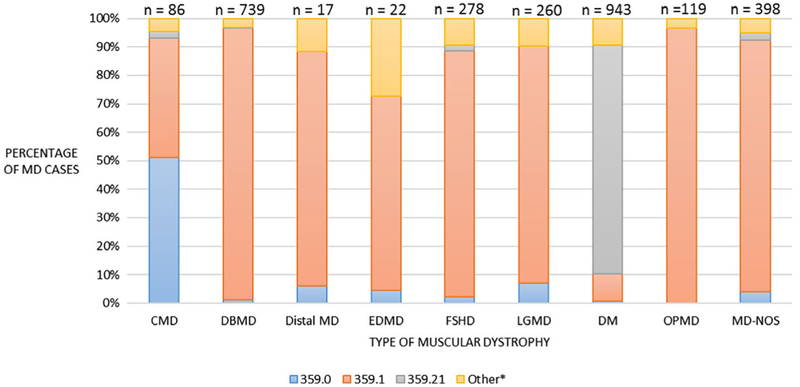
A total of 5,471 clinical and administrative records of 5,243 potential cases were reviewed. Table 1 describes the total number of potential and eligible records that were screened, by ICD code or other method. The DM code (ICD-9-CM 359.21) identified 78% of eligible MD cases whereas ICD-9-CM 359.0 identified only 18.1% of eligible cases. After screening, 2,862 cases were identified as eligible for the MD STARnet pilot. Figure 1 shows the utility of using ICD-9-CM codes in identifying MD cases. For all MD types except CMD and DM, the most common ICD-9-CM code used to identify MD cases was 359.1—hereditary progressive MD; this code identified 62.3% of eligible MD cases. The ICD-9-CM code for DM (359.21) was used to identify 83.4% of the DM cases. However, the code for congenital hereditary MD (359.0) was not the only ICD-9-CM code used for identifying CMD. About half (51.2%) of the CMD cases were identified by the code for congenital hereditary MD, while 41.9% were identified by the hereditary progressive MD code (359.1).
TABLE 1.
Potential and eligible records screened in the 2007–2011 MD STARnet pilot, by ICD code or other method
| Method of record identification | Records screened (n = 5,243) | Screened records of eligible cases (n = 2,862) | Percent of records deemed eligible |
|---|---|---|---|
| ICD-9-CM | |||
| 359.0 | 601 | 109 | 18.1 |
| 359.1 | 2,629 | 1,435 | 54.6 |
| 359.21 | 1,030 | 807 | 78.3 |
| ICD-10 | |||
| G71.0 | 71 | 17 | 23.9 |
| G71.1 | 44 | 18 | 40.9 |
| Othera | 1,096 | 665 | 50.5 |
| Totalb | 5,471 | 3,051 | 55.8 |
Note. ICD-9-CM codes listed are: 359.0 congenital hereditary muscular dystrophy, 359.1 hereditary progressive muscular dystrophy, 359.21 myotonic muscular dystrophy. ICD-10 codes listed are: G71.0 muscular dystrophy and G71.1 myotonic disorders. MD STARnet = muscular dystrophy surveillance, tracking and research network; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; ICD-10 = International Classification of Diseases, Tenth Revision.
Cases where ICD code was not documented were found through source lists that did not use ICD codes such as Duchenne or Becker muscular dystrophy cases already in the MD STARnet database and lists from sources that did not use ICD codes to identify cases.
Some cases were found at more than one source so number of records is greater than number of cases screened.
FIGURE 1.
Comparison of ICD-9-CM codes in the identification of major types of MDs from the 2007 to 2011 MD STARnet pilot. Codes listed are ICD-9-CM codes: 359.0: congenital hereditary MD (includes CMD), 359.1: hereditary progressive MD (includes DBMD, Distal, EDMD, FSHD, LGMD, OPMD, and MD-NOS), 359.21: myotonic muscular dystrophy (myotonic dystrophy). *Other indicates that cases were identified by ICD-10 code or other method. MD STARnet = muscular dystrophy surveillance, tracking and research network; MD = muscular dystrophy; CMD = congenital muscular dystrophy; DBMD = Duchenne and Becker muscular dystrophy; EDMD = Emery–Dreifuss muscular dystrophy; FSHD = facioscapulohumeral muscular dystrophy; LGMD = limb-girdle muscular dystrophy; DM = myotonic dystrophy; OPMD = oculopharyngeal muscular dystrophy; MD-NOS = muscular dystrophy-not otherwise specified
Table 2 provides a summary of the demographic characteristics for cases eligible for abstraction. The mean age of MD cases was 35.5 years (SD = 21.7). Mean age at the start of the study varied by MD type. For MDs that are generally childhood-onset, age ranged from 12.8 years for CMD to 23.2 years for EDMD. For MDs that are often adult-onset, age ranged from 38.4 years for DM to 65.7 years for OPMD. Conditions which are primarily X-linked, DBMD and EDMD, had larger percentages of affected males than the other MDs, which had more equal proportions of males to females. Most cases were male (63.6%), non-Hispanic (59.8%), and White/Caucasian (80.2%); 14.4% were deceased. Among all deceased cases, 8.5% were identified by ICD-10 codes in death certificate records.
TABLE 2.
Demographic characteristics of eligible cases from the 2007 to 2011 MD STARnet pilot (n = 2,862)
| Type of muscular dystrophy | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All MDs |
CMD |
DBMD |
Distal MD |
EDMD |
FSHD |
LGMD |
DM |
OPMD |
MD-NOS |
|||||||||||
| Characteristic | n = 2,862 | n = 86 | n = 739 | n = 17 | n = 22 | n = 278 | n = 260 | n = 943 | n = 119 | n = 398 | ||||||||||
| Age at start of projecta | ||||||||||||||||||||
| Number of casesb | 2,791 | 77 | 705 | 17 | 22 | 277 | 257 | 919 | 119 | 398 | ||||||||||
| Mean (SD) | 35.5 (21.7) | 14.8 (14.4) | 16.6 (12.6) | 43.9 (12.6) | 23.2 (14.7) | 43.8 (19.9) | 38.9 (21.5) | 39.5 (17.5) | 65.7 (9.8) | 46.9 (20.5) | ||||||||||
| Age at death | ||||||||||||||||||||
| Number of deaths | 411 | NR | 83 | NR | NR | 21 | 29 | 161 | 25 | 80 | ||||||||||
| Mean age (SD) | 51.6 (22.2) | 12.2 (7.5) | 25.8 (11.9) | NR | NR | 65.6 (8.5) | 67.0 (15.9) | 53.6 (16.3) | 77.0 (8.7) | 60.7 (20.6) | ||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Gender | ||||||||||||||||||||
| Female | 1,041 | 36.4 | 41 | 47.7 | 17 | 2.3 | NR | NR | NR | NR | 117 | 42.1 | 129 | 49.6 | 470 | 49.8 | 65 | 54.6 | 187 | 47.0 |
| Male | 1,821 | 63.6 | 45 | 52.3 | 722 | 97.7 | NR | NR | NR | NR | 161 | 57.9 | 131 | 50.4 | 473 | 50.2 | 54 | 45.4 | 211 | 53.0 |
| Race | ||||||||||||||||||||
| White/Caucasian | 2,294 | 80.2 | 66 | 76.7 | 578 | 78.2 | 12 | 70.6 | 21 | 95.5 | 228 | 82.0 | 202 | 77.7 | 804 | 85.3 | 75 | 63.0 | 308 | 77.4 |
| Black/African-American | 79 | 2.8 | NR | NR | 19 | 2.6 | NR | NR | NR | NR | NR | NR | 14 | 5.4 | 23 | 2.4 | 0 | 0.0 | 16 | 4.0 |
| Multiple/otherc | 211 | 7.4 | 12 | 14.0 | 86 | 11.6 | NR | NR | NR | NR | NR | NR | 16 | 6.2 | 44 | 4.7 | 12 | 10.9 | 22 | 5.5 |
| Unknown | 278 | 9.7 | NR | NR | 56 | 7.6 | NR | NR | NR | NR | 31 | 11.2 | 28 | 10.8 | 72 | 7.6 | 31 | 26.1 | 52 | 13.1 |
| Ethnicity | ||||||||||||||||||||
| Hispanic | 403 | 14.1 | 19 | 22.1 | 153 | 20.7 | NR | NR | NR | NR | 22 | 7.9 | 30 | 11.5 | 78 | 8.3 | 41 | 34.5 | 58 | 14.6 |
| Non-Hispanic | 1,712 | 59.8 | 58 | 67.4 | 495 | 67.0 | NR | NR | NR | NR | 159 | 57.9 | 143 | 55.0 | 590 | 62.6 | 31 | 26.1 | 207 | 52.0 |
| Unknown | 747 | 26.1 | 9 | 10.5 | 91 | 12.3 | NR | NR | NR | NR | 97 | 34.9 | 87 | 33.5 | 275 | 29.2 | 47 | 39.5 | 133 | 33.4 |
Note. MD STARnet = muscular dystrophy surveillance, tracking and research network; MD = muscular dystrophy; CMD = congenital muscular dystrophy; DBMD = Duchenne and Becker muscular dystrophy; EDMD = Emery– Dreifuss muscular dystrophy; FSHD = facioscapulohumeral muscular dystrophy; LGMD = limb-girdle muscular dystrophy; DM = myotonic dystrophy; OPMD = oculopharyngeal muscular dystrophy; MD-NOS = muscular dystrophy-not otherwise specified; SD = standard deviation; NR = not reported due to small case counts.
Age at start of project is defined as age on January 1, 2007.
Number of cases used to calculate age at start of project excludes cases that were born on or after January 1, 2007.
Multiple/other race includes American-Indian/Alaska Native, Asian, multiple races, Native Hawaiian/Pacific Islander, and other.
Table 3 shows the distribution of MD type by surveillance data attribute. Surveillance data abstracted for any MD were obtained largely or completely from clinical records (72–100%). For all MD types except DBMD and EDMD, clinical diagnosis was the most common diagnostic method ranging from 49.6% for FSHD to 77.7% for LGMD. For DBMD and EDMD, genetic diagnosis in self was the most common diagnostic method (69.3 and 50.0%, respectively). A positive family history ranged from 16.3% for CMD to 72.5% for DM.
TABLE 3.
Diagnostic and source characteristics of eligible cases from the 2007 to 2011 MD STARnet pilot (n = 2,862)
| Type of muscular dystrophy | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All MDs |
CMD |
DBMD |
Distal |
EDMD |
FSHD |
LGMD |
DM |
OPMD |
MD-NOS |
|||||||||||
|
n = 2,862 |
n = 86 |
n = 739 |
n = 17 |
n = 22 |
n = 278 |
n = 260 |
n = 943 |
n = 119 |
n = 398 |
|||||||||||
| Characteristic | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % |
| Method of diagnosis | ||||||||||||||||||||
| Clinical diagnosis | 1,565 | 54.7 | 54 | 62.8 | 174 | 23.5 | 11 | 64.7 | 9 | 40.9 | 138 | 49.6 | 202 | 77.7 | 512 | 54.3 | 70 | 58.8 | 395 | 99.2 |
| Genetic diagnosis in family | 179 | 6.3 | 2 | 2.3 | 51 | 6.9 | 0 | 0.0 | 2 | 9.1 | 25 | 9.0 | 8 | 3.1 | 84 | 8.9 | 6 | 5.0 | 1 | 0.3 |
| Genetic diagnosis in self | 1,118 | 39.0 | 30 | 34.9 | 514 | 69.6 | 6 | 35.3 | 11 | 50.0 | 115 | 41.4 | 50 | 19.2 | 347 | 36.8 | 43 | 36.1 | 2 | 0.5 |
| Family history present | 1,399 | 48.9 | 14 | 16.3 | 307 | 41.5 | 7 | 41.2 | 13 | 59.1 | 158 | 56.8 | 84 | 32.3 | 674 | 71.5 | 73 | 61.3 | 69 | 17.3 |
| Number of reporting source recordsa | 3,987 | 130 | 1,264 | 19 | 31 | 328 | 334 | 1,241 | 146 | 494 | ||||||||||
| Administrative data b | 530 | 13.3 | 20 | 15.4 | 107 | 8.5 | 0 | 0.0 | 3 | 9.7 | 24 | 7.3 | 39 | 11.7 | 165 | 12.3 | 35 | 24.0 | 137 | 27.7 |
| Clinical records c | 3,457 | 86.7 | 110 | 84.6 | 1,157 | 91.5 | 19 | 100.0 | 28 | 90.3 | 304 | 92.7 | 295 | 88.3 | 1,076 | 86.7 | 111 | 76.0 | 357 | 72.3 |
Note. MD STARnet = muscular dystrophy surveillance, tracking and research network; MD = muscular dystrophy; CMD = congenital muscular dystrophy; DBMD = Duchenne and Becker muscular dystrophy; EDMD = Emery– Dreifuss muscular dystrophy; FSHD = facioscapulohumeral muscular dystrophy; LGMD = limb-girdle muscular dystrophy; DM = myotonic dystrophy; OPMD = oculopharyngeal muscular dystrophy; MD-NOS = muscular dystrophy-not otherwise specified.
Cases may have data at one or more administrative or clinical sources.
Administrative data includes birth defects registries, healthcare administrative data (including accounting records), hospital discharge summaries, Medicaid claims, and vital records (birth and death certificates).
Clinical records include inpatient or outpatient facility with or without muscular dystrophy/neuromuscular clinic, genetic services, hospital records, and others.
4 ∣. DISCUSSION
To our knowledge, this is the first population-based surveillance system in the United States to capture the clinical, genetic, and demographic characteristics of several different types of MD. MDs are challenging for surveillance. There are nine major MD types with varying presentations and age of onset and each MD type is rare (Smith, Royer, Mann, & McDermott, 2017). As such, more effective approaches are needed to identify and describe true cases. We expanded our existing MD STARnet infrastructure, partnerships, and experience in conducting DBMD surveillance to capture demographic and key clinical characteristics of additional MD types. The lessons learned from this pilot were used to inform and guide current MD STARnet efforts.
The numbers of cases ascertained in our pilot show that the surveillance methods of MD STARnet can identify individuals with MDs. As expected, we observed the highest number of cases for the most common MDs—DM and DBMD followed by FSHD and LGMD. Multiple clinic and administrative data sources were used to ascertain cases and provide data to the system, enhancing the ability to identify cases within the surveillance regions.
We were able to assess the utility of using ICD-9-CM codes in identifying MD cases. Similar to another study (Smith, Royer, Mann, & McDermott, 2017), the diagnosis code for congenital hereditary MD (359.0) only identified about one-half of eligible CMD cases; most of the remaining eligible cases were identified under the code for hereditary progressive MD (359.1). In contrast, the code for DM (359.21) was able to identify over 80% of eligible cases. The most common code recorded was hereditary progressive MD (359.1), which can be used for identifying DBMD and other selected MDs (e.g., Distal, FSHD, LGMD). A large number of cases with ICD-9-CM 359.1 were classified with a diagnosis of MD-NOS, indicating a lack of diagnostic information in source records.
Because the results of our pilot suggest that distinguishing one type of MD from another type using solely administrative records is difficult, additional outpatient data sources may be needed to provide diagnostic data to classify cases and better characterize those who receive care locally and/or who may have less severe disease. To improve identification of MD cases in the future, billing codes would need to correspond to one MD type. Without specific ICD codes for each MD type, clinical record confirmation is needed to classify a type. Recently, ICD-10-CM codes specific for DBMD and FSHD have been approved. The codes will be in effect starting October 1, 2018 and may improve identification of these MD types. Death record searches using ICD-10 codes were conducted by Colorado, Iowa, and New York to identify potential MD cases who had died. Clinical sources were needed to determine eligibility of these cases and to provide demographic and clinical data.
Our pilot had several limitations. First, surveillance was conducted in only four geographic sites, so findings might be different in other geographic locations. Second, our pilot did not capture healthcare encounters that occurred outside the geographic sites; thus, data were incomplete for cases who resided in an MD STARnet site, but received treatment outside that site. Related to this, individuals who moved into an MD STARnet site from 2010 to 2011 or moved out from 2007 to 2008 might have less complete information compared to those who resided in an MD STARnet site throughout the study period. Third, because the available ICD-9-CM codes were not specific for each MD, data from clinical records were needed to confirm MD type. Fourth, sites did not have the time and resources during the pilot to complete case ascertainment by accessing medical records from other outpatient sources (e.g., providers identified through inpatient hospital records and private neurology offices). As a result, we did not report prevalence estimates. Cases identified through hospital discharge summaries might have been hospitalized for a condition unrelated to MD, so inpatient hospital records might not have contained enough information to determine the MD or diagnosis type. However, hospital records could contain information on where the case received care for their MD. Additionally, cases might not seek care in outpatient neuromuscular clinics, but instead receive care through private neurology practices. To better estimate prevalence and understand the full impact of MD, the inclusion of other sources of healthcare besides outpatient neuromuscular clinics is necessary.
To date, MD STARnet is the only population-based active surveillance system in the United States funded to study the major MDs. Although resource intensive, active case ascertainment using medical records enables confirmation of cases with MD, most of which cannot be identified through ICD codes alone. Cases are classified through a systematic case review by a committee of experienced clinicians. The methods used to build this cohort form the basis for future analyses on prevalence, disease progression, healthcare utilization and costs, disparities in access to care, and factors that influence outcomes for individuals with different types of MD. Currently, MD STARnet is conducting surveillance in six sites and is expanding the amount of clinical data collected for each of the nine MDs included in this study. MD STARnet is expected to contribute important epidemiologic and health outcome information about MDs. We anticipate that our experience will be useful for public health practitioners conducting surveillance of other rare diseases and for investigators interpreting data from MD STARnet.
ACKNOWLEDGMENTS
Work was carried out at each of the MD STARnet sites, with writing completed at the Centers for Disease Control and Prevention (CDC). This publication was supported by the Cooperative Agreement numbers, DD000830, DD000831, DD000832, DD000834, DD000835, DD000836, and DD000837 funded by CDC. The authors appreciate the work of Bailey Hill, for her assistance with data analysis. The authors also acknowledge with appreciation the work of the abstractors, local reviewers, data managers, and other MD STARnet personnel, without whom this article would not be possible.
Funding information
Centers for Disease Control and Prevention, Grant/Award Numbers: DD000830, DD000831, DD000832, DD000834, DD000835, DD000837, DD000836
Footnotes
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
DISCLAIMER
The findings and conclusions in this report are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Diseases Control and Prevention, the U.S. Department of Health and Human Services, or the authors’ affiliated institutions.
REFERENCES
- Andrews JG, Soim A, Pandya S, Westfield CP, Ciafaloni E, Fox DJ, Birnkrant DJ, Cunniff CM, Sheehan DW. (2016). Respiratory care received by individuals with Duchenne muscular dystrophy from 2000 to 2011. Respiratory Care 61 (10):1349–1359. doi: https://doi.org/10.4187/respcare.04676 [DOI] [PubMed] [Google Scholar]
- Caspers Conway K, Mathews KD, Paramsothy P, Oleszek J, Trout C, Zhang Y, Romitti PA. (2015). Neurobehavioral concerns among males with dystrophinopathy using population-based surveillance data from the Muscular Dystrophy Surveillance, Tracking, and Research Network. Journal of Developmental and Behavioral Pediatrics 36(6):455–463. doi: https://doi.org/10.1097/DBP.0000000000000177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2009). Prevalence of Duchenne/-Becker muscular dystrophy among males aged 5-24 years—Four states, 2007. Morbidity and Mortality Weekly Report 58(40):1119–1122. [PubMed] [Google Scholar]
- Ciafaloni E, Kumar A, Liu K, Pandya S, Westfield C, Fox DJ, Caspers Conway KM, Cunniff C, Mathews K, West N, Romitti PA, McDermott MP. (2016). Age at onset of first signs or symptoms predicts age at loss of ambulation in Duchenne and Becker muscular dystrophy: Data from the MD STARnet. Journal of Pediatric Rehabilitation Medicine 9(1):5—11. doi: https://doi.org/10.3233/PRM-160361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery AE. (1998). The muscular dystrophies. British Medical Journal (Clinical Research Ed) 317(7164):991–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox DJ, Kumar A, West NA, DiRienzo AG, James KA, Oleszek J. (2015). Trends with corticosteroid use in males with Duchenne muscular dystrophy born 1982–2001. Journal of Child Neurology 30(1):21–26. doi: https://doi.org/10.1177/0883073813517263 [DOI] [PubMed] [Google Scholar]
- Kim S, Campbell KA, Fox DJ, Matthews DJ, Valdez R. (2015). Corticosteroid treatments in males with Duchenne muscular dystrophy: Treatment duration and time to loss of ambulation. Journal of Child Neurology 30(10):1275–1280. doi: https://doi.org/10.1177/0883073814558120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb MM, West NA, Ouyang L, Yang M, Weitzenkamp D, James K, Ciafaloni E, Pandya S, DiGuiseppi C. (2016). Corticosteroid treatment and growth patterns in ambulatory males with Duchenne muscular dystrophy. The Journal of Pediatrics 173:207.e3–213.e3. doi: https://doi.org/10.1016/j.jpeds.2016.02.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah JK, Korngut L, Dykeman J, Day L, Pringsheim T, Jette N. (2014). A systematic review and meta-analysis on the epidemiology of Duchenne and Becker muscular dystrophy. Neuromuscular Disorders 24(6):482–491. doi: https://doi.org/10.1016/j.nmd.2014.03.008 [DOI] [PubMed] [Google Scholar]
- Mathews KD, Cunniff C, Kantamneni JR, Ciafaloni E, Miller T, Matthews D, Cwik V, Druschel C, Miller L, Meaney FJ, Sladky J, Romitti PA. (2010). Muscular dystrophy surveillance tracking and research network (MD STARnet): Case definition in surveillance for childhood-onset Duchenne/Becker muscular dystrophy. Journal of Child Neurology 25(9):1098–1102. doi: https://doi.org/10.1177/0883073810371001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LA, Romitti PA, Cunniff C, Druschel C, Mathews KD, Meaney FJ, Matthews D, Kantamneni J, Feng ZF, Zemblidge N, Miller TM, Andrews J, Fox D, Ciafaloni E, Pandya S, Montgomery A, Kenneson A. (2006). The muscular dystrophy surveillance tracking and research network (MD STARnet): Surveillance methodology. Birth Defects Research. Part A, Clinical and Molecular Teratology 76(11):793–797. doi: https://doi.org/10.1002/bdra.20279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya S, Andrews J, Campbell K, Meaney FJ. (2016). Rehabilitative technology use among individuals with Duchenne/Becker muscular dystrophy. Journal of Pediatric Rehabilitation Medicine 9(1):45–53. doi: https://doi.org/10.3233/PRM-160356 [DOI] [PubMed] [Google Scholar]
- Pandya SK, Campbell KA, Andrews JG, Meaney FJ, Ciafaloni E. (2016). Health services received by individuals with Duchenne/Becker muscular dystrophy. Muscle & Nerve 53(2):191–197. doi: https://doi.org/10.1002/mus.24727. [DOI] [PubMed] [Google Scholar]
- Reichard A, McDermott S, Ruttenber M, Mann J, Smith MG, Royer J, Valdez R. (2016). Testing the feasibility of a passive and active case ascertainment system for multiple rare conditions simultaneously: The experience in three US states. JMIR Public Health and Surveillance 2(2):e151. doi: https://doi.org/10.2196/publichealth.5516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romitti PA, Zhu Y, Puzhankara S, James KA, Nabukera SK, Zamba GK, Ciafaloni E, Cunniff C, Druschel CM, Mathews KD, Matthews DJ, Meaney FJ, Andrews JG, Conway KM, Fox DJ, Street N, Adams MM, Bolen J. (2015). Prevalence of Duchenne and Becker muscular dystrophies in the United States. Pediatrics 135(3):513–521. doi: https://doi.org/10.1542/peds.2014-2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MG, Royer J, Mann J, McDermott S, Valdez R. (2017). Capture-recapture methodology to study rare conditions using surveillance data for fragile X syndrome and muscular dystrophy. Orphanet Journal of Rare Diseases 12(1):76. doi: https://doi.org/10.1186/s13023-017-0628-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MG, Royer J, Mann JR, McDermott S. (2017). Using administrative data to ascertain true cases of muscular dystrophy: Rare disease surveillance. JMIR Public Health and Surveillance 3(1):e2. doi: https://doi.org/10.2196/publichealth.6720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnock BJ. (2009). Public health: What it is and how it works. Sudbury, MA: Jones & Bartlett Publishers, LLC; p 107–146. [Google Scholar]
- West NA, Yang ML, Weitzenkamp DA, Andrews J, Meaney FJ, Oleszek J, Miller LA, Matthews D, DiGuiseppi C. (2013). Patterns of growth in ambulatory males with Duchenne muscular dystrophy. The Journal of Pediatrics 163(6):1759–1763.e1751. doi: https://doi.org/10.1016/j.jpeds.2013.08.004. [DOI] [PubMed] [Google Scholar]