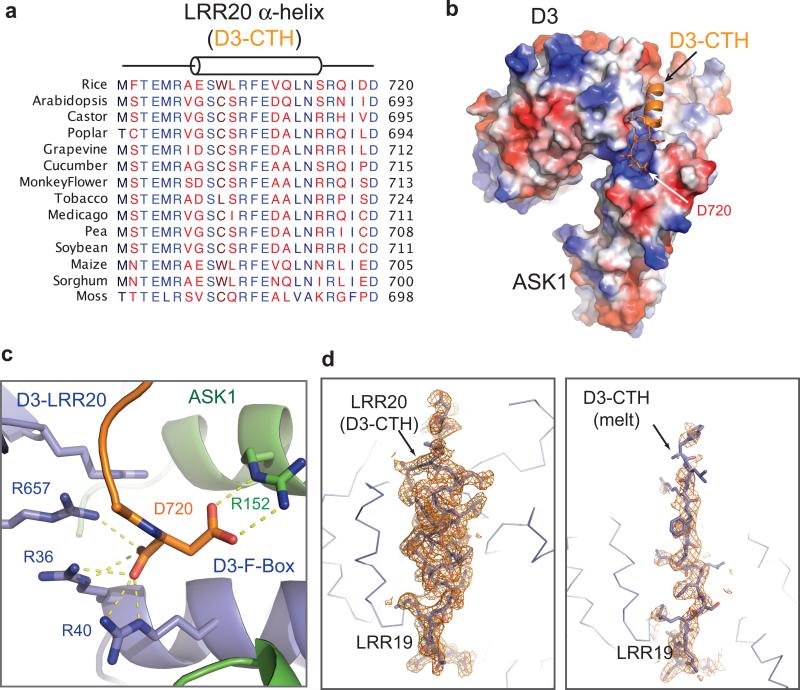
Extended Data Figure 1. Conservation and conformation of D3 C-terminal 𝛂-helix.
(a) Sequence alignment of the C-terminal regions of 14 MAX2/D3 orthologs. Highly conserved residues are colored in blue. (b) Electrostatic potential surface map of D3 with CTH shown in cartoon representation (orange). The C-terminus aspartic acid residue, Asp720, is anchored to a positively charged pocket. (c) Close-up view of the D3 extreme C-terminal residue, Asp720, and its interacting residues in D3 and ASK1. (d) Electron densities of the D3-CTH region in two different crystal forms, adopting either a regular helical conformation (left) or an extended conformation (right).