Summary
Left apical ballooning syndrome, also known as Takotsubo cardiomyopathy (TTC), characterized by transient left ventricular dysfunction is increasingly recognized worldwide. Predominantly affecting females, this condition mimics myocardial infarction and often occurs in the setting of emotional or physical stress. We report the case of a 77-year-old male who was admitted to the hospital for complete heart block and developed TTC after pacemaker implantation. To our knowledge, this is the first report of TTC development after pacemaker implantation in a male.
Keywords: Takotsubo cardiomyopathy, Left ventricular apical ballooning, Pacemaker, Male
Introduction
First described in Japan [1], left ventricular apical ballooning syndrome, or Takotsubo cardiomyopathy (TTC), is increasingly recognized worldwide [2]. Named after its resemblance to a takotsubo, or Japanese octopus trap, this condition is characterized by transient left apical and mid-ventricular systolic dysfunction with basal area hyperkinesis in the absence of significant coronary artery disease. TTC is often provoked by intense physical or emotional stress thus dubbing it the “broken heart syndrome” [3]. Patients presenting with this condition can be particularly alarming as their symptoms can mimic an acute coronary syndrome (often ST elevation myocardial infarction), with electrocardiographic (ECG) findings including ST elevations and T wave inversions, and elevations in cardiac-specific enzymes [2].
While the pathogenesis of this condition is not well-understood, theories including excess catecholamine stimulation, coronary artery spasm, and microvascular dysfunction have been proposed [3]. Also unexplained is the overwhelming predominance of this syndrome in elderly women as compared to men [3].
In recent years, a few reports have documented the occurrence of TTC after pacemaker implantation and have proposed TTC as a possible complication of this procedure [4], [5]. To our knowledge the case described below is the first case of TTC occurring in a male after pacemaker implantation.
Case report
A 77-year-old Dominican male with a history of diabetes mellitus, hypertension, and stroke presented to the emergency department after experiencing intermittent dizziness and increasing fatigue for the preceding two weeks. During this interval, the patient visited a doctor in the Dominican Republic on two occasions and was told to stop taking his anti-hypertensive medication (lisinopril and hydrochlorothiazide). On the day prior to presentation, the patient experienced near-syncope while arising from bed. The symptoms resolved, but the patient decided to come to the USA for further evaluation.
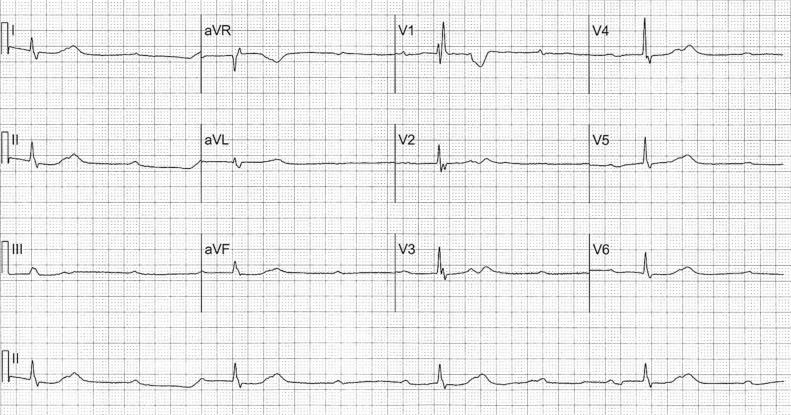
On presentation, the patient denied complaints of chest pain, shortness of breath, or dizziness. On examination his blood pressure was 150/57 mmHg with a heart rate of 25 beats per minute (bpm). He had no evidence of jugular venous distension or peripheral edema, his lungs were clear bilaterally and his heart examination was notable for bradycardia and a systolic flow murmur at the left lower sternal border. He was alert and oriented and in no apparent distress. A portable chest X-ray revealed no significant pulmonary edema or cardiomegaly. ECG revealed sinus rhythm with an atrial rate at 80 bpm, complete heart block, and a ventricular escape at 22 bpm with right bundle branch morphology (Fig. 1). Laboratory evaluation was remarkable only for a creatinine of 1.8 mg/dL. An echocardiogram to assess left ventricular (LV) function revealed normal LV systolic function [ejection fraction (EF) 76%] and no evidence of wall motion abnormalities (Fig. 2A and B).
Figure 1.
Baseline electrocardiogram revealed complete heart block with a ventricular escape at 22 bpm and right bundle branch morphology.
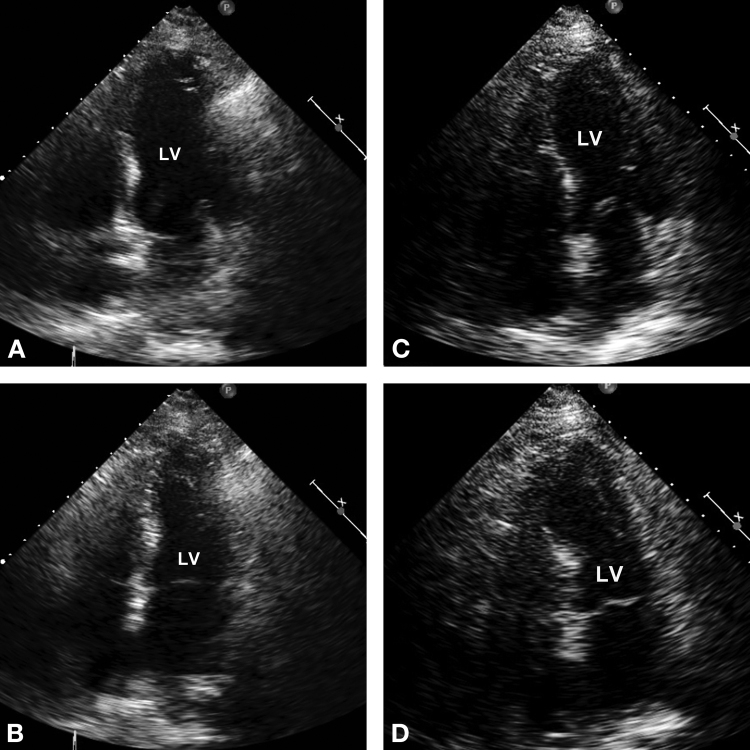
Figure 2.
Baseline echocardiogram at end-diastole (A) and end-systole (B) performed in the morning prior to pacemaker implantation revealed normal left ventricular ejection fraction (LVEF) (76%) and no wall motion abnormalities; echocardiogram post-pacemaker implantation at end-diastole (C) and end-systole (D) revealed an LVEF of 32% with mid-apical, interventricular, septal, apical, anterior, and inferior wall akinesis. The majority of these findings resolved within 24 h with complete resolution of all wall motion abnormalities on echocardiography performed at two-month follow-up.
The patient was asymptomatic and hemodynamically stable so he was referred for pacemaker implantation later that morning. A dual chamber pacemaker (atrial pacing lead, Guidant, # 4136, pulse generator, Boston Scientific, # 5603, St. Paul, MN, USA) was placed with a right atrial lead and a right ventricular septal lead. Conscious sedation was used during the implantation for patient comfort. Post-implantation ECG showed a paced rhythm at 80 bpm with a left bundle branch block pattern. The patient was transferred to the cardiac intensive care unit (CICU) for further observation.
Shortly after returning to the CICU the patient began complaining of extreme shortness of breath. Audible stridor was present without significant wheezing on lung exam. The patient became hypoxic despite receiving supplemental oxygen (100% FIO2 via nonrebreather mask) and became very agitated, noting pain in his throat and chest. The patient was intubated in light of his worsening respiratory status and a chest X-ray and echocardiogram were obtained. Chest X-ray revealed significant pulmonary edema. The echocardiogram was without evidence of pericardial effusion or tamponade, but was significant for mid-apical, interventricular, septal, apical, anterior and inferior wall akinesis and an EF of 32% (Fig. 2C and D). An ECG was performed and unchanged. Troponin-I and creatine kinase were 0.69 μg/L and 144 U/L, respectively. Later that day, the patient was transferred for cardiac catheterization in light of the above echocardiographic findings.
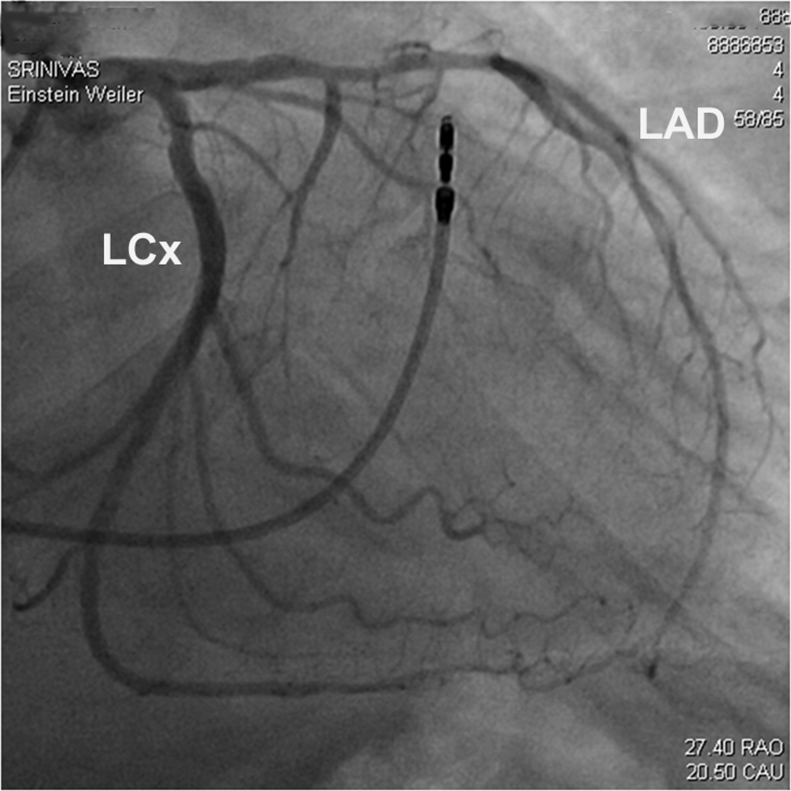
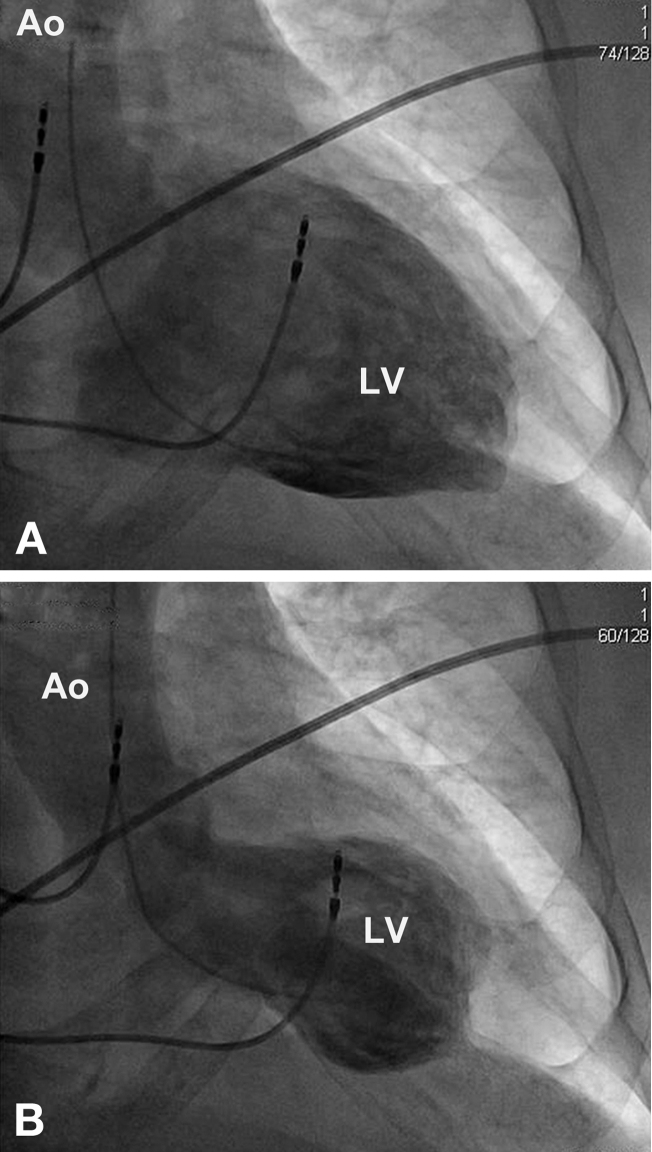
Cardiac catheterization showed LV end-diastolic pressure to be 20 mmHg, and non-obstructive stenosis of the mid-left anterior descending (LAD) artery with thrombolysis in myocardial infarction 3 flow (Fig. 3). Left ventriculogram showed overall preserved LV function with hyperdynamic basal LV segments (Fig. 4A and B).
Figure 3.
Cardiac angiography. Right anterior oblique view of the left anterior descending (LAD) artery revealed non-obstructive stenosis of the mid-LAD with thrombolysis in myocardial infarction-3 flow. LCx, left circumflex artery.
Figure 4.
Ventriculogram at end-diastole (A) and end-systole (B). Ao, aorta; LV, left ventricle.
The patient was extubated successfully soon after catheterization and mild diuresis. Echocardiography performed the following day revealed preserved LV systolic function with an EF of 55% and persistent mid to apical septal akinesis. Repeat cardiac biomarkers also trended toward normal. While hospitalized, the patient was initiated on aspirin, clopidogrel, simvastatin, furosemide, and lisinopril therapy, with the addition of metoprolol on the day prior to discharge. The patient remained hemodynamically stable and clinically euvolemic, and was discharged home six days after admission. A follow-up echocardiogram performed two months later revealed complete resolution of the wall motion abnormalities.
Discussion
As described in two prior reports [4], [5] the patient discussed above represents the development of TTC in the setting of pacemaker placement. This patient had a normal EF and LV wall motion on echocardiogram the morning of the procedure, after which he experienced severe respiratory distress and chest pain post-implantation with a marked decrease in EF and akinesis of the LV apical and inferior walls. The patient's respiratory distress was likely triggered by an acute onset of pulmonary edema in the setting of acute systolic dysfunction after pacemaker implantation.
This is the first case, to our knowledge, of the occurrence of TTC in a male after pacemaker implantation. TTC described in males is rare in any setting, as it occurs overwhelmingly in women [3]. A recent literature review evaluating the characteristics of TTC in males found that nearly three-quarters of reported cases occurred in the hospital setting, with more than half of those occurring peri-procedurally [6].
Additionally, the time to overall recovery of ventricular function in this case was remarkably rapid. The classical time frame for recovery of LV function is between 8 and 53 days [7], with a few reports of earlier contractile improvement [8], [9]. In this case, within less than 24 h from the episode onset, the EF and wall motion abnormalities had significantly improved.
The diagnostic criteria for TTC include transient LV hypokinesis or akinesis involving the apical and mid-ventricular segments, absence of obstructive coronary disease or acute plaque rupture on angiography, changes on ECG (including ST elevations or T wave inversion) or cardiac enzyme elevations (usually mildly to modestly increased), and the absence of other etiologies causing the clinical findings (including intracranial bleeding, pheochromocytoma and myocarditis) [8].
It is important to note that while this patient did have angiographic evidence of single-vessel coronary artery disease, the lesion's characteristics, the territory of LV dysfunction and rapid contractile improvement cannot be attributed to acute plaque rupture. In fact, there is increasing recognition of a subset of patients with TTC who have evidence of incidental coronary artery disease on angiography unrelated to the development of TTC [10].
As this case serves to highlight, the prevalence of this syndrome and conditions in which it occurs is probably more common than previously thought. Additionally, because the exact etiology of TTC is unclear, the treatment of this condition remains largely empirical and supportive with the use of traditional diuretics and vasodilator therapy. Further therapy including pressors, beta-agonists, and even selective beta-blockade remains controversial [11]. As supra-physiologic catecholamine levels and coronary artery spasm have been associated with the development of TTC, caution has been expressed with the use of such agents to prevent further catecholamine surge and unopposed alpha-stimulation (in the setting of beta-selective blockade) that may worsen myocardial function [11], [12]. Thus, while the overwhelming majority of TTC cases resolve spontaneously and with excellent prognosis, it is important to establish the appropriate diagnosis to avoid inappropriate interventions (including revascularization) and to aggressively manage their acute decompensation, as improvement in systolic function is to be expected [7].
References
- 1.Kawai S., Suzuki H., Yamaguchi H., Tanaka K., Sawada H., Aizawa T., Watanabe M., Tamura T., Umawatari K., Kawata M., Nakamura T., Yamanaka O., Okada R. Ampulla cardiomyopathy (“Takotsubo” cardiomyopathy) – reversible left ventricular dysfunction with ST segment elevation. Jpn Circ J. 2000;64:156–159. doi: 10.1253/jcj.64.156. [DOI] [PubMed] [Google Scholar]
- 2.Desmet W.J., Adriaenssens B.F., Dens J.A. Apical ballooning of the left ventricle: first series in white patients. Heart. 2003;89:1027–1031. doi: 10.1136/heart.89.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharkey S.W., Lesser J.R., Zenovich A.G., Maron M.S., Lindberg J., Longe T.F., Maron B.J. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111:472–479. doi: 10.1161/01.CIR.0000153801.51470.EB. [DOI] [PubMed] [Google Scholar]
- 4.Kurisu S., Inoue I., Kawagoe T., Ishihara M., Shimatani Y., Hata T., Nakama Y., Kijima Y., Kagawa E. Persistent left ventricular dysfunction in takotsubo cardiomyopathy after pacemaker implantation. Circ J. 2006;70:641–644. doi: 10.1253/circj.70.641. [DOI] [PubMed] [Google Scholar]
- 5.Chun S.G., Kwok V., Pang D.K., Lau T.K. Transient left ventricular apical ballooning syndrome (takotsubo cardiomyopathy) as a complication of permanent pacemaker implantation. Int J Cardiol. 2007;117:e27–e30. doi: 10.1016/j.ijcard.2006.11.125. [DOI] [PubMed] [Google Scholar]
- 6.Mendoza I., Zaitoun H., Novaro G. Takotsubo cardiomyopathy in men: different face, same disease. J Card Fail. 2010;16(Suppl. 1):S80. [abstract] [Google Scholar]
- 7.Gianni M., Dentali F., Grandi A.M., Sumner G., Hiralal R., Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006;27:1523–1529. doi: 10.1093/eurheartj/ehl032. [DOI] [PubMed] [Google Scholar]
- 8.Bybee K.A., Kara T., Prasad A., Lerman A., Barsness G.W., Wright R.S., Rihal C.S. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004;141:858–865. doi: 10.7326/0003-4819-141-11-200412070-00010. [DOI] [PubMed] [Google Scholar]
- 9.Kurisu S., Inoue I., Kawagoe T., Ishihara M., Shimatani Y., Nakama Y., Ohkawa K., Maruhashi T., Kagawa E., Dai K., Aokage T. Documentation of early improvement of left ventricular function in tako-tsubo cardiomyopathy. Int J Cardiol. 2006;114:e70–e72. doi: 10.1016/j.ijcard.2006.07.203. [DOI] [PubMed] [Google Scholar]
- 10.Kurisu S., Inoue I., Kawagoe T., Ishihara M., Shimatani Y., Nakama Y., Maruhashi T., Kagawa E., Dai K., Matsushita J., Ikenaga H. Prevalence of incidental coronary artery disease in tako-tsubo cardiomyopathy. Coron Artery Dis. 2009;20:214–218. doi: 10.1097/MCA.0b013e3283299260. [DOI] [PubMed] [Google Scholar]
- 11.Akashi Y.J., Goldstein D.S., Barbaro G., Ueyama T. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation. 2008;118:2754–2762. doi: 10.1161/CIRCULATIONAHA.108.767012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wittstein I.S., Thiemann D.R., Lima J.A., Baughman K.L., Schulman S.P., Gerstenblith G., Wu K.C., Rade J.J., Bivalacqua T.J., Champion H.C. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]