Abstract
Rats raised in an enriched condition (EC) show decreased stimulant self-administration relative to rats reared in an isolated condition (IC). However, few studies have examined the behavioral mechanisms underlying this environment-induced difference in self-administration. Because economic demand for drugs of abuse predicts addiction-like behavior in both humans and animals, we applied a behavioral economic analysis to cocaine self-administration data in EC and IC rats. During cocaine self-administration, the dose decreased across blocks of trials (0.75–0.003 mg/kg/inf), which allowed for a determination of demand intensity and demand elasticity. Demand intensity did not differ between EC and IC rats; however, cocaine was more elastic in EC rats relative to IC rats (i.e., EC rats were less willing to respond for cocaine as the unit price increased). When EC rats were placed in an isolated condition, demand elasticity decreased, whereas elasticity increased for IC rats placed in an enriched condition. Additionally, we applied behavioral economic analyses to previously published self-administration data and found that our results replicate past findings with cocaine and methylphenidate. To determine if differences in demand elasticity are specific to drug reinforcement, a separate group of rats was tested in sucrose or saccharin self-administration. Results showed that sucrose and saccharin were more elastic in EC rats relative to IC rats, and demand intensity was lower for saccharin in EC rats relative to IC rats. Overall, drug and nondrug reinforcers are more elastic in EC rats, which may account for the protective effects of environmental enrichment against stimulant self-administration.
Keywords: Behavioral economics, Cocaine, Environmental enrichment, Saccharin, Self-administration, Sucrose
1. Introduction
Environmental enrichment refers to animals raised in an environment with novel objects and social partners. To determine the effects of enrichment on physiological/behavioral processes, juvenile animals raised in an enriched condition (EC) are compared to juvenile animals raised in an isolated condition (IC; Bardo et al.2013; Simpson & Kelly 2011). Related to drug abuse, when tested as young adults, EC rats self-administer less amphetamine (Bardo et al. 2001; Green et al. 2002; Meyer & Bardo, 2015), cocaine (Puhl et al. 2012), and methylphenidate (Alvers et al. 2012) when either fixed ratio or progressive ratio schedules of reinforcement are used, and they acquire cocaine self-administration at a slower rate (Gipson et al. 2011) relative to IC rats. Thus, environmental enrichment protects against drug abuse vulnerability in rats.
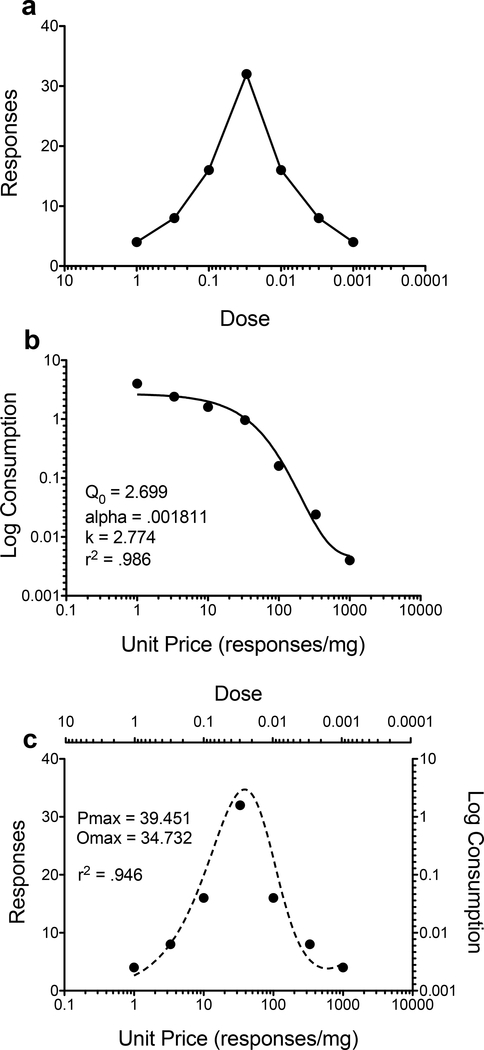
Studies assessing drug self-administration in EC and IC rats typically use procedures that measure the number of responses and/or infusions as the outcome variable (Alvers et al. 2012; Bardo et al. 2001; Green et al. 2002, 2010; Hofford et al. 2014; Meyer & Bardo 2015; Smith et al. 2009). Figure 1a shows an idealized dose response curve. Typically, manipulations that produce an upward shift or leftward shift in the dose response curve are considered to increase the reinforcing effects of the drug (Piazza et al. 2000). However, determining if an animal is more/less sensitive to the reinforcing effects of a drug can be difficult, as the term sensitivity is ambiguous in this context. For example, increased responding for drug reinforcement at each dose can be interpreted as increased sensitivity to that reinforcer because the animal is willing to work harder for that reinforcer, but an animal that emits fewer responses could be considered to be more sensitive to the drug because they need less drug to reach the hedonic set-point. This ambiguity can be observed when fixed ratio or PR schedules of reinforcement are used. To avoid the interpretational issues of using the term sensitivity to describe drug self-administration, behavioral economic principles can be used instead.
Figure 1.
Hypothetical dose response curve (a) and demand curve (b) generated from panel a. To generate the demand curve, consumption of a reinforcer is calculated by multiplying the number of responses (presented in a) by the drug dose. Unit price is calculated by dividing 1 by the dose. Higher Q0 values indicate greater consumption of a reinforcer at a minimally constrained price, and higher α values indicate reduced consumption of a reinforcer as price increases. These parameters can be used to derive the dose-response curve expressed as the number of responses for a reinforcer at each unit price (represented by dashed line; c). Pmax represents the price at which maximal responding occurs, and Omax represents the number of responses at Pmax. These values are calculated using the parameter estimates derived by the exponential demand function. Specifically, after applying the exponential demand function (see Introduction for the equation) to obtain Q0, k, and α, the following equation can be used to predict the number of responses from the demand curve: (10^(log(Q0)+k × (e(-αQ0C) – 1)))/10(C), inserting the parameters derived previously into the new equation.
Behavioral economics borrows from economic demand theory, which states that consumption of goods is often inversely related to their price (e.g., number of responses on a manipulandum; Hursh 1980). Specific to drug abuse, behavioral economics allows one to differentiate hedonic set-point (animal’s consumption of a drug when it is freely available) from essential value (how much of a good is consumed as its price increases; Oleson et al. 2011). In this case, unit price (the cost of obtaining a reinforcer) is influenced by both the response requirement and drug dose (Bickel et al. 1993). Generally, price of a reinforcer is manipulated by altering either the response requirement (Cosgrove & Carroll 2002; Koffarnus & Woods 2013) or adjusting the drug dose within a session (Hofford et al. 2016; Oleson & Roberts 2009; Zittel-Lazarini et al. 2007). By plotting consumption (calculated as responses × dose) as a function of price (calculated as 1 mg/dose), the traditional dose response plot presented in Figure 1a becomes transformed into the demand curve illustrated in Figure 1b. Behavioral economic analysis allows one to derive two measures from self-administration data: 1) demand intensity (denoted by Q0), which is consumption at a minimally constrained price and 2) demand elasticity (denoted by α), which is the rate at which consumption decreases as price increases (Hursh & Silberberg 2008). When a reinforcer is elastic, an organism will stop responding for it at a faster rate as the price increases. Additionally, breakpoints, the main dependent variable of PR tasks, can be extrapolated using demand curves (i.e., price at which consumption is 0).
The exponential demand function is defined as log Q = log(Q0) + k × (e(-αQ0C) – 1), where Q is consumption, Q0 is consumption at a minimally constrained price (intercept of function), C is unit price, k is a parameter that provides the best consumption range (in logarithmic units) for all individual subjects, and α is the inverse of essential value (slope of function). The parameters (Q0, k, and α) from the demand curve can then be used to derive the original dose response curve, including estimates of Pmax (price at which maximum consumption occurs) and Omax (amount of consumption that occurs at Pmax; Figure 1c).
One advantage of deriving economic demand curves for drugs is that we can predict addiction and relapse-like behavior in humans and animals. Greater demand for nicotine and opioids is associated with increased drug use during treatment Mackillop et al. 2016; Worley et al. 2015). In rats, economic demand for cocaine also predicts drug-seeking behavior in the absence of drug availability (Bentzley et al. 2014). Although these analyses show that essential value of drug stimuli can be altered by several manipulations, including performance on an impulsive choice task (Koffarnus & Woods 2013), exercise (Strickland et al. 2016), alternative monetary rewards (Greenwald & Steinmiller 2009), naltrexone treatment (Hofford et al. 2016), and self-administration history (Oleson & Roberts 2009), they have not been extensively applied to environmental enrichment studies.
The main purpose of this study was to determine if environmental enrichment during the juvenile period alters demand elasticity and/or intensity for cocaine, as well as to determine if any change in these parameters was permanent or reversible by switching the environmental context in adulthood (Experiment 1a). In addition, we applied behavioral economic analyses to previously published stimulant self-administration data from our laboratory (cocaine, methylphenidate, and methamphetamine) to determine generalizability across stimulants. Finally, to determine if changes in elasticity and/or intensity are specific to drug reinforcers, we tested rats in a self-administration paradigm for sucrose and saccharin reinforcement (Experiment 2). These studies, in addition to the reanalysis of previously collected data, were conducted to elucidate the behavioral mechanisms associated with environmental enrichment’s ability to attenuate drug abuse vulnerability. Considering other manipulations alter essential value of drug reinforcement without altering consumption at a minimally constrained price (e.g., Hofford et al. 2016; Koffarnus & Woods 2013; Strickland et al. 2016), we predicted that environmental enrichment would increase demand elasticity for drug and non-drug reinforcers but would not alter demand intensity.
2. Experimental Procedures
2.1. General Methods
2.1.1. Subjects.
A total of 40 male Sprague Dawley rats (Harlan Laboratories; Indianapolis, IN) arrived at postnatal day (PND) 21 and were housed in an EC or IC environment. EC rats were housed 8 per cage and were handled daily. EC rats were given an assortment of plastic objects (14 per cage), which varied in color, shape, and size, that were replaced and rearranged daily to maximize novelty. IC rats were single-housed in stainless steel hanging cages (17 × 24 × 20 cm) and were handled as briefly as possible during experimentation. EC rats were housed in large steel wire cages (122 × 61 × 45.5 cm), with solid steel floors and pine bedding, which were changed weekly. All rats were housed in a colony room held at constant temperature. Light and dark phases were on a 12:12-h cycle (lights on at 600; lights off at 1800), and all experimentation occurred during the light phase. Rats had unlimited access to food and water in their home cage during the entire experiment. Rats were cared for in accordance with the “Guide for the Care and Use of Laboratory Animals” (National Research Council 2011), and the Institutional Animal Care and Use Committee at the University of Kentucky approved all experimental procedures.
2.1.2. Apparatus.
Operant conditioning chambers (28 × 21 × 21 cm; ENV-008; MED Associates, St. Albans, VT) located inside sound-attenuating chambers (ENV-018M; MED Associates) were used. The front and back walls of the experimental chambers were made of aluminum, and the side walls were made of Plexiglas. There was either a recessed food tray (5 × 4.2 cm; Experiment 1) or dual cup liquid receptacle (5.1 × 5.1 cm; Experiment 2) located 2 cm above the floor in the bottom-center of the front wall. A 28-V white cue light was located 6 cm above each response lever. A white house light was mounted in the center of the back wall of the chamber. All responses and scheduled consequences were recorded and controlled by a computer interface. A computer controlled the experimental session using Med-IV software.
2.2. Experiment 1: Cocaine Self-Administration
2.2.1. Subjects.
A total of 24 rats (EC = 14; IC = 10) was used.
2.2.2. Drug.
Cocaine hydrochloride was obtained from NIDA (Bethesda, MD) and was dissolved in saline (0.9% NaCl).
2.2.3. Procedure.
Rats received one session of magazine training, in which sucrose-based pellets (45 mg dustless precision pellets; F0021; Bio-Serv, Frenchtown, NJ) were non-contingently delivered. For four sessions, rats responded on a lever (counterbalanced across rats) for a sucrose-based pellet according to a fixed ratio (FR) 1 schedule of reinforcement. Responses on the other lever were recorded but had no programmed consequences.
One day prior to surgery and 3 days afterwards, rats were treated with the non-opioid carprofen (5 mg/kg, s.c.). Rats were anesthetized with a mixture of ketamine (75 mg/kg; Butler Schein, Dublin OH)/xylazine (7.5 mg/kg; Akorn, Inc., Decatur IL)/acepromazine (0.75 mg/kg; Boehringer Ingelheim, St. Joseph, MO) delivered at 0.15 ml/100 g body weight (i.p.). A catheter was inserted into the right jugular vein, extended under the skin, and exited the body through an incision in the scalp. A cannula was attached to the end of the catheter and was secured to the skull using dental acrylic and four jeweler’s screws. Rats were given 7 days to recover, and catheter patency was checked by flushing a mixture of gentamicin (0.2 ml), heparin (0.6 ml), and saline each day.
For initial training, rats received five 110-min sessions of cocaine self-administration, in which a 0.1-ml infusion of cocaine (0.75 mg/kg/infusion delivered across 5.9 sec) was delivered to the jugular catheter via a silastic leash attached to the infusion pump. Each session began with illumination of the house light and extension of both levers. Responses on the active lever (counterbalanced across rats) were reinforced on an FR 1 schedule of reinforcement, whereas responses on the inactive lever were recorded but had no programmed consequences. During drug delivery, the stimulus lights were illuminated and remained on during the 20-s time-out period following drug delivery. Responses during the time-out period were recorded but had no programmed consequences.
Rats then received seven 110-min sessions of a threshold procedure (Oleson et al. 2011), in which the amount of cocaine delivered during each infusion decreased across eleven 10-min components in quarter log units (5.90, 3.32, 1.87, 1.05, 0.59, 0.33, 0.19, 0.11, 0.06, 0.03, 0.02 s), resulting in the following doses: 0.75, 0.42, 0.27, 0.15, 0.08, 0.05, 0.03, 0.02, 0.006, 0.003 mg/kg/inf and unit prices: 1.33, 2.38, 4.17, 7.14, 12.50, 20.00, 33.33, 50.00, 100.00, 166.67, 333.33 responses/mg. Responses on the active lever (FR 1) resulted in delivery of cocaine and illumination of both stimulus lights. Unlike the initial training sessions, there was no 20-s time-out period following a cocaine infusion. Following seven sessions, rats were placed in the opposite environmental housing condition (i.e., IC rats were placed in an enriched environment, and EC rats were placed in an isolated condition) and were immediately tested for seven additional sessions in the threshold procedure. Two EC rats did not complete all seven sessions after being placed in the opposite environment, and one EC rat and one IC rat did not complete all seven sessions in the original environment. Including these subjects did not affect the results because incomplete data have minimal impact on group parameter estimates when using maximum likelihood estimation of parameters (Pinheiro & Bates 2004). Similar to a previous report (Oleson & Roberts 2009), the final 10 doses were used in statistical analyses.
2.2.4. Statistical Analysis.
Because the focus of the current study was examining the effects of environmental enrichment on demand elasticity and/or demand intensity, only consumption data were analyzed. Cocaine consumption was calculated as: (infusions earned at each cocaine dose) × (cocaine dose) and was analyzed as a function of unit price (i.e., the number of responses necessary to receive 1 mg/kg cocaine). Exponential demand functions (Hursh & Silberberg 2008) were fit to individual data (averaged across the final three sessions during each phase of the experiment). The demand function was fit to the data via nonlinear mixed effects modeling (NLME; Hofford et al. 2016; Young et al. 2009) using the NLME package in the R statistical software package (Pinheiro et al. 2007). The NLME models defined Q0 and α as free parameters, k as a global constant (2.637), unit price as a fixed, continuous within-subjects factor, rearing condition as a fixed, nominal between-subjects factor, environmental switch as a nominal, within-subjects factor, and subject defined as a random factor. A significant interaction was probed with contrasts. To determine if changes in demand intensity and/or demand elasticity following environmental switch were associated with baseline (original environmental condition) Q0/α values, Pearson r correlation coefficients were calculated.
Statistical significance was defined as p <.05. Three rats (EC = 2; IC = 1) died during surgery, and one EC was excluded from data analyses because of catheter failure that occurred before starting the threshold procedure.
2.3. Applying Behavioral Economic Analyses to Previous Drug Self-Administration Results
2.3.1. Procedure.
Demand functions were applied to data from previously conducted self-administration studies: cocaine (Green et al. 2010), methylphenidate (Alvers et al. 2012), and methamphetamine (Hofford et al. 2014). In these previous studies, consumption for a dose was calculated across sessions, as opposed to Experiment 1a, in which dose was manipulated within session.
2.3.2. Statistical Analysis.
Analyses were conducted as described in Experiment 1a, with the exception that environmental switch was not included as a factor. The k constants associated with each study were 3.039, 1.901, and 2.327, respectively. Statistical significance was defined as p <.05.
In order to compare demand elasticity across each reinforcer, we normalized demand curves as previously described (Hursh and Winger 1995). See the Supplementary Materials for additional details.
2.4. Experiment 2: Sucrose and Saccharin Self-Administration
2.4.1. Subjects.
A total of 16 rats (EC = 8; IC = 8) was used.
2.4.2. Materials.
Sucrose and saccharin were purchased from Sigma Aldrich (St. Louis, MO).
2.4.3. Procedure.
Rats were tested for both sucrose (64%) and saccharin (1%) self-administration in counterbalanced order across treatment groups (EC vs. IC). Initially, rats received one session of magazine training, in which either sucrose (64%) or saccharin (1%) were delivered non-contingently to the receptacle for 5.9 s. Rats received 12 sessions in which responses on the active lever (FR 1) resulted in delivery of sucrose or saccharin. Responses on the inactive lever were recorded but had no programmed consequences.
Unlike Experiment 1, unit price during the threshold procedure was manipulated between sessions. This alteration was made because responses for sucrose and saccharin were minimal following the first couple of components of the within-session procedure. Even after decreasing the concentrations of sucrose (to 5%) and saccharin (to 0.05%), rats responded primarily during the first component. Rats were given one 10-min session at each unit price (1.00, 1.67, 3.33, 5.00, 10.00, 16.67, 33.33, 50.00, 100.00, 166.67, 333.33 responses/0.1 ml; in that order), and each session was separated by 24 h. The original sucrose (64%)/saccharin (1%) concentrations were used during the between-session manipulations of unit price. A single concentration was used because essential value of a reinforcer is not influenced by its magnitude (Hursh & Roma 2015). The concentrations were chosen because they have been shown to maintain operant responding in Sprague Dawley rats (Nolan et al. 2011; Sclafani & Ackroff 2003). Upon completion of the threshold procedure, rats were trained to respond for the other reinforcer.
2.4.4. Statistical Analysis.
The analyses were similar to those described in Experiment 1a. The NLME models defined unit price as a fixed, continuous within-subjects factor, rearing condition as a fixed, nominal between-subjects factor, reinforcer type as a fixed, nominal within-subjects factor, and subject defined as a random factor. The k constant was 2.690. A significant interaction was probed with contrasts. Statistical significance was defined as p <.05.
Additionally, we compared normalized consumption for sucrose/saccharin to drug reinforcers (see Supplementary Materials).
3. Results
3.1. Experiment 1
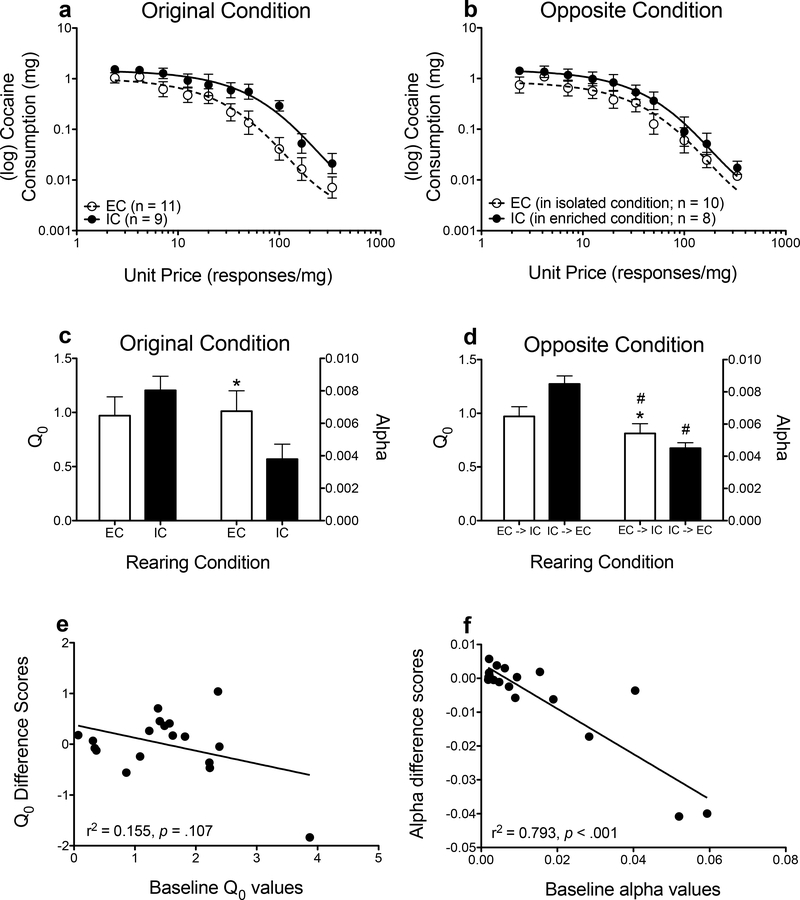
Figures 2a and 2b show demand functions, and Figures 2c and 2d show parameter estimates derived from the exponential demand function for rats raised in their original environment (Figs. 2a and 2c) and in the opposite environment (Figs. 2b and 2d). For demand elasticity, NLME analyses revealed main effects of rearing condition (F(1, 323) = 5.563, p =.019, environmental switch (F(1, 323) = 4.419 p =.036), and a rearing condition × environmental switch interaction (F(1, 323) = 4.928 p =.027; Figs. 2b and 2c). Overall, EC rats had higher α values, indicating that they were less willing to respond for cocaine as price increased. Placing EC rats into an isolated condition increased essential value for cocaine, whereas placing IC rats to an enriched condition had the opposite effect. Demand intensity did not differ across EC and IC rats, and moving rats to the opposite environments did not significantly affect Q0 values. Additionally, there was no interaction between environmental condition and environmental switch.
Figure 2.
Log mean consumption (± SEM) of cocaine in EC rats (n = 11 original condition; n = 10 opposite condition) and IC rats (n = 9 original condition; n = 8 opposite condition) in their original environment (a) and in the opposite environmental condition (b), plotted as a function of unit price. The lines (EC: dashed; IC: solid) indicate the best-fit curves from the demand function. Mean (± SEM) Q0 (left y-axis) and α (right y-axis) values derived from the demand function in the original (c) and opposite (d) environmental conditions. Correlations between baseline (in original environmental condition) values and differences scores (opposite environmental condition – original environmental condition) for Q0 (e) and α (f) values. *p<.05, relative to IC rats. #p <.05, relative to original housing condition.
To determine if changes in demand intensity and/or demand elasticity following environmental switch were associated with baseline (original environmental condition) Q0 (Fig. 2e) or α (Fig. 2f) values, Pearson r correlation coefficients were calculated on individual data. Although baseline Q0 values were not correlated with changes in demand intensity following environmental switch, baseline α values were negatively correlated with changes in demand elasticity following environmental switch (r(16) = −.891, r2 =.793, p <.001).
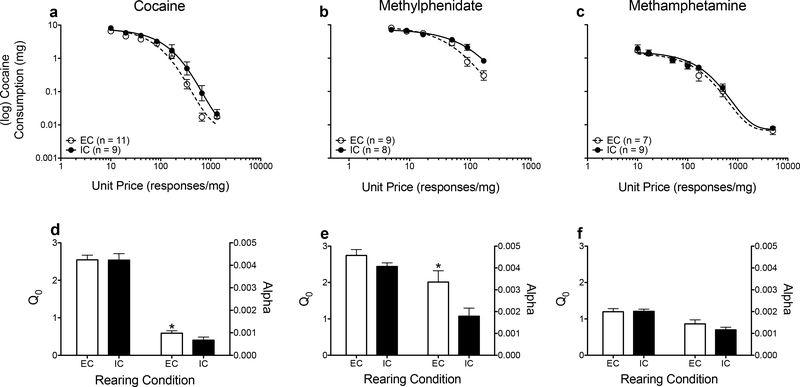
Figure 3 shows consumption of each stimulant reinforcer and corresponding demand functions derived from three previous experiments from our laboratory (panels a-c), as well as corresponding parameter estimates derived from the exponential demand function (panels d-f). For cocaine self-administration there were no significant differences in consumption in EC and IC rats (Fig. 3d), but cocaine was more elastic in EC rats relative to IC rats (F(1, 133) = 5.118, p =.025; Fig. 3d). Furthermore, methylphenidate was more elastic in EC rats relative to IC rats (F(1, 81) = 9.225, p =.003; Fig. 3e), but there were no differences in demand intensity (Fig. 3e). Demand intensity and elasticity did not differ in EC and IC rats for methamphetamine self-administration (Fig. 3f).
Figure 3.
Log mean consumption (± SEM) of cocaine (a), methylphenidate (b), and methamphetamine (c) in previous studies, plotted as a function of unit price. The lines (EC: dashed; IC: solid) indicate the best-fit curves from the demand function. Mean (± SEM) Q0 (left y-axis) and α (right y-axis) values derived from the demand function following self-administration of cocaine (d), methylphenidate (e) and methamphetamine (f). *p <.05, relative to IC rats. Note, panels were created using data from previously published work (Alvers et al. 2012; Green et al. 2010; Hofford et al. 2014). n = 7–11 per housing condition across experiments.
3.2. Experiment 2
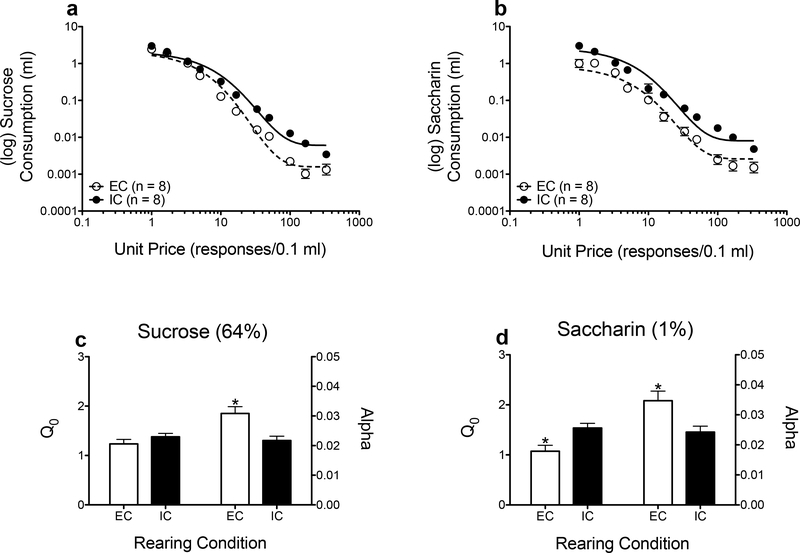
Figure 4 shows demand curves (Figs. 4a and 4b), as well as parameter estimates derived from the exponential demand function for sucrose (Fig. 4c) and saccharin (Fig. 4d). For demand elasticity, there was a main effect of rearing condition only (F(1, 320) = 16.119, p <.001). Demand elasticity for sucrose (Fig. 4c) and saccharin (Fig. 4d) was higher in EC rats relative to IC rats. Concerning demand intensity, NLME analyses revealed a significant rearing condition × reinforcer interaction (F(1, 320) = 6.987, p =.009). Contrasts showed that demand intensity for sucrose was similar across environmental conditions (Fig. 4c), but intensity was lower for saccharin in EC rats relative to IC rats (Fig. 4d).
Figure 4.
Log mean consumption (± SEM) of sucrose (a) and saccharin (b) self-administration in EC and IC rats (n = 8 each group), as plotted as a function of unit price. The lines (EC: dashed; IC: solid) indicate the best-fit curves from the demand function. Mean (± SEM) Q0 (left y-axis) and α (right y-axis) values derived from the demand function following sucrose (c) and saccharin (d) self-administration. *p <.05, relative to IC rats.
4. Discussion
4.1. Behavioral Economics of Drug Self-Administration
Previous studies show that EC rats respond less for drug reinforcement at low doses based on various stimulant dose-effect curves (Alvers et al. 2012; Bardo et al. 2001; Green et al. 2002, 2010; Meyer & Bardo 2015; Puhl et al. 2012). The results of the current study show that differential drug self-administration observed in EC and IC rats is driven primarily by demand elasticity, which is similar to the results we obtained after analyzing cocaine and methylphenidate self-administration data collected previously in our laboratory (i.e., Alvers et al. 2012; Green et al. 2010). Reversing housing conditions differentially changes the essential value, but not demand intensity, of cocaine in EC and IC rats. When EC rats are placed into an isolated environment, they respond more for cocaine, even as the price increases, indicating that the protective effects of enrichment are malleable and context-dependent. In addition, when IC rats are placed into an enriched environment, they respond less for cocaine as price increases, indicating that the detrimental effect of isolation is malleable. Although isolation increases cocaine self-administration in EC rats, elasticity is still greater for these rats relative to IC rats placed in an enriched condition, indicating that the protective effect of enrichment is retained to some extent when the contexts are reversed for one week. Collectively, these results suggest that the protective effects of enrichment are most effective when initiated during juvenile development, although a brief period of enrichment during adulthood is sufficient to reduce the negative consequences of developmental isolation rearing.
When EC rats are placed in an isolated condition, essential value of cocaine increases. These results are similar to previous research showing that animals raised in an enriched environment show increased cocaine-seeking behavior (i.e., decreased nose-pokes during extinction) 30 days after being placed in a standard environment (e.g., three rats housed together with no novel objects), whereas rats raised in a standard environment show decreased cocaine seeking after being placed in an enriched environment (Chauvet et al. 2009). In the current experiment, EC rats can be considered to live in an open economy where they trade goods (e.g., social interaction) with one another and have goods (e.g., novel toys) brought into the environment. Conversely, IC rats live in a closed economy, in which goods are not exchanged or brought into the environment. As such, switching environments can lead to changes in cross-price elasticity. When EC rats are placed in an isolated condition, they no longer have access to social partners or novel objects, which leads to an increase in demand for cocaine. However, demand for cocaine decreases for IC rats placed in an enriched environment because they now have access to other goods outside of the operant chamber (see Hursh 2000). Thus, it is possible that social interaction, novelty, or their combination serve as economic substitutes for stimulant reinforcers; future studies should explore this possibility further.
Although cocaine was more elastic in EC rats as evidenced by the current results and reexamination of previous data (Green et al. 2010), Hofford et al. (2016) did not observe differences in demand elasticity for cocaine in differentially reared rats. The methods used in the current study and the Hofford et al. (2016) study were similar, except for dose selections. Even though the current study used 11 doses with small percentage differences between each dose (0.75, 0.42, 0.27, 0.15, 0.08, 0.05, 0.03, 0.02, 0.01, 0.006, 0.003 mg/kg/inf), Hofford et al. (2016) used fewer doses with relatively large percentage differences between each dose (0.75, 0.27, 0.08, 0.03, 0.01, 0.003 mg/kg/inf), which may have prevented Hofford et al. (2016) from observing group differences in essential value for cocaine. Therefore, the demand function may be better suited when a larger number of unit prices (with smaller steps between each) is used.
In conclusion, the differences in self-administration observed in EC and IC rats are explained primarily by changes in demand elasticity of cocaine across each rearing condition. Viewed in microeconomic terms, cocaine is more elastic in EC rats relative to IC rats. As such, increases in unit price cause greater decreases in lever presses in EC rats relative to IC rats. However, when placed in an isolated condition during adulthood, the enrichment-induced increase in elasticity can be attenuated.
4.2. Behavioral Economics of Sucrose/Saccharin Self-Administration
The results of Experiment 2 show that essential value for sucrose and saccharin is also lower in EC rats relative to IC rats, and that EC rats respond less for saccharin at a minimally constrained price. These results indicate that the ability of environmental enrichment to decrease essential value of stimulant drugs generalizes to non-drug reinforcers. These results also provide evidence that differences in essential value for cocaine are not due to the direct motoric effects of the drug, as the pattern of responding observed in EC and IC rats is similar for sucrose and cocaine.
Whereas the results with sucrose match those obtained with cocaine, IC rats show increased demand intensity for saccharin. The discrepancy between the sucrose and saccharin results may be due to differences in caloric content between reinforcers. Because saccharin is non-caloric, rats should not become satiated as quickly as when consuming sucrose. As such, IC rats exhibit greater demand intensity compared to EC rats. The similar level of responding observed in EC and IC rats for sucrose reinforcement at a minimally constrained price may be due to a ceiling effect. Indeed, when the same within-session threshold procedure from Experiment 1a was used, a large proportion of rats did not respond after the second 10-min component, thus generating a demand curve was not possible. Despite this methodological change from Experiment 1a, we show a dissociation in demand intensity for sucrose and saccharin reinforcement. The major contribution of this experiment is that the results show the generalizability of environmental enrichment’s effects on non-drug reinforcement, both caloric and non-caloric. Overall, the current results show that environmental enrichment alters the essential value, but not necessarily hedonic set-point, of reinforcers. These findings may explain why enrichment is effective in reducing drug abuse vulnerability.
4.3. General Discussion
Social factors are an important research interest in the drug abuse field because evidence has implicated these factors with the initiation and maintenance of drug use, particularly in adolescents. Peer influences are known to influence the use of cigarettes/tobacco (Allen et al. 2003; Bahr et al. 2005), marijuana (Allen et al. 2003; Bahr et al. 2005) cocaine (Bahr et al. 1993), and alcohol (Allen et al. 2003; Bahr et al. 2005). Animal research shows that social stressors such as maternal separation (Cruz et al. 2008; Huot et al. 2001; Lewis et al. 2013), social confrontation/subordination (Haney et al. 1995; Miczek et al. 2011; Quadros & Miczek 2009), and social isolation (Bardo et al. 2001; Howes et al. 2000; Wolffgramm & Heyne 1991) increase drug abuse vulnerability (Miczek et al. 2008). By using animal models of social interaction, we can determine the underlying neurobehavioral mechanisms that may explain how certain social interactions, or lack of social interaction, alter susceptibility to substance use disorders.
By applying behavioral economic analyses to the current studies, as well as to previous studies assessing the effects of environmental enrichment on drug self-administration, we are able to show that enrichment increases demand elasticity for both drug and non-drug reinforcers. This alteration in elasticity provides an explanation for why enrichment is protective against stimulant reinforcers. Even when the price of obtaining a reinforcer is increased, IC rats are willing to defend that price. The increased elasticity observed in EC rats may be attributed primarily to the alternative reinforcement in the home cage, such as access to novel toys and social partners, suggesting that enrichment may decrease the demand for reinforcers outside of the home cage (an example of cross-price elasticity). This argument is strengthened by the finding that IC rats, after switching to an enriched environment, had α levels that were similar to EC rats housed in their original environment (~67% of the original EC values). Alternative reinforcement is known to decrease drug use in humans. For example, contingency management, in which individuals are provided alternative reinforcement (i.e., money or vouchers) for each drug-free urine sample provided, is effective in reducing drug use (see Walter & Petry 2016 for a recent review). Additionally, considering that measures of demand are associated with relapse-like behavior in humans (e.g., Mackillop et al. 2016; Worley et al. 2015) and rats (Bentzley et al. 2014), the current results can be used to explain why EC rats are more resistant to measures of reinstatement (e.g., Chauvet et al. 2009; Hofford et al. 2014).
One limitation of the current study, as well as the studies we reanalyzed, is the absence of additional groups of rats that were exposed to either social peers alone or novel toys alone. These comparison groups are important for determining if the decreased drug abuse vulnerability observed in EC rats is due to social interaction, exposure to novelty, or a combination of the two. Although we did not include these comparison groups, previous work shows that exposure to social cohorts, but not novelty itself, protects against the escalation of cocaine self-administration in rats (Gipson et al. 2011). Thus, we can infer that the protective effects of environmental enrichment are likely due, at least in part, to group housing.
Another limitation is the exclusive use of male rats in the current study. Because female rats tend to show increased drug self-administration (e.g., Lynch & Carroll 1999), determining if environmental enrichment differentially alters demand elasticity in males and females is of merit. Because most studies examining the effects of enrichment on drug self-administration include male rats only (Alvers et al. 2012; Gipson et al. 2011; Green et al. 2002; Hofford et al. 2014, 2016; Meyer & Bardo 2015; Puhl et al. 2012), we did not include female rats in these studies. Although there is evidence that environmental enrichment is effective in reducing amphetamine self-administration in both males and females (Bardo et al. 2001), future studies should focus on sex as a factor.
In conclusion, we applied microeconomic principles to drug self-administration data to explain why enrichment protects against drug abuse vulnerability. Our results show that EC rats treat both drug and non-drug reinforcers as elastic goods; as such, increases in price lead to decreased consumption of that good. In contrast, the same reinforcers are more inelastic in IC rats. Because there are no currently approved pharmacotherapies to treat cocaine use disorder, understanding the environmental factors and behavioral mechanisms that influence drug use is important for designing effective treatment options.
Figure Captions
Supplementary Material
Acknowledgements
The current study was supported by NIH grants DA05312, DA12964, DA033373, and DA016176. We would like to thank Emily Denehy and Travis McCuddy for technical assistance. We would also like to thank Kristin Alvers, Dr. Thomas Green, and Dr. Rebecca Hofford for allowing us to include data in the manuscript.
References
- Allen M, Donohue WA, Griffin A, Ryan D, Turner MM, 2003. Comparing the influence of parents and peers on the choice to use drugs: a meta-analytic summary of the literature. Crim. Justice Behav 30, 163–186. [Google Scholar]
- Alvers KM, Marusich JA, Gipson CD, Beckmann JS, Bardo MT, 2012. Environmental enrichment during development decreases intravenous self-administration of methylphenidate at low unit doses in rats. Behav. Pharmacol 23, 650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr SJ, Hawks RD, Wang G, 1993. Family and religious influences on adolescent substance abuse. Youth Soc. 24, 443–465. [Google Scholar]
- Bahr SJ, Hoffmann JP, Yang X, 2005. Parental and peer influences on the risk of adolescent drug use. J. Prim. Prev 26, 529–551. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C, 2001. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology 155, 278–284. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Neisewander JL, Kelly TH, 2013. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol. Rev 65, 255–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Jhou TC, Aston-Jones G, 2014. Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proc. Natl. Acad. Sci. U.S.A 111, 11822–11827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Higgins ST, 1993. Behavioral economics: a novel experimental approach to the study of drug dependence. Drug Alcohol Dep. 33, 173–192. [DOI] [PubMed] [Google Scholar]
- Chauvet C, Lardeux V, Goldberg SR, Jaber M, Solinas M, 2009. Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology 34, 2767–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Carroll ME, 2002. Effects of bremazocine on self-administration of smoked cocaine base and orally delivered ethanol, phencyclidine, saccharin, and food in rhesus monkeys: a behavioral economic analysis. J. Pharmacol. Exp. Ther 301, 993–1002. [DOI] [PubMed] [Google Scholar]
- Cruz FC, Quadros IM, Planeta CS, Miczek KA, 2008. Maternal separation stress in male mice: long-term increases in alcohol intake. Psychopharmacology 201, 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Beckmann JS, El-Maraghi S, Marusich JA, Bardo MT, 2011. Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology 214, 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Roybal CN, Winstanley CA, Theobald DE, Birnbaum SG, Graham AR, Unterberg S, Graham DL, Vialou V, Bass CE, Terwilliger EF, Bardo MT, Nestler EJ, 2010. Environmental enrichment produces a behavioral phenotype mediated by low cyclic adenosine monophosphate response element binding (CREB) activity in nucleus accumbens. Biol. Psychiatry 67, 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, Gehrke BJ, Bardo MT, 2002. Environmental enrichment decreases intravenous self-administration in rats: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology 162, 373–378. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Steinmiller CL, 2009. Behavioral economic analysis of opioid consumption in heroin-dependent individuals: effects of alternative reinforcer magnitude and post-session drug supply. Drug Alcohol Dep. 104, 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Maccari S, Le Moal M, Simon H, Piazza PV, 1995. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res. 698, 46–52. [DOI] [PubMed] [Google Scholar]
- Hofford RS, Beckmann JS, Bardo MT, 2016. Rearing environment differentially modulates cocaine self-administration after opioid pretreatment: a behavioral economic analysis. Drug Alcohol Dep. 167, 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofford RS, Darna M, Wilmouth CE, Dwoskin LP, Bardo MT, 2014. Environmental enrichment reduces methamphetamine cue-induced reinstatement but does not alter methamphetamine reward or VMAT2 function. Behav. Brain Res 270, 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes SR, Dalley JW, Morrison CH, Robbins TW, Everitt BJ, 2000. Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology 151, 55–63. [DOI] [PubMed] [Google Scholar]
- Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM, 2001. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology 158, 366–373. [DOI] [PubMed] [Google Scholar]
- Hursh SR, 1980. Economic concepts in the analysis of behavior. J. Exp. Anal. Behav 34, 219–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, 2000. Behavioral economic concepts and methods for studying health behavior, in Bickel WK, Vuchinich RE (Eds.), Reframing Health Behavioral Change with Behavioral Economics, Lawrence Erlbaum Associates, Mahwah, New Jersey, pp. 27–62. [Google Scholar]
- Hursh SR, Roma PG, 2015. Behavioral economics and the analysis of consumption and choice. Manage. Decis. Econ 37, 224–238. [Google Scholar]
- Hursh SR, Silberberg A, 2008. Economic demand and essential value. Psychol. Rev 115, 186–198. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Winger G, 1995. Normalized demand for drugs and other reinforcers. J. Exp. Anal. Behav 64, 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Woods JH, 2013. Individual differences in discount rate are associated with demand for self-administered cocaine, but not sucrose. Addict. Biol 18, 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CR, Staudinger K, Scheck L, Olive MF, 2013. The effects of maternal separation on adult methamphetamine self-administration, extinction, reinstatement, and MeCP2 immunoreactivity in the nucleus accumbens. Front. Psychiatry 4, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME, 1999. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology 144, 77–82. [DOI] [PubMed] [Google Scholar]
- Mackillop J, Murphy CM, Martin RA, Stojek M, Tidey JW, Colby SM, Rohsenow DJ, 2016. Predictive validity of a cigarette purchase task in a randomized controlled trial of contingent vouchers for smoking in individuals with substance use disorders. Nicotine Tob. Res 18, 531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AC, Bardo MT, 2015. Amphetamine self-administration and dopamine function: assessment of gene × environment interactions in Lewis and Fischer 344 rats. Psychopharmacology 232, 2275–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Nikulina EM, Shimamoto A, Covington HE 3rd, 2011. Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats. J. Neurosci 31, 9848–9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE, 2008. Social stress, therapeutics, and drug abuse: Preclinical models of escalated and depressed intake. Pharmacol. Ther 120, 102–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council, 2011. Guide for the care and use of laboratory animals. National Academies Press, Washington, DC. [Google Scholar]
- Nolan TA, Caudle RM, Neubert JK, 2011. Effect of caloric and non-caloric sweet reward solutions on thermal facial operant conditioning. Behav. Brain Res 216, 723–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Richardson JM, Roberts DC, 2011. A novel IV cocaine self-administration procedure in rats: differential effects of dopamine, serotonin, and GABA drug pre-treatments on cocaine consumption and maximal price paid. Psychopharmacology 214, 567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Roberts DCS, 2009. Behavioral economic assessment of price and cocaine consumption following self-administration histories which produce escalation of either final ratios or intake. Neuropsychopharmacology 34, 796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Moal ML 2000. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J. Neurosci 20, 4226–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM, 2004. Mixed effects models in S and S-Plus. Springer, New York, NY. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, 2007. Linear and nonlinear mixed effects models. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Puhl MD, Blum JS, Acosta-Torres S, Grigson PS, 2012. Environmental enrichment protects against the acquisition of cocaine self-administration in adult male rats, but does not eliminate avoidance of a drug-associated saccharin cue. Behav. Pharmacol 23, 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadros IMH, Miczek KA, 2009. Two modes of intense cocaine bingeing: increased persistence after social defeat stress and increased rate of intake due to extended access conditions in rats. Psychopharmacology 206, 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K, 2003. Reinforcement value of sucrose measured by progressive ratio operant licking in the rat. Physiol. Behav 79, 663–670. [DOI] [PubMed] [Google Scholar]
- Simpson J, Kelly JP, 2011. The impact of environmental enrichment in laboratory rats—behavioural and neurochemical aspects. Behav. Brain Res 222, 246–264. [DOI] [PubMed] [Google Scholar]
- Smith MA, Iordanou JC, Cohen MB, Cole KT, Gergans SR, Lyle MA, Schmidt KT, 2009. Effects of environmental enrichment on sensitivity to cocaine in female rats: importance of control rates of behavior. Behav. Pharmacol 20, 312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland JC, Abel JM, Lacy RT, Beckmann JS, Witte MA, Lynch WJ, Smith MA, 2016. The effects of resistance exercise on cocaine self-administration, muscle hypertrophy, and BDNF expression in the nucleus accumbens. Drug Alcohol Dep. 163, 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter KN, Petry NM, 2016. Motivation and contingency management treatments for substance use disorders. Curr. Top. Behav. Neurosci 27, 569–581. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J, Heyne A, 1991. Social behavior, dominance, and social deprivation of rats determine drug choice. Pharmacol. Biochem. Behav 38, 389–399. [DOI] [PubMed] [Google Scholar]
- Worley MJ, Shoptaw SJ, Bickel WK, Ling W, 2015. Using behavioral economics to predict opioid use during prescription opioid dependence treatment. Drug Alcohol Depend. 148, 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ME, Clark MH, Goffus A, Hoane MR, 2009. Mixed effects modeling of Morris water maze data: advantages and cautionary notes. Learn. Motiv 40, 160–177. [Google Scholar]
- Zittel-Lazarini A, Cador M, Ahmed SH, 2007. A critical transition in cocaine self-administration: behavioral and neurobiological implications. Psychopharmacology 192, 337–346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.