Summary
Legionella infection, although commonly seen as pneumonia, can also manifest systemic involvement. Here, we describe a case of sporadic Legionella infection coinciding with pneumonia, rhabdomyolysis, renal failure, and severe left ventricular dysfunction, which subsequently developed refractory septic shock. An endomyocardial biopsy revealed no findings of interstitial inflammatory infiltrates. After 3 days of intensive care, including percutaneous cardiopulmonary support, intraaortic balloon pumping, and continuous hemodialysis with endotoxin adsorption therapy, left ventricular wall motion improved spontaneously in accordance with a decrease in the concentration of inflammatory cytokines. Cardiac complications are rare but Legionella infection should be considered as a possible etiology of left ventricular dysfunction in patients with sepsis.
Keywords: Legionella, Left ventricular dysfunction, Cytokine
Introduction
Legionella infection is one of the common causes of community-acquired pneumonia, which becomes potentially fatal and develops rapidly. Cardiac complications during Legionella infection are rare, but can present as pericarditis, endocarditis, and/or myocarditis with decreased left ventricular wall motion. However, there have been only two documented adult cases that demonstrated an interstitial inflammatory reaction in the myocardium [1], [2], and the mechanisms of left ventricular dysfunction with Legionella infection remain unclear. Here, we report a case of severe left ventricular dysfunction caused by Legionella infection, which subsequently developed into refractory septic shock. In this case, the endomyocardial biopsy revealed no findings of interstitial inflammatory infiltrates and left ventricular wall motion improved spontaneously in accordance with a decrease in the concentration of inflammatory cytokines.
Case report
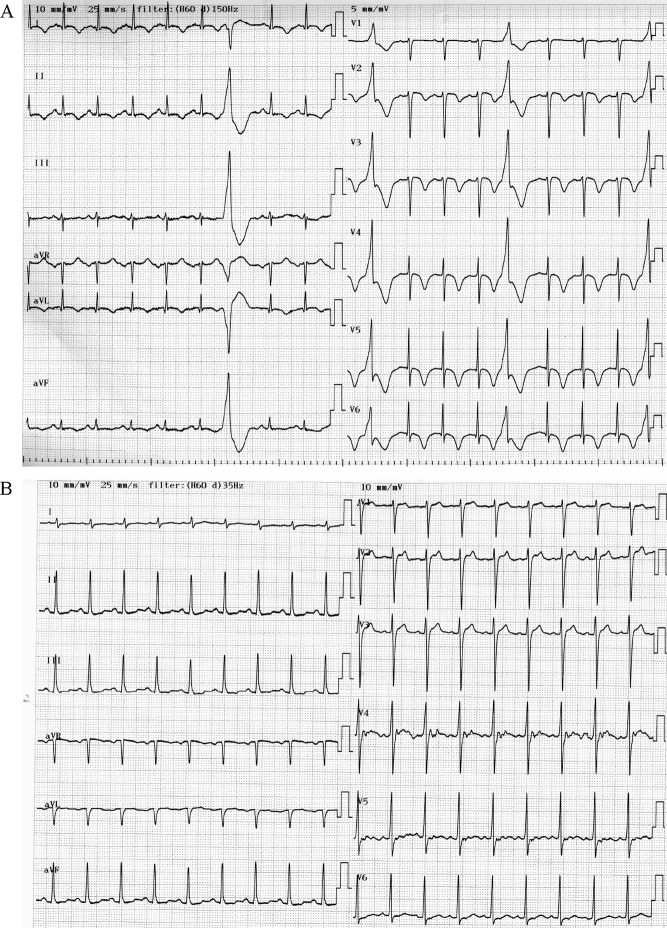
A previously healthy 58-year-old man was admitted due to a high fever and dyspnea. On physical examination, the patient had a temperature of 38.3 °C, with a regular heart rate of 108 beats/min, a blood pressure of 120/78 mmHg, a respiratory rate of 32 breaths/min, and an arterial oxygen saturation of 90% on room air. Laboratory investigations showed a white blood cell count of 16100/μl, C-reactive protein of 26.9 mg/dl, and a platelet count of 12.3 × 104/μl. Other significant values were as follows: aspartate transaminase, 2167 U/l; alanine aminotransferase, 488 U/l; total creatine kinase, 93320 U/l; creatine kinase MB isoform, 435 ng/ml; and creatinine 3.4 mg/dl. A chest radiograph on admission revealed dense right upper lung consolidation (Fig. 1). An electrocardiogram showed sinus tachycardia with T-wave inversions in the anterolateral leads and frequent ventricular premature beats (Fig. 2A). A trans-thoracic echocardiogram showed global hypokinesia of the left ventricle with an ejection fraction of 30%. Emergency cardiac catheterization demonstrated normal coronary arteries. The cardiac index was 2.7 l/min/m2. The endomyocardial biopsy from the right ventricle revealed findings of neither interstitial inflammatory infiltrates nor interstitial edema (Fig. 3). On day 2, the patient's condition deteriorated, and the cardiac index fell to 1.9 l/min/m2 with a state of shock despite intensive pharmacologic therapy. He required percutaneous cardiopulmonary support (PCPS), intraaortic balloon pumping (IABP), and continuous hemodialysis. A urinary antigen test for Legionella was positive and the patient was treated with imipenem, ciprofloxacin, and erythromycin. In addition, steroid pulse therapy (1000 mg/day, day 2–4), intravenous gamma globulin treatment (25 g/day, day 2–4), and endotoxin adsorption therapy (day 3) were started for severe Legionella infection. The trans-thoracic echocardiogram on day 3 showed severely decreased left ventricular wall motion with an ejection fraction of 20% (Fig. 4, Table 1, Video 1). On day 5, left ventricular wall motion, as demonstrated by echocardiography, began to improve spontaneously with an ejection fraction of 40% (Fig. 4, Table 1, Video 2). The patient became hemodynamically stable and could be weaned from the PCPS and IABP on day 5. Serum concentrations of tumor necrosis factor alpha (TNF-α) before and after endotoxin adsorption therapy were 15.6 pg/ml and 3.96 pg/ml, and those of interleukin (IL)-6 were 428 pg/ml and 68.5 pg/ml. The radiographic findings of the lungs slowly, but gradually improved with antibiotic treatment, and on the 15th hospital day, the patient was taken off the ventilator. On day 20, left ventricular wall motion returned to normal as shown by echocardiography (Table 1) and T-wave inversions in the anterolateral leads disappeared on the electrocardiogram (Fig. 2B). He could be weaned from hemodialysis on day 22 and discharged on day 128.
Figure 1.
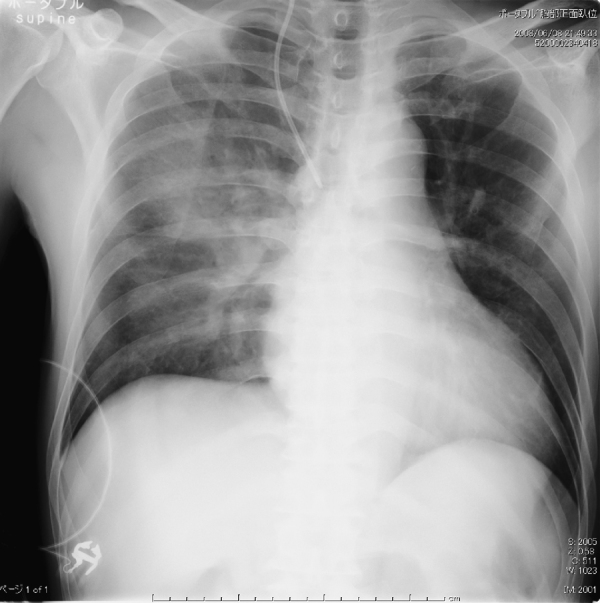
Chest radiograph showed lung consolidation in the right middle and upper lobe.
Figure 2.
(A) An electrocardiogram on admission indicated sinus tachycardia with T-wave inversions in the anterolateral leads and ventricular premature contractions. (B) An electrocardiogram on day 10.
Figure 3.
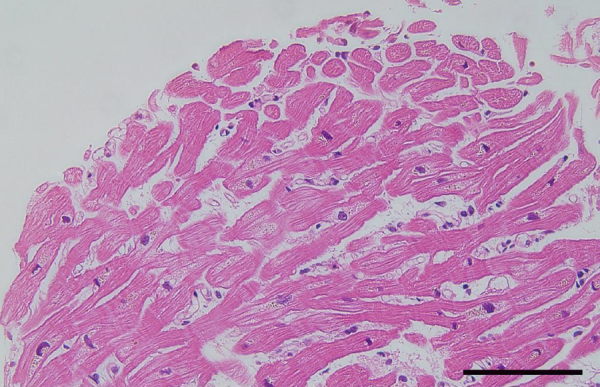
A histopathologic specimen showed neither infiltration of inflammatory cells nor interstitial edema (original magnification ×200, bar: 50 μm).
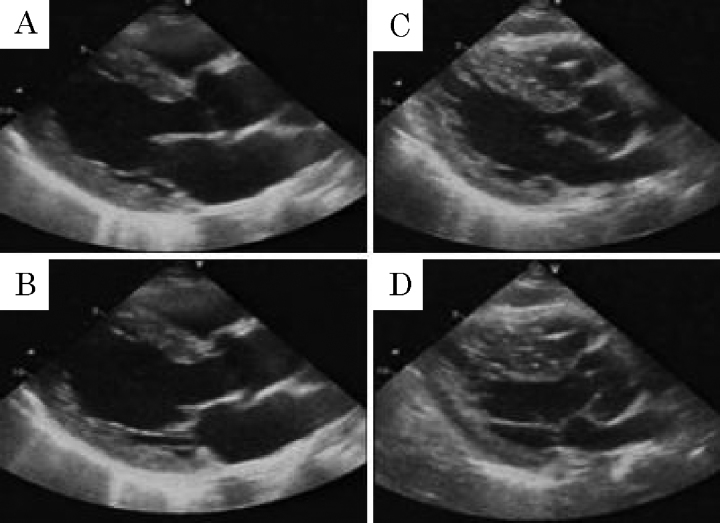
Figure 4.
The echocardiogram on day 3 showed severely decreased left ventricular wall motion (A: endo-diastolic phase, B: endo-systolic phase). On day 5, left ventricular wall motion improved spontaneously (C: endo-diastolic phase, D: endo-systolic phase).
Table 1.
Echocardiographic data.
| Day 3 | Day 5 | Day 20 | |
|---|---|---|---|
| LVEDd (cm) | 5.6 | 5.4 | 5.4 |
| LVEDs (cm) | 5.2 | 4.3 | 3.5 |
| LVEF (%) | 20 | 40 | 63 |
| IVS (cm) | 1.4 | 1.4 | 1.3 |
| PW (cm) | 1.3 | 1.3 | 1.3 |
| TVPG* (mmHg) | 20 | 25 | |
| IVC (cm) | 1.0 | 1.0 |
LVEDd, left ventricular end-diastolic diameter; LVEDs, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; IVS, interventricular septum; PW, posterior wall; TVPG, tricuspid valve pressure gradient; IVC, inferior vena cava.
TVPG was calculated from the peak TR velocity using the modified Bernoulli equation.
Discussion
Prompt and accurate diagnosis is mandatory to treat patients with severe sepsis. In this case, intensive care such as antibiotics, steroids, and gamma globulin treatment, and mechanical support including endotoxin adsorption therapy resulted in improvement in both hemodynamic status and left ventricular function. There are a few possible etiologies of transient left ventricular dysfunction in this case. First, myocarditis is a rare complication but can be caused by Legionella infection. There have been only two adult cases in which myocarditis and infiltration of inflammatory cells were documented in the myocardium [1], [2]. In this case, an endocardial biopsy specimen revealed no interstitial inflammatory infiltrates. Legionnaire's disease organisms also could not be seen in the biopsy samples. In addition, left ventricular wall thickness by echocardiography showed no significant change throughout his clinical course. Second, there has been accumulating evidence that myocardial depression is common in patients with sepsis [3], [4]. Septic shock typically produces a low systemic vascular resistance and elevated cardiac output. In addition, myocardial function is known to be depressed not only during the “hypodynamic” phase, but also during the “hyperdynamic” phase [3], [5]. Both human and animal studies have clearly demonstrated that decreased contractility and impaired myocardial compliance are major factors leading to myocardial dysfunction in septic patients. Although the pathogenesis of septic shock is extraordinarily complex, a number of mediators and pathways have been shown to be associated with myocardial depression [3], [4]. Circulatory myocardial depressant factors, such as cytokines, prostanoids, and endothelin are thought to play important roles in cardiac depression during sepsis, although the cellular mechanisms underlying cytokine-mediated cardiomyopathy are not entirely clear [6]. Our patient's status and left ventricular wall motion improved spontaneously in accordance with a decrease in the concentration of TNF-α and IL-6. Of note, a number of studies have demonstrated that cytokine synergy plays a key role in septic myocardial dysfunction [3], [4]. Endotoxin adsorption therapy is likely to have favorable effects on not only the hemodynamic condition but also on pathophysiological mediators in septic patients [7]. Thus, not due to myocarditis, but severe cytokinemia with Legionella infection is, at least in part, the etiology of left ventricular dysfunction in this case.
In summary, Legionella infection should be considered as a possible etiology of left ventricular dysfunction in patients with sepsis. Inflammatory cytokines may be responsible for sepsis-associated myocardial depression and therapy that decreases these factors will be helpful when treating these patients.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jccase.2011.01.002.
Appendix A. Supplementary data
Echocardiography on day 3 showed diffuse left ventricular systolic dysfunction with global myocardial severe hypokinesis.
Echocardiography on day 5 showed improvement of impaired left ventricular wall motion.
References
- 1.White H.J., Felton W.W., Sun C.N. Extrapulmonary histopathologic manifestations of Legionnaires’ disease: evidence for myocarditis and bacteremia. Arch Pathol Lab Med. 1980;104:287–289. [PubMed] [Google Scholar]
- 2.de Lassence A., Matsiota-Bernard P., Valtier B., Franc B., Jardin F., Nauciel C. A case of myocarditis associated with Legionnaires’ disease. Clin Infect Dis. 1994;18:120–121. doi: 10.1093/clinids/18.1.120. [DOI] [PubMed] [Google Scholar]
- 3.Merx M.W., Weber C. Sepsis and the heart. Circulation. 2007;116:793–802. doi: 10.1161/CIRCULATIONAHA.106.678359. [DOI] [PubMed] [Google Scholar]
- 4.Court O., Kumar A., Parrillo J.E., Kumar A. Myocardial depression in sepsis and septic shock. Crit Care. 2002;6:500–508. doi: 10.1186/cc1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parrillo J.E., Parker M.M., Natanson C., Suffredini A.F., Danner R.L., Cunnion R.E. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990;113:227–242. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- 6.Flynn A., Chokkalingam Mani B., Mather P.J. Sepsis-induced cardiomyopathy: a review of pathophysiologic mechanisms. Heart Fail Rev. 2010;15:605–611. doi: 10.1007/s10741-010-9176-4. [DOI] [PubMed] [Google Scholar]
- 7.Cruz D.N., de Cal M., Piccinni P., Ronco C. Polymyxin-B hemoperfusion and endotoxin removal: lessons from a review of the literature. Contrib Nephrol. 2010;167:77–82. doi: 10.1159/000315921. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Echocardiography on day 3 showed diffuse left ventricular systolic dysfunction with global myocardial severe hypokinesis.
Echocardiography on day 5 showed improvement of impaired left ventricular wall motion.