Summary
We encountered a case of hypothyroidism showing Brugada-type electrocardiogram (ECG). A 52-year-old man was referred to our hospital in August 2009. Past medical history showed that liver dysfunction and face edema of unknown origin had been pointed out 1 year earlier. He was diagnosed with primary hypothyroidism at this admission. ECG exhibited first-degree atrio-ventricular block (0.24 s) and showed Brugada-type ST-segment elevation ≥2 mm followed by a negative T wave (coved type) in the V1, V2 leads. On genetic analysis, the patient demonstrated three common variants in the SCN5A gene, L1988R (c.5963 T>G), H558R (c.1673 A>G), and R1193Q (c.3578 G>A).
Brugada-type ECG disappeared when the thyroid function normalized. We hypothesize that Brugada-type ECG in hypothyroidism is modified not only by a direct effect of thyroid hormone, but also due to SCN5A variants.
Some SCN5A gene polymorphisms or mutations will induce changes on ECG when ion channels are affected by hypothyroidism.
Keywords: Brugada-type ECG, Hypothyroidism, SCN5A genes, Late potential
Introduction
Brugada syndrome is an inherited disorder characterized by ST-segment elevation in V1, V2 leads and increased susceptibility to ventricular arrhythmias and sudden cardiac death. We encountered a case of hypothyroidism with Brugada-type electrocardiogram (ECG). Genetic examination showed three common variants in SCN5A gene.
Case report
A 52-year-old man with diarrhea and cold sweating was referred to our hospital in August 2009. Medical history showed that face edema was noted in June 2008. Mild pericardial effusion and left ventricular hypertrophy were then detected by echocardiography. He had been advised to take 20 mg furosemide, when he became concerned about his edema. He did not have a family history of sudden death or attacks of syncope or palpitation.
On admission in August 2009, height was 177 cm, weight 65 kg, and body temperature 36.5 °C. Blood pressure was 110/84 mm Hg and pulse rate 46 min−1 with regular rhythm. His voice was deep and husky, myxo-edematous face and bilateral legs were observed. Aurantiasis cutis was noted in the hands. The thyroid gland was not palpable. Lambert sign was observed in the Achilles’ tendon.
On laboratory examination white blood cell count was 3290/μl, red blood cell count 375 × 104/μl, aspartate transaminase (AST) 51 IU/L, alanine transaminase (ALT) 22 IU/L, lactate dehydrogenase (LDH) 354 IU/L, creatine kinase (CK) 1299 IU/L, total cholesterol 295 mg/dl, thyroid stimulating hormone (TSH) >100 μIU/L, free T3 0.99 pg/ml, free T4 0.5 mg/dl, TSH stimulating antibody (TSAB) 785%, anti thyroglobulin antibody 11.5 U/ml and TSH receptor antibody 8.1%. Technetium-99m uptake in the thyroid gland was very low (0.22%). We diagnosed the patient as having primary hypothyroidism.
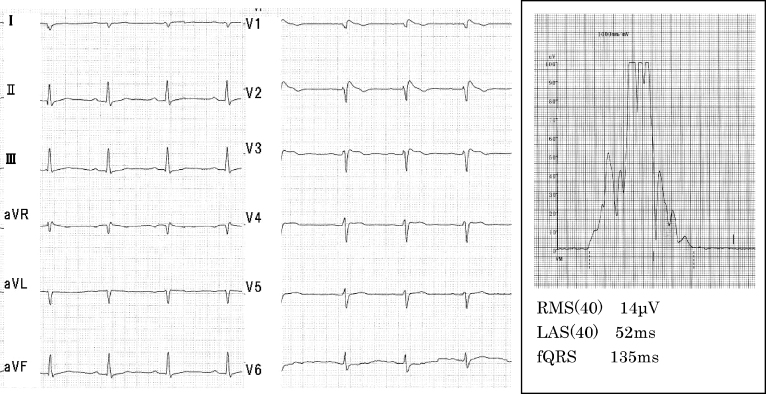
ECG exhibited sinus rhythm, right axis deviation with first-degree atrio-ventricular block (0.24 s) and showed Brugada-type ST-segment elevation of ≥2 mm followed by a negative T wave (coved type) in V1, V2 leads. The signal-averaged ECG (GE MAC5500, Waukesha, WI, USA) showed a positive value for the late potential. (Duration of low amplitude signals <40 μV in the terminal filtered QRS complex, 52 ms; root mean square voltage of the terminal 40 ms in the filtered QRS complex, 14 μV) (Fig. 1).
Figure 1.
Electrocardiogram and signal-averaged electrocardiogram on admission. RMS(40), root mean square voltage of the terminal 40 ms in the filtered QRS complex; LAS(40), duration of low amplitude signals <40 μV in the terminal filtered QRS complex; fQRS, duration of the filtered QRS.
The cardio-thoracic ratio was 54.6% on chest X-ray. Trans-thoracic echocardiography showed left ventricular hypertrophy (posterior wall of the left ventricle 12.5 mm, inter-ventricular septum 12.5 mm), and mild pericardial effusion. Compared with the findings on examination in July 2008, the volume of pericardial effusion had not changed but the ejection fraction had decreased from 74% to 67%.
The patient gave informed consent for genetic analysis, and demonstrated three common variants in the SCN5A gene, L1988R (c.5963 T>G), H558R (c.1673 A>G), and R1193Q (c.3578 G>A), which are identified in more than 1% of normal subjects.
After administration of levothyroxine sodium, thyroid and liver function improved over 2 months. ST-segment elevation of ECG and pericardial effusion also disappeared after 2 months of medication (Fig. 2). After 7 months of medication, the ECG changed again to Brugada-type, following administration of the classic anti-arrhythmic drug, pilsicainide (Fig. 3).
Figure 2.
Clinical course. AST, aspartate transaminase; ALT, alanine transaminase; LDH, lactate dehydrogenase; γGTP, γ-glutamyl transpeptidase; CK, creatine kinase; TSH, thyroid stimulating hormone.
Figure 3.
The electrocardiogram changed again to Brugada type after administration of the class Ic anti-arrhythmic drug, pilsicainide.
Discussion
We present a case report of Brugada-type ECG in a patient with hypothyroidism. We conjecture that the patient was already suffering from hypothyroidism in June 2008, judging from ECG or echocardiogram. The first report of Brugada-type ECG with hypothyroidism was published in 2008 [1]. The peculiar waveform disappeared with the normalization of thyroid function, the same as in our case. Although they did not perform genetic analysis in their patient, they suggested that changes in thyroid hormone had affected the ECG due to effects on myocardial ion channels.
The cause of the Brugada-type ECG is regarded to be a decrease in INa and relative increase in Ito. Thyroid hormone affects the action potential duration and repolarization currents in cardiac myocytes through both genomic and nongenomic mechanisms [2]. However, there is no evidence indicating that an alteration in INa current is caused by changes in thyroid hormone states. Voltage-gated potassium channels (Kv1.5, Kv4.2, Kv4.3) are also under T3 genomic regulation. Since the Kv4.2/4.3 genes and the Kv1.4 gene encode components that are required for the fast and slow inactivation of Ito, respectively, it is possible that thyroid function influenced Ito through the genomic pathway. Another route would be via the extra-nuclear nongenomic effects of thyroid hormone on cardiac myocytes. These effects of T3 can occur rapidly, inducing changes in various membrane ion channels for sodium, potassium, and calcium [1], [2]. However, most patients with hypothyroidism do not demonstrate Brugada-type ECG. Therefore, these data are not sufficient to explain Brugada-type ECG by genomic or nongenomic thyroid function.
Some SCN5A missense mutations in the sodium-channel gene were first identified by Priori et al. [3] in a group of patients with Brugada syndrome. In that paper, however, genetic analysis in the SCN5A gene could not demonstrate a relation to risk stratification.
Poelzing et al. [4] reported that the R282H-SCN5A mutation does not produce significant whole-cell sodium current and coexistence of the common H558R-SCN5A polymorphism with R282H-SCN5A mutation produces fully functional sodium currents. It is suggested that this common H558R-SCN5A polymorphism rescues a genetic characteristic among patients demonstrating Brugada syndrome with R282H-SCN5A mutation. On ECG, the common variant H558R G allele carrier had a shorter QRS duration and lower J point than the wild type of H558R genotype carrier with Brugada syndrome [5]. Deschênes et al. found that the R1432G-SCN5A mutation in a patient with Brugada syndrome showed abolition of sodium-channel expression using the patch clamp technique [6].
It currently remains unclear what selective mutation in the SCN5A gene causes the change to a Brugada-type ECG.
Recently, Bezzina et al. [7] reported that an ethnic-specific haplotype variant of SCN5A promoter modulates PR and QRS duration. They demonstrated that genetic variation is a key mediator of the variable effects of a sodium-channel blocker.
Alterable Brugada-type ECG can be seen under various conditions such as sodium-channel blocker administration or myocardial autonomic nervous dysfunction [8]. We suggest that these parameters may unmask the typical Brugada-type ECG in patients with some silent gene variants.
In our case, there were three common variants in the exon of the SCN5A gene. Brugada-type ECG was observed when only thyroid function became low. We hypothesize that these three common variants may have modified the Brugada-type ECG under the hypothyroid condition in the present patient.
Some SCN5A gene variations will induce changes on ECG when the ion channels are affected by hypothyroidism.
Further research is needed to fully understand the mechanism.
References
- 1.Kitahara A., Hirai R., Matsui Y., Ikeda Y., Nakamura H. A case of hypothyroidism with Brugada electrocardiographic waveforms. Endocr J. 2008;55:589–594. doi: 10.1507/endocrj.k07e-024. [DOI] [PubMed] [Google Scholar]
- 2.Klein I., Danzi S. Thyroid disease and the heart. Circulation. 2007;116:1725–1735. doi: 10.1161/CIRCULATIONAHA.106.678326. [DOI] [PubMed] [Google Scholar]
- 3.Priori S.G., Napolitano C., Gasparini M., Pappone C., Della Bella P., Giordano U., Bloise R., Giustetto C., De Nardis R., Grillo M., Ronchetti E., Faggiano G., Nastoli J. Natural history of Brugada syndrome. Circulation. 2002;105:1342–1347. doi: 10.1161/hc1102.105288. [DOI] [PubMed] [Google Scholar]
- 4.Poelzing S., Forleo C., Samodell M., Dudash L., Sorrentino S., Anaclerio M., Troccoli R., Iacoviello M., Romito R., Guida P., Chahine M., Pitzalis M., Deschênes I. SCN5A polymorphism restores trafficking of a Brugada syndrome mutation on a separate gene. Circulation. 2006;114:368–376. doi: 10.1161/CIRCULATIONAHA.105.601294. [DOI] [PubMed] [Google Scholar]
- 5.Lizotte E., Junttila M.J., Dube M.P., Hong K., DE Benito B., Zutter M., Henkens S., Sarkozy A., Huikuri H.V., Towbin J., Vatta M., Brugada P., Brugada J., Brugada R. Genetic modulation of Brugada syndrome by a common polymorphism. J Cardiovasc Electrophysiol. 2009;20:1137–1141. doi: 10.1111/j.1540-8167.2009.01508.x. [DOI] [PubMed] [Google Scholar]
- 6.Deschênes I., Baroudi G., Berthet M., Barde I., Chalvidan T., Denjoy I., Guicheney P., Chahine M. Electrophysiological characterization of SCN5A mutations causing long QT (E1784K) and Brugada (R1512W and R1432G) syndromes. Cardiovasc Res. 2000;46:55–65. doi: 10.1016/s0008-6363(00)00006-7. [DOI] [PubMed] [Google Scholar]
- 7.Bezzina C.R., Shimizu W., Yang P., Koopmann T.T., Tanck M.W., Miyamoto Y., Kamakura S., Roden D.M., Wilde A.A. Common sodium channel promoter haplotype in Asian subjects underlies variability in cardiac conduction. Circulation. 2006;113:338–344. doi: 10.1161/CIRCULATIONAHA.105.580811. [DOI] [PubMed] [Google Scholar]
- 8.Kawaguchi T., Nomura M., Tsujikawa T., Nakaya Y., Ito S. 123I-metaiodo-benzylguanidine myocardial scintigraphy in the Brugada-type ECG. J Med Invest. 2006;53:95–102. doi: 10.2152/jmi.53.95. [DOI] [PubMed] [Google Scholar]