Summary
A 74-year-old female was admitted to our hospital due to prolonged chest pain that had lasted about 2 h. An electrocardiogram revealed ST-elevation in leads I, aVL, and V3-6, with an increase in myocardial necrosis markers. Emergency coronary angiography was performed, and left ventriculography showed the typical features of apical ballooning, and so a diagnosis of Takotsubo cardiomyopathy (TC) was made. On the 10th day after admission, the patient suddenly went into cardiopulmonary arrest because of a blow-out type left ventricular (LV) free wall rupture. Despite extensive cardiopulmonary resuscitation, the patient died. The autopsy revealed hemopericardium and a perforating wound located in the anterior wall of the LV. It was revealed that the diagonal branch of the coronary artery was occluded, and so a diagnosis of TC coexisting with acute myocardial infarction (AMI) was made. No previous case of TC accompanied by AMI has been reported. We present its clinical course during hospitalization and the result of a histopathologic examination.
Keywords: Takotsubo cardiomyopathy, Acute myocardial infarction, Left ventricular rupture, Catecholamine cardiotoxicity
Introduction
Japanese authors initially referred to reversible left ventricular apical ballooning as Takotsubo cardiomyopathy (TC) [1], [2]. TC has subsequently been given many synonyms, such as stress-related cardiomyopathy and so forth, and has been reported worldwide. The risk of in-hospital mortality is low, and left ventricular function normalizes within a few weeks. Left ventricular free wall rupture has been reported, but these cases are rare [3], [4], [5], [6], [7]. We describe an autopsy case involving a fatal rupture of the left ventricular free wall associated with a combination of acute myocardial infarction (AMI) and stress-provoked cardiomyopathy (so-called TC).
Case report
A 74-year-old female with a history of dyslipidemia was admitted to our emergency department due to a prolonged chest pain that had lasted about 2 h on April 28, [2008]. The patient reported she had been to another clinic on the same day to seek orthopedic advice for her chronic lumbago. She had no history of cardiac disease, and no family history of heart disease or sudden death. On admission, her blood pressure was 169/79 mm Hg, and heart rate 98 bpm. Auscultation of the heart uncovered a pericardial squeak friction sound, and that of lung resulted in no abnormal findings. Abdominal and neurological examination findings were negative, and there was no peripheral edema. An electrocardiogram on admission revealed sinus rhythm, ST-elevation in leads I, aVL, and V3-6; ST-depression in leads II, III, aVF; and R-wave diminishment in leads V3 and V4. Her laboratory data on admission showed an increase in myocardial necrosis markers (troponin I: 0.92 μg/L, creatine phosphokinase: 306 IU/L, creatine kinase-MB: 41 IU/L). Chest radiography showed cardiomegaly (cardiothoracic ratio: 57%), but no pulmonary vascular congestion. Echocardiography showed akinesis in the apex of the left ventricle (LV) and hyperkinesis in the basal region of the LV, but no signs of pericardial effusion were found. She underwent an emergency cardiac catheterization, and coronary angiography revealed that the principal coronary arteries were intact (Fig. 1A and B). However, left ventriculography (LVG) showed the typical features of apical ballooning (Fig. 1C–F). Based on these findings, a diagnosis of TC was made. Her peak creatine phosphokinase level was 1069 IU/L (upper normal range threshold: 194 IU/L) at 12 h after admission and gradually normalized in follow-up laboratory examinations. Her systolic blood pressure was controlled in a range from 90 to 110 mm Hg with angiotensin-converting enzyme inhibitor. Unexpectedly, on May 7 (the 10th day after admission), the patient suddenly went into cardiopulmonary arrest during her first shower. Cardiopulmonary resuscitation was immediately started, and repeated intravenous epinephrine was administered, without recovery of ventricular mechanical activity. Echocardiography showed a moderate echo-free space with asystole in all heart chambers. Despite extensive resuscitation efforts, the patient died. On the basis of these findings and clinical evolution, a diagnosis of blow-out type cardiac rupture was made, and an autopsy examination was performed.
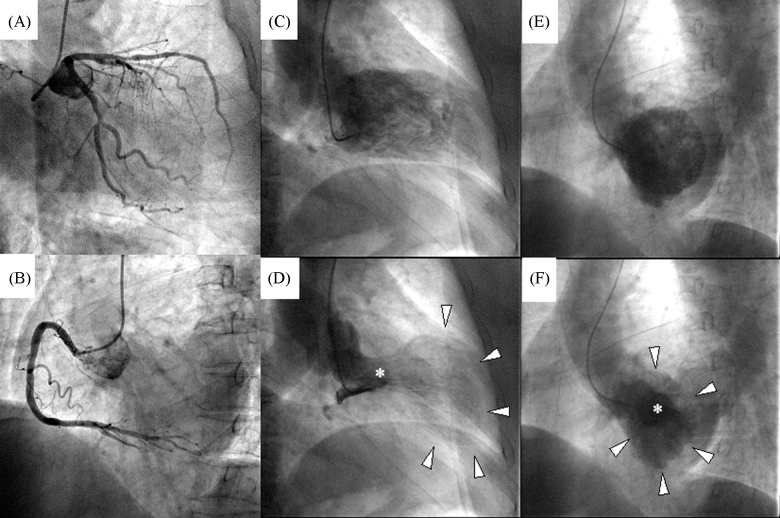
Figure 1.
Cardiac catheterization. Coronary angiography revealed intact left anterior descending, circumflex (A), and right coronary arteries (B). Left ventriculography in the right anterior oblique view showed the typical features of apical ballooning (arrowhead), and basal hyperkinetics (asterisk), and an end-diastolic volume of 145 mL, an end-systolic volume of 106 mL, an ejection fraction of 27% (C and D). Left ventriculography in left anterior oblique view (E and F) showed concentric circles caused by apical ballooning (arrowhead), and basal hyperkinetics (asterisk) in systolic phase (F).
Pathological investigations
The autopsy revealed hemopericardium with bloody pericardial effusion of 190 mL. The heart weight was 465 g. There was a perforating wound, measuring 11 mm in length along the short axis of the anterior LV wall and 30 mm above the apex (Fig. 2A). In a sectioned profile of the rupture site of the LV, a transmural myocardial necrotic zone (40 mm in diameter) was found, and the perforating wound was located on the necrotic side of the border between the necrotic and viable myocardium, not in the center of the thinning wall (Fig. 2B). There was a small mural thrombus inside the necrotic wall. The LV rupture site of the anterior-lateral wall showed inflammatory cell infiltrations, interstitial fibrosis, hemorrhage, and coagulation necrosis, but there was no evidence of contraction band necrosis (CBN) (Fig. 2C). There was no CBN in both infarct area and non-infarct area around the necrotic tissue (Fig. 2D). Similarly, the inferior-posterior wall, which revealed akinesis in the LVG and normal myocardium macroscopically during the autopsy, showed normal myocardial tissue but no evidence of CBN. These histopathological results are not contradictory to those on the 10th day after AMI. There was an obstructive lesion in the diagonal branch (D2 of American Heart Association classification) of the coronary arteries (Fig. 2E). Microscopically, the D2 ostial lesion showed severe stenosis due to plaque, dissection, and thrombus (Fig. 2F).
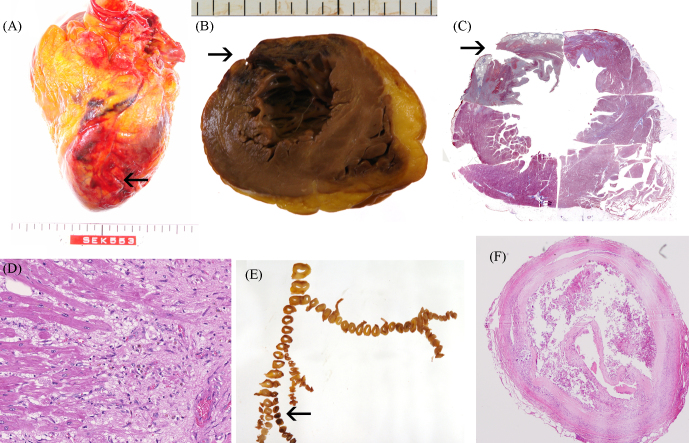
Figure 2.
Autopsy findings. The perforating wound is indicated by an arrow (A–C). In the sectioned profile of the rupture site at the apex (B), transmural necrosis was seen in the anterior-lateral wall. A low power view of the same section with B is indicated by masson-trichrome stain (C). Microscopically, there was no contraction band necrosis in the border zone between infarct and non-infarct tissue on hematoxylin-eosin stain (D). The coronary tree shows an occlusion in the 2nd diagonal branch (arrow in E), the occlusive lesion has fibrous plaque, dissection, and thrombus on hematoxylin-eosin stain (F).
Discussion
In the present case, we diagnosed TC on admission, sudden death caused by LV free wall rupture occurred on the 10th day after admission, and we made a final diagnosis as a combination of TC and anterior-lateral AMI due to occlusion of the diagonal branch based on the results of the autopsy. There are no formal diagnostic criteria for TC, some guidelines suggest that significant organic stenosis of the coronary artery must be excluded in the diagnosis of TC [8]. In theory, stress-related myocardial damage is one of the mechanisms through which TC could coexist with AMI, and it may be indistinguishable from TC and AMI. Although no such combination has been reported previously, it is known that both TC and AMI beget fatal cardiac rupture.
The overall incidence of cardiac rupture following myocardial infarction is estimated to be about 2–7%, but it accounts for as much as 15% of the in-hospital mortality after AMI [9]. On the other hand, the first reported case of ruptured TC was described by Akashi et al. [3], and 5 cases of free wall rupture have been reported since then [3], [4], [5], [6], [7]. Among these, the two studies by Ohara et al. [4] and Sacha et al. [6] included autopsy reports.
In coexisting cases of TC and AMI, as described in the current patient, could pathological investigation of the autopsy heart discriminate whether the ventricular free wall was ruptured by TC and/or AMI? The site of the transmural necrotic tissue revealed myocardial coagulation necrosis with hemorrhaging, inflammatory leukocyte infiltration, and interstitial fibrosis. The present findings are consistent with the 10th day of AMI. It is unclear whether the present findings agree with a rupture on the 10th day from the onset of TC because no previous cases have been reported. The necrotic zone was limited to the partial areas of the apical ballooning in our case, and the myocardial tissue surrounding the necrotic zone was both macroscopically and microscopically normal. These findings are in agreement with a report by Ohara et al. [4]. On the other hand, Sacha et al. [6] reported that LV functional abnormalities corresponded to the distribution and intensity of CBN. The common histological characteristics of TC are multiple foci of CBN extending beyond a single epicardial coronary distribution, and direct catecholamine cardiotoxicity [6], [8]. In a previous report of autopsied hearts in AMI, the distribution of CBN was mainly in the peripheral zone of the infarct area [10]. Sacha et al. [6] demonstrated CBN on the 2nd day after the onset of TC, but there was no evidence of CBN in the patient of Ohara et al. [4] (on the 7th day) or our present case (on the 10th day). In our present pathological analysis, it was hard to clarify the characterized phenomenon with regard to the cause of the cardiac rupture.
In conclusion, there are several reasons for incomplete antemortem diagnosis. First, because D2 occlusion is an ostial lesion, the entry of the branch was not imaged by coronary angiography. Second, the collateral flow was not imaged because of probably contributing to secondary ischemia caused by increased wall tension in the apical region [11]. And finally, the LV morphology in this case had noncoronary distribution wall motion abnormalities, extending beyond the territory of D2. TC is considered to have a favorable prognosis, so the predictors of and preventive therapeutics against cardiac rupture have not been fully investigated. Based on our experience, it is necessary in TC patients to frequently reappraise the diagnosis made on admission with electrocardiogram and echocardiography in order to perceive the signs of a cardiac rupture as early as possible.
References
- 1.Kawai S., Suzuki H., Yamaguchi H., Tanaka K., Sawada H., Aizawa T., Watanabe M., Tamura T., Umawatari K., Kawata M., Nakamura T., Yamanaka O., Okada R. Ampulla cardiomyopathy (‘Takotsubo’ cardiomyopathy) – reversible left ventricular dysfunction with ST segment elevation. Jpn Circ J. 2000;64:156–159. doi: 10.1253/jcj.64.156. [DOI] [PubMed] [Google Scholar]
- 2.Tsuchihashi K., Ueshima K., Uchida T., Oh-mura N., Kimura K., Owa M., Yoshiyama M., Miyazaki S., Haze K., Ogawa H., Honda T., Hase M., Kai R., Morii I. Angina Pectoris-Myocardial Infarction Investigations in Japan Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol. 2001;38:11–18. doi: 10.1016/s0735-1097(01)01316-x. [DOI] [PubMed] [Google Scholar]
- 3.Akashi Y.J., Tejima T., Sakurada H., Matsuda H., Suzuki K., Kawasaki K., Tsuchiya K., Hashimoto N., Musha H., Sakakibara M., Nakazawa K., Miyake F. Left ventricular rupture associated with Takotsubo cardiomyopathy. Mayo Clin Proc. 2004;79:821–824. doi: 10.4065/79.6.821. [DOI] [PubMed] [Google Scholar]
- 4.Ohara Y., Hiasa Y., Hosokawa S., Tomokane T., Yamaguchi K., Ogura R., Miyajima H., Ogata T., Yuba K., Suzuki N., Takahashi T., Kishi K., Ohtani R. Left ventricular free wall rupture in transient left ventricular apical ballooning. Circ J. 2005;69:621–623. doi: 10.1253/circj.69.621. [DOI] [PubMed] [Google Scholar]
- 5.Mafrici A., Proietti R., Fusco R., De Biase A., Klugmann S. Left ventricular free wall rupture in a Caucasian female with Takotsubo syndrome: a case report and a brief literature review. J Cardiovasc Med. 2006;7:880–883. doi: 10.2459/JCM.0b013e328010410c. [DOI] [PubMed] [Google Scholar]
- 6.Sacha J., Maselko J., Wester A., Szudrowicz Z., Pluta W. Left ventricular apical rupture caused by takotsubo cardiomyopathy – comprehensive pathological heart investigation. Circ J. 2007;71:982–985. doi: 10.1253/circj.71.982. [DOI] [PubMed] [Google Scholar]
- 7.Shinozaki K., Tamura A., Abe Y., Yano S., Kadota J. Left ventricular free wall rupture in takotsubo cardiomyopathy. Int J Cardiol. 2007;115:e3–e4. doi: 10.1016/j.ijcard.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 8.Bybee K.A., Prasad A. Stress-related cardiomyopathy syndromes. Circulation. 2008;118:397–409. doi: 10.1161/CIRCULATIONAHA.106.677625. [DOI] [PubMed] [Google Scholar]
- 9.Wehrens X.H., Doevendans P.A. Cardiac rupture complicating myocardial infarction. Int J Cardiol. 2004;95:285–292. doi: 10.1016/j.ijcard.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Matsuda M., Fujiwara H., Onodera T., Tanaka M., Wu D.J., Fujiwara T., Hamashima Y., Kawai C. Quantitative analysis of infarct size, contraction band necrosis, and coagulation necrosis in human autopsied hearts with acute myocardial infarction after treatment with selective intracoronary thrombolysis. Circulation. 1987;76:981–989. doi: 10.1161/01.cir.76.5.981. [DOI] [PubMed] [Google Scholar]
- 11.Stănescu C., Branidou K. Takotsubo cardiomyopathy. Rom J Intern Med. 2006;44:97–116. [PubMed] [Google Scholar]