Abstract
Human papillomavirus (HPV) is an important emerging etiology for head and neck cancers (HNCs) worldwide. Considering its impact on prognosis, it is important to understand the true prevalence of HPV-associated HNCs in India. This article reviews the prevalence of HPV-related HNCs across various studies in India where the population is predominantly tobacco users, and studies its outcomes with respect to HPV.
Keywords: Human papillomavirus, Head and neck cancers, Prevalence, India, Prognosis
Introduction
Head neck cancers (HNCs) are one of the most common cancers in India. Recently, emphasis has been laid on the role of the human papillomavirus (HPV) in the causation of HNCs. The International Agency of Cancer Research (IARC) concluded that there is sufficient evidence for a causal role of HPV 16 in the pathogenesis of oropharyngeal cancers and a smaller subset of oral cancers [1]. Oropharyngeal cancers are five times more likely to be caused by HPV as compared to oral, laryngeal, and other pharyngeal cancers [2]. Worldwide, there seems to be an epidemic of HPV-positive HNC, especially HPV-related oropharyngeal cancer. Chaturvedi et al. [3] reported an increase in the incidence of HPV-positive oropharyngeal cancer by 225% as compared to 50% decline in the incidence of HPV-negative oropharyngeal carcinoma between 1988 and 2004 in the USA. Mehanna et al. [4] have also reported a significant increase in HPV-positive oropharyngeal cancers from 40.5% before 2000 to 72.2% between 2005 and 2009, for an overall prevalence of 47.7%. But this “epidemic” does not clearly reflect the situation in India and other Asian countries. Chaturvedi et al. [3] did not find a similar increase in oropharyngeal cancer irrespective of HPV status in Asian countries including India. The true incidence of HPV cancers in India is not reported and it needs further research [4].
This review article makes an attempt to collate the data across Indian studies, looking specifically at the prevalence of HPV in HNCs and its impact in a predominantly tobacco-user population as well as outcomes with respect to HPV.
Understanding HPV Carcinogenesis
HPV is a DNA virus that infects the basal epithelial cells causing benign and malignant lesions of the mucosa of the upper aero-digestive tract. There are multiple strains of HPV, among which 40 are known to cause infections of the mucosa, and 9 strains have carcinogenic potential [5].The most common strains related to head and neck cancers are HPV 16 and 18. Most of the HPV infections go unnoticed; however, when there is an infection with a specific carcinogenic strain, the probability of cancer increases at the harbored site. HPV DNA virus comprise of late and early genes. They encode early proteins E1–E7 and late proteins L1–L2. The proteins E1–E5 are involved in transcription and replication, while E6 and E7 are involved in host cell transformation to the cancer cell. Late proteins are involved in capsid formation for the virion. E6 mainly targets p53, Bak, and Myc proteins which regulate apoptosis. In normal cells, during the period of stress, p53 undergoes post-transcriptional modification and gets detached from MDM2. After detachment, p53 causes cell cycle arrest and repairs DNA. If repair is possible, the cell cycle regains stability and restarts. However, when repair is not possible, the cell undergoes apoptosis to remove the damaged cells. The binding of E6 with p53 results in its ubiquitination. This blocks apoptosis and results in the uncontrolled division of the cells. E7 binds to Rb-E2Fcomplex, making Rb inactive. This leads to an increased DNA synthesis and cell proliferation. p16 is mainly inhibited by Rb-E2F complex, and disruption of this complex by E7 causes an increase in the level of p16. Thus, p16 is considered a surrogate marker of HPV carcinogenesis.
The Prevalence of HPV Cancers in India
The prevalence of HPV across various studies from India is given in Table 1. Due to the variable sample sizes, publication bias, different techniques of HPV detection, and reporting methods, there is a large variation in the published prevalence of HPV-related HNCs in India ranging from 7 to 78.7% [25, 26]. Moreover, this data is inconclusive in defining a definite time trend for any change in incidence unlike the Western literature. Most of the publications come from the North or Northeastern regions of the country, followed by the East and a few reports from the Southern parts of the country. Although most of the global data on HPV-associated HNCs is reported for oropharyngeal cancers, most of the Indian studies report on the prevalence of HPV in oral cavity cancers.
Table 1.
Prevalence of HPV in head and neck cancers across Indian studies
| Study | Subsite | n | Overall positivity (%) | Part of India | Method of assessment | Tobacco (%) | Smoking (%) | Tobacco + smoking (%) | Alcohol (%) |
|---|---|---|---|---|---|---|---|---|---|
| Balaram et al. (1995) [6] | Oral cavity | 91 | 73.6 | South | PCR amplified | 84.62 | 50.00 | ||
| D’Costa et al. (1998) [7] | Oral cavity | 100 | 15.0 | West | PCR amplified | ||||
| Jacob et al. (2002) [8] | Larynx | 44 | 34.1 | South | PCR amplified + IHC | ||||
| Nagpal et al. (2002) [9] | Oral cavity | 110 | 33.6 | East | PCR amplified | ||||
| Koppikar et al. (2005) [10] | Head and neck | 102 | 31 | West | PCR amplified | 38.46 | 23.08 | ||
| Mitra et al. (2007) [11] | Head and neck | 120 | 69 | East | PCR amplified | 61.40 | |||
| Gosh et al. (2009) [12] | Head and neck | 155 | 56 | East | PCR amplified + IHC | 48.4 | |||
| Gheit et al. (2009) [13] | Oral cavity | 65 | 27.5 | Central | PCR amplified/APEX assay | ||||
| Chaudhary et al. (2010) [14] | Oral cavity | 222 | 31.53 | North | PCR amplified/HC-II assay | 85.25 | 83.61 | 65.57 | |
| Jalouli et al. (2010) [15] | Oral cavity | 62 | 24 | North | PCR amplified | ||||
| Barwad et al. (2011) [16] | Head and neck | 111 | 32.4 | North | PCR amplified | ||||
| Kulkarni et al. (2011) [17] | Oral cavity | 34 | 70.6 | South | PCR amplified | ||||
| Elango et al. (2011) [31] | Oral Tongue | 60 | 48 | South | PCR amplified + IHC | 56.67 | |||
| Mondal et al. (2013) [18] | Oral cavity | 124 | 43.54 | East | PCR amplified | ||||
| Bahl et al. (2014) [19] | Oropharynx | 105 | 22.8 | North | PCR amplified | 33.33 | 83.33 | 29.17 | |
| Ramshankar et al. (2014) [20] | Oral Tongue | 167 | 51.2 | South | PCR amplified | 62.96 | |||
| Singh et al. (2015) [21] | Oral cavity | 250 | 9.2 | North | PCR amplified + IHC | 82.6 | 52.2 | 30.4 | |
| Sannigrahi et al. [32] | Head and neck | 226 | 29.7 | North | PCR amplified + IHC | 20.90 | 83.58 | 37.31 | |
| Kumar et al. [22] | Head and neck | 106 | 31.13 | North east | NMPCR amplified/HC-II assay | 60.61 | 30.30 | 54.55 | |
| Jitani et al. (2015) [23] | Oral cavity | 31 | 29 | North Eastern | Chromogenic ISH | 33.33 | 22.22 | ||
| Parshad et al. (2015) [24] | Oral cavity and Oropharynx | 50 | 42 | North | PCR amplified | ||||
| Ralli et al. (2016) [25] | Head and neck | 75 | 78.7 | North | IHC | ||||
| Singh et al. (2016) [26] | Oral cavity | 43 | 7 | North | PCR amplified | 8.33 | |||
| Murthy et al. (2016) [27] | Head and neck | 170 | 39.4 | Majority west | PCR amplified + IHC | ||||
| Verma et al. (2017) [28] | Oral cavity and Oropharynx | 135 | 22.9 | North | PCR amplified + IHC | 16.13 | 16.13 | 3.23 | |
| Gheit et al. (2017) [29] | Head and Neck | 364 | 13.7 | Central | PCR amplified + IHC | ||||
| AVERAGE | 51.51 | 57.02 | 40.84 | 31.48 |
HPV and Oral Cavity Cancers
Yete et al. observed 50 studies all around the world and reported the average prevalence of HPV-positive oral cancer as 24.4%. However, the prevalence in India was 36.6% [30] which is slightly higher than the global prevalence. A majority of the HPV-positive oral cancer patients were from Asia (33.77%) followed by America (19.65%), and Europe (16.19%), with the lowest prevalence in Australia (6.84%). It is important to note that maximum publications for HPV-related oral cancers came from India, i.e., 16 studies, whereas there are only two publications from the USA. If we look at individual Indian studies, Elango et al. [31] and Ramshankar et al. [20] reported a prevalence of 48% and 51.2%, respectively, in oral tongue cancers. We also reviewed the geographic distribution of HPV in India. Balaram et al. [6] reported a high prevalence of 73.6% in the South, while prevalence in the North and Northeast ranged from 7 to 29% [23, 26]. D’Costa et al. [7] reported 15% prevalence in the western region while it was 33.6% in the Eastern region [9]. The only study from Central India by Gheit et al. reported 27.5% prevalence [13]. Majority of HPV-positive cancers harbored HPV 16 more than HPV 18. Interestingly, though Elango et al. [31] detected HPV 16 in 48% by the PCR assay, the p16 IHC, which is commonly used as a surrogate marker, was detected in only 33% (n = 18) of the cases. HPV polymerase chain reaction (PCR) positive can be present due to infection and might not be truly responsible for carcinogenesis. It is also important to note that 15% of the cases with p16 overexpression were negative for HPV infection by PCR, suggesting that other causes like non-HPV 16 infections or the activation of alternate pathways might be responsible for the increase in protein expression.
Oropharynx and Larynx
Even though the literature on HPV-positive laryngeal and oropharyngeal cancers in India is limited, the only study that selectively included oropharyngeal cancer patients was published by Bhal et al. in 2003 and the reported prevalence of HPV was 22.5% (2013). Murthy et al. and Sannigrahi et al. reported a similar prevalence of 20% and 15% respectively. However, the more recent studies have shown higher prevalence rate of 78.7% [25]. Another study which selectively included laryngeal cancer patients by Jacob et al. found HPV prevalence of 34.1% [8]. As per Indian literature, the prevalence of HPV in laryngeal and hypopharyngeal cancer ranges from 5 to 20% [22, 27, 32].
Impact of Tobacco Usage in Patients with HPV-Related Head and Neck Cancers
Tobacco is an important known causative factor for head and neck cancers. It is known that use of tobacco decreases survival and treatment response in HPV-positive HNC patients. The risk of progression/mortality and second primary with oral squamous cell carcinoma (OSCC) increases by 1% and 1.5%, respectively, for each year of tobacco use after adjustment for HPV tumor status and other significant factors. It is important to note that if the patients smoke during radiation treatment, there is a two-times higher risk of mortality [33]. We reviewed various studies in Indian literature and found that the use of tobacco/alcohol in patients with HPV-related HNCs varied from 3.23 to 85.2%(Table 1). The mean prevalence of chewing tobacco, smoking tobacco, chewing and smoking tobacco, and alcohol use was 53.34%, 47.54%, 47.05%, and 31.48% respectively in patients with HPV-related HNC. The distribution of tobacco use in HPV-positive Indian cancer patients across various studies is given in Table 1. Murthy et al. analyzed the impact of tobacco on HPV-related cancers in Indian population. They found no difference in survival between p16-positive and p16-negative HNCs. However, when the analysis was done including only tobacco non-users, there was a definite improvement in overall survival and cause-specific survival which reached close to statistical significance (p = 0.08). It is clearly seen that the favorable prognosis associated with HPV cancer is tempered with the use of tobacco. Kumar et al. [22] have shown that that tobacco chewing and alcohol consumption may act as risk factors for HPV infection in HNCs for a population in the Northeast region of India. Nicotine modifying antigen-mediated signaling pathways leading to alterations in immune functions might be the reason for increased chances of viral infection in tobacco users. Herrero et al. [34] found that there is an additive effect of smoking and HPV E6/E7 seropositivity. When compared with never smokers who were negative for HPV16 E6 and E7, smokers who were negative for HPV16 E6 and E7 (OR = 11.2, 95% CI = 5.9 to 21.4), never smokers who were positive for HPV16 E6 or E7 (OR = 64.5, 95% CI = 18.3 to 226.7), and smokers who were positive for HPV16 E6 or E7 (OR = 56.2, 95% CI = 22.5 to 140.4) had an increased risk for cancer of the oropharynx. Thus, tobacco use increases the chances of HPV infection and it has an additive effect on carcinogenesis.
HPV Detection
Presence of HPV in HNCs has prognostic implications and treatment strategies may change in view of HPV positivity. Hence, it is important to accurately determine HPV as the etiologic agent in HNCs. To achieve this, a biopsy sample or cytology from the primary or the enlarged nodes often plays a significant role in the initial diagnosis. One of the widely used methods to detect HPV in tumor tissue is PCR or reverse transcriptase-PCR (RT-PCR) which could detect E6/E7 in fresh frozen tissue; however, it comes with a high set-up cost and turnaround time. Until recently, there was no consensus regarding the testing of HPV in tumor specimens. According to the recent AJCC eighth edition guidelines, the technique of choice should be immunohistochemistry (IHC) detection of overexpression of tumor suppressor protein p16, as a surrogate marker. Specifically, the cutoff point for p16 overexpression is diffuse (≥ 75%) tumor expression, with at least moderate (+ 2/3) staining intensity [35]. It has the advantage of universal availability and a straightforward interpretation, but false negatives confer a big challenge. Another highly specific method used is in situ hybridization (ISH) which can detect HPV in fresh tumor tissue and can detect integrated or episomal state, but it requires high viral loads and is technically challenging. Other methods such as Hybrid Capture II (HC-2) analysis, Cervista HPV HR test, Roche Cobas HPV test, and APTIMA HPV assay have been suggested but are not universally integrated.
Clinical Presentation of HPV-Positive HNCs
The clinical features of HPV-positive HNCs are distinctly different from those of HPV-negative HNCs. HPV-positive cancers present at a more advanced clinical stage with lower tumor size and aggressive nodal involvement compared to HPV-negative cancers. HPV-positive cancers are characterized by distinct histological features having moderate/poor tumor differentiation and non-keratinizing or basaloid pathology [36–38]. While reviewing the Indian literature on HPV-associated HNC, the distribution of T stage, N stage, and grade of differentiation was noted as shown in Table 2. We found that most of the patients had T3/T4 disease which is in contrast to the western literature. As far as N stage is concerned, complete details regarding nodal status was not available. We found that 37.1% were node negative and 62.88% were node positive. It was also seen that most of the tumors were well differentiated (51.38%) followed by moderate differentiation (39.21%) and few tumors had poor differentiation (33.97%). Thus, in contrast to the western literature, the clinical characteristics of Indian patients are different. This may be due to the higher usage of tobacco products in Indian patients as compared to western countries. There are multiple prospective and retrospective studies showing improved survival outcomes in HPV-positive oropharyngeal cancer as compared to HPV-negative oropharyngeal cancer with 38–80% reductions in the risk of mortality. As per Indian literature, Murthy et al. [27] found no survival difference between p16+ve and p16–ve HNC patients. This is again because of associated tobacco use which modifies the prognosis in HPV-positive HNC.
Table 2.
Distribution of T stage, N stage, and grade of differentiation in Indian patients with HPV-positive head and neck cancers
| Study | T1/T2 (%) | T3/T4 (%) | N0 (%) | N+ (%) | WD (%) | MD (%) | PD (%) |
|---|---|---|---|---|---|---|---|
| Balaram et al. (1995) [6] | 53.73 | 14.92 | 31.34 | ||||
| Nagpal et al. (2002) [9] | 22.72 | 14.54 | 62.74 | ||||
| Mitra et al. (2007) [11] | 38.98 | 35.59 | 11.86 | ||||
| Chaudhary et al. (2010) [14] | 36.03 | 63.06 | 29.72 | 70.27 | |||
| Elango et al. [31] | 72 | 21 | 7 | ||||
| Bahl et al. (2014) [19] | 12 | 88 | 20.83 | 79.17 | 12 | 84 | 4 |
| Ramshankar et al. (2014) [20] | 66.67 | 20.99 | 6.17 | ||||
| Sannigrahi et al. [32] | 9.4 | 90.6 | 31.2 | 68.8 | |||
| Kumar et al. [22] | 63.64 | 24.24 | 12.12 | ||||
| Jitani et al. (2015) [23] | 66.7 | 33.3 | 66.67 | 33.33 | |||
| Singh et al. (2015) [21] | 21.7 | 78.2 | 65.2 | 34.8 | 60.9 | 34.8 | 4.3 |
| Verma et al. (2017) [28] | 41.94 | 45.16 | 12.90 | ||||
| Average | 29.17 | 70.63 | 42.72 | 57.27 | 52.57 | 32.80 | 16.94 |
American Joint Committee on Cancer Eighth Edition
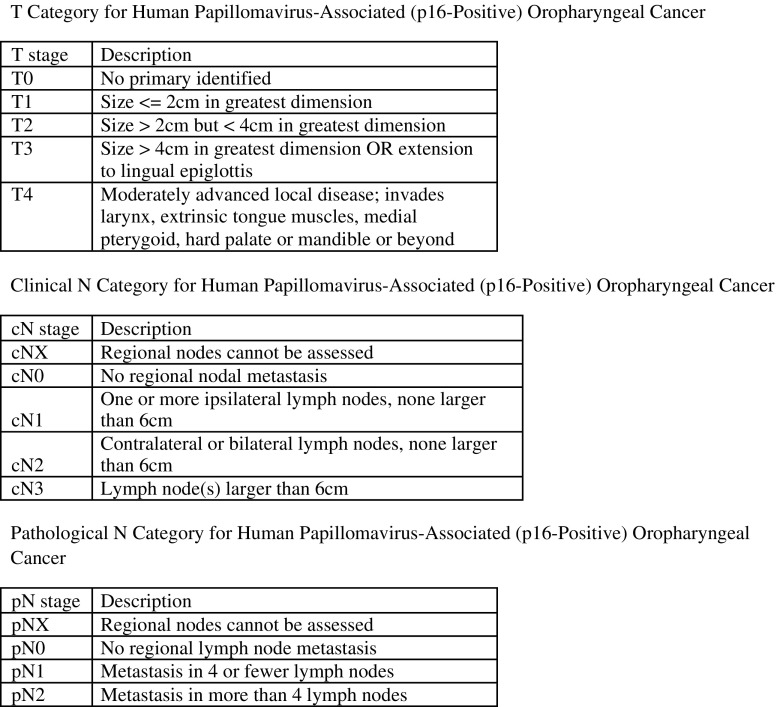
Considering the increasing prevalence of HPV in oropharyngeal cancer and its impact on survival, the seventh edition lost its ability to differentiate between stages. O′ Sullivan et al. [39] included 1907 patients with p16 in situ hybridization-positive oropharyngeal cancer from seven cancer centers and proposed a new ICON-S staging system for these patients. Haughey et al. [40] assembled data of 704 surgically managed p16-positive oropharyngeal cancer cohort (any T, any N, M0) from five cancer centers. He found that pathology-confirmed metastatic node count (4 versus 5) yielded three groups which had incrementally worse prognosis. These two studies laid the foundation for the changes in the oropharyngeal staging system (Fig. 1). As compared to previous version, the eighth edition has a separate staging system for HPV-positive and HPV-negative oropharyngeal cancers. The T classification is largely unchanged except for removal of Tis, i.e., carcinoma in situ and combining T4a and T4b oropharyngeal cancers. The N classification can be either pathological or clinical. Clinical staging is based on the laterality and size of nodes while the pathological N classification is based on the number of metastatic nodes in surgically treated patients. It is important to note that extracapsular spread is not considered a prognostic factor in the new classification. There is a drastic change in the overall stage classification where stage IV has been reserved only for metastatic disease.
Fig. 1.
New 8th AJCC staging system for HPV-positive oropharyngeal cancer
Quality of Life in HPV-Positive HNCs
Considering the improved prognosis in HPV-related head and neck cancers and longer survival in these patients, it is important to understand the impact of treatment on quality of life (QOL) of patients with HPV-related cancers. Sharma et al. [41] showed that pretreatment QOL was better in patients with HPV-related oral and oropharyngeal cancers. However, there was no difference in QOL post-treatment at 1 year. Thus, they showed a greater decline in QOL from pretreatment phase to immediate post-treatment period. Singh et al. [26] also showed no difference in QOL between patients with HPV-positive or HPV-negative oral cancers, but after 3 months post-treatment nearly all parameters showed the more average percentage change in HPV-positive cases. This rapid decline in QOL may be due to the high intensity of the treatment administered in this population. De-intensification of treatment was suggested for patients with HPV-positive oropharyngeal squamous carcinoma, as they are typically younger and have an improved survival rate, compared with other patients with the same disease. An international multicentric study by Spinato et al. [42] used the EORTC questionnaire for assessment and they too found no difference in QOL with respect to HPV status of the cancer patients.
Future Strategies in the Management of HPV-Related HNCs
- One of the important factors under investigation is “de-escalation” of treatment. It can be achieved by three methods:
- Replacing cisplatin with cetuximab to reduce the toxicity associated with conventional chemotherapy. There is a randomized phase III (RTOG 1016) study comparing radiation (RT) + cisplatin vs. RT + cetuximab in stage II–IV HPV-related OPCs. The primary aim is no survival difference in both the groups with acute toxicity in the cetuximab/RT arm will be reduced by at least 50% [43].
- Another method of de-intensification of treatment is reducing the dose of radiation to 52–54 Gy. The preliminary result of one of these trials (ECOG 1308) [44] has been recently presented. Eighty stage III/IV HPV-related OPSCC patients (p16 and ISH positive) were enrolled and received induction chemotherapy (cisplatin, cetuximab, and paclitaxel). Only those patients that demonstrated a complete response (78% of the cohort) received de-escalated treatment (54 Gy combined with cetuximab). The de-escalated group demonstrated a 1-year progression-free survival of 91% compared to 87% in the non/partial response group treated with standard treatment. These initials results are particularly promising as only 17% of the de-escalated group developed any post-treatment dysphagia.
- The third method of de-escalation is the use of minimally invasive surgery like TORS for early cancers (T1, 2 N0). The main advantage of surgery is the short duration of treatment and proper staging of the patients which will define the requirement of adjuvant radiation/concurrent chemo-radiation. There are three trials (PATHOS, ADEPT, and ECOG 3311) currently underway to evaluate the same [45].
Vaccines: The population-level burden of HPV-positive HNCs is currently not clear in India and may have important implications for cancer prevention, potentially through HPV vaccination. Currently, the bivalent (HPV 16, 18), Cervarix (GSK) and a quadrivalent vaccine (HPV16, 18, 6, 11) Gardasil (MERCK) are two prophylactic vaccines which are commercially available. Even though there is no level I evidence showing the effectiveness of the vaccine in prevention of oropharyngeal cancers, they appear promising as vaccinated individuals show a high titer of neutralizing HPV 16 antibodies [46]. However, long-term benefits of these vaccines need to be evaluated.
Conclusion
Similar to western world, prevalence of HPV in Indian HNC patient is increasing. The true impact of HPV on survival and its association with tobacco in Indian population needs to be studied thoroughly. HPV-associated HNC is different in the Indian population with an advanced T stage at presentation. The survival outcomes also seem to be poorer as compared to the western world. We need to determine whether these differences are just perceived or actual. The way forward is to have prospective data collated across Indian centers with respect to HPV prevalence, treatments, and survival outcomes, in order to draw meaningful conclusion on the impact of HPV in Indian population.
References
- 1.IARC Monographs- Monographs available in PDF format. http://monographs.iarc.fr/ENG/Monographs/vol90/index.php. Accessed 27 Feb 2018
- 2.Combes J-D, Franceschi S. Role of human papillomavirus in non-oropharyngeal head and neck cancers. Oral Oncol. 2014;50:370–379. doi: 10.1016/j.oraloncology.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M, Cozen W, Liu L, Lynch CF, Wentzensen N, Jordan RC, Altekruse S, Anderson WF, Rosenberg PS, Gillison ML. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehanna H, Beech T, Nicholson T, el-Hariry I, McConkey C, Paleri V, Roberts S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer--systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35:747–755. doi: 10.1002/hed.22015. [DOI] [PubMed] [Google Scholar]
- 5.Bharti AH, Chotaliya K, Marfatia YS. An update on oral human papillomavirus infection. Indian J Sex Transm Dis. 2013;34:77–82. doi: 10.4103/0253-7184.120533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balaram P, Nalinakumari KR, Abraham E, et al. Human papillomaviruses in 91 oral cancers from Indian betel quid chewers--high prevalence and multiplicity of infections. Int J Cancer. 1995;61:450–454. doi: 10.1002/ijc.2910610403. [DOI] [PubMed] [Google Scholar]
- 7.D’Costa J, Saranath D, Dedhia P, et al. Detection of HPV-16 genome in human oral cancers and potentially malignant lesions from India. Oral Oncol. 1998;34:413–420. doi: 10.1016/S1368-8375(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 8.Jacob SE, Sreevidya S, Chacko E, Pillai MR. Cellular manifestations of human papillomavirus infection in laryngeal tissues. J Surg Oncol. 2002;79:142–150. doi: 10.1002/jso.10075. [DOI] [PubMed] [Google Scholar]
- 9.Nagpal JK, Patnaik S, Das BR. Prevalence of high-risk human papilloma virus types and its association with P53 codon 72 polymorphism in tobacco addicted oral squamous cell carcinoma (OSCC) patients of Eastern India. Int J Cancer. 2002;97:649–653. doi: 10.1002/ijc.10112. [DOI] [PubMed] [Google Scholar]
- 10.Koppikar P, deVilliers EM, Mulherkar R. Identification of human papillomaviruses in tumors of the oral cavity in an Indian community. Int J Cancer. 2005;113:946–950. doi: 10.1002/ijc.20664. [DOI] [PubMed] [Google Scholar]
- 11.Mitra S, Banerjee S, Misra C, et al. Interplay between human papilloma virus infection and p53 gene alterations in head and neck squamous cell carcinoma of an Indian patient population. J Clin Pathol. 2007;60(9):1040–1047. doi: 10.1136/jcp.2005.034835.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh S, Ghosh A, Maiti GP, Alam N, Roy A, Roy B, Roychoudhury S, Panda CK. Alterations of 3p21.31 tumor suppressor genes in head and neck squamous cell carcinoma: Correlation with progression and prognosis. Int J Cancer. 2008;123(11):2594–2604. doi: 10.1002/ijc.23834. [DOI] [PubMed] [Google Scholar]
- 13.Gheit T, Vaccarella S, Schmitt M, Pawlita M, Franceschi S, Sankaranarayanan R, Sylla BS, Tommasino M, Gangane N. Prevalence of human papillomavirus types in cervical and oral cancers in Central India. Vaccine. 2009;27:636–639. doi: 10.1016/j.vaccine.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhary AK, Pandya S, Mehrotra R, Bharti AC, Singh M, Singh M. Comparative study between the Hybrid Capture II test and PCR based assay for the detection of human papillomavirus DNA in oral submucous fibrosis and oral squamous cell carcinoma. Virol J. 2010;7:253. doi: 10.1186/1743-422X-7-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jalouli J, Ibrahim SO, Mehrotra R, Jalouli MM, Sapkota D, Larsson PA, Hirsch JM. Prevalence of viral (HPV, EBV, HSV) infections in oral submucous fibrosis and oral cancer from India. Acta Otolaryngol. 2010;130(11):1306–1311. doi: 10.3109/00016481003782041. [DOI] [PubMed] [Google Scholar]
- 16.Barwad A, Sood S, Gupta N, Rajwanshi A, Panda N, Srinivasan R. Human papilloma virus associated head and neck cancer: A PCR based study. Diagn Cytopathol. 2012;40(10):893–897. doi: 10.1002/dc.21667. [DOI] [PubMed] [Google Scholar]
- 17.Kulkarni SS, Kulkarni SS, Vastrad PP, Kulkarni BB, Markande AR, Kadakol GS, Hiremath SV, Kaliwal S, Patil BR, Gai PB. Prevalence and distribution of high risk human papillomavirus (HPV) Types 16 and 18 in Carcinoma of cervix, saliva of patients with oral squamous cell carcinoma and in the general population in Karnataka, India. Asian Pac J Cancer Prev. 2011;12(3):645–648. [PubMed] [Google Scholar]
- 18.Mondal R, Ghosh SK, Choudhury JH, Seram A, Sinha K, Hussain M, Laskar RS, Rabha B, Dey P, Ganguli S, Nathchoudhury M, Talukdar FR, Chaudhuri B, Dhar B. Mitochondrial DNA copy number and risk of oral cancer: a report from Northeast India. PLoS One. 2013;8(3):e57771. doi: 10.1371/journal.pone.0057771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bahl A, Kumar P, Dar L, Mohanti BK, Sharma A, Thakar A, Karthikeyan V, Sikka K, Singh C, Poo K, Lodha J. Prevalence and trends of human papillomavirus in oropharyngeal cancer in a predominantly north Indian population. Head Neck. 2014;36(4):505–510. doi: 10.1002/hed.23317. [DOI] [PubMed] [Google Scholar]
- 20.Ramshankar V, Soundara VT, Shyamsundar V, Ramani P, Krishnamurthy A. Risk stratification of early stage oral tongue cancers based on HPV status and p16 immunoexpression. Asian Pac J Cancer Prev APJCP. 2014;15:8351–8359. doi: 10.7314/APJCP.2014.15.19.8351. [DOI] [PubMed] [Google Scholar]
- 21.Singh V, Husain N, Akhtar N, Kumar V, Tewari S, Mishra S, Misra S, Khan MY. Do Human Papilloma Viruses Play Any Role in Oral Squamous Cell Carcinoma in North Indians? Asian Pac J Cancer Prev. 2015;16(16):7077–7084. doi: 10.7314/APJCP.2015.16.16.7077. [DOI] [PubMed] [Google Scholar]
- 22.Kumar R, Rai AK, Das D, Das R, Kumar RS, Sarma A, Sharma S, Kataki AC, Ramteke A. Alcohol and tobacco increases risk of high risk HPV infection in head and neck cancer patients: study from north-east region of India. PLoS One. 2015;10:e0140700. doi: 10.1371/journal.pone.0140700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jitani AK, Raphael V, Mishra J, et al. Analysis of human papilloma virus 16/18 DNA and its correlation with p16 expression in oral cavity squamous cell carcinoma in North-Eastern India: a chromogenic in-situ hybridization based study. J Clin Diagn Res. 2015;9:EC04-07. doi: 10.7860/JCDR/2015/13022.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parshad S, Nandi S, Marwah N, et al. Human papillomavirus 16 and 18 in squamous cell carcinoma of oral cavity and sexual practices: A pilot study at a Tertiary Care Hospital of North India. Natl J Maxillofac Surg. 2015;6(2):185–189. doi: 10.4103/0975-5950.183857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ralli M, Singh S, Yadav SPS, Sharma N, Verma R, Sen R. Assessment and clinicopathological correlation of p16 expression in head and neck squamous cell carcinoma. J Cancer Res Ther. 2016;12:232–237. doi: 10.4103/0973-1482.151447. [DOI] [PubMed] [Google Scholar]
- 26.Singh AK, Kushwaha JK, Anand A, Sonkar AA, Husain N, Srivastava K, Singh S. Human papilloma virus in oral cavity cancer and relation to change in quality of life following treatment-a pilot study from northern India. Indian J Surg Oncol. 2016;7:386–391. doi: 10.1007/s13193-016-0559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murthy V, Swain M, Teni T, Pawar S, Kalkar P, Patil A, Chande A, Ghonge S, Laskar SG, Gupta T, Budrukkar A, Agrawal J. Human papillomavirus/p16 positive head and neck cancer in India: prevalence, clinical impact, and influence of tobacco use. Indian J Cancer. 2016;53:387–393. doi: 10.4103/0019-509X.200668. [DOI] [PubMed] [Google Scholar]
- 28.Verma G, Vishnoi K, Tyagi A, et al. Characterization of key transcription factors as molecular signatures of HPV-positive and HPV-negative oral cancers. Cancer Med. 2017;6(3):591–604. doi: 10.1002/cam4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gheit T, Anantharaman D, Holzinger D, Alemany L, Tous S, Lucas E, Prabhu PR, Pawlita M, Ridder R, Rehm S, Bogers J, Maffini F, Chiocca S, Lloveras B, Kumar RV, Somanathan T, de Sanjosé S, Castellsagué X, Arbyn M, Brennan P, Sankaranarayanan R, Pillai MR, Gangane N, Tommasino M, HPV-AHEAD study group Role of mucosal high-risk human papillomavirus types in head and neck cancers in central India. Int J Cancer. 2017;141(1):143–151. doi: 10.1002/ijc.30712.. [DOI] [PubMed] [Google Scholar]
- 30.Yete S, D’Souza W, Saranath D. High-risk human papillomavirus in oral cancer: clinical implications. Oncology. 2017;94:133–141. doi: 10.1159/000485322. [DOI] [PubMed] [Google Scholar]
- 31.Elango KJ, Suresh A, Erode EM, Subhadradevi L, Ravindran HK, Iyer SK, Iyer SK, Kuriakose MA. Role of human papilloma virus in oral tongue squamous cell carcinoma. Asian Pac J Cancer Prev APJCP. 2011;12:889–896. [PubMed] [Google Scholar]
- 32.Sannigrahi MK, Singh V, Sharma R, Panda NK, Radotra BD, Khullar M. Detection of active human papilloma virus-16 in head and neck cancers of Asian North Indian patients. Oral Dis. 2016;22:62–68. doi: 10.1111/odi.12382. [DOI] [PubMed] [Google Scholar]
- 33.Gillison ML, Zhang Q, Jordan R, Xiao W, Westra WH, Trotti A, Spencer S, Harris J, Chung CH, Ang KK. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30:2102–2111. doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herrero R, Castellsagué X, Pawlita M, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 35.Würdemann N, Wagner S, Sharma SJ, Prigge ES, Reuschenbach M, Gattenlöhner S, Klussmann JP, Wittekindt C (2017) Prognostic impact of AJCC/UICC 8th edition new staging rules in oropharyngeal squamous cell carcinoma. Front Oncol 7. 10.3389/fonc.2017.00129 [DOI] [PMC free article] [PubMed]
- 36.Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ang KK, Sturgis EM. Human papillomavirus as a marker of the natural history and response to therapy of head and neck squamous cell carcinoma. Semin Radiat Oncol. 2012;22:128–142. doi: 10.1016/j.semradonc.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Gillison ML. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin Oncol. 2004;31:744–754. doi: 10.1053/j.seminoncol.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 39.O’Sullivan B, Huang SH, Su J, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016;17:440–451. doi: 10.1016/S1470-2045(15)00560-4. [DOI] [PubMed] [Google Scholar]
- 40.Haughey BH, Sinha P, Kallogjeri D, Goldberg RL, Lewis JS, Jr, Piccirillo JF, Jackson RS, Moore EJ, Brandwein-Gensler M, Magnuson SJ, Carroll WR, Jones TM, Wilkie MD, Lau A, Upile NS, Sheard J, Lancaster J, Tandon S, Robinson M, Husband D, Ganly I, Shah JP, Brizel DM, O’Sullivan B, Ridge JA, Lydiatt WM. Pathology-based staging for HPV-positive squamous carcinoma of the oropharynx. Oral Oncol. 2016;62:11–19. doi: 10.1016/j.oraloncology.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma A, Méndez E, Yueh B, Lohavanichbutr P, Houck J, Doody DR, Futran ND, Upton MP, Schwartz SM, Chen C. Human papillomavirus-positive oral cavity and oropharyngeal cancer patients do not have better quality-of-life trajectories. Otolaryngol Head Neck Surg. 2012;146:739–745. doi: 10.1177/0194599811434707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spinato G, Stellin M, Azzarello G, Bonazza D, Zanconati F, Politi D, Cocuzza S, di Mauro P, Ausoni S, Tonoli G, Costantini G, Maiolino L, Spinato R, da Mosto MC, Baboci L, del Mistro A, Serra A, Tirelli G. Multicenter research into the quality of life of patients with advanced oropharyngeal carcinoma with long-term survival associated with human papilloma virus. Oncol Lett. 2017;14:185–193. doi: 10.3892/ol.2017.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wirth LJ. Cetuximab in human papillomavirus–positive oropharynx carcinoma. J Clin Oncol. 2016;34:1289–1291. doi: 10.1200/JCO.2015.65.1414. [DOI] [PubMed] [Google Scholar]
- 44.Marur S, Li S, Cmelak AJ, Gillison ML, Zhao WJ, Ferris RL, Westra WH, Gilbert J, Bauman JE, Wagner LI, Trevarthen DR, Balkrishna J, Murphy BA, Agrawal N, Colevas AD, Chung CH, Burtness B. E1308: phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx— ECOG-ACRIN Cancer Research Group. J Clin Oncol. 2016;35:490–497. doi: 10.1200/JCO.2016.68.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mirghani H, Blanchard P. Treatment de-escalation for HPV-driven oropharyngeal cancer: where do we stand? Clin Transl Radiat Oncol. 2018;8:4–11. doi: 10.1016/j.ctro.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008;113:3036–3046. doi: 10.1002/cncr.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]