Abstract
Involvement of the bladder by colorectal cancer is sufficiently rare to be encountered by an individual surgeon on an infrequent basis. Extirpative procedures for advanced colorectal cancers can involve partial/total bladder resections. In patients without evidence of distant metastatic disease, a reasonable therapeutic effect can be expected when negative surgical margins are obtained. The decision to perform a bladder-sparing procedure or a total pelvic exenteration (TPE) will be based on the extent of the primary lesion as well as patient characteristics. In this study, we report our experience in the management of operable locally advanced colorectal carcinomas involving the urinary bladder. We retrospectively reviewed the hospital records of all patients with advanced colorectal cancer invading the urinary bladder. The age, gender, clinical presentation, physical examination findings, and imaging records were noted. Colonoscopy reports and images were noted and biopsy findings recorded. Similarly, cystoscopy findings and biopsy reports were noted and analyzed. Eight (88%) patients had a primary sigmoid tumor and one (11%) had primary rectal tumor. The clinical staging of the primary tumor was T3 in three (33%) and T4 in six (66%). A biopsy taken during cystoscopy confirmed the malignant lesion in all the nine patients. Four (44%) patients received neoadjuvant chemotherapy with 5-fluorouracil. Eight (88%) patients underwent bladder-sparing resection and the remaining one underwent total pelvic exenteration with ileal conduit for urinary drainage. The mean overall survival was 44 months. The wide spectrum of possible bladder involvement by colorectal cancer requires individual patient-specific and disease-specific approaches. En bloc bladder resection for adherent or invading colorectal cancers achieves good local control and prognosis. The potential for cure in completely excised, node-negative tumors is good. Bladder reconstruction is achievable in most patients.
Keywords: Colorectal cancer, Local control, Urinary bladder, Bladder sparing
Introduction
Locally advanced colorectal tumors are known to constitute about 5–22% of all colorectal cancers at the time of presentation [1]. These sub-classes of colorectal tumors are characterized by aggressive local behavior in the form of invasion of adjacent organs or structures with no distant metastasis at presentation. The survival in these groups of patients is similar to those undergoing conventional resections [1]. The genitourinary tract (bladder, prostate, ureters, or vagina) is known to be infiltrated in 3–10% of cases [2–4]. The most common locations for colorectal cancer to involve the urinary bladder are the distal sigmoid followed by the rectum.
Determination of the extent of loco-regional extension with the use of pelvic computerized tomography (CT), magnetic resonance imaging (MRI), and endorectal ultrasound remains an area of active debate and research [5]. In cases wherein the bladder is involved, there exists an option between total pelvic exenteration (TPE) with urinary diversion or wide local excision with bladder-sparing procedures in select cases [6, 7]. However, several retrospective studies examining the oncologic efficacy of TPE versus bladder-sparing procedures have come out with conflicting conclusions. One consistent finding that was noted was obtaining negative surgical microscopic margins was the key to preventing local recurrences [5, 8]. The single best predictor of overall survival in patients undergoing aggressive surgical resection was the lymph node status (5-year overall survival 70% R0N0 vs 35% R0N+) followed by margin status of specimen (5-year overall survival 61% R0 vs 17% R1) [8–10]. We report our experience in the management of operable locally advanced colorectal carcinomas involving the urinary bladder.
Materials and Methods
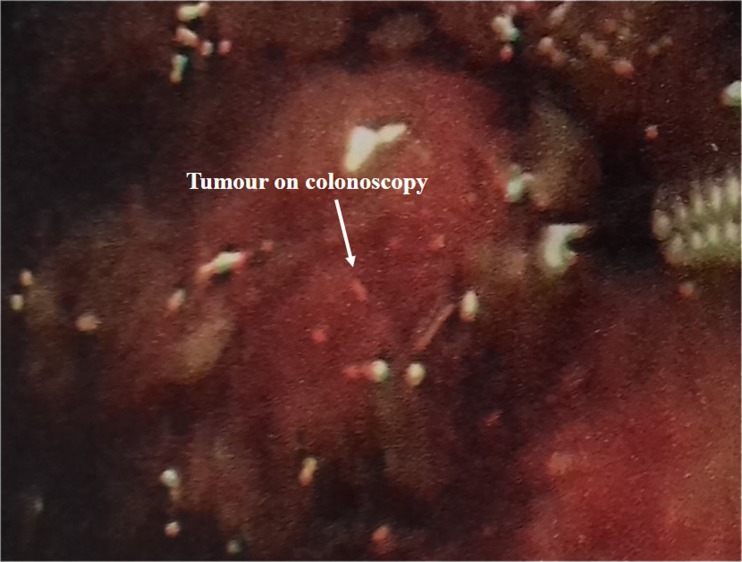
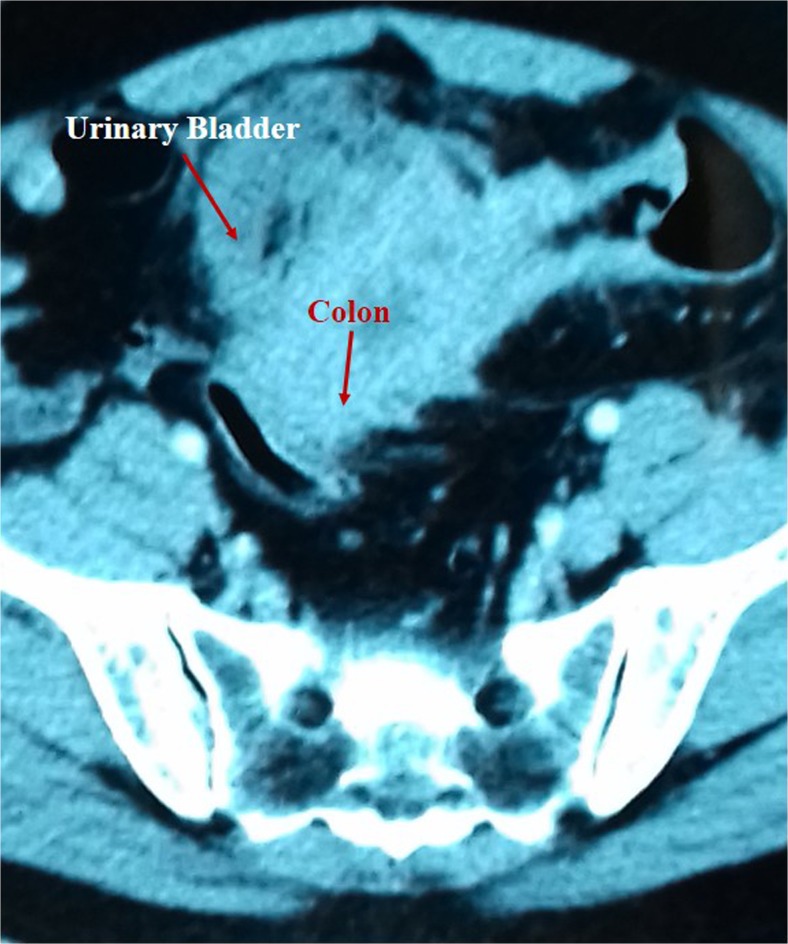
We retrospectively reviewed the hospital records of all patients with advanced (≥ T3) colorectal cancer invading the urinary bladder. The age, gender, clinical presentation, physical examination findings, and imaging records were noted. Colonoscopy (Fig. 1) reports and images (Fig. 2) were noted and biopsy findings recorded. Similarly, cystoscopy findings and biopsy reports were noted and analyzed.
Fig. 1.
Colonoscopy revealing a growth in the sigmoid colon. Biopsy is being taken
Fig. 2.
CT images showing adherence between the urinary bladder and the sigmoid colon
Pathologic staging of the primary tumor was performed using the 2010 modification of the TNM system as outlined by the American Joint Committee on Cancer [11], and the presence of carcinoma involving the bladder was determined. Postoperative tumor surveillance was comprised of a history, physical examination, chest X-ray, and liver function and alkaline phosphatase tests, which were performed every 3–4 months for the first 2 years, every 6 months for the next 2 years, and yearly thereafter. Abdominal imaging was performed at 6-month intervals at the surgeon’s discretion.
Results
During the study period Jan 2001 to Dec 2016, nine patients (7 men and 2 women) with a mean age of 57.91 ± 9.57 years (range 38–74) were diagnosed to have bladder infiltration from a locally advanced colorectal cancer. Eight (88%) patients had a primary sigmoid tumor and one (11%) had primary rectal tumor. The clinical staging of the primary tumor was T4a in three (33%) and T4b in six (66%).
The common presenting symptoms in these patients were altered bowel movements, passing of blood per annum, hematuria, dysuria, and urinary tract infections. On examination, an abdominal mass was palpable in six of the patients. Four patients were grossly anemic. Colonoscopy revealed growths in sigmoid in eight of the patients and rectal growth in one. Biopsies from the lesions confirmed the colorectal growths. CT abdomen revealed circumferential mural thickening of the sigmoid/rectum with an exophytic heterogeneously enhancing mass lesion. Cystoscopy revealed the extension of the colorectal growths in all the patients. Cystoscopy results were considered positive for neoplastic involvement of the bladder if edema or macroscopic tumor involvement was noted. Simple displacement of the bladder was not considered sufficient evidence of tumor involvement. A biopsy taken during cystoscopy confirmed the malignant lesion in all the nine patients. Four (44%) patients received neoadjuvant chemotherapy with 5-fluorouracil. None of the patients had radiological evidence of lymph node involvement or distant metastases.
During surgery, the bladder and pelvic sidewalls were examined for tumor involvement before the colon/rectum and tumor were dissected away from the bladder and ureters. The decision whether to perform a cystectomy and ileal conduit urinary diversion was made at the time of surgery based on the findings at surgery or after frozen-section evaluation of the surgical margin. Eight (88%) patients underwent bladder-sparing resection (Figs. 3 and 4) and the remaining one underwent TPE with ileal conduit for urinary drainage. The patient who underwent TPE was the patient with rectal cancer and nearly the whole of the posterior surface of the bladder including trigone and prostate was densely adherent with the rectal wall. In our series, the final pathologic examination showed bladder involvement in all the patients (100%). Hundred percent of these patients had negative histologic margins on final pathologic evaluation. Histopathological studies revealed all patients had P4 tumors.
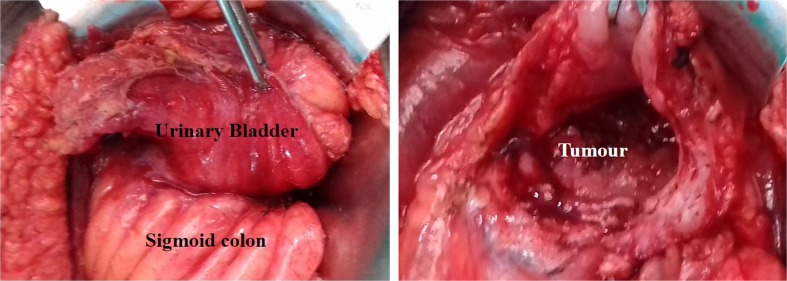
Fig. 3.
Intraoperative photograph showing a adhesions between the bladder and the colon, and b the bladder opened to reveal colonic tumor infiltration into the bladder
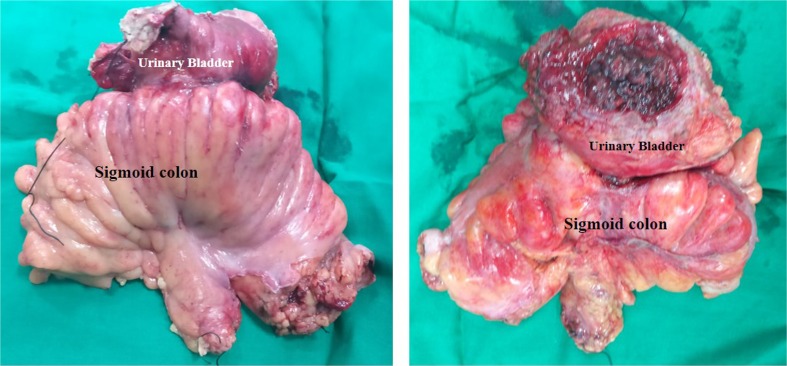
Fig. 4.
Resected specimen of the sigmoid colon with the urinary bladder
Of the eight patients who underwent bladder-sparing resection, the bladder capacity as determined intra-operatively was roughly 250 ml in five patients and around 150 ml in the remaining three. Three patients developed wound infection, which subsided on proper wound management. The mean hospital stay was 16 days. One-month postoperative mortality was 0%. None of the patients had complications related to bowel anastomosis. The disease-free survival was 28 ± 1.09 months. In the follow-up period, patients with bladder preservation voided via naturalis and were happy with the outcome. Two of these patients had complaints of frequency but was not bothersome.
All the patients received adjuvant chemotherapy. The mean overall survival was 44.2 ± 7.09 months. Histopathological examination details were moderately differentiated adenocarcinoma in seven patients and highly undifferentiated adenocarcinoma in two patients.
Discussion
It is estimated that 10 to 20% of the cases of colonic cancer in the USA represent locally advanced disease, with tumors extending through the colon wall with perforation and/or invasion of adjacent organs or structures [1–5]. These are classified as T4 lesions by the American Joint Committee on Cancer staging schema [11]. It is important to identify patients with advanced lesions so as to plan a proper surgical procedure. Patients with bladder involvement present clinically with dysuria and hematuria. Advanced lesions tend to be larger and are often palpable on physical examination. Colonoscopy may reveal annular or constrictive lesions. Radiographic imaging with computed tomography (CT) frequently indicates possible malignant fistula; however, the finding may be subtle. CT scan cannot differentiate peritumoral inflammation from direct tumor infiltration. Cystoscopy and examination under anesthesia will help in proper preoperative planning and can be used in conjunction with the above modalities. Even with a combination of these diagnostic modalities, some patients will have multivisceral involvement suspected only at the time of surgery. Ultimately, the surgeon needs to decide intra-operatively whether or not an extended resection is necessary.
Surgical resection remains the primary and most effective treatment for advanced colorectal cancers [12]. Individual patient selection, preoperative counseling, and the availability of a multidisciplinary surgical team are vital to providing an oncologically sound operation with curative intent. Due to the complex nature of these cases, a single standard surgical treatment option for all patients is not possible. The decision to perform TPE versus bladder-sparing surgery (partial cystectomy with reconstruction) must be made within the operating room. When possible, the possibility of urinary reconstruction or diversion should be discussed in great detail preoperatively. Meticulous bladder-sparing techniques have been shown to have equivalent oncologic outcomes with possibly less morbidity [4]. Of course, complete resection of involved tissues remains paramount in achieving these desired results.
When removing a portion of the bladder for malignant infiltration, the bladder either can be closed primarily or with the use of a bowel augmentation (enterocystoplasty). Enterocystoplasty should be considered in patients with poor compliance as assessed preoperatively or symptomatic bladder overactivity due to radiation therapy, as well as in patients with significant reduction of bladder volume after resection. Volume of bladder resected is a subjective indication and is difficult to quantitate at the time of surgery. There will be some patients who are closed primarily (without augmentation) and who will develop symptomatic storage symptoms and may require subsequent augmentation. Several authors recommend an initial conservative approach, due to the complications associated with augmentation and the feasibility of secondary augmentation (at a later date) [13]. Obtaining negative oncologic margin is of paramount importance, and at times, the bladder may be reduced to a capacity of 50 to 100 ml after partial cystectomy. In extreme cases, only the bladder neck and trigone may be left intact (supra-trigonal cystectomy). Enterocystoplasty may be a reasonable alternative to urinary diversion in these cases. Patients should be warned of this possibility before surgery and demonstrate a willingness and dexterity to self-catheterize. Also, it is important to confirm the adequacy of the urethral sphincteric function preoperatively so as to ensure dryness following surgery. Although augmentation can increase bladder compliance and functional capacity, it can also be a cause for voiding dysfunction necessitating self-catheterization.
In the setting of advanced (T3–T4) colorectal malignancy, exenteration or total cystectomy may become necessary along with urinary diversion. Numerous factors need to be considered when determining the type of urinary diversion. Patient expectations, comorbidities, surgical history, and the surgeon’s experience with the numerous diversion techniques are some of the factors that are considered when choosing a specific urinary diversion. Quality of life studies are inconsistent with respect to choosing a superior urinary diversion [14]. The non-continent urinary diversions (ileal or colon conduits) are commonly performed even today. This is especially true in complex patients such as those with advanced non-urologic cancers requiring pelvic exenteration and possible radiation. Even at many tertiary high-volume referral centers, the ileal conduit is performed on greater than 75% of patients undergoing radical cystectomy [15].
Nyam et al. [16] reported on 27 patients who underwent concomitant partial cystectomy for advanced colorectal carcinoma. Histological invasion into the bladder was found in only 26% of the cases. Eighty-five percent of the patients with carcinomatous invasion had preoperative urological symptoms. Twenty (74%) patients were alive without evidence of local or distant metastasis with a median follow-up of 40.2 months. Winter et al. [8] evaluated the outcome of combined bladder resection in 63 patients with colorectal carcinomas. The operative morbidity and mortality rates were 18 and 1.5% respectively. Histological staging demonstrated bladder adherence in 46% and invasion in 54%. Overall, disease-specific survival was 54% with a mean follow-up of 7.6 years. Five-year survival for margin-negative patients was 72 and 27% for positive margins. The bladder was closed primarily in 48 patients, with enterocystoplasty in five and ten patients required urinary diversion. Kobayashi et al. [3] retrospectively reviewed the clinical records of 580 patients with colorectal carcinoma. Seventeen (2.9%) had a diagnosis of urinary bladder invasion intra-operatively. Our study too shows the feasibility of combined bladder resection with resection of colorectal lesion. Bladder preservation was possible in eight of the nine patients, with negative surgical margins. We have been using cystoscopic guidance for resecting the bladder portion as described by us elsewhere [17].
Conclusions
Locally advanced primary colorectal cancer involving the bladder poses treatment challenges for the surgeon. Complete resection is a requisite for long-term survival. In the case of locally advanced colorectal cancer, differentiating malignant invasion of the bladder from benign adhesion is often not possible on imaging or at times even in the operating room. Because dissection of a malignant fistula and violating tumor planes are associated with tumor spillage and a worse outcome, en bloc resection of involved structures is recommended. In some cases, this may require extensive resection including the whole of the bladder; however, cure/control is possible if all disease is excised. Bladder preservation is possible in most of the cases. The postoperative complications, morbidity, and quality of life are better in patients with preservation of bladder. Neoadjuvant protocols utilizing conventional and biologic chemotherapy hold promise for downsizing tumors, facilitating complete resection, and prolonging survival.
References
- 1.Gebhardt C, Mayer W, Rukriegel S, Merier U. Multivisceral resection of advanced colorectal carcinoma. Langenbeck's Arch Surg. 1999;384:194–199. doi: 10.1007/s004230050191. [DOI] [PubMed] [Google Scholar]
- 2.Eldar S, Kemeny MM, Terz JJ. Extended resections for carcinoma of the colon and rectum. Surg Gynecol Obstet. 1985;161(4):319–322. [PubMed] [Google Scholar]
- 3.Kobayashi T, Kamoto T, Sugino Y, Takeuchi H, Habuchi T, Ogawa O. High incidence of urinary bladder involvement in carcinomas of the sigmoid and rectum: a retrospective review of 580 patients with colorectal carcinoma. J Surg Oncol. 2003;84(4):209–214. doi: 10.1002/jso.10322. [DOI] [PubMed] [Google Scholar]
- 4.Carne PWG, Frye JNR, Kennedy-Smith A, et al. Local invasion of the bladder with colorectal cancers: surgical management and patterns of local recurrence. Dis Colon Rectum. 2004;47(1):44–47. doi: 10.1007/s10350-003-0011-z. [DOI] [PubMed] [Google Scholar]
- 5.McKenzie SP, Barnes SL, Schwartz RW. An update on the surgical management of rectal cancer. Curr Surg. 2005;62(4):407–411. doi: 10.1016/j.cursur.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Balbay MD, Slaton JW, Trane N, Skibber J, Dinney CP. Rationale for bladder-sparing surgery in patients with locally advanced colorectal carcinoma. Cancer. 1999;86(11):2212–2216. doi: 10.1002/(SICI)1097-0142(19991201)86:11<2212::AID-CNCR6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein RP, Grob BM, Pachter EM, Soloway S, Fair WR. Partial cystectomy during radical surgery for nonurological malignancy. J Urol. 2001;166(1):79–81. doi: 10.1016/S0022-5347(05)66081-8. [DOI] [PubMed] [Google Scholar]
- 8.Winter DC, Walsh R, Lee G, Kiely D, O’Riordain MG, O’Sullivan GC. Local involvement of the urinary bladder in primary colorectal cancer: outcome with en-bloc resection. Ann Surg Oncol. 2007;14(1):69–73. doi: 10.1245/s10434-006-9031-y. [DOI] [PubMed] [Google Scholar]
- 9.Sasson AR, Sigurdson ER. Management of locally advanced rectal cancer. Surg Oncol. 2000;9(4):193–204. doi: 10.1016/S0960-7404(01)00012-3. [DOI] [PubMed] [Google Scholar]
- 10.Ike H, Shimada H, Yamaguchi S, Ichikawa Y, Fujii S, Ohki S. Outcome of total pelvic exenteration for primary rectal cancer. Dis Colon Rectum. 2003;46(4):474–480. doi: 10.1007/s10350-004-6585-2. [DOI] [PubMed] [Google Scholar]
- 11.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 12.Lehnert T, Methner M, Pollok A, Schaible A, Hinz U, Herfarth C. Multivisceral resection for locally advanced primary colon and rectal cancer: an analysis of prognostic factors in 201 patients. Ann Surg. 2002;235(2):217–225. doi: 10.1097/00000658-200202000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delacroix SE, Winters JC. Bladder reconstruction and diversion during colorectal surgery. Clin Colon Rectal Surg. 2010;23:113–118. doi: 10.1055/s-0030-1254298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sogni F, Brausi M, Frea B, et al. Morbidity and quality of life in elderly patients receiving ileal conduit or orthotopic neobladder after radical cystectomy for invasive bladder cancer. Urology. 2008;71(5):919–923. doi: 10.1016/j.urology.2007.11.125. [DOI] [PubMed] [Google Scholar]
- 15.Lowrance WT, Rumohr JA, Clark PE, Chang SS, Smith JA, Jr, Cookson MS. Urinary diversion trends at a high volume, single American tertiary care center. J Urol. 2009;182(5):2369–2374. doi: 10.1016/j.juro.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 16.Nyam DCNK, Seow-Choen F, Ho MS, Goh HS. Bladder involvement in patients with colorectal carcinoma. Singap Med J. 1995;36:525–526. [PubMed] [Google Scholar]
- 17.Nerli RB, Reddy M, Koura AC, Prabha V, Ravish IR, Amarkhed S. Cystoscopy assisted laparoscopic partial cystectomy. J Endourol. 2008;22:83–86. doi: 10.1089/end.2007.0105. [DOI] [PubMed] [Google Scholar]