Abstract
Malignant peripheral nerve sheath tumor (MPNST) refers to spindle cell sarcomas arising from or separating in the direction of cells of peripheral nerve sheath. The MPNST of the parotid gland is an extremely rare tumor, accounts for < 5% of all soft tissue sarcomas, and carries a poor prognosis. In this article, we report a case of MPNST of parotid gland in a 45-year-old male and review its diagnostic and therapeutic challenges. A 45-year-old male presented with right parotid swelling for 2 years with rapid increase in size over the last 3 months. He underwent right total conservative parotidectomy with selective neck dissection. Reconstruction was done with microvascular anterolateral thigh flap. On immunohistochemistry, the tumor cells expressed CD 56 diffusely and S 100 focally. Tumor was immunonegative for CK, Desmin, SOX – 10, and SMA consistent with MPNST. The MPNSTs arising as parotid mass are very rare and aggressive tumors. The role of IHC is paramount in establishing the diagnosis. Multimodal management including wide surgical resection, neck dissection, and adjuvant chemoradiotherapy is the choice of treatment.
Keywords: Malignant, Nerve sheath tumor, Facial nerve, Parotid gland
Introduction
Mesenchymal tumors comprise about 2 to 5% of all salivary gland tumors with over 95% involving the major salivary glands. Malignant tumors are outnumbered by benign mesenchymal tumors. Ratio of benign to malignant lesions described in literature is 2.4:1 to 18:1 [1–3]. Incidence of malignant peripheral nerve sheath tumor (MPNST) in parotid gland is very rare, about 0.01% [4]. The differential diagnosis includes other spindle cell tumors of parotid like myoepithelial carcinoma, spindle cell carcinoma, malignant melanoma, and fibrosarcoma. These are easily ruled out when histomorphological characteristics are combined with immunohistochemistry features, and hence, awareness of these is imperative for differentiating this rare entity from other spindle cell tumors. Wide surgical excision with possible preservation of facial nerve along with neck dissection and adjuvant chemoradiotherapy is the mainstay of treatment. MPNST of parotid gland has a very aggressive course and a very bad prognosis with a survival rate of 5 to 7 years [1]. We report a MPNST of parotid gland in a 45-year-old male and review its diagnostic details including histopathologic and immunohistochemical features.
Case Report
A 45-year-old male presented with a history of right-sided parotid gland swelling of 2 years duration which was rapidly increasing in size for last 3 months. The swelling was associated with pain. On examination, a mass of size 10 × 6 cm was found in the right-sided parotid region. The mass was fungating into overlying skin and was adherent to the deeper structures. There was no facial paralysis. There was no parapharyngeal extension, Fig. 1. Fine needle aspiration done elsewhere revealed an angiofibromatous tumor of the parotid gland. Magnetic resonance imaging done revealed a well-defined lobulated oval heterogenous lesion appearing slightly hypointense on both T1- and T2-weighted images, measuring approximately 95 × 63 × 48 mm in the right temporal and parotid region abutting the right parotid gland as well as masseter muscle. Visualized muscles and soft tissues also appear normal. Multiple lymph nodes were seen in the draining area. There was no history suggestive of neurofibromatosis. He underwent right total conservative parotidectomy with selective neck dissection (level II to IV lymph nodes). Reconstruction was done with microvascular anterolateral thigh flap. Intraoperative, there was a 13 × 5 cm soft tissue mass arising from the right parotid superficial lobe infiltrating into the skin. There were multiple enlarged lymph nodes in the neck. Gross specimen 13 × 7.5 × 5.5 composed of tumor mass with overlying skin. The cut surface showed a circumscribed gray-white firm tumor with areas of necrosis and hemorrhage surrounded by a thick fibrous capsule, Fig. 2. A spindle cell tumor with cells arranged in long intersecting fascicles with both hyper and hypocellular areas with hyalanization and myxoid changes. The tumors cells had spindled wavy appearance with moderate amount of eosinophilic cytoplasm tapered at ends, with oval to stellate-shaped nuclei with dispersed chromatin and inconspicuous nucleoli with mild to moderate nuclear pleomorphism. Mitotic activity of 10 to 12 per high power field was seen with interspersed areas of necrosis. Tumor mass was seen pushing the unremarkable salivary gland at the periphery. There was no lymphovascular or perineural invasion and resection margins were free. All lymph nodes were free of disease. On immunohistochemistry (IHC), the tumor cells expressed CD 56 and S 100 and were negative for CK, Desmin, SOX – 10, and SMA consistent with MPNST, Fig. 3. The postoperative course was uneventful except grade 1 infranuclear facial palsy. The patient was advised adjuvant chemoradiotherapy and was referred to medical and radiation oncology. Chemotherapy involved 6 cycles of doxorubicin at a dose of 60 mg/m2, every 21 days and ifosfamide at a dose of 2500 mg/m2, given at days 1 to 3. Radiation therapy was administered at a dose of 50–66 Gy, from 1.8 to 2 Gy per day. He is doing fine till 6 months follow-up, Fig. 4.
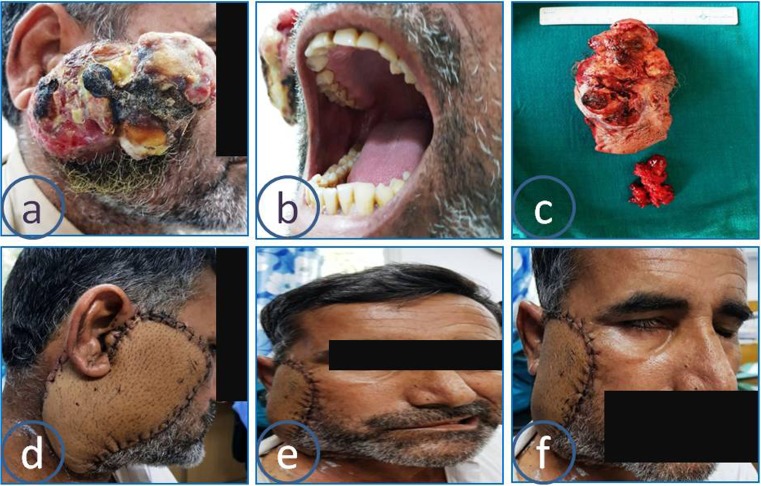
Fig. 1.
Large tumor of the right parotid gland (a), no intraoral extension (b), gross tumor and lymph node specimen (c), flap reconstruction (d), facial nerve palsy (e, f)
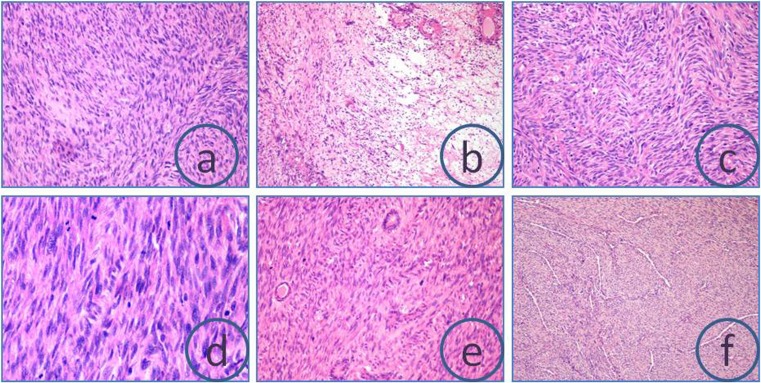
Fig. 2.
Hematoxylin & Eosin-stained slides depicting spindled cells arranged in fascicles (a), hyper and hypocellular areas with hyalinization and myxoid changes (b), herringbone pattern (c), frequent mitosis (d), glands (e), and necrosis and perivascular plump cells (f)
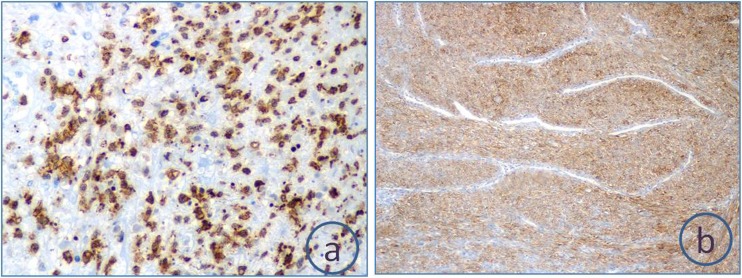
Fig. 3.
Immunohistochemistry revealing strong focal S 100 and CD 56 positivity
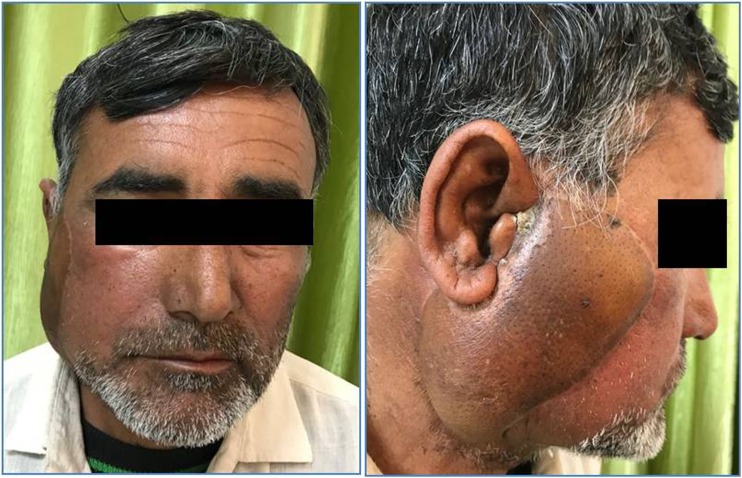
Fig. 4.
Follow-up images after chemoradiotherapy
Discussion
The MPNST, otherwise known as malignant schwannoma or neurogenic sarcoma, is an aggressive entity, with an origin from any cell of the nerve sheath: Schwann cell, perineural fibroblast, or endoneurial fibroblast. They arise primarily in the extremities or trunk. It accounts for about 5–10% of soft tissue sarcomas, of which, in general, only about 8–16% occur in the head and neck region with very rare presentation [5].
Malignant peripheral nerve sheath tumor is typically a disease of adult life as most tumors occur in 20–50 years of age. Our patient was a 45-year-old male. The lesions usually present as a rapidly enlarging mass, usually noted several months before diagnosis and may present with pain. Our case had rapidly increasing swelling over the right parotid region for last 3 months.
The MPNST can arise from pre-existing neurofibroma or de novo. Especially, patients with type 1 neurofibromatosis (NF 1) have a great tendency to develop malignant peripheral nerve sheath tumors. Etiopathogenesis of MPNST with NF 1 is believed to be associated with loss of chromosomal arm 17q sequence including complete inactivation of NF-1 gene [6, 7]. The present case had a history of parotid mass and no other mass throughout his body. Moreover, his family members did not have any history of neurofibromatosis. This indicates the tumor in the present case has de novo origin in parotid.
A sarcoma is assumed to be malignant peripheral nerve sheath tumor if one of the 3 criteria are met, with tumor arises from a peripheral nerve, tumor arises from a pre-existing benign nerve sheath tumor, neurofibroma, and or tumor displaying constellation of histological features of Schwann cell differentiation [1, 8]. Other subtle and less-specific features such as nuclear palisading, increased perivascular cellularity, storiform or herringbone, and curlicue growth patterns may also be seen. Areas of geographic necrosis may be present. The most striking feature of MPNST is its degree of morphological heterogeneity, not only in terms of cellular pleomorphism and nuclear atypia but also in its abrupt transition of patterns, cellularity, and grade. Certain histological variants such as epithelioid, glandular, rhabdomyosarcomatous (malignant triton tumor), pigmented, and perineural have also been described in the literature but have little effect on prognosis. Diagnosis of MPNSTs is usually based on histopathology aided by IHC, which reflects the Schwann cell differentiation in this neoplasm. Approximately 50–90% of MPNSTs are positive for S-100 protein [9, 10]. The present case displayed all histologic and immunohistochemistry features suggestive of MPNST. Focal positivity for S 100 and negativity for SMA and CK ruled out the possibility of sarcomatoid or spindle cell carcinoma in the present case.
Surgery including wide surgical excision with neck dissection is the treatment of choice followed by adjuvant chemoradiotherapy. Conservative excision with facial nerve preservation wherever possible improves cosmetic and functional outcomes. Despite comprehensive treatment with complete surgical resection and radiotherapy, the tumor displays a highly aggressive course, with local recurrence in 54% of cases and distant metastasis to lungs and bone in 65% cases [5, 11]. According to Wise et al., the prognosis of MPNSTs of head and neck region is poor and have a 5-year survival rate of only 20–50% [12]. Large tumor size (> 5 cm), presence of neurofibromatosis, and total resection are most important prognostic indicators for MPNST. Other prognostic factors described in literature are cellularity, pleomorphism, and mitotic activity. [5, 13]. The size of tumor in the present case was 13 cm and exhibited all poor prognostic features in histology. Some of the MPNSTs can have epithelioid morphology and is an aggressive variant of malignant peripheral nerve sheath tumor with a high rate of recurrence and poor prognosis [14].
Conclusion
The MPNSTs arising as parotid mass are very rare. The pathologists and surgeons should keep this in mind when dealing with a patient of parotid mass. Role of IHC is paramount in establishing the diagnosis. Wide surgical resection and adjuvant chemoradiotherapy are the choices of treatment.
Contributor Information
Garima Daga, Phone: +91-9930484364, Email: narsinghbaba@yahoo.co.in.
Rajiv Paul, Email: drrajivpaulms2013@gmail.com.
Ghanashyam Mandal, Email: ghanashyam009@gmail.com.
Rajeev Kumar, Email: rku66@hotmail.com.
References
- 1.Biswa P, Kai A, Moharty L, Pattaik K, Nayak M (2012) Malignant peripheral nerve sheath tumor in parotid gland-a rare and challenging case. J Clin Case Rep 3(1)
- 2.Ellis GL, Auclair PL, Gnepp DR. LiVolsi Major problems in pathology. Philadelphia, PA: Saunders; 1991. Surgical pathology of the salivary glands. [Google Scholar]
- 3.Seifert G, Miehlke A, Haubrich J. Diseases of the salivary glands. Stuttgart: George Thieme; 1986. [Google Scholar]
- 4.Imamura SI, Suzuki H, Usami SI, Koda E, Yoshizawa A. Malignant peripheral nerve sheath tumor of the parotid gland. Ann Otol, Rhinol Laryngol. 2003;112(7):637–643. doi: 10.1177/000348940311200711. [DOI] [PubMed] [Google Scholar]
- 5.Gogate BP, Anand M, Deshmukh SD, Purandare SN. Malignant peripheral nerve sheath tumor of facial nerve: presenting as parotid mass. J Oral Maxillofac Pathol: JOMFP. 2013;17(1):129–131. doi: 10.4103/0973-029X.110708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986;57:2006–2021. doi: 10.1002/1097-0142(19860515)57:10<2006::AID-CNCR2820571022>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Başaran B, Orhan KS, Polat B, Mete O, Başerer N. Malignant peripheral nerve sheath tumor in the parotid gland developed on the basis of neurofibromatosis type 1. Kulak burun bogaz ihtisas dergisi: KBB= Journal of ear, nose, and throat. 2011;21(4):220–224. doi: 10.5606/kbbihtisas.2011.030. [DOI] [PubMed] [Google Scholar]
- 8.Weiss SW, Goldblum JR. Enzinger and Weiss’s soft tissue tumors. 5th edn. St. Louis: Mosby; 2008. pp. 903–941. [Google Scholar]
- 9.Sham ME, Ghorpande, Shetty A, et al. Malignant peripheral nerve cell tumor. J Maxillofac Oral Surg. 2009;9:68–71. doi: 10.1007/s12663-010-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel S, Pathak J, Dekate K, Mohanty N. Malignant peripheral nerve sheath tumour (MPNST) of mandible: solving the perplexity. BMJ Case Reports. 2015;2015:bcr2014207790. doi: 10.1136/bcr-2014-207790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canor G, Ozkok G, Guler G, Gul E, Ziya M. Malignant peripheral nerve sheath tumour presenting as a parotid mass. InKBB Forum. 2007;6:76–78. [Google Scholar]
- 12.Wise JB, Patel SG, Shah JP. Management issues in massive pediatric facial plexiform neurofibroma with neurofibromatosis type 1. Head Neck. 2002;24:207–211. doi: 10.1002/hed.10001. [DOI] [PubMed] [Google Scholar]
- 13.Colreavy MP, Lacy PD, Hughes J, Bouchier-Hayes D, Brennan P, O’Dwyer AJ, et al. Head and neck schwannomas: a 10 year review. J Laryngol Otol. 2000;114:119–124. doi: 10.1258/0022215001905058. [DOI] [PubMed] [Google Scholar]
- 14.De Stefano A, Kulamarva G, Citraro L, Borgia L, Croce A. Malignant peripheral nerve sheath tumour (malignant epithelioid Schwannoma) of the parotid gland. Bratislavske lekarske listy. 2012;113(10):628–631. doi: 10.4149/bll_2012_143. [DOI] [PubMed] [Google Scholar]