Summary
We report a case of cardiac memory following recurrent episodes of monomorphic idiopathic ventricular tachycardia and explain how it could be helpful in localizing the site of origin of the arrhythmia.
Keywords: T wave memory, Monomorphic ventricular tachycardia
Introduction
“Cardiac memory” is a variety of electrophysiological remodeling, resulting in T wave changes, which usually follows a period of abnormal ventricular activation [1]. Cardiac memory, different from memory in neurons (which is a gain of function), is a loss of function and a transition of cardiac cells into a neonatal status. Usually separated into 2 main categories (short- and long-term cardiac memory), the short-term process (usually lasting minutes to hours) is associated with a loss of Ito channels induced by myocardial stretching and increase in angiotensin II concentration. The long-term process (usually lasting weeks to months) is related to a complex change in transcription factors and in a further loss of function of Ito and K channels [2].
Cardiac memory has been associated with right ventricular pacing, left bundle branch block, ventricular pre-excitation, ventricular widening after flecainide intoxication, and ventricular tachycardia [2]. We report a case of cardiac memory following recurrent episodes of monomorphic idiopathic ventricular tachycardia. We invite the readers to observe carefully the electrocardiograms (ECGs) displayed in Figure 1, Figure 2. In “Discussion” section, we explained what is the peculiarity of our report and how T wave vector may help in localizing the site of origin of a monomorphic ventricular tachycardia.
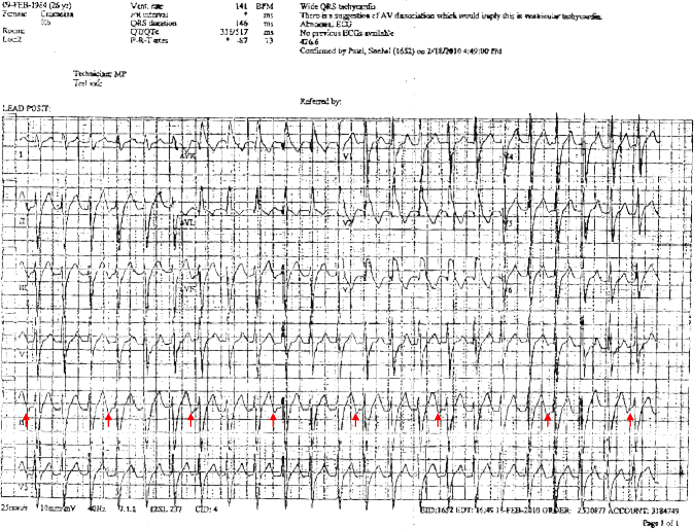
Figure 1.
12-Lead electrocardiogram showing a monomorphic ventricular tachycardia with right bundle branch morphology and left axis deviation. Red arrows display P waves in lead II. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
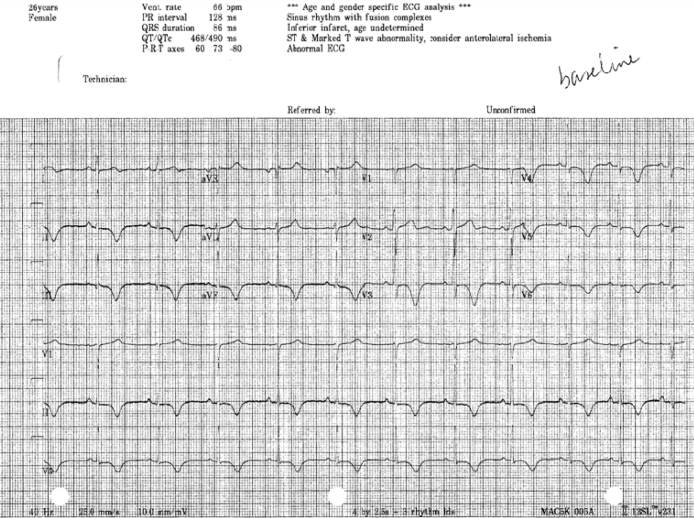
Figure 2.
12-Lead baseline electrocardiogram showing T wave memory.
Case report
We present the case of a 26 year-old woman, with a history of anxiety disorder and hypothyroidism, who was referred to our hospital for an ablation of monomorphic ventricular tachycardia. The patient had been suffering from regular and sudden-onset fast palpitations for several years. Noteworthy, about 9 months before the admission to our hospital, she was brought to an emergency room where an ECG revealed a wide QRS complex tachycardia with a heart rate of 145 bpm, right bundle branch morphology, and a left-axis deviation (automatic detection of the QRS main vector showed a value of −87) (Fig. 1). Adenosine, which was given twice, was ineffective while verapamil ultimately terminated the arrhythmia. The presence of a ventriculo-atrial dissociation (Fig. 1, arrows) and the resistance to adenosine oriented the diagnosis toward a monomorphic ventricular tachycardia. She was initially treated with low dosages of metoprolol and thereafter with the combination of verapamil and propafenone: nevertheless, the patient continued to experience daily episodes of palpitations. An event recorder confirmed the wide complex tachycardia as the cause of the cited episodes of palpitations (no information on the burden of ventricular tachycardia has been given in the Holter ECG report). The patient denied any episode of syncope, chest pain, and/or dyspnea and had no family history of sudden death. An echocardiogram showed normal left ventricular wall thickness and systolic function with an ejection fraction of 55%. Cardiac magnetic resonance imaging showed normal right and left ventricular volumes and dimensions and no evidence of delayed enhancement in either ventricle.
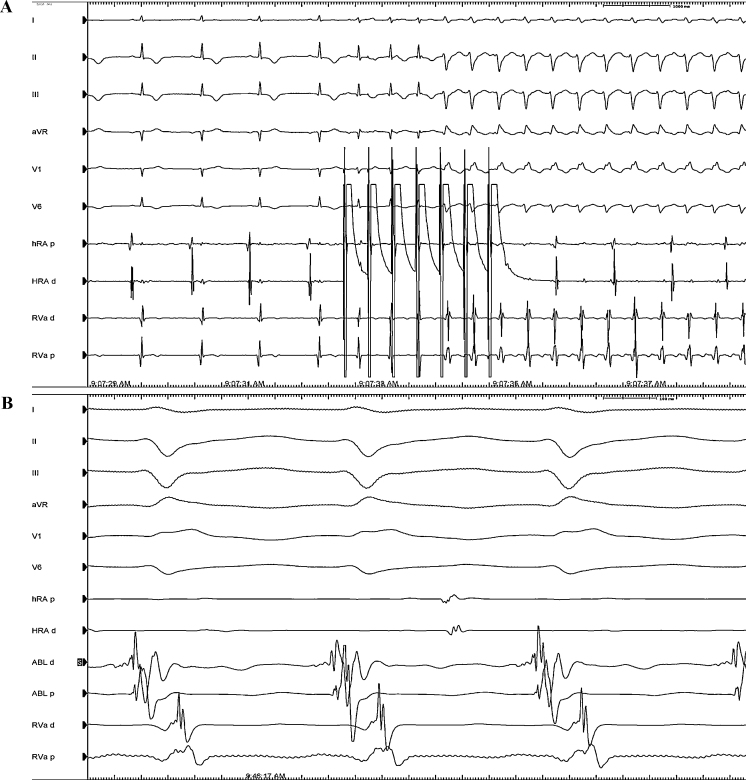
Upon presentation in our department, her vital signs were temperature 36.5 °C, blood pressure 100/70 mmHg, oxygen saturation 100% on room air, heart rate 65 bpm. Physical examination was unremarkable. Her medication included bupropion HCl 150 mg/daily, lamotrigine 100 mg/daily, levothyroxine 150 μg/daily, propafenone 225 mg twice a day, and verapamil 120 mg/daily. The ECG (Fig. 2) showed sinus rhythm with a heart rate of 66 bpm, a PR interval of 128 ms, and a QRS duration of 86 ms with a QTc of 490 ms. Interestingly, there was T wave inversion in the inferior leads and in the left precordial leads (V3–V6), a biphasic T wave in lead I and a positive T wave in aVR, in aVL, and in V1 and V2. T wave vector pointed “north-east” with a value of −80. The patient underwent an electrophysiological study. A quadripolar catheter was positioned in the apex of the right ventricle and a decapolar one in the coronary sinus. No accessory pathway was noticed at baseline and after differential pacing manoeuvres from the atrium and the ventricle. The tachycardia was easily and repeatedly induced with atrial bursts (Fig. 3A). Ventricular to atrial dissociation proved a ventricular origin of the arrhythmia. Right bundle branch morphology and a left upward axis oriented the diagnosis toward a monomorphic idiopathic ventricular tachycardia originating from the posterior fascicle of the left bundle branch. The left ventricular septum was mapped with identification of Purkinje spikes and presystolic potentials up to 56 ms before onset of the QRS complex (located at mid posterior septum). Radiofrequency catheter ablation was performed successfully with a retrograde approach in the infero-septal medial portion of the left ventricle (Fig. 3B). Propafenone and verapamil have been weaned off after the ablation procedure.
Figure 3.
(A) Induction of ventricular tachycardia with atrial bursts from the high right atrium (HRA). From the top to the bottom electrocardiogram (ECG) leads I, II, III, aVR, V1, and V6 are displayed with electrograms from proximal and distal HRA (HRAd and HRAp) and proximal and distal right ventricular apex (RVAd and RVAp). (B) Site of successful ablation. From the top to the bottom ECG leads I, II, III, aVR, V1, and V6 are displayed with electrograms from HRAd and HRAp, proximal and distal ablation catheter (ABLp and ABLd) and RVAd and RVAp.
Which is the most reasonable explanation for cardiac memory in this patient and why ischemic reperfusion (Wellens’ syndrome) [3] and transient left bundle branch block might be a priori ruled out?
Discussion
The answer to our question might be found in the correspondence between the T wave vector in sinus rhythm and the QRS vector during tachycardia in all leads, both limb and precordial ones. Indeed, the QRS vector during tachycardia and the vector of the T wave in sinus rhythm were very close to each other (−87 and −80, respectively). Cardiac ischemia can be ruled out since the patient had no history of coronary artery disease, no clinical signs of ischemia, and normal levels of troponin T at the admission. Furthermore, as demonstrated by Shvilkin et al. [4], the presence of a positive T wave in aVL and of a positive/isoelectric T wave in lead I is strongly predictive of cardiac memory following right ventricular apical pacing and excludes myocardial ischemia. Left bundle branch block can be excluded in this case also. Although the patient presented with a positive T wave in aVL, the presence of a positive T wave in V1 and V2 ruled out this option. The presence of a positive T wave in the right precordial leads is usually secondary to an activation of the ventricles from the posterior to the anterior wall and this is exactly the opposite of what happens as a consequence of a left bundle branch block or right ventricular pacing, when one should expect a negative T wave in these leads linked to an activation of the ventricles from the anterior to the posterior wall.
The most interesting and simplest finding of our case report is that T waves in sinus rhythm resembled completely the aspect of the QRS complexes during the tachycardia. Taking this in mind, one can deduce the morphology of the tachycardia even not having the chance to induce the tachycardia itself. Therefore we propose a possible additional use of cardiac memory. Even if in our case the arrhythmia was easily inducible, inducibility of a ventricular arrhythmia can be challenging. Bearing in mind what happened in our case, we propose cardiac memory as a guide to both diagnosing the type of arrhythmia and localizing the site of origin of the arrhythmia when the arrhythmia itself cannot be induced.
We recognize that our case has several limitations. First of all, one should bear in mind that different ventricular tachycardias originating from different but contiguous anatomical areas might cause the same shift in the T wave vector. Secondly, one should consider that T wave memory might derive from different clinical conditions such as intermittent posteroseptal accessory pathways, intermittent RBBB with left anterior hemiblock, and bradycardia with the escape rhythm from left posterior fascicle. It is mandatory to exclude all of them before making any conclusion. Moreover, T wave memory does not help in recognizing the mechanism of the ventricular tachycardia and therefore cannot guide the therapeutic decision-making process. Finally, the influence of drugs such as bupropion and lamotrigine in modifying cardiac repolarization cannot be a priori ruled out.
References
- 1.Rosenbaum M.B., Blanco H.H., Elizari M.V., Lázzari J.O., Davidenko J.M. Electrotonic modulation of the T wave and cardiac memory. Am J Cardiol. 1982;50:213–222. doi: 10.1016/0002-9149(82)90169-2. [DOI] [PubMed] [Google Scholar]
- 2.Ozgen N., Rosen M.R. Cardiac memory: a work in progress. Heart Rhythm. 2009;6:564–570. doi: 10.1016/j.hrthm.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Rhinehardt J., Brady W.J., Perron A.D., Mattu A. Electrocardiographic manifestations of Wellens’ syndrome. Am J Emerg Med. 2002;20:638–643. doi: 10.1053/ajem.2002.34800. [DOI] [PubMed] [Google Scholar]
- 4.Shvilkin A., Ho K.K.L., Rosen M.R., Josephson M.E. T-vector direction differentiates postpacing from ischemic T-wave inversion in precordial leads. Circulation. 2005;111:969–974. doi: 10.1161/01.CIR.0000156463.51021.07. [DOI] [PubMed] [Google Scholar]