Summary
A 69-year-old man with a history of paroxysmal atrial fibrillation (PAF) and subsequent cerebral infarction was referred to our hospital because of an abnormal accumulation of 18F-fluorodeoxyglucose (FDG) in the left atrial appendage (LAA) that was detected on positron emission tomography-computed tomography (PET-CT) imaging during a health screening. Transesophageal echocardiography (TEE) demonstrated thrombus formation in the LAA. Even after the thrombus disappeared by strictly guided oral anticoagulant therapy, intense abnormal FDG uptake in the LAA on PET-CT imaging persisted. In low-risk patients such as this, inflammation may have played some role in LAA thrombus formation.
Keywords: Paroxysmal atrial fibrillation, Thrombus, 18F-fluorodeoxyglucose, Left atrial appendage, Positron emission tomography-computed tomography, Inflammation
Introduction
Intra-atrial thrombus formation in patients with atrial fibrillation (AF) is known to be a potent risk factor for thromboembolic events such as stroke. Thrombus formation is preferentially found in the left atrial appendage (LAA) and has been thought to be induced by blood stasis associated with AF. However, there is an apparent link between thrombogenesis and inflammation [1].
CHADS2 (congestive heart failure, hypertension, age, diabetes, stroke) score is a convenient and accurate classification for estimating stroke risk in patients who have non-rheumatic AF [2]. In low-risk patients, the thromboembolic stroke risk can be as high as 1.5% per year and is associated with a 1.8-fold increase in mortality [2]. We report a case demonstrating a low stroke risk, in which 18F-fluorodeoxyglucose (FDG) accumulation in the LAA was detected on positron emission tomography-computed tomography (PET-CT).
Case report
In July 2008, a 69-year-old man was admitted to our hospital because of cerebral infarction presenting with aphonia, right hemiplegia, and paroxysmal atrial fibrillation (PAF) (Fig. 1A). He was started on medication with oral intake of warfarin 3.5 mg/day and pilsicainide 150 mg/day. In November 2008, he was re-admitted to our hospital because an abnormal accumulation of FDG in the LAA was detected on PET-CT imaging (Discovery LS, General Electric Medical Systems, Waukesha, WI, USA) during health screening. PET-CT was performed after fasting for at least 4 h and image acquisition was initiated 50 min after intravenous injection of 185 MBq of FDG. Whole-body emission scanning was performed from the head to the inguinal region with 2 min per bed position (7–8 bed positions). A transmission scan with CT for attenuation correction was performed prior to the emission scan. Using emission and transmission scan data, image reconstruction was performed and the reconstructed images were converted to standardized uptake value (SUV) image using the patient's body weight and the injected dose of FDG.
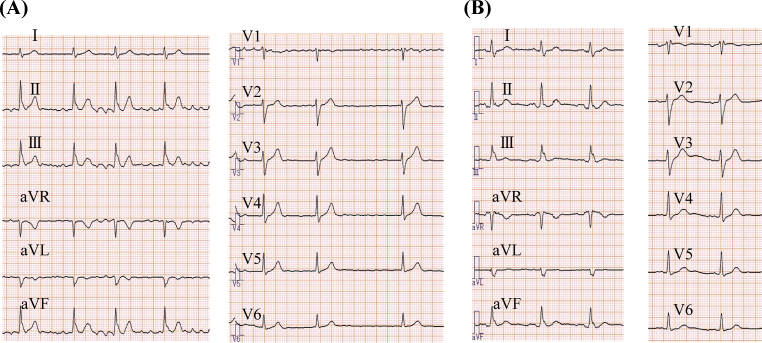
Figure 1.
Serial 12-lead electrocardiograms at the time of stroke show atrial fibrillation (A), which changed to sinus rhythm 5 months later (B). During sinus rhythm, P wave was biphasic in lead V1.
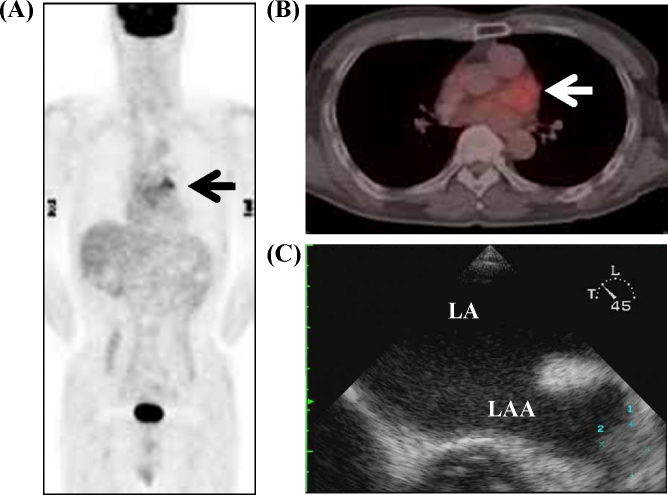
Laboratory examination showed prothrombin time-international normalized ratio (PT-INR) 2.27 (normal range < 1.14), brain natriuretic peptide (BNP) 177.4 pg/ml (normal range <18 pg/ml), highly sensitive C-reactive protein (hs-CRP) 1.18 mg/L, and d-dimer level 1.9 μg/ml (normal range < 1.0). Twelve-lead electrocardiogram (ECG) showed sinus rhythm and a biphasic P wave in lead V1 (Fig. 1B). The patient was diagnosed as having PAF because of the ECG changes and frequent episodes of palpitation. Health screening PET-CT showed accumulation of FDG in the LAA (Fig. 2A and B) and the SUV was 3.4. Transesophageal echocardiography (TEE) demonstrated thrombus formation in the LAA (Fig. 2C). The thrombus diameter was 12 mm × 11 mm, and the LAA flow was 85 cm/s and 82 cm/s (>20). Therefore, oral anticoagulant therapy was increased to warfarin 4.5 mg/day under strict monitoring, and sinus rhythm was maintained with pilsicainide 150 mg/day.
Figure 2.
Positron emission tomography-computed tomography showed abnormal uptake in the left atrial appendage (LAA) (arrow), and the standardized uptake value was 3.4 (A and B). Transesophageal echocardiography showed thrombus formation in the LAA (C). LA, left atria.
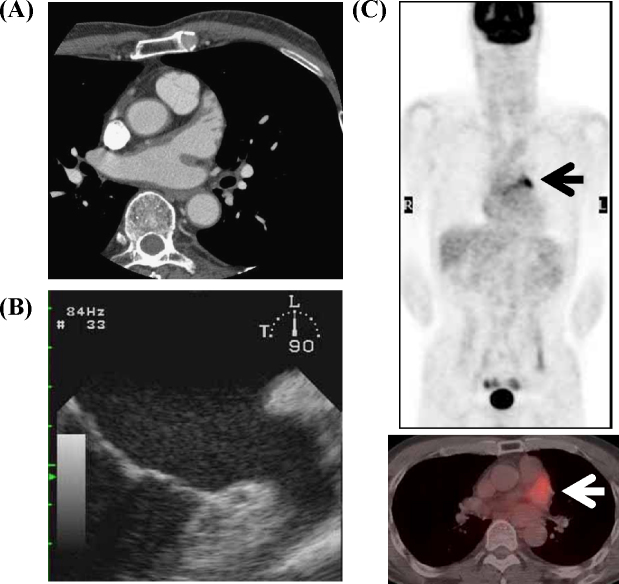
After strict anticoagulant therapy, the thrombus became unclear and there was no tumor lesion detected on computed tomography (Fig. 3A) Laboratory examination showed PT-INR 3.14, and the thrombus in the LAA disappeared on TEE (Fig. 3B). Health screening PET-CT was performed again 1 year 2 months later under the same conditions. Intense abnormal FDG uptake in the LAA persisted on PET-CT imaging (Fig. 3C), and the SUV was 4.76. Similarly this time, there was no apparent thrombus in the LAA on TEE.
Figure 3.
Computed tomography of the chest (A) and transesophageal echocardiography (B) showing disappearance of thrombus in the left atrial appendage (LAA). Positron emission tomography-computed tomography showed abnormal uptake in the LAA (arrow), and the standardized uptake value was 4.76 (C).
Discussion
We encountered a patient with PAF who showed intense abnormal accumulation of FDG in the LAA, which remained on PET-CT imaging even after the thrombus disappeared, by strictly guided oral anticoagulant therapy.
A previous case report showed FDG uptake in the LAA in patients with chronic AF [3], but on PET imaging alone. We had PET-CT, which helps to specify the LAA with more accuracy, also TEE, and follow-up PET-CT performed additionally. Also in the present case, an intensive positive FDG uptake in the LAA was recorded on PET-CT during a health screening, so we had confirmation on the patient's history of PAF, and also any malignancy and thrombus were ruled out, and such a case was not reported previously.
FDG is a glucose analog that traces the transmembranous transport and hexokinase-mediated phosphorylation of glucose, and deoxyglucose combines with glucose transporter in inflammatory cells and malignant cells [4]. In previous case reports, cardiac sarcoidosis or pericarditis showed abnormal FDG uptake representing inflammatory activity, apart from its physiological uptake 5, 6.
A recent report from the stroke prevention in atrial fibrillation (SPAF)-III study demonstrated that CRP positively correlated with stroke risk, related stroke risk factors, and prognosis in 880 patients with AF [7]. In addition, a previous study demonstrated that there is a possible link between CRP and left atrial thrombus [1]. There are some reports that active adhesion and recruitment of macrophages across the endocardium occur in human fibrillating atria suggesting the presence of local immunologic inflammatory responses around the atrial endocardium of AF [8]. Therefore, it is possible that some inflammatory alterations in the endocardium may modulate the LAA endothelial function leading to a thrombogenic status in patients with AF. We speculate that inflammation may play some role in LAA thrombus formation in this case.
Fujii et al. reported FDG uptake in the right atrial wall in a patient with AF [9], and it was speculated that it was due to an increased metabolism. It is known that thrombus form in the LAA, and in the present case, an intensive positive FDG uptake was recorded on PET-CT in the LAA, not in the right atrial wall. We also similarly speculate that the increased accumulation of FDG was caused by an increased metabolism, but more specifically an inflammatory mechanism both in the LAA as in the right atrial wall.
CHADS2 score is an easy to use classification scheme that estimates the risk of stroke in elderly patients with AF. CHADS2 score could facilitate risk stratification and selection of antithrombotic therapy based on a patient-specific risk of stroke [2]. Gage et al. suggested that by applying a classification scheme, clinicians could be able to identify AF patients who are at low risk for stroke even without warfarin therapy. This patient had low risk of thrombus formation with a CHADS2 score 0 before the referring stroke in 2007. Despite anti-coagulation medication and normal LAA flow velocity, this patient demonstrated an enlarged thrombus and an accumulation of FDG in the LAA in 2008. Although PAF is a disease that can account for the formation of thrombus, the most remarkable feature of this case was that there was an accumulation of FDG only in the LAA, and that it persisted after the thrombus had disappeared by anti-coagulation therapy.
The efficacy of oral anticoagulant therapy in reducing the risk of thromboembolic events has been demonstrated in patients with AF. To optimize the intensity of anticoagulation, as indicated by the INR, a target INR needs to achieve the optimal balance between the prevention of thromboembolic events and the occurrence of bleeding complications. In this case, the INR value was effective. However, even though the INR value was maintained within the range recommended in the guidelines, we found that this case became complicated by the LAA thrombus.
Our report emphasizes that, in case of an abnormal FDG accumulation in the LAA found during a health screening, we should examine not only by PET imaging but also by PET-CT, to screen for thrombosis specifically in the LAA.
The presence of inflammation, as evident by FDG accumulation, may also help to further stratify even low-risk patients for LAA thrombus formation and possibly stroke and peripheral embolism in non-rheumatic AF patients. PET-CT may be helpful for detecting inflammatory lesions related to AF in low embolic risk patients. In patients with FDG accumulation in the LAA, strict anticoagulant therapy should be administered. However, whether the local inflammation is a cause or a result of AF could not be determined in this case and should be investigated in future studies.
References
- 1.Maehama T., Okura H., Imai K., Saito K., Yamada R., Koyama T., Hayashida A., Neishi Y., Kawamoto T., Yoshida K. Systemic inflammation and left atrial thrombus in patients with non-rheumatic atrial fibrillation. J Cardiol. 2010;56:118–124. doi: 10.1016/j.jjcc.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Gage B.F., Waterman A.D., Shannon W., Boechler M., Rich M.W., Radford M.J. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen B.D. PET demonstration of left atrial appendage in chronic atrial fibrillation. Clin Nucl Med. 2005;30:177–179. doi: 10.1097/00003072-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Kubota R., Yamada S., Kubota K., Ishikawa K., Tamahashi N., Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med. 1992;33:1972–1980. [PubMed] [Google Scholar]
- 5.Okumura W., Iwasaki T., Toyama T., Iso T., Arai M., Oriuchi N., Endo K., Yokoyama T., Suzuki T., Kurabayashi M. Usefulness of fasting 18F-FDG PET in identification of cardiac sarcoidosis. J Nucl Med. 2004;45:1989–1998. [PubMed] [Google Scholar]
- 6.Testempassi E., Kubota K., Morooka M., Ito K., Masuda-Miyata Y., Yamashita H., Ito K., Mimori A., Kuroki H. Constrictive tuberculous pericarditis diagnosed using 18F-fluorodeoxyglucose positron emission tomography: a report of two cases. Ann Nucl Med. 2010;24:421–425. doi: 10.1007/s12149-010-0365-y. [DOI] [PubMed] [Google Scholar]
- 7.Lip G.Y., Patel J.V., Hughes E., Hart R.G. High-sensitivity C-reactive protein and soluble CD40 ligand as indices of inflammation and platelet activation in 880 patients with nonvalvular atrial fibrillation: relationship to stroke risk factors, stroke risk stratification schema, and prognosis. Stroke. 2007;38:1229–1237. doi: 10.1161/01.STR.0000260090.90508.3e. [DOI] [PubMed] [Google Scholar]
- 8.Yamashita T., Sekiguchi A., Iwasaki Y.K., Date T., Sagara K., Tanabe H., Suma H., Sawada H., Aizawa T. Recruitment of immune cells across atrial endocardium in human atrial fibrillation. Circ J. 2010;74:262–270. doi: 10.1253/circj.cj-09-0644. [DOI] [PubMed] [Google Scholar]
- 9.Fujii H., Yasuda S., Ide M., Takahashi W., Shohtsu A., Kubo A. Increased fluorine-18 fluorodeoxyglucose uptake in the right atrial wall in a patient with atrial fibrillation. Clin Nucl Med. 1999;24:136–137. doi: 10.1097/00003072-199902000-00020. [DOI] [PubMed] [Google Scholar]