Abstract
Background
Denosumab inhibits the receptor activator of nuclear factor κB (RANK) pathway and is used to treat osteoporosis. Emerging evidence suggests RANK-blockade may play a role in mammary tumourigenesis. Thus, we undertook a population-based study of denosumab use and breast cancer risk in a large cohort of postmenopausal women.
Methods
We included women 67+ years with prior bisphosphonate use who filled a first prescription for denosumab. They were matched on age, date, cumulative prior use of and time since last use of a bisphosphonate to women with no history of denosumab. Cox proportional hazards was used to estimate the hazard ratio (HR) of breast cancer with denosumab use.
Results
A total of 100,368 women were included in the analysis with 1271 incident breast cancer events. Denosumab use was associated with a 13% decreased breast cancer risk (HR = 0.87; 95% CI 0.76–1.00). There was no relationship between increasing number of denosumab doses and breast cancer risk (P-trend = 0.15).
Conclusion
These findings suggest a potential protective effect of ever denosumab use on breast cancer risk in a cohort of older women previously treated with bisphosphonates.
Subject terms: Breast cancer, Risk factors
Introduction
The drug denosumab is an anti-RANKL monoclonal antibody which is used to treat osteoporosis and prevent skeletal damage caused by breast cancer metastases.1 RANK (receptor activator of nuclear factor κB) and its ligand (RANKL) are known for their involvement in bone metabolism.2 Binding of RANKL to RANK on osteoclast precursors induces osteoclast maturation and activation, thereby stimulating bone resorption. In contrast, binding of RANKL either pharmacologically or by osteoprotegerin (OPG --the endogenous decoy receptor for RANKL) inhibits the RANKL-mediated signaling pathway, consequently inhibiting bone resorption and maintaining bone density. A large trial of over 7000 older women with osteoporosis demonstrated that denosumab significantly reduced the risk of fractures.3 Based on those findings, denosumab was added to the Ontario Drug Benefits formulary in 2012 for the treatment of osteoporosis under restricted criteria that requires prior exposure to, or hypersensitivity following oral bisphosphonate use.4 It is estimated that 1% of older women in Ontario have initiated this drug annually since its addition to the provincial drug formulary.5 The typical schedule for denosumab is one subcutaneous injection every 6 months.
There is emerging evidence that progesterone-mediated up-regulation of the RANK/RANKL also plays a critical role in mammary gland epithelial cell proliferation, in mammary stem cell expansion, and in mammary carcinogenesis.6–10 Furthermore, preliminary findings from the adjuvant denosumab in breast cancer (ABCSG 18) double-blind, placebo-controlled trial showed improved disease-free survival among the women randomized to denosumab injected subcutaneously twice a year.11,12 The impact of denosumab on the incidence of a second primary cancer has yet to be reported. Targeting of RANK-signaling may be particularly relevant for women at a high risk of developing breast cancer attributed to an inherited mutation in BRCA1.6–10
The current landscape of chemoprevention for women at high-risk of developing breast cancer consists of either selective estrogen receptor modulators (i.e., SERMs) or aromatase inhibitors and is dependent on menopausal status.13 Given the suboptimal uptake of the current prevention options, it is important to identify novel and highly effective therapeutic cancer prevention strategies.14 Given the seminal preclinical evidence supporting a role of aberrant RANK-signaling in the development of breast cancer, it is of interest to evaluate whether denosumab with a relatively safe toxicity profile, is a potential candidate.15 To our knowledge, there are no studies that have specifically evaluated the relationship between denosumab use and breast cancer risk. The objective of the current study was to utilize large healthcare administrative databases to evaluate the incidence of breast cancer in a large cohort of postmenopausal women following denosumab initiation.
Materials and Methods
Study design and data sources
We conducted a population-based matched cohort study using healthcare administrative databases in Ontario, Canada. The databases contain records for all individuals eligible for the province’s universal health coverage. The databases included in this analysis were: the Registered Persons Database files for demographic information (e.g., birth date, death date, sex); the Ontario Cancer Registry (OCR)16 to identify invasive breast cancer and cancer history; the Canadian Institute for Health Information Hospital Discharge Abstract Database (CIHI-DAD/) for information regarding hospital admissions; the National Ambulatory Care Reporting System (NACRS) for emergency department visits and day surgeries; the Ontario Drug Benefit (ODB) database for prescription drug claims records as all residents of Ontario aged 65 years and older are eligible for provincial drug coverage through the ODB;17,18 and the Ontario Health Insurance Plan (OHIP) for information about physician service claims including mammography history. These datasets were linked using unique encoded identifiers and analyzed at the Institute for Clinical Evaluative Sciences (ICES).
This study was approved by the Research Ethics Board of the Sunnybrook Health Sciences Centre and Women’s College Hospital.
Study eligibility
Given that eligibility for ODB coverage of denosumab is predominantly restricted to women with a history of oral bisphosphonate use, and breast cancer risk is suggested to be lower among women with osteoporosis and also following bisphosphonate exposure;19–21 we restricted inclusion to women with prior oral bisphosphonate use to isolate effects of denosumab. Three oral bisphosphonates are approved for osteoporosis in Canada (alendronate, etidronate, and risedronate), and have been available through the ODB program since 1996.22 Bisphosphonate use prior to the date of study entry (i.e., index date as defined below) was obtained for each subject and the cumulative use and time since most recent use was calculated. Women were excluded if they were over the age of 85 at index date, ineligible for OHIP at any point in the two years preceding the index date, had no ODB claims in the two year preceding the index date, had a history of any cancer (excluding non-melanoma skin cancer, ICD-10 any C44) at any point prior to the index date, had a history of any conditions that would impact bone quality at any point prior to the index date (e.g., celiac disease, Cushing’s syndrome, hypercalcemia, hyperparathyroidism, organ transplant, osteomalacia, osteopetrosis, Paget’s disease or renal disease), had a death date preceding/on the index date and had a history of living in a long-term care setting.
Denosumab exposure
We identified all women aged 67 or more years who received a first prescription of denosumab between 29 February 2012 (first date on ODB formulary) and 30 April 2016. The index date was the first date of dispensation of denosumab. The total number of denosumab prescriptions dispensed at least four months from each other ( ≥ 120 days) were then categorized as 1–2 doses, 3–4 doses, and ≥ 5 doses. All women meeting our inclusion criteria with oral bisphosphonate exposure since 1996 were assigned a random index date based on the distribution of index dates among the eligible exposed subjects.
Covariates
We also collected information on the following covariates: age, resident location (i.e., rural vs. urban) determined by linking postal codes to census data, income status based on neighborhood income quintile, number of primary care visits, emergency department (ED) visits or acute care hospitalizations in the previous year, screening mammogram in the two years prior to the index date and comorbidity using the John Hopkins aggregated diagnosis groups (ADG) score in the two years prior to the index date,23 as well as history of pathologic or other fractures, documentation of osteoporosis, and prior use of other drugs that may impact bone health (i.e., calcitonin, raloxifene).
Matching
All women aged 67 or more years with oral bisphosphonate exposure since 1996 were assigned a random index date based on the distribution of index dates among the eligible exposed subjects; this ensured that exposed women could serve unexposed time prior to denosumab initiation. We conducted 3:1 matching of unexposed to exposed subjects. Subjects were matched on age ( ± 2 years), index date ( ± 1 year), cumulative use of bisphosphonates ( ± 1 year), time since last use of a bisphosphonate ( ± 1 year) and propensity score. Propensity scores were generated using variables for urban vs. rural residence, historic ED and inpatient visits, ADGs, history of fracture, and historic use of any anti-estrogen therapy, estrogen therapy, or aromatase inhibitors. We conducted propensity score calliper matching using a calliper of 0.2 times the standard deviation of the propensity score.
Outcomes
The primary outcome of interest was a diagnosis of incident invasive breast cancer documented in OCR in the follow-up period. Incident breast cancers included any malignant neoplasms of the breast (ICD-10 any C50). Benign neoplasms of the breast (ICD-10 any D24), carcinomas in situ of the breast (ICD-10 any D05) and neoplasms of uncertain behavior of the breast (ICD-10 D485 or D486) were not included.
Statistical analysis
Baseline descriptive characteristics of the two groups were compared using standardized differences. A standardized difference of <0.10 was used to determine comparability between the groups for each covariate of interest.24 Cox proportional hazards models, stratified on matched pairs was used to estimate the adjusted hazard ratio (HR) and 95% confidence intervals (CI) for denosumab exposure, as well as increasing dose and time since last bisphosphonate use, and the risk of breast cancer. Women were followed from their index date to either: (1) an incident breast cancer diagnosis, (2) other cancer diagnosis (excluding non-melanoma skin cancer), (3) death, or (4) end of follow-up (31 August 2017), whichever occurred first. Unexposed subjects who eventually received denosumab in the follow-up were also censored (n = 2,201).
All statistical analyses were performed using SAS software version 9.3.
Results
A total of 100,368 women with a history of bisphosphonate use were included in the final analysis. Of these, 25,092 women were denosumab users and 75,276 were matched non-users. Prior to matching, users and non-users of denosumab differed with respect to age, residential location, bisphosphonate use, history of fall-related injuries, fractures, primary care visits, emergency department visits and acute care hospitalizations in the past year, as well as, total aggregated diagnosis groups. However, following matching for age, index date, bisphosphonate use and propensity score, the two groups of women were similar with respect to all the baseline characteristics (Table 1). The mean number of denosumab doses in the exposed group was 4.8 (standard deviation = 2.9; range 1–12).
Table 1.
Characteristics of propensity score matched cohort, among all women and by denosumab use
| Variable, n (%) unless otherwise noted | Value | Total | No denosumab | Denosumab | Standardized difference |
|---|---|---|---|---|---|
| N = 100,368 | N = 75,276 | N = 25,092 | |||
| Age at index date | Mean ± SD | 76.3 ± 4.9 | 76.3 ± 4.9 | 76.3 ± 4.9 | 0.01 |
| Median (IQR) | 76 (72 – 80) | 76 (72 – 80) | 77 (72 – 80) | 0.01 | |
| 67 – 69 | 13,778 (14%) | 10,406 (14%) | 3372 (13%) | 0.01 | |
| 70 – 74 | 26,574 (26%) | 20,142 (27%) | 6432 (26%) | 0.03 | |
| 75 – 79 | 32,714 (33%) | 24,353 (32%) | 8361 (33%) | 0.02 | |
| 80 + | 27,302 (27%) | 20,375 (27%) | 6927 (28%) | 0.01 | |
| Resident location | Urban | 93,279 (93%) | 70,017 (93%) | 23,262 (93%) | 0.01 |
| Rural | 7089 (7%) | 5259 (7%) | 1830 (7%) | 0.01 | |
| Income quintile | Missing | 283 (0%) | 224 (0%) | 59 (0%) | 0.01 |
| 1 – Lowest | 18,816 (19%) | 14,391 (19%) | 4425 (18%) | 0.04 | |
| 2 | 21,022 (21%) | 15,791 (21%) | 5231 (21%) | 0 | |
| 3 | 19,808 (20%) | 14,919 (20%) | 4889 (19%) | 0.01 | |
| 4 | 20,839 (21%) | 15,542 (21%) | 5297 (21%) | 0.01 | |
| 5 – Highest | 19,600 (20%) | 14,409 (19%) | 5191 (21%) | 0.04 | |
| Years taking bisphosphonate | Mean ± SD | 4.9 ± 3.7 | 4.9 ± 3.7 | 4.9 ± 3.7 | 0 |
| Median (IQR) | 4 (2 – 8) | 4 (2 – 8) | 4 (2 – 8) | 0 | |
| <1 year | 17,988 (18%) | 13,390 (18%) | 4598 (18%) | 0.01 | |
| 1 – 2 years | 20,693 (21%) | 15,654 (21%) | 5039 (20%) | 0.02 | |
| 3 – 5 years | 25,880 (26%) | 19,388 (26%) | 6492 (26%) | 0 | |
| 6 – 9 years | 24,454 (24%) | 18,354 (24%) | 6100 (24%) | 0 | |
| 10 + years | 11,353 (11%) | 8490 (11%) | 2863 (11%) | 0 | |
| Years since last bisphosphonate use | Mean ± SD | 1.1 ± 2.0 | 1.1 ± 2.0 | 1.1 ± 2.0 | 0.02 |
| Median (IQR) | 0 (0 – 1) | 0 (0 – 1) | 0 (0 – 1) | 0.21 | |
| <1 year | 71,959 (72%) | 54,211 (72%) | 17,748 (71%) | 0.03 | |
| 1 – 2 years | 14,950 (15%) | 10,959 (15%) | 3991 (16%) | 0.04 | |
| 3 – 5 years | 9402 (9%) | 7056 (9%) | 2346 (9%) | 0 | |
| 6 + years | 4057 (4%) | 3050 (4%) | 1007 (4%) | 0 | |
| Primary care visit(s) in the previous year | Yes | 95,732 (95%) | 71,441 (95%) | 24,291 (97%) | 0.1 |
| Mean ± SD | 6.0 ± 5.1 | 5.9 ± 5.0 | 6.3 ± 5.2 | 0.07 | |
| Median (IQR) | 5 (3 – 8) | 5 (3 – 8) | 5 (3 – 8) | 0.09 | |
| Emergency department visit(s) in the previous year | Yes | 27,275 (27%) | 20,389 (27%) | 6886 (27%) | 0.01 |
| Mean ± SD | 0.5 ± 1.1 | 0.5 ± 1.1 | 0.5 ± 1.3 | 0 | |
| Median (IQR) | 0 (0 – 1) | 0 (0 – 1) | 0 (0 – 1) | 0.01 | |
| Acute care hospitalization(s) in the previous year | Yes | 5612 (6%) | 4230 (6%) | 1382 (6%) | 0 |
| Mean ± SD | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.01 | |
| Median (IQR) | 0 (0 – 0) | 0 (0 – 0) | 0 (0 – 0) | 0 | |
| Mammogram(s) in the previous 2 years | Yes | 39,165 (39%) | 28,803 (38%) | 10,362 (41%) | 0.06 |
| Mean ± SD | 0.5 ± 0.6 | 0.4 ± 0.6 | 0.5 ± 0.6 | 0.07 | |
| Median (IQR) | 0 (0 – 1) | 0 (0 – 1) | 0 (0 – 1) | 0.07 | |
| No mammograms | 61,203 (61%) | 46,473 (62%) | 14,730 (59%) | 0.06 | |
| 1 mammogram | 32,353 (32%) | 23,925 (32%) | 8428 (34%) | 0.04 | |
| 2 mammograms | 6702 (7%) | 4807 (6%) | 1895 (8%) | 0.05 | |
| 3+ mammograms | 110 (0%) | 71 (0%) | 39 (0%) | 0.02 | |
| Aggregated Diagnosis Groups (ADGs) (2 year lookback) | Mean ± SD | 7.4 ± 3.3 | 7.4 ± 3.3 | 7.5 ± 3.3 | 0.03 |
| Median (IQR) | 7 (5 – 10) | 7 (5 – 10) | 7 (5 – 10) | 0.02 | |
| 0 – 4 ADGs | 19,668 (20%) | 14,821 (20%) | 4847 (19%) | 0.01 | |
| 5 – 9 ADGs | 55,259 (55%) | 41,545 (55%) | 13,714 (55%) | 0.01 | |
| 10 + ADGs | 25,441 (25%) | 18,910 (25%) | 6531 (26%) | 0.02 | |
| Fall-related Injury | Yes | 7846 (8%) | 5814 (8%) | 2032 (8%) | 0.01 |
| Nonfall-related injury | Yes | 4811 (5%) | 3615 (5%) | 1196 (5%) | 0 |
| Any fracture diagnosis | Yes | 4083 (4%) | 3026 (4%) | 1057 (4%) | 0.01 |
| Concurrent medication use (excluding bisphosphonates and denosumab) | Mean ± SD | 3.2 ± 2.8 | 3.2 ± 2.8 | 3.1 ± 2.7 | 0.05 |
| Median (IQR) | 3 (1 – 5) | 3 (1 – 5) | 3 (1 – 5) | 0.05 | |
| 0 medications | 16,966 (17%) | 12,701 (17%) | 4265 (17%) | 0 | |
| 1 – 4 medications | 55,974 (56%) | 41,610 (55%) | 14,364 (57%) | 0.04 | |
| 5 – 9 medications | 24,348 (24%) | 18,577 (25%) | 5771 (23%) | 0.04 | |
| 10 + medications | 3080 (3%) | 2388 (3%) | 692 (3%) | 0.02 | |
| Follow up in years | Mean ± SD | 2.8 ± 1.5 | 2.8 ± 1.5 | 2.8 ± 1.5 | 0.02 |
| Median (IQR) | 3 (1 – 4) | 3 (1 – 4) | 3 (1 – 4) | 0.02 | |
| Breast cancer diagnosisa | Yes | 1271 (1%) | 986 (1%) | 285 (1%) | 0.02 |
| Other cancer diagnosisa | Yes | 3022 (3%) | 2261 (3%) | 761 (3%) | 0 |
| Deatha | Yes | 5306 (5%) | 3970 (5%) | 1336 (5%) | 0 |
aNote: censoring not included in these outcomes. Outcomes are based on experiencing the event at any point in follow-up.
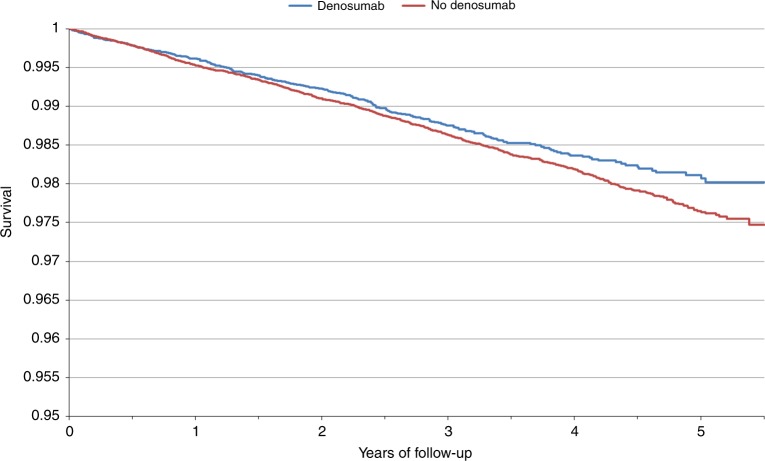
A total of 1271 women were diagnosed with breast cancer over the follow-up period with 285 (1.1%) cases diagnosed among the denosumab users and 986 (1.3%) cases among the non-users (Table 1). Women were followed for an average of 2.8 years, reflecting an overall protective effect with denosumab exposure (HR = 0.87, 95% CI 0.76–1.00) (Table 2). The 5-year cumulative incidence of breast cancer was 1.9% in denosumab users and 2.4% among the non-users (P – log rank = 0.04), Figure 1. The relationship did not vary by increasing cumulative dose (P – trend = 0.15) or time since last use of bisphosphonates (P – interaction = 0.52) (Table 2).
Table 2.
The association between denosumab use and breast cancer risk
| Strata | BC events | Univariate | ||
|---|---|---|---|---|
| No denosumab | Denosumab | HR (95% CI) | P | |
| Ever/never denosumab use | 957 | 281 | 0.87 (0.76–1.00) | 0.04 |
| Cumulative dose | ||||
| Per injection | 0.98 (0.94–1.01) | 0.15a | ||
| 1–2 injections | - | 124 | 0.82 (0.68–1.00) | 0.04 |
| 3–4 injections | - | 71 | 0.88 (0.69–1.13) | 0.32 |
| 5+injections | - | 86 | 0.95 (0.75–1.20) | 0.65 |
aP – Trend
Fig. 1.
Kaplan-Meier curve of breast cancer-free survival over follow-up time by denosumab use
The characteristics of the breast cancer patients are summarized in Table 3 by history of denosumab use. On average, women in the denosumab group had slightly more nodal involvement compared to women in the no denosumab group (mean nodal involvement 1.1 vs. 0.6); however, this was only different for the continuous variable (number of nodes) and not the type of nodal involvement (positive or negative). The two groups were similar with respect to various factors including stage, size, grade, hormone-receptor status or HER2 status of the incident breast cancers.
Table 3.
Breast cancer characteristics of all women and by denosumab use
| Variable, n (%) unless otherwise noted | Value | Total | No denosumab | Denosumab | P |
|---|---|---|---|---|---|
| N=1238 (%) | N=957 (%) | N=281 (%) | |||
| Diagnosis year | 2012 | 47 (4) | 37 (4) | 10 (4) | 0.14 |
| 2013 | 117 (9) | 81 (8) | 36 (13) | ||
| 2014 | 177 (14) | 139 (15) | 38 (14) | ||
| 2015 | 263 (21) | 205 (21) | 58 (21) | ||
| 2016 | 376 (30) | 284 (30) | 92 (33) | ||
| 2017 | 258 (21) | 211 (22) | 47 (17) | ||
| Stage | Unknown | 307 (25) | 242 (25) | 65 (23) | |
| I | 405 (44) | 316 (44) | 89 (41) | 0.28 | |
| II | 350 (38) | 267 (37) | 83 (38) | ||
| III | 113 (12) | 80 (11) | 33 (15) | ||
| IV | 63 (7) | 52 (7) | 11 (5) | ||
| Tumor size (continuous) (cm) | Mean±SD | 2.6±2.0 | 2.6±2.0 | 2.7±2.0 | 0.50 |
| Median (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–4) | 0.45 | |
| Tumor size (categorical) (cm) | Unknown | 532 (43) | 428 (45) | 104 (37) | |
| <1 cm | 103 (15) | 75 (14) | 28 (16) | 0.57 | |
| 1–2 cm | 222 (31) | 174 (33) | 48 (27) | ||
| 2–3 cm | 161 (23) | 120 (23) | 41 (23) | ||
| 3–5 cm | 145 (21) | 108 (20) | 37 (21) | ||
| 5+ cm | 75 (11) | 52 (10) | 23 (13) | ||
| Nodes involved (continuous) | Mean±SD | 0.7±2.2 | 0.6±1.6 | 1.1±3.3 | 0.03 |
| Median (IQR) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.37 | |
| Nodes involved (categorical) | Unknown | 640 (52) | 509 (53) | 131 (47) | |
| Node negative | 421 (70) | 317 (71) | 104 (69) | 0.74 | |
| Node positive | 177 (30) | 131 (29) | 46 (31) | ||
| Grade | Unknown | 651 (53) | 522 (55) | 129 (46) | |
| Low grade | 126 (21) | 94 (22) | 32 (21) | 0.98 | |
| Medium grade | 306 (52) | 227 (52) | 79 (52) | ||
| High grade | 155 (26) | 114 (26) | 41 (27) | ||
| ER status | Unknown | 574 (46) | 462 (48) | 112 (40) | |
| Negative | 89 (13) | 64 (13) | 25 (15) | 0.54 | |
| Positive | 575 (87) | 431 (87) | 144 (85) | ||
| PR status | Unknown | 582 (47) | 467 (49) | 115 (41) | |
| Negative | 158 (24) | 117 (24) | 41 (25) | 0.83 | |
| Positive | 498 (76) | 373 (76) | 125 (75) | ||
| HER2 status | Unknown | 602 (49) | 477 (50) | 125 (44) | |
| Borderline | 165 (26) | 127 (26) | 38 (24) | 0.62 | |
| Negative | 417 (66) | 315 (66) | 102 (65) | ||
| Positive | 54 (8) | 38 (8) | 16 (10) |
In a sensitivity analysis, the inclusion cohort was re-matched such that the index date for denosumab users was 6 months following their initial injection to allow for a 6 month lag period between denosumb use and breast cancer risk. The findings did not change considerably. For example, the HR comparing users vs. non-users was 0.86 (95% CI 0.74–0.99; P = 0.04)(data not shown).
Discussion
In this large, population-based study of older women with a history of oral bisphosphonate exposure we observed that use of denosumab was associated with a modestly significant 13% decreased risk of subsequent breast cancer. After 5 years of follow-up, the cumulative incidence of breast cancer was significantly lower in the denosumab users vs. the non-users (1.9% vs. 2.4%). There was no dose-response relationship and the association did not vary by time since last bisphosphonate use. Except for nodal involvement, pathologic, and histologic characteristics of the breast tumors did not vary by history of denosumab exposure. Although these findings suggest a protective effect of denosumab exposure, they should be interpreted with caution given the relatively short duration of follow-up in this analysis as well as the median age of the population (~76 years). It is of interest to confirm our findings in other studies and to further evaluate the relationship between denosumab and breast cancer in younger patients and with additional years of follow-up.
Our cohort consisted of women from the general population with a history of bisphosphonate use, and thus, are not representative of women in the general population. Although we found little evidence of a dose-response association between denosumab exposure and subsequent breast cancer risk, our findings with any denosumab use do not preclude a potential chemoprevention role of this drug among women without a history of osteoporosis including those under the age of 67. Indeed, our results suggests that a short-course of denosumab has the potential to offer long-term protection against breast cancer which is analogous to the cancer protective effects conferred by a later age at menarche and breastfeeding, intrinsic, and transient exposures that significantly reduce the risk of breast cancer.25 On the other hand, we cannot rule-out some residual confounding related to denosumab initiation. For example, women treated with denosumab (a new drug to market) may be healthier in terms of diet, alcohol consumption and exercise – aware of the new pharmacological option and broaching the discussion with their physician, or treated by attentive physicians encouraging preventive health behaviors.
Despite the inclusion of an older population in the current study, one cannot preclude a potential breast cancer protective effect of denosumab (or other RANKL inhibitors) in younger high-risk populations, particularly among women with an inherited BRCA1 mutation. Pre-clinical findings from various seminal publications have collectively elucidated a pivotal role of the RANK-pathway in brca1 mammary carcinogenesis.6,8,10,26,27 Specifically, Nolan et al., demonstrated that RANKL inhibition resulted in a significant delay in mammary tumor onset and incidence in a brca1 deficient mouse model, and furthermore, that treatment of premenopausal women with denosumab resulted in a substantial reduction in breast epithelial cellular proliferation based on Ki67 expression27 and confirmed by an independent research group.26 These findings are of particular relevance for women with a BRCA1 mutation given their high lifetime risk of developing breast cancer, the very limited data regarding tamoxifen use for primary prevention, along with the suboptimal uptake of tamoxifen since most BRCA mutation carriers opt for yearly screening with MRI.28 Randomized trials or observational intervention trials in this specific population are highly anticipated. We did not have information on family history or BRCA mutation status, and thus, were not able to assess risk in these subgroups.
The prevention and treatment of postmenopausal osteoporosis have historically included the use of bisphosphonates, a class of drugs that inhibits osteoclast-mediated bone resorption.29 Intravenous bisphosphonates are also prescribed to breast (and other) cancer patients to prevent treatment-induced skeletal complications including bone loss and bone metastases.30 Evidence from earlier, epidemiologic studies suggested a possible reduction in breast cancer risk among postmenopausal women who used bisphosphonates;20,21 however, a results from a recent prospective cohort of 64,438 French postmenopausal women and 2407 incident cases, reported no significant association between bisphosphonate use and breast cancer risk (HR = 0.98, 95% CI 0.85–1.12).31
There are several limitations to our study. First, the duration of follow-up was short (on average ~2.8 years). This was purely attributed to the fairly recent introduction of denosumab for the treatment of osteoporosis. We wanted to ensure accurate information regarding prescribed denosumab use, and thus, only included women who were 67 years of age or older who had at least 2 prior years of coverage under the ODB program. However, it should be noted that denosumab for the treatment of osteoporosis was not added to the Ontario provincial formulary until February 2012 and is only provided in special circumstances. This may have resulted in some misclassification including under-capturing if patients received drug coverage through other mechanisms (e.g., out-of-pocket, private insurance).32 Given that more than half of the breast cancers in Canada are diagnosed prior to age 69, we did not capture the full population of interest. The women included in the current analysis were limited to older (on average 76 years of age) women with a history of bisphosphonate use, and likely not representative of the larger number of women at risk of developing breast cancer. Although we did not have information on various breast cancer risk factors including family history, we were able to demonstrate that both groups of women were similar with respect to screening (Table 1) and use of chemopreventive drugs such as raloxifene and tamoxifen (data not shown). Furthermore, reproductive and hormonal risk factors are unlikely to differ based on initiation of denosumab.
Despite these limitations, our study had several strengths, in particular, the use of large provincial administrative datasets, allowing for well-powered analyses and matching on relevant confounders. Our exposed and unexposed groups were similar with respect to most demographic characteristics, prior history of bisphosphonate use and other medications that may impact bone health (e.g., estrogen therapy, calcitonin), comorbidities, as well as, health care utilization patterns. Comprehensive ODB drug data permits capture of oral bisphosphonate therapy since 1996, matching on age and calendar time helps control for changes in osteoporosis management and therapy over time.
In conclusion, we found a small inverse relationship between denosumab exposure and breast cancer incidence in this large population-based study of older women residing in Ontario, Canada. To our knowledge, this represents the first report of denosumab use and subsequent breast cancer risk. Further studies with a longer follow-up period, as well as the inclusion of younger women or cohorts of high-risk women, are necessary to delineate the role of RANK-inhibition in the prevention of breast cancer. It is of importance to establish whether denosumab, administered subcutaneously as a semi-annual injection with a safe toxicity profile, has the potential to be used in the primary prevention setting.
Author contributions
J.K. conceived and designed the study, obtained funding, and was involved in data analysis and manuscript preparation. V.G. designed the study, conducted the data analysis and was involved in manuscript preparation. All authors were involved in study design, interpretation of data, and manuscript preparation.
Competing interests
This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred.
Data availability
The data analyzed for the current study are available upon reasonable request from the corresponding author.
Ethics approval and consent to participate
This study was approved by the Research Ethics Boards at Sunnybrook Health Sciences Centre and Women’s College Hospital. Given the retrospective nature of this study, informed consent was waived. This study was performed in accordance with the Declaration of Helsinki
Funding
JK is the recipient of a Tier II Canada Research Chair. SAN is the recipient of a Tier I Canada Research Chair. VG is supported by the Canadian Institutes of Health Research (CIHR) Frederick Banting and Charles Best Doctoral Research Award.
Consent for publication
Not applicable.
References
- 1.Hanley DA, Adachi JD, Bell A, Brown V. Denosumab: mechanism of action and clinical outcomes. Int. J. Clin. Pract. 2012;66:1139–1146. doi: 10.1111/ijcp.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagy V, Penninger JM. The RANKL-RANK Story. Gerontology. 2015;61:534–542. doi: 10.1159/000371845. [DOI] [PubMed] [Google Scholar]
- 3.Cummings SR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 4.Burden AM, Tadrous M, Calzavara A, Cadarette SM. Uptake and characteristics of zoledronic acid and denosumab patients and physicians in Ontario, Canada: impact of drug formulary access. Osteoporos. Int. 2015;26:1525–1533. doi: 10.1007/s00198-014-3023-8. [DOI] [PubMed] [Google Scholar]
- 5.Ban J. K., et al editors. Over 63,700 ontario seniors have initiated denosumab: user characteristics and persistence with therapy. J. Popul. Ther. Clin. Pharmacol.24(3):e51–e78; Ontario 26, 2017. Page 31. 2017 (Abstract).
- 6.Fata JE, et al. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell. 2000;103:41–50. doi: 10.1016/S0092-8674(00)00103-3. [DOI] [PubMed] [Google Scholar]
- 7.Schramek D, et al. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature. 2010;468:98–102. doi: 10.1038/nature09387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez-Suarez E, et al. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;468:103–107. doi: 10.1038/nature09495. [DOI] [PubMed] [Google Scholar]
- 9.Asselin-Labat ML, et al. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465:798–802. doi: 10.1038/nature09027. [DOI] [PubMed] [Google Scholar]
- 10.Joshi PA, et al. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465:803–807. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- 11.Gnant M, et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:433–443. doi: 10.1016/S0140-6736(15)60995-3. [DOI] [PubMed] [Google Scholar]
- 12.Gnant M. P. G., et al, on behalf of the Austrian Breast and Colorectal Cancer StudyGroup. The impact of adjuvant denosumab on disease-free survival: results from 3,425 postmenopausal patients of the ABCSG-18 trial. San Antonio Breast Cancer Symposium; 2015.
- 13.Cuzick J. Preventive therapy for cancer. Lancet Oncol. 2017;18:e472–e482. doi: 10.1016/S1470-2045(17)30536-3. [DOI] [PubMed] [Google Scholar]
- 14.Smith SG, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Ann. Oncol. 2016;27:575–590. doi: 10.1093/annonc/mdv590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotsopoulos J, Singer C, Narod SA. Can we prevent BRCA1-associated breast cancer by RANKL inhibition? Breast Cancer Res. Treat. 2017;161:11–16. doi: 10.1007/s10549-016-4029-z. [DOI] [PubMed] [Google Scholar]
- 16.Robles SC, Marrett LD, Clarke EA, Risch HA. An application of capture-recapture methods to the estimation of completeness of cancer registration. J. Clin. Epidemiol. 1988;41:495–501. doi: 10.1016/0895-4356(88)90052-2. [DOI] [PubMed] [Google Scholar]
- 17.Cadarette SM, Jaglal SB, Raman-Wilms L, Beaton DE, Paterson JM. Osteoporosis quality indicators using healthcare utilization data. Osteoporos. Int. 2011;22:1335–1342. doi: 10.1007/s00198-010-1329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Care OMoHaL-T. Ontario Public Drug Programs - Exceptional Access Program. Available from http://health.gov.on.ca/en/pro/programs/drugs.
- 19.Qu X, et al. Bone mineral density and risk of breast cancer in postmenopausal women. Breast Cancer Res. Treat. 2013;138:261–271. doi: 10.1007/s10549-013-2431-3. [DOI] [PubMed] [Google Scholar]
- 20.Rennert G, Pinchev M, Rennert HS. Use of bisphosphonates and risk of postmenopausal breast cancer. J. Clin. Oncol. 2010;28:3577–3581. doi: 10.1200/JCO.2010.28.1113. [DOI] [PubMed] [Google Scholar]
- 21.Newcomb PA, Trentham-Dietz A, Hampton JM. Bisphosphonates for osteoporosis treatment are associated with reduced breast cancer risk. Br. J. Cancer. 2010;102:799–802. doi: 10.1038/sj.bjc.6605555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cadarette SM, et al. Osteoporosis medication prescribing in British Columbia and Ontario: impact of public drug coverage. Osteoporos. Int. 2012;23:1475–1480. doi: 10.1007/s00198-011-1771-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Austin PC, Shah BR, Newman A, Anderson GM. Using the Johns Hopkins’ Aggregated Diagnosis Groups (ADGs) to predict 1-year mortality in population-based cohorts of patients with diabetes in Ontario, Canada. Diabet. Med. 2012;29:1134–1141. doi: 10.1111/j.1464-5491.2011.03568.x. [DOI] [PubMed] [Google Scholar]
- 24.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat. Med. 2007;26:734–753. doi: 10.1002/sim.2580. [DOI] [PubMed] [Google Scholar]
- 25.Anderson KN, Schwab RB, Martinez ME. Reproductive risk factors and breast cancer subtypes: a review of the literature. Breast Cancer Res. Treat. 2014;144:1–10. doi: 10.1007/s10549-014-2852-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sigl V, et al. RANKL/RANK control Brca1 mutation-driven mammary tumors. Cell Res. 2016;26:761–774. doi: 10.1038/cr.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nolan E, et al. RANK ligand as a potential target for breast cancer prevention in BRCA1-mutation carriers. Nat. Med. 2016;22:933–939. doi: 10.1038/nm.4118. [DOI] [PubMed] [Google Scholar]
- 28.Metcalfe KA, et al. International variation in rates of uptake of preventive options in BRCA1 and BRCA2 mutation carriers. International journal of cancer. J. Int. Cancer. 2008;122:2017–2022. doi: 10.1002/ijc.23340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gronich N, Rennert G. Beyond aspirin-cancer prevention with statins, metformin and bisphosphonates. Nat. Rev. Clin. Oncol. 2013;10:625–642. doi: 10.1038/nrclinonc.2013.169. [DOI] [PubMed] [Google Scholar]
- 30.Aapro M, et al. Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel. Annals of oncology: official journal of the European Society for. Ann. Oncol. 2008;19:420–432. doi: 10.1093/annonc/mdm442. [DOI] [PubMed] [Google Scholar]
- 31.Fournier A, et al. Use of bisphosphonates and risk of breast cancer in a French cohort of postmenopausal women. J. Clin. Oncol. 2017;35:3230–3239. doi: 10.1200/JCO.2016.71.4337. [DOI] [PubMed] [Google Scholar]
- 32.Gamble JM, McAlister FA, Johnson JA, Eurich DT. Restrictive drug coverage policies can induce substantial drug exposure misclassification in pharmacoepidemiologic studies. Clin. Ther. 2012;34:1379–1386 e3. doi: 10.1016/j.clinthera.2012.04.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analyzed for the current study are available upon reasonable request from the corresponding author.