Summary
Posterior reversible encephalopathy syndrome (PRES) is characterized clinically by headache, altered mental status, visual loss, and seizures. PRES is associated with neuroradiological findings characterized by white matter abnormalities, predominantly in the parieto-occipital regions of the brain. PRES is most often described in cases of hypertensive encephalopathy, eclampsia, renal failure, and immunosuppressive or anticancer therapy. We report a case of PRES associated with severe hypertension in the setting of a progressive renovascular hypertension from bilateral atherosclerotic renal artery stenosis. The pathogenesis of PRES is discussed and the importance of a prompt diagnosis and treatment is emphasized.
Keywords: Reversible encephalopathy, Renovascular hypertension, Magnetic resonance imaging
Introduction
Posterior reversible encephalopathy syndrome (PRES) is a disorder predominantly affecting the white matter in the posterior portion of the cerebral hemispheres. Magnetic resonance imaging (MRI) shows diffuse signal abnormalities mainly involving the subcortical white matter in the parieto-occipital regions of the brain; the temporal and frontal lobes, cerebellum, basal ganglia, and brainstem may also be involved [1], [2]. Headache, seizures, decreased alertness, altered mental status, and visual loss are frequent clinical symptoms. PRES is most often described in cases of hypertensive encephalopathy, preeclampsia and eclampsia, renal failure, or following immunosuppressive or anticancer therapy [1]. Other reported causes include autoimmune diseases, thrombotic thrombocytopenic purpura, human immunodeficiency virus syndrome, acute intermittent porphyria, organ transplantation [2], hypercalcemia [3], and sepsis [4]. In most cases, an abrupt rise in blood pressure is thought to be the cause of PRES by means of acute disruption of the blood brain barrier. However, some cases of PRES in normotensive patients have been described as well [3], [5]. Although this condition was earlier reported to be reversible, it bears a significant risk of permanent cerebral injury if treatment is delayed [1], [2].
Renal artery stenosis, from either fibrodysplasia or atherosclerosis, is a common cause of severe or resistant hypertension. Herein we describe a case of renovascular hypertension complicated by PRES associated with severe hypertensive encephalopathy.
Case report
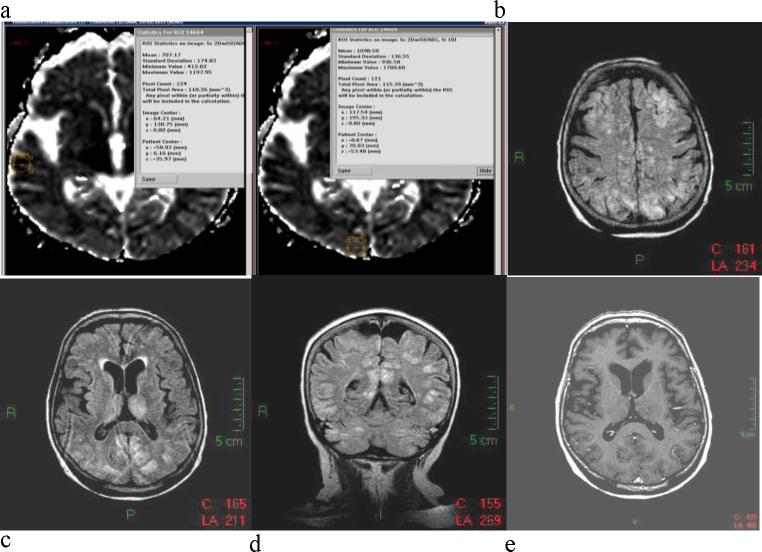
A 76-year-old hypertensive woman was referred to the local emergency department because of sudden onset of headache, visual disturbance, and vomiting on the previous day. She had been found to be hypertensive at the age of 65 years. In the past 3 years, she had been treated with enalapril 20 mg od and hydrochlorothiazide 25 mg od. Home blood pressure measurements had shown values never exceeding 160/90 mm Hg. Her past medical history was otherwise unremarkable except a previous vertebroplasty for osteoporotic lumbar spine fractures, and neither head trauma nor seizure disorders were reported. In the morning before her admission, she presented with disorientation; blood pressure values turned out to be 218/124 mm Hg. On the way to the hospital by ambulance, the patient's impairment of consciousness deteriorated up to coma on arrival at the emergency department. On admission, patient's blood pressure was 220/120 mm Hg, heart rate 78 beats per minute, body temperature 36.6 °C, and oxygen saturation 95% on low-flow oxygen. The patient's pupils were equal and reactive, and fundoscopy did not show any papilledema. Electrocardiography was unremarkable. Blood tests showed plasma glucose of 168 mg/dl, hemoglobin 14.3 g/dl, white cell count 9830 mm−3, creatinine 0.8 mg/dl, sodium 136 mmol/l, and potassium 3.9 mmol/l, the high-sensitivity reactive C protein was 0.92 mg/dl (normal value: 0.05–0.30 mg/dl). Rapidly, the patient developed generalized seizures, which ended following i.v. midazolam administration. A head non-contrast computed tomography scan did not reveal any remarkable changes except for some ill-defined hypodense lesions in the white matter, which were interpreted as remote ischemic insults. Magnetic resonance imaging (MRI) revealed a severe pattern of bilateral and somewhat symmetrical patchy lesions, involving both the gray and white matter, including the thalami. The altered signal was characterized by hyperintensity on long TR sequences, mainly seen on fluid-attenuated inversion recovery (FLAIR), and located in both the supra- and infra-tentorial compartments. The findings were especially evident in the posterior circulation territories, mostly in the watershed zones of the hemispheres (Fig. 1). They did not cause any change in diffusion apart from a small patchy area of restriction in the right parietal lobe; in addition, there was no contrast enhancement.
Figure 1.
Imaging at admission. (a) Quantification of apparent diffusion coefficient values derived from diffusion weighted imaging acquisition on magnetic resonance imaging (MRI). (b–d) MRI fluid-attenuated inversion recovery images in axial and coronal planes showing extensive hyperintensities involving gray and white matter in both hemispheres; the pattern is bilateral and symmetric and the thalami and cerebellar hemispheres are also involved. (e) No contrast enhancement is evident in the sites of lesion.
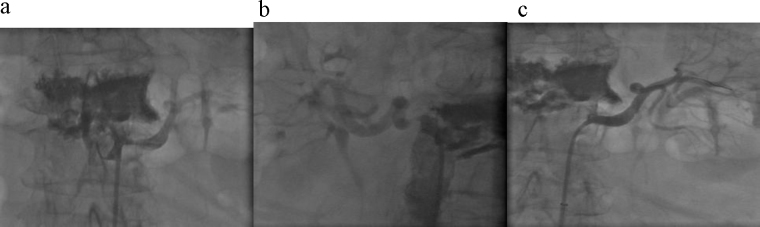
Based on the clinical and radiological findings, PRES and infective/parainfective lesions were considered in the differential diagnosis. The patient partly regained consciousness 3 h after admission, and neurological examination showed right hemiparesis and dysarthria. Cardiovascular, respiratory and abdominal examinations were unremarkable apart from an epigastric aortic bruit. The cerebrospinal fluid did not show any alteration. Blood pressure values were still very high (215/125 mm Hg), and a second attack of epileptic seizures occurred, which was treated with i.v. midazolam. Considering the absence of any evidence of infection, a diagnosis of hypertensive encephalopathy with PRES on MRI was established. Antihypertensive therapy with urapidil i.v. was started, and quickly interrupted because of an abrupt drop of blood pressure to 100/50 mm Hg associated with a decrease in urinary flow. On day 2 of admission, the serum creatinine was 1.6 mg/dl with an estimated glomerular filtration rate of 35 ml/min. A renal duplex ultrasonography showed in both renal arteries an increase in peak systolic velocity (PSV) at the proximal segment, associated with turbulent flow in the post-stenotic tract. These alterations were more prominent at the left renal artery, with a PSV of 4 m/s and an intrarenal resistance index (RI) of 0.58, compared to a PSV of 3 m/s and a RI of 0.65 at the right renal artery. The kidney size was 8.7 cm and 9.2 cm on the left and right side respectively. Moreover, the plasma renin activity (PRA) was 12.95 ngAng1/ml/h. On day 3 of admission, the patient was completely conscious and did not show any significant neurological deficits. Arterial angiography confirmed bilateral atherosclerotic renal artery stenosis leading to 85% luminal reduction on the left side and 65% on the right side. Percutaneous transluminal angioplasty plus stenting on the left side was performed with satisfactory angiographic results (Fig. 2). In the next few days, treatment with amlodipine at a dose of 10 mg od resulted in normalization of blood pressure. Serum creatinine was found to be 0.8 mg/dl, and PRA was 1.20 ngAng1/ml/h. A brain MRI performed 14 days later showed a near-complete reversal of the abnormalities: the hyperintense areas almost completely disappeared on FLAIR sequence, DWI sequence turned out to be completely normal, the swelling of the convexity sulci disappeared; no contrast enhancement was still evident (Fig. 3). On day 15 the patient was discharged in good condition.
Figure 2.
Renal angiography. (a) Left renal artery with stenosis 85%. (b) Right renal artery with stenosis 65%. (c) Post-stenting left renal artery.
Figure 3.
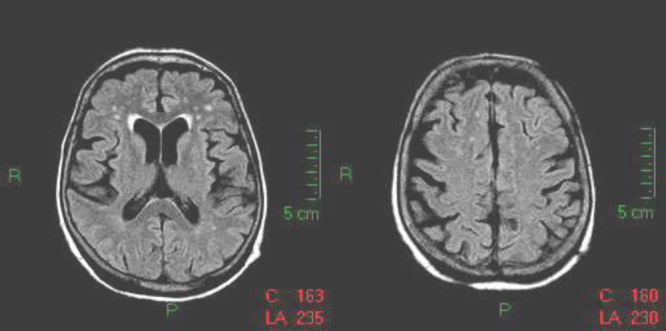
Magnetic resonance imaging (MRI) 14 days after admission; MRI fluid-attenuated inversion recovery sequence on axial plane. Complete recovery with disappearance of the hyperintense lesions previously involving gray and white matter. There is no more swelling of the sulci.
Discussion
We report a case of PRES in the setting of a severe renovascular hypertension from bilateral atherosclerotic renal artery stenosis.
Renovascular hypertension represents one of the most frequent forms of secondary hypertension. It is more commonly caused by atherosclerotic renal artery stenosis, and less frequently by fibromuscular dysplasia or other conditions [6]. Anatomical progression of renal artery stenosis may occur in about 20% of patients [7]. Hemodynamically significant renal artery stenosis usually results in severe and refractory hypertension or progressive renal insufficiency; clinical criteria for suspecting renovascular hypertension have been reported [8]. Angiotensin-converting enzyme inhibitors are contraindicated in the setting of bilateral renal artery stenosis [8]. Our patient was on chronic anti-hypertensive treatment with enalapril, and achieved a satisfactory blood pressure control without any renal dysfunction. In this case, the abrupt onset of PRES was clearly caused by progression of a silent bilateral renal artery stenosis to a hemodynamically significant status on the left side.
Moreover, it is noteworthy that, in our patient, i.v. infusion of alpha-1 blocker urapidil, performed in the emergency department before the diagnosis of bilateral renal artery stenosis, caused an abrupt fall in blood pressure with consequent renal hypoperfusion. The choice of urapidil was made according to local hospital guidelines about the treatment of hypertensive emergencies. However, we acknowledge that in hypertensive encephalopathy, as in our case, a less rapid reduction of blood pressure would be advisable eventually with the drip, slow i.v. infusion of a calcium antagonist.
PRES is a syndrome [1] characterized by rapid-onset neurological changes, which may quickly resolve when treated early. They encompass headache, altered consciousness, nausea and vomiting, seizures, and visual symptoms including cortical blindness; focal or lateralizing neurological signs may also occur. Despite PRES being frequently associated with an acute rise in blood pressure, it may also occur in association with mild elevations in blood pressure and even in normotensive patients [5]. Although the pathogenesis of PRES remains poorly defined, the most widely accepted theory states that a rapid increase in blood pressure may exceed the upper limit of cerebral autoregulation with abrupt dilation of cerebral arterioles resulting in interstitial extravasation of serum protein and fluid, that is a vasogenic edema. In the normotensive cases occurring after immunosuppressive or cytotoxic treatment, a toxic effect on vascular endothelium is hypothesized [2]. Vasogenic edema is mainly detectable in the white matter of the occipito-parietal regions, but may extend to the surrounding gray matter. Moreover, as in our case, the edematous lesions may also involve the brain stem, cerebellum, and basal ganglia [1]. In our patient (Fig. 1), quantification of apparent diffusion coefficient (ADC) values derived from diffusion weighted imaging acquisition demonstrated marked increase in diffusivity suggestive for vasogenic edema and not for cytotoxic edema. Vasogenic edema is typically encountered in PRES, resulting probably from failure of the blood–brain barrier leading to water extravasation in the interstitial spaces and is considered a reversible pathologic situation. In our case this was confirmed by the subsequent normalization of the findings in follow up MRI. On the other hand the appearance of cytotoxic edema, characterized by water diffusivity restriction and low ADC values, suggests a serious cell metabolic failure leading to irreversible damage. In such a situation alternative diagnosis should be considered, mainly ischemia, and in any case a more aggressive treatment is needed [9]. It is noteworthy, however, that a few exceptions have been described where restricted diffusion in PRES was almost partially reversible [10].
Although most cases of PRES are reversible, delay in diagnosis and treatment may lead to irreversible lesions [2]; therefore, early recognition and correction of the condition underlying PRES is the recommended treatment for this disorder.
To our knowledge, only one other case of PRES with the presentation of bilateral renal artery stenosis has been reported to date [11]. Our case is noteworthy because an abrupt rise of blood pressure caused severe hypertensive encephalopathy with PRES in the setting of an ongoing progression of renal artery stenosis. The prompt diagnosis and treatment of that underlying condition resulted in the complete resolution of PRES along with normalization of blood pressure and increase in renal function.
In conclusion, this case illustrates a severe PRES caused by either an abrupt onset or a progression of renovascular hypertension. We believe that clinicians should be aware of PRES in the context of renovascular hypertension, since an early diagnosis and aggressive treatment may result in complete resolution.
References
- 1.Hinchey J., Chaves C., Appignani B., Breen J., Pao L., Wang A., Pessin M.S., Lamy C., Mas J.L., Caplan L.R. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 2.Garg R.K. Posterior leukoencephalopathy syndrome. Postgrad Med J. 2001;77:24–28. doi: 10.1136/pmj.77.903.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kastrup O., Maschke M., Wanke I., Diener H.C. Posterior reversible encephalopathy syndrome due to severe hypercalcemia. J Neurol. 2002;249:1563–1566. doi: 10.1007/s00415-002-0895-x. [DOI] [PubMed] [Google Scholar]
- 4.Fugate J.E., Claassen D.O., Cloft H.J., Kallmes D.F., Kozak O.S., Rabinstein A.A. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85:427–432. doi: 10.4065/mcp.2009.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ay H., Buonanno F.S., Schaefer P.W., Le D.A., Wang B., Gonzalez R.G., Koroshetz W.J. Posterior leukoencephalopathy without severe hypertension: utility of diffusion-weighted MRI. Neurology. 1998;51:1369–1376. doi: 10.1212/wnl.51.5.1369. [DOI] [PubMed] [Google Scholar]
- 6.Dworkin L.D., Cooper C.J. Renal artery stenosis. N Engl J Med. 2009;361:1972–1978. doi: 10.1056/NEJMcp0809200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis R.P., Pearce J.D., Craven T.E., Moore P.S., Edwards M.S., Godshall C.J., Hansen K.J. Atherosclerotic renovascular disease among hypertensive adults. J Vasc Surg. 2009;50:564–570. doi: 10.1016/j.jvs.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soulez G., Oliva V.L., Turpin S., Lambert R., Nicolet V., Therasse E. Imaging of renovascular hypertension: respective values of renal scintigraphy, renal doppler US, and MR angiography. Radiographics. 2000;20:1355–1368. doi: 10.1148/radiographics.20.5.g00se131355. [DOI] [PubMed] [Google Scholar]
- 9.Doelken M., Lanz S., Rennert J., Alibek S., Richter G., Doerfler A. Differentiation of cytotoxic and vasogenic edema in a patient with reversible posterior leukoencephalopathy syndrome using diffusion-weighted MRI. Diagn Interv Radiol. 2007;13:125–128. [PubMed] [Google Scholar]
- 10.Benziada-Boudour A., Schmitt E., Kremer S., Foscolo S., Rivière A.S., Tisserand M., Boudour A., Bracard S. Posterior reversible encephalopathy syndrome: a case of unusual diffusion-weighted MR images. J Neuroradiol. 2009;36:102–105. doi: 10.1016/j.neurad.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Parker R., Ostermann M., Reidy J., Goldsmith D. Posterior leuko-encephalopathy syndrome as the presentation of renal artery stenosis. J Renovasc Dis. 2003;2:48–53. [Google Scholar]