Summary
A 21-year-old African American male presented to the emergency department after an episode of unexplained syncope. He had no significant past medical history. The initial physical examination was within normal limits, and his 12-lead electrocardiogram showed no signs of ischemia or evidence of chamber enlargement. The patient received a transthoracic echocardiogram which demonstrated mild global left ventricular dysfunction with an estimated ejection fraction of 45%. Coronary angiography was next performed and found no evidence of atherosclerotic coronary artery disease but did reveal hypoplasia of the left circumflex and right coronary arteries, with intraluminal diameters of approximately 1 mm. The left anterior descending coronary artery was small distally. Cardiac magnetic resonance imaging with gadolinium enhancement found no evidence of myocardial scar. The patient was ultimately diagnosed with aborted sudden cardiac death due to hypoplastic coronary artery disease (HCAD). The patient received an implantable cardioverter-defibrillator (ICD) prior to hospital discharge for secondary prevention of sudden cardiac death. One year after this presentation, the patient has been asymptomatic, with no ICD discharges.
Keywords: Hypoplastic coronary artery disease, Sudden cardiac death, Implantable cardioverter-defibrillator, Congenital coronary artery anomalies
Introduction
Hypoplastic coronary artery disease (HCAD) is a rare condition in which the coronary arteries are abnormally small. Sudden cardiac death (SCD) has been reported as an initial presentation of this condition, and opportunities for treatment are rare. We present a case in which a previously asymptomatic young male was diagnosed with HCAD following a syncopal episode and was treated with a prophylactic implantable cardioverter-defibrillator (ICD).
Case report
A 21-year-old male presented to the emergency department after losing consciousness while walking in the park. He experienced dyspnea and palpitations just before the episode, but no chest pain. Bystanders witnessed no tonic clonic movements suggestive of seizure activity. The patient had never previously experienced a syncopal episode. He had no significant past medical history and took no medications. He reported some tobacco use, but no history of alcohol or illicit drug abuse. He did report a family history of an “enlarged heart” in a brother, as well as a history of sudden death in several great aunts.
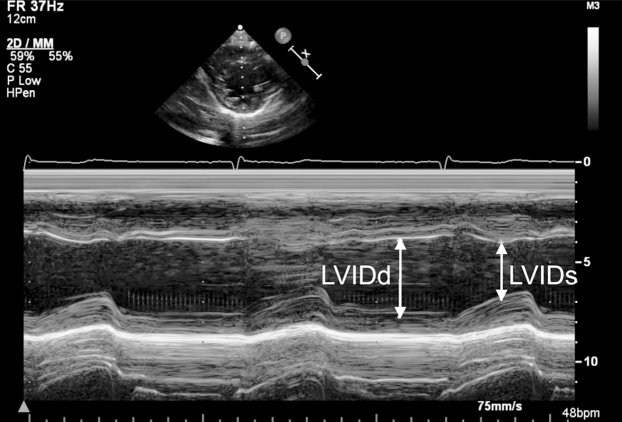
The patient's physical exam was unremarkable. He was a thin male in no distress and was fully alert and oriented. Cardiac auscultation was unremarkable. Laboratory studies revealed electrolytes within the normal range, a blood glucose of 77 mg/dL, and a normal hemoglobin level and white cell count. Toxicology screen for illicit drugs was negative. His 12-lead electrocardiogram (ECG) showed mild sinus bradycardia at 52 beats per minute but no ST segment abnormalities or chamber enlargement. The patient received a transthoracic echocardiogram which demonstrated mild global left ventricular dysfunction with an estimated ejection fraction of 45% as well as a moderately dilated right ventricle with mildly reduced performance (Fig. 1). There was no significant chamber hypertrophy, intracardiac shunt, or evidence of valvular disease.
Figure 1.
M-mode echocardiography using fractional shortening (FS) to estimate left ventricular function. This image reveals FS of 26%, consistent with mild left ventricular dysfunction. Lines with arrows indicate left ventricular internal dimension in systole (LVIDs) and left ventricular internal dimension in diastole (LVIDd).
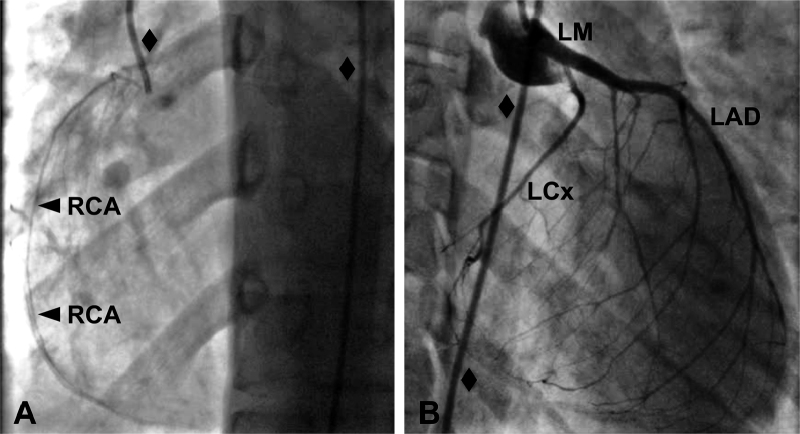
Due to these abnormal echocardiogram findings, cardiac catheterization was performed, revealing narrowed lumens of all three of the patient's major epicardial coronary artery branches (Fig. 2). The right coronary artery had a normal ostium but a maximal diameter of approximately 1 mm along its entire length. The left coronary artery ostium was also without abnormality, and the left main artery had a normal caliber of approximately 4 mm. The left circumflex coronary artery, however, had a maximal diameter of approximately 1 mm. The left anterior descending artery (LAD) was of normal caliber proximally but was small distally, with a diameter of under 2 mm in its distal segment. A myocardial bridge was identified at the mid-portion of the LAD. Intraluminal infusion of nitroglycerin did not change the caliber of the arteries. Computed tomography angiography confirmed the above findings; subsequent cardiac magnetic resonance imaging with gadolinium enhancement found no evidence of scar. A 24-h Holter monitor recorded no abnormalities.
Figure 2.
Coronary artery angiograms. (A) Left anterior oblique view of the patient's hypoplastic right coronary artery (RCA, with arrows). (B) Right anterior oblique view of the patient's normal left main (LM), hypoplastic left circumflex (LCx), and distally small left anterior descending (LAD) arteries. In each panel, the angiography catheter, indicated by black diamonds, is 2 mm in caliber.
The abnormally small coronary arteries were consistent with HCAD. In the absence of any other identified cause of the patient's syncope, he was diagnosed with aborted SCD due to insufficient myocardial perfusion, and an ICD was implanted. Prior to implantation, the patient underwent an electrophysiology study with various pacing maneuvers; no inducible arrhythmias were noted. However, based on the clinical context, it was determined that the patient would still benefit from ICD placement for the secondary prevention of SCD. The patient was discharged on metoprolol and lisinopril for his systolic dysfunction and one year later, the patient is doing well, with no episodes of syncope, symptoms of cardiac ischemia, heart failure symptoms, or ICD discharges.
Discussion
HCAD is a rare congenital malformation in which at least one of the major epicardial artery branches is underdeveloped. Coronary artery anomalies have an incidence of approximately 1% in the general population, and one postmortem survey identified HCAD in 2.2% of patients with coronary artery anomalies [1], [2]. In a postmortem analysis of athletes who experienced sudden death, hypoplastic coronary arteries were identified in less than 5% of total patients and comprised less than 33% of observed congenital coronary artery anomalies [3]. The hypoplasia of HCAD can manifest as a narrowed luminal diameter or a shortened course, and is typically present in one or two of the three major epicardial branches. A luminal diameter of less than 1.5 mm with no nearby compensatory branches has been proposed as a criterion for diagnosis [4]. The case we present here appears to be particularly severe: the right and left circumflex coronary arteries were markedly hypoplastic, while the left anterior descending artery was small distally.
SCD is a common presentation of the condition, likely from arrhythmia triggered by myocardial ischemia [4]. In some of these cases, symptoms such as syncope, palpitations, dyspnea, and chest pain are present beforehand, while in many cases SCD is the first manifestation. HCAD in cases of SCD is typically diagnosed during postmortem autopsy [5]. The mechanisms of myocardial ischemia in coronary artery anomalies are not well elucidated, and ischemia in such patients is difficult to predict and to reproduce during stress testing [1]. HCAD may be no different: thrombosis, coronary artery spasm, a milking effect by a myocardial bridge, and exertion may exacerbate limitations to flow already imposed by narrowed coronary artery lumens [1], [4].
Uncommonly, HCAD is diagnosed in living patients. In one of these cases, HCAD was diagnosed in an 11-year-old boy during a coronary angiogram following symptoms of myocardial infarction; he experienced SCD 4 years later during intense physical exertion [6], [7]. In another case, a 20-year-old man with myocardial infarction was found to have HCAD [8].
Few options are available for the treatment of HCAD in such cases. Bypass surgery is not feasible due to the diffuse distribution of disease, although transmyocardial revascularization may be an alternative method of improving myocardial perfusion in HCAD patients. Heart transplant can be undertaken when HCAD is accompanied by advanced heart failure. Due to the heightened risk of arrhythmia and SCD, ICD implantation has been suggested for HCAD; the benefits of ICDs in older adults at risk for ventricular arrhythmia may extend to younger patients with congenital heart disease [6], [9]. In no prior reported cases in the literature has the implantation of an ICD for SCD prevention been used for HCAD. In the case of the 11-year-old boy mentioned above, pediatric ICD implantation was not routinely performed at the hospital of treatment. An ICD was not used in the case of the 20-year-old man mentioned above either, as the authors noted that he did not have persistent myocardial ischemia; periodic electrophysiologic monitoring was instead chosen.
In the case presented here, it was felt that the benefit from reduction of SCD risk outweighed the adverse effects of ICD implantation, even given a lack of definitive proof that aborted SCD had occurred. Further testing, such as a vasospasm provocation and stress perfusion testing, may have provided insight into the mechanisms of ischemia in this patient. Given the poorly defined triggers of ischemia and arrhythmia in HCAD, however, ICD implantation was felt to be necessary regardless of the results of such tests. Indeed, recent guidelines state that ICD implantation is reasonable in congenital heart disease, including coronary artery malformations, in patients with unexplained episodes of syncope who also have impaired ventricular function and are on long-term treatment for heart failure [10]. Until our understanding of coronary artery anomalies and the means by which they cause SCD are better elucidated, ICD placement may be a prudent choice for some patients with HCAD.
References
- 1.Angelini P., Velasco J.A., Flamm S. Coronary anomalies: incidence, pathophysiology, and clinical relevance. Circulation. 2002;105:2449–2454. doi: 10.1161/01.cir.0000016175.49835.57. [DOI] [PubMed] [Google Scholar]
- 2.Ogden J.A. Congenital anomalies of the coronary arteries. Am J Cardiol. 1970;25:474–479. doi: 10.1016/0002-9149(70)90016-0. [DOI] [PubMed] [Google Scholar]
- 3.Maron B.J., Shirani J., Poliac L.C., Mathenge R., Roberts W.C., Mueller F.O. Sudden death in young competitive athletes: clinical, demographic, and pathological profiles. JAMA. 1996;276:199–204. [PubMed] [Google Scholar]
- 4.Zugibe F.T., Zugibe F.T., Costello J.T., Breithaupt M.K. Hypoplastic coronary artery disease within the spectrum of sudden unexpected death in young and middle age adults. Am J Forensic Med Pathol. 1993;14:276–283. doi: 10.1097/00000433-199312000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Smith E.T., Davis G.J. Sudden cardiac death associated with hypoplasia of the coronary arteries and conduction system alteration. Am J Forensic Med Pathol. 1997;18:189–193. doi: 10.1097/00000433-199706000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Amabile N., Fraisse A., Quilici J. Hypoplastic coronary artery disease: report of one case. Heart. 2005;91:e12. doi: 10.1136/hrt.2004.047621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraisse A., Quilici J., Canavy I., Savin B., Aubert F., Bory M. Myocardial infarction in children with hypoplastic coronary arteries. Circulation. 2000;101:1219–1222. doi: 10.1161/01.cir.101.10.1219. [DOI] [PubMed] [Google Scholar]
- 8.Sim D.S., Jeong M.H., Choi S., Yoon N.S., Yoon H.J., Moon J.Y., Hong Y.J., Kim K.H., Park H.W., Kim J.H., Ahn Y., Cho J.G., Park J.C., Kang J.C. Myocardial infarction in a young man due to a hypoplastic coronary artery. Korean Circ J. 2009;39:163–167. doi: 10.4070/kcj.2009.39.4.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gradaus R., Wollmann C., Köbe J., Hammel D., Kotthoff S., Block M., Breithardt G., Böcker D. Potential benefit from implantable cardioverter-defibrillator therapy in children and young adolescents. Heart. 2004;90:328–329. doi: 10.1136/hrt.2003.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zipes D.P., Camm A.J., Borggrefe M., Buxton A.E., Chaitman B., Fromer M., Gregoratos G., Klein G., Moss A.J., Myerburg R.J., Priori S.G., Quinones M.A., Roden D.M., Silka M.J., Tracy C. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e385–e484. doi: 10.1161/CIRCULATIONAHA.106.178233. [DOI] [PubMed] [Google Scholar]