Summary
We present a case of a giant fenestration and a fibrous strand rupture of the aortic valve without massive regurgitation. A 56-year-old woman, was referred for coronary revascularization, had II–III degree aortic regurgitation without symptoms of heart failure. On the intraoperative direct view, the non coronary cusp (NCC) had the giant fenestration and the left coronary cusp (LCC) had the fibrous strand rupture. There was no severe inflammation, thrombi, or vegetation. Finally, she had coronary artery bypass surgery and aortic valve replacement.
Although fenestration of the aortic valve is not rare, it is hard to determine its configuration preoperatively. When the echocardiographic findings indicate an eccentric regurgitation flow despite the absence of prolapse, we should perform examinations with the possibility of coexisting aortic valve fenestration in mind. Massive regurgitation does not necessarily correspond to a giant fenestration and a fibrous strand rupture.
We report a rare case of the unusually large fenestration and the rupture of the fenestrated fibrous strand of the aortic valve without massive regurgitation.
Keywords: Aortic valve, Aortic regurgitation, Giant fenestration, Fibrous strand rupture
Case report
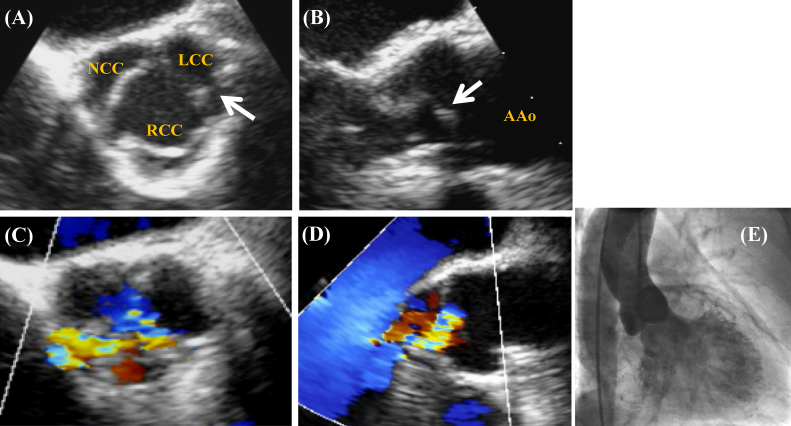
A 56-year-old woman, diagnosed as having silent myocardial ischemia with three-vessel disease by coronary angiography, was referred to our hospital for coronary revascularization. She had hypertension, diabetes mellitus, hyperlipidemia, and a nine-year history of hemodialysis therapy. The New York Heart Association classification was class II. Cardiac auscultation revealed a grade 3/6 diastolic murmur at the 3rd left sternal border. B-type natriuretic peptide was mildly elevated (81 pg/ml). Transthoracic echocardiogram (TTE) showed mild eccentric hypertrophy [left ventricular (LV) end-diastolic diameter 54 mm, interventricular septal thickness 12 mm, posterior wall thickness 9 mm], a normal ejection fraction (EF) (65%), and moderate aortic regurgitation (AR) from the closing edge between the right coronary cusp (RCC) and the non coronary cusp (NCC) (pressure half-time 571 msec, regurgitant jet height/LV outflow tract height 30%). Transesophageal echocardiogram (TEE) showed the tricuspid aortic valve (AV) with border irregularity and partial thickness without calcification, prolapse, and perforation. A mobile fibrous strand (length 7 mm) was detected at the left coronary cusp (LCC) commissure. The eccentric AR flow was detected not only at the coaptation site between the RCC and the NCC but also the margin of the LCC (Fig. 1). Left ventriculography showed mild LV enlargement (end-diastolic volume index 96 ml/m2) and a normal EF (59%). Aortography showed II–III degree AR without enlargement of the ascending aorta (Fig. 1).
Figure 1.
Transesophageal echocardiogram and aortography. (A, B) A mobile fibrous strand (white arrow) at the left coronary cusp commissure in the 2-dimensional image. (C) Aortic regurgitation (AR) from not only the coaptation site between the right coronary cusp and the non coronary cusp (NCC) but also the margin of the left coronary cusp in the color Doppler image. (D) A suction flow located on the NCC side from the center and the eccentric AR flow to the interventricular septum (A, C) short-axis view, (B, D) long-axis view, (E) Aortography showed II–III degree AR. LCC: left coronary cusp; RCC: right coronary cusp; AAo: ascending aorta.
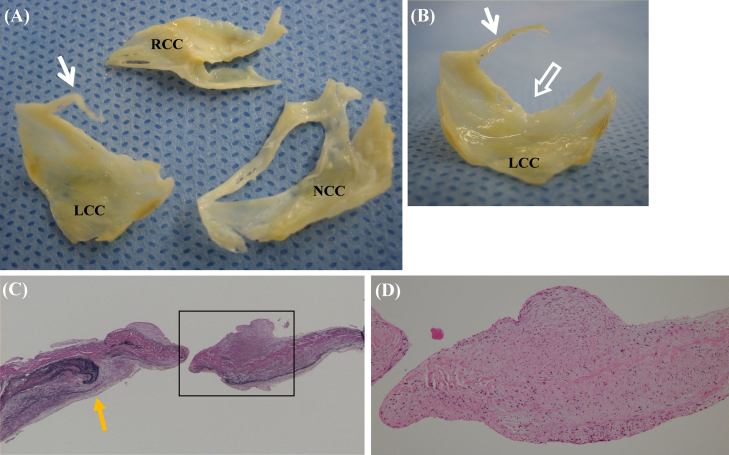
On the intraoperative direct view, the NCC had a giant fenestration (size 17 mm × 3 mm) and the LCC had a fibrous strand rupture, namely, a torn strand of its fenestrated cusp (length 11 mm) (Fig. 2). There were no valvular anomalies and no acute or healed infective endocarditis. The pathological findings showed rupture of elastic fibers and spindle-cell proliferation with myxoid matrix in the perforated site of the NCC, and degeneration of collagen fibers and lipomatous metaplasia in the basal NCC (Fig. 2). There was no severe inflammation, thrombi, nor vegetation. Finally, she had coronary artery bypass graft (CABG) surgery and AV replacement (AVR) (St. Jude Medical Regent valve 19 mm; St. Paul, MN, USA). Her postoperative course was uneventful. Thereafter, she was discharged without significant complications.
Figure 2.
Resected and tissue specimens of the aortic valve. (A, B) The non coronary cusp with a giant fenestration (size 17 mm × 3 mm) and the left coronary cusp with a fibrous strand (length 11 mm) (white arrow) and an open fenestration (open arrow). (C) Torn elastic fibers (yellow arrow) in the perforated site of the non coronary cusp. (Elastica Van Gieson stain, ×40). (D) Spindle-cell proliferation with myxoid matrix, without inflammatory cell infiltrate (hematoxylin & eosin stain, ×100). LCC: left coronary cusp; RCC: right coronary cusp; NCC: non coronary cusp. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Discussion
Fenestration of the AV is not a rare malformation. It is considered a congenital anomaly during the embryonic phase [1] and a form of atrophy which may begin in early childhood, or even in the fetus, and that aging, dilatation of the ring and increased intravascular pressure are contributing and modifying factors [2]. The characteristics are mentioned as follows: (1) located in the coaptation zone of the para-commissural area 1, 2, 3, 4, 5, 6, 7; (2) higher in males [2]; (3) with myxomatous degeneration [6]; (4) with hypertrophy 2, 4, 5; (5) with root dilatation 2, 4. Concerning these latter three points, there are some controversial reports 6, 7. Strand rupture of the AV has 2 types: one is a strand rupture of a fenestrated cusp, and the other between the aortic cusp and the aortic wall [8]. This case was the former. Fenestration rarely produces significant valvular regurgitation because of its location 1, 2, 3, 4, 5, 6. However, an unusually large fenestration and a rupture of the fenestrated fibrous strand, which regardless of annulus dilates, prolapsed cusp and bicuspid, can lead to massive regurgitation 5, 6. Although the present case had both the giant fenestration and the fibrous strand rupture, she did not have massive valvular regurgitation. We speculated that there was just moderate regurgitation derived from both the giant fenestration and the strand rupture because the giant fenestration was located in the coaptation zone of the para-commissural area and it was covered by the aortic cusp coaptation in the diastolic phase. Many studies have reported that the primary cause of rupture of the fenestrated fibrous strand was not infection and inflammation, but degeneration. In this case there was no sign of either infection or inflammation. On the pathological findings, the NCC became thin with myxomatous degeneration, which was similar to cases reported by Akiyama et al. [6]. Some reports have suggested that myxoid degeneration of the AV is common and, in many cases, may be secondary to long-standing systemic hypertension [9]. Meanwhile, others have suggested fenestration occurs in the presence of normal configuration of aortic cusps.
We were not able to elucidate the accurate configuration of the AV by either TTE or TEE preoperatively. Preoperative echocardiographic diagnosis is important in that AV plasty for fenestration without concomitant root dilatation in the tricuspid AV can be a candidate [10], although this case had AVR. Despite moderate AR without overt heart failure, the case had simultaneous operation: AVR and CABG in consideration of following points: (1) she had coronary disease needing CABG; (2) with mild LV dilatation; (3) we were concerned about the valvular disease progressing along with her history of hemodialysis therapy and the risk of a second surgery with diabetes mellitus. When the echocardiographic findings indicate an eccentric AR flow despite the absence of prolapse, we should perform examinations with the possibility of coexisting aortic valve fenestration in mind. Furthermore, massive regurgitation does not necessarily correspond with a giant fenestration and a fibrous strand rupture. In recent years, three-dimensional TEE has come into use. Therefore, it is expected that this newly-introduced method will promote better understanding of complicated configurations at an early stage of diagnosis.
References
- 1.Foxe A.N. Fenestrations of the semiluna valves. Am J Pathol. 1929;5:179–182. [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman B., Hathaway B.M. Fenestration of the semilunar cusps, and functional aortic and pulmonic valve insufficiency. Am J Med. 1958;24:549–558. doi: 10.1016/0002-9343(58)90295-x. [DOI] [PubMed] [Google Scholar]
- 3.Bailey C.P., Brest A.N., Dontas N., Uricchio J.F. Successful surgical repair of aortic insufficiency due to valvular fenestration. Circulation. 1959;20:587–589. doi: 10.1161/01.cir.20.4.587. [DOI] [PubMed] [Google Scholar]
- 4.Symbas P.N., Walter P.F., Hurst J.W., Schlant R.C. Fenestration of aortic cusps causing aortic regurgitation. J Thorac Cardiovasc Surg. 1969;57:464–470. [PubMed] [Google Scholar]
- 5.Blaszyk H., Witkiewicz A.J., Edwards W.D. Acute aortic regurgitation due to spontaneous rupture of a fenestrated cusp: report in a 65-year-old man and review of seven additional cases. Cardiovasc Pathol. 1999;8:213–216. doi: 10.1016/s1054-8807(99)00009-5. [DOI] [PubMed] [Google Scholar]
- 6.Akiyama K., Hirota J., Taniyasu N., Maisawa K., Kobayashi Y., Tsuda M. Pathogenetic significance of myxomatous degeneration in fenestration-related massive aortic regurgitation. Circ J. 2004;68:439–443. doi: 10.1253/circj.68.439. [DOI] [PubMed] [Google Scholar]
- 7.Schäfers H.J., Langer F., Glombitza P., Kunihara T., Fries R., Aicher D. Aortic valve reconstruction in myxomatous degeneration of aortic valves: are fenestrations a risk factor for repair failure? J Thorac Cardiovasc Surg. 2009;17:1–5. doi: 10.1016/j.jtcvs.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 8.Nakajima M., Tsuchiya K., Naito Y., Hibino N., Inoue H. Aortic regurgitation caused by rupture of a well-balanced fibrous strand suspending a degenerative tricuspid aortic valve. J Thorac Cardiovasc Surg. 2002;124:843–844. doi: 10.1067/mtc.2002.124296. [DOI] [PubMed] [Google Scholar]
- 9.Allen W.M., Matloff J.M., Fishbein M.C. Myxoid degenerarion of the aortic valve and isolated severe aortic regurgitation. Am J Cardiol. 1985;55:439–444. doi: 10.1016/0002-9149(85)90390-x. [DOI] [PubMed] [Google Scholar]
- 10.Schäfers H.J., Langer F., Glombitza P., Kunihara T., Fries R., Aicher D. Aortic valve reconstruction in myxomatous degeneration of aortic valve: are fenestration a risk for repair failure? J Thorac Cardiovasc Surg. 2010;139:660–664. doi: 10.1016/j.jtcvs.2009.06.025. [DOI] [PubMed] [Google Scholar]