Abstract
Streptococcus suis is a major pathogen of swine, responsible for a number of chronic and acute infections, and is also emerging as a major zoonotic pathogen, particularly in South-East Asia. Our study of a diverse population of S. suis shows that this organism contains both Type I and Type III phase-variable methyltransferases. In all previous examples, phase-variation of methyltransferases results in genome wide methylation differences, and results in differential regulation of multiple genes, a system known as the phasevarion (phase-variable regulon). We hypothesized that each variant in the Type I and Type III systems encoded a methyltransferase with a unique specificity, and could therefore control a distinct phasevarion, either by recombination-driven shuffling between different specificities (Type I) or by biphasic on-off switching via simple sequence repeats (Type III). Here, we present the identification of the target specificities for each Type III allelic variant from S. suis using single-molecule, real-time methylome analysis. We demonstrate phase-variation is occurring in both Type I and Type III methyltransferases, and show a distinct association between methyltransferase type and presence, and population clades. In addition, we show that the phase-variable Type I methyltransferase was likely acquired at the origin of a highly virulent zoonotic sub-population.
INTRODUCTION
Streptococcus suis is a major veterinary pathogen, causing a range of chronic and severe diseases in pigs such as arthritis and septicaemia (1). These diseases are responsible for major economic losses to agriculture globally. Streptococcus suis is also a major cause of zoonotically acquired bacterial meningitis in humans, particularly in South-East Asia (2) where it is an endemic public health concern, with high mortality rates associated with a Streptococcal septic/toxic shock like syndrome (3). Although human-to-human transmission appears rare, an increasing incidence of zoonotically acquired disease is associated with increasing human populations and intense agricultural practices (4). Risk factors for S. suis infection in humans appear to be prolonged contact with pigs, and consumption of undercooked pork and pork-products (5). The majority of human cases of S. suis infection appear to be associated with serogroup 2, although other serogroups have been reported to cause disease (6–8). A recent study has shown that there are no consistent genomic differences between strains that cause disease in humans versus those that cause disease in pigs, and that strains isolated from humans are confined to a single lineage that emerged in the 1920s when pig production was intensified (9).
Many host adapted bacterial pathogens contain phase-variable genes. Phase variation is the random on-off switching of gene expression. This process often occurs by the variability in simple sequence repeats (SSRs) within a gene or promoter, or by the shuffling of sequences by homologous recombination between expressed and silent loci via inverted repeats (IRs) present in these loci (10). Phase variation is often associated with genes that encode bacterial surface factors such as adhesins (11–13), pili (14), flagella (15) iron-acquisition proteins (16,17), and lipo-oligosaccharide (18,19). However, many host-adapted bacterial pathogens contain phase-variable methyltransferases, associated with restriction-modification (R-M) systems, that are able to phase-vary (20,21), and are involved in regulation of multiple genes via epigenetic mechanisms, in a system known as the phasevarion (for phase-variable regulon) (22). These systems control gene regulation in a number of important human pathogens, including non-typeable Haemophilus influenzae (23), Moraxella catarrhalis (24), pathogenic Neisseria (25) and Streptococcus pneumoniae (26). All these organisms, with the exception of S. pneumoniae, contain Type III methyltransferases that contain SSRs, with variation in SSR number leading to biphasic on-off switching of methyltransferase expression. Streptococcus pneumoniae is the first example of a Gram-positive organism containing a phasevarion, and also the first example of a phasevarion controlled by a Type I methyltransferase (26). In contrast to the biphasic on/off phasevarions previously characterized in Type III methyltransferases, shuffling of specificity domains between expressed and silent loci leads to six different methyltransferase specificities. This results in a six-phase switch, and consequently six different phenotypic states within a pneumococcal population (26). All phasevarions characterized to date regulate putative vaccine candidates and virulence factors (22–27). This has clear implications in the development of stable vaccines and treatment strategies: it is only possible to define the stably expressed protein profile in organisms containing phasevarions by studying the genes regulated by randomly switching methyltransferases in these organisms. Here we report the identification and analysis of multiple phase-variable methyltransferases in S. suis.
MATERIALS AND METHODS
Bacterial strains and growth conditions
Streptococcus suis isolates were grown on Brain-Heart Infusion (BHI) agar or Sheep-blood agar (Oxoid), with liquid cultures grown in Todd-Hewitt broth containing 0.2% yeast extract (Oxoid) at 37°C. Escherichia coli DH5α and BL21 were grown in Luria–Bertani media supplemented with 100 μg/ml ampicillin where appropriate, at 37°C with 200 rpm shaking. Prototype S. suis strains used in this study are detailed in Table 1.
Table 1.
Prototype strains used in this study
| S. suis strain | Phase-variable methyltransferase locus | Allelic variant | Genbank locus tag (accession number) | Additional information |
|---|---|---|---|---|
| P1/7§ | Type I | allele B | SSU1271-SSU1274 (AM946016) | |
| S735 | Type I | allele B | YYK_06100- YYK_06115 (CP003736) | population also contains minor variants A, C, D (Figure 1) |
| T15§ | Type III | modS1 | T15_1305 (CP006246.1) | modS1 locus first observed in strain T15* |
| LSS89# | Type III | modS1 | (FIJA01000010.1) | |
| SS1028# | Type III | modS2 | (FIJX01000003.1) | |
| LSS66 | Type III | modS2 | (FIHP01000004.1) | also contains two Type I signatures and Dam (Table 3) |
| YS77# | Type III | modS3 | (ALNH01000046.1) |
*described in reference (39).
#gene was cloned from these strains and the methyltransferase expressed heterologously in E. coli, as described in ‘Material and Methods’ section.
§locus first identified in these strains/sequences used to search custom database.
Molecular biology
Standard molecular biology techniques were used throughout (28). Genomic DNA was prepared using the Sigma GenElute bacterial genomic DNA kit according to manufacturer's instructions. Plasmid DNA for SMRT sequencing was prepared using the Promega Wizard mini prep kit according to manufacturer's instructions (Promega). Oligonucleotide primers were purchased from Integrated DNA Technologies, and are detailed in Table 2. Polymerase chain reaction (PCR) was carried out using GoTaq polymerase (Promega), or KOD HotStart polymerase (Merck) according to manufacturer's instructions. Restriction enzymes were purchased from New England Biolabs, and used according to manufacturer’s instructions. DNA fragment length analysis was carried out at the Griffith University DNA Sequencing Facility (GUDSF; Brisbane, Australia), or the Australian Genome Research Facility (AGRF; Brisbane, Australia). Sequencing was carried out using Big Dye 3.1 (Perkin Elmer), using PCR products purified using the Qiagen PCR purification kit according to the manufacturer's instructions. Samples were analysed by GUDSF or AGRF.
Table 2.
Oligonucleotides used in this study
| Name | Sequence |
|---|---|
| Ssu-T1-For-FAM | 5′-FAM-CCAAATGATGAGCCTGCAAGTGAAC |
| Ssu-T1-Rev | 5′-CCTTAATCCCAACCTGCTCTTTTAG |
| SsuT3-F-FAM | 5′-FAM-CATCAAAAACGGCTTGACAGCC |
| SsuT3-R | 5′-GCAATGTTGTCTGATAAAACATCTTTTG |
| SsuT3-oE-F | 5′-AGTCAGCCATGGGGGAGCAGAAGCTTGGAGACTACACTCAAG |
| SsuT3-oE-R | 5′-AGTCAGGGATCCCTACACCACCTTCACTTTGGTACC |
| RT-16S-Ssu-F | ATGGACCTGCGTTGTATTAGC |
| RT-16S-Ssu-R | CATTGCCGAAGATTCCCTAC |
| RT-SsuT1-5′_1-F | GGT TTT GGA GAT ACA CTT CTC C |
| RT-SsuT1-5′_2-F | CCA TTC GTA GCA CAA ACT CAA C |
| RT-SsuT1-3′_1-R | GAA GAG TCC AAT TTC TCG GAA TC |
| RT-SsuT1-3′_2-R | GTA GCA CCA AGA ACT AGG AAG |
*16s rRNA primer pair sequences from (51).
Type I allele quantification
The variant alleles of hsdS in S. suis strain S735 were quantified utilising FAM labelled PCR coupled to a restriction digest in a method similar to that used previously for S. pneumoniae (26). The whole hsdS locus was PCR amplified (3.02 kb) from extracted genomic DNA prepared from an entire plate of S. suis strain S735 grown overnight on BHI agar. PCR was carried out using GoTaq DNA polymerase (Promega) according to the manufacturer’s instructions, and utilising primers that bind outside the area of recombination (Ssu-T1-For-FAM and Ssu-T1-Rev). The PCR products were then digested in a double-digest with DraI and NciI (New England Biolabs). This digestion was predicted to yield different sized FAM-labelled fragments for each of the variant forms. The pool of restriction fragments was run on an ABI prism Gene Analyser (Life Technologies). The area of the peak given by each labelled fragment, each corresponding to the prevalence of one of the variant forms, was quantified using Peak Scanner v1.0.
Type III allele quantification
Fragment length analysis of the phase-variable Type III methyltransferase repeat tract was conducted using the fluorescently labelled forward primer SsuT3-F-FAM and the reverse primer SsuT3-R, and fragments were analysed by the Australian Genome Research Facility (AGRF, Brisbane, Australia). PCR was carried out using cell suspensions using the relevant S. suis strain grown over-night on BHI agar.
RNA preparation and semi-quantitative RT-PCR
Whole cell RNA preps were made from S. suis strain S735 using cells grown overnight on BHI plates, so as to replicate the growth conditions used for the Type I allele quantification assay (above; Figure 1). RNA was prepared using Trizol reagent (Thermo-Fisher) according to the manufacturer’s instructions. Prior to reverse transcription (RT), RNA was treated with heat-labile dsDNAse (Thermo Fisher catalogue number EN0771) according to manufacturer’s instructions, and used directly in the RT reaction. cDNA was synthesized from DNAse-treated RNA using Protoscript II Reverse Transcriptase (New England Biolabs) according to manufacturer’s instructions. Negative controls were carried out by omitting the RT from the cDNA synthesis reaction. Semi-quantitative PCR was carried out in a multiplex reaction using GoTaq DNA polymerase (Promega) in a standard PCR reaction with primers specific for 16s rRNA, and one of four Type I allele-specific primer pairs (alleles A, B, C or D; Table 2). 16s rRNA primers have been described previously (51).
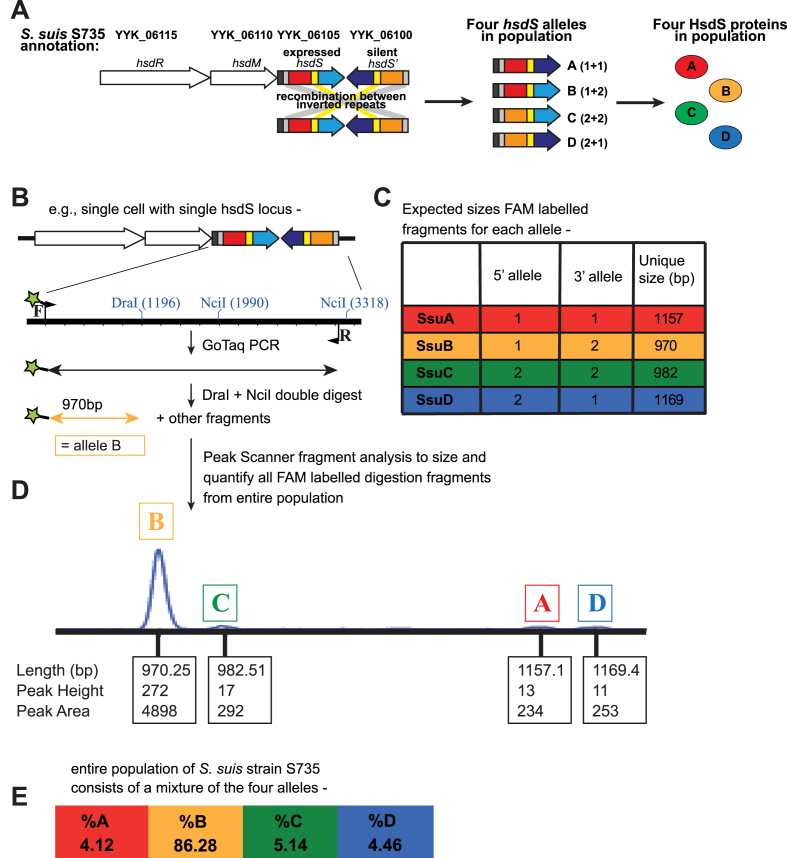
Figure 1.
Demonstration that Streptococcus suis contains a Type I hsd locus that is able to phase-vary. (A) Illustration of the Type I hsd R-M locus from S. suis strain S735 containing duplicate, variable hsdS specificity loci that contain IRs (yellow and grey boxes). By shuffling between variable 5′ (red and orange regions) and 3′ (blue and purple) TRDs, four unique hsdS genes can be encoded at the hsdS expressed locus immediately downstream of the hsdM gene. This results in the expression of four unique allelic variants of the HsdS protein, all of which are predicted to lead to different methyltransferase specificities; (B) Illustration of FAM-labelled PCR assay used to type and quantify hsdS alleles present in the expressed locus in the S. suis population, with FAM label illustrated as green star on forward primer, and (C) predicted sizes of each allelic variant based on in silico analysis. (D) Following PCR and restriction digest, FAM-labelled fragments are separated and quantified using a Gene Analyser (Life Technologies) and quantified used Peak Scanner software in order to determine the percentage of each allele present in a S. suis population; (E) quantification of hsdS alleles A–D present in a population of S. suis strain S735 following Peak Scanner analysis.
Cloning and over-expression of Type III methyltransferase variants
PCR products for cloning into the NcoI/BamHI site of pET15b cloning vector (Merck) were prepared using KOD Hot-start DNA polymerase (Merck) according to manufacturer's instructions. Primers specific for the Type III system were designed to clone the gene containing only one GAGCA repeat so as to lock-on the methyltransferase (SsuT3-oE-F and SsuT3-oE-R). We cloned the three unique mod alleles from S. suis strains LSS89 (modS1), SS1028 (modS2) and YS77 (modS3). A vector containing an non-methyltransferase, siaB, used previously (23) served as a non-methylating control. Over-expression of each protein was carried out using E. coli BL21 cells, which were induced by the addition of Isopropyl β-D-1-thiogalactopyranoside (IPTG) to a final concentration of 0.5 mM overnight at 37°C with shaking at 200 rpm.
Single-molecule, real-time (SMRT) sequencing and methylome analysis
Mini-preps from E. coli cells expressing each methyltransferase and a negative control expressing an the non-methyltransferase siaB, described previously (23) were prepared using the Promega wizard plasmid mini kit according to the manufacturer’s instructions. Genomic DNA from S. suis strain LSS66 was prepared from an overnight culture in Todd Hewitt broth (Oxoid) +0.2% Yeast Extract (Fisher Scientific) and cells lysed according to the Genomic DNA Handbook (Qiagen) using 100 mg/ml Lysozyme (Sigma) supplemented with 250 units/ml Mutanolysin (Sigma). High-molecular-weight genomic DNA was isolated using Genomic-tip 100/G columns (Qiagen) according to the manufacturer's instructions and DNA resuspended in 10 mM Tris–Cl, pH 8.5 (Qiagen) overnight at 4°C. A phenol:chloroform:isoamylalcohol (Sigma) clean-up was then performed before finally resuspending DNA in 10 mM Tris–Cl, pH 8.5 (Qiagen) overnight at 4°C. SMRT sequencing and methylome analysis was carried out as previously (29,30). Briefly, DNA was sheared to an average length of ∼0.5–2kb (plasmids) or 5–10 kb (genomic DNA) using g-TUBEs (Covaris, Woburn, MA, USA) and SMRTbell template sequencing libraries were prepared using sheared DNA. DNA was end repaired, then ligated to hairpin adapters. Incompletely formed SMRTbell templates were degraded with a combination of Exonuclease III (New England Biolabs; Ipswich, MA, USA) and Exonuclease VII (USB; Cleveland, OH, USA). Primer was annealed and samples were sequenced on the PacBio Sequel system (Menlo Park, CA, USA) using standard protocols for long insert libraries. SMRT sequencing and methylome analysis was carried out at the Yale Centre for Genome Analysis (plasmid vectors) or the Wellcome Sanger Institute (genomic DNA; Cambridge, UK). Initial methylation analysis of DNA from strain L66 using the standard SMRT analysis pipeline showed a very high noise level, so it was reanalysed using whole-genome amplified DNA as a methylation-negative control.
Bioinformatic analysis
The strain collection used to determine the Type I and Type III methyltransferase distribution was described previously (9,31). This collection comprises of isolates from different countries (UK, Vietnam, China, the Netherlands) and are associated with different clinical phenotypes of pigs. Non-clinical isolates come from the tonsils and upper-respiratory tract of pigs without signs of S. suis disease, respiratory isolates come from the lungs of pigs with signs of pneumonia, and systemic isolates come from the blood, brain or joints of a pig with signs of systemic disease. Sequencing was performed after isolation on Columbia agar (Oxoid Ltd. Basingstoke, UK) containing 5% (v/v) sheep blood (TCS biosciences Ltd., Bucks, UK) as described previously (9). We took the entire hsd region from S. suis P1/7 (Genbank locus tags SSU1271-SSU1274) and the mod locus from S. suis T15 (T15_1305) and used blastn against a custom blast database containing the collection and published complete genomes (Supplementary Data 1) to score presence and absence. A core genome of the collection was produced by extracting the protein sequences from S. suis strain P1/7 and using these as a tblastn query against a custom blast database containing the collection and published complete genomes (Supplementary Data 1). All sequences that showed a minimum of 80% amino acid identity across 80% of the length of the protein to the S. suis P1/7 (Genbank ID: AM946016) and which were present in every isolate were included in the core genome. A neighbour-joining tree of S. suis was made from this core genome using a pairwise distance matrix created with the ‘K80’ model (32) of sequence evolution in the package ape in R (33). Bayesian populations (BAPS groups) were taken from Weinert et al. (9). The neighbour joining tree with associated meta data presented in Figure 3 was created using the interactive Tree of Life (34).
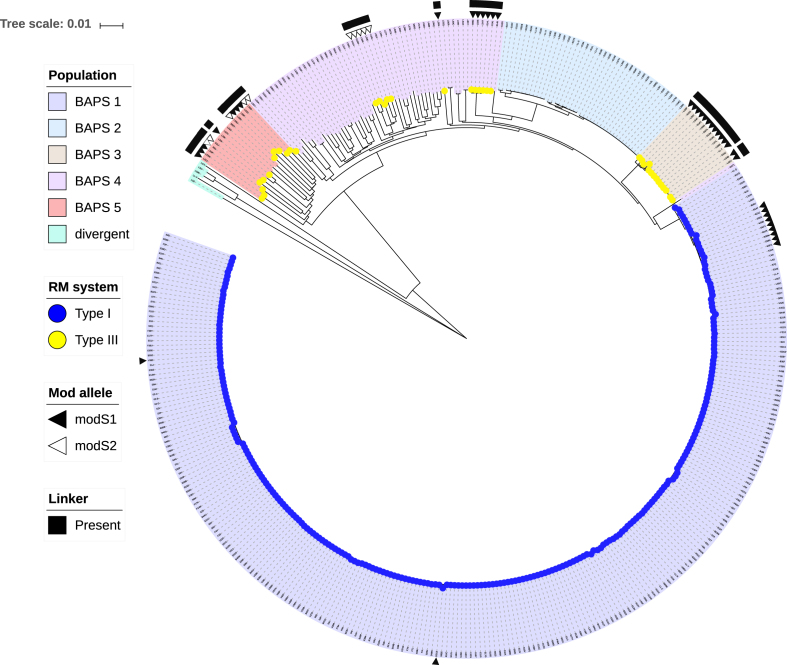
Figure 3.
The distribution of the phase-variable Type I and Type III methyltransferases in Streptococcus suis. A neighbour joining tree showing the genetic distance between isolates based on all sites in a core genome of S. suis taken from Weinert et al. (9). BAPS are represented by coloured clades and the presence of the phase-variable Type I methyltransferase (blue) and the presence of the phase-variable Type III methyltransferase (yellow) are indicated by coloured tips. The Type I methyltransferase is restricted to BAPS population 1, which has been shown to be a virulent zoonotic population (9). The Type III methyltransferase (modS) is more interspersed, being found in three different populations, with two populations containing a mixture of the modS1 (filled triangle) and modS2 (empty triangle) alleles (shown as the second column). The Type III methyltransferase with modS1 allele is also found within population 1 but all isolates lack the 119 bp ‘linker’ sequence (presence shown as filled square in the first column). The scale bar indicates the genetic distance (number of substitutions per site).
RESULTS
Distribution and variation in the phase-variable methyltransferases of S. suis
We examined a large and diverse collection of S. suis genome sequences that has been described previously (n = 377) (9). We also examined all those strains in Genbank with annotated genomes (n = 16; therefore total number of strains = 393). This analysis examined the presence of both phase-variable Type I and Type III methyltransferases within S. suis genomes. Of these 393 S. suis strains, 262 strains contained a phase-variable Type I system, characterized by duplicate, variable hsdS loci containing IRs (see Figure 1A) (35) and 41 strains contained a phase-variable Type III mod gene, characterized by locus located SSRs (see Figure 2A) (20). Ninety strains contained neither a Type I nor a Type III phase-variable methyltransferase. All strain data is presented in Supplementary Data 1.
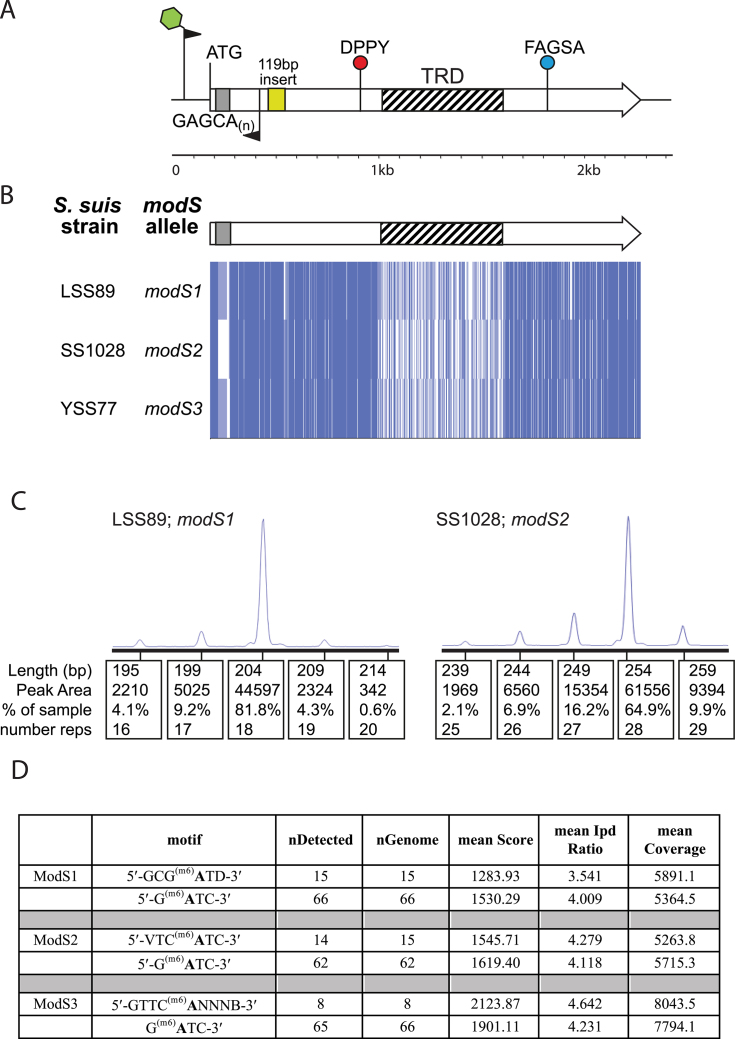
Figure 2.
Streptococcus suis contains three allelic variants of the new phase-variable Type III modS gene. (A) Illustration of the Type III mod gene, modS, present in multiple strains of S. suis, showing the location of key features; DPPY—catalytic motif; FXGXG—AdoMet substrate binding motif; repeat tract at 5′ end of gene shown by a grey box and GAGCA(n); 119 bp ‘insert’ sequence shown by yellow box; location of FAM-labelled primer pair used to carry out fragment length analysis (results in panel C) shown, with FAM label illustrated as green star on forward primer; (B) alignment of the three modS alleles identified in this study, alleles modS1, modS2 and modS3, from S. suis strains LSS89, SS1028 and YS77, respectively. Alignments were carried out using ClustalW, and visualized in JalView overview feature; (C) Fragment length analysis PCR and quantification of fragment sizes using a Gene Analyser (Life Technologies) and quantified used Peak Scanner software. Percentage of each peak area indicates the percentage of the total bacterial population containing the number of repeats indicated by that particular peak size (bp); and (D) SMRT sequencing and methylome analysis results of plasmid vectors from Escherichia coli BL21 strains over-expressing one of the three ModS allelic variants, demonstrating the methyltransferase specificity for ModS1 (5′-GCG(m6)ATD-3′), ModS2 (5′-VTC(m6)ATC-3′) and ModS3 (5′-GTTC(m6)ANNNB-3′). Dam methylation (5′-G(m6)ATC-3′) from the same vector is shown underneath the respective ModS data in the table. nDetected = number of sites called as methylated in each plasmid vector; nGenome = number of sites present in each plasmid vector; IPD = interpulse duration. Only motifs that had an IPD > 3.5 are determined to be significant as methylated. Nucleotide codes: N = any nucleotide; B = G, C or T; D = A, G or T; V = A, G or C.
Most S. suis strains contain a Type I methyltransferase with duplicated hsdS specificity loci
Type I R-M systems have three subunits, containing restriction (HsdR), methyltransferase (HsdM) and specificity (HsdS) components (Figure 1A), with the HsdS subunits dictating where the restriction enzymes methylate and cleave DNA (36). Each complete HsdS protein is made up of two half-target recognition domains (TRDs), each contributing half to the overall specificity. In our previous studies with S. pneumoniae (26), we demonstrated that duplicated, inverted silent hsdS loci provide a repository for alternate specificity proteins that can be generated by recombination with the expressed hsdS locus. Upon examination of Type I R-M systems in S. suis, we observed that many contained a Type I R-M system with duplicated hsdS loci (Figure 1), and that the sequence of the expressed hsdS gene, located immediately downstream of the hsdM and hsdR components, showed a variability in sequence when different strains of S. suis were compared. Examination of these loci showed the presence of IRs that would allow homologous recombination to occur, resulting in shuffling of the TRDs between the expressed hsdS locus and the downstream silent hsdS′ (Figure 1A). Analysis of these hsdS sequences showed that there were two variable 5′-TRD sequences, and two variable 3′-TRD sequences, meaning four unique hsdS genes can be produced, which we have named hsdS alleles A-D (Figure 1A; allele A contains 5′-TRD-1 and 3′-TRD-1; allele B contains 5′-TRD-1 and 3′-TRD-2; allele C contains 5′-TRD-2 and 3′-TRD-2; and allele D contains 5′-TRD-2 and 3′-TRD-1). Upon examination of the sequence of the 262 strains containing a Type I locus with duplicated hsdS genes, we discovered that the majority (260/262) of Type I alleles present in the expressed hsdS locus of the Type I system consist of 5′-TRD-1 and 3′-TRD-2, which we have designated allele B. Only two strains contain allele A, consisting of 5′-TRD-1 and 3′-TRD-1. No strains contained allele C (5′-TRD-2 and 3′-TRD-2) or allele D (5′-TRD-2 and 3′-TRD-1) in the expressed hsdS locus immediately downstream of the hsdM gene. However, this apparent lack of either allele C or allele D in the expressed hsdS locus of S. suis genomes could be a result of the genome annotation process – minority alleles do exist in a population but this locus will only be annotated as the majority allele in the final sequence annotation. In order to investigate if these duplicate, ‘tail-to-tail’ hsdS genes could undergo homologous recombination to produce variable hsdS sequences in the expressed locus immediately downstream of the hsdM gene, even in the absence of a locus-associated recombinase, we designed a fluorescent PCR reaction coupled to a restriction digest in order to type and quantify the alleles present within a population of S. suis (Figure 1B–E). Using S. suis strain S735 (Type I locus YYK_06100- YYK_06115; Table 1), this assay shows that the majority of the population (86.27%) of this strain contains hsdS allele B (5′-TRD-1 and 3′-TRD-2) in the expressed hsdS locus (Figure 1D and E), confirming the annotated genome sequence (accession number CP003736.1). However, 4.12% of the population contains hsdS allele A (5′-TRD-1 and 3′-TRD-1), 5.14% of the population hsdS allele C (5′-TRD-2 and 3′-TRD-2) (Figure 1D and E), and 4.46% of the population hsdS allele D (5′-TRD-2 and 3′-TRD-1) (Figure 1D and E). To demonstrate that all four of these encoded hsdS alleles are expressed, we carried out semi-quantitative RT-PCR to demonstrate the presence of RNA encoding all four alleles within a population of S. suis. Using RNA prepared from strain S735, we show that the majority of the population express RNA encoding allele B (Supplementary Figure S1), and that there is also the presence of RNA encoding each of the three minor variants (alleles A, C and D) which we show are present in the genomic DNA isolated from strain S735 grown under the same conditions (Figure 1 and Supplementary Figure S1). This demonstrates that these encoded minor variants are expressed within a population. We have also carried out analysis on cells grown in liquid media (BHI broth) and observe similar allele distributions as those shown in Figure 1 (data not shown).
Our examination of genome sequences of S. suis also demonstrated the presence of two additional Type I R-M loci (YYK_03085-YYK_03100 and YYK_07615-YYK_07625 in strain S735). Previous in depth analysis of Type I R-M systems in S. suis has shown that homologues of these loci are distributed across the majority of S. suis strains (37). This work also demonstrated the presence of the phase-variable Type I R-M system studied here, but did not show phase-variation within the population of an individual strain as we have.
Multiple alleles of a phase-variable Type III methyltransferase are present in S. suis
Examination of the 41 strains that contained a phase-variable Type III mod gene showed the presence of two unique coding sequences, encoding two distinct Type III methyltransferase enzymes. Sequence alignment of these genes demonstrated that they are highly conserved in their 5′ and 3′ regions (>95% nucleotide identity), with high diversity observed in the central TRD (<25% nucleotide identity), which dictates the DNA sequence to be methylated (38) (Figure 2). Therefore, these two genes are allelic variants of the same gene, rather than two separate genes. Of the 41 genes containing a Type III mod gene with 5′-GAGCA(n) repeats, 29 contain an identical gene to the one we identified in a single strain of S. suis, strain T15, in our systematic study of all SSR-containing Type III mod genes in REBASE (39). We chose strain LSS89 as our prototype strain of S. suis containing this allele. The remaining 12 strains contained a different allele of the same gene (Figure 2B). We chose strain SS1028 as our prototype strain of S. suis containing this allele. In order to examine if any other new alleles of this newly identified phase-variable methyltransferase were present in S. suis, but not captured in our strain collection, we performed a BLAST search of all whole genome shotgun contigs present in Genbank. This analysis identified a third allele, present in a single S. suis isolate, strain YS77 (Figure 2B), isolated in China. In order to demonstrate that phase-variation of the GAGCA(n) repeat tract occurs in these genes, leading to variable expression of the mod gene, we performed FAM-labelled PCR coupled to fragment length analysis, over the repeat tract of the alleles identified in strains LSS89 and SS1028, as we have used previously to characterize and quantify expression of phase-variable genes (18,23). This analysis demonstrated both a difference in repeat numbers present in these genes (Figure 2C; major peaks of 18 repeats in LSS89, 28 repeats in SS1028), and minor populations of each strain containing variable numbers of repeats around the major peak size, highly indicative of ON-OFF switching within the population of each individual strain (Figure 2C). We also observe a high variability in GAGCA(n) repeat tract length between each of the 41 Type III mod genes in our S. suis strain collection; this is also an excellent indication that phase-variable expression of this gene is occurring (Supplementary Data 1), as this phenomenon is seen in every other example of a Type III mod gene that has been demonstrated to be phase-variable (20,39).
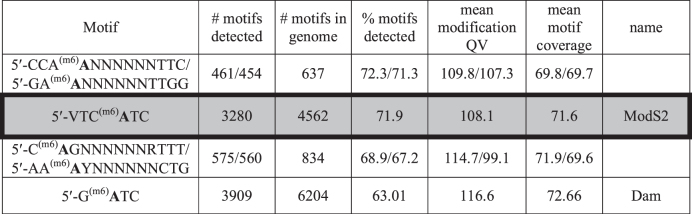
In line with our previous studies (23,25,27,40) we hypothesized that the three identified mod alleles in S. suis methylate different DNA target sequences, and therefore control different phasevarions, as they all encode for different TRDs. Single-molecule, real-time (SMRT) sequencing and methylome analysis using plasmid DNA isolated from E. coli BL21 over-expressing each allelic variant demonstrated that these three alleles all methylate different DNA target sequences (Figure 2D). The allele from strain LSS89, identical to that initially identified in strain T15, methylates the sequence 5′-GCG(m6)ATD-3′ (Figure 2D). The second allele identified in our S. suis strain collection (from strain SS1028) methylates the sequence 5′-VTC(m6)ATC-3′ (Figure 2D). The third allele, from strain YS77, methylates the sequence 5′-GTTC(m6)ANNNB-3′ (Figure 2D). Dam methyltransferase activity (5′-G(m6)ATC-3′) occurs in all three vectors, as would be expected in DNA isolated from E. coli (Figure 2D). We could detect only Dam methylation in our non-methylating control (not shown). In order to align naming conventions with current phase-variable Type III methyltransferases, we propose naming these alleles modS1 (LSS89), modS2 (SS1028) and modS3 (YS77) (39), with systematic names based on REBASE conventions (41) of S.Ssu89I (ModS1), S.Ssu1028I (ModS2) and S.Ssu77I (ModS3). We also carried out PacBio SMRT sequencing and methylome analysis using genomic DNA prepared from S. suis strain LSS66 containing allele modS2. Our analysis of the genome sequence generated previously (9) showed that this strain contained GAGCA[6] in the modS2 ORF, which would lead to the gene being in frame (ON). This low number of repeats is highly stable, with low rates of phase-variation predicted based on previous analyses of SSR length and phase-variation rate (42–44). modS2 was the only annotated Type III mod gene present in the genome of strain LSS66, so we predicted that any Type III methyltransferase signatures detected could be attributed to ModS2 methylation. Our methylome analysis detected a single Type III signature, 5′-VTC(m6)ATC-3′ (Table 3), identical to that identified above using plasmid DNA isolated from a strain over-expressing ModS2 from a different strain of S. suis, SS1028 (Figure 2D). This not only confirmed the sequence recognized and methylated by ModS2, but also showed that the same allele has the same specificity in different strains of S. suis, and therefore is highly likely to regulate the same phasevarion. The LSS66 methylome demonstrated the presence of two Type I motifs in this strain (Table 3), which we predict to be the sequence specificities of the two non-phase-variable Type I loci previously reported to be present in all strains of S. suis (37), but we cannot confidently assign these signatures to particular methyltransferases without significant additional analysis, which is outside the scope of the current work. An examination of REBASE (41) shows that these two Type I motifs have not previously been reported. All methylome data from strain LSS66 is presented in Table 3.
Table 3.
PacBio SMRT sequencing and methylome data from S. suis strain LSS66
|
An interesting finding from the analysis of our S. suis strain collection was that strains containing a Type I locus with duplicated hsdS genes also contained a GAGCA(n) immediately upstream of a putative Type III mod gene. However, closer examination of this region showed that a functional Type III mod gene containing SSR’s only occurred if a unique 119 bp sequence was present between the SSRs and the putative mod gene (shown schematically in Figure 2A), linking the two regions into a single, continuous open reading frame. Where this 119 bp ‘linker’ sequence was present, resulting in a phase-variable mod gene, those strains did not contain a Type I locus with duplicated hsdS genes. In those strains containing duplicated hsdS genes, and by implication a phase-variable Type I methyltransferase, GAGCA(n) repeats were present, sometimes in very high numbers (Supplementary Data 1), but the 119 bp ‘linker’ sequence was not (filled square in Figure 3). Therefore, it appears that a single strain of S. suis cannot contain both phase-variable Type I and Type III methyltransferases (yellow and blue coloured tips in Figure 3).
A clear segregation exists between phase-variable methyltransferases and population structure in S. suis
Our previous studies with S. suis defined five genetic lineages (BAPS) based on a Bayesian clustering method (9) as well as an additional ‘divergent’ lineage of S. suis (Baig et al. (15)). Upon examination of BAPS group (9) and the presence of each of the distinct types of methyltransferase, a clear segregation was evident (Figure 3). The phase-variable Type I system was only present in BAPS group 1, with the majority of these being systemic isolates (Table 4), and serotype 2, and containing all the S. suis isolates from humans (Supplementary Data 1). All members of this virulent zoonotic group contained the Type I system indicating it was acquired at the origin of the BAPS group 1 lineage. Phase-variable Type III mod genes were found in variable numbers of isolates from BAPS group 3, 4 and 5, and within a variety of serotypes, suggesting that it has been independently acquired and/or lost multiple times. In contrast to the Type I system, Table 4 shows that the Type III system is under-represented in systemic isolates but over-represented in respiratory and non-clinical isolates, although further work is needed to verify if this association is robust or a reflection of sampling bias (the most obvious bias being the oversampling of isolates from the virulent zoonotic population BAPS group 1). The majority of the isolates containing a phase-variable Type III mod gene are from strains isolated in the UK (39/41; 95%). Only strains T15 (Netherlands) and R61 (China) were not isolated in the UK. Interestingly, all strains from BAPS group 2 contained neither phase-variable methyltransferase, with a large proportion of BAPS group 4 (44/57; 77.2%) also containing neither system (Figure 3 and Supplementary Data 1). In summary, we predict that the phase-variable Type I methyltransferase arose once at the origin of the virulent zoonotic population of S. suis (BAPS group 1), but that the phase-variable Type III methyltransferase has either been independently acquired at least three times in different populations, or alternatively been lost multiple times from isolates of S. suis.
Table 4.
The association between phase-variable Type I and Type III methyltransferases and the clinical phenotype of S. suis isolates
| Clinical phenotype | |||||
|---|---|---|---|---|---|
| Systemic | Respiratory | Non-clinical | Not known | Total | |
| Phase-variable Type I | 208 | 8 | 34 | 12 | 262 |
| Phase-variable Type III | 3 | 10 | 20 | 8 | 41 |
| neither | 13 | 23 | 41 | 13 | 90 |
| All isolates | 224 | 41 | 95 | 33 | 393 |
DISCUSSION
Phase variation of single genes by bacterial pathogens is well studied, and is typically associated with surface factors (11,12,14,18,19). When a bacterial strain contains several individual phase-variable genes, the population as a whole will contain a broad and diverse array of phenotypes (10). The discovery of phasevarions in a diverse array of bacterial species further compounds the problem of phenotypic diversity from a vaccine and therapeutic development point of view. Whilst it is easy to identify individual phase-variable genes by in silico analysis due to the genetic features associated with them (SSRs, IRs), and thus exclude them as stably expressed antigens, the identification of members of a phasevarion can only be accomplished by studying the strains that contain phase-variable methyltransferases (21,45). The first major steps in characterizing a new phasevarion is to prove phase variation occurs then to identify the target sites of each phase-variable methyltransferase, in order to identify regions that are methylated and to correlate this with an expression profile analysis of strains containing each distinct methyltransferase state: ON or OFF for phase-variable Type III methyltransferases, or each distinct HsdS expression state for phase-variable Type I methyltransferases. We have demonstrated that there are different allelic variants of hsdS genes present in different strains of the expressed locus of this system, and that variable SSR tract lengths exist in different strains containing a phase-variable Type III mod gene. To confirm that phase-variation occurs within a bacterial population, we demonstrated that switching of hsdS genes occurs within S. suis strain S735, by showing that in addition to the major expressed hsdS allele (allele B), the three minor variants (alleles A, C and D) could all be detected using our bespoke FAM-labelled PCR assay (Figure 1). For the phase-variable Type III methyltransferase, we demonstrate that variable tract lengths exist in this locus using prototype strains containing the two most common allelic variants of what we have termed modS, modS1 (strain LSS89) and modS2 (strain SS1028) (Figure 2C). Phase-variation by slipped-strand mis-pairing over SSRs appears to be much more prevalent in Gram-negative bacteria than in Gram-positives (46), although there are several examples of phase- and antigenic-variation in Gram-positive organisms (13,15,46–48). Our demonstration of the presence and functionality of a phase-variable Type III methyltransferase in S. suis is the first example of SSRs resulting in phase-variable expression of a methyltransferase in a Gram-positive organism.
The advent of PacBio SMRT sequencing technology coupled to methylome analysis has revolutionized the identification of methyltransferase specificity, and allows the analysis of the complete methylome of bacterial strains, whilst simultaneously allowing precise identification and methylation state characterization of each target site in the genome. This has allowed us to identify the methylation specificities from all three modS allelic variants present in S. suis (Figure 2D). The specificities of the four Type I hsdS alleles are being determined in ongoing studies addressing gene expression profile analysis of S. suis strain S735 expressing these alleles.
Our demonstration of a distinct lineage-specific segregation of phase-variable methyltransferases in S. suis is the first description of its type. The segregation we see with the phase-variable Type I and Type III methyltransferases is reminiscent of the mutually exclusive expression of the adhesins HMW and Hia in non-typeable Haemophilus influenzae, where it is proposed that each adhesin was acquired in separate events followed by divergence (49). In the case of phase-variable methyltransferases in S. suis, it is possible that too much phase-variable methylation, and consequent variation in gene expression levels is deleterious—a balance must be achieved between adaptability and amount of variability generated by epigenetic gene regulation. Here we present the first characterized example of individual bacterial strains of any species having both phase-variable Type I and Type III methyltransferases, and thereby multiple phasevarions. There have been previous reports of strains with multiple phase-variable Type III methyltrasferases in certain bacterial species: for example, Neisseria meningitidis can encode modA, modB and modD (50), and strains of Helicobacter pylori can also encode three different phase-variable Type III mod genes (27,39). The presence of phase-variable Type III methyltransferases has been systematically analysed in large genome collections (39), but there has been no equivalent systematic search for phase-variable Type I R-M systems. Thus, frequency of the presence of multiple phase-variable Type I and Type III methyltransferases in bacterial genomes remains to be determined. The exact individual events that have contributed to strains of S. suis containing only either a Type I or a Type III phase-variable methyltransferase are therefore still open to investigation since the generation of a reliable phylogenetic tree of the S. suis core genome is impossible given the evidence of widespread recombination (Weinert et al. (9)). Nevertheless, it is tempting to speculate a role for the phase-variable Type I methyltransferase in the disease phenotype of S. suis given the likely acquisition of the Type I system at the origin of the globalized highly virulent zoonotic lineage in the 1920s (Weinert et al. (9)). However, an alternative scenario is that different genetic elements are responsible for the disease phenotype and that the Type I system is in syntenic linkage with these elements. The phase-variable Type III methyltransferase shows a stronger association with respiratory and non-clinical isolates suggesting a possible role for colonization (Table 4) but again, phylogenetic non-independence complicates this finding. Further sampling of S. suis for presence/absence of phase-variable Type I and the Type III methyltransferases may give us the necessary statistical power to see an association that remains robust after controlling for S. suis relatedness. As we always see the presence of GAGCA(n) repeats in the genomes of our S. suis strain collection, but these are not always linked to the downstream mod gene due to the apparent requirement of the observed 119 bp ‘linker’ sequence we observed in the 41 strains containing a phase-variable Type III mod gene, it may be that acquisition of a phase-variable Type I locus leads to subsequent inactivation of the Type III mod gene by loss of the 119 bp ‘linker’ required for phase-variation of the Type III locus, but this would require subsequent investigation to confirm.
Our demonstration of the presence of several unique phase-variable methyltransferases in S. suis leads to the possibility of multiple phasevarions in this species. The presence of phasevarions complicates the development of vaccines and therapeutics, as the stably expressed protein profile of S. suis can only be defined by dissecting which genes are controlled by phasevarions in this species.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the Yale Centre for Genome Analysis (YCGA), and Karen Oliver at the Sanger Institute, for carrying out Pacific Biosciences Single-Molecule, Real Time (SMRT) sequencing and methylome analysis.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Australian Research Council (ARC) Discovery Project [180100976 to J.M.A., P.J.B.]; National Health and Medical Research Council (NHMRC; Australia) Program Grant [1071659 to M.P.J.]; Principal Research Fellowship [1138466 to M.P.J.]; Wellcome Grant [098051 to J.P.]; Dorothy Hodgkin Fellowship funded by the Royal Society [DH140195 to L.A.W.]; Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society [109385/Z/15/Z to L.A.W.]. Funding for open access charge: NHMRC Program grant 1071659.
Conflict of interest statement. None declared.
REFERENCES
- 1. Gottschalk M. Zimmerman J, Karriker L, Rameriz A, Schwartz K, Stevenson G. Diseases of Swine. 2010; 10:Ames, IA: Wiley-Blackwell; 841–855. [Google Scholar]
- 2. Wertheim H.F., Nghia H.D., Taylor W., Schultsz C.. Streptococcus suis: an emerging human pathogen. Clin. Infect. Dis. 2009; 48:617–625. [DOI] [PubMed] [Google Scholar]
- 3. Tang J., Wang C., Feng Y., Yang W., Song H., Chen Z., Yu H., Pan X., Zhou X., Wang H. et al. . Streptococcal toxic shock syndrome caused by Streptococcus suis serotype 2. PLoS Med. 2006; 3:e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goyette-Desjardins G., Auger J.P., Xu J., Segura M., Gottschalk M.. Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerg. Microbes Infect. 2014; 3:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nghia H.D., Tu le T.P., Wolbers M., Thai C.Q., Hoang N.V., Nga T.V., Thao le T.P., Phu N.H., Chau T.T., Sinh D.X. et al. . Risk factors of Streptococcus suis infection in Vietnam. A case-control study. PLoS One. 2011; 6:e17604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vilaichone R.K., Vilaichone W., Nunthapisud P., Wilde H.. Streptococcus suis infection in Thailand. J. Med. Assoc. Thai. 2002; 85(Suppl. 1):S109–S117. [PubMed] [Google Scholar]
- 7. Arends J.P., Zanen H.C.. Meningitis caused by Streptococcus suis in humans. Rev. Infect. Dis. 1988; 10:131–137. [DOI] [PubMed] [Google Scholar]
- 8. Nghia H.D., Hoa N.T., Linh le D., Campbell J., Diep T.S., Chau N.V., Mai N.T., Hien T.T., Spratt B., Farrar J. et al. . Human case of Streptococcus suis serotype 16 infection. Emerg. Infect. Dis. 2008; 14:155–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weinert L.A., Chaudhuri R.R., Wang J., Peters S.E., Corander J., Jombart T., Baig A., Howell K.J., Vehkala M., Valimaki N. et al. . Genomic signatures of human and animal disease in the zoonotic pathogen Streptococcus suis. Nat. Commun. 2015; 6:6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moxon R., Bayliss C., Hood D.. Bacterial contingency loci: the role of simple sequence DNA repeats in bacterial adaptation. Ann. Rev. Genet. 2006; 40:307–333. [DOI] [PubMed] [Google Scholar]
- 11. Atack J.M., Winter L.E., Jurcisek J.A., Bakaletz L.O., Barenkamp S.J., Jennings M.P.. Selection and counter-selection of Hia expression reveals a key role for phase-variable expression of this adhesin in infection caused by non-typeable Haemophilus influenzae. J. Infect. Dis. 2015; 212:645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Åberg A., Gideonsson P., Vallström A., Olofsson A., Öhman C., Rakhimova L., Borén T., Engstrand L., Brännström K., Arnqvist A.. A repetitive DNA element regulates expression of the Helicobacter pylori sialic acid binding adhesin by a Rheostat-like mechanism. PLoS Pathog. 2014; 10:e1004234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ziebuhr W., Heilmann C., Gotz F., Meyer P., Wilms K., Straube E., Hacker J.. Detection of the intercellular adhesion gene cluster (ica) and phase variation in Staphylococcus epidermidis blood culture strains and mucosal isolates. Infect. Immun. 1997; 65:890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blyn L.B., Braaten B.A., Low D.A.. Regulation of pap pilin phase variation by a mechanism involving differential dam methylation states. EMBO J. 1990; 9:4045–4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Simon M., Zieg J., Silverman M., Mandel G., Doolittle R.. Phase variation: evolution of a controlling element. Science. 1980; 209:1370–1374. [DOI] [PubMed] [Google Scholar]
- 16. Ren Z., Jin H., Whitby P.W., Morton D.J., Stull T.L.. Role of CCAA nucleotide repeats in regulation of hemoglobin and hemoglobin-haptoglobin binding protein genes of Haemophilus influenzae. J. Bacteriol. 1999; 181:5865–5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Richardson A.R., Stojiljkovic I.. HmbR, a hemoglobin-binding outer membrane protein of Neisseria meningitidis, undergoes phase variation. J. Bacteriol. 1999; 181:2067–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fox K.L., Atack J.M., Srikhanta Y.N., Eckert A., Novotny L.A., Bakaletz L.O., Jennings M.P.. Selection for phase variation of LOS biosynthetic genes frequently occurs in progression of non-typeable Haemophilus influenzae infection from the nasopharynx to the middle ear of human patients. PLoS One. 2014; 9:e90505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poole J., Foster E., Chaloner K., Hunt J., Jennings M.P., Bair T., Knudtson K., Christensen E., Munson R.S. Jr, Winokur P.L. et al. . Analysis of nontypeable Haemophilus influenzae phase variable genes during experimental human nasopharyngeal colonization. J. Infect. Dis. 2013; 208:720–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Atack J.M., Tan A., Bakaletz L.O., Jennings M.P., Seib K.L.. Phasevarions of bacterial pathogens: methylomics sheds new light on old enemies. Trends Microbiol. 2018; 26:715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tan A., Atack J.M., Jennings M.P., Seib K.L.. The capricious nature of bacterial pathogens: phasevarions and vaccine development. Front. Immunol. 2016; 7:586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Srikhanta Y.N., Maguire T.L., Stacey K.J., Grimmond S.M., Jennings M.P.. The phasevarion: a genetic system controlling coordinated, random switching of expression of multiple genes. Proc. Natl. Acad. Sci. U.S.A. 2005; 102:5547–5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Atack J.M., Srikhanta Y.N., Fox K.L., Jurcisek J.A., Brockman K.L., Clark T.A., Boitano M., Power P.M., Jen F.E.C., McEwan A.G. et al. . A biphasic epigenetic switch controls immunoevasion, virulence and niche adaptation in non-typeable Haemophilus influenzae. Nat. Commun. 2015; 6:7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blakeway L.V., Power P.M., Jen F.E., Worboys S.R., Boitano M., Clark T.A., Korlach J., Bakaletz L.O., Jennings M.P., Peak I.R. et al. . ModM DNA methyltransferase methylome analysis reveals a potential role for Moraxella catarrhalis phasevarions in otitis media. FASEB J. 2014; 28:5197–5207. [DOI] [PubMed] [Google Scholar]
- 25. Srikhanta Y.N., Dowideit S.J., Edwards J.L., Falsetta M.L., Wu H.-J., Harrison O.B., Fox K.L., Seib K.L., Maguire T.L., Wang A.H.J. et al. . Phasevarions mediate random switching of gene expression in pathogenic Neisseria. PLoS Pathog. 2009; 5:e1000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Manso A.S., Chai M.H., Atack J.M., Furi L., De Ste Croix M., Haigh R., Trappetti C., Ogunniyi A.D., Shewell L.K., Boitano M. et al. . A random six-phase switch regulates pneumococcal virulence via global epigenetic changes. Nat. Commun. 2014; 5:5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Srikhanta Y.N., Gorrell R.J., Steen J.A., Gawthorne J.A., Kwok T., Grimmond S.M., Robins-Browne R.M., Jennings M.P.. Phasevarion mediated epigenetic gene regulation in Helicobacter pylori. PLoS One. 2011; 6:e27569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sambrook J., Fritsch E.F., Maniatis T.. Molecular Cloning: A Laboratory Manual. 1989; 2nd ednNY: Cold Spring Harbour Laboratory Press. [Google Scholar]
- 29. Clark T.A., Murray I.A., Morgan R.D., Kislyuk A.O., Spittle K.E., Boitano M., Fomenkov A., Roberts R.J., Korlach J.. Characterization of DNA methyltransferase specificities using single-molecule, real-time DNA sequencing. Nucleic Acids Res. 2012; 40:e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murray I.A., Clark T.A., Morgan R.D., Boitano M., Anton B.P., Luong K., Fomenkov A., Turner S.W., Korlach J., Roberts R.J.. The methylomes of six bacteria. Nucleic Acids Res. 2012; 40:11450–11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baig A., Weinert L.A., Peters S.E., Howell K.J., Chaudhuri R.R., Wang J., Holden M.T.G., Parkhill J., Langford P.R., Rycroft A.N. et al. . Whole genome investigation of a divergent clade of the pathogen Streptococcus suis. Front. Microbiol. 2015; 6:1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980; 16:111–120. [DOI] [PubMed] [Google Scholar]
- 33. Paradis E., Claude J., Strimmer K.. APE: analyses of phylogenetics and evolution in R language. Bioinformatics. 2004; 20:289–290. [DOI] [PubMed] [Google Scholar]
- 34. Letunic I., Bork P.. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016; 44:W242–W245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. De Ste Croix M., Vacca I., Kwun M.J., Ralph J.D., Bentley S.D., Haigh R., Croucher N.J., Oggioni M.R.. Phase-variable methylation and epigenetic regulation by type I restriction–modification systems. FEMS Microbiol. Rev. 2017; 41:S3–S15. [DOI] [PubMed] [Google Scholar]
- 36. Loenen W.A.M., Dryden D.T.F., Raleigh E.A., Wilson G.G.. Type I restriction enzymes and their relatives. Nucleic Acids Res. 2014; 42:20–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Willemse N., Schultsz C.. Distribution of Type I restriction–modification systems in Streptococcus suis: an outlook. Pathogens. 2016; 5:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rao D.N., Dryden D.T., Bheemanaik S.. Type III restriction-modification enzymes: a historical perspective. Nucleic Acids Res. 2014; 42:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Atack J.M., Yang Y., Seib K.L., Zhou Y., Jennings M.P.. A survey of Type III restriction-modification systems reveals numerous, novel epigenetic regulators controlling phase-variable regulons; phasevarions. Nucleic Acids Res. 2018; 46:3532–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Seib K.L., Jen F.E., Tan A., Scott A.L., Kumar R., Power P.M., Chen L.T., Wu H.J., Wang A.H., Hill D.M. et al. . Specificity of the ModA11, ModA12 and ModD1 epigenetic regulator N6-adenine DNA methyltransferases of Neisseria meningitidis. Nucleic Acids Res. 2015; 43:4150–4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roberts R.J., Vincze T., Posfai J., Macelis D.. REBASE-a database for DNA restriction and modification: enzymes, genes and genomes. Nucleic Acids Res. 2015; 43:D298–D299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bayliss C.D., Bidmos F.A., Anjum A., Manchev V.T., Richards R.L., Grossier J.-P., Wooldridge K.G., Ketley J.M., Barrow P.A., Jones M.A. et al. . Phase variable genes of Campylobacter jejuni exhibit high mutation rates and specific mutational patterns but mutability is not the major determinant of population structure during host colonization. Nucleic Acids Res. 2012; 40:5876–5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cox E.C. Bacterial mutator genes and the control of spontaneous mutation. Annu. Rev. Genet. 1976; 10:135–156. [DOI] [PubMed] [Google Scholar]
- 44. Farabaugh P.J., Schmeissner U., Hofer M., Miller J.H.. Genetic studies of the lac repressor. J. Mol. Biol. 1978; 126:847–863. [DOI] [PubMed] [Google Scholar]
- 45. Srikhanta Y.N., Fox K.L., Jennings M.P.. The phasevarion: phase variation of type III DNA methyltransferases controls coordinated switching in multiple genes. Nat. Rev. Micro. 2010; 8:196–206. [DOI] [PubMed] [Google Scholar]
- 46. van der Woude M.W., Baumler A.J.. Phase and antigenic variation in bacteria. Clin. Microbiol. Rev. 2004; 17:581–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pericone C.D., Bae D., Shchepetov M., McCool T., Weiser J.N.. Short-sequence tandem and nontandem DNA repeats and endogenous hydrogen peroxide production contribute to genetic instability of Streptococcus pneumoniae. J. Bacteriol. 2002; 184:4392–4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rasmussen M., Bjorck L.. Unique regulation of SclB—a novel collagen-like surface protein of Streptococcus pyogenes. Mol. Microbiol. 2001; 40:1427–1438. [DOI] [PubMed] [Google Scholar]
- 49. St Geme J.W. 3rd, Kumar V.V., Cutter D., Barenkamp S.J.. Prevalence and distribution of the hmw and hia genes and the HMW and Hia adhesins among genetically diverse strains of nontypeable Haemophilus influenzae. Infect. Immun. 1998; 66:364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tan A., Hill D.M., Harrison O.B., Srikhanta Y.N., Jennings M.P., Maiden M.C., Seib K.L.. Distribution of the type III DNA methyltransferases modA, modB and modD among Neisseria meningitidis genotypes: implications for gene regulation and virulence. Sci. Rep. 2016; 6:21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Feng L., Zhu J., Chang H., Gao X., Gao C., Wei X., Yuan F., Bei W.. The CodY regulator is essential for virulence in Streptococcus suis serotype 2. Sci. Rep. 2016; 6:21241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.