Abstract
Alzheimer’s disease (AD) is the most common neurodegenerative disease and is the leading form of dementia. AD entails chronic inflammation, impaired synaptic integrity and reduced neurogenesis. The clinical and molecular onsets of the disease do not temporally overlap and the initiation phase of the cellular changes might start with a complex causativeness between chronic inflammation, reduced neural stem cell plasticity and neurogenesis. Although the immune and neuronal aspects in AD are well studied, the neural stem cell-related features are far less investigated. An intriguing question is, therefore, whether a stem cell can ever be made proliferative and neurogenic during the prevalent AD in the brain. Recent findings affirm this hypothesis and thus a plausible way to circumvent the AD phenotypes could be to mobilize the endogenous stem cells by enhancing their proliferative and neurogenic capacity as well as to provide the newborn neurons the potential to survive and integrate into the existing circuitry. To address these questions, zebrafish offers unprecedented information and tools, which can be effectively translated into mammalian experimental systems.
Keywords: zebrafish, Alzheimer’s disease, neural stem/progenitor cells, regeneration, neurogenesis
The Re-Rise of Stem Cell Aspect for Neurodegenerative Diseases
Stem cells are the main reservoir for production of new cells. Understanding the basic biology of how stem cells are specified, maintained and regulated has been an exciting focus of research for many decades. Yet, there are still missing pieces especially on how stem cells could be utilized for neurodegenerative diseases (Jebelli et al., 2015; Tincer et al., 2016; Wyss-Coray, 2016). Stem cells offer great promises for medicine, as they can be the golden way to a “regenerative therapy” (Doetsch and Scharff, 2001; Lopez-Toledano and Shelanski, 2004; Rodriguez and Verkhratsky, 2011; Kizil et al., 2012b; Gage and Temple, 2013; Hong et al., 2014; Lilja et al., 2015). By using the endogenous stem cells, tissue loss could be reverted or the integrity of the existing tissues could be enhanced. Such outcomes would have ramifications in several human diseases but possibly among the most interesting is neurodegeneration (Tincer et al., 2016; Wyss-Coray, 2016). Indeed, since the generic term of “neurodegeneration” denotes the state of losing cells of the nervous system and in particular the neurons, transplantation of stem cells into the brain to get more neurons produced from these stem cells were one of the first treatment options (Dantuma et al., 2010; Mu and Gage, 2011; Rodriguez and Verkhratsky, 2011; van Tijn et al., 2011; Ager et al., 2015; Lee et al., 2015; Tincer et al., 2016; Wyss-Coray, 2016; Espuny-Camacho et al., 2017), for instance, injection of fetal NSCs for treating Parkinsonism (Baetge, 1993; Dunnett et al., 1997; Svendsen et al., 1997; Brundin and Bjorklund, 1998; Studer et al., 1998). These efforts could not gain spotlight as the transplanted stem cells or progenitors could not survive or could not form the desired cell types. For a couple of decades, the main focus in neurodegenerative diseases has been to prevent the neuronal death and synaptic failure (Carter and Lippa, 2001; Selkoe, 2003; Iqbal et al., 2016; Wyss-Coray, 2016). In Alzheimer’s disease (AD) – where the main culprit of the pathology is accumulation of Amyloid plaques and neurofibrillary tangles that lead to the loss of mostly cholinergic innervations in the brain – preventing the loss of synaptic degeneration and reduction in the neurotransmitter acetylcholine was prioritized as a therapy option (Fischer et al., 1987; Tuszynski et al., 1990; Nagele et al., 2002; Park et al., 2012; Gu et al., 2015). Several current drugs on the market for AD are blockers of the enzyme choline acetyltransferase, which degrades the cholinergic neurotransmitters in the brain. These drugs also failed to cure the disease despite causing meager slowdown in the cognitive decline in Alzheimer’s patients (Schneider et al., 2014). Similarly, physically destroying the plaques causes a cognitive advantage while does not fully restore the disease-associated symptoms (Takeda and Morishita, 2015). All these hypotheses and failures tell us a lesson: Alzheimer’s is not only a neuronal disease but also a complex mixture of malfunctioning in various cell types. An array of different cell types was implicated in the onset and progression of AD (De Strooper and Karran, 2016). These include changes in immune components (Amor et al., 2010; Heneka et al., 2015; Heppner et al., 2015), neurovascular niche (Kirkitadze et al., 2002; De Strooper and Karran, 2016), NSCs (Tong et al., 2015; Tincer et al., 2016), astrocytes (Attems and Jellinger, 2014; Lian and Zheng, 2016), and oligodendrocytes (Bartzokis, 2011; Ettle et al., 2016), suggesting a multifactorial influence on the initiation of AD. It can even be hypothesized that the loss of neurons – which is relatively a late symptom of the disease – might be the consequence of the yet-elusive real cause. When we generate a temporal onset of various symptoms of AD – mostly in animal models – we see that the first changes in the brain are the deterioration of the immune system balance, gliotic response from astrocytes and reduction in neural stem cell proliferation (Aguzzi and Haass, 2003; Selkoe, 2003; Blennow et al., 2006; Harman, 2006; Chai, 2007; Arendt, 2009; Hardy, 2009; Huang and Mucke, 2012; De Strooper and Karran, 2016; Dzamba et al., 2016; Tincer et al., 2016). As Amyloid deposition and neurofibrillary tangles occur, an inflammatory reaction manifests and becomes chronic in time. Concomitant to this reaction, NSCs also reduce their proliferation rate and produce less neurons long before the myelin breakdown, synaptic degeneration and neuronal cell death manifest (Demars et al., 2010; Tincer et al., 2016). Therefore, it is a plausible hypothesis to think that the inflammatory environment is negatively affecting the brain homeostasis in Alzheimer’s conditions not only by eliciting a chronic inflammation that is detrimental for synapses on its own but also by reducing the capacity of the brain to produce more neurons – an ability that could have been utilized to replace the lost neurons. These questions seem to have opened a wide research realm focusing on the role of immune system in AD (Akiyama et al., 2000; Heneka et al., 2005, 2015; Wyss-Coray, 2006; Amor et al., 2010; Aguzzi et al., 2013; Heppner et al., 2015; Kizil et al., 2015). Many reports documenting the effects of inflammation on AD pathology and the role of immune cells on the progression of the disease emerged. It is quite likely that coming years will bring important paradigm shifts in the relationship of immune system and the AD. However, a largely overlooked phenomenon in this context is the NSCs. Can NSCs and neurogenesis be the key to the cure for neurodegeneration? (Ziabreva et al., 2006; Waldau and Shetty, 2008; Taupin, 2009; Dantuma et al., 2010; Rodriguez and Verkhratsky, 2011; Tincer et al., 2016). This is where zebrafish could contribute to the answer of this provocative question.
Zebrafish and the Hope for Stem Cell-Based Regenerative Therapies
No existing model for AD recapitulates the full spectrum of the disease, and existing mouse models are not exceptions (LaFerla and Green, 2012). These models can be considered at best the tools to study the early onset stages of Alzheimer’s (De Strooper and Karran, 2016). Although mouse models provided invaluable information on the pathology of AD, these mammalian models are not ideal to study “regeneration” as they do not have regenerative ability at first place (Goss, 1991). Zebrafish, an animal model that can regenerate its neurons offers unprecedented hope for restoring lost neurons in AD (Kizil et al., 2012b; Cosacak et al., 2015; Tincer et al., 2016; Kizil, 2018).
Mammals fail to regenerate amputated limbs, cardiac tissue, brain or spinal cord due to their restricted and limited regenerative potential (Tanaka and Ferretti, 2009; Poss, 2010). Current studies focus to improve methods or develop novel approach that can induce regenerative programs into the mammalian systems (Antos and Tanaka, 2010; Gemberling et al., 2013; Cosacak et al., 2015). One approach is to induce regeneration by activating endogenous regeneration programs. Zebrafish could serve as a model to understand those molecular cues as many “regeneration” programs were identified in zebrafish and they serve as interesting candidates toward this aim (Raya et al., 2003; Zupanc, 2008; Kizil et al., 2009, 2012a,b,c; Millimaki et al., 2010; Kyritsis et al., 2012; Diotel et al., 2013; Berberoglu et al., 2014; Cosacak et al., 2015; Alunni and Bally-Cuif, 2016; Bhattarai et al., 2016; Katz et al., 2016; Mokalled et al., 2016; Kizil, 2018; Than-Trong et al., 2018). Hence, the remarkable feature of regeneration in zebrafish deserves a closer attention for translational ramifications.
The exciting yet provocative argument of “zebrafish can teach us” could be challenged from another perspective: it could also be argued that the reduced capability of regeneration in rodents makes them better models as they are closer to the human situation. It is surely true that a model, which is as close as possible to human condition, would be ideal to work out reductionist aspects of a disease and indeed the mouse models offered invaluable knowledge on AD pathology. However, regenerating organisms endow a novel perspective of stem cell plasticity and regenerative ability that might be harnessed for therapeutic ramifications in humans but may not be investigated in mammalian systems. If nature has evolved a set of molecular programs that enable regenerative output of NSCs in AD conditions, zebrafish and other regenerating organisms but not mammals could teach us these programs. In the long run, those programs must be tested in mammals to investigate if they are evolutionarily conserved and whether they are sufficient to elicit a stem cell response similar to that of zebrafish. This could be the step where zebrafish could come in handy: identification of naturally occurring “candidate” programs that might underlie a regenerative touch to the old problem of AD. It is also necessary to mention that the nature of regenerative ability and why it is lost evolutionarily in mammals are still unknowns. Therefore, the applicability of the knowledge from zebrafish to humans needs further studies, which will shed more light onto the extent of parallelism between mammals and zebrafish in disease conditions.
Neural Stem Cells and Neuronal Regeneration
Mammalian nervous system contains NSCs that give rise to newborn neurons during development as well as adulthood (Doetsch et al., 1999; Gage, 2000; Conti and Cattaneo, 2010; Gage and Temple, 2013). The ability of NSCs to form neurons however varies and is still controversial (Kronenberg et al., 2003; Galvan and Jin, 2007; Kempermann et al., 2008, 2018; Ernst et al., 2014; Magnusson et al., 2014; Urban and Guillemot, 2015; Magnusson and Frisen, 2016; Boldrini et al., 2018; Sorrells et al., 2018). During development, NSCs give rise to all neuronal subtypes (Gage, 2000; Temple, 2001; Doetsch, 2003; Kriegstein and Alvarez-Buylla, 2009; Hansen et al., 2010; Pacary et al., 2012; Urban and Guillemot, 2015). But, during the adulthood, the NSCs are restrictive and limited to fewer areas – the subventricular zone (SVZ) of the lateral ventricle and the dentate gyrus of the hippocampus (Doetsch and Scharff, 2001; Alvarez-Buylla et al., 2002; Spalding et al., 2013; Kempermann et al., 2018). Though constitutive neurogenesis occurs in these neurogenic regions, upon injury they fail to achieve neuronal repair due to lack of neurogenic inputs (Silver and Miller, 2004; Rolls et al., 2009; Costa et al., 2010). For instance, in case of mammalian traumatic injury model, there is absence of permissive environment for NSCs to react effectively.
Unlike mammals, zebrafish can successfully regenerate the injured part of its brain (Chapouton et al., 2007; Zupanc, 2008; Kroehne et al., 2011; Baumgart et al., 2012; Kishimoto et al., 2012; Kizil et al., 2012a,b,c; Kyritsis et al., 2012; Marz et al., 2012; Barbosa et al., 2015; Cosacak et al., 2015; Bhattarai et al., 2016; Kizil, 2018). This ability is possible because of the stem cell niches and the neurogenic regions that harbors proliferative neural progenitor cells (Adolf et al., 2006; Grandel et al., 2006; Chapouton et al., 2007; Kaslin et al., 2009). However, there is more to it. The regenerative ability after neuronal loss in zebrafish brain relies on the activation of specific molecular mechanisms that do not exist in normal homeostatic state or even during development of those structures (Zupanc, 2008; Kaslin et al., 2009; Fleisch et al., 2010; Kizil et al., 2012b; Cosacak et al., 2015; Alunni and Bally-Cuif, 2016; Kizil, 2018; Shimizu et al., 2018). There is still a long way to understand the complete picture that makes the zebrafish brain special, yet the path is quite promising. Can we understand in zebrafish how new neurons are made and can we harness this information for humans to effectively regenerate our brains when needed – for instance in AD?
Addressing Stem Cell Potential in Alzheimer’s Disease Model in Adult Zebrafish Brain
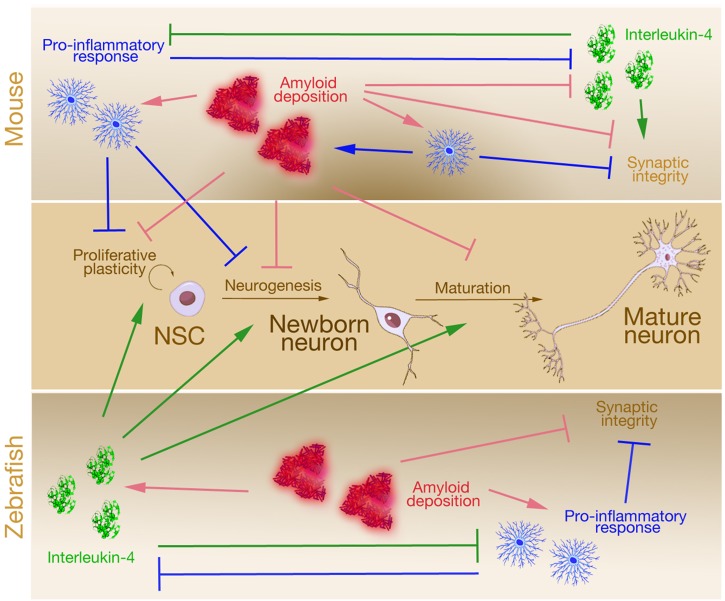
One of the hallmarks of AD is accumulation of amyloid plaques that are made up of the short peptide Amyloid-beta42 (Aβ42) (Yang et al., 1995; Duff et al., 1996; Younkin, 1998). In mammals, plaques elicit chronic inflammation and together with the plaques lead to synaptic failure, reduced neural stem cell plasticity and neurogenesis (Figure 1). We recently developed a microinjection-based method to generate an Aβ42 model in adult zebrafish that displayed AD-like phenotypes (Bhattarai et al., 2016, 2017a,b). Aβ42 aggregation in adult zebrafish brain led to phenotypes reminiscent of human AD pathophysiology: neuronal death, inflammation, synaptic degeneration, memory and learning deficits. In addition, this model also induced regenerative response by activation of NSCs and subsequent neurogenesis to compensate the neuronal insult (Figure 1). Therefore, this Aβ42 toxicity model in adult zebrafish offers an opportunity to study the molecular mechanisms how NSCs can be activated to form neurons and induce regeneration in AD condition. Interestingly, this regenerative neurogenesis response upon Aβ42 in adult zebrafish brain was mediated by a crosstalk between the immune system and the NSCs via an unexpected mediator: Interleukin-4 (IL4), an anti-inflammatory cytokine (Figure 1). Although the role of IL4 in suppressing the inflammatory response and in turn relieving the suppressive effects of inflammation on the neural stem cell proliferation in mammalian Alzheimer’s models were known, the direct regulation of the inflammatory environment on NSCs – which are the only non-immune cell types that express the receptor for IL4 – was a novel finding. Even with known molecules, zebrafish could provide novel understanding and ideas on how crosstalk mechanisms between the neurodegenerative milieu and the NSCs in the adult zebrafish brain could induce regenerative response (Bhattarai et al., 2016; Kizil, 2018). These studies also proposed that neural stem cell activity might be key to a successful recovery from neurodegeneration.
FIGURE 1.
A simplified comparison of the effects of Alzheimer’s disease on neural stem cell plasticity in mouse and zebrafish. In mouse, Amyloid deposition initiates pro-inflammatory response that potentiates Amyloid toxicity that impairs neural stem cell proliferation, neurogenesis, neuronal maturation, and synaptic integrity. This chronic inflammation suppresses anti-inflammatory factor Interleukin-4, which is beneficial for neuronal survival and synaptic integrity. In zebrafish, although Amyloid deposition follows a toxicity cascade similar to that of the mouse (activation of pro-inflammatory response and hampered synaptic integrity), Amyloid also leads to induction of anti-inflammatory factor Interleukin-4, which enhances neural stem cell proliferation, neurogenesis, and neuronal maturation. The effects of Interleukin-4 counteracts synaptic degeneration and reduced neural stem cell plasticity.
Can Alzheimer’s Be Treated With Increased Neurogenesis?
The role of neurogenesis in Alzheimer’s pathology and whether new neurons could really rescue the symptoms of Alzheimer’s is quite controversial and some researchers are skeptical toward this approach because the effects of Amyloid deposition on stem cell proliferation are beneficial or detrimental in a context dependent manner (Haughey et al., 2002; Lopez-Toledano and Shelanski, 2007; Diaz-Moreno et al., 2013; He et al., 2013; Lee et al., 2013; Bhattarai et al., 2016). Since neurogenesis cannot be equated with functional integration into the circuitry, the need to detect the effects of newborn neurons on circuit integrity has not been met sufficiently (Wen et al., 2004; Yamasaki et al., 2007; Blurton-Jones et al., 2009; Gomez-Nicola et al., 2014). However, when we scrutinize the course of manifestation of AD, we see that the NSCs are affected during the neurodegenerative conditions in all mammalian model systems tested: a progressive decline in neural stem cell pool during the course of neurodegeneration (Haughey et al., 2002; Ziabreva et al., 2006; Waldau and Shetty, 2008; Rodriguez and Verkhratsky, 2011; He et al., 2013; Martinez-Canabal, 2014; De Strooper and Karran, 2016; Dzamba et al., 2016; Tincer et al., 2016). But in case of Aβ42-mediated neurodegeneration in zebrafish, increased neuronal death was followed by increased proliferation of NSCs (Bhattarai et al., 2016, 2017a,b). Zebrafish brain reacted to neurodegeneration by utilizing neuro-inflammatory crosstalk to mediate the regenerative response. It indicates that the molecular mechanism regulating the regenerative response after amyloid-mediated neurodegeneration was pathology-induced plasticity response, and could be helpful to alleviate the symptoms of AD (Kizil, 2018). In fact, supporting evidence to this hypothesis came from comparative studies in a tissue mimetic 3D human NSCs plasticity assays and neuronal cultures as 3D systems are emerging as promising surrogates for human brain disease modeling (Justice et al., 2009; Haycock, 2011; Tang-Schomer et al., 2014; Zhang et al., 2014; Pasca et al., 2015; Ravi et al., 2015; Choi et al., 2016; Murphy et al., 2017; Papadimitriou et al., 2018). To test whether IL4 would act, similarly, in humans during AD – and therefore can be used as a regenerative paradigm, we developed an in vitro 3D culture system to grow mature human cortical neurons and networks from human NSCs (Papadimitriou et al., 2018). This system provides an in-vivo like environment including the essential components of the extracellular matrix, which are dynamically produced by the cultured cells and allows experimentation on a wide spectrum of human brain physiology: from neural stem cell plasticity to neuronal differentiation, from neuronal maturation to integration of neurons into existing networks. Adapting a glycosaminoglycan-based, cell-responsive hydrogel platform, we stimulated primary human neural stem cells (NSCs) from human cortex to form extensive neuronal networks in vitro. The 3D cultures exhibited neurotransmitter responsiveness, electrophysiological activity, tissue-specific extracellular matrix (ECM) deposition, and the expression of pro-neural genes and cortical neuronal markers that are undetectable in conventional 2D cultures. Importantly, those cultures formed from primary (human fetal) cortical cells, closely resemble the human physiology, which is critical for any disease modeling or therapeutic drug discovery efforts. The 3D cultures displayed a robust neural stem cell proliferation and neuronal differentiation, which is essential for a self-sustaining germinal niche of the human brain. After being formed in situ, our cultures express mature cortical neuronal markers showing a tissue-mimetic development (Papadimitriou et al., 2018). In this system, we modeled Amyloid toxicity as in adult zebrafish brain and found that the 3D culture system nicely recapitulated the major Alzheimer’s phenotypes such as the synaptic degeneration, loss of network connectivity, reduced neural stem cell proliferation and Tauopathies in a highly reproducible manner (Papadimitriou et al., 2018). Interestingly, treatment with IL4 under high Amyloid burden restored the neural stem cell proliferation, neurogenesis, network formation and functional integration of neurons into the existing circuitry, suggesting that increasing the neurogenesis in Alzheimer’s conditions could rescue the symptoms and might be a plausible way to cure this disease. In fact, a recent in vivo study found that increasing adult neurogenesis in Alzheimer’s model of mice increases the cognitive abilities and generated a healthier brain microenvironment in AD conditions (Choi et al., 2018), suggesting that the role of neurogenesis in conjunction with inflammation is a charming research realm in AD.
Notwithstanding with the ease of charting this interaction, realizing an immune-stem cell crosstalk in human brains that will lead to a real recuperation seems like a sci-fi novel. However, we know quite a bit on how inflammation is affecting the AD brain (Akiyama et al., 2000; Sastre et al., 2006; Amor et al., 2010; Glass et al., 2010; Aguzzi et al., 2013; Heneka et al., 2013, 2015; Heppner et al., 2015). Chronic phase of inflammation impinges on stem cell plasticity and synaptic integrity while resolution of inflammation provides a relief on the inflammatory burden and affected cell types may regain their potentials. An example of this regulation pertaining to our findings is the effects of Interleukin-4. After experimental models of inflammation, microglial dynamics were shown to be regulated by Interleukin-4 (e.g., pro-inflammatory cytokine release and the extent of initial inflammatory response) and this had an effect on neurogenesis dynamics and neuronal activity (e.g., long term potentiation in hippocampus, neural stem cell proliferation and neuroprotection) (Maher et al., 2005; Nolan et al., 2005; Lyons et al., 2007, 2009; Clarke et al., 2008; Nunan et al., 2014; Barrett et al., 2015). These “beneficial” effects of IL4 was considered to be because of its anti-inflammatory roles. However, in mouse brains, a direct interaction between anti-inflammatory factors and NSCs was not shown. In zebrafish and 3D cultures of human brains, on the other hand, IL4 seems to be directly affecting neural stem/progenitor cells by enhancing their neurogenic output (Bhattarai et al., 2016; Papadimitriou et al., 2018). This proposes an alternative approach to neuroinflammation research where we may need to decouple the microglial inflammation dynamics and direct interaction of immune factors with NSCs, which may be a collateral by-stander effect. In one hypothetical scenario, we may need to investigate which molecules partake in the direct crosstalk between immune system and NSCs in zebrafish and see whether those molecules are able to activate NSCs directly in mammals. Given that even though an immune-related factor would be available in AD brains, its effect is limited to those cells that can receive the signal. The by-stander effects of immune factors could be used to design a stage-specific modulation of NSCs in disease conditions. By a hypothetical scenario, we can appreciate why the immune-related signaling in neuronal compartment and in NSC niche can give us alternative treatment options in humans. For example, in a scenario, an immune factor could turn out to be beneficial for NSC plasticity in AD conditions, but this molecule would be an anti-inflammatory factor (e.g., IL4). Therefore, this factor would prevail only when there is a resolution of inflammation, which is not the case in AD. Therefore, the human NSCs would not be able to increase their proliferation simply due to the stage of the disease (they could otherwise do). Then, a drug can be designed to activate the immune-type signaling in NSCs regardless of the inflammation conditions and this can help elicit a neurogenic contribution from NSCs even if the inflammation is not resolved. When combined with strategies to increase the survival of newborn neurons, such a “nudge” on NSCs could contribute to the remedy of the disease, which could otherwise not happen naturally. Therefore, understanding the direct interaction of immune system with NSCs by using zebrafish and other appropriate models is important to establish deeper knowledge on the crosstalk between various cell types and NSCs. Additionally, activating the neural stem cell proliferation and neurogenesis in AD conditions must definitely be re-visited as an effective way of tackling this horrendous disease. The neural stem cell aspect of the AD could also provide us new ways for clinical therapies and may help to overcome the inefficient drug discovery efforts for Alzheimer’s so far.
Limitations and Promises Ahead
Although zebrafish could be an excellent tool from which we could understand how NSCs could be utilized to revert the symptoms of AD, there are experimental and physiological limitations we have to consider (Table 1). Zebrafish is a vertebrate and has evolutionary similarities to humans; however, it is still different than the human brains in terms of complexity, molecular structure, and physiology. Given that even mouse models of Alzheimer’s cannot be perfect surrogates for human disease, it would be naive to assume that zebrafish brain would fully recapitulate the AD in human brains. This is an aspect where the disease models could be refined in fish and could be made more compatible with human situation. By doing so, zebrafish could also be in part used for early phase pre-clinical studies to test drug efficiency. Additionally, the neurodegenerative disease models should be diversified in zebrafish in order to match the versatility of disease causing proteins. A future perspective for AD modeling in zebrafish could be to generate transgenic animals that display a more chronic and steady accumulation of disease hallmarks that persist throughout the adult stages. Several examples of those efforts are emerging (Malaga-Trillo et al., 2011; Xi et al., 2011; Schmid and Haass, 2013; Cosacak et al., 2017; Lopez et al., 2017; Kizil, 2018). Additionally, using comparative mammalian assays such as organoids or 3D culture systems (Choi et al., 2014, 2016; Fatehullah et al., 2016; Mansour et al., 2018; Papadimitriou et al., 2018) could be a way to check the stringency of conclusion from zebrafish as to whether or not they would hold true in mammalian brains.
Table 1.
Comparison of zebrafish and rodent models in Alzheimer’s disease research.
| Zebrafish | Rodents |
|---|---|
| Zebrafish advantegeous over rodents | |
| Amyloid-mediated neuronal death | No neuronal death |
| Neuroregenerative capacity | No neuroregenerative capacity |
| Stem cell plasticity for neurogenesis | Stem cells reduce plasticity and neurogenesis |
| Cost efficient generation and maintenance | Expensive generation and maintenance |
| High number of animals testable | Limited number of animals testable |
| 3R strategies developed | 3R strategies to be developed |
| Zebrafish and rodents equal | |
| Synaptic degeneration | Synaptic degeneration |
| Cognitive decline with Amyloidosis | Cognitive decline with Amyloidosis |
| Genetic tools available | Genetic tools available |
| Does not reflect the entire biology of the human disease | Does not reflect the entire biology of the human disease |
| Rodents advantegeous over zebrafish | |
| Non-mammalian physiology | Mammalian physiology |
| Need for adaptation to preclinical studies | Suitable for preclinical studies |
| Limited number of models expressing disease-related proteins | Variety of models expressing disease-related proteins |
Despite its disadvantages listed above, zebrafish holds up well with the handiness of the rodent models of AD in several aspects such as the diversity of genetic tools and the ability of modeling disease hallmarks such as synaptic degeneration and cognitive decline (Table 1). Nevertheless, neither mouse models nor zebrafish models can recapitulate the whole pathophysiological biology of AD as in human brains, which suggests that those models are useful insofar as their strengths in particular aspects were found. For instance, zebrafish is quite advantageous over rodents in many aspects (Table 1). These include (1) the pathological outcomes that resemble the human brain such as the ability of Amyloid depositions to lead to neuronal death, (2) regenerative ability owing to the capacity of NSCs to respond to tissue loss by enhanced plasticity and neuro-regenerative outcome, (3) cost of experimental studies, (4) number of animals that can be tested in a laboratory setting, and (5) the availability and possibility of 3R-friendly experimentation schemes.
Outlook
Although zebrafish lags behind the mammalian models in certain aspects, it already outperforms in many (Table 1). For instance, stem cell biology in zebrafish offers unprecedented information on the molecular programs that enable stem cell-based regeneration. Many research reports contributed to understanding of how NSCs in adult zebrafish brain function, are affected by external cues and respond to loss of neurons through recruitment of diverse signaling pathways including Notch, Wnt, Fgf, Bmp, and chemokine signaling (Mueller et al., 2004; Adolf et al., 2006; Grandel et al., 2006; Chapouton et al., 2007, 2011; Pellegrini et al., 2007; Zupanc, 2008; Diotel et al., 2010, 2013; Kroehne et al., 2011; Rothenaigner et al., 2011; Baumgart et al., 2012; Coolen et al., 2012, 2013; Kishimoto et al., 2012; Kizil et al., 2012a,b,c; Kyritsis et al., 2012; Marz et al., 2012; Alunni et al., 2013; Salta et al., 2014; Barbosa et al., 2015; Rodriguez Viales et al., 2015; Than-Trong and Bally-Cuif, 2015; Alunni and Bally-Cuif, 2016; Bhattarai et al., 2016; Kizil, 2018; Shimizu et al., 2018; Than-Trong et al., 2018). This large repertoire of knowledge will be instrumental in comparing the neuro-regenerative aptitude of zebrafish NSCs to human conditions and would help to find out how a successful proliferation-neurogenesis cascade could be elicited in mammals. Especially in the AD condition, which is the focus of this Perspective Article, such information could be instrumental and be a game-changer by providing an alternative approach to the disease mechanism and to its treatment (Figure 1). The role of neurogenesis in the manifestation of AD and its cure is rising to the spotlight again and zebrafish could offer valuable information on how our NSCs could be made “regenerative” using endogenous molecular programs and possibly by tweaking the immune system. Together with chemical/genetic screens, gene targeting and advances in research methodology; zebrafish stands out as an influential model that could drive preclinical findings toward novel clinically relevant discoveries.
Author Contributions
CK formulated the perspective. PB and CK wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by German Center for Neurodegenerative Diseases (DZNE) and Helmholtz Association (VH-NG-1021), Deutsche Forschungsgemeinschaft (DFG) (KI1524/6; KI1524/10 and KI1524/11), Center for Regenerative Therapies Dresden and TU Dresden (FZ-111, 043_261518) (CK).
References
- Adolf B., Chapouton P., Lam C. S., Topp S., Tannhauser B., Strahle U., et al. (2006). Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev. Biol. 295 278–293. 10.1016/j.ydbio.2006.03.023 [DOI] [PubMed] [Google Scholar]
- Ager R. R., Davis J. L., Agazaryan A., Benavente F., Poon W. W., LaFerla F. M., et al. (2015). Human neural stem cells improve cognition and promote synaptic growth in two complementary transgenic models of Alzheimer’s disease and neuronal loss. Hippocampus 25 813–826. 10.1002/hipo.22405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguzzi A., Barres B. A., Bennett M. L. (2013). Microglia: scapegoat, saboteur, or something else? Science 339 156–161. 10.1126/science.1227901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguzzi A., Haass C. (2003). Games played by rogue proteins in prion disorders and Alzheimer’s disease. Science 302 814–818. 10.1126/science.1087348 [DOI] [PubMed] [Google Scholar]
- Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G. M., et al. (2000). Inflammation and Alzheimer’s disease. Neurobiol. Aging 21 383–421. 10.1016/S0197-4580(00)00124-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alunni A., Bally-Cuif L. (2016). A comparative view of regenerative neurogenesis in vertebrates. Development 143 741–753. 10.1242/dev.122796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alunni A., Krecsmarik M., Bosco A., Galant S., Pan L., Moens C. B., et al. (2013). Notch3 signaling gates cell cycle entry and limits neural stem cell amplification in the adult pallium. Development 140 3335–3347. 10.1242/dev.095018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A., Seri B., Doetsch F. (2002). Identification of neural stem cells in the adult vertebrate brain. Brain Res. Bull. 57 751–758. 10.1016/S0361-9230(01)00770-5 [DOI] [PubMed] [Google Scholar]
- Amor S., Puentes F., Baker D., van der Valk P. (2010). Inflammation in neurodegenerative diseases. Immunology 129 154–169. 10.1111/j.1365-2567.2009.03225.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antos C. L., Tanaka E. M. (2010). Vertebrates that regenerate as models for guiding stem cels. Adv. Exp. Med. Biol. 695 184–214. 10.1007/978-1-4419-7037-4_13 [DOI] [PubMed] [Google Scholar]
- Arendt T. (2009). Synaptic degeneration in Alzheimer’s disease. Acta Neuropathol. 118 167–179. 10.1007/s00401-009-0536-x [DOI] [PubMed] [Google Scholar]
- Attems J., Jellinger K. A. (2014). The overlap between vascular disease and Alzheimer’s disease–lessons from pathology. BMC Med. 12:206. 10.1186/s12916-014-0206-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetge E. E. (1993). Neural stem cells for CNS transplantation. Ann. N. Y. Acad. Sci. 695 285–291. 10.1111/j.1749-6632.1993.tb23068.x [DOI] [PubMed] [Google Scholar]
- Barbosa J. S., Sanchez-Gonzalez R., Di Giaimo R., Baumgart E. V., Theis F. J., Gotz M., et al. (2015). Neurodevelopment. Live imaging of adult neural stem cell behavior in the intact and injured zebrafish brain. Science 348 789–793. 10.1126/science.aaa2729 [DOI] [PubMed] [Google Scholar]
- Barrett J. P., Minogue A. M., Jones R. S., Ribeiro C., Kelly R. J., Lynch M. A. (2015). Bone marrow-derived macrophages from AbetaPP/PS1 mice are sensitized to the effects of inflammatory stimuli. J. Alzheimers Dis. 44 949–962. 10.3233/JAD-142076 [DOI] [PubMed] [Google Scholar]
- Bartzokis G. (2011). Alzheimer’s disease as homeostatic responses to age-related myelin breakdown. Neurobiol. Aging 32 1341–1371. 10.1016/j.neurobiolaging.2009.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart E. V., Barbosa J. S., Bally-Cuif L., Gotz M., Ninkovic J. (2012). Stab wound injury of the zebrafish telencephalon: a model for comparative analysis of reactive gliosis. Glia 60 343–357. 10.1002/glia.22269 [DOI] [PubMed] [Google Scholar]
- Berberoglu M. A., Dong Z., Li G., Zheng J., Trejo Martinez Ldel. C.,et al. (2014). Heterogeneously expressed fezf2 patterns gradient Notch activity in balancing the quiescence, proliferation, and differentiation of adult neural stem cells. J. Neurosci. 34 13911–13923. 10.1523/JNEUROSCI.1976-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai P., Thomas A. K., Cosacak M. I., Papadimitriou C., Mashkaryan V., Froc C., et al. (2016). IL4/STAT6 signaling activates neural stem cell proliferation and neurogenesis upon amyloid-beta42 aggregation in adult zebrafish brain. Cell Rep. 17 941–948. 10.1016/j.celrep.2016.09.075 [DOI] [PubMed] [Google Scholar]
- Bhattarai P., Thomas A. K., Cosacak M. I., Papadimitriou C., Mashkaryan V., Zhang Y., et al. (2017a). Modeling Amyloid-β42 toxicity and neurodegeneration in adult zebrafish brain. J. Vis. Exp. 128:e56014. 10.3791/56014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai P., Thomas A. K., Zhang Y., Kizil C. (2017b). The effects of aging on Amyloid-β42-induced neurodegeneration and regeneration in adult zebrafish brain. Neurogenesis 4:e1322666. 10.1080/23262133.2017.1322666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K., de Leon M. J., Zetterberg H. (2006). Alzheimer’s disease. Lancet 368 387–403. 10.1016/S0140-6736(06)69113-7 [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M., Kitazawa M., Martinez-Coria H., Castello N. A., Müller F.-J., Loring J. F., et al. (2009). Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 106 13594–13599. 10.1073/pnas.0901402106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M., Fulmore C. A., Tartt A. N., Simeon L. R., Pavlova I., Poposka V., et al. (2018). Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 22:e585. 10.1016/j.stem.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundin P., Bjorklund A. (1998). Survival of expanded dopaminergic precursors is critical for clinical trials. Nat. Neurosci. 1:537. 10.1038/2773 [DOI] [PubMed] [Google Scholar]
- Carter J., Lippa C. F. (2001). Beta-amyloid, neuronal death and Alzheimer’s disease. Curr. Mol. Med. 1 733–737. 10.2174/1566524013363177 [DOI] [PubMed] [Google Scholar]
- Chai C. K. (2007). The genetics of Alzheimer’s disease. Am. J. Alzheimers Dis. Other Demen. 22 37–41. 10.1177/1533317506295655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapouton P., Jagasia R., Bally-Cuif L. (2007). Adult neurogenesis in non-mammalian vertebrates. Bioessays 29 745–757. 10.1002/bies.20615 [DOI] [PubMed] [Google Scholar]
- Chapouton P., Webb K. J., Stigloher C., Alunni A., Adolf B., Hesl B., et al. (2011). Expression of hairy/enhancer of split genes in neural progenitors and neurogenesis domains of the adult zebrafish brain. J. Comp. Neurol. 519 1748–1769. 10.1002/cne.22599 [DOI] [PubMed] [Google Scholar]
- Choi S. H., Bylykbashi E., Chatila Z. K., Lee S. W., Pulli B., Clemenson G. D., et al. (2018). Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 361:eaan8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. H., Kim Y. H., Hebisch M., Sliwinski C., Lee S., D’Avanzo C., et al. (2014). A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature 515 274–278. 10.1038/nature13800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. H., Kim Y. H., Quinti L., Tanzi R. E., Kim D. Y. (2016). 3D culture models of Alzheimer’s disease: a road map to a “cure-in-a-dish”. Mol. Neurodegener. 11:75. 10.1186/s13024-016-0139-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke R. M., Lyons A., O’Connell F., Deighan B. F., Barry C. E., Anyakoha N. G., et al. (2008). A pivotal role for interleukin-4 in atorvastatin-associated neuroprotection in rat brain. J. Biol. Chem. 283 1808–1817. 10.1074/jbc.M707442200 [DOI] [PubMed] [Google Scholar]
- Conti L., Cattaneo E. (2010). Neural stem cell systems: physiological players or in vitro entities? Nat. Rev. Neurosci. 11 176–187. 10.1038/nrn2761 [DOI] [PubMed] [Google Scholar]
- Coolen M., Katz S., Bally-Cuif L. (2013). miR-9: a versatile regulator of neurogenesis. Front. Cell. Neurosci. 7:220. 10.3389/fncel.2013.00220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen M., Thieffry D., Drivenes O., Becker T. S., Bally-Cuif L. (2012). miR-9 controls the timing of neurogenesis through the direct inhibition of antagonistic factors. Dev. Cell 22 1052–1064. 10.1016/j.devcel.2012.03.003 [DOI] [PubMed] [Google Scholar]
- Cosacak M. I., Papadimitriou C., Kizil C. (2015). Regeneration, plasticity, and induced molecular programs in adult zebrafish brain. Biomed. Res. Int. 2015:769763. 10.1155/2015/769763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosacak M. I., Bhattarai P., Bocova L., Dzewas T., Mashkaryan V., Papadimitriou C., et al. (2017). Human TAUP301L overexpression results in TAU hyperphosphorylation without neurofibrillary tangles in adult zebrafish brain. Sci. Rep. 7:12959. 10.1038/s41598-017-13311-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M. R., Gotz M., Berninger B. (2010). What determines neurogenic competence in glia? Brain Res. Rev. 63 47–59. 10.1016/j.brainresrev.2010.01.002 [DOI] [PubMed] [Google Scholar]
- Dantuma E., Merchant S., Sugaya K. (2010). Stem cells for the treatment of neurodegenerative diseases. Stem Cell Res. Ther. 1:37. 10.1186/scrt37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B., Karran E. (2016). The cellular phase of alzheimer’s disease. Cell 164 603–615. 10.1016/j.cell.2015.12.056 [DOI] [PubMed] [Google Scholar]
- Demars M., Hu Y. S., Gadadhar A., Lazarov O. (2010). Impaired neurogenesis is an early event in the etiology of familial Alzheimer’s disease in transgenic mice. J. Neurosci. Res. 88 2103–2117. 10.1002/jnr.22387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Moreno M., Hortiguela R., Goncalves A., Garcia-Carpio I., Manich G., Garcia-Bermudez E., et al. (2013). Abeta increases neural stem cell activity in senescence-accelerated SAMP8 mice. Neurobiol. Aging 34 2623–2638. 10.1016/j.neurobiolaging.2013.05.011 [DOI] [PubMed] [Google Scholar]
- Diotel N., Vaillant C., Gabbero C., Mironov S., Fostier A., Gueguen M. M., et al. (2013). Effects of estradiol in adult neurogenesis and brain repair in zebrafish. Horm. Behav. 63 193–207. 10.1016/j.yhbeh.2012.04.003 [DOI] [PubMed] [Google Scholar]
- Diotel N., Vaillant C., Gueguen M. M., Mironov S., Anglade I., Servili A., et al. (2010). Cxcr4 and Cxcl12 expression in radial glial cells of the brain of adult zebrafish. J. Comp. Neurol. 518 4855–4876. 10.1002/cne.22492 [DOI] [PubMed] [Google Scholar]
- Doetsch F. (2003). The glial identity of neural stem cells. Nat. Neurosci. 6 1127–1134. 10.1038/nn1144 [DOI] [PubMed] [Google Scholar]
- Doetsch F., Caille I., Lim D. A., Garcia-Verdugo J. M., Alvarez-Buylla A. (1999). Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97 703–716. 10.1016/S0092-8674(00)80783-7 [DOI] [PubMed] [Google Scholar]
- Doetsch F., Scharff C. (2001). Challenges for brain repair: insights from adult neurogenesis in birds and mammals. Brain Behav. Evol. 58 306–322. 10.1159/000057572 [DOI] [PubMed] [Google Scholar]
- Duff K., Eckman C., Zehr C., Yu X., Prada C. M., Perez-tur J., et al. (1996). Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature 383 710–713. 10.1038/383710a0 [DOI] [PubMed] [Google Scholar]
- Dunnett S. B., Kendall A. L., Watts C., Torres E. M. (1997). Neuronal cell transplantation for Parkinson’s and Huntington’s diseases. Br. Med. Bull. 53 757–776. 10.1093/oxfordjournals.bmb.a011646 [DOI] [PubMed] [Google Scholar]
- Dzamba D., Harantova L., Butenko O., Anderova M. (2016). Glial cells – The key elements of Alzheimer’s disease. Curr. Alzheimer Res. 13 894–911. 10.2174/1567205013666160129095924 [DOI] [PubMed] [Google Scholar]
- Ernst A., Alkass K., Bernard S., Salehpour M., Perl S., Tisdale J., et al. (2014). Neurogenesis in the striatum of the adult human brain. Cell 156 1072–1083. 10.1016/j.cell.2014.01.044 [DOI] [PubMed] [Google Scholar]
- Espuny-Camacho I., Arranz A. M., Fiers M., Snellinx A., Ando K., Munck S., et al. (2017). Hallmarks of Alzheimer’s disease in stem-cell-derived human neurons transplanted into mouse brain. Neuron 93:e1068. 10.1016/j.neuron.2017.02.001 [DOI] [PubMed] [Google Scholar]
- Ettle B., Schlachetzki J. C. M., Winkler J. (2016). Oligodendroglia and myelin in neurodegenerative diseases: more than just bystanders? Mol. Neurobiol. 53 3046–3062. 10.1007/s12035-015-9205-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatehullah A., Tan S. H., Barker N. (2016). Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 18 246–254. 10.1038/ncb3312 [DOI] [PubMed] [Google Scholar]
- Fischer W., Wictorin K., Björklund A., Williams L. R., Varon S., Gage F. H. (1987). Amelioration of cholinergic neuron atrophy and spatial memory impairment in aged rats by nerve growth factor. Nature 329 65–68. 10.1038/329065a0 [DOI] [PubMed] [Google Scholar]
- Fleisch V. C., Fraser B., Allison W. T. (2010). Investigating regeneration and functional integration of CNS neurons: lessons from zebrafish genetics and other fish species. Biochim. Biophys. Acta 1812 364–380. 10.1016/j.bbadis.2010.10.012 [DOI] [PubMed] [Google Scholar]
- Gage F. H. (2000). Mammalian neural stem cells. Science 287 1433–1438. 10.1126/science.287.5457.1433 [DOI] [PubMed] [Google Scholar]
- Gage F. H., Temple S. (2013). Neural stem cells: generating and regenerating the brain. Neuron 80 588–601. 10.1016/j.neuron.2013.10.037 [DOI] [PubMed] [Google Scholar]
- Galvan V., Jin K. (2007). Neurogenesis in the aging brain. Clin. Interventions Aging 2 605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemberling M., Bailey T. J., Hyde D. R., Poss K. D. (2013). The zebrafish as a model for complex tissue regeneration. Trends Genet. 29 611–620. 10.1016/j.tig.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass C. K., Saijo K., Winner B., Marchetto M. C., Gage F. H. (2010). Mechanisms underlying inflammation in neurodegeneration. Cell 140 918–934. 10.1016/j.cell.2010.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Nicola D., Suzzi S., Vargas-Caballero M., Fransen N. L., Al-Malki H., Cebrian-Silla A., et al. (2014). Temporal dynamics of hippocampal neurogenesis in chronic neurodegeneration. Brain 137 2312–2328. 10.1093/brain/awu155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss R. J. (1991). The Natural History (and Mystery) of Regeneration. Cambridge: Cambridge University Press. [Google Scholar]
- Grandel H., Kaslin J., Ganz J., Wenzel I., Brand M. (2006). Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell fate. Dev. Biol. 295 263–277. 10.1016/j.ydbio.2006.03.040 [DOI] [PubMed] [Google Scholar]
- Gu G., Zhang W., Li M., Ni J., Wang P. (2015). Transplantation of NSC-derived cholinergic neuron-like cells improves cognitive function in APP/PS1 transgenic mice. Neuroscience 291 81–92. 10.1016/j.neuroscience.2015.01.073 [DOI] [PubMed] [Google Scholar]
- Hansen D. V., Lui J. H., Parker P. R., Kriegstein A. R. (2010). Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 464 554–561. 10.1038/nature08845 [DOI] [PubMed] [Google Scholar]
- Hardy J. (2009). The amyloid hypothesis for Alzheimer’s disease: a critical reappraisal. J. Neurochem. 110 1129–1134. 10.1111/j.1471-4159.2009.06181.x [DOI] [PubMed] [Google Scholar]
- Harman D. (2006). Alzheimer’s disease pathogenesis: role of aging. Ann. N. Y. Acad. Sci. 1067 454–460. 10.1196/annals.1354.065 [DOI] [PubMed] [Google Scholar]
- Haughey N. J., Liu D., Nath A., Borchard A. C., Mattson M. P. (2002). Disruption of neurogenesis in the subventricular zone of adult mice, and in human cortical neuronal precursor cells in culture, by amyloid beta-peptide: implications for the pathogenesis of Alzheimer’s disease. Neuromol. Med. 1 125–135. 10.1385/NMM:1:2:125 [DOI] [PubMed] [Google Scholar]
- Haycock J. W. (2011). 3D cell culture: a review of current approaches and techniques. Methods Mol. Biol. 695 1–15. 10.1007/978-1-60761-984-0_1 [DOI] [PubMed] [Google Scholar]
- He N., Jin W.-L., Lok K.-H., Wang Y., Yin M., Wang Z.-J. (2013). Amyloid-β(1-42) oligomer accelerates senescence in adult hippocampal neural stem/progenitor cells via formylpeptide receptor 2. Cell Death Dis. 4:e924. 10.1038/cddis.2013.437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka M. T., Carson M. J., El Khoury J., Landreth G. E., Brosseron F., Feinstein D. L., et al. (2015). Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 14 388–405. 10.1016/S1474-4422(15)70016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka M. T., Kummer M. P., Stutz A., Delekate A., Schwartz S., Vieira-Saecker A., et al. (2013). NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493 674–678. 10.1038/nature11729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka M. T., Sastre M., Dumitrescu-Ozimek L., Dewachter I., Walter J., Klockgether T., et al. (2005). Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J. Neuroinflamm. 2:22. 10.1186/1742-2094-2-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner F. L., Ransohoff R. M., Becher B. (2015). Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 16 358–372. 10.1038/nrn3880 [DOI] [PubMed] [Google Scholar]
- Hong J. Y., Lee S. H., Lee S. C., Kim J. W., Kim K. P., Kim S. M., et al. (2014). Therapeutic potential of induced neural stem cells for spinal cord injury. J. Biol. Chem. 289 32512–32525. 10.1074/jbc.M114.588871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Mucke L. (2012). Alzheimer mechanisms and therapeutic strategies. Cell 148 1204–1222. 10.1016/j.cell.2012.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K., Liu F., Gong C. X. (2016). Tau and neurodegenerative disease: the story so far. Nat. Rev. Neurol. 12 15–27. 10.1038/nrneurol.2015.225 [DOI] [PubMed] [Google Scholar]
- Jebelli J., Su W., Hopkins S., Pocock J., Garden G. A. (2015). Glia: guardians, gluttons, or guides for the maintenance of neuronal connectivity? Ann. N. Y. Acad. Sci. 1351 1–10. 10.1111/nyas.12711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice B. A., Badr N. A., Felder R. A. (2009). 3D cell culture opens new dimensions in cell-based assays. Drug Discov. Today 14 102–107. 10.1016/j.drudis.2008.11.006 [DOI] [PubMed] [Google Scholar]
- Kaslin J., Ganz J., Geffarth M., Grandel H., Hans S., Brand M. (2009). Stem cells in the adult zebrafish cerebellum: initiation and maintenance of a novel stem cell niche. J. Neurosci. 29 6142–6153. 10.1523/JNEUROSCI.0072-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S., Cussigh D., Urban N., Blomfield I., Guillemot F., Bally-Cuif L., et al. (2016). A nuclear role for miR-9 and argonaute proteins in balancing quiescent and activated neural stem cell states. Cell Rep. 17 1383–1398. 10.1016/j.celrep.2016.09.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G., Gage F. H., Aigner L., Song H., Curtis M. A., Thuret S., et al. (2018). Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell 23 25–30. 10.1016/j.stem.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G., Krebs J., Fabel K. (2008). The contribution of failing adult hippocampal neurogenesis to psychiatric disorders. Curr. Opin. Psychiatry 21 290–295. 10.1097/YCO.0b013e3282fad375 [DOI] [PubMed] [Google Scholar]
- Kirkitadze M. D., Bitan G., Teplow D. B. (2002). Paradigm shifts in Alzheimer’s disease and other neurodegenerative disorders: the emerging role of oligomeric assemblies. J. Neurosci. Res. 69 567–577. 10.1002/jnr.10328 [DOI] [PubMed] [Google Scholar]
- Kishimoto N., Shimizu K., Sawamoto K. (2012). Neuronal regeneration in a zebrafish model of adult brain injury. Dis. Model. Mech. 5 200–209. 10.1242/dmm.007336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizil C. (2018). Mechanisms of pathology-induced neural stem cell plasticity and neural regeneration in adult zebrafish brain. Curr. Pathobiol. Rep. 6 71–77. 10.1007/s40139-018-0158-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizil C., Dudczig S., Kyritsis N., Machate A., Blaesche J., Kroehne V., et al. (2012a). The chemokine receptor cxcr5 regulates the regenerative neurogenesis response in the adult zebrafish brain. Neural Dev. 7:27. 10.1186/1749-8104-7-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizil C., Kaslin J., Kroehne V., Brand M. (2012b). Adult neurogenesis and brain regeneration in zebrafish. Dev. Neurobiol. 72 429–461. 10.1002/dneu.20918 [DOI] [PubMed] [Google Scholar]
- Kizil C., Kyritsis N., Dudczig S., Kroehne V., Freudenreich D., Kaslin J., et al. (2012c). Regenerative neurogenesis from neural progenitor cells requires injury-induced expression of Gata3. Dev. Cell 23 1230–1237. 10.1016/j.devcel.2012.10.014 [DOI] [PubMed] [Google Scholar]
- Kizil C., Kyritsis N., Brand M. (2015). Effects of inflammation on stem cells: together they strive? EMBO Rep. 16 416–426. 10.15252/embr.201439702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizil C., Otto G. W., Geisler R., Nusslein-Volhard C., Antos C. L. (2009). Simplet controls cell proliferation and gene transcription during zebrafish caudal fin regeneration. Dev. Biol. 325 329–340. 10.1016/j.ydbio.2008.09.032 [DOI] [PubMed] [Google Scholar]
- Kriegstein A., Alvarez-Buylla A. (2009). The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 32 149–184. 10.1146/annurev.neuro.051508.135600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroehne V., Freudenreich D., Hans S., Kaslin J., Brand M. (2011). Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development 138 4831–4841. 10.1242/dev.072587 [DOI] [PubMed] [Google Scholar]
- Kronenberg G., Reuter K., Steiner B., Brandt M. D., Jessberger S., Yamaguchi M., et al. (2003). Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J. Comp. Neurol. 467 455–463. 10.1002/cne.10945 [DOI] [PubMed] [Google Scholar]
- Kyritsis N., Kizil C., Zocher S., Kroehne V., Kaslin J., Freudenreich D., et al. (2012). Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science 338 1353–1356. 10.1126/science.1228773 [DOI] [PubMed] [Google Scholar]
- LaFerla F. M., Green K. N. (2012). Animal models of Alzheimer disease. Cold Spring Harbor Perspect. Med. 2:a006320. 10.1101/cshperspect.a006320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I.-S., Jung K., Kim I.-S., Lee H., Kim M., Yun S., et al. (2015). Human neural stem cells alleviate Alzheimer-like pathology in a mouse model. Mol. Neurodegener. 10:38. 10.1186/s13024-015-0035-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I.-S., Jung K., Kim I.-S., Park K. I. (2013). Amyloid-β oligomers regulate the properties of human neural stem cells through GSK-3β signaling. Exp. Mol. Med. 45:e60. 10.1038/emm.2013.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian H., Zheng H. (2016). Signaling pathways regulating neuron-glia interaction and their implications in Alzheimer’s disease. J. Neurochem. 136 475–491. 10.1111/jnc.13424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilja A. M., Malmsten L., Röjdner J., Voytenko L., Verkhratsky A., Ögren S. O., et al. (2015). Neural stem cell transplant-induced effect on neurogenesis and cognition in alzheimer Tg2576 mice is inhibited by concomitant treatment with amyloid-lowering or cholinergic α7 nicotinic receptor drugs. Neural Plast. 2015:370432. 10.1155/2015/370432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A., Lee S. E., Wojta K., Ramos E. M., Klein E., Chen J., et al. (2017). A152T tau allele causes neurodegeneration that can be ameliorated in a zebrafish model by autophagy induction. Brain 140 1128–1146. 10.1093/brain/awx005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Toledano M. A., Shelanski M. L. (2004). Neurogenic effect of beta-amyloid peptide in the development of neural stem cells. J. Neurosci. 24 5439–5444. 10.1523/JNEUROSCI.0974-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Toledano M. A., Shelanski M. L. (2007). Increased neurogenesis in young transgenic mice overexpressing human APP(Sw, Ind). J. Alzheimers Dis. 12 229–240. 10.3233/JAD-2007-12304 [DOI] [PubMed] [Google Scholar]
- Lyons A., Griffin R. J., Costelloe C. E., Clarke R. M., Lynch M. A. (2007). IL-4 attenuates the neuroinflammation induced by amyloid-beta in vivo and in vitro. J. Neurochem. 101 771–781. 10.1111/j.1471-4159.2006.04370.x [DOI] [PubMed] [Google Scholar]
- Lyons A., McQuillan K., Deighan B. F., O’Reilly J. A., Downer E. J., Murphy A. C., et al. (2009). Decreased neuronal CD200 expression in IL-4-deficient mice results in increased neuroinflammation in response to lipopolysaccharide. Brain Behav. Immun. 23 1020–1027. 10.1016/j.bbi.2009.05.060 [DOI] [PubMed] [Google Scholar]
- Magnusson J. P., Frisen J. (2016). Stars from the darkest night: unlocking the neurogenic potential of astrocytes in different brain regions. Development 143 1075–1086. 10.1242/dev.133975 [DOI] [PubMed] [Google Scholar]
- Magnusson J. P., Goritz C., Tatarishvili J., Dias D. O., Smith E. M., Lindvall O., et al. (2014). A latent neurogenic program in astrocytes regulated by Notch signaling in the mouse. Science 346 237–241. 10.1126/science.346.6206.237 [DOI] [PubMed] [Google Scholar]
- Maher F. O., Nolan Y., Lynch M. A. (2005). Downregulation of IL-4-induced signalling in hippocampus contributes to deficits in LTP in the aged rat. Neurobiol. Aging 26 717–728. 10.1016/j.neurobiolaging.2004.07.002 [DOI] [PubMed] [Google Scholar]
- Malaga-Trillo E., Salta E., Figueras A., Panagiotidis C., Sklaviadis T. (2011). Fish models in prion biology: underwater issues. Biochim. Biophys. Acta 1812 402–414. 10.1016/j.bbadis.2010.09.013 [DOI] [PubMed] [Google Scholar]
- Mansour A. A., Goncalves J. T., Bloyd C. W., Li H., Fernandes S., Quang D., et al. (2018). An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol. 36 432–441. 10.1038/nbt.4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Canabal A. (2014). Reconsidering hippocampal neurogenesis in Alzheimer’s disease. Front. Neurosci. 8:147. 10.3389/fnins.2014.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marz M., Schmidt R., Rastegar S., Strahle U. (2012). Regenerative response following stab injury in the adult zebrafish telencephalon. Dev. Dyn. 240 2221–2231. 10.1002/dvdy.22710 [DOI] [PubMed] [Google Scholar]
- Millimaki B. B., Sweet E. M., Riley B. B. (2010). Sox2 is required for maintenance and regeneration, but not initial development, of hair cells in the zebrafish inner ear. Dev. Biol. 338 262–269. 10.1016/j.ydbio.2009.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokalled M. H., Patra C., Dickson A. L., Endo T., Stainier D. Y., Poss K. D. (2016). Injury-induced ctgfa directs glial bridging and spinal cord regeneration in zebrafish. Science 354 630–634. 10.1126/science.aaf2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y., Gage F. H. (2011). Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol. Neurodegener. 6:85. 10.1186/1750-1326-6-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller T., Vernier P., Wullimann M. F. (2004). The adult central nervous cholinergic system of a neurogenetic model animal, the zebrafish Danio rerio. Brain Res. 1011 156–169. 10.1016/j.brainres.2004.02.073 [DOI] [PubMed] [Google Scholar]
- Murphy A. R., Laslett A., O’Brien C. M., Cameron N. R. (2017). Scaffolds for 3D in vitro culture of neural lineage cells. Acta Biomater. 54 1–20. 10.1016/j.actbio.2017.02.046 [DOI] [PubMed] [Google Scholar]
- Nagele R. G., D’Andrea M. R., Anderson W. J., Wang H. Y. (2002). Intracellular accumulation of beta-amyloid(1-42) in neurons is facilitated by the alpha 7 nicotinic acetylcholine receptor in Alzheimer’s disease. Neuroscience 110 199–211. 10.1016/S0306-4522(01)00460-2 [DOI] [PubMed] [Google Scholar]
- Nolan Y., Maher F. O., Martin D. S., Clarke R. M., Brady M. T., Bolton A. E., et al. (2005). Role of interleukin-4 in regulation of age-related inflammatory changes in the hippocampus. J. Biol. Chem. 280 9354–9362. 10.1074/jbc.M412170200 [DOI] [PubMed] [Google Scholar]
- Nunan R., Sivasathiaseelan H., Khan D., Zaben M., Gray W. (2014). Microglial VPAC1R mediates a novel mechanism of neuroimmune-modulation of hippocampal precursor cells via IL-4 release. Glia 62 1313–1327. 10.1002/glia.22682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacary E., Martynoga B., Guillemot F. (2012). Crucial first steps: the transcriptional control of neuron delamination. Neuron 74 209–211. 10.1016/j.neuron.2012.04.002 [DOI] [PubMed] [Google Scholar]
- Papadimitriou C., Celikkaya H., Cosacak M. I., Mashkaryan V., Bray L., Bhattarai P., et al. (2018). 3D culture method for alzheimer’s disease modeling reveals interleukin-4 rescues abeta42-induced loss of human neural stem cell plasticity. Dev. Cell 46:e108. 10.1016/j.devcel.2018.06.005 [DOI] [PubMed] [Google Scholar]
- Park D., Joo S. S., Kim T. K., Lee S. H., Kang H., Lee H. J., et al. (2012). Human neural stem cells overexpressing choline acetyltransferase restore cognitive function of kainic acid-induced learning and memory deficit animals. Cell Transplant. 21 365–371. 10.3727/096368911X586765 [DOI] [PubMed] [Google Scholar]
- Pasca A. M., Sloan S. A., Clarke L. E., Tian Y., Makinson C. D., Huber N., et al. (2015). Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 12 671–678. 10.1038/nmeth.3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini E., Mouriec K., Anglade I., Menuet A., Le Page Y., Gueguen M. M., et al. (2007). Identification of aromatase-positive radial glial cells as progenitor cells in the ventricular layer of the forebrain in zebrafish. J. Comp. Neurol. 501 150–167. 10.1002/cne.21222 [DOI] [PubMed] [Google Scholar]
- Poss K. D. (2010). Advances in understanding tissue regenerative capacity and mechanisms in animals. Nat. Rev. Genet. 11 710–722. 10.1038/nrg2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi M., Paramesh V., Kaviya S. R., Anuradha E., Solomon F. D. (2015). 3D cell culture systems: advantages and applications. J. Cell. Physiol. 230 16–26. 10.1002/jcp.24683 [DOI] [PubMed] [Google Scholar]
- Raya A., Koth C. M., Buscher D., Kawakami Y., Itoh T., Raya R. M., et al. (2003). Activation of Notch signaling pathway precedes heart regeneration in zebrafish. Proc. Natl. Acad. Sci. U.S.A. 100(Suppl. 1), 11889–11895. 10.1073/pnas.1834204100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. J., Verkhratsky A. (2011). Neurogenesis in Alzheimer’s disease. J. Anat. 219 78–89. 10.1111/j.1469-7580.2011.01343.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Viales R., Diotel N., Ferg M., Armant O., Eich J., Alunni A., et al. (2015). The helix-loop-helix protein id1 controls stem cell proliferation during regenerative neurogenesis in the adult zebrafish telencephalon. Stem Cells 33 892–903. 10.1002/stem.1883 [DOI] [PubMed] [Google Scholar]
- Rolls A., Shechter R., Schwartz M. (2009). The bright side of the glial scar in CNS repair. Nat. Rev. Neurosci. 10 235–241. 10.1038/nrn2591 [DOI] [PubMed] [Google Scholar]
- Rothenaigner I., Krecsmarik M., Hayes J. A., Bahn B., Lepier A., Fortin G., et al. (2011). Clonal analysis by distinct viral vectors identifies bona fide neural stem cells in the adult zebrafish telencephalon and characterizes their division properties and fate. Development 138 1459–1469. 10.1242/dev.058156 [DOI] [PubMed] [Google Scholar]
- Salta E., Lau P., Sala Frigerio C., Coolen M., Bally-Cuif L., De Strooper B. (2014). A self-organizing miR-132/Ctbp2 circuit regulates bimodal notch signals and glial progenitor fate choice during spinal cord maturation. Dev. Cell 30 423–436. 10.1016/j.devcel.2014.07.006 [DOI] [PubMed] [Google Scholar]
- Sastre M., Klockgether T., Heneka M. T. (2006). Contribution of inflammatory processes to Alzheimer’s disease: molecular mechanisms. Int. J. Dev. Neurosci. 24 167–176. 10.1016/j.ijdevneu.2005.11.014 [DOI] [PubMed] [Google Scholar]
- Schmid B., Haass C. (2013). Genomic editing opens new avenues for zebrafish as a model for neurodegeneration. J. Neurochem. 127 461–470. 10.1111/jnc.12460 [DOI] [PubMed] [Google Scholar]
- Schneider L. S., Mangialasche F., Andreasen N., Feldman H., Giacobini E., Jones R., et al. (2014). Clinical trials and late-stage drug development for Alzheimer’s disease: an appraisal from 1984 to 2014. J. Int. Med. 275 251–283. 10.1111/joim.12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D. J. (2003). Folding proteins in fatal ways. Nature 426 900–904. 10.1038/nature02264 [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Ueda Y., Ohshima T. (2018). Wnt signaling regulates proliferation and differentiation of radial glia in regenerative processes after stab injury in the optic tectum of adult zebrafish. Glia 66 1382–1394. 10.1002/glia.23311 [DOI] [PubMed] [Google Scholar]
- Silver J., Miller J. H. (2004). Regeneration beyond the glial scar. Nat. Rev. Neurosci. 5 146–156. 10.1038/nrn1326 [DOI] [PubMed] [Google Scholar]
- Sorrells S. F., Paredes M. F., Cebrian-Silla A., Sandoval K., Qi D., Kelley K. W., et al. (2018). Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 555 377–381. 10.1038/nature25975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding K. L., Bergmann O., Alkass K., Bernard S., Salehpour M., Huttner H. B., et al. (2013). Dynamics of hippocampal neurogenesis in adult humans. Cell 153 1219–1227. 10.1016/j.cell.2013.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer L., Tabar V., McKay R. D. (1998). Transplantation of expanded mesencephalic precursors leads to recovery in parkinsonian rats. Nat. Neurosci. 1 290–295. 10.1038/1105 [DOI] [PubMed] [Google Scholar]
- Svendsen C. N., Caldwell M. A., Shen J., ter Borg M. G., Rosser A. E., Tyers P., et al. (1997). Long-term survival of human central nervous system progenitor cells transplanted into a rat model of Parkinson’s disease. Exp. Neurol. 148 135–146. 10.1006/exnr.1997.6634 [DOI] [PubMed] [Google Scholar]
- Takeda S., Morishita R. (2015). Ultrasound attacks Alzheimer’s disease? Ann. Transl. Med. 3:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka E. M., Ferretti P. (2009). Considering the evolution of regeneration in the central nervous system. Nat. Rev. Neurosci. 10 713–723. 10.1038/nrn2707 [DOI] [PubMed] [Google Scholar]
- Tang-Schomer M. D., White J. D., Tien L. W., Schmitt L. I., Valentin T. M., Graziano D. J., et al. (2014). Bioengineered functional brain-like cortical tissue. Proc. Natl. Acad. Sci. U.S.A. 111 13811–13816. 10.1073/pnas.1324214111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P. (2009). Adult neurogenesis, neural stem cells and Alzheimer’s disease: developments, limitations, problems and promises. Curr. Alzheimer Res. 6 461–470. 10.2174/156720509790147151 [DOI] [PubMed] [Google Scholar]
- Temple S. (2001). The development of neural stem cells. Nature 414 112–117. 10.1038/35102174 [DOI] [PubMed] [Google Scholar]
- Than-Trong E., Bally-Cuif L. (2015). Radial glia and neural progenitors in the adult zebrafish central nervous system. Glia 63 1406–1428. 10.1002/glia.22856 [DOI] [PubMed] [Google Scholar]
- Than-Trong E., Ortica-Gatti S., Mella S., Nepal C., Alunni A., Bally-Cuif L. (2018). Neural stem cell quiescence and stemness are molecularly distinct outputs of the Notch3 signalling cascade in the vertebrate adult brain. Development 145:dev161034. 10.1242/dev.161034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tincer G., Mashkaryan V., Bhattarai P., Kizil C. (2016). Neural stem/progenitor cells in Alzheimer’s disease. Yale J. Biol. Med. 8923–35. [PMC free article] [PubMed] [Google Scholar]
- Tong L. M., Fong H., Huang Y. (2015). Stem cell therapy for Alzheimer’s disease and related disorders: current status and future perspectives. Exp. Mol. Med. 47:e151. 10.1038/emm.2014.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski M. H., U H. S., Amaral D. G., Gage F. H. (1990). Nerve growth factor infusion in the primate brain reduces lesion-induced cholinergic neuronal degeneration. J. Neurosci. 10 3604–3614. 10.1523/JNEUROSCI.10-11-03604.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban N., Guillemot F. (2015). Neurogenesis in the embryonic and adult brain: same regulators, different roles. Front. Cell. Neurosci. 8:160 10.3389/fncel.2015.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tijn P., Kamphuis W., Marlatt M. W., Hol E. M., Lucassen P. J. (2011). Presenilin mouse and zebrafish models for dementia: focus on neurogenesis. Prog. Neurobiol. 93 149–164. 10.1016/j.pneurobio.2010.10.008 [DOI] [PubMed] [Google Scholar]
- Waldau B., Shetty A. K. (2008). Behavior of neural stem cells in the Alzheimer brain. Cell Mol. Life Sci. 65 2372–2384. 10.1007/s00018-008-8053-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen P. H., Hof P. R., Chen X., Gluck K., Austin G., Younkin S. G., et al. (2004). The presenilin-1 familial Alzheimer disease mutant P117L impairs neurogenesis in the hippocampus of adult mice. Exp. Neurol. 188 224–237. 10.1016/j.expneurol.2004.04.002 [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T. (2006). Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat. Med. 12 1005–1015. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T. (2016). Ageing, neurodegeneration and brain rejuvenation. Nature 539 180–186. 10.1038/nature20411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Y., Noble S., Ekker M. (2011). Modeling neurodegeneration in zebrafish. Curr. Neurol. Neurosci. Rep. 11 274–282. 10.1007/s11910-011-0182-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki T. R., Blurton-Jones M., Morrissette D. A., Kitazawa M., Oddo S., LaFerla F. M. (2007). Neural stem cells improve memory in an inducible mouse model of neuronal loss. J. Neurosci. 27 11925–11933. 10.1523/JNEUROSCI.1627-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A. J., Knauer M., Burdick D. A., Glabe C. (1995). Intracellular A beta 1-42 aggregates stimulate the accumulation of stable, insoluble amyloidogenic fragments of the amyloid precursor protein in transfected cells. J. Biol. Chem. 270 14786–14792. 10.1074/jbc.270.24.14786 [DOI] [PubMed] [Google Scholar]
- Younkin S. G. (1998). The role of A beta 42 in Alzheimer’s disease. J. Physiol. Paris 92 289–292. 10.1016/S0928-4257(98)80035-1 [DOI] [PubMed] [Google Scholar]
- Zhang D., Pekkanen-Mattila M., Shahsavani M., Falk A., Teixeira A. I., Herland A. (2014). A 3D Alzheimer’s disease culture model and the induction of P21-activated kinase mediated sensing in iPSC derived neurons. Biomaterials 35 1420–1428. 10.1016/j.biomaterials.2013.11.028 [DOI] [PubMed] [Google Scholar]
- Ziabreva I., Perry E., Perry R., Minger S. L., Ekonomou A., Przyborski S., et al. (2006). Altered neurogenesis in Alzheimer’s disease. J. Psychosom. Res. 61 311–316. 10.1016/j.jpsychores.2006.07.017 [DOI] [PubMed] [Google Scholar]
- Zupanc G. K. (2008). Adult neurogenesis and neuronal regeneration in the brain of teleost fish. J. Physiol. Paris 102 357–373. 10.1016/j.jphysparis.2008.10.007 [DOI] [PubMed] [Google Scholar]