Summary
Cardiac allograft rejection can be accompanied by diastolic dysfunction, but the hemodynamic change is usually compensated and hard to be recognized noninvasively. Here we report on two transplanted patients who showed electrocardiogram (ECG) changes suggesting right ventricular overload. Hemodynamic measurement revealed increased right ventricular pressure and endomyocardial biopsy confirmed grade 3R rejection. After rejection was treated with steroid pulse, the ECG alterations were reversed and right ventricular pressure was normalized. In such cases, asymptomatic rejection may be diagnosed by ECG changes that are reversible along with the treatment of rejection, although those ECG changes are apparently non-specific.
Keywords: Cardiac rejection, Heart transplantation, Diastolic dysfunction, Right axis deviation, Electrocardiogram
Introduction
The improvement in surgical techniques and advances in immunosuppressant therapy after heart transplantation (HTx) have increased survival rates among transplant recipients, but acute allograft rejection is still a concern. Earlier detection of cardiac rejection is important to avoid graft failure, but the diagnostic methods are limited. Thus far, endomyocardial biopsy has been the only established way to detect acute cellular rejection, and other noninvasive methods are considered to have limitations. Even though Allomap has recently been developed as a promising alternative [1], it can only suggest the absence of rejection.
Acute cellular rejection in a moderate form occasionally accompanies diastolic dysfunction [2], albeit the hemodynamic change is usually compensated in terms of cardiac output. An increase in pulmonary capillary wedge (PCW) pressure resulted from left ventricular (LV) diastolic dysfunction may lead to an elevation of right ventricular (RV) systolic pressure, whereas RV diastolic dysfunction is often associated with increased RV end-diastolic pressure. However, moderate rejection is rarely complicated with LV systolic dysfunction, and the above-mentioned diastolic dysfunction is difficult to be recognized by noninvasive methods such as routine echocardiography [3]. Therefore, our cases reported here were unique since we were able to diagnose rejection by electrocardiographic (ECG) changes suggesting RV overload.
Case report
The first patient was a 25-year-old female with refractory heart failure resulting from postpartum cardiomyopathy. After implantation of an extracorporeal LV assist device for 2 years, she received HTx in August 2008. She had had rejection with International Society of Heart and Lung Transplantation grade 2R twice that had been treated with methylpredonisolone pulse each time, but her last endomyocardial biopsy in August 2009 showed no evidence of rejection (Fig. 1D). Because ECG changes were observed at the routine visit on our outpatient clinic in April 2010, she was warranted unplanned hospitalization, although asymptomatic. She had been treated with a combination of cyclosporine, mycophenolate mofetil, everolimus, low dose of prednisolone, enalapril, spironolactone, and pravastatin.
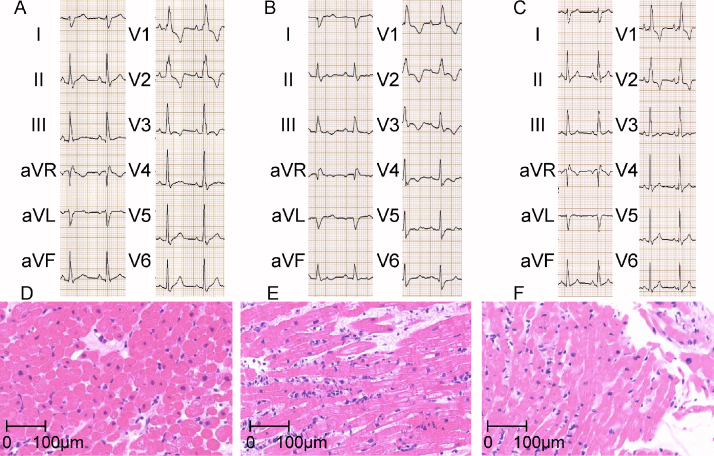
Figure 1.
(A–C) Electrocardiogram (ECG) obtained on 4 September 2009 (before), on 16 April 2010 (during), and on 30 April 2010 (after rejection) in case 1 are shown, respectively. (D–F) Endomyocardial biopsy obtained from right ventricle on 4 September 2009 (before), on 16 April 2010 (during), and on 30 April 2010 (after rejection) in case 1 are shown, respectively. Each biopsy showed International Society of Heart and Lung Transplantation grade 0, 3R, 1R, respectively.
Her 12-lead ECG showed complete right bundle branch block (RBBB) since HTx (Fig. 1A), but on admission it showed disappearance of R waves in I, aVL, and prolongation of PR intervals with wider QRS duration and deeper S waves in V5,6 compared with the previous ones (Fig. 1A and B). ST depression and T wave inversion in II, III, aVF, and V4–6 was also observed. Echocardiography revealed normal ejection fraction with marginally elevated E/e′ but no pericardial effusion. Plasma level of B-type natriuretic peptide (BNP) was increased by more than 2-fold (Table 1). We performed hemodynamic measurement and endomyocardial biopsy from RV. RV systolic, RV diastolic, right atrial (RA) as well as PCW pressures were all markedly elevated compared with her previous hemodynamic data (Table 1). Endomyocardial biopsy showed grade 3R cellular rejection without any evidence of humoral rejection (Fig. 1E). After treatment with steroid pulse (1000 mg of methylpredonisolone daily for 3 days), the changes in ECG and hemodynamics were reversed (Fig. 1C, Table 1), and the biopsy specimen from RV endomyocardium showed grade 1R cellular rejection (Fig. 1F). Intravascular ultrasound (IVUS) showed only mild plaque without any changes in the proximal portion of left anterior descending coronary artery in August 2009 (before this episode) and November 2010 (after this episode).
Table 1.
Echocardiographic data, hemodynamic parameters and laboratory data before and after treatment of allograft rejection in case 1.
| 4 September 2009 | 16 April 2010 | 30 April 2010 | |
|---|---|---|---|
| ISHLT rejection grade | 0 | 3R | 1R |
| Echocardiographic data | |||
| LVDd (mm) | 41 | 44 | 41 |
| LVDs (mm) | 27 | 28 | 27 |
| Ejection fraction (%) | 65 | 65 | 64 |
| E wave (cm/s) | 66.1 | 71.5 | 65.9 |
| A wave (cm/s) | 31.8 | 33.3 | 29.2 |
| Ea wave (cm/s) | 15.1 | 7.2 | 15.3 |
| Deceleration time (ms) | 183 | 200 | 189 |
| E/A ratio | 2.08 | 2.15 | 2.26 |
| E/Ea ratio | 4.38 | 9.93 | 4.31 |
| eRVsP (mmHg) | 37 | 42 | 39 |
| Hemodynamic parameters | |||
| RA a/v/mean (mmHg) | 5/5/2 | 12/12/13 | 2/4/1 |
| RV systolic/end-diastolic (mmHg) | 22/5 | 34/15 | 21/3 |
| PCW a/v/mean (mmHg) | 7/7/4 | 14/14/12 | 7/8/6 |
| Cardiac output (L/min) | 4.00 | 4.37 | 4.93 |
| Cardiac index (L/min/m2) | 2.36 | 2.73 | 3.08 |
| Laboratory data | |||
| BNP (pg/mL) | 140.6 | 332.6 | 256.2 |
| SaO2 (%) | 99 | 98 | 98 |
| CTR of chest X-ray | 49.8 | 56.0 | 50.4 |
LVDd/Ds, left ventricular end-diastolic/-systolic diameter; E wave, early diastolic mitral inflow wave; A wave, late diastolic mitral inflow wave; Ea wave, early diastolic mitral annular motion velocity; eRVsP, estimated right ventricular systolic pressure; RA, right atrium; RV, right ventricle; PA, pulmonary artery; PCW, pulmonary capillary wedge; BNP, B-type natriuretic peptide; CTR, cardiothracic ratio; SaO2, arterial oxygen saturation.
The second patient was a 48-year-old male, who had suffered obstruction of the left main trunk coronary artery and extensive myocardial infarction due to the dissection of ascending aorta in 2004. Bypass surgery had failed to rescue him, and subsequently he had been implanted with an extracorporeal left ventricular assist device. He had received HTx in 2006, and had been treated with tacrolimus, mycophenolate mofetil, enalapril, and atorvastatin without any event of rejection for 4 years, including the last result of biopsy in June 2010 (Fig. 2D). Although he denied any symptoms, his ECG was found abnormal in the outpatient clinic and he made an unscheduled admission to our hospital in October 2010.
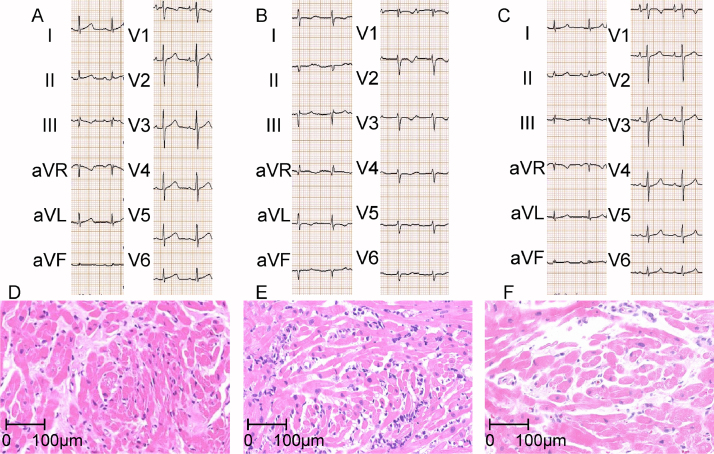
Figure 2.
(A–C) Electrocardiogram obtained on 10 June 2010 (before), on 1 October 2010 (during), and on 15 October 2010 (after rejection) in case 2 are shown, respectively. (D–F) Endomyocardial biopsy obtained from right ventricle on 10 June 2010 (before), on 1 October 2010 (during), and on 15 October 2010 (after rejection) in case 2 are shown, respectively. Each biopsy showed International Society of Heart and Lung Transplantation grade 0, 3R, 1R, respectively.
His ECG had been normal since HTx (Fig. 2A), but the ECG on admission showed deep S wave in I, II, aVF, poor R progression in all anterior precordial leads, marked prolongation of PR intervals, and deeper S wave in V4–6 (Fig. 2B). ST depression and T wave inversion in I, aVL, and V2–6 were also identified. Echocardiography showed preserved LV systolic function with no pericardial effusion, but tissue Doppler analysis showed markedly elevated E/e′ that strongly suggested LV diastolic dysfunction. Plasma BNP level was increased by approximately 6-fold (Table 2). Performed hemodynamic study revealed increases in both PCW pressure and RV systolic pressure, but elevations of RV diastolic pressure and RA pressure were modest (Table 2). IVUS at that time showed only mild plaque in mid portion of left anterior descending coronary artery. Endomyocardial biopsy showed grade 3R cellular rejection without any evidence of humoral rejection (Fig. 2E). After methylpredonisolone pulse twice, his 12-lead ECG and hemodynamic data considerably reversed (Fig. 2C, Table 2) with grade 1R rejection confirmed by endomyocardial biopsy (Fig. 2F).
Table 2.
Echocardiographic data, hemodynamic parameters and laboratory data before and after treatment of allograft rejection in case 2.
| 10 June 2010 | 1 October 2010 | 15 October 2010 | |
|---|---|---|---|
| ISHLT rejection grade | 0 | 3R | 1R |
| Echocardiographic data | |||
| LVDd (mm) | 43 | 44 | 45 |
| LVDs (mm) | 22 | 25 | 25 |
| Ejection fraction (%) | 81 | 75 | 77 |
| E wave (cm/s) | 83.3 | 102 | 78.3 |
| A wave (cm/s) | 21.7 | 38.9 | 26.2 |
| Ea wave (cm/s) | 16.8 | 4.0 | – |
| Deceleration time (ms) | 114 | 136 | 180 |
| E/A ratio | 3.04 | 2.62 | 2.99 |
| E/Ea ratio | 4.96 | 25.5 | – |
| eRVsP (mmHg) | 28 | 31 | 32 |
| Hemodynamic parameters | |||
| RA a/v/mean (mmHg) | 3/3/2 | 5/4/3 | 5/5/3 |
| RV systolic/end-diastolic (mmHg) | 17/3 | 27/5 | 21/3 |
| PCW a/v/mean (mmHg) | 6/7/5 | 11/12/9 | 12/10/7 |
| Cardiac output (L/min) | 4.44 | 4.51 | 3.90 |
| Cardiac index (L/min/m2) | 2.34 | 2.37 | 2.05 |
| Laboratory data | |||
| BNP (pg/mL) | 28.1 | 187.3 | 96.6 |
| SaO2 (%) | 97 | 96 | 96 |
| CTR of chest X-ray | 49.2 | 52.8 | 51.0 |
LVDd/Ds, left ventricular end-diastolic/-systolic diameter; E wave, early diastolic mitral inflow wave; A wave, late diastolic mitral inflow wave; Ea wave, early diastolic mitral annular motion velocity; eRVsP, estimated right ventricular systolic pressure; RA, right atrium; RV, right ventricle; PA, pulmonary artery; PCW, pulmonary capillary wedge; BNP, B-type natriuretic peptide; CTR, cardiothracic ratio; SaO2, arterial oxygen saturation.
Discussion
We experienced two cases of reversible ECG abnormalities during treatment of cardiac rejection. These ECG changes appeared to be consequences of RV overload that resulted from diastolic dysfunction of LV and/or RV. These two patients were totally asymptomatic and had normal systolic function in echocardiography, but we were able to diagnose their rejection by ECG changes. As far as we know, this is the first report describing a close relationship among ECG changes, acute rejection, and hemodynamic abnormalities.
There are few reports presenting reversibility of ECG changes after treatment of allograft rejection. Jones et al. reported a loss of pre-excitation during acute cardiac rejection that recovered after treatment [4]. However, it is uncommon that donor hearts have pre-excitation. Several groups have tried to establish the relationship between RBBB and allograft rejection after HTx, but failed [5]. Only one group reported that progressive RBBB after HTx was related to cardiac rejection when progression was defined as QRS duration becoming wider by 20 ms than before [6]. Although the widening of QRS duration at the time of allograft rejection did not meet their criteria in our first case, the narrowing of QRS width as well as the shortening of PR intervals after steroid pulse may suggest the causal relationship between conduction disturbance and cardiac rejection. Reversible atrioventricular conduction was also observed in the second case.
Apart from conduction disturbance by rejection, allograft rejection occasionally associates with diastolic dysfunction [2]. In both cases, ECG changes such as deepening of S wave in I and anterolateral leads suggested RV overload that was consistent with diastolic dysfunction caused by rejection. According to the hemodynamic data, there was biventricular diastolic dysfunction in the first case. On the other hand, the second case appeared to be complicated solely with LV diastolic dysfunction. As was observed in both cases, E/e′ [3] as well as within-individual increases in BNP levels [7] may also be useful to detect rejection.
Though no ECG changes specific for rejection have been reported thus far, the changes described above, which may reflect real hemodynamic abnormalities, can be a diagnostic tool for rejection.
References
- 1.Pham M.X., Teuteberg J.J., Kfoury A.G., Starling R.C., Deng M.C., Cappola T.P., Kao A., Anderson A.S., Cotts W.G., Ewald G.A., Baran D.A., Bogaev R.C., Elashoff B., Baron H., Yee J. Gene-expression profiling for rejection surveillance after cardiac transplantation. N Engl J Med. 2010;362:1890–1900. doi: 10.1056/NEJMoa0912965. [DOI] [PubMed] [Google Scholar]
- 2.Skowronski E.W., Epstein M., Ota D., Hoagland P.M., Gordon J.B., Adamson R.M., McDaniel M., Peterson K.L., Smith S.C., Jr., Jaski B.E. Right and left ventricular function after cardiac transplantation. Changes during and after rejection. Circulation. 1991;84:2409–2417. doi: 10.1161/01.cir.84.6.2409. [DOI] [PubMed] [Google Scholar]
- 3.Mena C., Wencker D., Krumholz H.M., McNamara R.L. Detection of heart transplant rejection in adults by echocardiographic diastolic indices: a systematic review of the literature. J Am Soc Echocardiogr. 2006;19:1295–1300. doi: 10.1016/j.echo.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 4.Jones D.G., Bougard R.S., Burke M.M., Banner N.R. Reversible loss of pre-excitation as a sign of acute cardiac rejection. J Heart Lung Transplant. 2009;28:647–650. doi: 10.1016/j.healun.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Golshayan D., Seydoux C., Berguer D.G., Stumpe F., Hurni M., Ruchat P., Fischer A., Mueller X., Sadeghi H., von Segesser L., Goy J.J. Incidence and prognostic value of electrocardiographic abnormalities after heart transplantation. Clin Cardiol. 1998;21:680–684. doi: 10.1002/clc.4960210914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osa A., Almenar L., Arnau M.A., Martínez-Dolz L., Rueda J., Morillas P., Palencia M. Is the prognosis poorer in heart transplanted patients who develop a right bundle branch block? J Heart Lung Transplant. 2000;19:207–214. doi: 10.1016/s1053-2498(99)00122-9. [DOI] [PubMed] [Google Scholar]
- 7.Kittleson M.M., Skojec D.V., Wittstein I.S., Champion H.C., Judge D.P., Barouch L.A., Halushka M., Hare J.M., Kasper E.K., Russell S.D. The change in B-type natriuretic peptide levels over time predicts significant rejection in cardiac transplant recipients. J Heart Lung Transplant. 2009;28:704–709. doi: 10.1016/j.healun.2009.04.019. [DOI] [PubMed] [Google Scholar]