Abstract
Key points
Intrauterine growth restriction (IUGR) increases offspring risk of chronic diseases later in life, including cardiovascular dysfunction.
Our prior studies suggest biventricular cardiac dysfunction and vascular impairment in baboons who were IUGR at birth because of moderate maternal nutrient reduction.
The current study reveals changes in artery sizes, distensibility, and blood flow pattern in young adult IUGR baboons, which may contribute to cardiac stress.
The pattern of abnormality observed suggests that vascular redistribution seen with IUGR in fetal life may continue into adulthood.
Abstract
Maternal nutrient reduction induces intrauterine growth restriction (IUGR), increasing risks of chronic diseases later in life, including cardiovascular dysfunction. Using ultrasound, we determined regional blood flow, blood vessel sizes, and distensibility in IUGR baboons (8 males, 8 females, 8.8 years, similar to 35 human years) and controls (12 males, 12 females, 9.5 years). The measured blood vessels were larger in size in the males compared to females before but not after normalization to body surface area. Smaller IUGR normalized blood vessel sizes were observed in the femoral and external iliac arteries but not the brachial or common carotid arteries and not correlated significantly with birth weight. Mild decrease in distensibility in the IUGR group was seen in the iliac but not the carotid arteries without between‐sex differences. In IUGR baboons there was increased carotid arterial blood flow velocity during late systole and diastole. Overall, our findings support the conclusion that region specific vascular and haemodynamic changes occur with IUGR, which may contribute to the occurrence of later life cardiac dysfunction. The pattern of alteration observed suggests vascular redistribution efforts in response to challenges in the perinatal period may persist into adulthood. Further studies are needed to determine the life course progression of these changes.
Keywords: baboon, developmental programming, intrauterine growth restriction (IUGR), maternal nutrient reduction, nonhuman primates, distensibility, blood vessels, arteries, blood flow
Key points
Intrauterine growth restriction (IUGR) increases offspring risk of chronic diseases later in life, including cardiovascular dysfunction.
Our prior studies suggest biventricular cardiac dysfunction and vascular impairment in baboons who were IUGR at birth because of moderate maternal nutrient reduction.
The current study reveals changes in artery sizes, distensibility, and blood flow pattern in young adult IUGR baboons, which may contribute to cardiac stress.
The pattern of abnormality observed suggests that vascular redistribution seen with IUGR in fetal life may continue into adulthood.
Abbreviations
- BSA
body surface area
- IUGR
intrauterine growth restriction
- MAP
mean arterial pressure
- MNR
maternal nutrient reduction
- MRI
magnetic resonance imaging
- NS
not significant
- PI
pulsatility index
- RI
resistivity index
Introduction
Cardiovascular function can be programmed by challenges during perinatal development (Godfrey et al. 2007). Low birth weight (a hallmark of intrauterine growth restriction, IUGR) has been linked to increased incidence of heart disease, vascular disease, stroke, and hypertension in humans (Fowden et al. 2006). In a study of 15,000 Swedish men and women over a span of more than 60 years, mortality from ischaemic heart disease was strongly correlated with low birth weight (Leon et al. 1998). In animal studies, IUGR leads to profound impairment of post‐ischaemic recovery of cardiac function (Xu et al. 2006). Various components of those programming changes have been replicated in animal models, suggestive of a causal relationship (Armitage et al. 2004). Yet, the mechanisms underlying these predispositions remain incompletely understood.
In our non‐human primate model of IUGR resulting from moderate maternal undernutrition during pregnancy and lactation (30% reduction in global nutrient intake), we demonstrated impaired cardiac function using magnetic resonance imaging (MRI) (Kuo et al. 2016, 2017a). Impaired filling function of the cardiac ventricles was seen along with reduction in biventricular ejection fraction, as well as changes in cardiac morphology, indicating reduced cardiac reserve and resembling subclinical heart failure. Importantly, our investigation yielded a constellation of findings suggesting elevated afterload, a known major contributor to diastolic predominant cardiac dysfunction (Leite‐Moreira et al. 1999). While cardiac programming changes across various animal models are not always congruent, our findings share many similarities to results from other IUGR methods, and may have risen from reduction in cardiomyocyte endowment, contractility, or metabolic regulation as described in the literature (Botting et al. 2012; Blackmore & Ozanne, 2015). We have also shown both decreased size and distensibility in the distal thoracic aorta of this IUGR cohort, suggesting the presence of vascular structure and function alteration (Kuo et al. 2017b). Given the intricate relationship between the systemic vasculature and afterload, we sought to further investigate regional vascular structure and blood flow in the IUGR offspring.
The concurrent presence of cardiac and vascular abnormalities has been reported in many programming models, including hypoxia‐induced IUGR chick (Salinas et al. 2014), placental embolization‐induced IUGR sheep (Bubb et al. 2007), and protein restriction‐induced IUGR rat (Menendez‐Castro et al. 2011). The function of the vasculature is greatly dependent on its size (Rodbard, 1975). Children born of small stature are known to possess proportionally sized vasculature (Walther et al. 1986). If compensatory growth of the body is not accompanied by matching extent of vascular expansion in the postnatal period, a mismatch of vascular supply and systemic demand will likely develop, which can lead to changes in haemodynamics and contribute to cardiac stress and peripheral organ dysfunction (Norman, 2008). Supporting the importance of matching vascular function to overall size, when postnatal catch‐up growth in IUGR animal models is decreased by concurrent postnatal undernutrition, a more benign cardiovascular phenotype is observed than when catch‐up growth occurs (Huxley et al. 2000; Cleal et al. 2007). IUGR is associated with a host of circulatory abnormalities in the perinatal period (Arbeille, 1997; Galan et al. 2005). Left uncorrected, the distorted fetal haemodynamics can lead to adult pathology. Based on our previous findings suggestive of increased afterload, we hypothesize that vascular mismatch is present in IUGR baboon offspring, which partially underlies the previously described impairment in cardiac function. More specifically, we aim to detect changes in regional blood vessel size, distensibility, and blood flow in the adult IUGR baboons due to prior maternal nutrient reduction.
Methods
Ethical approval
All procedures were approved by the Texas Biomedical Research Institute Institutional Animal Care and Use Committees (IACUC) and conducted in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care. The IACUC is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. This work complies with the animal ethical principles under which The Journal of Physiology operates and with the checklist outlined by Grundy (Grundy, 2015).
Animal model
Baboons (Papio species, Southwest National Primate Research Center, Texas Biomedical Research Institute, San Antonio, TX, USA) were housed and maintained in an outdoor, group social environment and fed using an individual feeding system (Schlabritz‐Loutsevitch et al. 2004; VandeBerg et al. 2009). Healthy gravid female baboons of similar age and weight were randomly assigned to an ad libitum diet during pregnancy and lactation or a globally reduced diet regimen consisting of 70% of feed eaten by control ad libitum fed mothers from 0.16 gestation (G) to end of lactation (Li et al. 2017). The offspring baboons were fully weaned at 9 months of age and moved to juvenile group housing, where ad libitum diet was given. The diet was Monkey Diet 5038 (Purina LabDiets, St Louis, MO, USA), which contains 13% calories from fat, 18% calories from protein, 69% calories from carbohydrates, mineral and vitamin additives, and a metabolizable energy content of 3.22 kcal g−1. Unrestricted access to water was provided.
Vascular ultrasonography
Vascular ultrasonography was performed on two groups of baboons, IUGR (8 male, 8 female, age = 8.8 ± 1.2 years; mean ± SD) and age‐matched control baboons (CTL, 12 male, 12 female, age = 9.5 ± 1.7 years). To account for potential diurnal and prandial effects on vascular regulation, the studies were conducted during the same time of the day, in the morning (09.00–11.00) after overnight fast. Subject baseline data are shown in Table 1.
Table 1.
Baseline characteristics and vital data of subjects studied (mean ± SEM)
| Control | IUGR | ||||
|---|---|---|---|---|---|
| Group | M | F | M | F | ANOVA |
| Number | 12 | 12 | 8 | 8 | — |
| Age (years) | 9.5 ± 0.5 | 9.6 ± 0.5 | 9.1 ± 0.4 | 8.6 ± 0.5 | NS |
| Weight (kg) | 31.0 ± 1.4 | 17.3 ± 0.8 | 27.5 ± 0.8 | 16.9 ± 0.9 | M > F*** |
| Calculated body surface area (m2) | 0.76 ± 0.02 | 0.51 ± 0.01 | 0.70 ± 0.01 | 0.50 ± 0.02 | M > F*** |
| Birth weight (kg) | 0.92 ± 0.03 | 0.82 ± 0.04 | 0.82 ± 0.03 | 0.74 ± 0.05 | M > F*
CTL > IUGR* |
| Systolic BP (mmHg) | 110 ± 6 | 124 ± 7 | 116 ± 5 | 116 ± 4 | NS |
| Diastolic BP (mmHg) | 60 ± 4 | 71 ± 6 | 66 ± 6 | 70 ± 4 | NS |
| Pulse BP (mmHg) | 50 ± 3 | 53 ± 2 | 51 ± 6 | 47 ± 2 | NS |
| MAP (mmHg) | 77 ± 5 | 89 ± 7 | 83 ± 5 | 85 ± 4 | NS |
| Heart rate (beats min−1) | 102 ± 5 | 114 ± 5 | 97 ± 4 | 122 ± 7 | F > M** |
ANOVA main effects reported. No significant sex‐group interaction was found. * P < 0.05; ** P < 0.01; *** P < 0.001; NS, not significant.
Evaluation of all subjects was performed on the same portable Terason Ultrasound System 2000 (Teratech Corp., Burlington, MA, USA) using the Terason 10L5 transducer (linear transducer, 5–10 MHz, 128 elements) without administration of intravascular contrast. Ultrasound was performed cage‐side under anaesthesia following removal of hair in the lateral cervical, medial arm, inguinal, and medial thigh regions by an electric razor. Anaesthesia was induced with single dose of ketamine hydrochloride (10 mg kg−1, i.m.), which lasts approximately 25 min in duration. Aquasonic 100 Ultrasound Transmission Gel (Parker Laboratories, Inc., Fairfield, NJ, USA) was used as coupling medium to improve contact and image quality. Longitudinal and axial cine images were obtained in triplicate from the (a) bilateral common carotid arteries 3 cm proximal to the carotid bifurcation, (b) bilateral brachial arteries at the level of the mid‐humerus, (c) bilateral external iliac arteries 1 cm proximal to the inguinal fold, and (d) bilateral femoral arteries at the level of the mid‐femur. The arteries were differentiated from the adjacent veins by either presence of visible pulsatile movement or arterial waveform by Doppler evaluation. Depth of view was set at 2 cm with focus zone set at the level of the vessel of interest, which allowed visualization of the arteries in all animals (Fig. 1). Additionally, Doppler waveform of the bilateral common carotid arteries were obtained approximately 3 cm proximal to the carotid bifurcation with angle correction maintained at or below 60 deg and sampling size of 2 mm (Fig. 2 A). Upon completion of the study, visual assessment of the animal for respiration, mucosal coloration, and movement was performed at regular intervals until the animal was alert, in a sternal position, and demonstrated control of movement. After full recovery, baboons were returned to their group cages.
Figure 1. Measurement of vessel luminal area.
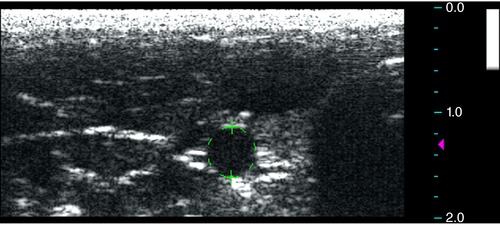
Measurements of the arterial lumen area were obtained using cine axial images with focus zone set at the level of the vessel and depth of 2 cm using the built‐in software. The arteries were differentiated from the adjacent veins by either the presence of visible pulsatile movement or arterial Doppler waveform evaluations. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 2. Measurement and analysis of common carotid Doppler waveform.
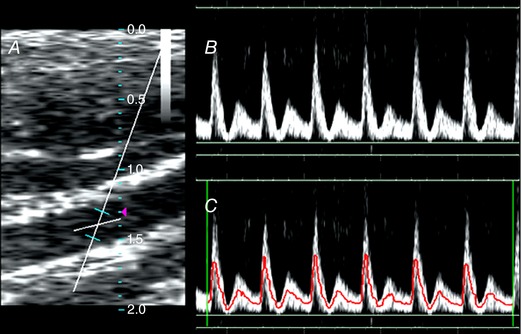
A, Doppler waveforms of the bilateral common carotid arteries were obtained with angle correction maintained at or below 60 deg and sampling size of 2 mm. B and C, the automatic tracing algorithm of the built‐in processing software then was applied on the obtained spectra (B) to generate intensity‐weighted mean frequency velocity tracings (C). The various velocities were measured from the tracings. [Color figure can be viewed at http://wileyonlinelibrary.com]
Blood pressure
Blood pressure measurements were acquired from an Omron HBP‐1300 sphygmomanometer in the left upper arm, using a small (17–22 cm) or a medium (22–32 cm) cuff. Measurements were obtained with the baboons in the supine position. Three measurements were obtained and averaged. Pulse pressure is defined as the difference between systolic and diastolic blood pressure. Mean arterial pressure (MAP) was approximated by the formula:
as conventionally defined.
Image processing and analysis
Image processing and measurement were performed using the Terason Ultrasound System 2000 built‐in image processing software. The diastolic and systolic vessel lumen dimensions were obtained using the axial images by measuring the minimal and maximal anechoic area of each heartbeat, respectively. The values reported were averages of the systolic and diastolic lumen areas. Triplicate measurements were obtained and averaged. In a few limited instances where the vessel edge was not clearly delineated on axial images, longitudinal images were used to calculate the lumen dimension assuming circular geometry. Distensibility was calculated using the formula:
where ΔP is the pulse pressure.
To analyse carotid arterial waveforms, the built‐in automatic tracing algorithm was applied to the obtained spectra (Fig. 2 B) to generate intensity‐weighted mean frequency velocity tracings to counter the effects of spectral broadening and minimize measurement error (Fig. 2 C). Peak systolic velocity and end‐diastolic velocities were measured from the tracings. Time‐average velocity (intensity‐weighted mean frequency) was calculated by the machine software. Resistivity index (RI) and pulsatility index (PI) were calculated as:
and
respectively, as conventionally defined.
Normalization
The dimensional measurements were also normalized to the body surface area (BSA), using weight based models previously described for baboons (Glassman et al. 1984; VandeBerg et al. 2009). For females,
and in males,
where weight is measured in kilograms, and the calculated BSA has unit of metres squared (m2).
Statistical analysis
Data were analysed using GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA). Grubbs’ test (extreme Studentized deviate) was used to evaluate for statistical outliers. Normality of distribution was assessed by the d'Agostino‐Pearson test. Two‐way ANOVA was used to evaluate the null hypotheses that there were no differences between the factors group and sex and no significant interactions. Given no group‐sex interaction was detected in any of the measurements, ANOVA main effects were reported without further post hoc analysis. In cases of vessels area and distensibility where statistically significant differences were seen between groups, correlation analyses between the measurements and birth weight were performed to see whether the extent of the abnormality could be linked to the degree of IUGR.
Data are presented as means ± standard error of the mean (SEM). Number of animals used in the final analysis is indicated as N. Statistical significance was set at P < 0.05 for all tests.
Results
Subject baseline characteristics and vital data
Baseline morphometric and vital data of the baboon cohorts are shown in Table 1. Male baboons demonstrated higher body weights than females (P < 0.001), a known physiological difference. Similarly, birth weight (P = 0.03) and estimated body surface area (P < 0.001) were higher in the males compared to the females. IUGR birth weights were reduced compared to age‐matched controls (P = 0.02).
The systolic blood pressure (P = 0.9), diastolic blood pressure (P = 0.7), pulse pressure (P = 0.4), and mean arterial blood pressure (P = 0.9) were not different between groups or sexes. Heart rate was higher in female baboons compared to males (P = 0.002), a known physiological difference in humans (Umetani et al. 1998).
Common carotid artery measurements
The common carotid artery measurements are summarized in Table 2. Group‐sex interaction was not seen in any of the common carotid artery measurements. The common carotid artery peak systolic velocity was not different between groups (Fig. 3 A, P = 0.5). Male baboons demonstrated higher peak systolic velocity (P < 0.001) than females. Increased end‐systolic velocity (17.8 ± 1.1 cm s−1 vs. 12.5 ± 1.7 cm s−1, P = 0.02, Fig. 3 B) and end‐diastolic velocity (17.3 ± 0.9 cm s−1 vs. 15.0 ± 0.8 cm s−1, P = 0.04, Fig. 3 C) were seen in the IUGR group compared to controls (CTL). The change in time‐average velocity between groups did not reach significance (27.6 ± 1.3 cm s−1 vs. 25.6 ± 1.1 cm s−1, P = 0.2, Fig. 3 D). Similarly, the resistive index (IUGR 75.2 ± 1.1% vs. CTL 77.7 ± 1.0%, P = 0.1) and pulsatility index (IUGR 1.95 ± 0.09 vs. CTL 2.10 ± 0.08, P = 0.2) decreased in the IUGR group without reaching significance. The common carotid artery lumen area was larger in males before but not after normalization to BSA and not different between groups (Fig. 3 E). Overall, these changes led to a trend of increased normalized common carotid artery flow rate in the IUGR group that did not reach significance (47.8 ± 2.9 mm3 s−1 m−2 vs. 42.4 ± 2.1 mm3 s−1 m−2, P = 0.1).
Table 2.
Common carotid artery measurements (mean ± SEM)
| Group | Control (N = 24) | IUGR (N = 16) | ANOVA |
|---|---|---|---|
| Absolute values | |||
| Common carotid artery lumen area (mm2) | 10.4 ± 0.4 | 10.5 ± 0.7 | M > F*** |
| Male | 12.1 ± 0.5 | 12.5 ± 0.7 | |
| Female | 8.8 ± 0.3 | 8.5 ± 0.4 | |
| Common carotid artery flowrate (mm3 s−1) | 26.9 ± 1.8 | 29.5 ± 2.7 | M > F*** |
| Male | 32.7 ± 2.3 | 37.7 ± 2.7 | |
| Female | 21.2 ± 1.4 | 21.3 ± 2.1 | |
| Common carotid artery distensibility (10−3 mmHg−1) | 5.68 ± 0.41 | 6.19 ± 0.72 | NS |
| Male | 5.47 ± 0.57 | 5.09 ± 0.77 | |
| Female | 5.90 ± 0.62 | 7.28 ± 1.14 | |
| Peak systolic velocity (cm s−1) | 68.2 ± 3.0 | 71.3 ± 4.2 | M > F*** |
| Male | 73.5 ± 4.5 | 83.0 ± 3.7 | |
| Female | 62.9 ± 3.4 | 59.6 ± 4.7 | |
| End systolic velocity (cm s−1) | 12.5 ± 1.7 | 17.8 ± 1.1 | IUGR > CTL* |
| Male | 10.2 ± 3.1 | 18.3 ± 1.4 | |
| Female | 14.7 ± 1.1 | 17.4 ± 1.7 | |
| End diastolic velocity (cm s−1) | 15.0 ± 0.8 | 17.3 ± 0.9 |
M > F**
IUGR > CTL* |
| Male | 16.2 ± 1.3 | 19.3 ± 0.9 | |
| Female | 13.8 ± 0.7 | 15.4 ± 1.2 | |
| Time‐average velocity (cm s−1) | 25.6 ± 1.1 | 27.6 ± 1.3 | M > F* |
| Male | 27.1 ± 1.8 | 30.2 ± 1.4 | |
| Female | 24.0 ± 1.2 | 25.1 ± 1.8 | |
| Resistive index (RI, ratio in %) | 77.7 ± 1.0 | 75.2 ± 1.1 | NS |
| Male | 77.7 ± 1.5 | 76.5 ± 1.2 | |
| Female | 77.6 ± 1.5 | 73.9 ± 1.9 | |
| Pulsatility index (PI, ratio) | 2.10 ± 0.08 | 1.95 ± 0.09 | NS |
| Male | 2.14 ± 0.11 | 2.13 ± 0.13 | |
| Female | 2.05 ± 0.11 | 1.77 ± 0.10 | |
| Normalized to body surface area (BSA) | |||
| Common carotid artery luminal area/BSA (mm2 m−2) | 16.6 ± 0.4 | 17.3 ± 0.6 | NS |
| Male | 16.0 ± 0.7 | 17.7 ± 1.0 | |
| Female | 17.2 ± 0.5 | 16.9 ± 0.9 | |
| Common carotid artery flowrate/BSA (mm3 s−1 m−2) | 42.4 ± 2.1 | 47.8 ± 2.9 | NS |
| Male | 43.5 ± 3.6 | 53.3 ± 3.1 | |
| Female | 41.4 ± 2.2 | 42.3 ± 4.1 | |
ANOVA main effects reported. No significant sex‐group interaction was found. * P < 0.05; ** P < 0.01; *** P < 0.001; NS, not significant.
Figure 3. Common carotid artery measurements.
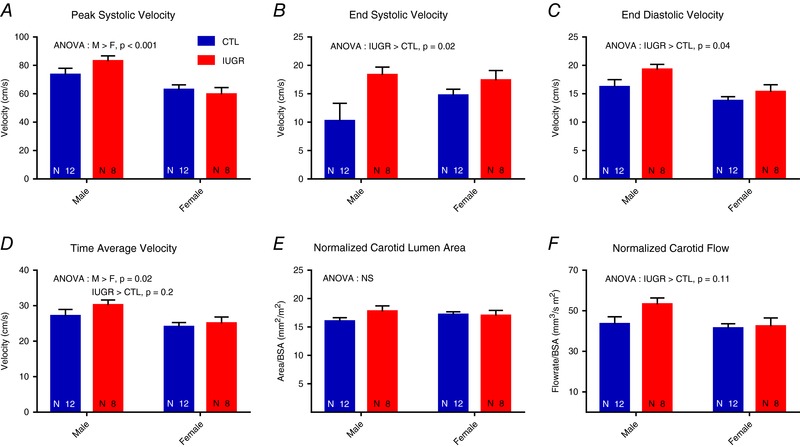
No group‐sex interaction was detected by ANOVA. A, the peak systolic velocity of the common carotid artery was higher in the males compared to the females without group differences. B and C, increased end‐systolic velocity (B) and end‐diastolic velocity (C) were seen in the IUGR group compared to controls (CTL) without between‐sex differences. D, time‐average velocity was not significantly different between groups. E, the lumen area was not different between groups. F, a trend of increased normalized carotid blood flow was observed in IUGR without reaching significance. [Color figure can be viewed at http://wileyonlinelibrary.com]
Brachial, external iliac, and femoral artery measurements
Brachial, external iliac, and femoral artery measurements are summarized in Table 3. Results of ANOVA did not reveal any group‐sex interactions in the measurements. Arterial lumen in all three arteries was larger in size in males than females before but not after normalization to BSA. The normalized brachial artery size was not different between groups (P = 0.1, Fig. 4 A). After normalization to BSA, the femoral (10.4 ± 0.6 mm2 m−2 vs. 13.1 ± 1.0 mm2 m−2, P < 0.05, Fig. 4 B) and external iliac (21.0 ± 1.2 mm2 m−2 vs. 25.3 ± 1.5 mm2 m−2, P < 0.05, Fig. 4 C) artery lumen areas were higher in the CTL group. Higher distensibility of the external iliac artery was seen in the CTL group (4.5 ± 0.5 × 10−3 mmHg−1 vs. 5.6 ± 0.3 10−3 mmHg−1, P < 0.05, Fig. 4 D). Correlation analysis revealed no correlation between birth weight and normalized iliac artery lumen size (P = 0.16), normalized femoral artery lumen size (P = 0.45), or iliac distensibility (P = 0.5).
Table 3.
Brachial, external iliac, and femoral artery measurements (mean ± SEM)
| Group | Control (N = 24) | IUGR (N = 16) | ANOVA |
|---|---|---|---|
| Absolute values | |||
| Brachial artery lumen area (mm2) | 6.5 ± 0.4 | 5.6 ± 0.5 |
M > F***
CTL > IUGR* |
| Male | 7.8 ± 0.4 | 6.9 ± 0.5 | |
| Female | 5.2 ± 0.1 | 4.4 ± 0.5 | |
| External iliac artery lumen area (mm2) | 16.2 ± 1.3 | 12.9 ± 4.2 |
M > F***
CTL > IUGR* |
| Male | 19.5 ± 2.0 | 16.3 ± 1.0 | |
| Female | 12.8 ± 1.1 | 9.4 ± 0.7 | |
| Femoral artery lumen area (mm2) | 8.4 ± 0.8 | 6.4 ± 0.6 |
M > F***
CTL > IUGR* |
| Male | 10.3 ± 1.2 | 8.0 ± 0.7 | |
| Female | 6.5 ± 0.7 | 4.8 ± 0.4 | |
| External iliac artery distensibility (10−3 mmHg−1) | 5.6 ± 0.3 | 4.5 ± 0.5 | CTL > IUGR* |
| Male | 5.8 ± 0.4 | 4.2 ± 0.8 | |
| Female | 5.4 ± 0.4 | 4.8 ± 0.7 | |
| Normalized to body surface area (BSA) | |||
| Brachial artery lumen area/BSA (mm2 m−2) | 10.2 ± 0.3 | 9.3 ± 0.6 | NS |
| Male | 10.3 ± 0.6 | 9.8 ± 0.7 | |
| Female | 10.2 ± 0.3 | 8.8 ± 0.9 | |
| External iliac artery lumen area/BSA (mm2 m−2) | 25.3 ± 1.5 | 21.0 ± 1.2 | CTL > IUGR* |
| Male | 25.5 ± 2.3 | 23.1 ± 1.3 | |
| Female | 25.0 ± 2.0 | 18.9 ± 1.6 | |
| Femoral artery lumen area/BSA (mm2 m−2) | 13.1 ± 1.0 | 10.4 ± 0.6 | CTL > IUGR* |
| Male | 13.6 ± 1.5 | 11.3 ± 0.9 | |
| Female | 12.7 ± 1.2 | 9.6 ± 0.7 | |
ANOVA main effects reported. No significant sex‐group interaction was found. * P < 0.05; ** P < 0.01; *** P < 0.001; NS, not significant.
Figure 4. Brachial, femoral, and iliac artery measurements.
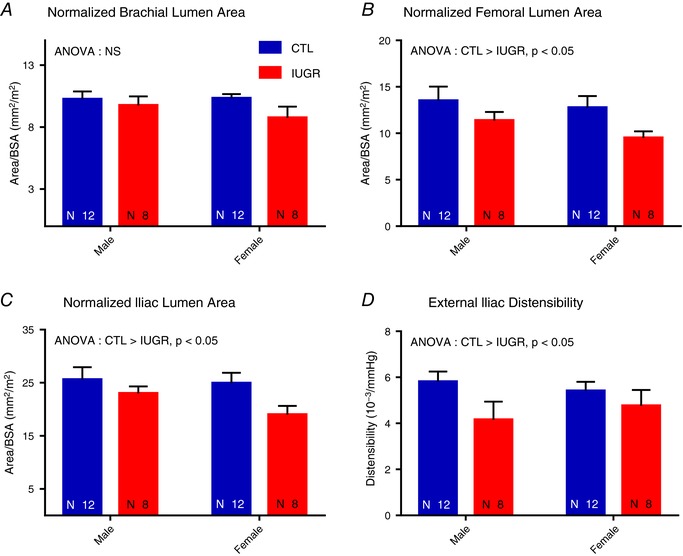
ANOVA detected no group‐sex interaction. A, the normalized brachial artery lumen area was not different between groups. B and C, decreased femoral (B) and iliac (C) artery lumen sizes are seen in the IUGR groups both before and after normalization to BSA. D, decreased distensibility of the iliac artery was seen in the IUGR group. [Color figure can be viewed at http://wileyonlinelibrary.com]
Discussion
While impaired cardiac function results from IUGR in both animal and human studies, the origin of the cardiac dysfunction is less clear. We uncovered vascular changes, more marked in the lower extremities than the carotid flow. More specifically, we identified decreased size and distensibility of the external iliac artery and decreased size of the femoral artery after adjustment for BSA without changes in size of the brachial or common carotid arteries. Increased end‐systolic and end‐diastolic IUGR common carotid artery velocities combined with unchanged carotid artery size suggest a subtle alteration of blood flow to the cerebral circulation in the IUGR group. These findings support indications of altered haemodynamics of the adult circulation with IUGR found in our previous studies on the performance of the left and right ventricles (Kuo et al. 2016, 2017a) and contribute to previous reports of increased incidence of vascular disease with IUGR, such as stroke and hypertension. To the best of our knowledge, this is the first study to report alterations in vascular health in a primate model exposed to developmental programming via maternal nutrient reduction.
This study addressed the premise that underdevelopment of the vascular tree in IUGR offspring alters vascular structure and regional blood flow with potential long‐lasting consequences. In this cohort, the previously reported reduced distal thoracic aorta diameter at 5–6 years (Kuo et al. 2017b), as well as the decreased external iliac artery and the femoral artery normalized diameters reported hereafter, are consistent with prior reports of decreased aortic size in pre‐adolescent (8–13 years) children born small for gestation age (Ley et al. 1997; Jiang et al. 2006; Bradley et al. 2010) and smaller aorta and popliteal arteries in adolescents (16–19 years) with clinical history of IUGR (Brodszki et al. 2005). Other studies reported values that demonstrate similar but non‐significant trends in the aorta of low birth weight pre‐adolescents (7–11 years) (Martin et al. 2000) and newborns (Akira & Yoshiyuki, 2006; Mori et al. 2006), as well as positive correlation between adult common femoral artery diameter and birth weight (mean age = 36 years) (te Velde et al. 2004). One complicating factor in some human studies is the inclusion of subjects from both term and pre‐term births since gestational age is associated with differences in aorta diameter (Bonamy et al. 2005; Schubert et al. 2011; Ciccone et al. 2013). The change in large artery size with IUGR has not been consistently demonstrated across animal models, and may be partially attributable to both variation in the method of IUGR induction and inter‐species difference. In rats, for example, the aorta size is unchanged with IUGR induced by hypoxia (Rueda‐Clausen et al. 2009) or maternal nutrient restriction (Ozaki et al. 2001). In hypoxic incubation of IUGR chick embryos, however, smaller ascending aortic lumens were seen (Rouwet et al. 2002). Smaller aortas were noted in maternal hyperthermia‐induced IUGR sheep (thoracic and abdominal segments) (Dodson et al. 2014) and maternal uterine artery ligation‐induced IUGR guinea‐pigs (descending thoracic segment) (Briscoe et al. 2004). We suspect timing of the evaluation contributes to the disparity of findings reported in the literature. In humans, larger abdominal aorta diameter was reported in IUGR compared to controls prenatally, but this relationship reverses by 18 months of age (Cosmi et al. 2009). Our results indicate that IUGR induced by maternal undernutrition results in decreased size of aorta and lower extremity arteries in primates in early to mid‐adulthood with no, or much less, effect on the cranially directed vessels.
Interestingly, we failed to observe similar decrease in size of the common carotid or brachial arteries, which may hint at the mechanism underpinning the reduction in vascular size with IUGR. The lack of association between low birth weight and brachial artery diameter has been documented in human children (8–13 years) (Franco et al. 2006), adolescents (13–19 years) (Singhal et al. 2001; Brodszki et al. 2005), and young adults (20–28 years) (Goodfellow et al. 1998; Leeson et al. 2001). Likewise, in most studies of IUGR humans, a difference in common carotid artery dimension has not been found (Martin et al. 2000; Bonamy et al. 2005; Brodszki et al. 2005; Mori et al. 2006). It has been hypothesized that the differential effects of IUGR on vessel size may be due to the relative sensitivity of elastic artery growth to IUGR compared to muscular arteries (Karatza & Varvarigou, 2013). Supporting this hypothesis, early studies on the rat aorta revealed that even though elongation of the aorta proceeds rapidly to 12 weeks of age (Berry et al. 1972), the increase in concentration of elastin of the aorta ceases at 18 days of age and decreases thereafter (Looker & Berry, 1972). Arguably, if deposition of elastin is reduced in the perinatal period, growth of elastic arteries may be impaired later in life. In line with this notion, in patients with decreased production of the elastin protein or microdeletion of the elastin gene, narrower arteries and obstructive arteriopathy are observed (Morris, 1998; Morris & Mervis, 2000). Further, it has been proposed that IUGR‐related decrease in size of the popliteal artery, a muscular artery, may be due to the popliteal artery being more similar in behaviour to a central elastic artery (Debasso et al. 2004). Yet, the decreased sizes of the external iliac and femoral arteries in our study suggest that the distinction between elastic and muscular arteries may not be the dominant determinant of arterial size in IUGR, given that both of those arteries are muscular arteries by both histology and function (Meyer et al. 1980).
Instead, we suspect decreased sizes of the aorta and lower extremity arteries in IUGR may be a long‐lasting consequence of blood flow redistribution in response to challenges that occur in fetal life, such as hypoxia (Cohn et al. 1974). Even though cardiac output is preserved, or even increased, in growth‐restricted fetuses, volume blood flow is decreased in the descending aorta (Gardiner et al. 2002), consistent with redirection of blood flow towards the key organs for survival, brain and heart, and away from the abdomen, pelvis, and lower extremities. This effect is thought to be protective in nature and serves to preserve cerebral and coronary blood flow, and is at least partially mediated by decreased vascular resistance in the internal carotid artery and increased vascular resistance in the descending aorta of the IUGR subjects (Wladimiroff et al. 1986, 1987; Baschat et al. 2001; Giussani, 2016).
The dependence of remodelling and growth of the vasculature on blood flow (Chapman, 1918), even after accounting for effects of hypoxia, has been accepted for nearly 100 years (Männer et al. 1995). Indeed, later studies have since determined that blood flow, via its effect on shear stress among other factors, plays an essential role in vascular growth, remodelling, and determination of vessel diameter (Jones et al. 2006). For example, a 70% reduction in blood flow through the common carotid artery in rabbits triggered a 21% decrease in vessel diameter within 2 weeks (Langille & O'Donnell, 1986). Similarly, a 35% blood flow reduction in rats resulted in 10% decrease in vessel diameter (Guyton & Hartley, 1985). Earlier studies in fetal lambs suggest a greater than 50% reduction in blood flow to non‐preserved organs such as intestines, spleen, and kidneys can result from fetal stress induced redistribution (Cohn et al. 1974). Given vascular flow to the lower body is decreased in IUGR fetuses during the critical window of development, under‐development/under‐remodelling of the vasculature would be expected to occur in these vascular beds. If no significant subsequent remodelling occurs, asymmetric vessel size would likely persist into adulthood, as we observed in these IUGR baboons.
The increased common carotid end‐systolic and end‐diastolic velocities led to a trend (P = 0.1) of increased blood flow to the cerebral circulation when combined with the unchanged artery size. The altered flow pattern is concerning. High velocity is indicative of turbulent blood flow (Nichols et al. 2011). Together with anatomic factors of non‐unidirectional flow at the carotid bifurcation and focal luminal dilatation of the carotid bulb, an increase in carotid blood flow and altered flow mechanics potentiate atherosclerosis (Glagov et al. 1988) in a population already at increased risk because of a higher incidence of metabolic abnormalities. While the ultrasound scanner used in this study did not allow for adequate assessment of the carotid intima‐media wall thickness as a marker of atherosclerosis, we note that carotid atherosclerosis has been reported as a sequela of low birth weight in some studies (Lamont et al. 2000; Gale et al. 2006; Crispi et al. 2010). However, not all studies reported the same association (Tilling et al. 2004; Painter et al. 2007), possibly due to the requirement of either severe IUGR or exaggerated postnatal growth before significant atherosclerosis is evident (Oren et al. 2004).
The pattern of increased end‐systolic and end‐diastolic velocity is reminiscent of reduced cerebral vascular resistance seen in IUGR fetuses as a component of the ‘brain‐sparing effect’ (Groenenberg et al. 1989). Our findings are the first to suggest that the vasodilatory mechanisms responsible for the change in cerebral blood flow during the prenatal period may have long‐term effects persisting into adulthood. We suspect that sometime in the postnatal period systemic demand of blood flow increased in the IUGR animals, resulting in some degree of normalization of vascular supply in most organs but leading to mild over‐perfusion of the cerebral circulation. To our best knowledge, carotid blood flow in adults with histories of IUGR has not been examined in either human or animal studies. Increased carotid artery blood flow was previously reported in an IUGR fetal sheep model induced by umbilical artery ligation, which is thought to be responsible for the increased brain to body weight ratio (Miller et al. 2007). However, the functional consequence of that finding in both fetal life and postnatally is unknown. One study in IUGR sheep shows the increased fetal cerebral blood flow is uneven, with increased blood flow to the temporal lobe (Poudel et al. 2015). It is possible that vascular abnormality underlies the known association between IUGR and some neuropsychological conditions, such as impaired executive function in human young adults (Tideman et al. 2007). In future studies, we propose to correlate cognitive function using the Cambridge Neuropsychological Test Automated Battery (CANTAB) system with blood flow data obtained by MRI.
The decreased distensibility of the external iliac artery shown in the current study combined with our previous report of decreased aortic distensibility (Kuo et al. 2017b) is consistent with increased arterial stiffness of IUGR animals. Using measurement of diameter change, increased local arterial stiffness has been reported in the aorta of IUGR newborns (Akira & Yoshiyuki, 2006; Mori et al. 2006) and children (8–13 years) (Levent et al. 2009; Bradley et al. 2010), with some studies showing a similar trend of increase in the aorta (Ley et al. 1997; Brodszki et al. 2005) and popliteal arteries (Brodszki et al. 2005) that did not reach significance. Using pulse wave velocity, increased regional arterial stiffness has been reported in the brachioradial segment of IUGR infants (9 months old) (Cheung et al. 2000), both the brachioradial segment (Cheung et al. 2004) and aorta (Bradley et al. 2010) of IUGR children (8 years and 8–13 years), and the brachioankle segment of low birth weight adults (mean age 36 years) (Mzayek et al. 2009). Further, analysis of aortic pressure waveform revealed increased systemic arterial stiffness in IUGR pre‐adolescents (14 years) (Chan et al. 2010). The decrease in distensibility was previously thought to originate from deficient elastin deposition secondary to IUGR (Martyn & Greenwald, 1997, 2001), similar to the process leading to decreased compliance of the IUGR lungs (Joss‐Moore et al. 2011). However, more recent work in hyperthermia‐induced IUGR sheep, which demonstrated aorta stiffening, suggests the mechanism of arterial stiffening is more complex, involving changes in both composition and organization of the extracellular matrix, and is likely divergent in different arterial segments (Dodson et al. 2014). Interestingly, we did not observe vascular stiffening in the carotid arteries. In line with our results, increase in carotid stiffness is either not seen in IUGR children (Bonamy et al. 2005; Brodszki et al. 2005) or only present in a sub‐group of the subjects with increased aortic stiffness (Mori et al. 2006). Possibly related to this finding, when regional arterial stiffness is assessed across the carotid‐radial segment, no difference is seen between IUGR and control adolescents (Rossi et al. 2011). The preservation of carotid distensibility may originate from the preservation in carotid blood flow. Mechanical stimuli simulating blood flow trigger synthesis of extracellular matrix components in vitro (Leung et al. 1976). In vivo sheep studies reveal that changes in elastin deposition correlate with changes in blood flow in the perinatal period (Bendeck & Langille, 1991).
We acknowledge a few limitations to this study. First, the use of ultrasound precluded adequate evaluation of intracranial vessels, thoracic/abdominal aorta, renal, and smaller arteries of the extremities, which would give a more comprehensive assessment of the vascular health. Second, due to animal availability, there is a noticeable but not significant difference in the age of CTL vs. IUGR animals. We note that given the normal baboon lifespan of 25–30 years, this age gap (less than 1 year) is relatively small. When the data are re‐analysed after removal of the oldest animals from the control groups, which further shortens the age difference, there is no change in the results. Next, it remains to be determined in a primate model whether the observed changes result from microstructural alterations as previously mentioned or arise from other irregularities such as variations in sympathetic tone (Lee et al. 1998). The use of ketamine, in particular, raises question as to whether differential vascular effects of ketamine may partially account for the differences observed. While investigation into this possibility is needed, we note that the literature suggests that, in cases of mild sedation, baseline systemic haemodynamics are not significantly impacted by ketamine (Gooding et al. 1977, Hickey et al. 1985), and ketamine is known to produce fewer cardiovascular changes than some other anaesthetics (Stowe et al. 1992). From the practical standpoint, some form of anaesthesia will always be necessary for studies involving non‐human subjects with the assumption that effects of the anaesthetic will be similar in the control and treatment groups. Thus, this issue is not unique to this study. Last, we note that this study examines only a brief time point in the IUGR life‐course and longitudinal studies will help inform our understanding of the interplay between ageing and IUGR physiology that result in later life development of cardiovascular co‐morbidities.
Overall, the findings in this study suggest that IUGR results in abnormal vasculature in early adulthood in the baboons observed predominantly in the lower extremity arteries. This pattern of involvement raises suspicion for the long‐term consequences of vascular redistribution seen in the perinatal period, particularly fetal life in response to programming challenges such as hypoxia. Abnormality of the arterial vasculature may contribute to the previously noted cardiac changes with IUGR. Subtle changes in distribution of the carotid blood flow with IUGR suggest that the cerebral circulation may be affected, and this circumstance warrants further study. Future studies will be required to determine whether the extracellular and other cellular changes of IUGR arterial vasculature, reported in non‐primate animal studies, also are present and can explain the changes observed in non‐human primates in this study.
Additional information
Competing interests
The authors have no potential conflict of interest, financial or otherwise, to disclose.
Author contributions
A.H.K. participated in the design of the work, acquisition, analysis, and interpretation of data, and drafting the manuscript and revising it critically for important intellectual content. C.L. participated in the design of the work, data acquisition, and revising the manuscript critically for important intellectual content. H.F.H. participated in the design of the work, data acquisition and analysis, and revising the manuscript critically for important intellectual content. G.D.C. participated in the conception and design of the work, data interpretation and analysis, and revising the manuscript critically for important intellectual content. P.W.N. participated in the conception and design of the work, data interpretation, and revising the manuscript critically for important intellectual content. All authors approved the final version of the manuscript, agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved, and all persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by the National Institutes of Health 5P01HD021350 (P.W.N.), 5R24OD011183 (P.W.N.), and 1R25EB016631 (A.H.K.). NIH grant OD P51 OD011133 was from the Office of Research Infrastructure Programs/Office of the Director. This work was also supported in part by funding from the EU FP 7/HEALTH/GA No.: 279281: BrainAge‐ Impact of Prenatal Stress on BRAINAGEing.
Acknowledgements
The authors thank the Joe R. and Teresa Lozano Long University of Texas School of Medicine at San Antonio HEB Clinical Skills Center for the use of the ultrasound machine. The authors recognize Dr Robert Lanford and the Southwest National Primate Center staff for their ongoing support of the baboon research program described in this article. The authors also acknowledge the technical support of Steven Rios, Sam Vega, Susan Jenkins, and McKenna Considine, as well as the administrative support of Karen Moore.
Edited by: Laura Bennet & Janna Morrison
This is an Editor's Choice article from the 1 December 2018 issue.
References
- Akira M & Yoshiyuki S (2006). Placental circulation, fetal growth, and stiffness of the abdominal aorta in newborn infants. J Pediatr 148, 49–53. [DOI] [PubMed] [Google Scholar]
- Arbeille P (1997). Fetal arterial Doppler‐IUGR and hypoxia. Eur J Obstet Gynecol Reprod Biol 75, 51–53. [DOI] [PubMed] [Google Scholar]
- Armitage JA, Khan IY, Taylor PD, Nathanielsz PW & Poston L (2004). Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals?. J Physiol 561, 355–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baschat A, Gembruch U & Harman C (2001). The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet Gynecol 18, 571–577. [DOI] [PubMed] [Google Scholar]
- Bendeck MP & Langille BL (1991). Rapid accumulation of elastin and collagen in the aortas of sheep in the immediate perinatal period. Circ Res 69, 1165–1169. [DOI] [PubMed] [Google Scholar]
- Berry CL, Looker T & Germain J (1972). The growth and development of the rat aorta. I. Morphological aspects. J Anat 113, 1–16. [PMC free article] [PubMed] [Google Scholar]
- Blackmore HL & Ozanne SE (2015). Programming of cardiovascular disease across the life‐course. J Mol Cell Cardiol 83, 122–130. [DOI] [PubMed] [Google Scholar]
- Bonamy AE, Bendito A, Martin H, Andolf E, Sedin G & Norman M (2005). Preterm birth contributes to increased vascular resistance and higher blood pressure in adolescent girls. Pediatr Res 58, 845–849. [DOI] [PubMed] [Google Scholar]
- Botting KJ, Wang K, Padhee M, McMillen I, Summers‐Pearce B, Rattanatray L, Cutri N, Posterino G, Brooks D & Morrison J (2012). Early origins of heart disease: low birth weight and determinants of cardiomyocyte endowment. Clin Exp Pharmacol Physiol 39, 814–823. [DOI] [PubMed] [Google Scholar]
- Bradley TJ, Potts JE, Lee SK, Potts MT, De Souza AM & Sandor GG (2010). Early changes in the biophysical properties of the aorta in pre‐adolescent children born small for gestational age. J Pediatr 156, 388–392. [DOI] [PubMed] [Google Scholar]
- Briscoe TA, Rehn AE, Dieni S, Duncan JR, Wlodek ME, Owens JA & Rees SM (2004). Cardiovascular and renal disease in the adolescent guinea pig after chronic placental insufficiency. Obstet Gynecol 191, 847–855. [DOI] [PubMed] [Google Scholar]
- Brodszki J, Lanne T, Marsal K & Ley D (2005). Impaired vascular growth in late adolescence after intrauterine growth restriction. Circulation 111, 2623–2628. [DOI] [PubMed] [Google Scholar]
- Bubb KJ, Cock ML, Black MJ, Dodic M, Boon W, Parkington HC, Harding R & Tare M (2007). Intrauterine growth restriction delays cardiomyocyte maturation and alters coronary artery function in the fetal sheep. J Physiol 578, 871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PY, Morris JM, Leslie GI, Kelly PJ & Gallery ED (2010). The long‐term effects of prematurity and intrauterine growth restriction on cardiovascular, renal, and metabolic function. Int J Pediatr 2010, 280402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman W (1918). The effect of the heart‐beat upon the development of the vascular system in the chick. Am J Anat 23, 175–203. [Google Scholar]
- Cheung Y, Taylor MJ, Fisk NM, Redington AN & Gardiner HM (2000). Fetal origins of reduced arterial distensibility in the donor twin in twin‐twin transfusion syndrome. Lancet 355, 1157–1158. [DOI] [PubMed] [Google Scholar]
- Cheung YF, Wong KY, Lam BC & Tsoi NS (2004). Relation of arterial stiffness with gestational age and birth weight. Arch Dis Child 89, 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccone MM, Scicchitano P, Salerno C, Gesualdo M, Fornarelli F, Zito A, Filippucci L, Riccardi R, Cortese F, Pini F, Angrisani L, Di Mauro A, Schettini F & Laforgia N (2013). Aorta structural alterations in term neonates: the role of birth and maternal characteristics. Biomed Res Int 2013, 459168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleal JK, Poore KR, Boullin JP, Khan O, Chau R, Hambidge O, Torrens C, Newman JP, Poston L, Noakes DE, Hanson MA & Green LR (2007). Mismatched pre‐ and postnatal nutrition leads to cardiovascular dysfunction and altered renal function in adulthood. Proc Natl Acad Sci USA 104, 9529–9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn HE, Sacks EJ, Heymann MA & Rudolph AM (1974). Cardiovascular responses to hypoxemia and acidemia in fetal lambs. Obstet Gynecol 120, 817–824. [DOI] [PubMed] [Google Scholar]
- Cosmi E, Visentin S, Fanelli T, Mautone AJ & Zanardo V (2009). Aortic intima media thickness in fetuses and children with intrauterine growth restriction. Obstet Gynecol 114, 1109–1114. [DOI] [PubMed] [Google Scholar]
- Crispi F, Bijnens B, Figueras F, Bartrons J, Eixarch E, Le Noble F, Ahmed A & Gratacos E (2010). Fetal growth restriction results in remodeled and less efficient hearts in children. Circulation 121, 2427–2436. [DOI] [PubMed] [Google Scholar]
- Debasso R, Åstrand H, Bjarnegård N, Ahlgren ÅR, Sandgren T & Länne T (2004). The popliteal artery, an unusual muscular artery with wall properties similar to the aorta: implications for susceptibility to aneurysm formation? J Vasc Surg 39, 836–842. [DOI] [PubMed] [Google Scholar]
- Dodson RB, Rozance PJ, Petrash CC, Hunter KS & Ferguson VL (2014). Thoracic and abdominal aortas stiffen through unique extracellular matrix changes in intrauterine growth restricted fetal sheep. Am J Physiol Heart Circ Physiol 306, H429–H437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowden AL, Giussani DA & Forhead AJ (2006). Intrauterine programming of physiological systems: causes and consequences. Physiology (Bethesda) 21, 29–37. [DOI] [PubMed] [Google Scholar]
- Franco MC, Christofalo DM, Sawaya AL, Ajzen SA & Sesso R (2006). Effects of low birth weight in 8‐ to 13‐year‐old children: implications in endothelial function and uric acid levels. Hypertension 48, 45–50. [DOI] [PubMed] [Google Scholar]
- Galan HL, Anthony RV, Rigano S, Parker TA, de Vrijer B, Ferrazzi E, Wilkening RB & Regnault TR (2005). Fetal hypertension and abnormal Doppler velocimetry in an ovine model of intrauterine growth restriction. Obstet Gynecol 192, 272–279. [DOI] [PubMed] [Google Scholar]
- Gale CR, Jiang B, Robinson SM, Godfrey KM, Law CM & Martyn CN (2006). Maternal diet during pregnancy and carotid intima‐media thickness in children. Arterioscler Thromb Vasc Biol 26, 1877–1882. [DOI] [PubMed] [Google Scholar]
- Gardiner H, Brodszki J, Eriksson A & Marl K (2002). Volume blood flow estimation in the normal and growth‐restricted fetus. Ultrasound Med Biol 28, 1107–1113. [DOI] [PubMed] [Google Scholar]
- Giussani DA (2016). The fetal brain sparing response to hypoxia: physiological mechanisms. J Physiol 594, 1215–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glagov S, Zarins C, Giddens DP & Ku DN (1988). Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med 112, 1018–1031. [PubMed] [Google Scholar]
- Glassman DM, Coelho AM Jr, Carey KD & Bramblett CA (1984). Weight growth in savannah baboons: a longitudinal study from birth to adulthood. Growth 48, 425–433. [PubMed] [Google Scholar]
- Godfrey KM, Lillycrop KA, Burdge GC, Gluckman PD & Hanson MA (2007). Epigenetic mechanisms and the mismatch concept of the developmental origins of health and disease. Pediatr Res 61, 5R–10R. [DOI] [PubMed] [Google Scholar]
- Goodfellow J, Bellamy MF, Gorman ST, Brownlee M, Ramsey MW, Lewis MJ, Davies DP & Henderson AH (1998). Endothelial function is impaired in fit young adults of low birth weight. Cardiovasc Res 40, 600–606. [DOI] [PubMed] [Google Scholar]
- Gooding JM, Dimick AR, Tavakoli M & Corssen G (1977). A physiologic analysis of cardiopulmonary responses to ketamine anesthesia in noncardiac patients. Anesth Analg 56, 813–816. [PubMed] [Google Scholar]
- Groenenberg IA, Wladimiroff JW & Hop WC (1989). Fetal cardiac and peripheral arterial flow velocity waveforms in intrauterine growth retardation. Circulation 80, 1711–1717. [DOI] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology . J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton JR & Hartley CJ (1985). Flow restriction of one carotid artery in juvenile rats inhibits growth of arterial diameter. Am J Physiol Heart Circ Physiol 248, H540–H546. [DOI] [PubMed] [Google Scholar]
- Hickey PR, Hansen DD, Cramolini GM, Vincent RN & Lang P (1985). Pulmonary and systemic hemodynamic responses to ketamine in infants with normal and elevated pulmonary vascular resistance. Anesthesiology 62, 287–293. [DOI] [PubMed] [Google Scholar]
- Huxley RR, Shiell AW & Law CM (2000). The role of size at birth and postnatal catch‐up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens 18, 815–831. [DOI] [PubMed] [Google Scholar]
- Jiang B, Godfrey KM, Martyn CN & Gale CR (2006). Birth weight and cardiac structure in children. Pediatrics 117, e257–e261. [DOI] [PubMed] [Google Scholar]
- Jones EA, le Noble F & Eichmann A (2006). What determines blood vessel structure? Genetic prespecification vs. hemodynamics. Physiology (Bethesda) 21, 388–395. [DOI] [PubMed] [Google Scholar]
- Joss‐Moore LA, Wang Y, Yu X, Campbell MS, Callaway CW, McKnight RA, Wint A, Dahl MJ, Dull RO, Albertine KH & Lane RH (2011). IUGR decreases elastin mRNA expression in the developing rat lung and alters elastin content and lung compliance in the mature rat lung. Physiol Genomics 43, 499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatza AA & Varvarigou A (2013). Intrauterine growth restriction and the developing vascular tree In Early Life Nutrition and Adult Health and Development: Lessons from Changing Dietary Patterns, Famines and Experimental Studies, pp. 331–351. Nova Science Publishers, New York, NY. [Google Scholar]
- Kuo AH, Li C, Huber HF, Schwab M, Nathanielsz PW & Clarke GD (2017a). Maternal nutrient restriction during pregnancy and lactation leads to impaired right ventricular function in young adult baboons. J Physiol 595, 4245–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo AH, Li C, Li J, Huber HF, Nathanielsz PW & Clarke GD (2016). Cardiac remodelling in a baboon model of intrauterine growth restriction mimics accelerated ageing. J Physiol 595, 1093–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo AH, Li J, Li C, Huber HF, Nathanielsz PW & Clarke GD (2017b). Poor perinatal growth impairs baboon aortic windkessel function. J Dev Orig Health Dis (in press; 10.1017/S2040174417000770). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont D, Parker L, White M, Unwin N, Bennett SM, Cohen M, Richardson D, Dickinson HO, Adamson A, Alberti KG & Craft AW (2000). Risk of cardiovascular disease measured by carotid intima‐media thickness at age 49–51: lifecourse study. BMJ 320, 273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langille BL & O'Donnell F (1986). Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium‐dependent. Science 231, 405–407. [DOI] [PubMed] [Google Scholar]
- Lee J, Park K, Hwang J, Park M & Yum M (1998). Chaotic and periodic heart rate dynamics in uncomplicated intrauterine growth restricted fetuses. Early Hum Dev 53, 121–128. [DOI] [PubMed] [Google Scholar]
- Leeson CP, Kattenhorn M, Morley R, Lucas A & Deanfield JE (2001). Impact of low birth weight and cardiovascular risk factors on endothelial function in early adult life. Circulation 103, 1264–1268. [DOI] [PubMed] [Google Scholar]
- Leite‐Moreira AF, Correia‐Pinto J & Gillebert TC (1999). Afterload induced changes in myocardial relaxation: a mechanism for diastolic dysfunction. Cardiovasc Res 43, 344–353. [DOI] [PubMed] [Google Scholar]
- Leon DA, Lithell HO, Vagero D, Koupilova I, Mohsen R, Berglund L, Lithell UB & McKeigue PM (1998). Reduced fetal growth rate and increased risk of death from ischaemic heart disease: cohort study of 15 000 Swedish men and women born 1915–29. BMJ 317, 241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DY, Glagov S & Mathews MB (1976). Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science 191, 475–477. [DOI] [PubMed] [Google Scholar]
- Levent E, Atik T, Darcan Ş, Ülger Z, Gökşen D & Özyürek AR (2009). The relation of arterial stiffness with intrauterine growth retardation. Pediatr Int 51, 807–811. [DOI] [PubMed] [Google Scholar]
- Ley D, Stale H & Marsal K (1997). Aortic vessel wall characteristics and blood pressure in children with intrauterine growth retardation and abnormal foetal aortic blood flow. Acta Paediatrica 86, 299–305. [DOI] [PubMed] [Google Scholar]
- Li C, Jenkins S, Mattern V, Comuzzie AG, Cox LA, Huber HF & Nathanielsz PW (2017). Effect of moderate, 30 percent global maternal nutrient reduction on fetal and postnatal baboon phenotype. J Med Primatol 46, 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looker T & Berry CL (1972). The growth and development of the rat aorta. II. Changes in nucleic acid and scleroprotein content. J Anat 113, 17–34. [PMC free article] [PubMed] [Google Scholar]
- Männer J, Seidl W & Steding G (1995). Formation of the cervical flexure: an experimental study on chick embryos. Acta Anat (Basel) 152, 1–10. [DOI] [PubMed] [Google Scholar]
- Martin H, Hu J, Gennser G & Norman M (2000). Impaired endothelial function and increased carotid stiffness in 9‐year‐old children with low birthweight. Circulation 102, 2739–2744. [DOI] [PubMed] [Google Scholar]
- Martyn C & Greenwald S (2001). A hypothesis about a mechanism for the programming of blood pressure and vascular disease in early life. Clin Exp Pharmacol Physiol 28, 948–951. [DOI] [PubMed] [Google Scholar]
- Martyn C & Greenwald S (1997). Impaired synthesis of elastin in walls of aorta and large conduit arteries during early development as an initiating event in pathogenesis of systemic hypertension. Lancet 350, 953–955. [DOI] [PubMed] [Google Scholar]
- Menendez‐Castro C, Fahlbusch F, Cordasic N, Amann K, Münzel K, Plank C, Wachtveitl R, Rascher W, Hilgers KF, Hartner A (2011). Early and late postnatal myocardial and vascular changes in a protein restriction rat model of intrauterine growth restriction. PLoS One 6, e20369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer WW, Walsh SZ & Lind J (1980). Functional morphology of human arteries during fetal and postnatal development In Structure and Function of the Circulation, pp. 95–379. Springer. [Google Scholar]
- Miller SL, Chai M, Loose J, Castillo‐Melendez M, Walker DW, Jenkin G & Wallace EM (2007). The effects of maternal betamethasone administration on the intrauterine growth‐restricted fetus. Endocrinology 148, 1288–1295. [DOI] [PubMed] [Google Scholar]
- Mori A, Uchida N, Inomo A & Izumi S (2006). Stiffness of systemic arteries in appropriate‐ and small‐for‐gestational‐age newborn infants. Pediatrics 118, 1035–1041. [DOI] [PubMed] [Google Scholar]
- Morris C & Mervis C (2000). Williams syndrome and related disorders. Annu Rev Genomics Hum Genet 1, 461–484. [DOI] [PubMed] [Google Scholar]
- Morris CA (1998). Genetic aspects of supravalvular aortic stenosis. Curr Opin Cardiol 13, 214–219. [PubMed] [Google Scholar]
- Mzayek F, Sherwin R, Hughes J, Hassig S, Srinivasan S, Chen W & Berenson GS (2009). The association of birth weight with arterial stiffness at mid‐adulthood: the Bogalusa Heart Study. J Epidemiol Community Health 63, 729–733. [DOI] [PubMed] [Google Scholar]
- Nichols W, O'Rourke M & Vlachopoulos C (2011). McDonald's Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. CRC Press, USA. [Google Scholar]
- Norman M (2008). Low birth weight and the developing vascular tree: a systematic review. Acta Paediatrica 97, 1165–1172. [DOI] [PubMed] [Google Scholar]
- Oren A, Vos LE, Uiterwaal CS, Gorissen WH, Grobbee DE & Bots ML (2004). Birth weight and carotid intima‐media thickness: new perspectives from the atherosclerosis risk in young adults (ARYA) study. Ann Epidemiol 14, 8–16. [DOI] [PubMed] [Google Scholar]
- Ozaki T, Nishina H, Hanson M & Poston L (2001). Dietary restriction in pregnant rats causes gender‐related hypertension and vascular dysfunction in offspring. J Physiol 530, 141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R, De Rooij S, Hutten B, Bossuyt P, De Groot E, Osmond C, Barker D, Bleker O & Roseboom T (2007). Reduced intima media thickness in adults after prenatal exposure to the Dutch famine. Atherosclerosis 193, 421–427. [DOI] [PubMed] [Google Scholar]
- Poudel R, McMillen IC, Dunn SL, Zhang S & Morrison JL (2015). Impact of chronic hypoxemia on blood flow to the brain, heart, and adrenal gland in the late‐gestation IUGR sheep fetus. Am J Physiol Regul Integr Comp Physiol 308, R151–R162. [DOI] [PubMed] [Google Scholar]
- Rodbard S (1975). Vascular caliber. Cardiology 60, 4–49. [DOI] [PubMed] [Google Scholar]
- Rossi P, Tauzin L, Marchand E, Boussuges A, Gaudart J & Frances Y (2011). Respective roles of preterm birth and fetal growth restriction in blood pressure and arterial stiffness in adolescence. J Adolesc Health 48, 520–522. [DOI] [PubMed] [Google Scholar]
- Rouwet EV, Tintu AN, Schellings MW, van Bilsen M, Lutgens E, Hofstra L, Slaaf DW, Ramsay G & Le Noble FA (2002). Hypoxia induces aortic hypertrophic growth, left ventricular dysfunction, and sympathetic hyperinnervation of peripheral arteries in the chick embryo. Circulation 105, 2791–2796. [DOI] [PubMed] [Google Scholar]
- Rueda‐Clausen CF, Morton JS & Davidge ST (2009). Effects of hypoxia‐induced intrauterine growth restriction on cardiopulmonary structure and function during adulthood. Cardiovasc Res 81, 713–722. [DOI] [PubMed] [Google Scholar]
- Salinas CE, Blanco CE, Villena M & Giussani DA (2014). High‐altitude hypoxia and echocardiographic indices of pulmonary hypertension in male and female chickens at adulthood. Circ J 78, 1459–1464. [DOI] [PubMed] [Google Scholar]
- Schlabritz‐Loutsevitch NE, Howell K, Rice K, Glover EJ, Nevill CH, Jenkins SL, Bill Cummins L, Frost PA, McDonald TJ & Nathanielsz PW (2004). Development of a system for individual feeding of baboons maintained in an outdoor group social environment. J Med Primatol 33, 117–126. [DOI] [PubMed] [Google Scholar]
- Schubert U, Müller M, Bonamy AE, Abdul‐Khaliq H & Norman M (2011). Aortic growth arrest after preterm birth: a lasting structural change of the vascular tree. J Dev Orig Health Dis 2, 218–225. [DOI] [PubMed] [Google Scholar]
- Singhal A, Kattenhorn M, Cole TJ, Deanfield J & Lucas A (2001). Preterm birth, vascular function, and risk factors for atherosclerosis. Lancet 358, 1159–1160. [DOI] [PubMed] [Google Scholar]
- Stowe DF, Bosnjak ZJ & Kampine JP (1992). Comparison of etomidate, ketamine, midazolam, propofol, and thiopental on function and metabolism of isolated hearts. Anesth Analg 74, 547–558. [DOI] [PubMed] [Google Scholar]
- te Velde SJ, Ferreira I, Twisk JW, Stehouwer CD, van Mechelen W, Kemper HC & Amsterdam Growth and Health Longitudinal Study (2004). Birthweight and arterial stiffness and blood pressure in adulthood‐results from the Amsterdam Growth and Health Longitudinal Study. Int J Epidemiol 33, 154–161. [DOI] [PubMed] [Google Scholar]
- Tideman E, Maršál K & Ley D (2007). Cognitive function in young adults following intrauterine growth restriction with abnormal fetal aortic blood flow. Ultrasound Obstet Gynecol 29, 614–618. [DOI] [PubMed] [Google Scholar]
- Tilling K, Smith GD, Chambless L, Rose K, Stevens J, Lawlor D & Szklo M (2004). The relation between birth weight and intima‐media thickness in middle‐aged adults. Epidemiology 15, 557–564. [DOI] [PubMed] [Google Scholar]
- Umetani K, Singer DH, McCraty R & Atkinson M (1998). Twenty‐four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol 31, 593–601. [DOI] [PubMed] [Google Scholar]
- VandeBerg JL, Williams‐Blangero S & Tardif SD (2009). The Baboon in Biomedical Research. Springer Science & Business Media. [Google Scholar]
- Walther F, Siassi B, King J & Wu P (1986). Echocardiographic measurements in normal preterm and term neonates. Acta Paediatrica 75, 563–568. [DOI] [PubMed] [Google Scholar]
- Wladimiroff J, Tonge H & Stewart P (1986). Doppler ultrasound assessment of cerebral blood flow in the human fetus. Br J Obstet Gynaecol 93, 471–475. [PubMed] [Google Scholar]
- Wladimiroff J, vd Wijngaard J, Degani S, Noordam M & Tonge H (1987). Cerebral and umbilical arterial blood flow velocity waveforms in normal and growth‐retarded pregnancies. Obstet Gynecol 69, 705–709. [PubMed] [Google Scholar]
- Xu Y, Williams SJ, O'Brien D & Davidge ST (2006). Hypoxia or nutrient restriction during pregnancy in rats leads to progressive cardiac remodeling and impairs postischemic recovery in adult male offspring. FASEB J 20, 1251–1253. [DOI] [PubMed] [Google Scholar]


