Abstract
Key points
The placental insulin‐like growth factor (IGF) system is critical for normal fetoplacental growth, which is dysregulated following several pregnancy perturbations including uteroplacental insufficiency and maternal obesity. We report that the IGF system was altered in placentae of mothers born growth restricted compared to normal birth weight mothers, with maternal diet‐ and fetal sex‐specific responses.
Additionally, we report increased body weight and plasma IGF1 concentrations in fetuses from chow‐fed normal birth weight mothers that exercised prior to and continued during pregnancy compared to sedentary mothers.
Exercise initiated during pregnancy, on the other hand, resulted in placental morphological alterations and increased IGF1 and IGF1R protein expression, which may in part be modulated by reduced Let 7f‐1 miRNA abundance.
Growth restriction of mothers before birth and exercise differentially regulate the placental IGF system with diet‐ and sex‐specific responses, probably as a means to improve fetoplacental growth and development, and hence neonatal survival. This increased neonatal survival may prevent adult disease onset.
Abstract
The insulin‐like growth factor (IGF) system regulates fetoplacental growth and plays a role in disease programming. Dysregulation of the IGF system is implicated in several pregnancy perturbations associated with altered fetal growth, including intrauterine growth restriction and maternal obesity. Limited human studies have demonstrated that maternal exercise enhances fetoplacental growth and decreases cord IGF ligands, which may restore the placental IGF system in complicated pregnancies. This study investigated the impact maternal exercise has on the placental IGF system in placentae from mothers born growth restricted and if these outcomes are dependent on maternal diet or fetal sex. Uteroplacental insufficiency (Restricted) or sham (Control) surgery was induced on embryonic day (E) 18 in Wistar–Kyoto rats. F1 offspring were fed a chow or high‐fat diet from weaning, and at 16 weeks were randomly allocated an exercise protocol: Sedentary, Exercised prior to and during pregnancy (Exercise), or Exercised during pregnancy only (PregEx). Females were mated (20 weeks) with placentae associated with F2 fetuses collected at E20. The placental IGF system mRNA abundance and placental morphology was altered in mothers born growth restricted. Exercise increased fetal weight and Control plasma IGF1 concentrations, and decreased female placental weight. PregEx did not influence fetoplacental growth but increased placental IGF1 and IGF1R (potentially modulated by reduced Let 7f‐1 miRNA) and decreased placental IGF2 protein. Importantly, these placental IGF system changes occurred with sex‐specific responses. These data highlight that exercise differently influences fetoplacental growth and the placental IGF system depending on maternal exercise initiation, which may prevent the transgenerational transmission of deficits and dysfunction.
Keywords: growth restriction, placenta, fetal programming, exercise, IGF‐system
Key points
The placental insulin‐like growth factor (IGF) system is critical for normal fetoplacental growth, which is dysregulated following several pregnancy perturbations including uteroplacental insufficiency and maternal obesity. We report that the IGF system was altered in placentae of mothers born growth restricted compared to normal birth weight mothers, with maternal diet‐ and fetal sex‐specific responses.
Additionally, we report increased body weight and plasma IGF1 concentrations in fetuses from chow‐fed normal birth weight mothers that exercised prior to and continued during pregnancy compared to sedentary mothers.
Exercise initiated during pregnancy, on the other hand, resulted in placental morphological alterations and increased IGF1 and IGF1R protein expression, which may in part be modulated by reduced Let 7f‐1 miRNA abundance.
Growth restriction of mothers before birth and exercise differentially regulate the placental IGF system with diet‐ and sex‐specific responses, probably as a means to improve fetoplacental growth and development, and hence neonatal survival. This increased neonatal survival may prevent adult disease onset.
Introduction
The insulin‐like growth factor (IGF) system is involved in a myriad of physiological pathways that promote fetoplacental growth and development by the binding of IGF ligands (IGF1 and IGF2) to their receptors (IGF1R and IGF2R). Specifically, either ligand binding to IGF1R activates pathways that promote cellular proliferation, differentiation and survival. IGF2, on the other hand, has a higher affinity to bind to IGF2R, where it promotes placental growth and increases IGF2 clearance (Han et al. 1996). The IGF system also consists of IGF binding proteins (IGFBPs) that have a higher affinity of binding IGF ligands than IGF receptors, thus sequestering their ability to act on their receptors. Recent studies demonstrate that the IGF system, specifically IGF1 and IGF1R, can be modulated by the action of Let 7f‐1 miRNA. Specifically, increased Let 7f‐1 miRNA abundance is associated with reduced IGF1 and IGF1R protein expression (Hu et al. 2014). IGF system knockout mouse models clearly demonstrate the essential role of this system in fetoplacental growth, whereby Igf1 −/−, Igf2 −/− and Igf1r −/− mice have a 40–55% reduction in birth weight (DeChiara et al. 1990; Baker et al. 1993; Liu et al. 1993; Woods et al. 1996; Lupu et al. 2001).
Uteroplacental insufficiency is the leading cause of fetal growth restriction in Western populations, and impairs oxygen and nutrient delivery to the growing fetus. It is therefore not surprising that dysregulation of the IGF system has been implicated in the pathogenesis of fetal growth restriction. Clinically, growth‐restricted human fetuses have lower IGF1, IGF2 and IGFBP3 cord blood concentrations, along with elevated IGFBP1 concentrations (Langford et al. 1994; Spencer et al. 1995), probably due to reduced placental development (Koutsaki et al. 2011). Similar findings are reported in experimental animal models of growth restriction, whereby fetal plasma IGF1 and tissue‐specific IGF1 concentrations are reduced in naturally occurring growth‐restricted rabbits (Thakur et al. 2000) and in several models of growth‐restricted sheep (Bauer et al. 1995; Kind et al. 1995; de Vrijer et al. 2006; Gentili et al. 2009). These data clearly highlight that dysregulation of the placental IGF system following pregnancy perturbations influences fetoplacental development. However, limited studies have characterized if similar changes in the placental IGF system are observed in placentae associated with the second (F2) generation whose mother was born small, which may contribute to the F2 cardiometabolic dysfunction we have previously reported in our animal model of uteroplacental insufficiency (Gallo et al. 2012, 2013).
Concerningly, 40% of pregnancies in Australia are complicated by maternal overweight/obesity that has negative effects on maternal and fetal health, which is in part due to its chronic inflammatory state. Of importance, individuals that were born growth restricted have an increased susceptibility to developing obesity (Cottrell & Ozanne, 2008). Maternal obesity increases the risk of pregnancy complications, including gestational diabetes mellitus that is similarly observed in growth‐restricted individuals, and can alter fetoplacental growth and development (Leddy et al. 2008; Higgins et al. 2011). Maternal obesity in humans reduces cord blood IGFBP1 and IGFBP3 concentrations and increases cord IGF2 concentrations and birth weight (Jansson et al. 2008; Hoyo et al. 2012), which may in part be due to increased nutrient availability. In the mouse, maternal consumption of a high‐fat/high‐sugar diet increases placental Igf2 mRNA abundance, increases placental nutrient transportation and alters placental morphology (Sferruzzi‐Perri et al. 2013; Rosario et al. 2016). It is therefore possible that maternal obesity in growth‐restricted females may further compound alterations in the placental IGF system.
Physical activity is associated with several positive health benefits including improved cardiovascular and metabolic health (Petersen & Pedersen, 2005; Bruun et al. 2006; O'Gorman et al. 2006). Exercise during pregnancy in humans improves maternal cardiometabolic outcomes, reducing the incidence of gestational diabetes mellitus and enhancing fetoplacental growth (Clapp et al. 2000, 2002; Clapp, 2006), which may in part be due to the IGF system (Vega et al. 2011). It is important to note, however, that research on the impact maternal exercise has on fetoplacental growth is contradictory with other studies reporting no change in birth weight (Hopkins et al. 2010), which highlights the need for additional studies. Only one study to date has characterized the IGF system following maternal exercise, which reported reduced cord blood concentrations of IGF ligands (IGF1 and IGF2) (Hopkins et al. 2010). However, it is important to note that the impact of exercise on maternal health and fetoplacental outcomes are dependent on the intensity, duration and timing of exercise initiation. Despite limited evidence of maternal exercise altering the placental IGF system, there is a large body of evidence that demonstrates that exercise in non‐pregnant individuals can modulate the IGF system (Borer, 1995; Raastad et al. 2000; Turgut et al. 2006). Therefore, it is possible that maternal exercise may restore changes in the placental IGF system associated with mothers born small and following consumption of a high‐fat diet, which could break the transgenerational disease cycle.
Therefore, this study first aimed to determine changes in the placental IGF system of F2 fetuses whose mother was born growth restricted and the period of exercise initiation (prior to or during pregnancy) that is most beneficial in preventing these alterations. We next aimed to determine whether maternal high‐fat diet consumption exacerbated these changes in the placental IGF system associated with mothers born growth restricted within each exercise group. As previous studies have demonstrated that male‐ and female‐associated placentae respond differently to several pregnancy perturbations, we additionally characterized whether these responses were different in male‐ and female‐associated placentae (Cuffe et al. 2014, 2017; Gardebjer et al. 2014).
Methods
Animals
All experiments were approved by The University of Melbourne's animal experimentation ethics sub‐committee (AEC: 1212639) following the National Health and Medical Research Councils (NHMRC) Australian code for the care and use of animals for scientific purposes. Female Wistar‐Kyoto (WKY) rats (8 weeks of age) were acquired from the biological resource facility at the University of Melbourne and were housed in an environmentally controlled room (19–22°C) under a 12 h light–dark cycle with ad libitum access to standard rat chow and water. Rats were mated and surgery was performed on day 18 of gestation (term = 22 days) as described previously (Wlodek et al. 2005). Briefly, F0 female rats were anaesthetized with 4% isoflurane and 650 ml/min oxygen flow (reduced to 3.2% isoflurane and 250 ml/min oxygen flow when suturing to aid in the animal's recovery), with 0.125% bupivacaine administered to the skin and muscle layers prior to closure. Pregnant females were randomly allocated to undergo uteroplacental insufficiency surgery, by bilateral uterine vessel ligation (offspring termed Restricted) or sham surgery (offspring termed Control) and dams were allowed to deliver naturally at term. At weaning, on postnatal day 35 (PN35), litter mate F1 normal birth weight (Control) and growth‐restricted (Restricted) females were randomly allocated to a Chow (AIN93G; Specialty Feeds, Glen Forrest, WA, Australia) or High‐fat diet (SF03‐020 and SF01‐028; Specialty Feeds) that were matched for micro‐ and macronutrients. At 16 weeks, F1 female offspring were further randomly allocated to one of the following exercise regimes: remained Sedentary, exercised before and during pregnancy (Exercise; from 16 to 24 weeks of age) or exercised only during pregnancy [PregEx; Sedentary prior to and in the first week of pregnancy, then exercised from embryonic day (E)7 to E19]. At 20 weeks of age, F1 females were mated with normal males as illustrated in Fig. 1 A. All animals were generated concurrently.
Figure 1. Flow chart of experimental exercise and diet protocol.
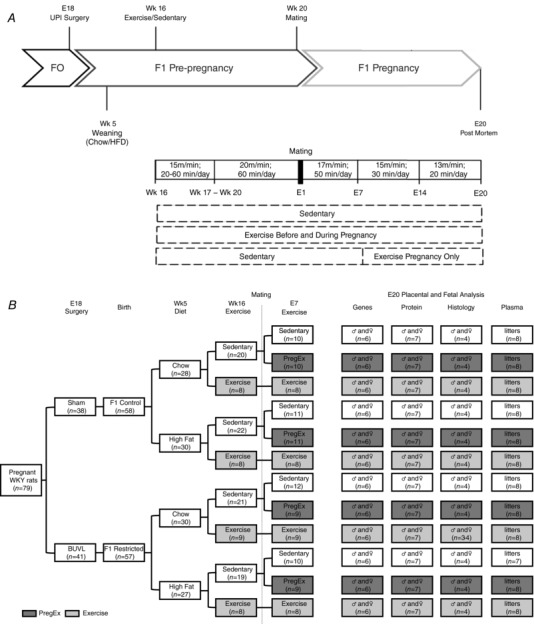
A, flow chart of the experimental protocol indicating the allocation of pregnant rats between the different treatments, diet and exercise groups. B, flow chart of exercise protocol. E, embryonic day; HFD, high‐fat diet; UPI, uteroplacental insufficiency; Wk, week.
Exercise training
The exercise regime is outlined in Fig. 1 B. Briefly, starting at 16 weeks of age, F1 females allocated to the Exercise group exercised for 5 days/week on a motorized treadmill (Columbus Instruments, Columbus, OH, USA) followed by 2 days of rest. On the first day of training, rats allocated to the Exercise group ran for 20 min at a speed of 15 m/min. On each subsequent day an additional 10 min per day was added to the running time until on day 5 of week 1 the rats were exercised for 60 min. On day 1 of week 2 and thereafter until mating, the rats were exercised for 60 min/day at a speed of 20 m/min, as previously described (Laker et al. 2011, 2012; Wadley et al. 2016; Asif et al. 2018). The day after mating (on E1), for week 1 of pregnancy rats were exercised for 50 min/day at a speed of 17 m/min, for week 2 of pregnancy (on E8) rats were exercised for 30 min/day at a speed of 13 m/min and for week 3 of pregnancy (on E15) rats were exercised for 20 min/day at a speed of 11 m/min. Females allocated to the PregEx group remained Sedentary prior to mating and for the first week of pregnancy, and underwent exercise for 5 days/week on a motorized treadmill followed by 2 days of rest from E8 at the same intensities and durations as per the Exercise group. Sedentary rats were placed on a stationary treadmill for the same duration as the exercising rats. Rats were encouraged to run by blowing compressed air near the base of their tail.
Post‐mortem
At E20, F1 females were anaesthetized with a 1:1 mixed solution of ketamine (100 mg/kg; Parnell Laboratories; Alexandria, NSW, Australia) and ilium xylazil (30 mg/kg; Troy Laboratories; Smithfield, NSW, Australia) and their uterus exposed. F2 fetuses were weighed, sexed by visual inspection of the anogenital distance and then killed by decapitation. Fetal plasma was collected and pooled into litters, with fetal tails collected to verify fetal sex. The placentae were excised, weighed and fixed whole in 10% neutral buffered formalin (NBF) or separated into labyrinth and junctional regions then frozen immediately in liquid nitrogen and stored at −80°C for subsequent analysis. For tissue analyses (morphology and gene/protein expression) placentae associated with one male and one female from each litter were chosen with a fetal and placental weight closest to the litter average, with each sample representing a single animal (i.e. n = 1). The dam was then killed by cardiac puncture. Fetal sex was confirmed by quantitative PCR (qPCR) to determine the presence/absence of the sex‐determining region Y (SRY) in DNA extracted from fetal tails using a commercially available Taqman probe (Rn04224592_u1; NM_012772.1) (Life Technologies, Scoresby, VIC, Australia) as previously described (Cuffe et al. 2012).
Placental morphology
Fixed placentae were processed into paraffin blocks, sectioned at 5 μm and stained with haemotoxylin and eosin (n = 3–4 dams/group with one male and female analysed per dam). Five sections per placenta were analysed for whole placental, labyrinth and junctional zone cross‐sectional areas using the Aperio ScanScope system (Aperio Technologies, Vista, CA, USA) and Image Scope software (Leica Microsystems, Mt Waverly, VIC, Australia).
Placental gene abundance
RNA and miRNA were extracted from the placental labyrinth (nutrient transport) region using a Precellys 24 homogenizer (Bertin Technologies, Aix en Provence, France) with CK14 ceramic beads using a commercially available kit with on‐column DNase digestion (miRNA easy mini kit; Qiagen, Chadstone, VIC, Australia) (Cheong et al. 2016). First‐strand cDNA was generated from 1 μg of RNA using the High Capacity cDNA kit (for mRNA; Life Technologies) and the Taqman MicroRNA Reverse Transcription Kit (for miRNA; Life Technologies) according to the manufacturer's instructions. qPCR was then conducted using Taqman mastermix (Life Technologies). PCR primers were purchased from Life Technologies for the following IGF system targets of interest: Igf1 (Rn00710306_m1; NM_178866.4), Igf2 (Rn01454518_m1; NM_031511.2), Igf1r (Rn00583837_m1; NM_052807.2), Igf2r (Rn01636937_m1; NM_012756.1) and Igfbp3 (Rn00561416_m1; NM_012588.2) mRNA as well as Let7f‐1 miRNA (Mm04238181_s1; NR_029731.1). To compensate for variations in RNA input amounts and reverse transcriptase efficiency, mRNA and miRNA abundance of the genes of interest were normalized to the geometric mean of two reference RNA or miRNA genes: TATA box binding protein (Tbp, Rn01455646_m1; NM_001004198.1) and β Actin (Actb, Rn00667869_m1; NM_031144.3) were selected for mRNA and 191 miRNA (Hs04231511_s1; NR_029690.1) and U6 snRNA (001973; NR_004394) were selected for Let 7f‐1 miRNA. HotStart DNA Taq Polymerase was activated by heating the mixture to 95°C for 10 min, then qPCRs were run for 40 cycles of 95°C for 15 s and 60°C for 60 s. Relative changes in mRNA and miRNA abundance was quantified using the 2ΔΔCT method and reported in arbitrary units normalized to Control Sedentary Chow male values. Tbp, Actb, miRNA 191 and U6 snRNA were not different between treatments, diets, exercises or sexes.
Protein extraction and Western blot analysis
Protein was extracted from 50 mg of placental labyrinth tissue using RIPA buffer (Cuffe et al. 2011). Twenty micrograms of protein lysate was loaded onto a 4–15% Tris‐glycine extended (TGX) Stain‐Free gel (Bio‐Rad Laboratories, Gladesville, NSW, Australia) and transferred onto a nitrocellulose membrane (Bio‐Rad Laboratories) (Gardebjer et al. 2014). As it was not possible to include all samples on an individual gel, multiple gels were run concurrently with cross gel calibrators included. The PregEx samples were ran on a separate gel (with a Sedentary Chow Control male sample used as an absolute control). Membranes were probed with antibodies against IGF1 (1:1000, Abcam, Melbourne, VIC, Australia), IGF2 (1:1000, Abcam) or IGF1R (1:1000, Abcam). Densitometric analysis was performed using a ChemiDoc MP with ImageLab Software (Bio‐Rad Laboratories). Protein expression was normalized relative to Stain‐Free total protein in each well (Parviainen et al. 2013) allowing us to control for all of our experimental conditions and expressed as values relative to Control Sedentary Chow males. All gels contained a Control Sedentary Chow male sample for normalization.
Fetal plasma IGF1 analysis
IGF1 concentrations in pooled fetal plasma were analysed using an enzyme‐linked immunosorbent assay (ELISA) following the manufacturer's protocol (R&D Systems, Minneapolis, MN, USA) with a minimum detection limit of 3.5 pg/ml, and intra‐ and inter‐assay coefficients of variation of 4.3% and 6.0%, respectively.
Statistical analysis
A two‐way ANOVA was first conducted to identify differences between Treatment and Exercise within each Diet and Sex. If a main Exercise effect was present, a one‐way ANOVA with a Duncan's post hoc test was used to identify Exercise differences. If an interaction was observed, the data were further split to identify Treatment effects within each Exercise using a Student's unpaired t‐test and a one‐way ANOVA determined Exercise effects in Control and Restricted groups. To determine differences between Diets, the data were split by Sex and Exercise and a two‐way ANOVA conducted to report main Diet effects within Treatments in each Exercise. To identify any Sex differences, a Student's unpaired t‐test was used. There were minimal Diet‐ and Sex‐specific effects. ANOVA statistical analysis was performed using SPSS Statistics 22 (IBM, St Leonards, NSW, Australia) and Student's unpaired t‐tests were performed using Excel (Microsoft, North Ryde, NSW, Australia). All data are presented as means ± SEM and statistical significance was set at P < 0.05.
Results
Fetal and placental outcomes
Effects of F1 maternal growth restriction prior to birth on F2 fetal and placental outcomes
Chow‐fed mothers that were growth restricted prior to birth had normal weight fetuses compared to the Chow‐fed normal birth weight (Control) mothers (Fig. 2 A and B). Chow‐fed mothers that were growth restricted prior to birth (Restricted) increased placental efficiency in F2 males from Sedentary mothers and decreased placental efficiency in F2 males from Exercise mothers compared to their respective Chow‐fed normal birth weight (Control) mothers (Fig. 2 E; Student's t‐test P = 0.012), despite no changes in placental weight (Fig. 2 C). There were no changes observed in placental weight or placental efficiency in F2 females from mothers that were growth restricted prior to birth (Restricted) compared to normal birth weight (Control) mothers (Fig. 2 D and F).
Figure 2. Fetal and placental weights.
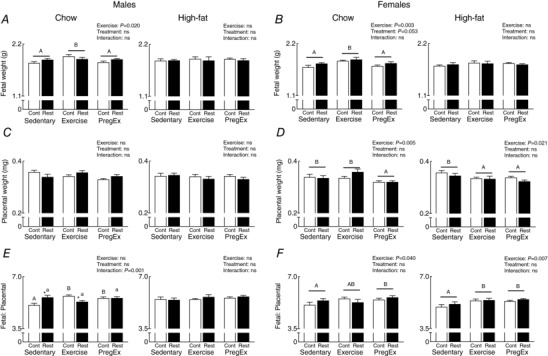
Fetal (A and B) and placental (C and D) weight along with fetal‐placental ratio (E and F) (n = 8–12 litters in each group) for male and female fetuses whose mothers were Control (open bars) or Restricted (black bars) and consumed a Chow (left panel) or High‐fat diet (right panel). Data were analysed by a two‐way ANOVA and presented as mean ± SEM, where ‘ns’ is not significant. * P<0.05 vs. Control and differences across exercises are denoted by different letters where ‘a/A’ is different from ‘b/B’ but not ‘ab/AB’.
Representative sections were taken from F2 female‐associated placentae whose F1 mother was of normal birth weight (Control) and growth restricted prior to birth (Restricted) that Exercised (Fig. 3 B) to highlight the effect F1 maternal growth restriction prior to birth has on placental morphology. F1 mothers that were growth restricted prior to birth and consumed a Chow diet, irrespective of Exercise, had increased total placental, junctional zone and labyrinth cross‐sectional areas in F2 females compared to normal birth weight (Control) mothers (Fig. 3 D, F and H, respectively; two‐way ANOVA). No differences in placental morphology was reported in F2 male‐associated placentae (Fig. 3 C, E and G).
Figure 3. Placental histology parameters.
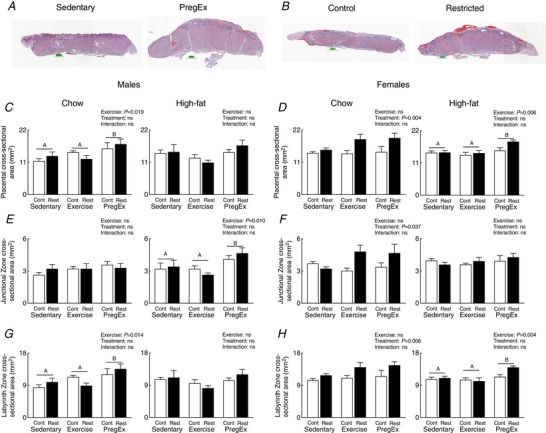
Representative whole placental images demonstrating morphological changes following PregEx compared to Sedentary (male placentae from Chow‐fed mothers) (A) and in Restricted compared to Control (B) placentae (female placentae from Exercise mothers). Whole placental (C and D), junctional zone (E and F) and labyrinth (G and H) cross‐sectional areas in male‐ and female‐associated placentae whose mothers were Control (open bars) or Restricted (black bars) and consumed a Chow (left panel) or High‐fat diet (right panel) (n = 3–4 in each group/sex n = 1 representing one pup from one litter). Data were analysed by a two‐way ANOVA and presented as mean ± SEM. Differences across exercises are denoted by different letters where ‘A’ is different from ‘B’, but not ‘AB’. [Color figure can be viewed at http://wileyonlinelibrary.com]
F1 maternal exercise effects on F2 placental and fetal outcomes
Exercise in F1 Chow‐fed mothers, irrespective of maternal birth weight, increased F2 male and female fetal weights compared to Sedentary and PregEx mothers, with no changes in F1 mothers fed a High‐fat diet (Fig. 2 A and B; two‐way ANOVA). Placental weight was reduced in F2 females whose F1 mother underwent Exercise (High‐fat only) and PregEx, irrespective of maternal birth weight, compared to F1 Sedentary mothers (Fig. 2 D; two‐way ANOVA). Exercise and PregEx in F1 Chow‐fed normal birth weight (Control) mothers increased F2 male placental efficiency compared to F1 Chow‐fed normal birth weight (Control) Sedentary mothers (Fig. 2 E; one‐way ANOVA), with no exercise effects observed in F2 male‐associated placentae from F1 mothers that were growth restricted prior to birth. In F2 females, placental efficiency was increased with Exercise (High‐fat mothers only) and PregEx (Chow and High‐fat fed mothers), irrespective of maternal birth weight, compared to F1 Sedentary mothers (Fig. 2 F; one‐way ANOVA).
Representative sections were taken from F2 male‐associated placentae whose F1 mother was Sedentary and PregEx (Fig. 3 A) to highlight exercise effects on placental morphology. PregEx increased placental and labyrinth zone cross‐sectional areas in F2 males (Chow‐fed mothers only) and F2 females (High‐fat fed mothers only) (Figs 3 C, D, G and 2H; two‐way ANOVA), and increased junctional zone cross‐sectional area in F2 males (High‐fat fed mothers only; Fig. 3 E, two‐way ANOVA), irrespective of maternal birth weight, compared to F1 Sedentary mothers.
Fetal plasma IGF1 concentrations
Effects of F1 maternal growth restriction prior to birth on F2 fetal plasma outcomes
F1 mothers that were growth restricted prior to birth (Restricted) had F2 fetuses with reduced IGF1 plasma concentrations (pooled) in Chow‐fed Exercise mothers (Fig. 4; Student's unpaired t‐test) compared to F1 normal birth weight (Control) Chow‐fed mothers that Exercised. IGF1 plasma concentrations were not altered in F2 fetuses from mothers that were growth restricted prior to birth (Restricted) that consumed a High‐fat diet.
Figure 4. Fetal IGF1 concentrations.
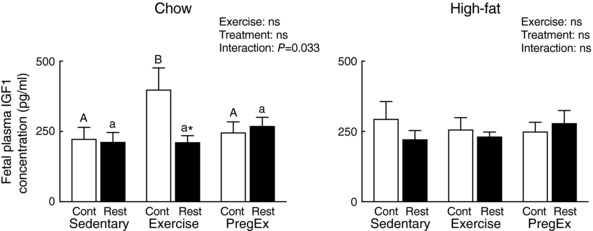
Pooled plasma IGF1 concentrations in fetuses from Chow (left) and High‐fat (right) fed mothers (n = 8–10 litters in each group). Data were analysed by a two‐way ANOVA and presented as mean ± SEM, where ‘ns’ is not significant. * P<0.05 vs. Control and differences across exercises are denoted by different letters where ‘a/A’ is different from ‘b/B’.
F1 maternal exercise effects on F2 fetal plasma outcomes
Exercise in Chow‐fed F1 normal birth weight (Control) mothers increased pooled F2 fetal IGF1 plasma concentrations compared to F1 Sedentary Chow‐fed normal birth weight (Control) mothers, with no Exercise changes reported in mothers that were growth restricted prior to birth (Restricted, Fig. 4; one‐way ANOVA). Exercise in F1 mothers that consumed a High‐fat diet did not alter pooled F2 fetal plasma IGF concentrations (Fig. 4).
Placental IGF1
Effects of F1 maternal growth restriction prior to birth on F2 placental IGF1
F1 mothers that were growth restricted prior to birth (Restricted) and consumed a High‐fat diet had increased Let7f‐1 miRNA abundance in placentae of F2 males, irrespective of maternal exercise, compared to F1 normal birth weight (Control) mothers that consumed a High‐fat diet (Fig. 5 A; two‐way ANOVA), with no changes in IGF1 protein expression (Fig. 5 E). No changes in placental Let 7f‐1 miRNA, Igf1 mRNA or IGF1 protein were detected in F2 female‐associated placentae whose mother was growth restricted prior to birth (Restricted) that consumed a Chow or High‐fat diet compared to normal birth weight (Control) mothers (Fig. 5 B, D and F).
Figure 5. Placental IGF1 expression.
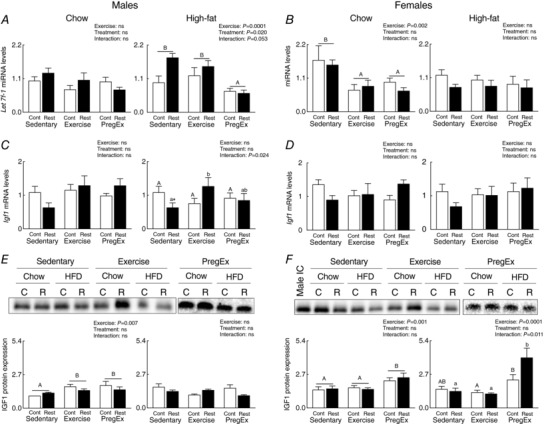
Let 7f‐1 miRNA abundance (A and B), Igf1 mRNA abundance (C and D) (n = 6 in each group/sex n = 1 representing one pup from one litter) and IGF1 protein expression (E and F) (n = 6–7 in each group/sex n = 1 representing one pup from one litter) in male‐ and female‐associated placentae whose mothers were Control (open bars) or Restricted (black bars) and consumed a Chow (left panel) or High‐fat diet (right panel). Data were analysed by a two‐way ANOVA and presented as mean ± SEM, where ‘ns’ is not significant. * P<0.05 vs. Control and differences across exercises are denoted by different letters where ‘a/A’ is different from ‘b/B’ but not ‘ab/AB’.
F1 maternal exercise effects on F2 placental IGF1
PregEx in F1 High‐fat fed mothers, irrespective of maternal birth weight, reduced Let 7f‐1 miRNA abundance in F2 male‐associated placentae compared to F1 Sedentary and PregEx High‐fat fed mothers (Fig. 5 A; two‐way ANOVA). In F2 female‐associated placentae, Exercise and PregEx in F1 Chow‐fed mothers reduced Let7f‐1 miRNA abundance, irrespective of maternal birth weight, compared to F1 Chow‐fed Sedentary mothers (Fig. 5 B; two‐way ANOVA). Exercise in F1 mothers that were growth restricted prior to birth (Restricted) and consumed a High‐fat diet had increased placental Igf1 mRNA abundance in F2 males compared to Restricted Sedentary mothers (Fig. 5 C; one‐way ANOVA), which did not translate to changes in IGF1 protein abundance (Fig. 5 E). Despite no changes in Igf1 mRNA abundance in Chow‐fed F2 male‐associated placentae (Fig. 5 C), Exercise and PregEx increased IGF1 protein expression, irrespective of maternal birth weight, compared to F1 Chow‐fed Sedentary mothers (Fig. 5 E; two‐way ANOVA). Similarly, despite no changes in Igf1 mRNA abundance in F2 female‐associated placentae (Fig. 5 C), consumption of a High‐fat diet in PregEx F1 mothers that were growth restricted prior to birth (Restricted) increased IGF1 protein expression in F2 female‐associated placentae compared to F1 Sedentary and Exercise Restricted mothers (Fig. 5 F; one‐way ANOVA). With PregEx in High‐fat fed normal birth weight (Control) mothers, we observed increasing IGF1 protein expression in F2 female‐associated placentae compared to Exercise in F1 normal birth weight (Control) mothers (Fig. 5 F).
Placental IGF1R
Effects of F1 maternal growth restriction prior to birth on F2 placental IGF1R
F1 mothers that were growth restricted prior to birth (Restricted) and consumed a Chow diet, irrespective of maternal exercise, had reduced Igf1r mRNA abundance in F2 male‐associated placenta compared to normal birth weight (Control) mothers (Fig. 6 A, two‐way ANOVA). No effects were observed in F2 male‐associated placentae if their F1 mother was growth restricted prior to birth (Restricted) and consumed a High‐fat diet (Fig. 6 A). In F2 female associated placentae, PregEx in F1 mothers growth restricted prior to birth (Restricted) that consumed a Chow diet reduced Igf1r mRNA abundance compared to PregEx in F1 Chow‐fed normal birth weight (Control) mothers (Fig. 6 B; Student's unpaired t‐test). No effects were observed in F2 female‐associated placentae if their F1 mother was growth restricted prior to birth (Restricted) and consumed a High‐fat diet (Fig. 6B). However, IGF1R protein expression was not affected by F1 mothers that were growth restricted prior to birth (Restricted; Fig. 6 C and D).
Figure 6. Placental IGF1R expression.
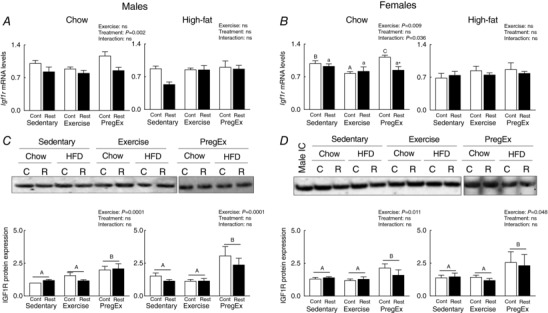
IGF1R mRNA abundance (A and B) (n = 6 in each group/sex n = 1 representing one pup from one litter) and protein expression (C and D) (n =6–7 in each group/sex n = 1 representing one pup from one litter) in male‐ and female‐associated placentae whose mothers were Control (open bars) or Restricted (black bars) and consumed a Chow (left panel) or High‐fat diet (right panel). Data were analysed by a two‐way ANOVA and presented as mean ± SEM, where ‘ns’ is not significant. * P<0.05 vs. Control and differences across exercises are denoted by different letters where ‘a/A’ is different from ‘b/B’ and ‘c/C’ but not different from ‘ab/AB’.
F1 maternal exercise effects on F2 placental IGF1R
No exercise effects were observed in Igf1r mRNA abundance in F2 male‐associated placentae whose F1 mother consumed a Chow or High‐fat diet (Fig. 6 A). In F1 normal birth weight (Control) mothers on a Chow diet, Exercise reduced Igf1r mRNA abundance and PregEx increased Igf1r mRNA abundance in F2 females (Fig. 6 B; one‐way ANOVA) compared to F1 normal birth weight (Control) Sedentary Chow‐fed mothers. No exercise effects were observed in Igf1r mRNA abundance in F2 female‐associated placentae if their F1 mother consumed a High‐fat diet (Fig. 6 B). Interestingly, PregEx increased IGF1R protein expression, irrespective of maternal birth weight, compared to Sedentary in F2 male‐ and female‐associated placenta if their F1 mother consumed a Chow or High‐fat diet (Fig. 6 C and D; two‐way ANOVA).
Placental IGF2
Effect of F1 maternal growth restriction prior to birth on F2 placental IGF2
Exercise in F1 mothers growth restricted prior to birth (Restricted) that consumed a Chow diet reduced Igf2 mRNA abundance in F2 male‐associated placentae compared to F1 Chow‐fed normal birth weight (Control) mothers that Exercised (Fig. 7 A; Student's unpaired t‐test). Additionally, PregEx in F1 mothers growth restricted prior to birth (Restricted) that consumed a Chow diet increased Igf2 mRNA abundance in F2 male‐associated placentae compared to PregEx in F1 Chow‐fed normal birth weight (Control) mothers (Fig. 7 A; Student's unpaired t‐test). No effects were observed in Igf2 mRNA abundance in F2 female‐associated placentae if their F1 mother was growth restricted prior to birth (Restricted) and consumed a Chow or High‐fat diet (Fig. 7 B). Consumption of a High‐fat diet in F1 mothers growth restricted prior to birth (Restricted) increased Igf2 mRNA abundance in F2 male‐ and female‐associated placentae, irrespective of maternal exercise, compared to F1 normal birth weight (Control) mothers (Fig. 7 A and B; two‐way ANOVA). No effects were observed in IGF2 protein expression in F2 male‐ or female‐associated placentae if their F1 mother was growth restricted prior to birth (Restricted) and consumed a Chow or High‐fat diet (Fig. 7 C and D).
Figure 7. Placental IGF2 expression.
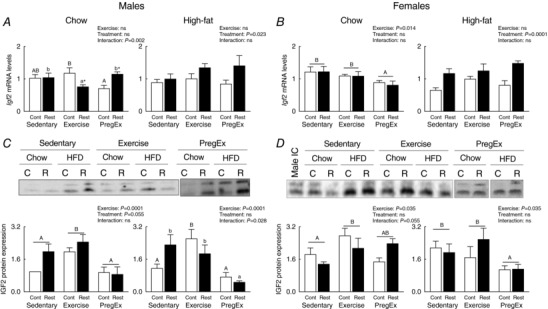
IGF2 mRNA abundance (A and B) (n = 6 in each group/sex n = 1 representing one pup from one litter) and protein expression (C and D) (n = 6–7 in each group/sex n = 1 representing one pup from one litter) in male‐ and female‐associated placentae whose mothers were Control (open bars) or Restricted (black bars) and consumed a Chow (left panel) or High‐fat diet (right panel). Data were analysed by a two‐way ANOVA and presented as mean ± SEM, where ‘ns’ is not significant. * P<0.05 vs. Control and differences across exercises are denoted by different letters where ‘a/A’ is different from ‘b/B’ and ‘c/C’ but not different to ‘ab/AB’.
F1 maternal exercise effects on F2 placental IGF2
In F2 Chow‐fed mothers, Exercise in mothers growth restricted prior to birth (Restricted) caused a reduction in placental Igf2 mRNA abundance compared to Sedentary and PregEx F1 Chow‐fed mothers that were growth restricted prior to birth (Restricted; Fig. 7 A, one‐way ANOVA). Exercise in normal birth weight (Control) mothers increased Igf2 mRNA abundance in F2 male‐associated placentae compared to PregEx in F1 Chow‐fed normal birth weight (Control) mothers (Fig. 7 A, one‐way ANOVA). In F2 female‐associated placentae, PregEx, irrespective of maternal birth weight, reduced Igf2 mRNA abundance compared to F1 Chow‐fed Sedentary and Exercise mothers (Fig. 7 B, two‐way ANOVA). No exercise effects were observed in F2 male‐ or female‐associated placentae, irrespective of maternal birth weight, if their F1 mother consumed a High‐fat diet (Fig. 7 A and B).
Exercise in F1 Chow‐fed mothers, irrespective of maternal birth weight, increased placental IGF2 protein expression in F2 male‐associated placentae compared to F2 Chow‐fed mothers that were Sedentary or PregEx (Fig. 7 C, one‐way ANOVA). Similarly, Exercise in F1 normal birth weight (Control) mothers that consumed a High‐fat diet increased IGF2 protein expression in F2 male‐associated placentae compared to F1 normal birth weight (Control) mothers that were Sedentary or PregEx (Fig. 7 C, one‐way ANOVA). In F2 female‐associated placentae, Exercise in F1 Chow‐fed mothers, irrespective of maternal birth weight, increased IGF2 protein expression compared to F1 Chow‐fed Sedentary mothers (Fig. 7 D, two‐way ANOVA). By contrast, PregEx in F1 High‐fat mothers, irrespective of maternal birth weight, reduced IGF2 protein expression in F2 female‐associated placentae compared to F1 High‐fat fed mothers that were Sedentary or PregEx (Fig. 7 D, one‐way ANOVA).
Placental IGF2R
Effect of F1 maternal growth restriction prior to birth on placental IGF2R
F1 mothers growth restricted prior to birth (Restricted) that consumed a Chow diet reduced Igf2r mRNA abundance in F2 male‐associated placentae, irrespective of maternal exercise, compared to Chow‐fed F1 normal birth weight (Control) mothers (Fig. 8 A; two‐way ANOVA). PregEx in mothers that were growth restricted prior to birth (Restricted) and consumed a High‐fat diet caused a reduction in Igf2r mRNA abundance in F2 female‐associated placentae compared to PregEx in Chow‐fed F1 normal birth weight (Control) mothers (Fig. 8 B; Student's unpaired t‐test). No effects were observed in F2 male‐ or female‐associated placentae, irrespective of maternal exercise, if their F2 mother was growth restricted prior to birth (Restricted) and consumed a High‐fat diet (Fig. 8 A and B).
Figure 8. Placental Igf2r and Igfbp3 mRNA abundance.
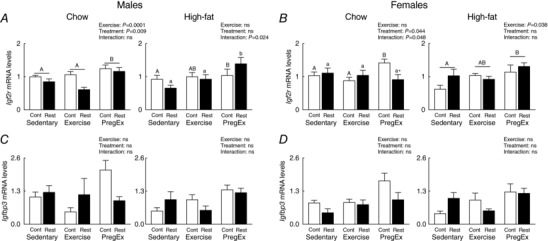
Igf2r (A and B) and Igfbp3 (C and D) mRNA abundance in male‐ and female‐associated placentae whose mothers were Control (open bars) or Restricted (black bars) and consumed a Chow (left panel) or High‐fat diet (right panel). Data were analysed by a two‐way ANOVA and presented as mean ± SEM, where ‘ns’ is not significant (n = 6 in each group/sex n = 1 representing one pup from one litter). * P < 0.05 vs. Control and differences across exercises are denoted by different letters where ‘a/A’ is different from ‘b/B’ but not ‘ab/AB’.
F1 maternal exercise effects on F2 placental IGF2R
PregEx, irrespective of maternal birth weight, increased Igf2r mRNA abundance in Chow‐fed F2 male‐associated placentae compared to Chow‐fed F1 mothers that were Sedentary or Exercised (Fig. 8 A; one‐way ANOVA). With High‐fat feeding, PregEx in F1 normal birth weight (Control) mothers increased Igf2r mRNA abundance in F2 male‐associated placentae compared to F1 normal birth (Control) weight Sedentary mothers (Fig. 8 A, one‐way ANOVA). PregEx in F1 mothers that were growth restricted prior to birth (Restricted) and consumed a High‐fat diet caused an increase in Igf2r mRNA abundance in F2 male‐associated placentae compared to Sedentary and Exercised F1 mothers growth restricted prior to birth (Restricted; Fig. 8 A, one‐way ANOVA). In F2 female‐associated placenta from F1 normal birth weight (Control) mothers that consumed a Chow diet, Igf2r mRNA abundance was increased compared to F1 normal birth weight (Control) mothers that were Sedentary or that Exercised (Fig. 8 B, one‐way ANOVA). High‐fat feeding in PregEx F1 mothers, irrespective of maternal birth weight, increased Igf2r mRNA abundance in F2 female‐associated placentae compared to Sedentary F1 mothers (Fig. 8 B; one‐way ANOVA).
Placental IGFBP3
No effects were observed in Igfbp3 mRNA abundance in F2 male‐ or female‐associated placentae if their mother was growth restricted prior to birth (Restricted) or exercised on either diet (Chow and High‐fat; Fig. 8 C and D).
Discussion
This study has, for the first time, demonstrated that the placental IGF system is independently influenced by maternal birth weight and exercise. Furthermore, these responses are dependent upon the maternal diet and fetal sex. We have previously demonstrated that F1 mothers born growth restricted, and that develop glucose intolerance only during pregnancy, transmit β‐cell deficits to F2 male offspring (Cheong et al. 2016), which may increase their susceptibility to metabolic disease with additional lifestyle challenges. This disease transmission is likely, in part, to be due to placental programming, which this study highlights may not be due to changes in the placental IGF system. Given that exercise is beneficial for maternal and fetal health, it is possible that maternal exercise in ‘at‐risk’ women may prevent the transgenerational transmission of disease, which requires further investigation. However, based on the current study it is likely that any benefits of exercise are not due to improvements in the placental IGF system, as we report minimal exercise effects in F1 mothers born growth restricted or following high‐fat feeding. Additionally, as growth‐restricted women are susceptible to becoming obese, which is independently linked to poor childhood health and increased adult disease susceptibility, changes in the placental IGF system in F1 mothers that were born growth restricted may be more adversely impacted by maternal obesity.
Impact of F1 maternal growth restriction prior to birth
Several studies have demonstrated that the IGF system is dysregulated following fetal growth restriction due to both uteroplacental insufficiency (Laviola et al. 2005) and maternal undernutrition (Coan et al. 2010). This study has, for the first time, demonstrated that this system is similarly dysregulated in placentae of the next generation (F2), which probably contributes to the transgenerational disease programming we have previously reported (Gallo et al. 2012, 2013; Cheong et al. 2016). In the current study F2 male‐associated placentae from F1 Chow‐fed mothers born growth restricted had reduced Igf2r mRNA abundance compared to F1 normal birth weight mothers, which may increase placental IGF2 abundance. As placental IGF2 regulates nutrient handling or partitioning by influencing placental labyrinth morphology and nutrient transport efficiency (Constancia et al. 2002), this probably explains the increased placental efficiency in Sedentary Chow‐fed F1 mothers born growth restricted compared to Sedentary normal birth weight mothers, which is maintaining normal F2 fetoplacental growth. It thus appears that F2 male fetuses of Chow‐fed F1 mothers born growth restricted have an intrinsic adaptation that aims to normalize F2 fetal growth and development by optimizing placental efficiency through IGF2 signalling, by reducing Igf2r mRNA abundance. If this change in Igf2r mRNA abundance results in increased protein expression it would limit the amount of placental IGF2 available to bind to IGF1R, thus inhibiting growth of the F2 fetoplacental unit (Wylie et al. 2003; Harris et al. 2011). Despite similar alterations in IGF2 signalling in F2 male‐associated placentae of F1 mothers born growth restricted that Exercised, this adaptation is inadequate as placental efficiency is reduced compared to Exercise in Chow‐fed F1 normal birth weight mothers, which is probably due to the high maternal metabolically demanding environment. Specifically, Exercise in Chow‐fed F1 mothers born growth restricted would result in the reallocation of nutrients to favour the maternal metabolic system (Mottola & Christopher, 1991), reducing placental nutrient transport capacity (and hence placental efficiency) and could explain the reduced pooled F2 fetal plasma IGF1 concentrations due to impaired placental IGF1 secretion (as IGF1 protein expression is increased), which requires further investigation. Therefore, this reduction in placental nutrient uptake capacity by F2 male fetuses, whose mother was born growth restricted and Exercised, may compromise F2 male fetal development via the IGF pathway. However, additional studies are required to identify if this compromises birth weight and long‐term offspring health. Given that maternal obesity is associated with excess nutrition, it is not surprising that minimal alterations in the IGF system were found in F2 male‐associated placentae of High‐fat fed F1 mothers born growth restricted.
As several studies report sex‐specific responses in the placenta following several pregnancy perturbations (Clifton, 2005; Cuffe et al. 2011, 2012), it is not surprising that placentae of F2 fetuses responded differently if their F1 mother was born growth restricted. Specifically, placentae of F2 female fetuses whose F1 mother was born growth restricted and consumed a Chow diet have significant morphometric adaptations, which probably ensures normal fetoplacental growth by increasing hormone and nutrient storage (junctional zone) and nutrient transportation (labyrinth). These morphological changes would facilitate increased nutrient delivery to the F2 female fetus, which may influence fetal weight; however, no changes in F2 female fetal weight were reported. It is possible that the reduction in pooled IGF1 plasma concentrations measured in F2 fetuses of F1 mothers born growth restricted that Exercised may be due to impaired fetal and/or placental nutrient availability in F2 male fetuses and is not a true representation of what occurred in each sex, which may mask any subtle differences between sexes. Thus, additional studies are required to quantify sex‐specific responses in F2 plasma IGF1 concentrations during pregnancy and at birth along with placental nutrient transporter expression.
Impact of F1 maternal exercise
To our knowledge we are the first to report dynamic changes in F2 fetal plasma IGF1 concentrations following maternal exercise and to identify that changes in the placental IGF system are dependent on the timing of exercise initiation. Similar to studies in humans who perform weight‐bearing exercise prior and during pregnancy (Clapp et al. 2002), Exercise in F1 Chow‐fed mothers, irrespective of maternal birth weight, increased F2 fetal weight, which appears to be caused by different mechanisms for each sex. Specifically, in F2 male‐associated placentae, Exercise in F1 Chow‐fed mothers increased the expression of IGF ligands (IGF1 and IGF2), which stimulates fetoplacental growth by increasing placental nutrient transport capacity (Fowden, 2003) and through their receptor‐specific signalling cascades by stimulating growth, differentiation and proliferation (Chitnis et al. 2008). This potential increase in placental nutrient transportation following Exercise in F1 Chow‐fed mothers may explain the increased pooled IGF1 concentrations in F2 fetuses of normal birth weight mothers, but not in mothers born growth restricted, due to the aforementioned reduced F2 male placental efficiency, which requires further investigation. In F2 female‐associated placentae, however, the increased F2 fetal weight in Chow‐fed F1 mothers is likely to be due to the increased placental IGF2 protein expression, which is known to increase fetal growth via regulation of placental morphology and nutrient partitioning (Constancia et al. 2002; Kent et al. 2012). Nevertheless, it remains to be determined whether this increased F2 female fetal weight in Chow‐fed mothers is beneficial or detrimental to birth weight and/or long‐term offspring health, especially as maternal High‐fat feeding did not alter fetal weight.
Interestingly, more dynamic changes in the placental IGF system were reported following PregEx compared to Exercise, which may be due to the exercise being initiated after implantation and the placenta was required to adapt to the high metabolically demanding environment to ensure normal F2 fetoplacental growth. In line with this suggestion, PregEx, irrespective of maternal birth weight, increased placental IGF receptors (IGF1R and Igf2r) and IGF1 protein expression in both F2 male‐ and female‐associated placentae, which the aim of increasing nutrient delivery to the fetus (Sferruzzi‐Perri et al. 2006). This finding is consistent with studies in humans who underwent low‐intensity exercise during mid‐gestation where placental vascular volume and surface area are increased, indicating a placental adaptation to increase nutrient transfer via increased blood flow (Jackson et al. 1995). It is interesting to note that these increases in IGF1 and IGF1R protein expression in PregEx may be due to reduced Let 7f‐1 miRNA abundance compared to Sedentary in F2 male‐ (High‐fat diet; IGF1R only) and female‐ (Chow diet) associated placentae. This is of interest as Let 7f‐1 miRNA is a known regulator of IGF1 and IGF1R gene and protein expression (Hu et al. 2014). To our knowledge, this is the first study to demonstrate that Let 7f‐1 miRNA is increased in F2 male‐associated placentae from F1 mothers that were born growth restricted and consumed a High‐fat diet and that Let 7f‐1 miRNA is reduced following exercised prior to and during pregnancy (F2 male‐ and female‐associated placenta whose mother consumed a high‐fat and chow diet, respectively) or during pregnancy only (F2 female‐associated placentae whose mother consumed a chow diet). However, as changes in Let 7f‐1 miRNA abundance did not always correlate with alterations in IGF1 or IGF1R gene/protein expression, this suggests that other post‐transcriptional regulators may be involved in the regulation of the placental IGF system in the current study. As such, future studies should characterize other Let 7 cluster miRNAs as potential modulators of IGF1/IGF1R regulation in F2 placentae of F1 mothers born growth restricted and following maternal exercise and high‐fat feeding.
Surprisingly both Exercise (High‐fat fed mothers only) and PregEx (Chow and High‐fat feeding) reduced F2 female‐associated placental weight, irrespective of maternal birth weight, compared to F1 Sedentary mothers, the mechanisms of which are dependent on the timing of exercise initiation. Specifically, the reduction in F2 placental IGF2 protein expression and increased Igf2r mRNA abundance following PregEx in High‐fat fed mothers, irrespective of maternal birth weight, would limit the amount of placental IGF2 binding IGF1R, thus reducing F2 placental weight. Exercise in High‐fat fed mothers, by contrast, reduces F2 placental weight independently of the IGF system and is probably due to another pathway, such as placental growth factor. Despite PregEx in F1 High‐fat fed mothers reducing F2 IGF2 protein expression (mothers born growth restricted only) and increasing Igf2r mRNA abundance in F2 placentae associated with males, junctional zone cross‐sectional area was increased, irrespective of maternal birth weight, with no change in placental weight, which may be an adaptation to increase nutrient storage or increase placental hormone production to facilitate normal fetoplacental growth (Burton & Fowden, 2012). However, additional studies are required to characterize alterations in placental nutrient handling and nutrient partitioning following maternal exercise.
Study limitations
A strength of the current study is that it allows direct comparison of the impact that F1 maternal growth restriction and maternal diet consumption have on the placental IGF system in both male and female F2 fetuses, thus improving our understanding of the impact these factors have on the transgenerational programming of disease. However, it should be noted that to address this research question required the generation of a large number of groups and, as such, the sample sizes used throughout limits the power of the analysis to be able to statistically compare how these parameters (treatment, exercise, diet and fetal sex) in combination influence the placental IGF system (i.e. four‐way ANOVA). Instead analysis prioritized each of the major effects in isolation.
Conclusion
This study demonstrates that the placental IGF system is differentially regulated in F1 mothers born growth restricted and following maternal exercise, with responses dependent on maternal diet and fetal sex, which probably aims to improve F2 fetoplacental growth in the face of adverse in utero environments. F2 fetuses of F1 mothers born growth restricted have structural placental alterations that would facilitate increased nutrient delivery (females) and increase placental IGF2 signalling (males) both of which would promote fetoplacental growth. However, this adaptation in F2 males is inadequate if the growth‐restricted mother consumes a Chow diet and Exercises as fetoplacental efficiency is impaired. Therefore, F2 female fetuses of mothers born growth restricted are able to withstand additional pregnancy challenges that can influence fetoplacental growth via the placental IGF system and may explain why F2 males have compromised organ development (Cheong et al. 2016).
Maternal exercise, specifically PregEx, resulted in profound changes in the placental IGF system that increases the expression of IGF ligands and their receptors to maintain normal fetoplacental growth, which mostly occurred in both mothers with a normal and small birth weight. However, this adaptation is inadequate in F2 female‐associated placentae whereby placental weight is reduced due to limited IGF2 availability (Chow‐fed mothers) or in a manner that is independent of the IGF system (High‐fat fed mothers). Importantly, reductions in Let 7f‐1 miRNA abundance with PregEx may play a role in the regulation of the placental IGF system as it coincided with increased IGF1 (female‐associated placenta whose mother consumed a chow diet) and IGF1R (male‐ and female‐associated placenta whose mother consumes a high‐fat and chow diet, respectively). However, the exact effects that these alterations in the placental IGF system following maternal exercise have on F2 offspring birth weight, development and long‐term health are unknown and future studies are required.
Additional information
Competing interests
The authors declare no conflicts of interest.
Author contributions
M.E.W. and K.M.M. designed the study. Y.T.M.M., J.S.M.C., J.F.B. and S.H. performed all experiments. D.M., K.A. and A.J.J performed the animal work, with assistance from Y.T.M.M. Y.T.M.M., J.S.M.C., J.F.B. and S.H. analysed the data. All authors participated in the interpretation of the results and contributed to writing the manuscript. All authors approved the submission of this version to the Journal of Physiology.
Funding
This research was supported by the National Health and Medical Research Council (NHMRC) of Australia (M.E.W.; 1045602), 2013 Diabetes Australia Research Trust Research Project (M.E.W), J.F.B. holds an Elizabeth and Vernon Puzey Postdoctoral Fellowship at the University of Melbourne, J.S.M.C. held a Postdoctoral Research Fellowship at Griffith University, Y.T.M.M. and K.A. hold a La Trobe University Post Graduate Award and D.M. has a Malaysia Government Scholarship.
Biography
Yeukai Mangwiro is a PhD candidate in the Department of Physiology, Anatomy and Microbiology at La Trobe University, Melbourne, Australia, in collaboration with the Fetal, postnatal & adult physiology & disease laboratory of Professor Mary Wlodek at the University of Melbourne. Her research is centred on the effects of exercise during pregnancy on F2 fetal and placental outcomes including the growth factor system from mothers born growth restricted exposed to a high‐fat diet. She plans to continue her research in the programming field, researching methods to better understand the effects of lifestyle interventions on placental programming in complicated pregnancies.

Edited by: Laura Bennet & Janna Morrison
Linked articles This article is highlighted in a Perspectives article by Gatford. To read this article, visit http://doi.org/10.1113/JP276679.
References
- Asif Y, Wlodek ME, Black MJ, Russell AP, Soeding PF & Wadley GD (2018). Sustained cardiac programming by short‐term juvenile exercise training in male rats. J Physiol 596, 163–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J, Liu JP, Robertson EJ & Efstratiadis A (1993). Role of insulin‐like growth factors in embryonic and postnatal growth. Cell 75, 73–82. [PubMed] [Google Scholar]
- Bauer MK, Breier BH, Harding JE, Veldhuis JD & Gluckman PD (1995). The fetal somatotropic axis during long term maternal undernutrition in sheep: evidence for nutritional regulation in utero. Endocrinology 136, 1250–1257. [DOI] [PubMed] [Google Scholar]
- Borer KT ( 1995). The effects of exercise on growth. Sports Medicine 20, 375–397. [DOI] [PubMed] [Google Scholar]
- Bruun JM, Helge JW, Richelsen B & Stallknecht B (2006). Diet and exercise reduce low‐grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab 290, E961–E967. [DOI] [PubMed] [Google Scholar]
- Burton GJ & Fowden AL (2012). Review: The placenta and developmental programming: balancing fetal nutrient demands with maternal resource allocation. Placenta 33, S23–S27. [DOI] [PubMed] [Google Scholar]
- Cheong JN, Cuffe JS, Jefferies AJ, Moritz KM & Wlodek ME (2016). Adrenal, metabolic and cardio‐renal dysfunction develops after pregnancy in rats born small or stressed by physiological measurements during pregnancy. J Physiol 594, 6055–6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis MM, Yuen JSP, Protheroe AS, Pollak M & Macaulay VM (2008). The type 1 insulin‐like growth factor receptor pathway. Clin Cancer Res 14, 6364. [DOI] [PubMed] [Google Scholar]
- Clapp JF ( 2006). Influence of endurance exercise and diet on human placental development and fetal growth. Placenta 27, 527–534. [DOI] [PubMed] [Google Scholar]
- Clapp JF III, Kim H, Burciu B & Lopez B (2000). Beginning regular exercise in early pregnancy: effect on fetoplacental growth. Am J Obstet Gynecol 183, 1484–1488. [DOI] [PubMed] [Google Scholar]
- Clapp JF III, Kim H, Burciu B, Schmidt S, Petry K & Lopez B (2002). Continuing regular exercise during pregnancy: effect of exercise volume on fetoplacental growth. Am J Obstet Gynecol 186, 142–147. [DOI] [PubMed] [Google Scholar]
- Clifton VL ( 2005). Sexually dimorphic effects of maternal asthma during pregnancy on placental glucocorticoid metabolism and fetal growth. Cell Tissue Res 322, 63–71. [DOI] [PubMed] [Google Scholar]
- Coan PM, Vaughan OR, Sekita Y, Finn SL, Burton GJ, Constancia M & Fowden AL (2010). Adaptations in placental phenotype support fetal growth during undernutrition of pregnant mice. J Physiol 588, 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constancia M, Hemberger M, Hughes J, Dean W, Ferguson‐Smith A, Fundele R, Stewart F, Kelsey G, Fowden A, Sibley C & Relk W (2002). Placental‐specific IGF‐ II is a major modulator of placental and fetal growth. Nature 417, 945–948. [DOI] [PubMed] [Google Scholar]
- Cottrell EC & Ozanne SE (2008). Early life programming of obesity and metabolic disease. Physiol Behav 94, 17–28. [DOI] [PubMed] [Google Scholar]
- Cuffe JS, O'Sullivan L, Simmons DG, Anderson ST & Moritz KM (2012). Maternal corticosterone exposure in the mouse has sex‐specific effects on placental growth and mRNA expression. Endocrinology 153, 5500–5511. [DOI] [PubMed] [Google Scholar]
- Cuffe JS, Walton SL, Steane SE, Singh RR, Simmons DG & Moritz KM (2014). The effects of gestational age and maternal hypoxia on the placental renin angiotensin system in the mouse. Placenta 35, 953–961. [DOI] [PubMed] [Google Scholar]
- Cuffe JSM, Dickinson H, Simmons DG & Moritz KM (2011). Sex specific changes in placental growth and MAPK following short term maternal dexamethasone exposure in the mouse. Placenta 32, 981–989. [DOI] [PubMed] [Google Scholar]
- Cuffe JSM, Saif Z, Perkins AV, Moritz KM & Clifton VL (2017). Dexamethasone and sex regulate placental glucocorticoid receptor isoforms in mice. J Endocrinol 234, 89–100. [DOI] [PubMed] [Google Scholar]
- de Vrijer B, Davidsen ML , Wilkening RB, Anthony RV & Regnault TR (2006). Altered placental and fetal expression of IGFs and IGF‐binding proteins associated with intrauterine growth restriction in fetal sheep during early and mid‐pregnancy. Pediatr Res 60, 507–512. [DOI] [PubMed] [Google Scholar]
- DeChiara TM, Efstratiadis A & Robertson EJ (1990). A growth‐deficiency phenotype in heterozygous mice carrying an insulin‐like growth factor II gene disrupted by targeting. Nature 345, 78–80. [DOI] [PubMed] [Google Scholar]
- Fowden A ( 2003). The insulin‐like growth factors and feto‐placental growth. Placenta 24, 803–812. [DOI] [PubMed] [Google Scholar]
- Gallo LA, Tran M, Cullen‐McEwen LA, Denton KM, Jefferies AJ, Moritz KM & Wlodek ME (2013). Transgenerational programming of fetal nephron deficits and sex‐specific adult hyptertension in rats. Reprod Fertil Dev 26, 1032–1043. [DOI] [PubMed] [Google Scholar]
- Gallo LA, Tran M, Cullen‐McEwen LA, Mortiz KM & Wlodek ME (2012). Low maternal birth weight is associated with transmission of nephron deficits and high blood pressure in male rats. J Hypertens 30, e26. [Google Scholar]
- Gardebjer EM, Cuffe JS, Pantaleon M, Wlodek ME & Moritz KM (2014). Periconceptional alcohol consumption causes fetal growth restriction and increases glycogen accumulation in the late gestation rat placenta. Placenta 35, 50–57. [DOI] [PubMed] [Google Scholar]
- Gentili S, Morrison JL & McMillen IC (2009). Intrauterine growth restriction and differential patterns of hepatic growth and expression of IGF1, PCK2, and HSDL1 mRNA in the sheep fetus in late gestation. Biol Reprod 80, 1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han VK, Bassett N, Walton J & Challis JR (1996). The expression of insulin‐like growth factor (IGF) and IGF‐binding protein (IGFBP) genes in the human placenta and membranes: evidence for IGF‐IGFBP interactions at the feto‐maternal interface. J Clin Endocrinol Metab 81, 2680–2693. [DOI] [PubMed] [Google Scholar]
- Harris LK, Crocker IP, Baker PN, Aplin JD & Westwood M (2011). IGF2 actions on trophoblast in human placenta are regulated by the insulin‐like growth factor 2 receptor, which can function as both a signaling and clearance receptor. Biol Reprod 84, 440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins L, Greenwood SL, Wareing M, Sibley CP & Mills TA (2011). Obesity and the placenta: a consideration of nutrient exchange mechanisms in relation to aberrant fetal growth. Placenta 32, 1–7. [DOI] [PubMed] [Google Scholar]
- Hopkins SA, Baldi JC, Cutfield WS, McCowan L & Hofman PL (2010). Exercise training in pregnancy reduces offspring size without changes in maternal insulin sensitivity. J Clin Endocrinol Metab 95, 2080–2088. [DOI] [PubMed] [Google Scholar]
- Hoyo C, Fortner K, Murtha AP, Schildkraut JM, Soubry A, Demark‐Wahnefried W, Jirtle RL, Kurtzberg J, Forman MR, Overcash F, Huang Z & Murphy SK (2012). Association of cord blood methylation fractions at imprinted insulin‐like growth factor 2 (IGF2), plasma IGF2, and birth weight. Causes Cancer Control 23, 635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Li T, Hu R, Wu L, Li M & Meng X (2014). MicroRNA let‐7a and let‐7f as novel regulatory factors of the sika deer (Cervus nippon) IGF‐1R gene. Growth Factors 32, 27–33. [DOI] [PubMed] [Google Scholar]
- Jackson MR, Gott P, Lye SJ, Ritchie JW & Clapp JF III (1995). The effects of maternal aerobic exercise on human placental development: placental volumetric composition and surface areas. Placenta 16, 179–191. [DOI] [PubMed] [Google Scholar]
- Jansson N, Nilsfelt A, Gellerstedt M, Wennergren M, Rossander‐Hulthen L, Powell TL & Jansson T (2008). Maternal hormones linking maternal body mass index and dietary intake to birth weight. Am J Clin Nutr 87, 1743–1749. [DOI] [PubMed] [Google Scholar]
- Kent LN, Ohboshi S & Soares MJ (2012). Akt1 and insulin‐like growth factor 2 (Igf2) regulate placentation and fetal/postnatal development. Int J Dev Biol 56, 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kind KL, Owens JA, Robinson JS, Quinn KJ, Grant PA, Walton PE, Gilmore RS & Owens PC (1995). Effect of restriction of placental growth on expression of IGFs in fetal sheep: relationship to fetal growth, circulating IGFs and binding proteins. J Endocrinol 146 23–34. [DOI] [PubMed] [Google Scholar]
- Koutsaki M, Sifakis S, Zaravinos A, Koutroulakis D, Koukoura O & Spandidos DA (2011). Decreased placental expression of hPGH, IGF‐I and IGFBP‐1 in pregnancies complicated by fetal growth restriction. Growth Horm IGF Res 21, 31–36. [DOI] [PubMed] [Google Scholar]
- Laker RC, Gallo LA, Wlodek ME, Siebel AL, Wadley GD & McConell GK (2011). Short‐term exercise training early in life restores deficits in pancreatic β‐cell mass associated with growth restriction in adult male rats. Am J Physiol Endocrinol Metab 301, E931–E940. [DOI] [PubMed] [Google Scholar]
- Laker RC, Wlodek ME, Wadley GD, Gallo LA, Meikle PJ & McConell GK (2012). Exercise early in life in rats born small does not normalize reductions in skeletal muscle PGC‐1α in adulthood. Am J Physiol Endocrinol Metab 302, E1221–E1230. [DOI] [PubMed] [Google Scholar]
- Langford K, Blum W, Nicolaides K, Jones J, McGregor A & Miell J (1994). The pathophysiology of the insulin‐like growth factor axis in fetal growth failure: a basis for programming by undernutrition? Eur J Clin Invest 24, 851–856. [DOI] [PubMed] [Google Scholar]
- Laviola L, Perrini S, Belsanti G, Natalicchio A, Montrone C, Leonardini A, Vimercati A, Scioscia M, Selvaggi L, Giorgino R, Greco P & Giorgino F (2005). Intrauterine growth restriction in humans is associated with abnormalities in placental insulin‐like growth factor signaling. Endocrinology 146, 1498–1505. [DOI] [PubMed] [Google Scholar]
- Leddy MA, Power ML & Schulkin J (2008). The impact of maternal obesity on maternal and fetal health. Rev Obstet Gynecol 1, 170–178. [PMC free article] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ & Efstratiadis A (1993). Mice carrying null mutations of the genes encoding insulin‐like growth factor 1 (Igf‐1) and type 1 IGF receptor (Igf1r). Cell 75, 59–72. [PubMed] [Google Scholar]
- Lupu F, Terwilliger JD, Lee K, Segre GV & Efstratiadis A (2001). Roles of growth hormone and insulin‐like growth factor 1 in mouse postnatal growth. Dev Biol 229, 141–162. [DOI] [PubMed] [Google Scholar]
- Mottola MF & Christopher PD (1991). Effects of maternal exercise on liver and skeletal muscle glycogen storage in pregnant rats. J App Physiol (1985) 71, 1015–1019. [DOI] [PubMed] [Google Scholar]
- O'Gorman DJ, Karlsson HK, McQuaid S, Yousif O, Rahman Y, Gasparro D, Glund S, Chibalin AV, Zierath JR & Nolan JJ (2006). Exercise training increases insulin stimulated glucose disposal and GLUT4 (SLC2A4) protein content in patients with type 2 diabetes. Diabetologia 49, 2983–2992. [DOI] [PubMed] [Google Scholar]
- Parviainen VI, Joenväärä S, Tohmola N & Renkonen R (2013). Label‐free mass spectrometry proteome quantification of human embryonic kidney cells following 24 hours of sialic acid overproduction. Proteome Sci 11, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AM & Pedersen BK (2005). The anti‐inflammatory effect of exercise. J Appl Physiol (1985) 98, 1154–1162. [DOI] [PubMed] [Google Scholar]
- Raastad T, Bjoro T & Hallen J (2000). Hormonal responses to high‐ and moderate‐intensity strength exercise. Eur J Appl Physiol 82, 121–128. [DOI] [PubMed] [Google Scholar]
- Rosario FJ, Powell TL & Jansson T (2016). Activation of placental insulin and mTOR signaling in a mouse model of maternal obesity associated with fetal overgrowth. Am J Physiol Regul Integr Comp Physiol 310, R87–R93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sferruzzi‐Perri AN, Owens JA, Pringle KG, Robinson JS & Roberts CT (2006). Maternal insulin‐like growth factors‐I and ‐II act via different pathways to promote fetal growth. Endocrinology 147, 3344–3355. [DOI] [PubMed] [Google Scholar]
- Sferruzzi‐Perri AN, Vaughan OR, Haro M, Cooper WN, Musial B, Charalambous M, Pestana D, Ayyar S, Ferguson‐Smith AC, Burton GJ, Constancia M & Fowden AL (2013). An obesogenic diet during mouse pregnancy modifies maternal nutrient partitioning and the fetal growth trajectory. FASEB J 27, 3928–3937. [DOI] [PubMed] [Google Scholar]
- Spencer JA, Chang TC, Jones J, Robson SC & Preece MA (1995). Third trimester fetal growth and umbilical venous blood concentrations of IGF‐1, IGFBP‐1, and growth hormone at term. Arch Dis Child Fetal Neonatal Ed 73, F87–F90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur A, Sase M, Lee JJ, Thakur V & Buchmiller TL (2000). Ontogeny of insulin‐like growth factor 1 in a rabbit model of growth retardation. J Surg Res 91, 135–140. [DOI] [PubMed] [Google Scholar]
- Turgut S, Kaptanoglu B, Emmungil G & Turgut G (2006). Increased plasma levels of growth hormone, insulin‐like growth factor (IGF)‐I and IGF‐binding protein 3 in pregnant rats with exercise. Tohoku J Exp Med 208, 75–81. [DOI] [PubMed] [Google Scholar]
- Vega SR, Kleinert J, Sulprizio M, Hollmann W, Bloch W & Struder HK (2011). Responses of serum neurotrophic factors to exercise in pregnant and postpartum women. Psychoneuroendocrinology 36, 220–227. [DOI] [PubMed] [Google Scholar]
- Wadley GD, Laker RC, McConell GK & Wlodek ME (2016). Endurance training in early life results in long‐term programming of heart mass in rats. Physiol Rep 4, e12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodek ME, Westcott KT, O'Dowd R, Serruto A, Wassef L, Moritz KM & Moseley JM (2005). Uteroplacental restriction in the rat impairs fetal growth in association with alterations in placental growth factors including PTHrP. Am J Physiol Regul Integr Comp Physiol 288, R1620–R1627. [DOI] [PubMed] [Google Scholar]
- Woods KA, Camacho‐Hubner C, Savage MO & Clark AJL (1996). Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin‐like growth factor I gene. N Engl J Med 335, 1363–1367. [DOI] [PubMed] [Google Scholar]
- Wylie AA, Pulford DJ, McVie‐Wylie AJ, Waterland RA, Evans HK, Chen Y‐T, Nolan CM, Orton TC & Jirtle RL (2003). Tissue‐specific inactivation of murine M6P/IGF2R. Am J Pathol 162, 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]


