Abstract
Key points
We evaluated the effect of magnesium sulphate (MgSO4) on seizures induced by asphyxia in preterm fetal sheep.
MgSO4 did not prevent seizures, but significantly reduced the total duration, number of seizures, seizure amplitude and average seizure burden.
Saline‐asphyxia male fetuses had significantly more seizures than female fetuses, but male fetuses showed significantly greater reduction in seizures during MgSO4 infusion than female fetuses.
A circadian profile of seizure activity was observed in all fetuses, with peak seizures seen around 04.00–06.00 h on the first and second days after the end of asphyxia.
This study is the first to demonstrate that MgSO4 has utility as an anti‐seizure agent after hypoxia–ischaemia. More information is needed about the mechanisms mediating the effect of MgSO4 on seizures and sexual dimorphism, and the influence of circadian rhythms on seizure expression.
Abstract
Seizures are common in newborns after asphyxia at birth and are often refractory to anti‐seizure agents. Magnesium sulphate (MgSO4) has anticonvulsant effects and is increasingly given to women in preterm labour for potential neuroprotection. There is limited information on its effects on perinatal seizures. We examined the hypothesis that MgSO4 infusion would reduce fetal seizures after asphyxia in utero. Preterm fetal sheep at 0.7 gestation (104 days, term = 147 days) were given intravenous infusions of either saline (n = 14) or MgSO4 (n = 12, 160 mg bolus + 48 mg h−1 infusion over 48 h). Fetuses underwent umbilical cord occlusion (UCO) for 25 min, 24 h after the start of infusion. The start time for seizures did not differ between groups, but MgSO4 significantly reduced the total number of seizures (P < 0.001), peak seizure amplitude (P < 0.05) and seizure burden (P < 0.005). Within the saline‐asphyxia group, male fetuses had significantly more seizures than females (P < 0.05). Within the MgSO4‐asphyxia group, although both sexes had fewer seizures than the saline‐asphyxia group, the greatest effect of MgSO4 was on male fetuses, with reduced numbers of seizures (P < 0.001) and seizure burden (P < 0.005). Only 1 out of 6 MgSO4 males had seizures on the second day post‐UCO compared to 5 out of 6 MgSO4 female fetuses (P = 0.08). Finally, seizures showed a circadian profile with peak seizures between 04.00 and 06.00 h on the first and second day post‐UCO. Collectively, these results suggest that MgSO4 may have utility in treating perinatal seizures and has sexually dimorphic effects.
Keywords: magnesium sulphate, asphyxia, seizures
Key points
We evaluated the effect of magnesium sulphate (MgSO4) on seizures induced by asphyxia in preterm fetal sheep.
MgSO4 did not prevent seizures, but significantly reduced the total duration, number of seizures, seizure amplitude and average seizure burden.
Saline‐asphyxia male fetuses had significantly more seizures than female fetuses, but male fetuses showed significantly greater reduction in seizures during MgSO4 infusion than female fetuses.
A circadian profile of seizure activity was observed in all fetuses, with peak seizures seen around 04.00–06.00 h on the first and second days after the end of asphyxia.
This study is the first to demonstrate that MgSO4 has utility as an anti‐seizure agent after hypoxia–ischaemia. More information is needed about the mechanisms mediating the effect of MgSO4 on seizures and sexual dimorphism, and the influence of circadian rhythms on seizure expression.
Introduction
There is strong clinical and preclinical evidence in adults that MgSO4 has significant anticonvulsant effects, primarily by binding to a specific site on the N‐methyl‐d‐aspartate (NMDA) receptor in a voltage‐dependent manner (Traynelis et al. 2010). MgSO4 may also reduce excitation of the glutaminergic kainate and α‐amino‐3‐hydroxy‐5‐methylisoxazole‐4‐propionic acid (AMPA) receptors (Hallak et al. 2000). It has been used for many years to reduce the risk of maternal seizures during moderate to severe eclampsia, and may be more effective than anticonvulsant agents such as phenytoin (Duley et al. 2010; Roy et al. 2013). Meta‐analysis suggests that MgSO4 may also be effective for non‐eclamptic refractory epilepsy and status epilepticus (Zeiler et al. 2015). Similarly, animal experiments have confirmed that MgSO4 has marked anticonvulsant effects in seizures induced by NMDA, electrical stimulation and eclampsia, including reducing seizure duration and number (Wolf et al. 1990; Hallak et al. 1992; Cotton et al. 1993; Standley et al. 1995a; Decollogne et al. 1997; Oliveira et al. 2011; Liu et al. 2013; Huang et al. 2014).
In newborn infants, hypomagnesaemia is associated with seizures, and it is standard practice to correct this with MgSO4 infusions (Silverstein & Jensen, 2007). However, there is little direct evidence about the effect of MgSO4 on other neonatal seizures. Hypoxic–ischaemic encephalopathy (HIE) at birth is the commonest cause of seizures in newborns (Silverstein & Jensen, 2007). The effect of boluses of MgSO4 (250 mg kg−1 or 125 mg kg−1) given in the days after birth in infants with HIE had either no anti‐seizure effect (Groenendaal et al. 2002; Ichiba et al. 2002; Bhat et al. 2009), or a modest effect (Gathwala et al. 2010). Levene and colleagues observed that bolus doses produced a rapidly tapering plasma concentration, and that cerebrospinal fluid levels are lower, with a delay in transfer of MgSO4 from blood to the brain (Levene et al. 1995). Broadly consistent with these findings, MgSO4 (250 mg kg−1) given within 24 h of birth did not reduce the levels of excitatory amino acids in cerebrospinal fluid in term infants with HIE (Khashaba et al. 2006).
MgSO4 is increasingly given to women at risk of preterm labour because it may provide neuroprotection (Galinsky et al. 2014; Shepherd et al. 2017). Extremely preterm infants have much higher risks of perinatal HIE than term infants (Manuck et al. 2016), with correspondingly high risk of clinical seizures (Gale et al. 2017). Further, it is important to note that a much higher proportion of seizures in preterm infants are subclinical than at term (Glass et al. 2017). At present, there is limited information on the effect of antenatal MgSO4 treatment on preterm seizures after a hypoxic–ischaemic insult.
Thus, the primary aim of the current study was to examine the effect of antenatal MgSO4 on the development of seizures after acute asphyxia induced by complete umbilical cord occlusion in preterm fetal sheep at 0.7 of gestation. At this gestational age, the neural maturation of fetal sheep is broadly equivalent to 28–32 weeks of human development (Barlow, 1969). Based on the evidence from adult animal experiments, we hypothesised that MgSO4 would significantly reduce the total time spent seizing and seizure number. A secondary aim of the study was to examine whether there were sex‐specific effects of MgSO4 on seizure activity. There is sexual dimorphism in seizure‐control networks within the brain, and males have a greater susceptibility to seizures (Akman et al. 2014). Indeed, limited data suggest that MgSO4 may be more effective in males (Standley et al. 1995b).
Methods
Ethical approval
All animal procedures and animal facilities were approved by the Animal Ethics Committee of The University of Auckland, New Zealand, in accordance with the Code of Ethical Conduct of The University of Auckland, and the New Zealand Animal Welfare Act 1999. These studies conform to the principles and regulations described by Grundy (2015).
Animals and surgical instrumentation
Romney ewes were time mated with Suffolk rams and pregnancies and parity were identified using ultrasound. Ewes were brought to the University laboratory a week before surgery to allow acclimatisation to the laboratory. Ewes and their fetuses underwent surgical procedures between 97 and 98 days gestation (term = 147 days) (Quaedackers et al. 2004; Galinsky et al. 2017). Food, but not water, was withdrawn 12–18 h before surgery to reduce aspiration during surgery. Prior to surgery, ewes were weighed and given an intramuscular injection of the antibiotic oxytetracycline (20 mg kg−1, Phoenix Pharm, Auckland, New Zealand) for prophylaxis. Anaesthesia was induced by intravenous injection of propofol (5 mg kg−1; AstraZeneca Limited, Auckland, New Zealand), the ewes intubated, and anaesthesia maintained using 2–3% isoflurane in O2 (Bomac Animal Health, NSW, Australia). During surgery, ewes received isotonic saline (∼250 ml h−1) by intravenous drip to maintain fluid balance. All anaesthetic protocols were undertaken and monitored by trained anaesthetic technicians.
Instrumentation
Following maternal laparotomy and a uterine incision the fetus was partially exteriorised for instrumentation. Two pairs of electroencephalograph (EEG) electrodes (AS633‐5SSF; Cooner Wire, Chatsworth, CA, USA) were placed through burr holes onto the dura over the parasagittal parietal cortex (5 and 10 mm anterior to, and 5 mm lateral to, bregma) and secured with cyanoacrylate glue. A reference electrode was sewn over the occiput. Additional electrodes were placed across the fetal chest to measure the fetal electrocardiogram (ECG) to derive fetal heart rate. Polyvinyl catheters (SteriHealth, Dandenong South, VIC, Australia) were inserted in the right femoral artery, and right brachial artery and vein for measurement of blood pressure and for fetal blood sampling and drug administration. A catheter was placed in the amniotic cavity for measurement of amniotic pressure. An inflatable silicone occluder (In Vivo Metric, Healdsburg, CA, USA) was placed loosely around the umbilical cord to facilitate cord occlusion at a later time. The fetus was returned to the uterus, the uterus and abdominal wounds closed, and antibiotics (80 mg gentamycin; Rousell Ltd, Auckland, New Zealand) administered into the amniotic sac. The maternal midline skin incision was infiltrated with a local analgesic (10 ml 0.5% bupivacaine plus adrenaline, AstraZeneca Ltd). All fetal leads were exteriorised through the maternal flank and a maternal long saphenous vein to provide access for post‐operative care and humane killing.
Post‐surgical care
When surgery was completed the ewes were taken off anaesthesia, extubated and returned to their home cages where they were continuously monitored until they were stable and had been observed to stand and eat and drink. Sheep were housed together in separate metabolic cages with access to water and food ad libitum in a temperature‐controlled room (16 ± 1°C, humidity 50 ± 10%) with a 12 h:12 h light–dark cycle (lights off at 18.00 h). Fetuses were allowed a 4–5 days post‐operative recovery period before experiments commenced. During this time welfare monitoring was undertaken several times each day and ewes received intravenous antibiotics daily for 4 days (benzylpenicillin sodium; 600 mg; Novaris, Auckland, New Zealand and gentamycin; 80 mg). Fetal catheters were maintained patent by continuous infusion of heparinised saline (20 IU ml−1) at a rate of 0.2 ml h−1 and the maternal catheters were flushed daily with heparinised saline.
Experimental recordings
Fetal mean arterial blood pressure (MAP), corrected for movement by subtraction of amniotic pressure, EEG and ECG were recorded continuously for offline analysis using custom data acquisition software (LabView for Windows, National Instruments, Austin, TX, USA). The blood pressure signal was recorded using a Novatrans II, MX860 pressure transducer (Medex Inc., Hilliard, OH, USA) collected at 64 Hz and low‐pass filtered at 30 Hz. The raw ECG signal was used to derive fetal heart rate (FHR) and was analogue filtered with a first order high‐pass filter set at 0.05 Hz and an 8th order low‐pass Bessel filter set at 100 Hz and digitised at 512 Hz. EEG signals were recorded via leads through a head‐stage with an overall gain of 10,000. Signals were then processed with a 6th order low‐pass Butterworth filter set to 500 Hz. The EEG signal was low‐pass filtered with a cutoff frequency set with the −3 dB point at 30 Hz, and digitised at a sampling rate of 1024 Hz for seizure analysis (Davidson et al. 2011; Koome et al. 2013; Abbasi et al. 2017).
Experimental protocol
At 104 days fetuses were randomly allocated to receive an intravenous infusion of magnesium plus asphyxia (magnesium sulphate heptahydrate, MgSO4.7H2O, 500 mg ml−1; Phebra, NSW, Australia; n = 12: 6 females and 6 males), or isotonic saline plus asphyxia (n = 14: 7 females and 7 males). Fetuses were infused with MgSO4 from 24 h before until 24 h after fetal asphyxia (Galinsky et al. 2016). The sheep placenta rapidly metabolises magnesium and thus to achieve a clinically relevant steady‐state fetal plasma concentration MgSO4 was given directly to the fetus. Fetuses received a 160 mg loading dose over 5 min followed by a 48 mg h−1 maintenance infusion for 48 h. By 24 h, this produced a steady‐state plasma concentration of around 1.88 mmol l−1 (Galinsky et al. 2016), consistent with the neonatal plasma levels after antenatal treatment for neuroprotection (Borja‐Del‐Rosario et al. 2014).
Fetal asphyxia was induced by rapid, complete inflation of the umbilical cord occluder for 25 min (Quaedackers et al. 2004). Umbilical cord occlusion (UCO) was started at 09.30 h. Successful occlusion was confirmed by the rapid onset of bradycardia, a rise in MAP and changes in blood chemistry. Fetal blood samples were taken from the brachial artery for analysis of MgSO4 (Roche/Hitachi 902 clinical chemistry analyser, Hoffman‐La Roche, Basel, Switzerland) 15 min before MgSO4 or saline infusion started (baseline), and 1, 4, 6 and 24 h before asphyxia, then 1, 2, 4, 6, 24, 48 and 72 h after asphyxia. Pre‐ductal blood was also taken for pH, blood gas (ABL 800, Radiometer, Copenhagen, Denmark), glucose and lactate measurements (model 2300, YSI, Yellow Springs, OH, USA) before the start of MgSO4 infusion, 1 h pre‐asphyxia, 17 min of asphyxia, and 1, 2, 4, 6, 24, 48 and 72 h post‐asphyxia. Ewes and fetuses were killed 3 days post‐asphyxia by an overdose of pentobarbitone sodium to the ewe (9 g, Pentobarb 300; Chemstock International, Christchurch, New Zealand).
Data analysis and statistics
Off‐line physiological data analysis was performed using Labview‐based customised programs. Seizure analysis was undertaken blinded to the treatment group (V. Draghi and L. Bennet). Each minute of the raw EEG traces of all fetuses was assessed for the appearance of high amplitude stereotypic evolving seizures consisting of rhythmic repetitive waves occurring for at least 10 s, and varying in frequency or amplitude as the seizure progresses (Shellhaas & Clancy, 2007; Davidson et al. 2012; Koome et al. 2013). Data were averaged hourly for analysis. The numbers of high amplitude seizures per hour, the individual duration of seizures, their peak amplitude, the duration spent seizing per hour (seizure burden), and the total duration of seizures (from first to last seizure) was calculated for the groups as a whole and by sex.
Hourly averages were used to evaluate temporal changes in activity, and analysed in 6 h bins to account for temporal changes. Data were compared within and between groups using two‐way analysis of variance (ANOVA, SPSS 22.0 for Windows (SPSS, Chicago, Il, USA) followed by a Fisher's protected least‐significant difference (LSD) post hoc test when a significant effect of group was found. The data were also averaged over the total period (total group data) for group, and by sex and group, and these and the blood chemistry data were analysed by one‐way ANOVA with group as the independent factor. Statistical significance was accepted when P < 0.05. Data are median (interquartile range) or mean ± SEM as appropriate.
Results
General group data and plasma magnesium
The effects of the MgSO4 infusion on fetal plasma magnesium have been reported earlier for a subset of the fetuses in the present study (Galinsky et al. 2017). MgSO4 infusion increased fetal plasma magnesium rapidly after the start of infusion, and levels remained significantly elevated throughout the experiment, and for 2 days after the end of the infusion. Peak concentrations occurred on the day of occlusion (Galinsky et al. 2017). There were no differences between groups in pH, blood gases, glucose and lactate before or during the occlusion (Table 1). There was a small reduction in during recovery in the MgSO4‐asphyxia group (Table 1), but there were no other significant differences between groups. The nadir of FHR in the final minute of UCO was 57 ± 2.7 beats min−1 in the vehicle group vs. 56.0 ± 2.1 beats min−1 in magnesium (not significantly different (NS)). The nadir of MAP was 11.2 ± 0.7 mmHg vs. 11.3 ± 0.6 mmHg (NS).
Table 1.
Fetal arterial pH, blood gases, glucose and lactate concentrations before and after UCO before magnesium sulphate infusion begins (−24 h), 1 hour prior to UCO (−60 min), 17 min during umbilical cord occlusion, and at designated time points during recovery from occlusiona
| Time | −24 h | −60 min | 17 min | +1 h | +2 h | +4 h | +6 h | +24 h | +48 h | +72 h | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | Saline | 7.37 ± 0.01 | 7.37 ± 0.01 | 6.80 ± 0.01 | 7.29 ± 0.2 | 7.33 ± 0.03 | 7.40 ± 0.01 | 7.39 ± 0.01 | 7.36 ± 0.01 | 7.37 ± 0.1 | 7.35 ± 0.1 |
| MgSO4 | 7.36 ± 0.01 | 7.34 ± 0.01 | 6.82 ± 0.01* | 7.29 ± 0.01 | 7.32 ± 0.01 | 7.38 ± 0.01 | 7.38 ± 0.01 | 7.36 ± 0.01 | 7.36 ± 0.1 | 7.34 ± 0.2 | |
| (mmHg) | Saline | 48.5 ± 2.0 | 46.5 ± 0.3 | 135.0 ± 2.1* | 50.1 ± 2.6 | 49.5 ± 1.7 | 46.2 ± 0.9 | 47.9 ± 1.7 | 48.3 ± 1.1 | 46.2 ± 0.7 | 49.4 ± 0.7 |
| MgSO4 | 45.7 ± 1.1 | 44.1 ± 1.2 | 129.5 ± 4.6* | 41.5 ± 1.6* | 42.0 ± 2.0* | 42.3 ± 1.3* | 43.4 ± 1.6* | 44.6 ± 1.3 | 42.3 ± 1.3* | 44.5 ± 2.0 | |
| (mmHg) | Saline | 24.2 ± 0.6 | 25.2 ± 1.8 | 6.9 ± 0.8* | 28.7 ± 2.4 | 25.0 ± 1.9 | 24.1 ± 1.4 | 24.4 ± 1.6 | 25.3 ± 1.7 | 25.8 ± 1.4 | 26.0 ± 1.6 |
| MgSO4 | 24.6 ± 1.1 | 24.6 ± 1.5 | 6.5 ± 1.1* | 30.5 ± 2.5 | 28.6 ± 1.6 | 27.1 ± 2.1 | 27.0 ± 1.5 | 30.1 ± 1.6 | 30.1 ± 3.0 | 28.1 ± 2.7 | |
| Lactate (mmol l−1) | Saline | 0.8 ± 0.1 | 1.0 ± 0.1 | 6.8 ± 0.3* | 4.2 ± 0.6 | 4.0 ± 0.8 | 2.6 ± 0.6 | 2.3 ± 0.5 | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| MgSO4 | 0.9 ± 0.1 | 0.9 ± 0.1 | 6.3 ± 0.3* | 4.1 ± 0.3 | 3.5 ± 0.2 | 2.0 ± 0.2 | 1.9 ± 0.2 | 1.3 ± 0.3 | 1.0 ± 0.1 | 0.9 ± 0.1 | |
| Glucose (mmol l−1) | Saline | 1.1 ± 0.1 | 1.0 ± 0.1 | 0.7 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 |
| MgSO4 | 1.0 ± 0.0 | 0.9 ± 0.1 | 0.7 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.0 | 1.2 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
Data are mean ± SEM, * P < 0.05 saline vs. MgSO4. aMagnesium sulphate infusion ended after the +24 h sample.
General seizure analysis
The start time for seizures after UCO was not significantly different between groups (9.7 (7.4–14.5) h vs. 6.7 (5.6–7.7) h post‐UCO, MgSO4‐asphyxia vs. saline‐asphyxia, P = 0.08, Fig. 1 A). MgSO4 reduced the total time spent seizing (14.6 (8.0–36.7) h vs. 39 (36.0–46.0) h, P < 0.005, Fig. 1 B). MgSO4 also significantly reduced the total number of seizures (30.0 (17.0–37.0) vs. 138.0 (100–168.5), P < 0.001, Fig. 2 A), peak seizure amplitude (86.0 (70.0–118.0) μV vs. 120.0 (90–156.0) μV, P < 0.05, Fig. 2 B), and seizure burden (124.0 (92.3–156.0) s h−1 vs. 201.0 (159.1–267.0) s h−1, P < 0.005, Fig. 2 C). However, there was no significant effect on the mean duration of individual seizures (68.0 (56.4–85.0) s vs. 61.1 (53.2–77.5) s, P = 0.33, data not shown).
Figure 1. The onset time of seizures after the end of umbilical cord occlusion (A) and the total time spent seizing from first to last seizure (B) in saline‐asphyxia (open circles) and MgSO4‐asphyxia (filled circles) treated fetuses.
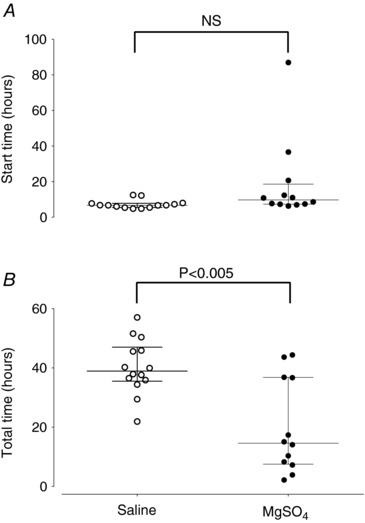
Data are median (interquartile ranges). NS, not significant.
Figure 2. The total number of seizures (A), peak seizure amplitude (B) and seizure burden (C) in saline‐asphyxia (open circles) and MgSO4‐asphyxia (filled circles) treated fetuses.
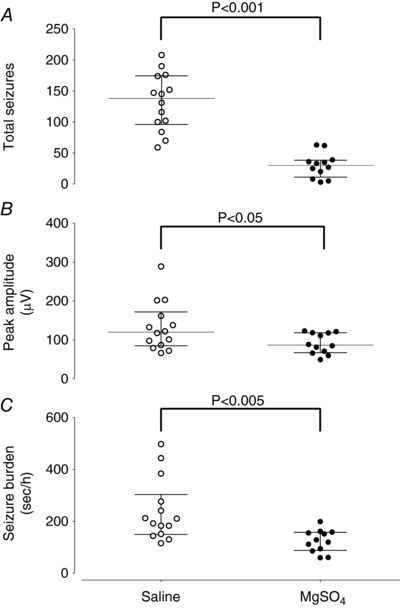
Data are median (interquartile ranges).
Sex differences in seizure activity
Data for sex and treatment are shown in Table 2. There were no significant sex differences in the onset time of seizures (Fig. 3 A). In the saline‐asphyxia group, males had more seizures than females (P < 0.05). Overall, both MgSO4 females and males had significantly fewer seizures than their saline‐treated counterparts (P < 0.001, Fig. 4 A), and reduced total seizure burden (P < 0.05, Fig. 4 C). The total duration of seizures was not different between saline‐asphyxia and MgSO4‐asphyxia females (Fig. 3 B), whereas it was shorter in MgSO4‐asphyxia males than saline‐asphyxia males (P < 0.001, Fig. 3 B) and MgSO4‐asphyxia females (P < 0.005). There were no significant sex differences for individual seizure duration (Table 2), or seizure amplitude (Table 2, Fig. 4 B).
Table 2.
Seizure activity grouped by sex and treatment
| Female saline‐asphyxia | Female MgSO4‐asphyxia | Male saline‐asphyxia | Male MgSO4‐asphyxia | |
|---|---|---|---|---|
| Start (h) | 6.8 (6.4–10.0) | 10.1 (7.2–18.6) | 6.7 (5.3–7.0) | 9.7 (7.8–11.0) |
| Total time (h) | 40.0 (35.1–51.3) | 36.8 (22.2–42.0) | 40.2 (37.0–48.1) |
|
| Seizure number |
|
|
174 (152.3–183) |
|
| Average duration (s) | 73 (58.1–79.4) | 69 (61.0–88.2) | 58.1 (57.2–61.1) | 64.1 (60.1–79.1) |
| Peak amplitude (μV) | 138.1 (110.1–202.3) | 118.2 (91.0–121.0) | 101.4 (90.5–126) | 78.0 (62.0–87.5) |
| Seizure burden (s h−1) | 192.0 (110–202.3) | 125.5 (99–148.4) | 212.2 (190.3–313.0) |
|
Data are median (interquartile range).
Figure 3. Start time of seizures after the end of umbilical cord occlusion (A) and the total time spent making seizures (B) in female and male saline‐asphyxia fetuses (open and filled circles), and female and male MgSO4‐asphyxia fetuses (open and filled squares).
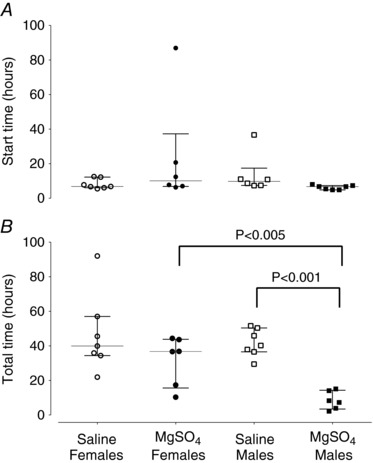
Data are median (interquartile ranges).
Figure 4. The total number of seizures (A), peak seizure amplitude (B) and seizure burden (C) in female and male saline‐asphyxia fetuses (open and filled circles), and female and male MgSO4‐asphyxia fetuses (open and filled squares).
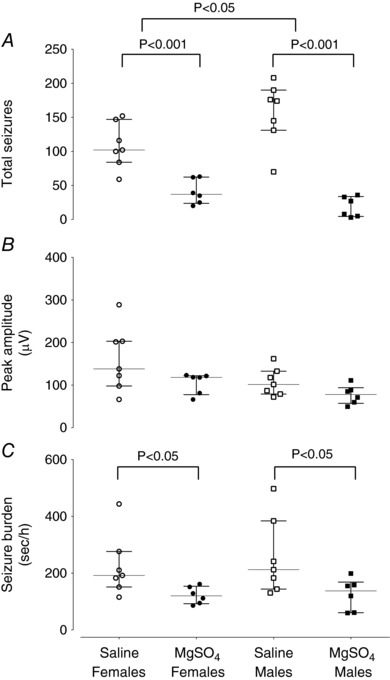
Data are median (interquartile ranges).
Temporal pattern of seizures
The saline‐asphyxia group showed two distinct phases of seizures in the first and the second 24 h after asphyxia (Fig. 5). During the first phase, seizure number and burden peaked around 21 h post‐UCO (06.30 h). Seizures then progressively fell until around 36 h post‐UCO, before increasing again to a second peak at around 43 h post‐UCO (04.30 h). In the saline‐asphyxia group, seizure amplitude was higher in the first 12 h compared to subsequent 12 h time bins (P < 0.05, Fig. 5) and the individual duration of a seizure was longer in the first 12 h than in subsequent 12 h time bins (P < 0.05, data not shown). There was a significant decrease in seizure number in the MgSO4‐asphyxia group between 6 and 37 h (P < 0.001, Fig. 5 A), peak seizure amplitude between 6 and 18 h (P < 0.05, Fig. 5 B), and seizure burden between 6 and 36 h (P < 0.005, Fig. 5 C). The individual length of a seizure was significantly longer between 7 and 20 h in the saline‐asphyxia group vs. MgSO4‐asphyxia group (data not shown).
Figure 5. Time sequence of changes in numbers of seizures (A), peak seizure amplitude (B) and seizure burden (C) in saline‐asphyxia (open bars) and MgSO4‐asphyxia (filled bars) fetuses.
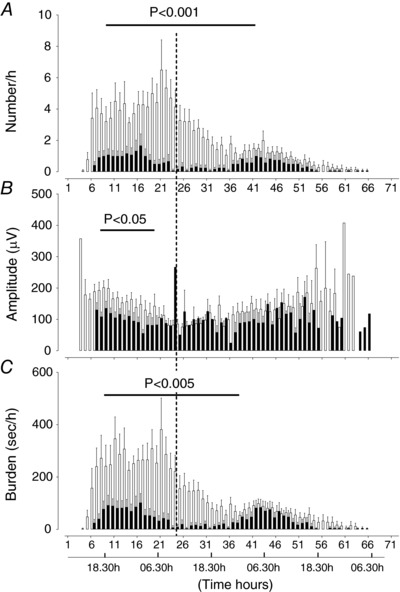
Data are mean ± SEM.
Compared to saline‐asphyxia female fetuses, MgSO4‐asphyxia female fetuses had significantly fewer seizures between 7 and 20 h (P < 0.005, Fig. 6 A), reduced seizure amplitude between 6 and 18 h (P < 0.05, Fig. 6 B), and reduced seizure burden between 7 and 20 h (P < 0.005, Fig. 6 C). Compared to saline‐asphyxia males, MgSO4‐asphyxia male fetuses had a significant reduction in seizure number between 18 and 36 h (P < 0.001, Fig. 7 A), peak seizure amplitude between 6 and 18 h (P < 0.05, Fig. 7 B), and seizure burden between 12 and 25 h (P < 0.005, Fig. 7 C).
Figure 6. Time sequence of changes in number of seizures (A), peak seizure amplitude (B) and seizure burden (C) in female saline‐asphyxia fetuses (open bars) and female MgSO4‐asphyxia fetuses (filled bars).
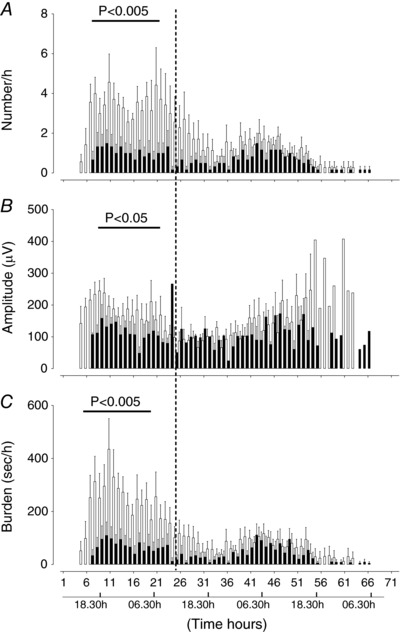
Data are mean ± SEM.
Figure 7. Time sequence of changes in number of seizures (A), peak seizure amplitude (B) and seizure burden (C) in male saline‐asphyxia fetuses (open bars) and male MgSO4‐asphyxia fetuses (filled bars).
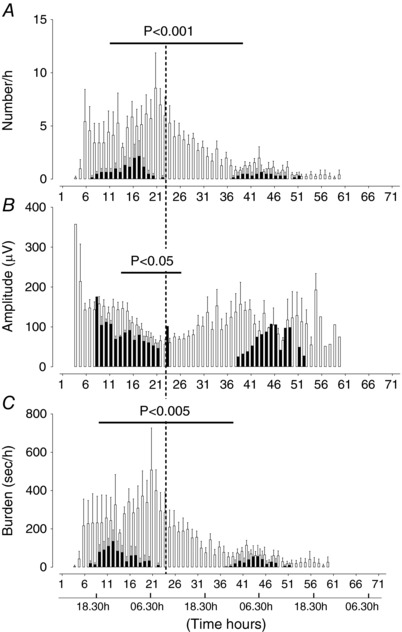
Data are mean ± SEM.
Saline‐asphyxia female fetuses had total fewer seizures than saline‐asphyxia male fetuses between 13 and 34 h (P < 0.05), but there were no other differences between sexes in the saline‐asphyxia group (Table 2). MgSO4‐asphyxia males had a shorter total duration of seizures compared to MgSO4‐asphyxia females (P < 0.005, Table 2) and fewer seizures (P < 0.05, Table 2). It is notable that MgSO4‐asphyxia male fetuses had no seizures between 23 and 30 h (Fig. 7), whereas 3 out of 6 MgSO4‐asphyxia female fetuses had seizures during this period (Fig. 7). Only one male from the MgSO4‐asphyxia group had seizures in the second phase of seizures compared to 5 out of 6 female MgSO4‐asphyxia fetuses (P = 0.08, Fisher exact test).
Discussion
This study is the first to dissect in detail the effects of a clinically relevant plasma concentration of MgSO4 on seizures in preterm fetuses after an acute asphyxial insult in utero. The data show that while MgSO4 did not prevent or delay the onset of seizures, it significantly reduced the duration of seizures, seizure burden, and numbers and amplitude of seizures. Further, we have made the novel observation that while MgSO4 was beneficial for both sexes, it had a greater impact on male fetuses. Unexpectedly, the effects of MgSO4 were time dependent, with the maximal effect of MgSO4 seen on the ramp up to peak seizure numbers. A second key observation was that there were two distinct peaks in seizure activity on the first and second days after occlusion, occurring around the same time in the early hours of the morning. A circadian pattern in seizure activity has been observed in adults, but this is the first report in the fetus.
The asphyxia protocol used in the present study is associated with subcortical neuronal injury and diffuse white matter loss, but no cortical neuronal injury (Bennet et al. 2006; Dean et al. 2006b; Galinsky et al. 2017). Using this protocol, we have recently shown that MgSO4 is not neuroprotective, and thus any effects on seizures would be independent of changes in neuronal survival (Galinsky et al. 2017). Seizures in the current study in all fetuses were single, brief and relatively infrequent events as demonstrated previously (Quaedackers et al. 2004). The primary phase of seizures in the saline‐asphyxia group occurred in the first 24 h after asphyxia. While little is known about the temporal evolution of preterm seizures, the present data are consistent with limited clinical data on the evolution of seizure activity in normothermic term infants, which show seizures are maximal in the first 24 h after a hypoxic–ischaemic insult (McBride et al. 2000; Lynch et al. 2012).
Consistent with the overall reduction of delayed seizures, we have previously observed a reduction in pre‐seizure epileptiform transient activity during MgSO4 infusion (Lakadia et al. 2016). MgSO4 primarily ameliorates seizures by binding to glutamate receptors as demonstrated in adult rodent models (Hallak et al. 1992; Cotton et al. 1993; Mason et al. 1994; Decollogne et al. 1997). Glutamate plays a key role in mediating seizures in fetal sheep (Tan et al. 1992; Dean et al. 2006a). The immature brain is considered more susceptible to seizures due to the density of calcium permeable, GluR2‐subunit‐deficient AMPA receptors, which contribute to a lower threshold for seizures, and expression of NMDA glutamate receptor subunits (GluN) such as GluN2B, which mediate longer‐duration excitatory postsynaptic potentials, and GluN3A, which are relatively magnesium insensitive (Jensen, 2009). Further, hypoxia can change the proportional expression of NMDA receptor subunits, and so promote increased neuronal excitability and seizures in the immature brain (Dean et al. 2005; Rakhade et al. 2008; Zhou et al. 2009, 2015).
γ‐Aminobutyric acid (GABA) may also play a role, as chloride efflux promotes depolarisation instead of hyperpolarisation as seen in the adult brain (Nardou et al. 2013). While the GABAergic promotion of excitation is important for development of neural circuitry, it can increase susceptibility to seizures (Nardou et al. 2013). Moreover, in neonatal rodents, depolarisation of GABA receptors can facilitate the removal of the voltage‐dependent magnesium block from NMDA channels (Leinekugel et al. 1999).
The time‐dependent anticonvulsant effects of MgSO4 in the present study likely reflected the voltage‐dependent nature of magnesium binding. Under physiological conditions, NMDA receptor activation requires glutamate and glycine plus initial membrane depolarisation, typically through AMPA receptor activation, to relieve the magnesium mediated voltage‐dependent NMDA receptor channel block (Traynelis et al. 2010; Nikolaev et al. 2012). The influx of calcium plus internal calcium release during glutamatergic activation prolongs depolarisation and further extends the initial release of magnesium blockade (Traynelis et al. 2010; Nikolaev et al. 2012). At the onset of seizures in the present study, seizures were relatively infrequent but each event was of greater magnitude than subsequent events. Thus, speculatively, the greater magnitude of depolarisation‐elevated levels of magnesium were insufficient to completely maintain the magnesium block, although the extracellular levels attained during infusion were at a level that facilitated seizure modulation. As seizures evolved, their amplitude fell, likely sufficient to allow magnesium to further block NMDA receptors.
In the current study, fetal sex affected both seizure expression in saline‐asphyxia animals and the impact of MgSO4. Strikingly, MgSO4 not only significantly attenuated the occurrence of seizures in the first phase of seizures (first 24 h), but in the second period of seizures (i.e. the second 24 h), only one male fetus developed further seizures, compared with nearly all of the female fetuses. These data suggest that the effect of MgSO4 on males was not dependent on circulating plasma levels of MgSO4, but rather that MgSO4 had a sustained effect on the mechanisms that increase the threshold for seizure induction. In the saline‐asphyxia group, female fetuses had significantly fewer seizures than males, consistent with observations in neonates (Jensen, 2009; Giorgi et al. 2014).
Sex‐specific differences in seizures in the saline‐asphyxia group may relate to GABA receptors. Numerous studies show that GABA switches from depolarisation to hyperpolarisation, as seen in adults, much sooner in females (Galanopoulou & Moshe, 2003; Veliskova et al. 2004; Nunez & McCarthy, 2007; Stafstrom, 2008; Murguia‐Castillo et al. 2013). This is due to an earlier switch to the potassium chloride cotransporter 2 (KCC2), which is active in transporting chloride out of the cell (Galanopoulou & Moshe, 2003; Veliskova et al. 2004; Nunez & McCarthy, 2007; Stafstrom, 2008; Murguia‐Castillo et al. 2013). However, GABA receptors, like glutamate receptors, can be labile during seizures and limited data suggest that repetitive seizures may cause GABA to switch from initiating neuronal depolarisation to inducing hyperpolarisation, and this may occur earlier in males (Stafstrom, 2008).
There are surprisingly few studies on sex differences in glutamate receptors in the developing brain. Newborn rodent studies show that there are developmental changes in NMDA receptor subunit composition with a switch from a predominance of NR2B‐containing to NR2A‐containing receptors (Monyer et al. 1994; Jensen, 2009; Traynelis et al. 2010). Damborsky and colleagues reported that neonatal male rodents have a higher expression of GluN2A, and lower mRNA expression of GluN2B, compared to females (Damborsky & Winzer‐Serhan, 2012), which would lead to shorter NMDA receptor current durations (Matta et al. 2011). Further, excitotoxic brain damage in neonatal mice was associated with greater long‐term motor and cognitive deficits in males than females, which were alleviated by MgSO4 (Daher et al. 2017), supporting the possibility of sex‐specific neuroprotection. In the current study, while individual seizures tended to be shorter in MgSO4 treated males, this was not significant. Potentially, a sex dependent switch to more GluN2A, and a reduction in other receptor subtypes such as GluN3A, would increase the threshold to seizures leading to fewer seizures and the sex dependent reduction in seizure burden seen in males.
Alternatively, a role for sex hormones should also be considered. Our data on the effect of magnesium on male fetuses are consistent with a study in adult rats, which showed that intracerebroventricular administration of NMDA caused greater seizures in males, but magnesium treatment reduced total seizure duration and number only in males (Standley et al. 1995b). In this study, the authors speculated that there may be a role for sex hormones in modulating these differences, as is described in adults (McCarthy, 2008; Reddy, 2017). In neonatal rat pups, androgens exacerbate seizures induced in males by administration of the GABA agonist muscimol; this effect is prevented by castration (Giorgi et al. 2007). Oestradiol, metabolised from testosterone, further reduces KCC2, thus exacerbating chloride clearance (Galanopoulou & Moshe, 2003). Additionally, androgens are associated with reduced clearance of calcium after a seizure, and a sustained calcium response to subsequent seizures in neonatal pups (Nunez & McCarthy, 2008).
In adults, testosterone has both pro‐ and anticonvulsant effects, and the pro‐convulsant effect may in part be due to aromatisation of testosterone to oestrogen (Reddy, 2017). Oestradiol can potentiate NMDA receptor activity through sex‐specific mechanisms, with oestradiol increasing release and sensitivity to glutamate in males (Oberlander & Woolley, 2016). Further, seizures can induce neurosteroid oestradiol synthesis, which in turn escalates seizure activity (Sato & Woolley, 2016). Testosterone aromatase activity can be downregulated in the presence of increased magnesium (Balthazart et al. 2001) and thus magnesium may have modulated seizures by acting as an aromatase inhibitor. There is considerable, unsurprising, evidence that testosterone is elevated in fetal life in males compared to females (Pomerantz & Nalbandov, 1975; Roselli et al. 2011).
Finally, we report the novel observation that post‐asphyxial seizure activity in the preterm fetal sheep shows an apparently circadian profile, with a ramp‐up in seizures late in the evening/early morning and a peak around 04.00–06.00 h. In the context of the present study, it is not possible to rule out the possibility that it might be partly related to differential evolution of progressive secondary mitochondrial failure between regions of the brain (Bennet et al. 2006). Future studies could examine how the seizure profile is modulated by initiating asphyxia at night for example instead of during the day. Although this is the first description of a circadian pattern of seizures in the immature brain, diurnal changes in seizure activity were first described in postnatal life by Gowers in 1885. Subsequent studies in adults have shown that seizures can be either nocturnal or diurnal, depending on their type or seizure locus (Anderson et al. 2015; van Campen et al. 2015), and a similar pattern has been observed in children (Loddenkemper et al. 2011).
The mechanisms mediating nocturnal seizures are multifactorial, but may relate to changes in GABA and associated glutamate release (Avoli et al. 2013; Moore et al. 2017). In adults, GABA is a key circadian time‐keeper, and within the suprachiasmatic nucleus (SCN) exhibits bipolar activity, promoting excitability during the day and inhibition at night (Wagner et al. 1997; Choi et al. 2008). However, deficits in GABAergic inhibition are associated with abnormal activity and within a hyperexcitable network GABA can promote seizure activity (Moore et al. 2017).
The fetus exhibits circadian patterns in behaviour, which are entrained by maternal rhythms and melatonin passage through the placenta (Dalton et al. 1977; Simonetta et al. 1991; Breen et al. 1996; Seron‐Ferre et al. 2012). Maternal signals are in part co‐ordinated by signals from the fetal central and peripheral clocks (Seron‐Ferre et al. 2012). In the immature brain, activity in the neurons in the SCN are more active during the day than night, with SCN activity potentially suppressed by melatonin (Breen et al. 1996).
In the current study, fetuses do not yet show sleep‐state cycling (Mellor et al. 2005). Nevertheless, the preterm fetal brain activity does change during the night, with loss of quiescent periods such as interburst intervals (Davidson et al. 2011). Further, we have demonstrated increased neural cytochrome oxidase activity at night, suggesting greater neural metabolic activity (Bennet et al. 2006). Neural circadian activity in the immature fetus is poorly understood but, like adults (Raven et al. 2017), the fetus may preferentially use night‐time to promote neural network development. Certainly, sleep is important for normal perinatal maturation of the brain (Mirmiran et al. 2003) and GABA, along with synergistic glutamate activity, is postulated to play a key role in mediating development of the perinatal neural network (Dehorter et al. 2012). Speculatively, in our experiments, in a setting where there is high risk of epileptiform activity, increased release of GABA and glutamate at night may promote greater seizure activity.
Finally, there is a potential dose effect to consider. MgSO4 did not modulate overall seizure activity in the second 24 h period, particularly in female fetuses who showed less effect during the first seizure phase. This may reflect falling levels of MgSO4 after the end of infusion at 24 h post‐UCO (Galinsky et al. 2017). Our data suggest that not only is there a need to maintain treatment until the risk of seizures has abated, but that a higher dose will likely be required to optimise control of seizures. Clinically, there are safety concerns with increasing the dose of MgSO4 for both mother and infant (Levene et al. 1995; Zeng et al. 2016), including potential for increased risk of intraventricular haemorrhage and neonatal morbidity (Narasimhulu et al. 2017). Studies of MgSO4 given to newborns suggest that bolus doses of between 125 and 250 mg kg−1 given repeatedly every 24 h have little effect on seizures, possibly because of the relatively limited transfer of MgSO4 from blood to the brain (Ichiba et al. 2002; Gathwala et al. 2010). Further, in term infants with hypoxic–ischaemic encephalopathy, increasing the dose to 400 mg was associated with hypotension and respiratory depression (Levene et al. 1995). In the current study we have demonstrated the efficacy of a clinically relevant dose, given over a longer duration of treatment, on seizure activity without fetal hypotension or any evidence of mortality (Galinsky et al. 2016, 2017). Potentially, a relatively modest increase in the dose may have provided better control of seizures. Alternatively, we may speculate that antenatal MgSO4 treatment continued into the postnatal period at the current dose may facilitate better seizure management with anti‐epileptic therapies.
In conclusion, our study has demonstrated for the first time that MgSO4 may have utility as an anti‐seizure agent after an acute fetal hypoxic–ischaemic insult. Importantly, our study has shown that seizures in the immature brain exhibit an apparently circadian profile, and that while both sexes benefitted from MgSO4 treatment, the effect on male fetuses was greater. Further work is now required to determine the optimal therapeutic anti‐seizure dose regime for MgSO4, and to examine whether MgSO4 given after seizures start can stop seizure activity.
Perspective
Seizures are common after perinatal hypoxic–ischaemic insults, and it is recognised that they have been significantly under‐reported, in part due to a lack of EEG monitoring and training (Boylan et al. 2013; Hellstrom‐Westas et al. 2015). Whether all seizures increase brain injury, and thus should be treated, remains debated (Shetty, 2015). However, resolving this issue is difficult because anti‐seizure treatments for neonates are limited in number, potentially injurious themselves, and for many infants simply ineffective or effectiveness is lost over time (Hellstrom‐Westas et al. 2015; Shetty, 2015; Thoresen & Sabir, 2015). There is a need for more evidence‐based studies to guide neonatal seizure management (Hellstrom‐Westas et al. 2015).
Importantly, however, as highlighted in this paper, to make advances in designing new treatments requires greater information to address significant gaps in our knowledge about the mechanisms mediating perinatal seizures, how they change over time and as a function of factors such as the seizures themselves, perinatal age and sex, time of day, and other clinical conditions and treatments. Collectively, this information, in turn, will inform us about which drugs may be effective, when they are effective and who will benefit, including those treated before birth.
Additional information
Competing interests
None declared.
Author contributions
L.B., A.J.G. and R.G. conceived and designed the experiments; V.D., R.G., C.A.L., J.O.D. and L.B. collected the data; L.B. and V.D. analysed the data; L.B., C.P.U. and A.J.G. interpreted the data and drafted the article. R.G., V.D., C.A.L., J.O.D. and C.P.U. contributed to drafting the article and/or revising it critically for intellectual content. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
These studies were supported by the Health Research Council of New Zealand, grants 17/601 and 12/613, and the Auckland Medical Research Council (grant 1213003).
Biography
Laura Bennet is head of the Department of Physiology and co‐director of the Fetal Physiology and Neuroscience Group in the Department of Physiology, at the University of Auckland. She is a fetal systems physiologist with a specialist interest in preterm fetal and neonatal cardiovascular and neurophysiological adaptations to common in utero insults such as asphyxia and infection, interactions between insults and with other standard clinical therapies such as glucocorticoids. Her work encompasses both pre‐clinical and clinical studies and her current work includes assessment of EEG waveforms as a biomarker for predicting injury processes in the preterm infant, and the development of treatments designed to help the immature brain repair itself after injury.

Edited by: Kim Barrett & Dino Giussani
References
- Abbasi H, Bennet L, Gunn AJ & Unsworth CP (2017). Robust wavelet stabilized “footprints of uncertainty” for fuzzy system classifiers to automatically detect sharp waves in the EEG after hypoxia ischemia. Int J Neural Syst 27, 1650051. [DOI] [PubMed] [Google Scholar]
- Akman O, Moshe SL & Galanopoulou AS (2014). Sex‐specific consequences of early life seizures. Neurobiol Dis 72, 153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CT, Tcheng TK, Sun FT & Morrell MJ (2015). Day‐night patterns of epileptiform activity in 65 patients with long‐term ambulatory electrocorticography. J Clin Neurophysiol 32, 406–412. [DOI] [PubMed] [Google Scholar]
- Avoli M, de Curtis M & Kohling R (2013). Does interictal synchronization influence ictogenesis? Neuropharmacology 69, 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Baillien M & Ball GF (2001). Rapid and reversible inhibition of brain aromatase activity. J Neuroendocrinol 13, 63–73. [DOI] [PubMed] [Google Scholar]
- Barlow RM (1969). The foetal sheep: morphogenesis of the nervous system and histochemical aspects of myelination. J Comp Neurol 135, 249–262. [DOI] [PubMed] [Google Scholar]
- Bennet L, Roelfsema V, Pathipati P, Quaedackers J & Gunn AJ (2006). Relationship between evolving epileptiform activity and delayed loss of mitochondrial activity after asphyxia measured by near‐infrared spectroscopy in preterm fetal sheep. J Physiol 572, 141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat MA, Charoo BA, Bhat JI, Ahmad SM, Ali SW & Mufti MU (2009). Magnesium sulfate in severe perinatal asphyxia: a randomized, placebo‐controlled trial. Pediatrics 123, e764–769. [DOI] [PubMed] [Google Scholar]
- Borja‐Del‐Rosario P, Basu SK, Haberman S, Bhutada A & Rastogi S (2014). Neonatal serum magnesium concentrations are determined by total maternal dose of magnesium sulfate administered for neuroprotection. J Perinat Med 42, 207–211. [DOI] [PubMed] [Google Scholar]
- Boylan GB, Stevenson NJ & Vanhatalo S (2013). Monitoring neonatal seizures. Semin Fetal Neonatal Med 18, 202–208. [DOI] [PubMed] [Google Scholar]
- Breen S, Rees S & Walker D (1996). The development of diurnal rhythmicity in fetal suprachiasmatic neurons as demonstrated by fos immunohistochemistry. Neuroscience 74, 917–926. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Lee CJ, Schroeder A, Kim YS, Jung SH, Kim JS, Kim DY, Son EJ, Han HC, Hong SK, Colwell CS & Kim YI (2008). Excitatory actions of GABA in the suprachiasmatic nucleus. J Neurosci 28, 5450–5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton DB, Hallak M, Janusz C, Irtenkauf SM & Berman RF (1993). Central anticonvulsant effects of magnesium sulfate on N‐methyl‐D‐aspartate‐induced seizures. Am J Obstet Gynecol 168, 974–978. [DOI] [PubMed] [Google Scholar]
- Daher I, Le Dieu‐Lugon B, Dourmap N, Lecuyer M, Ramet L, Gomila C, Ausseil J, Marret S, Leroux P, Roy V, El Mestikawy S, Daumas S, Gonzalez B, Leroux‐Nicollet I & Cleren C (2017). Magnesium sulfate prevents neurochemical and long‐term behavioral consequences of neonatal excitotoxic lesions: Comparison between male and female mice. J Neuropathol Exp Neurol 76, 883–897. [DOI] [PubMed] [Google Scholar]
- Dalton KJ, Dawes GS & Patrick JE (1977). Diurnal, respiratory, and other rhythms of fetal heart rate in lambs. Am J Obstet Gynecol 127, 414–424. [DOI] [PubMed] [Google Scholar]
- Damborsky JC & Winzer‐Serhan UH (2012). Effects of sex and chronic neonatal nicotine treatment on Na2+/K+/Cl− co‐transporter 1, K+/Cl− co‐transporter 2, brain‐derived neurotrophic factor, NMDA receptor subunit 2A and NMDA receptor subunit 2B mRNA expression in the postnatal rat hippocampus. Neuroscience 225, 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JO, Green CR, Nicholson LF, O'Carroll SJ, Fraser M, Bennet L & Gunn AJ (2012). Connexin hemichannel blockade improves outcomes in a model of fetal ischemia. Ann Neurol 71, 121–132. [DOI] [PubMed] [Google Scholar]
- Davidson JO, Quaedackers JS, George SA, Gunn AJ & Bennet L (2011). Maternal dexamethasone and EEG hyperactivity in preterm fetal sheep. J Physiol 589, 3823–3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JM, Fraser M, Shelling AN, Bennet L, George S, Shaikh S, Scheepens A & Gunn AJ (2005). Ontogeny of AMPA and NMDA receptor gene expression in the developing sheep white matter and cerebral cortex. Mol Brain Res 139, 242–250. [DOI] [PubMed] [Google Scholar]
- Dean JM, George SA, Wassink G, Gunn AJ & Bennet L (2006a). Suppression of post hypoxic‐ischemic EEG transients with dizocilpine is associated with partial striatal protection in the preterm fetal sheep. Neuropharmacology 50, 491–503. [DOI] [PubMed] [Google Scholar]
- Dean JM, Gunn AJ, Wassink G, George S & Bennet L (2006b). Endogenous α2‐adrenergic receptor‐mediated neuroprotection after severe hypoxia in preterm fetal sheep. Neuroscience 142, 615–628. [DOI] [PubMed] [Google Scholar]
- Decollogne S, Tomas A, Lecerf C, Adamowicz E & Seman M (1997). NMDA receptor complex blockade by oral administration of magnesium: comparison with MK‐801. Pharmacol Biochem Behav 58, 261–268. [DOI] [PubMed] [Google Scholar]
- Dehorter N, Vinay L, Hammond C & Ben‐Ari Y (2012). Timing of developmental sequences in different brain structures: physiological and pathological implications. Eur J Neurosci 35, 1846–1856. [DOI] [PubMed] [Google Scholar]
- Duley L, Henderson‐Smart DJ & Chou D (2010). Magnesium sulphate versus phenytoin for eclampsia. Cochrane Database Syst Rev, CD000128. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS & Moshe SL (2003). Role of sex hormones in the sexually dimorphic expression of KCC2 in rat substantia nigra. Exp Neurol 184, 1003–1009. [DOI] [PubMed] [Google Scholar]
- Gale C, Statnikov Y, Jawad S, Uthaya SN & Modi N (2017). Neonatal brain injuries in England: population‐based incidence derived from routinely recorded clinical data held in the National Neonatal Research Database. Arch Dis Child Fetal Neonatal Ed 10.1136/archdischild-2017-313707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinsky R, Bennet L, Groenendaal F, Lear CA, Tan S, van Bel F, Juul SE, Robertson NJ, Mallard C & Gunn AJ (2014). Magnesium is not consistently neuroprotective for perinatal hypoxia‐ischemia in term‐equivalent models in preclinical studies: A systematic review. Dev Neurosci 36, 73–82. [DOI] [PubMed] [Google Scholar]
- Galinsky R, Davidson JO, Drury PP, Wassink G, Lear CA, van Den Heuij LG, Gunn AJ & Bennet L (2016). Magnesium sulphate and cardiovascular and cerebrovascular adaptations to asphyxia in preterm fetal sheep. J Physiol 594, 1281–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinsky R, Draghi V, Wassink G, Davidson JO, Drury PP, Lear CA, Gunn AJ & Bennet L (2017). Magnesium sulfate reduces EEG activity but is not neuroprotective after asphyxia in preterm fetal sheep. J Cereb Blood Flow Metab 37, 1362–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathwala G, Khera A, Singh J & Balhara B (2010). Magnesium for neuroprotection in birth asphyxia. J Pediatr Neurosci 5, 102–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi FS, Galanopoulou AS & Moshe SL (2014). Sex dimorphism in seizure‐controlling networks. Neurobiol Dis 72, 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi FS, Veliskova J, Chudomel O, Kyrozis A & Moshe SL (2007). The role of substantia nigra pars reticulata in modulating clonic seizures is determined by testosterone levels during the immediate postnatal period. Neurobiol Dis 25, 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass HC, Shellhaas RA, Tsuchida TN, Chang T, Wusthoff CJ, Chu CJ, Cilio MR, Bonifacio SL, Massey SL, Abend NS & Soul JS (2017). Seizures in preterm neonates: A multicenter observational cohort study. Pediatr Neurol 72, 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowers WR (1885). Epilepsy and Other Chronic Convulsive Diseases: Their Causes, Symptoms and Treatment. William Wood and Company, New York, USA. [Google Scholar]
- Groenendaal F, Rademaker CM, Toet MC & de Vries LS (2002). Effects of magnesium sulphate on amplitude‐integrated continuous EEG in asphyxiated term neonates. Acta Paediatr 91, 1073–1077. [DOI] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology . J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallak M, Berman RF, Irtenkauf SM, Evans MI & Cotton DB (1992). Peripheral magnesium sulfate enters the brain and increases the threshold for hippocampal seizures in rats. Am J Obstet Gynecol 167, 1605–1610. [DOI] [PubMed] [Google Scholar]
- Hallak M, Hotra JW, Custodio D & Kruger ML (2000). Magnesium prevents seizure‐induced reduction in excitatory amino acid receptor (kainate and alpha‐amino‐3‐hydroxy‐5‐methylisoxazole‐4‐propionic acid) binding in pregnant rat brain. Am J Obstet Gynecol 183, 793–798. [DOI] [PubMed] [Google Scholar]
- Hellstrom‐Westas L, Boylan G & Agren J (2015). Systematic review of neonatal seizure management strategies provides guidance on anti‐epileptic treatment. Acta Paediatr 104, 123–129. [DOI] [PubMed] [Google Scholar]
- Huang Q, Liu L, Hu B, Di X, Brennecke SP & Liu H (2014). Decreased seizure threshold in an eclampsia‐like model induced in pregnant rats with lipopolysaccharide and pentylenetetrazol treatments. PLoS One 9, e89333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiba H, Tamai H, Negishi H, Ueda T, Kim TJ, Sumida Y, Takahashi Y, Fujinaga H & Minami H (2002). Randomized controlled trial of magnesium sulfate infusion for severe birth asphyxia. Pediatr Int 44, 505–509. [DOI] [PubMed] [Google Scholar]
- Jensen FE (2009). Neonatal seizures: an update on mechanisms and management. Clin Perinatol 36, 881–900, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khashaba MT, Shouman BO, Shaltout AA, Al‐Marsafawy HM, Abdel‐Aziz MM, Patel K & Aly H (2006). Excitatory amino acids and magnesium sulfate in neonatal asphyxia. Brain Dev 28, 375–379. [DOI] [PubMed] [Google Scholar]
- Koome ME, Davidson JO, Drury PP, Mathai S, Booth LC, Gunn AJ & Bennet L (2013). Antenatal dexamethasone after asphyxia increases neural injury in preterm fetal sheep. PLoS One 8, e77480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakadia MJ, Abbasi H, Gunn AJ, Unsworth CP & Bennet L (2016). Examining the effect of MgSO4 on sharp wave transient activity in the hypoxic‐ischemic fetal sheep model. Conf Proc IEEE Eng Med Biol Soc 2016, 908–911. [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Khalilov I, McLean H, Caillard O, Gaiarsa JL, Ben‐Ari Y & Khazipov R (1999). GABA is the principal fast‐acting excitatory transmitter in the neonatal brain. Adv Neurol 79, 189–201. [PubMed] [Google Scholar]
- Levene M, Blennow M, Whitelaw A, Hanko E, Fellman V & Hartley R (1995). Acute effects of two different doses of magnesium sulphate in infants with birth asphyxia. Arch Dis Child Fetal Neonatal Ed 73, F174–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Liu H, Huang Q, Brennecke S & Hu B (2013). PP024. Effects of intravenous magnesium sulfate on the characteristics of eclamptic seizures induced by electrical stimuli in a rat preeclampsia/eclampsia model. Pregnancy Hypertens 3, 76. [DOI] [PubMed] [Google Scholar]
- Loddenkemper T, Vendrame M, Zarowski M, Gregas M, Alexopoulos AV, Wyllie E & Kothare SV (2011). Circadian patterns of pediatric seizures. Neurology 76, 145–153. [DOI] [PubMed] [Google Scholar]
- Lynch NE, Stevenson NJ, Livingstone V, Murphy BP, Rennie JM & Boylan GB (2012). The temporal evolution of electrographic seizure burden in neonatal hypoxic ischemic encephalopathy. Epilepsia 53, 549–557. [DOI] [PubMed] [Google Scholar]
- McBride MC, Laroia N & Guillet R (2000). Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology 55, 506–513. [DOI] [PubMed] [Google Scholar]
- McCarthy MM (2008). Estradiol and the developing brain. Physiol Rev 88, 91–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck TA, Rice MM, Bailit JL, Grobman WA, Reddy UM, Wapner RJ, Thorp JM, Caritis SN, Prasad M, Tita AT, Saade GR, Sorokin Y, Rouse DJ, Blackwell SC & Tolosa JE; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal‐Fetal Medicine Units Network (2016). Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. Am J Obstet Gynecol 215, 103.e1–103.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BA, Standley CA, Irtenkauf SM, Bardicef M & Cotton DB (1994). Magnesium is more efficacious than phenytoin in reducing N‐methyl‐D‐aspartate seizures in rats. Am J Obstet Gynecol 171, 999–1002. [DOI] [PubMed] [Google Scholar]
- Matta JA, Ashby MC, Sanz‐Clemente A, Roche KW & Isaac JT (2011). mGluR5 and NMDA receptors drive the experience‐ and activity‐dependent NMDA receptor NR2B to NR2A subunit switch. Neuron 70, 339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor DJ, Diesch T, Gunn AJ & Bennet L (2005). The importance of ‘awareness’ for understanding fetal pain. Brain Res Rev 49, 455–471. [DOI] [PubMed] [Google Scholar]
- Mirmiran M, Maas YG & Ariagno RL (2003). Development of fetal and neonatal sleep and circadian rhythms. Sleep Med Rev 7, 321–334. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B & Seeburg PH (1994). Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12, 529–540. [DOI] [PubMed] [Google Scholar]
- Moore YE, Kelley MR, Brandon NJ, Deeb TZ & Moss SJ (2017). Seizing control of KCC2: a new therapeutic target for epilepsy. Trends Neurosci 40, 555–571. [DOI] [PubMed] [Google Scholar]
- Murguia‐Castillo J, Beas‐Zarate C, Rivera‐Cervantes MC, Feria‐Velasco AI & Urena‐Guerrero ME (2013). NKCC1 and KCC2 protein expression is sexually dimorphic in the hippocampus and entorhinal cortex of neonatal rats. Neurosci Lett 552, 52–57. [DOI] [PubMed] [Google Scholar]
- Narasimhulu D, Brown A, Egbert NM, Rojas M, Haberman S, Bhutada A, Minkoff H & Rastogi S (2017). Maternal magnesium therapy, neonatal serum magnesium concentration and immediate neonatal outcomes. J Perinatol 37, 1297–1303. [DOI] [PubMed] [Google Scholar]
- Nardou R, Ferrari DC & Ben‐Ari Y (2013). Mechanisms and effects of seizures in the immature brain. Semin Fetal Neonatal Med 18, 175–184. [DOI] [PubMed] [Google Scholar]
- Nikolaev MV, Magazanik LG & Tikhonov DB (2012). Influence of external magnesium ions on the NMDA receptor channel block by different types of organic cations. Neuropharmacology 62, 2078–2085. [DOI] [PubMed] [Google Scholar]
- Nunez JL & McCarthy MM (2007). Evidence for an extended duration of GABA‐mediated excitation in the developing male versus female hippocampus. Dev Neurobiol 67, 1879–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL & McCarthy MM (2008). Androgens predispose males to GABAA‐mediated excitotoxicity in the developing hippocampus. Exp Neurol 210, 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander JG & Woolley CS (2016). 17β‐Estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females. J Neurosci 36, 2677–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira LD, Oliveira RW, Futuro Neto Hde A & Nakamura‐Palacios EM (2011). The role of magnesium sulfate in prevention of seizures induced by pentylenetetrazole in rats. Arq Neuropsiquiatr 69, 349–355. [DOI] [PubMed] [Google Scholar]
- Pomerantz DK & Nalbandov AV (1975). Androgen level in the sheep fetus during gestation. Proc Soc Exp Biol Med 149, 413–416. [DOI] [PubMed] [Google Scholar]
- Quaedackers JS, Roelfsema V, Heineman E, Gunn AJ & Bennet L (2004). The role of the sympathetic nervous system in post‐asphyxial intestinal hypoperfusion in the preterm sheep fetus. J Physiol 557, 1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhade SN, Zhou C, Aujla PK, Fishman R, Sucher NJ & Jensen FE (2008). Early alterations of AMPA receptors mediate synaptic potentiation induced by neonatal seizures. J Neurosci 28, 7979–7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven F, Van der Zee EA, Meerlo P & Havekes R (2017). The role of sleep in regulating structural plasticity and synaptic strength: Implications for memory and cognitive function. Sleep Med Rev (in press; 10.1016/j.smrv.2017.05.002. [DOI] [PubMed] [Google Scholar]
- Reddy DS (2017). The neuroendocrine basis of sex differences in epilepsy. Pharmacol Biochem Behav 152, 97–104. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Estill CT, Stadelman HL, Meaker M & Stormshak F (2011). Separate critical periods exist for testosterone‐induced differentiation of the brain and genitals in sheep. Endocrinology 152, 2409–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy J, Mitra JK & Pal A (2013). Magnesium sulphate versus phenytoin in eclampsia – Maternal and foetal outcome – A comparative study. Australas Med J 6, 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato SM & Woolley CS (2016). Acute inhibition of neurosteroid estrogen synthesis suppresses status epilepticus in an animal model. Elife 5 e12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seron‐Ferre M, Mendez N, Abarzua‐Catalan L, Vilches N, Valenzuela FJ, Reynolds HE, Llanos AJ, Rojas A, Valenzuela GJ & Torres‐Farfan C (2012). Circadian rhythms in the fetus. Mol Cell Endocrinol 349, 68–75. [DOI] [PubMed] [Google Scholar]
- Shellhaas RA & Clancy RR (2007). Characterization of neonatal seizures by conventional EEG and single‐channel EEG. Clin Neurophysiol 118, 2156–2161. [DOI] [PubMed] [Google Scholar]
- Shepherd E, Salam RA, Middleton P, Makrides M, McIntyre S, Badawi N & Crowther CA (2017). Antenatal and intrapartum interventions for preventing cerebral palsy: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev 8, CD012077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty J (2015). Neonatal seizures in hypoxic‐ischaemic encephalopathy – risks and benefits of anticonvulsant therapy. Dev Med Child Neurol 57, 40–43. [DOI] [PubMed] [Google Scholar]
- Silverstein FS & Jensen FE (2007). Neonatal seizures. Ann Neurol 62, 112–120. [DOI] [PubMed] [Google Scholar]
- Simonetta G, Walker DW & McMillen IC (1991). Effect of feeding on the diurnal rhythm of plasma cortisol and adrenocorticotrophic hormone concentrations in the pregnant ewe and sheep fetus. Exp Physiol 76, 219–229. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE (2008). When it comes to GABAergic responses and neonatal seizures – sex matters! Epilepsy Curr 8, 166–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standley CA, Irtenkauf SM & Cotton DB (1995a). Anticonvulsant effects of magnesium sulfate in hippocampal‐kindled rats. J Biomed Sci 2, 57–62. [DOI] [PubMed] [Google Scholar]
- Standley CA, Mason BA & Cotton DB (1995b). Differential regulation of seizure activity in the hippocampus of male and female rats. Am J Obstet Gynecol 173, 1160–1165. [DOI] [PubMed] [Google Scholar]
- Tan WK, Williams CE, Gunn AJ, Mallard CE & Gluckman PD (1992). Suppression of postischemic epileptiform activity with MK‐801 improves neural outcome in fetal sheep. Ann Neurol 32, 677–682. [DOI] [PubMed] [Google Scholar]
- Thoresen M & Sabir H (2015). Epilepsy: Neonatal seizures still lack safe and effective treatment. Nat Rev Neurol 11, 311–312. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ & Dingledine R (2010). Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62, 405–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Campen JS, Valentijn FA, Jansen FE, Joels M & Braun KP (2015). Seizure occurrence and the circadian rhythm of cortisol: a systematic review. Epilepsy Behav 47, 132–137. [DOI] [PubMed] [Google Scholar]
- Veliskova J, Claudio OI, Galanopoulou AS, Lado FA, Ravizza T, Velisek L & Moshe SL (2004). Seizures in the developing brain. Epilepsia 45, 6–12. [DOI] [PubMed] [Google Scholar]
- Wagner S, Castel M, Gainer H & Yarom Y (1997). GABA in the mammalian suprachiasmatic nucleus and its role in diurnal rhythmicity. Nature 387, 598–603. [DOI] [PubMed] [Google Scholar]
- Wolf G, Keilhoff G, Fischer S & Hass P (1990). Subcutaneously applied magnesium protects reliably against quinolinate‐induced N‐methyl‐D‐aspartate (NMDA)‐mediated neurodegeneration and convulsions in rats: Are there therapeutical implications? Neurosci Lett 117, 207–211. [DOI] [PubMed] [Google Scholar]
- Zeiler FA, Matuszczak M, Teitelbaum J, Gillman LM & Kazina CJ (2015). Magnesium sulfate for non‐eclamptic status epilepticus. Seizure 32, 100–108. [DOI] [PubMed] [Google Scholar]
- Zeng X, Xue Y, Tian Q, Sun R & An R (2016). Effects and safety of magnesium sulfate on neuroprotection: a meta‐analysis based on PRISMA guidelines. Medicine (Baltimore) 95, e2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Jensen FE & Sucher NJ (2009). Altered development of glutamatergic synapses in layer V pyramidal neurons in NR3A knockout mice. Mol Cell Neurosci 42, 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Sun H, Klein PM & Jensen FE (2015). Neonatal seizures alter NMDA glutamate receptor GluN2A and 3A subunit expression and function in hippocampal CA1 neurons. Front Cell Neurosci 9, 362. [DOI] [PMC free article] [PubMed] [Google Scholar]


