Abstract
Placental amino acid transfer is a complex process that is essential for fetal development. Impaired amino acid transfer causes fetal growth restriction, which may have lifelong health consequences. Transepithelial transfer of amino acids across the placental syncytiotrophoblast requires accumulative, exchange and facilitated transporters on the apical and basal membranes to work in concert. However, transporters alone do not determine amino acid transfer and factors that affect substrate availability, such as blood flow and metabolism, may also become rate‐limiting for transfer. In order to determine the rate‐limiting processes, it is necessary to take a systems approach which recognises the interdependence of these processes. New technologies have the potential to deliver targeted interventions to the placenta and help poorly growing fetuses. While many factors are necessary for amino acid transfer, novel therapies need to target the rate‐limiting factors if they are going to be effective. This review will outline the factors which determine amino acid transfer and describe how they become interdependent. It will also highlight the role of computational modelling as a tool to understand this process.
Keywords: trans-epithelial transport, fetal growth restriction, computational modelling
Introduction
Placental transfer provides the amino acids required for fetal growth and metabolism. The fetus requires amino acids for protein accretion, metabolic processes and biosynthetic pathways but can only obtain these from the placenta. Understanding the process of placental transfer is important as it provides the amino acids required for appropriate growth and development of the fetus. Indeed, impaired placental amino acid transfer is associated with reduced fetal growth, which is itself associated with complications in the perinatal period and elevated rates of chronic disease in later life. To improve outcomes from fetal growth restriction (FGR), we need to appreciate how placental amino acid transfer operates as an integrated system. This integrated understanding will allow targeting of the right mechanisms to prevent or treat impaired placental amino acid transfer.
The human placenta mediates net transfer of amino acids to the fetus, with amino acid concentrations being higher in fetal plasma compared to maternal plasma, indicating an active transfer process across the placenta (Cetin et al. 1996). The most notable exception to this is the amino acid glutamate for which there is net placental uptake from the fetus. Amino acid transport across the placenta is a complex process that is determined by multiple interacting factors. These include transporter characteristics, placental structure, maternal and fetal blood flow and the amino acid concentrations within the maternal, placental and fetal compartments.
Multiple amino acid transporters are differentially expressed in the maternal facing microvillous (MVM) and fetal facing basal membranes (BM) of the placental syncytiotrophoblast. Transporter activity is not simply determined by the protein expression levels, but also by the factors that control substrate levels on both sides of the membrane (Panitchob et al. 2016). The factors which affect amino acid levels include blood flow, metabolism and the action of multiple amino acid transporters. The trafficking of amino acid transporter protein to the plasma membrane is also an important factor in the regulation of amino acid transfer (Chen et al. 2015, 2017). Any effective intervention needs to target the rate‐limiting factors in the system, but the interaction and interdependency between these factors makes identifying the rate‐limiting factors difficult.
Placental amino acid transfer, must be thought of as a system rather than simply focusing on individual mechanisms (Lofthouse et al. 2015). A computational modelling approach is helping us to predict the rate‐limiting factors in this system so that these can be the focus of future intervention‐based studies (Panitchob et al. 2015, 2016; Lofthouse et al. 2016). Placental amino acid transfer can be measured experimentally in the perfused placenta (Cleal et al. 2011) and uptake studies can be performed in villous fragments or purified trophoblast preparations (Rosario et al. 2013; Ditchfield et al. 2015). The isolation of purified MVM and BM vesicles can also be used to study transporters in these membranes in isolation (Speake et al. 2003; Panitchob et al. 2015). This review will provide an overview of the current knowledge of amino acid transfer across the human placenta. We will discuss the mechanisms of amino acid transfer, how these interact and how computational modelling can be used to identify rate‐limiting steps. Identifying the rate‐limiting steps is crucial if clinical interventions aimed at improving placental transfer and fetal growth are to be successful.
Computational modelling of amino acid transfer
The complex and interdependent nature of the interactions underlying placental amino acid transfer mean it is hard to identify the rate‐limiting processes. Computational modelling provides a tool to interrogate the complex activity of the system. Modelling is most useful when it is based on experimental data and makes predictions that can be tested in future experiments. The typical model parameters of a compartmental model are outlined in Table 1. Crucial data for placental modelling include both maternal and fetal venous‐arterial differences and tissue levels; otherwise mass balance cannot be validated. An assumption of compartmental models is that the compartments are well mixed and it is necessary to consider the time scales over which the relevant processes occur. We assume transporter behaviour follows the principles of carrier‐mediated models, but phenomenological transport models can be applied depending on the data available (Widdows et al. 2015). Placental perfusions provide the best system for modelling transfer and the combination of perfusions with modelling has provided unexpected insight into placental amino acid, fatty acid and cortisol transport (Lofthouse et al. 2015; Panitchob et al. 2016; Perazzolo et al. 2017a; Stirrat et al. 2018). Recent work modelling oxygen transfer has recently been reviewed elsewhere (Nye et al. 2018). This combined modelling and experimental approach can also be applied to transporter function and in combination with experiments in MVM membrane vesicles has demonstrated that the LAT2 transporter is not an obligate exchanger (Panitchob et al. 2015; Widdows et al. 2015).
Table 1.
Typical parameters for a compartmental model of placental transfer in the perfused placenta
| Parameters | Compartments | Source from which values are typically determined |
|---|---|---|
| Compartment volume | Intervillous space; placenta; fetal capillary volume | Literature or experimentally determined |
| Flow rates | Maternal arterial; fetal arterial | Experimental design |
| Initial concentration (for each substrate) | Maternal intervillous space; fetal capillary | Equal to initial perfusion buffer |
| Arterial input concentration (for each substrate) | Maternal artery; fetal artery | Experimental design |
| Membrane transport: diffusion or transporter model, substrate specific | Maternal ↔ placental; placental ↔ fetal | Determined by the model based on experimental data |
| Metabolic rate | Placental tissue | Predicted by the model based on experimental data |
| Paracellular diffusion | Maternal ←→ fetal | Experimental measurements, e.g. creatinine transfer |
These approaches allow us to test our understanding of the physiology underlying placental amino acid transfer. In doing so, we will be able to more accurately predict those factors that are likely to be rate‐limiting and those factors that are not. Understanding which factors (e.g. structure, blood flow, transporter expression, etc.) are likely to be rate‐limiting will help focus future research and clinical interventions.
A strength of modelling is that when used in combination with experimental data it allows our biological understanding to be tested. If a model is able to fit experimental data across a range of different experimental conditions then it is likely that the assumptions underlying it are reasonable. A model that is not able to fit the experimental data is still informative as it suggests that the underlying assumptions are incorrect or at least incomplete. This was the case in our work on amino acid and lipid transport where initial models failed because they did not include metabolism (Lofthouse et al. 2016; Perazzolo et al. 2017a). The limitation of models comes when they are not closely linked to experimental data or where modelling is based on a limited set of experimental data. Models are also limited where there are too many unknown parameters, which cannot be uniquely determined by the model. For this reason, it is better to start with simple models and test them thoroughly before building on these.
Current mechanistic models of placental transfer do not reflect how hormonal or feedback regulation may affect the system. As regulatory pathways become better defined these too can be modelled and integrated with mechanistic models. Modelling could also address other interactions, such as the way in which spatial differences in oxygen tension within the lobule may affect energy metabolism and so amino acid transfer. Ultimately, the aim should be to develop a virtual placenta as part of a virtual mother and fetus.
Placental structure and blood flow
The placenta has a complex structure and specific structural features may be particularly important for the efficiency of amino acid transport (Fig. 1). These features include those affecting blood flow and mixing of amino acids in the blood within the intervillous spaces of the placenta, the surface area available for exchange, the distance over which diffusion occurs and paracellular leak back to the mother. Although these features will affect solute transfer generally, they must have a specific effect on amino acids for them to be rate‐limiting to amino acid transfer.
Figure 1. The processes determining placental amino acid transfer operate across a range of scales.
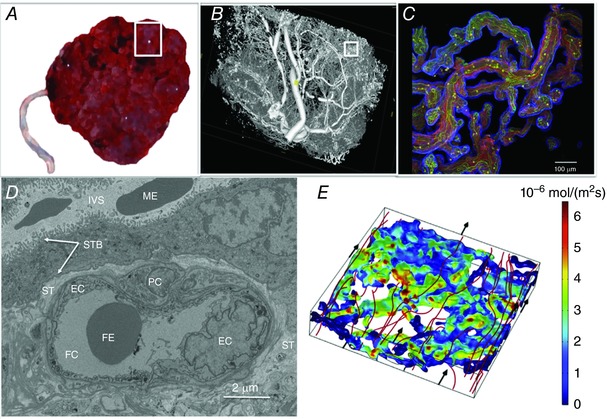
Amino acids within maternal blood mix effectively between the placental villi before being transported across the apical and basal membranes of the syncytiotrophoblast, diffusing through the stroma and then through the junctions between endothelial cells. A, a whole placenta approximately 20 cm across. The white box indicates one placental lobule (∼3 cm across). B, micro‐computed tomography image of the region shown in the white box in A, illustrating the extent and complexity of the fetal vasculature. The white box in B indicates a region of placental villi. C, the region shown by the white box in B shown as a projection of a whole mount confocal image stack, with the trophoblast stained blue, connective tissue red and endothelium green. D, an electron microscopy image of a cross section of a terminal villi. E, computational 3D simulation results modelling streamlines of maternal blood flow (m s−1) through the intervillous space surrounding placental villi, with streamlines in red depicting the main flow routes (flow direction indicated by black arrows). Colour on the villous surface represents uptake of substrate in that area with red indicating highest solute flux through the villous barrier (mol m−2 s−1); predicted using the computational model. EC, endothelial cell; FC, fetal capillary; FE, fetal erythrocyte; IVS, intervillous space; ME, maternal erythrocyte; PC, pericyte; ST, villous stroma; STB, syncytiotrophoblast (the microvillous membrane and basal plasma membranes of the syncytiotrophoblast are indicted by the white arrows).
The size of the placenta and more importantly the surface area of the MVM, which mediates the uptake of amino acids from the maternal circulation, is a key consideration. Classic studies in the P0 knockout mouse suggest that a smaller placenta may become rate‐limiting for amino acid transfer (Constancia et al. 2002). In the P0 mouse model, a smaller placenta was initially compensated for by increasing placental transporter activity to maintain fetal weight. However, as gestation progressed fetal growth restriction was observed despite transporter upregulation, indicating that at a certain threshold transporter regulation can no longer compensate for reduced placental size (Sferruzzi‐Perri et al. 2011). A decrease in the number and surface area of terminal villi and capillaries has also been reported in placentas from intrauterine growth restricted pregnancies (Mayhew et al. 2003).
The interaction between structure and the mixing of maternal blood within the placental intervillous space could also affect amino acid transfer. Low blood flow, or inefficient mixing, can cause regions of low substrate concentration to form in the intervillous space (Perazzolo et al. 2017b). Amino acid transfer may be susceptible to inefficient mixing and if substrate gradients are not maintained, exchange transporters may mediate reverse transport of specific amino acids (Panitchob et al. 2016). The effect of maternal and fetal blood flow on placental phenylalanine transfer has been studied in the isolated perfused placenta (Lofthouse et al. 2016). Neither maternal nor fetal flow was found to be limiting for amino acid transfer within the range of flows tested. However, at very low substrate concentrations maternal flow was demonstrated to be limiting for amino acid uptake (though not transfer). So, while flow does not appear to be limiting for placental amino acid transfer within the physiological range, this study illustrates interdependence between flow and substrate availability.
Diffusion through the stoma or endothelium is unlikely to be rate‐limiting to amino acid transfer as perfusion data indicate that movement of solute from the fetal circulation to the BM and back occurs rapidly (Lofthouse et al. 2016). There is limited evidence that transplacental amino acid transfer is also rapid (Sengers et al. 2010). As such, we do not believe that stromal diffusion or the endothelium is likely to be limiting for amino acid transfer.
Amino acid transfer is susceptible to the effects of paracellular transfer as amino acid concentrations are higher in the fetal circulation, and so net diffusion would be back to the maternal circulation. There is significant paracellular diffusion of small solutes, including amino acids, across the placenta but the pathway by which this occurs is unclear. While rates of paracellular diffusion are fairly constant in normal placentas, this may vary in disease states, and paracellular diffusion is correlated to area of syncytial damage (Brownbill et al. 2000).
Placental amino acid transport
All of the amino acid transporters in the placenta are members of the SLC superfamily (Table 2; Hediger et al. 2013). The expression of these transporters at RNA and protein levels is becoming clear with next generation sequencing and proteomic approaches. However, whether specific amino acid transporters are localised in the MVM, the BM or other placental cell types often remains uncertain, with differences reported between immunological and functional localisation (Cleal et al. 2011; Widdows et al. 2015).
Table 2.
Amino acid transporter systems in the placenta
| Human gene name (protein/system) | Expression and localisation of mRNA expression from Simner et al. (2017) | Mechanism | Substrates |
|---|---|---|---|
|
SLC1A1 (EAAT3/XAG) SLC1A2 (EAAT2/XAG) SLC1A3 (EAAT1/XAG) SLC1A6 (EAAT4/XAG) SLC1A3 (EAAT5/XAG) |
mRNA; activity on MVM and BM (cannot distinguish family member); SLCA1,2,3 protein expressed but location unclear (Moe & Smith, 1989; Hoeltzli et al. 1990; Noorlander et al. 2004) | 3Na+/H+/AA cotransport/K+ exchange | D, E (Kanai et al. 2013) |
| SLC1A4 (ASCT1/ASC) | A, S, C, T | ||
| mRNA; activity on BM (Cleal et al. 2011) | Na+ dependant exchanger | ||
| SLC1A5 (ASCT2/ASC) | A, C, Q, S, T, N (Kanai et al. 2013) | ||
|
SLC3A1 (rBAT) SLC3A2 (4F2hc) |
mRNA mRNA; protein on MVM and BM (Palacin & Kanai, 2004) |
Chaperone subunit for specific SLC7 transporters | |
| SLC6A6 (TAUT) | mRNA; activity and protein on MVM (Roos et al. 2004; Desforges et al. 2013) | Na+/Cl− dependant cotransporter | Taurine (Pramod et al. 2013) |
|
SLC7A1 (CAT1/y+) SLC7A2 (CAT2B/y+) SLC7A3P (CAT3/y+) |
mRNA; activity on MVM and BM; CAT1 protein on BM (Speake et al. 2003). | Electrogenic uniporter | R, H, K (Fotiadis et al. 2013) |
|
SLC7A5 (LAT1/L) SLC7A8 (LAT2/L) |
mRNA; LAT2 activity on MVM and LAT1 activity on BM; both proteins on MVM and BM (Cleal et al. 2011; Widdows et al. 2015) | Exchanger; requires 4F2hc (SLC3A2) | F, Y, W, M, V, I, L, H, BCH L, A, S, T, C, F, Y, W, BCH, N, H, I, M, V, Q, G (Fotiadis et al. 2013) |
|
SLC7A7 (y+LAT1/y+L) SLC7A6 (y+LAT2/y+L) |
mRNA; activity on MVM and BM but cannot distinguish family member (Ayuk et al. 2000; Cleal et al. 2011) | Exchanger; requires 4F2hc (Na+ dependant for neutral amino acids) | R, H, K, M, AL (Fotiadis et al. 2013) |
| SLC7A9 (b0,+AT) | mRNA; activity inconclusive. | Requires rBAT (SLC3A1) | R, H, K, F, Y, W, T, M, V, I, L (Fotiadis et al. 2013) |
| SLC7A10 (asc1/asc) | mRNA; no activity on BM (Cleal et al. 2011) | Exchanger; requires 4F2hc (SLC3A2) | G, A, S, T, C (Fotiadis et al. 2013) |
| SLC7A11 (xCT/Xc‐) | mRNA | Exchanger; requires 4F2hc (SLC3A2) | Cystine, E (Fotiadis et al. 2013) |
| SLC16A10 (TAT1) | mRNA; activity and protein on BM (Cleal et al. 2011) | Facilitated diffusion | F, W, Y A, L (Ramadan et al. 2006) |
|
SLC38A1 (SNAT1/A) SLC38A2 (SNAT2/A) SLC38A4 (SNAT4/A) |
mRNA; activity and protein on MVM (Desforges et al. 2009) | Na+/AA cotransporter; Na/AA cotransport, H antiport | Q, A, N, C, H, S A, N, C, Q, G, H, M, P, S A, N, C, G, S, T (Schioth et al. 2013) |
| SLC38A3 (SNAT3/N) | mRNA | Na/AA cotransport, H antiport | Q, H, A, N |
| SLC38A5 (SNAT5/N) | mRNA; activity and protein on MVM (Day et al. 2013) | Q, H, N, S (Schioth et al. 2013) | |
|
SLC43A1 (LAT3) SLC43A2 (LAT4) |
mRNA; activity and protein on BM (Cleal et al. 2011) | Facilitated diffusion | L, I, V, F, M, BCH (Bodoy et al. 2013) |
BCH, 2‐aminobicyclo‐(2,2,1)‐heptane‐2‐carboxylic acid. l‐Alanine (A), l‐arginine (R), l‐asparagine (N), l‐aspartate (D), l‐cysteine (C), l‐glutamate (E), l‐glutamine (Q), glycine (G), l‐histidine (H), l‐isoleucine (I), l‐leucine (L), l‐lysine (K), l‐methionine (M), l‐phenylalanine (F), l‐proline (P), l‐serine (S), l‐threonine (T), l‐tryptophan (W), l‐tyrosine (Y), l‐valine (V). AL: y+L, influx but not efflux of l‐Leucine (Chillaron et al.).
Amino acid transporters are divided into three functional classes based on their mode of operation: accumulative, exchange and facilitated transporters (Cleal et al. 2011). In order to transport the full range of amino acids to the fetus, the placenta requires the different classes of amino acid transporter to work together on both the MVM and BM of the syncytiotrophoblast. To understand how transporters work together to function as a system, it is important to understand how the activities of the different classes complement each other on the MVM and the BM.
Accumulative transporters such as SLC1 and SLC7 (Table 2) mediate net uptake of specific amino acids across the MVM into the syncytiotrophoblast. This creates transmembrane amino acid gradients across the MVM which drive the uptake of other extracellular amino acids via amino acid exchange transporters (Fig. 2). The exchange transporters on the MVM include the SLC7 family and they swap intracellular amino acids for other exchange transporter specific amino acids in the maternal plasma (Fig. 2). Therefore, amino acid exchangers alter the composition (i.e. concentration fractions) of their amino acids substrates within the placenta, but not the overall quantity of amino acids. Neither the accumulative transporters nor the exchangers alone can provide the placenta with all the amino acids it requires, and they must work together to achieve this.
Figure 2. A cartoon showing the location of transporter classes within the MVM and BM of the placental syncytiotrophoblast, and the factors that determine amino acid transfer.
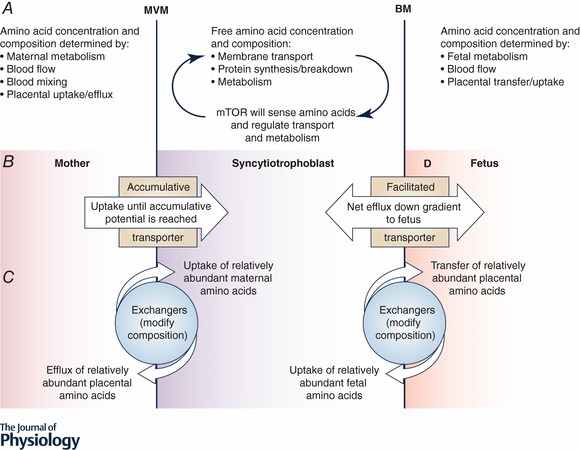
A, maternal, placental and fetal metabolism, blood flow and transport control the gradients which determine transporter activity. B, accumulative transporters mediate uptake until their accumulative potential is reached. Functionally this means their activity is affected by low extracellular concentrations and that higher extracellular concentrations above the V max will no longer increase uptake. C, exchangers will mediate net influx of abundant external substrates in exchange for efflux of relatively higher abundance intracellular substrates. This means that transfer of one substrate will decrease the levels of another. Within physiological limits this will be independent of concentration. D, facilitated transporters on the BM will mediate efflux of substrates down the concentration gradient built up by other transporters. Fetal consumption will increase the concentration gradient and increase transfer to the fetus.
On the BM of the syncytiotrophoblast, the facilitated transporters (SLC16A10 and SLC43; Table 2) mediate net efflux of specific amino acids across the BM into the fetal circulation down their concentration gradient (Cleal et al. 2011). These amino acids can be exchanged for other amino acids via exchangers to ensure all the necessary amino acids are transferred to the fetus (Fig. 2). Placental perfusion experiments indicate a pattern of amino acid exchange consistent with Systems ASC and L, but not Systems asc and b0,+AT activity on the BM (Cleal et al. 2011).
Accumulative transporters, including members of the SLC1 and SCL38 families, are also present on the BM and will mediate uptake of fetal amino acids into the placenta (Hoeltzli et al. 1990; Desforges et al. 2006). The glutamate and aspartate transporting SLC1s are highly active on the fetal BM and mediate net uptake of fetal glutamate into the placenta where it is metabolised and can act as a counter ion for organic anion exchangers (Day et al. 2013; Lofthouse et al. 2015). The physiological role of other accumulative transporters on the BM, such as SLC38 family members, is unclear (Desforges et al. 2006).
In terms of amino acid transporters working together as a system, we need to understand when membrane transport will become rate‐limiting for amino acid transfer across the placenta. Whether membrane transport is rate‐limiting will depend on the amount of transporter, its mechanism of action and the free amino acid concentrations on both sides of the membrane (Fig. 2).
Accumulative amino acid transporters are secondary active transporters driven by previously established chemical and electrical gradients. The Na+, K+ and electrical gradients driving uptake are maintained by the Na+/K+ ATPase, and the H+ gradient is also important for specific transporters (Table 2). These electrochemical potential gradients determine how much substrate can be accumulated within the cell. Accumulation of amino acids creates an outwardly directed gradient and influx will only continue while the inward force of the driving electrochemical gradient is greater than that of the substrate being accumulated within the cell. It should be noted that in a physiological system the balance of activity between uptake and efflux means it is unlikely that the system will reach these limits at steady state (Panitchob et al. 2016). Membrane transport via accumulative transporters will become rate‐limiting when the driving gradients are depleted or there is just too little transporter in the membrane relative to other transporter classes.
Exchange transporters transport one solute into the cell in exchange for another leaving the cell. Amino acid exchangers are regarded as being obligate exchangers where influx of extracellular amino acids is always coupled to efflux of intracellular amino acids. However, we have recently demonstrated that the exchange transporter LAT2 does not function exclusively as an obligate exchanger (Widdows et al. 2015). These transporters mediate net transport until the relative composition (i.e. concentration fractions) of their substrates are the same inside and outside the cell (Fig. 2). This means that at equilibrium, if serine makes up 20% of all the substrates of a transporter outside the cell it will also make up 20% of all these substrates inside the cell regardless of any concentration differences between these pools. The exchangers will mediate a degree of futile cycling, whereby an amino acid is swapped for the same amino acid creating no overall change.
Facilitated transporters mediate transport of solute in both directions across the membrane; net transport will occur down the concentration gradient until an equal concentration of all substrates is achieved on both sides of the membrane. Facilitated transporters become limiting where insufficient transporter is expressed or the transmembrane gradient is reduced. For individual amino acids, too much of a competing substrate could also become limiting.
Modelling of the interactions between the different transporter classes in the placenta is therefore needed, and indeed demonstrates the likely complexity of this system. From this work it is clear that simply increasing or decreasing the activity of one transporter cannot always be expected to have a corresponding effect on overall transfer (Panitchob et al. 2016). Indeed there are examples, particularly with exchangers, where increased activity of a transporter will decrease the transfer of its substrates (Fig. 3). Changing the activity of a transporter will have different effects on different substrates depending on what other transporters also transport each substrate. This model also makes clear that increasing the activity of a transporter has no effect on transfer if another transporter is limiting. An experimental example of these complex interactions may come from a study where SCL38 transporters were inhibited in vivo in rat pregnancy (Cramer et al. 2002). Inhibition of SCL38 transporters was shown to alter the activity of SLC1 transporters despite the fact that they do not share any substrates.
Figure 3. The effect of changing the activity of accumulative (A), MVM exchange (B), BM exchange (C) and facilitative (D) transporters on amino acid transfer to the fetus (fetal venous–arterial difference).
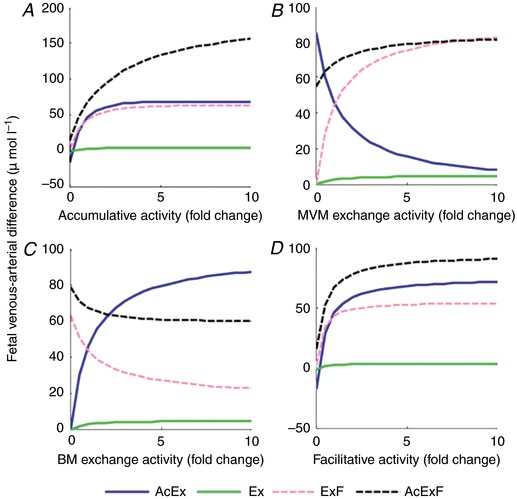
For simplicity amino acids have been grouped by the classes of transporter which transport them: those transported by accumulative and exchange transporters (AcEx), those transported by exchangers only (Ex), those transported by exchange and facilitated transporters (AcF) and those substrates transported by all three classes of transporter (AcExF). Results represent the sum of all amino acids in each group. Note how in many cases increasing transporter activity has little effect on overall transfer and how increasing exchanger activity can decrease transfer of some substrates (Panitchob et al. 2016).
It should also be noted that the measured K M of a transporter is not a constant but is an apparent K M and dependent on the transmembrane substrate gradient (Panitchob et al. 2015). For this reason differences in the reported apparent K M in different systems, for instance cells and membrane vesicles, should be expected due to the differences in intracellular substrate concentration.
Metabolism and placental amino acid transfer
As we outline above, amino acid transporter activity depends on the total concentration of amino acids available for transport and on their relative concentrations. Metabolism can therefore influence placental amino acid transfer through its effects on both total and relative concentrations. In addition to metabolism within the placenta, maternal and fetal metabolism will influence arterial plasma amino acid concentrations and so the gradients which determine uptake and efflux. For example, fetal amino acid consumption will increase the maternal–fetal gradient, potentially coupling fetal demand to placental delivery. Here we will focus on placental protein synthesis, breakdown and amino acid interconversion. The use of amino acids as precursors of biosynthetic pathways may also be relevant but is less well understood (Bonnin & Levitt, 2011).
Placental protein synthesis will reduce the pool of amino acids available for transport while the breakdown of protein will increase their availability. In a recent study, we provide evidence that protein synthesis is likely to be a major determinant of placental amino acid transfer (Lofthouse et al. 2016). This study suggests that phenylalanine taken up by the placenta is not all available for transfer to the fetus because it has been converted to protein. The effect of metabolism was such that it was not possible to fit a mathematical model to the data unless metabolism was also modelled. Amino acids that enter the placental protein pool are not lost to the fetus but will not be available until that protein is recycled. This delay while amino acids are held within the placental protein pool has implications for the interpretation of tracer studies using amino acids (as opposed to non‐metabolisable amino acid analogues). For amino acids the rate of transfer will be underestimated until the tracer has equilibrated within all metabolic pools, including protein.
Amino acid oxidation within the placenta is coupled to interconversion, preventing the release of toxic ammonia by forming a new amino acid such as alanine or glutamine. As such, oxidation will not significantly reduce overall amino acid concentrations but will change the amino acid composition and thus the gradients which drive their transfer. The human placenta makes glutamine from substrates including glutamate and leucine, and this glutamine is primarily released into the maternal circulation (Day et al. 2013). As glutamine is a substrate of many placental amino acid exchangers, the synthesis of glutamine will create gradients that drive uptake of other extracellular amino acids (Day et al. 2013).
Amino acid metabolism, and its regulation, have the potential to be rate‐limiting determinants of placental amino acid transfer and so need to be considered when seeking to understand the basis of fetal growth restriction.
Regulation of placental amino acid transfer
Understanding the regulation of placental amino acid transfer is the key to designing effective intervention strategies to modulate fetal growth. Many studies report stimuli that can regulate specific mechanisms underlying amino acid transfer such as transport and metabolism. However, as we outline in this review the way in which these mechanisms interact is complex and it is only when rate‐limiting processes are targeted that amino acid transfer will be affected.
Regulation of placental amino acid transfer is likely to be based on amino acid sensing and sensing of overall metabolic state. The mechanistic target of rapamycin (mTOR) pathway is central to the regulation of protein metabolism and amino acid transporter activity and is likely to be a key central regulator of placental amino acid transfer (Goberdhan et al. 2016). Specifically, intracellular leucine levels are sensed by binding to Sestrin 2 which activates the GATOR2 complex and activates the mTOR complex 1 (mTORC1) pathway (Wolfson et al. 2016). mTORC1, which is downregulated in intrauterine growth restriction (Roos et al. 2005), modifies plasma membrane trafficking of System A and System L members, suggesting that mTOR regulates fetal growth by modulating specific placental amino acid transporters (Rosario et al. 2013).
Regulation of amino acid transporters in the placenta has been demonstrated in response to nutrients and hormones. For example, the MVM expresses receptors for leptin, insulin‐like growth factor‐1 and insulin, which are factors that stimulate System A activity (von Versen‐Hoynck et al. 2009; Jones et al. 2010). The gene expression of specific placental amino acid transporters can be regulated by maternal factors such as diet, smoking and vitamin D levels (Cleal et al. 2015; Day et al. 2015; Chen et al. 2017). Epigenetic regulation may also be involved in the regulation of amino acid transporter expression in the placenta (Simner et al. 2017).
The interrelationships between the determinants of transfer of an amino acid have implications for our understanding of its regulation. A regulatory stimulus that just affects one transporter or metabolic process is unlikely to have a significant effect on the system as a whole unless that target of regulation is the rate‐limiting factor in the system. We suggest that regulatory stimuli will be more effective where they affect the activity of a range of mechanistic targets to up‐ or down‐regulate amino acid transfer.
Novel therapies, health implications and a systems biology approach
An appreciation of how placental amino acid transfer operates as an integrated system will allow us to target the right mechanisms to prevent or treat impaired placental amino acid transfer and therefore improve outcomes from poor fetal growth. Reduced placental amino acid transfer or transporter activity is associated with reduced fetal growth in vivo (Cramer et al. 2002). Studies in rodents suggest that decreased amino acid transfer precedes, and may therefore cause, growth restriction (Jansson et al. 2006). In humans pregnancies with FGR there is generally decreased transporter activity, and decreased transfer of specific amino acids has been demonstrated using tracers (Paolini et al. 2001).
Technologies are being developed which deliver targeted drug or gene therapy to the placenta (King et al. 2016; Beards et al. 2017; David, 2017). These new approaches have great promise, although promising interventions have proved ineffective in the past (Poston et al. 2011). To be effective interventions need to target the aspects of placental function that are rate‐limiting for fetal growth; increasing maternal amino acid levels will only be effective if maternal amino acid levels are an initial problem (Brown et al. 2011). Identifying the rate‐limiting processes within the placenta is not always obvious, and computational modelling may provide key insights here. As there may be multiple causes of placental dysfunction, a personalised medicine approach may be necessary with a need to better characterise placental phenotypes and identify accessible biomarkers for this. Just as improving fetal growth may have lifelong benefits, an ill‐judged intervention could cause lifelong detriment (Hanson & Gluckman, 2014).
Conclusion
Amino acid transfer across the placenta is a complex process which is crucial for normal fetal growth and to create an intrauterine environment which predisposes to lifelong health. In poorly growing fetuses, intervening to improve placental amino acid transfer may improve the intrauterine environment and postnatal health. However, the complex interactions between the different mechanisms mediating amino acid transfer mean we must be careful that any interventions do not have unintended consequences. This will require us to understand the placenta as a system rather than simply focusing on individual mechanisms. The complexity of placental amino acid transfer as a system makes this hard to do at an intuitive level. However, the use of computational modelling can provide a mechanism by which this can be achieved.
Additional information
Competing interests
There are no competing interests.
Author contributions
All authors contributed to the writing of this review. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
The authors are supported by BBSRC grants BB/L020823/1 and BB/R002762/1.
Acknowledgements
We would like to thank the Biomedical Imaging Unit and μ‐VIS Centre for Computed Tomography at the University of Southampton for help producing images shown.
Biographies
The authors have been working as part of an interdisciplinary team to understand placental solute transfer in an integrated manner, including transport, metabolism, structure and flow. Jane Cleal's work demonstrated how amino acids cross the basal membrane of the human placenta and how the maternal environment regulates placental function and fetal growth. Her interests include how placental vitamin D is transferred and metabolised by the placenta.
Emma Lofthouse is a Postdoctoral Fellow who has worked on placental glutamate cycling and the effects of flow and metabolism on phenylalanine transfer.

Bram Sengers is an engineer whose work modelling placental solute transfer has allowed us to develop a much deeper understanding of these processes.
Rohan Lewis initiated this program of work in Southampton and has led the drive to develop an integrated approach to understanding placental function. He is developing 3D‐imaging approaches to enhance our models by including realistic spatial geometry.
Edited by: Ole Petersen & Dennis Brown
References
- Ayuk PT, Sibley CP, Donnai P, D'Souza S & Glazier JD (2000). Development and polarization of cationic amino acid transporters and regulators in the human placenta. Am J Physiol Cell Physiol 278, C1162–C1171. [DOI] [PubMed] [Google Scholar]
- Beards F, Jones LE, Charnock J, Forbes K & Harris LK (2017). Placental homing peptide‐microRNA inhibitor conjugates for targeted enhancement of intrinsic placental growth signaling. Theranostics 7, 2940–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodoy S, Fotiadis D, Stoeger C, Kanai Y & Palacin M (2013). The small SLC43 family: facilitator system l amino acid transporters and the orphan EEG1. Mol Aspects Med 34, 638–645. [DOI] [PubMed] [Google Scholar]
- Bonnin A & Levitt P (2011). Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience 197, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LD, Green AS, Limesand SW & Rozance PJ (2011). Maternal amino acid supplementation for intrauterine growth restriction. Front Biosci 3, 428–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownbill P, Mahendran D, Owen D, Swanson P, Thornburg KL, Nelson DM & Sibley CP (2000). Denudations as paracellular routes for alphafetoprotein and creatinine across the human syncytiotrophoblast. Am J Physiol Regul Integr Comp Physiol 278, R677–R683. [DOI] [PubMed] [Google Scholar]
- Cetin I, Ronzoni S, Marconi AM, Perugino G, Corbetta C, Battaglia FC & Pardi G (1996). Maternal concentrations and fetal‐maternal concentration differences of plasma amino acids in normal and intrauterine growth‐restricted pregnancies. Am J Obstet Gynecol 174, 1575–1583. [DOI] [PubMed] [Google Scholar]
- Chen YY, Powell TL & Jansson T (2017). 1,25‐Dihydroxy vitamin D3 stimulates system A amino acid transport in primary human trophoblast cells. Mol Cell Endocrinol 442, 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YY, Rosario FJ, Shehab MA, Powell TL, Gupta MB & Jansson T (2015). Increased ubiquitination and reduced plasma membrane trafficking of placental amino acid transporter SNAT‐2 in human IUGR. Clin Sci (Lond) 129, 1131–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chillaron J, Estevez R, Mora C, Wagner CA, Suessbrich H, Lang F, Gelpi JL, Testar X, Busch AE, Zorzano A & Palacin M (1996). Obligatory amino acid exchange via systems bo,+‐like and y+L‐like. A tertiary active transport mechanism for renal reabsorption of cystine and dibasic amino acids. J Biol Chem 271, 17761–17770. [DOI] [PubMed] [Google Scholar]
- Cleal JK, Day PE, Simner CL, Barton SJ, Mahon PA, Inskip HM, Godfrey KM, Hanson MA, Cooper C, Lewis RM & Harvey NC (2015). Placental amino acid transport may be regulated by maternal vitamin D and vitamin D‐binding protein: results from the Southampton Women's Survey. Br J Nutr 113, 1903–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleal JK, Glazier JD, Ntani G, Crozier SR, Day PE, Harvey NC, Robinson SM, Cooper C, Godfrey KM, Hanson MA & Lewis RM (2011). Facilitated transporters mediate net efflux of amino acids to the fetus across the basal membrane of the placental syncytiotrophoblast. J Physiol 589, 987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constancia M, Hemberger M, Hughes J, Dean W, Ferguson‐Smith A, Fundele R, Stewart F, Kelsey G, Fowden A, Sibley C & Reik W (2002). Placental‐specific IGF‐II is a major modulator of placental and fetal growth. Nature 417, 945–948. [DOI] [PubMed] [Google Scholar]
- Cramer S, Beveridge M, Kilberg M & Novak D (2002). Physiological importance of system A‐mediated amino acid transport to rat fetal development. Am J Physiol Cell Physiol 282, C153–C160. [DOI] [PubMed] [Google Scholar]
- David AL (2017). Maternal uterine artery VEGF gene therapy for treatment of intrauterine growth restriction. Placenta 59(Suppl 1), S44–S50. [DOI] [PubMed] [Google Scholar]
- Day PE, Cleal JK, Lofthouse EM, Goss V, Koster G, Postle A, Jackson JM, Hanson MA, Jackson AA & Lewis RM (2013). Partitioning of glutamine synthesised by the isolated perfused human placenta between the maternal and fetal circulations. Placenta 34, 1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day PE, Ntani G, Crozier SR, Mahon PA, Inskip HM, Cooper C, Harvey NC, Godfrey KM, Hanson MA, Lewis RM & Cleal JK (2015). Maternal factors are associated with the expression of placental genes involved in amino acid metabolism and transport. PLoS One 10, e0143653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges M, Ditchfield A, Hirst CR, Pegorie C, Martyn‐Smith K, Sibley CP & Greenwood SL (2013). Reduced placental taurine transporter (TauT) activity in pregnancies complicated by pre‐eclampsia and maternal obesity. Adv Exp Med Biol 776, 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges M, Lacey HA, Glazier JD, Greenwood SL, Mynett KJ, Speake PF & Sibley CP (2006). SNAT4 isoform of system A amino acid transporter is expressed in human placenta. Am J Physiol Cell Physiol 290, C305–C312. [DOI] [PubMed] [Google Scholar]
- Desforges M, Mynett KJ, Jones RL, Greenwood SL, Westwood M, Sibley CP & Glazier JD (2009). The SNAT4 isoform of the system A amino acid transporter is functional in human placental microvillous plasma membrane. J Physiol 587, 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditchfield AM, Desforges M, Mills TA, Glazier JD, Wareing M, Mynett K, Sibley CP & Greenwood SL (2015). Maternal obesity is associated with a reduction in placental taurine transporter activity. Int J Obes 39, 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotiadis D, Kanai Y & Palacin M (2013). The SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med 34, 139–158. [DOI] [PubMed] [Google Scholar]
- Goberdhan DC, Wilson C & Harris AL (2016). Amino acid sensing by mTORC1: intracellular transporters mark the spot. Cell Metab 23, 580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MA & Gluckman PD (2014). Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol Rev 94, 1027–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hediger MA, Clemencon B, Burrier RE & Bruford EA (2013). The ABCs of membrane transporters in health and disease (SLC series): introduction. Mol Aspects Med 34, 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeltzli SD, Kelley LK, Moe AJ & Smith CH (1990). Anionic amino acid transport systems in isolated basal plasma membrane of human placenta. Am J Physiol 259, C47–C55. [DOI] [PubMed] [Google Scholar]
- Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, Ganapathy V, Powell TL & Jansson T (2006). Down‐regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol 576, 935–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HN, Jansson T & Powell TL (2010). Full‐length adiponectin attenuates insulin signaling and inhibits insulin‐stimulated amino Acid transport in human primary trophoblast cells. Diabetes 59, 1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Clemencon B, Simonin A, Leuenberger M, Lochner M, Weisstanner M & Hediger MA (2013). The SLC1 high‐affinity glutamate and neutral amino acid transporter family. Mol Aspects Med 34, 108–120. [DOI] [PubMed] [Google Scholar]
- King A, Ndifon C, Lui S, Widdows K, Kotamraju VR, Agemy L, Teesalu T, Glazier JD, Cellesi F, Tirelli N, Aplin JD, Ruoslahti E & Harris LK (2016). Tumor‐homing peptides as tools for targeted delivery of payloads to the placenta. Sci Adv 2, e1600349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofthouse EM, Brooks S, Cleal JK, Hanson MA, Poore KR, O'Kelly IM & Lewis RM (2015). Glutamate cycling may drive organic anion transport on the basal membrane of human placental syncytiotrophoblast. J Physiol 593, 4549–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofthouse EM, Perazzolo S, Brooks S, Crocker IP, Glazier JD, Johnstone ED, Panitchob N, Sibley CP, Widdows KL, Sengers BG & Lewis RM (2016). Phenylalanine transfer across the isolated perfused human placenta: an experimental and modeling investigation. Am J Physiol Regul Integr Comp Physiol 310, R828–R836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew TM, Ohadike C, Baker PN, Crocker IP, Mitchell C & Ong SS (2003). Stereological investigation of placental morphology in pregnancies complicated by pre‐eclampsia with and without intrauterine growth restriction. Placenta 24, 219–226. [DOI] [PubMed] [Google Scholar]
- Moe AJ & Smith CH (1989). Anionic amino acid uptake by microvillous membrane vesicles from human placenta. Am J Physiol 257, C1005–C1011. [DOI] [PubMed] [Google Scholar]
- Noorlander CW, de Graan PN, Nikkels PG, Schrama LH & Visser GH (2004). Distribution of glutamate transporters in the human placenta. Placenta 25, 489–495. [DOI] [PubMed] [Google Scholar]
- Nye GA, Ingram E, Johnstone ED, Jensen OE, Schneider H, Lewis RM, Chernyavsky IL & Brownbill P (2018). Human placental oxygenation in late gestation: experimental and theoretical approaches. J Physiol 596, 5523–5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacin M & Kanai Y (2004). The ancillary proteins of HATs: SLC3 family of amino acid transporters. Pflugers Arch 447, 490–494. [DOI] [PubMed] [Google Scholar]
- Panitchob N, Widdows KL, Crocker IP, Hanson MA, Johnstone ED, Please CP, Sibley CP, Glazier JD, Lewis RM & Sengers BG (2015). Computational modelling of amino acid exchange and facilitated transport in placental membrane vesicles. J Theor Biol 365, 352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panitchob N, Widdows KL, Crocker IP, Johnstone ED, Please CP, Sibley CP, Glazier JD, Lewis RM & Sengers BG (2016). Computational modelling of placental amino acid transfer as an integrated system. Biochim Biophys Acta 1858, 1451–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini CL, Marconi AM, Ronzoni S, Di Noio M, Fennessey PV, Pardi G & Battaglia FC (2001). Placental transport of leucine, phenylalanine, glycine, and proline in intrauterine growth‐restricted pregnancies. J Clin Endocrinol Metab 86, 5427–5432. [DOI] [PubMed] [Google Scholar]
- Perazzolo S, Hirschmugl B, Wadsack C, Desoye G, Lewis RM & Sengers BG (2017a). The influence of placental metabolism on fatty acid transfer to the fetus. J Lipid Res 58, 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perazzolo S, Lewis RM & Sengers BG (2017b). Modelling the effect of intervillous flow on solute transfer based on 3D imaging of the human placental microstructure. Placenta 60, 21–27. [DOI] [PubMed] [Google Scholar]
- Poston L, Igosheva N, Mistry HD, Seed PT, Shennan AH, Rana S, Karumanchi SA & Chappell LC (2011). Role of oxidative stress and antioxidant supplementation in pregnancy disorders. Am J Clin Nutr 94, 1980s–1985s. [DOI] [PubMed] [Google Scholar]
- Pramod AB, Foster J, Carvelli L & Henry LK (2013). SLC6 transporters: structure, function, regulation, disease association and therapeutics. Mol Aspects Med 34, 197–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan T, Camargo SM, Summa V, Hunziker P, Chesnov S, Pos KM & Verrey F (2006). Basolateral aromatic amino acid transporter TAT1 (Slc16a10) functions as an efflux pathway. J Cell Physiol 206, 771–779. [DOI] [PubMed] [Google Scholar]
- Roos S, Palmberg I, Saljo K, Powell TL & Jansson T (2005). Expression of placental mammalian target of rapamycin (mTOR) is altered in relation to fetal growth and mTOR regulates leucine transport. Placenta 26, A9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos S, Powell TL & Jansson T (2004). Human placental taurine transporter in uncomplicated and IUGR pregnancies: cellular localization, protein expression, and regulation. Am J Physiol Regul Integr Comp Physiol 287, R886–R893. [DOI] [PubMed] [Google Scholar]
- Rosario FJ, Kanai Y, Powell TL & Jansson T (2013). Mammalian target of rapamycin signalling modulates amino acid uptake by regulating transporter cell surface abundance in primary human trophoblast cells. J Physiol 591, 609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schioth HB, Roshanbin S, Hagglund MG & Fredriksson R (2013). Evolutionary origin of amino acid transporter families SLC32, SLC36 and SLC38 and physiological, pathological and therapeutic aspects. Mol Aspects Med 34, 571–585. [DOI] [PubMed] [Google Scholar]
- Sengers BG, Please CP & Lewis RM (2010). Computational modelling of amino acid transfer interactions in the placenta. Exp Physiol 95, 829–840. [DOI] [PubMed] [Google Scholar]
- Sferruzzi‐Perri AN, Vaughan OR, Coan PM, Suciu MC, Darbyshire R, Constancia M, Burton GJ & Fowden AL (2011). Placental‐specific Igf2 deficiency alters developmental adaptations to undernutrition in mice. Endocrinology 152, 3202–3212. [DOI] [PubMed] [Google Scholar]
- Simner C, Novakovic B, Lillycrop KA, Bell CG, Harvey NC, Cooper C, Saffery R, Lewis RM & Cleal JK (2017). DNA methylation of amino acid transporter genes in the human placenta. Placenta 60, 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speake PF, Glazier JD, Ayuk PT, Reade M, Sibley CP & D'Souza SW (2003). L‐Arginine transport across the basal plasma membrane of the syncytiotrophoblast of the human placenta from normal and preeclamptic pregnancies. J Clin Endocrinol Metab 88, 4287–4292. [DOI] [PubMed] [Google Scholar]
- Stirrat LI, Sengers BG, Norman JE, Homer NZM, Andrew R, Lewis RM & Reynolds RM (2018). Transfer and metabolism of cortisol by the isolated perfused human placenta. J Clin Endocrinol Metab 103, 640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Versen‐Hoynck F, Rajakumar A, Parrott MS & Powers RW (2009). Leptin affects system A amino acid transport activity in the human placenta: evidence for STAT3 dependent mechanisms. Placenta 30, 361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdows KL, Panitchob N, Crocker IP, Please CP, Hanson MA, Sibley CP, Johnstone ED, Sengers BG, Lewis RM & Glazier JD (2015). Integration of computational modeling with membrane transport studies reveals new insights into amino acid exchange transport mechanisms. FASEB J 29, 2583–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR & Sabatini DM (2016). Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 351, 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]


