Abstract
Over 30 years ago Professor David Barker first proposed the theory that events in early life could explain an individual's risk of non‐communicable disease in later life: the developmental origins of health and disease (DOHaD) hypothesis. During the 1990s the validity of the DOHaD hypothesis was extensively tested in a number of human populations and the mechanisms underpinning it characterised in a range of experimental animal models. Over the past decade, researchers have sought to use this mechanistic understanding of DOHaD to develop therapeutic interventions during pregnancy and early life to improve adult health. A variety of animal models have been used to develop and evaluate interventions, each with strengths and limitations. It is becoming apparent that effective translational research requires that the animal paradigm selected mirrors the tempo of human fetal growth and development as closely as possible so that the effect of a perinatal insult and/or therapeutic intervention can be fully assessed. The guinea pig is one such animal model that over the past two decades has demonstrated itself to be a very useful platform for these important reproductive studies. This review highlights similarities in the in utero development between humans and guinea pigs, the strengths and limitations of the guinea pig as an experimental model of DOHaD and the guinea pig's potential to enhance clinical therapeutic innovation to improve human health.
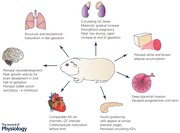
Keywords: DOHaD, guinea pig, animal models, fetus, placenta, pregnancy
Introduction
Originally describing the association between early life nutrition and the increased risk of early onset cardiovascular and metabolic diseases, Barker and others, such as Neel and Forsdahl, through the 1960s and into the 1980s developed and put forward the concept of early life reprogramming (Neel, 1962) and later specifically fetal programming (Forsdahl, 1977). Further to this, large‐cohort epidemiological studies not only confirmed but strengthened the argument (Barker, 2004; Gluckman & Hanson, 2004) ultimately leading to Barker's description of the developmental origins of health and disease (DOHaD) hypothesis (Barker et al. 1990, 2002; Barker, 2005). Today, it is widely acknowledged that suboptimal in utero or early life conditions result in permanent alterations to fetal developmental and growth trajectories due to structural, functional and epigenetic changes and that these are associated with increased metabolic disease risk later in the life cycle.
While epidemiological studies in human populations have provided robust evidence linking suboptimal growth in utero with adverse health outcomes in adult life, such associations cannot establish causality nor define the mechanisms through which these adult health consequences are mediated. Instead, the use of appropriate animal models of DOHaD has allowed us to understand some of the pathophysiological and mechanistic basis for the effect of early life perturbation on long‐term health and wellbeing (Barker et al. 1990, 2002; Barker, 2005; Dickinson et al. 2016). Additionally, prospective and interventional studies in humans would take decades to determine outcomes in only the F1 generation, further supporting the need for animal model systems with relatively short reproductive timelines. This latency between understanding the mechanistic basis of disease risk through to development of a therapeutic strategy and assessing its potential efficacy is far too long. Thus, animal models play important roles both in determining the mechanisms that link growth in utero and early life to health in adult life and in development of novel therapeutic interventions.
Although a range of species (sheep, rats, mice, spiny mouse) have been used to investigate DOHaD, in this review we will outline the strengths of using pregnant guinea pigs (Cavia porcellus), as well as potential limitations, as an experimental model for the study of DOHaD. We present how studies in this species have contributed and continue to contribute to our understanding of DOHaD, generating data to lead clinical innovation in this area.
DOHaD has multigenerational and transgenerational effects
Notably, the consequences of perinatal exposures are not restricted to the offspring in the F1 generation but also occur in the F2 and F3 generations (Fig. 1). For example, grand‐paternal food supply is associated with a grandson's mortality risk ratio whilst grand‐maternal food supply is associated with a granddaughter's mortality risk ratio (Pembrey et al. 2006). These persistent multigenerational effects reflect exposures of the granddaughter's oocyte, present within her mother when her mother was a fetus in her grandmother (Dickinson et al. 2016). Changes in metabolism in the F1 generation may also mediate some effects on subsequent generations, whilst epigenetic changes are proposed to underlie some persistent effects of early life exposures that are transgenerational as well as multigenerational (Fig. 1), including those transmitted through the male line (Burdge et al. 2007; McPherson et al. 2015).
Figure 1. DOHaD studies have shown that exposures in one generation can have multigenerational and intergenerational effects.
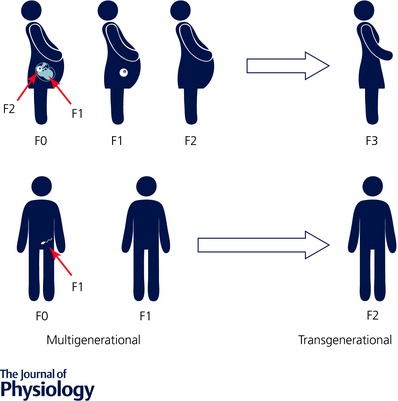
Changes in the grandmother (F0) or grandfather (F0) can influence the health of the granddaughter (F2) or son (F1), respectively. Transgenerational effects have also been identified that affect the great granddaughter (F3) and the grandson (F2). Adapted from Dickinson et al. (2016) with permission.
Mechanistic DOHaD studies require a clinically relevant animal model
The need for translational animal models of high‐risk pregnancy and adverse pregnancy outcomes remains a research priority, in part due to the relative paucity and ethical concerns of interventional human studies in this population. Understanding the mechanistic basis of multi‐ and transgenerational developmental programming requires studies of DOHaD in a small animal species with a relatively short lifespan compared with humans. Although large animal species such as the sheep are also widely used in DOHaD studies, sheep take longer to reach maturity and have additional housing and logistical requirements compared with small animal species.
For meaningful clinical translation, these animal models must satisfy several conditions. Firstly, that the in utero environment is comparable to humans. Secondly that fetal developmental timing is broadly similar to humans, so that in utero exposures occur at similar developmental stages to those of the human. As such, the translatability of studies performed in altricial species (mice and rats) should be approached with caution as many key stages of development that occur before birth in humans occur after birth in these species (Dobbing & Sands, 1970; Hunter et al. 2016). Thirdly, fetal number should not be excessive. Unlike humans, guinea pigs generally have more than one offspring, but their litter sizes of 1–5 are substantially lower than the 8–12 pups usually seen in rats or mice. Finally, it is crucial that animal models of DOHaD share similar patterns of adverse outcomes to those seen in human studies following the same exposure, so that any therapeutic intervention can be thoroughly evaluated (Shaw et al. 2016).
Fetal environment and developmental timing: similarities between guinea pig and humans
The guinea pig has a relatively long gestation (69–71 days) compared to other rodents such as the mouse and rat (19–21 days), yet shorter than that of the sheep (145–150 days). This longer gestation allows for better temporal resolution of developmental plasticity and identification of critical time periods for vulnerability to insults. Importantly, the timing of development of major organ systems that are impacted by in utero exposures or perinatal complications with adverse health outcomes in humans are similar in the guinea pig (Briscoe et al. 2004).
Placenta development
Placenta morphology
The guinea pig has a haemomonochorial placenta that is an excellent surrogate for the haemochorial human placenta (Fig. 2; Smith et al. 1999; Carter et al. 2006; Mess, 2007; Mitchell & Taggart, 2009). Importantly, the guinea pig placenta invades deeply into the decidua similar to the human, in contrast to the shallower invasion of other rodents (Kaufmann et al. 2003). The subplacenta is analogous to the cell columns of the anchoring chorionic villi of the human placenta (Carter et al. 2006; Mess, 2007) and contains proliferating trophoblast cells of similar subtypes to those of the human (Kaufmann et al. 2003; Carter et al. 2006) that differentiate and contribute to decidualisation of maternal endometrium and invasion of maternal blood vessels (Kaufmann et al. 2003; Mess, 2007). The labyrinthine region of the guinea pig placenta contains distinct units of maternal and fetal blood vessels separated by a fetal syncytiotrophoblast layer and functions to exchange oxygen/nutrient supply between these two circulations (Fig. 2). However, the guinea pig placenta functions as a countercurrent gas exchanger (Miglino et al. 2004), whereas the human placenta appears to function as a concurrent gas exchanger (Wilkening & Meschia, 1992; Lin et al. 2016).
Figure 2. Structural similarities between guinea pig and human placenta.
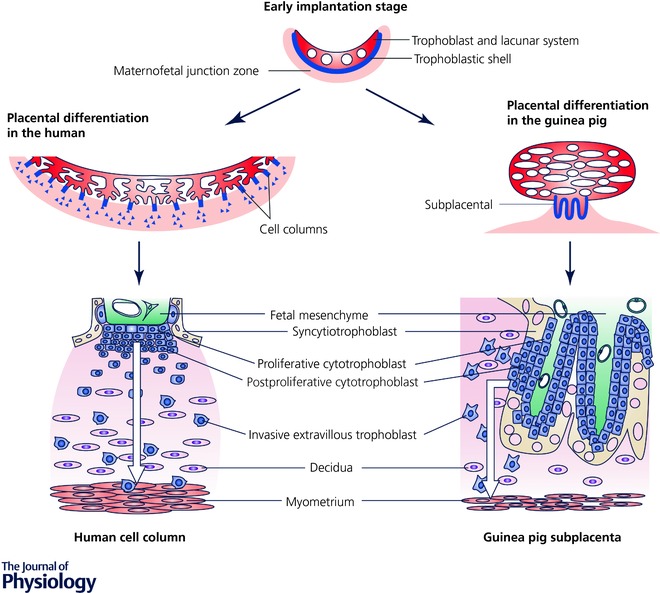
Reproduced with permission from Mess (2007).
Placental and reproductive endocrine function
Like humans, pregnant guinea pigs exhibit a luteo‐placental shift in hormone production so that the placenta acts as the major progesterone source throughout the major part of pregnancy (Heap & Deanesly, 1966). Similar to pregnancy in humans and non‐human primates, circulating progesterone remains elevated until term (Challis et al. 1971; Illingworth et al. 1971; Mitchell & Taggart, 2009). This contrasts with most other laboratory species, including mice, rats and sheep (Fig. 3 A), which are dependent on systemic progesterone withdrawal to initiate parturition (Mitchell & Taggart, 2009). Placental progesterone production in guinea pigs means that, similar to the human, maintenance of pregnancy is independent of the ovary in the second half of pregnancy (Csapo et al. 1981). Conversely, ovariectomy induces parturition and/or pregnancy loss in mice and rats throughout pregnancy (Fig. 3 B).
Figure 3. Circulating progesterone remains elevated throughout pregnancy in guinea pigs and humans.
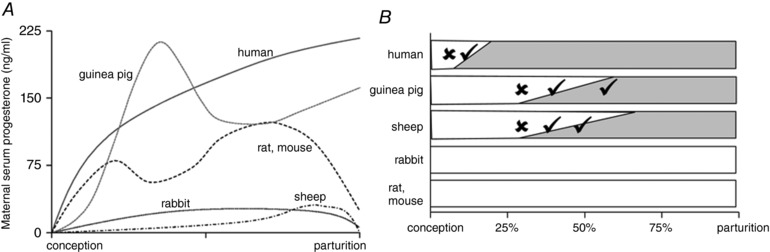
Circulating progesterone (A) and corpus luteum (CL)‐dependence of pregnancy maintenance (B) reflect species differences in placental progesterone production throughout pregnancy. Bars indicate main source of circulating progesterone (white: CL, shaded: placenta). Ticks indicate that pregnancy can be maintained after removal of the ovary or CL, crosses indicate that either the CL or exogenous progesterone is required to maintain pregnancy. A is reproduced from Mitchell & Taggart (2009 with permission. Data in B are from Csapo & Pulkkinen (1978), Csapo et al. (1981), Al‐Gubory et al. (2000) and Bazer (2015).
Human and guinea pig placentas share similarities in their response to glucocorticoids, allowing effects of endogenous and exogenous glucocorticoids on placental function and fetal programming to be investigated. Similar to the human placenta, the guinea pig placenta expresses multiple isoforms of the glucocorticoid receptor (Saif et al. 2016), and metabolises maternal glucocorticoids via 11β‐hydroxysteroid dehydrogenase type 2, with activity of this enzyme decreasing towards term, resulting in increased fetal exposure to endogenous glucocorticoids (Sampath‐Kumar et al. 1998; Murphy & Clifton, 2003). It also expresses P‐glycoprotein (Kalabis et al. 2009), which contributes to the placental glucocorticoid barrier via transport of glucocorticoids back into the maternal circulation (Soo et al. 2012; Hodyl et al. 2013; Bloise et al. 2016).
Placental metabolic function
The placenta of many species, including guinea pig, is incapable of gluconeogenesis (Bossi & Greenberg, 1972) and any newly synthesized glucose must be of either fetal or maternal origin (Dwyer & Stickland, 1994). Gluconeogenic enzymes are present in the guinea pig liver from gestational day (GD) 40. Similar to sheep and humans (Sadava et al. 1992; Houin et al. 2015), fetal gluconeogenesis does not take place until late gestation (Jones & Ashton, 1976), meaning the fetus is dependent on glucose delivery from the mother down a concentration gradient. As with glucose, amino acids cross the guinea pig placenta (Jansson & Persson, 1990) and essential and non‐essential amino acids and fatty acids can be transferred from the maternal to the fetal circulation against the feto‐maternal gradient (Thomas & Lowy, 1983). These observations are supportive of active amino acid transport mechanisms being present (Jones & Rolph, 1985), similar to human pregnancies. In guinea pigs, like humans but unlike other rodents, maternal circulating concentrations of insulin‐like growth factor 1 and 2 are substantial, increasing during pregnancy (Sohlstrom et al. 1998), and may have major influences on nutrient partitioning during pregnancy (Sferruzzi‐Perri et al. 2006).
Uteroplacental circulation
Radioactive microsphere studies have demonstrated a correlation between placental blood flow and fetal size even after correction for placental weight differences (Myers et al. 1982). This mirrors the human singleton pregnancy where total uterine artery blood volume flow in the first or early second trimesters is positively correlated to birth weight (McKelvey et al. 2017), and total uterine blood flow is reduced in fetal growth restricted (FGR) pregnancies (Konje et al. 2003). In contrast to humans, lower placental and fetal weights are found naturally in the middle pregnancy sites compared to either the tubal or cervical zones in guinea pigs and other rodents (Turner & Trudinger, 2000), reflecting preferential perfusion of the tubal and cervical uterine zones.
Fetal development
Metabolic tissues
Significant accumulation of adipose tissue in late gestation occurs in fetal guinea pigs and humans. Like the human fetus, but unlike most other species, the guinea pig fetus lays down both brown and white adipose tissue in utero and has a total fat content of ∼14% at birth (Mace et al. 2006), significantly higher than other rodents and the fetal sheep, but similar (∼10%) to the human (Carberry et al. 2010). Adipogenesis and fat cell hyperplasia are upregulated during early life but very low in the adult guinea pig (Castaneda‐Gutierrez et al. 2011). Similarly, in humans, adipocyte number is set before puberty with limited ability to form new adipocytes in adulthood (Spalding et al. 2008). Furthermore, the guinea pig develops large epicardial adipose depots (Rolph et al. 1982), making them a better model for studies of human cardiac–epicardial depot interactions than rats (Swifka et al. 2008).
Timing of myogenesis in the guinea pig also resembles that of humans and species such as sheep (Romero et al. 2013) with secondary myogenesis occurring around mid‐gestation, in contrast to rats where this occurs later in gestation. In the guinea pig, primary myotubes are observed at GD 30, secondary myotubes appear between GD30 and 35 and fibre hyperplasia is complete by GD50 (Dwyer et al. 1995).
Pancreatic development also occurs at similar prenatal stages in guinea pigs as in humans, with insulin‐producing cells apparent at ∼25% of gestation in human (Reddy & Elliott, 1988) and by ∼40% of gestation in guinea pig (Reddy et al. 1992). Drug transporters are present in the guinea pig liver in late gestation (Soo et al. 2012). Despite these similarities in developmental timing of metabolic tissues, the guinea pig is born relatively more metabolically mature than most other mammals, including humans, since, in addition to suckling milk, it is able to eat solid food from birth (Davis et al. 1979).
The timing for ovarian follicle development in guinea pigs is likewise more similar to that in humans than to other rodents. While primordial follicle assembly occurs prenatally between GD48 and 56 in guinea pigs (Bookhout, 1946) and beginning at gestational week 13 in humans (Forabosco & Sforza, 2007), this process occurs during the early post‐natal period in other rodents (Bookhout, 1946; Rajah et al. 1992; Forabosco & Sforza, 2007).
Cardiovascular system
There remain gaps in our knowledge regarding the developmental timing of the fetal guinea pig heart and its comparison to human heart development. The literature does, however, identify a mature fetal heart phenotype in guinea pigs in contrast to other rodent species and in some ways similar to the human fetus. In the fetal guinea pig heart, there is a linear increase in the fraction of cell volume occupied by mitochondria and myofibrils, and a decrease in the fractional volume of nuclei and sarcoplasmic reticulum over the last third of gestation (Rolph et al. 1982). These developmental changes are associated with an increase in the number of mitochondria and myofibrils and a decrease in nuclear number and sarcoplasmic reticulum volume, respectively. Increasing contractile properties in the developing heart are associated with increased organization of cardiac ultrastructure along with increased oxidative capacity of mitochondria (Schaper et al. 1985), maturation of sarcomere structure and myofibril organization (Racca et al. 2016).
The ultrastructure of the fetal guinea pig heart (Rolph et al. 1982) is consistent with the density, elongation and alignment of myofibrils measured in midterm (127 days’ gestation) fetal human hearts (Racca et al. 2016). The fetal guinea pig heart is more mature at birth in regards to compartmentation of metabolic pathways and organization of mitochondrial ultrastructure than fetal hearts of rat or mouse (Barrie & Harris, 1977; Hoerter et al. 1994; Hew & Keller, 2003). Further, the fetal guinea pig heart exhibits a greater reliance on transarcolemmal calcium (Hew & Keller, 2003), cellular compartmentation of creatine kinase (Hoerter et al. 1994) and advanced development of the sarcoplasmic reticulum toward term (Agata et al. 1994) providing more mature mechanisms for cardiac contraction. Lastly, the fetal guinea pig has increasing CaATPase activity associated with mitochondria and sarcoplasmic reticulum in late gestation (Rolph et al. 1982) and both sympathetic and parasympathetic innervation at the time of birth (De Champlain et al. 1970; Friedman, 1972; Hew & Keller, 2003) also identifying a mature cardiac phenotype that differs from other rodent species and is similar to humans. The guinea pig has been used to study functional properties of the heart because action potential configuration, ion channels and the QT interval characteristics are similar to those of the human heart and demonstrate comparable maturational changes during gestation and after birth (Shiotani et al. 2008; Nerbonne, 2016). For example, while similarities in K+ channel characteristics exist among animal species such as guinea pig, human, rat and rabbit (Nerbonne, 2016), developmental changes are complete in the late gestation fetal human and guinea pig heart, whereas this occurs postnatally in rats and mice (Agata et al. 1993, 1994).
Placenta–heart axis
Fetal heart development is influenced by haemodynamics of the feto‐placental circulation, since the fetal heart must eject a blood volume against a systemic impedance determined by downstream vascular beds, including the placenta (Thornburg et al. 2010). Thus, disorders of placental development may have effects on fetal cardiac function either directly or indirectly via altered haemodynamic and/or metabolic influences. As both guinea pig fetal heart and guinea pig placenta mirror many of the key phenotypic characteristics of the human fetal heart and placenta (Carter, 2007; Mess et al. 2007), experimentally induced placental dysfunction in the guinea pig (Turan et al. 2017) may contribute to our understanding of the ‘programming’ of later heart dysfunction in offspring of a high‐risk pregnancy.
Brain and nervous system
Using the timing for peaks in brain growth velocity as a marker of development, guinea pigs and sheep can be classified as prenatal brain developers, humans as perinatal brain developers, and other rodents as postnatal brain developers (McIntosh et al. 1979). The most rapid phase of synapse formation and myelination is initiated in the latter half of pregnancy in humans and guinea pigs, whilst predominantly occurring in the weeks after birth in other rodents (Dobbing & Sands, 1970; Nitsos & Rees, 1990; Nacher et al. 2000; Back et al. 2001). The anatomical development of the brain also correlates with its electrophysiological development as indicated by behavioural state maturation. Whereas guinea pigs, sheep and humans have well differentiated and relatively mature electrocortical patterns at birth, rats have a poorly differentiated electrocorticogram at birth (Szeto & Hinman, 1985; Szeto et al. 1985; Umans et al. 1985). This neurodevelopmental correlation, along with the high proportion of rapid eye movement‐like behavioural activity during early life, supports a role for behavioural state activity in the brain's development and more so in guinea pigs and humans prenatally in response to conditions during pregnancy than in rats (Richardson et al. 2014).
The immature brain exerts excitatory γ‐aminobutyric acid (GABA) activity that is involved in many processes of neurogenesis including neuronal proliferation, migration, differentiation, and oligodendrocyte and synaptic development. A transition from immature (excitatory) to mature (inhibitory) GABAergic activity occurs before birth in humans and primates (Khazipov et al. 2001; Sedmak et al. 2016), but postnatally in rodents (Rivera et al. 1999). Preliminary studies in guinea pigs indicate this transition occurs in the last third of pregnancy (Coleman et al. 2013), further supporting the use of guinea pigs to explore the neurodevelopmental impact of pregnancy complications.
Lung
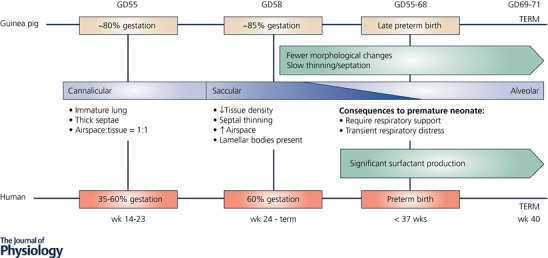
Comparable to humans, lung biochemical and morphological development occurs in late gestation in the guinea pig but after birth in rats (Hunt et al. 1991). At GD55 the fetal guinea pig's lungs are immature and ‘canalicular’ (seen in humans from 14 to 23 weeks of gestation; term, 40 weeks), with thick septae and an airspace to tissue ratio of approximately 1:1 (Fig. 4). Although type II alveolar epithelial cells that produce surfactant protein are present, their density increases linearly with age (Lin & Lechner, 1990). By GD58, the lungs have undergone rapid and significant maturation, representative of the ‘saccular’ stage of lung development (seen in humans from 24 weeks’ gestation, the cusp of human postnatal viability, through to term), characterised by decreased tissue density, marked septal thinning, and as a consequence, a marked increase in potential airspace (∼73%). Morphological changes from GD58 through to term are less significant, with slower thinning and septation giving rise to greater potential airspace (∼81%) by term. This similar lung developmental timing allows survival of preterm guinea pigs with support much like that provided to preterm neonates in the neonatal intensive care unit (NICU) (Sosenko & Frank, 1987; Kelly et al. 1991b; Berry et al. 2015).
Figure 4. Comparison of the stages of lung development in the human and guinea pig fetus.
Kidney
Although the guinea pig renal system is relatively less well characterised than other systems, key aspects of maturation and development appear to be similar to that of the human. For instance, like humans, but unlike other rodent species, nephrogenesis in the fetal guinea pig is completed during fetal life (Welling et al. 1989) with a trajectory of renin–angiotensin pathway activation that mirrors that of the human (Raimbach & Thomas, 1990).
Assessment of fetal size and wellbeing
As with human pregnancy, fetal number, growth trajectory and haemodynamics can be tracked throughout gestation using non‐invasive ultrasound techniques in guinea pigs. Optimal images are obtained with a narrow footprint high frequency ultrasound probe (7–15 Hz). Gestation sacs are visible from ∼GD20–25 and the fetal measurements obtained are similar to those used for dating and serial ultrasound assessment of human fetal growth and fetal growth in other rodents, ruminants and rabbits. Crown–rump length can be assessed up to GD35 but after that time the fetus is too long and flexed for accurate measurement (Santos et al. 2014; Swanson et al. 2017). Biparietal diameter, occipito snout length, head circumference and abdominal circumference can be measured from ∼GD25 and used to track changes in fetal size as gestation advances (Fig. 5; Turner & Trudinger, 2000, 2009). Blood flow in the umbilical artery, uterine artery and fetal heart can be measured using Doppler ultrasound (Herrera et al. 2016). In this way ultrasound can identify in vivo fetal growth and/or haemodynamic acute and longitudinal responses to experimentally induced environmental insults as well as to therapeutic intervention.
Figure 5. Ultrasound measurement of fetal size throughout gestation in the guinea pig.
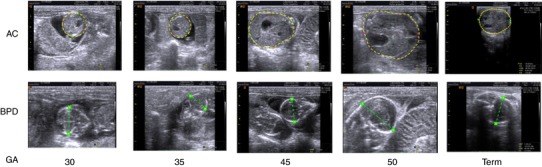
Images show measurement of abdominal circumference (AC, yellow lines) and biparietal diameter (BPD, green lines) from GD30 until term.
We have not found major differences in fetal outcomes in models of hypoxia or stress in terms of stillbirth, spontaneous abortion or preterm birth. For example, repeated maternal stress with a strobe light during early and mid‐gestation does not change reproductive parameters (Schopper et al. 2011), despite down‐regulation of the hypothalamic–pituitary–adrenal (HPA) axis and lower weight gain in treatment females. However, maternal undernutrition severe enough to result in fetal growth restriction can lead to preterm delivery (Elias et al. 2016; Nevin et al. 2018).
In addition, dams must remove the fetal membranes from around the face of each pup and thus, in large litters, she may not get to the last pup quickly enough. These postnatal deaths are not stillbirths but are primarily a reflection of litter size and the short inter‐pup delivery interval. Furthermore, we have not observed dystocia in dams that have had their first mating by 6–8 months of age.
Models of DOHaD in guinea pigs
The wealth of commonality between guinea pigs and humans in terms of the developmental trajectory of major organ systems illustrates why many researchers choose to work with guinea pigs as a translational model of DOHaD to understand the impact of early life conditions on diverse outcomes including neurodevelopmental, cardiovascular, respiratory, metabolic and reproductive (Table 1). Crucially, as occurs clinically, the pattern of deficit differs depending on the severity, timing and duration (chronic, acute or repeated) of the perinatal insult (Morrison, 2008). The ensuing functional deficits can be evaluated postnatally using established guinea pig assessment techniques (Bennett et al. 2016; Shaw et al. 2016). Many guinea pig models of clinically important perinatal perturbation have been established (Table 1) and we have summarised the knowledge that has been gained below. This has positioned the field to now pursue studies of potential therapeutic interventions to improve outcomes.
Table 1.
Guinea pig models used in DOHaD research
| Model to induce developmental programming | Specific protocol | Altered fetal or neonatal growth | Organ system investigated (fetal/postnatal time point of analysis) |
|---|---|---|---|
| Chronic maternal hypoxia | 10–16% O2 for varying durations in the second half of pregnancy | Dependent on duration and degree of hypoxic insult, e.g. 12% O2 for 2nd half of gestation or 10% O2 ≥ 10 days before term |
|
| Acute maternal hypoxia | 1 h at 35, 40, 45, 50, 55 or 60 days’ gestation; 7% O2 | No | Brain, fetal (Mishra & Delivoria‐Papadopoulos, 1988a,b; Mishra et al. 1988, 1995; Mishra & Delivoria‐Papadopoulos, 1989, 1992; Graham et al. 1993, 1995; Lampley et al. 1995; Razdan et al. 1996; Maulik et al. 1998, 1999, 2001, 2002, 2005, 2008; Buonocore et al. 1999; Zanelli et al. 1999, 2005; Katsetos et al. 2001; Qayyum et al. 2001; Abedin et al. 2005; Vibert et al. 2008) |
| Maternal hypobaric hypoxia | For the last 17–28 days before term; 380–420 torr | Yes | Pulmonary vasculature, postnatal (Murphy et al. 1986) |
| From 3 days post‐conception; 3962 or 1600 m | In 56 day cohort | Placenta, fetal (Rockwell et al. 2000) | |
| Maternal carbon monoxide exposure | 200 ppm carbon monoxide for 10 h/day from day 23–25 of gestation until term | No, but smaller at 4 days after birth | Respiratory control, postnatal (McGregor et al. 1998) |
| Unilateral uterine artery ligation | Performed at 28–32 days’ gestation | IUGR in 26.5–50% of pups from ligated horn |
|
| Progressive uterine artery occlusion | Ameroid constrictors implanted at 32–35 days’ gestation | Yes | |
| Ablation of radial arteries supplying each placenta | Performed at 30–35 days’ gestation | Yes | |
| Maternal nutrient restriction (global) | 70% average food intake per kg of body weight of the control animals 4 weeks before conception until mid‐pregnancy (35 days) increasing to 90% thereafter | Yes |
|
| F0 fed 0% of control food intake from gestational days 1–35 (early) or 36–70 (late); F1 females bred with control males | MNR late: yes; MNR early: F1 females heavier than control | ||
| Deprived of all food for 48 h from 50 days’ gestation | At 52 days’ gestation, but no difference in birth weight | ||
| 85% average food intake per kg of body weight of the control animals from at least 4 weeks before conception until fetal post‐mortem or birth | Yes | ||
| Fed 60% of food intake of control in beginning, late or throughout pregnancy | Yes | Muscle, fetal (Dwyer et al. 1995) | |
| Fed 50% of food intake of control from mid‐gestation | Yes | ||
| Maternal iron deficiency | Fed iron‐deficient diet from 3 weeks prior to mating to PN9 | No | Cochlea, postnatal (Yu et al. 2016) |
| Maternal periconceptional overnutrition | 160% food intake of control prior to mating and until 18 days’ gestation | Greater percentage of larger and smaller fetuses | Vasculature, fetal (Krause et al. 2016) |
| Maternal high fat diet | Diet contained 40% energy from fat from mating, through pregnancy until weaning (PN21) | No difference in birth weight, but greater adiposity | Adipose tissue and insulin signalling, postnatal (Castaneda‐Gutierrez et al. 2011) |
| Diet containing either maize oil or beef dripping in last weeks of gestation | No | ||
| Antenatal glucocorticoid therapy | Dexamethasone (1 mg kg−1) or vehicle on gestational days 40 and 41, 50 and 51, 60 and 61 (or until fetal post‐mortem) | No | |
| Dexamethasone (1 mg kg−1) or vehicle on gestational days 50 and 51 | No | Brain and HPA function, postnatal (Dean et al. 2001) | |
| Betamethasone (1 mg kg−1; phosphate–acetate mix) or vehicle on gestational days 40 and 41, 50 and 51, 60 and 61 (or until fetal post mortem) | No |
|
|
| Maternal stress | Exposure to high‐frequency strobe light for 2 h at 50, 51 and 52 or 60, 61 and 62 days’ gestation | No | Brain and HPA function, postnatal (Kapoor & Matthews, 2005, 2008, 2011; Kapoor et al. 2008, 2009) |
| Exposed to 1 of 4 stressors every 2nd day in from 32 to 66 days’ gestation and PN 1–25 (weaning) | No | Brain and HPA function, postnatal (Emack et al. 2008; Emack & Matthews, 2011) | |
| Exposure to high‐frequency strobe light for 2 h on gestational day 50, 55, 60 and 65 | No | ||
| F0 exposed to high‐frequency strobe light twice a day during gestational days −7, 0, 7, 14, 21, 28, 35, 42; F2 generated from either F1 male/control female or F1 female/control male | No | ||
| Chronic maternal ethanol exposure | 4 g ethanol per kg maternal body weight per day from 2 days’ gestation until fetal post‐mortem or 63 days’ gestation | Some find IUGR, some do not | |
| 3–4 g ethanol per kg maternal body weight per day for 5 days followed by 2 days without until term (∼68 days) | No | Folate, fetal (Hewitt et al. 2011) | |
| In utero infection (Group B Streptococci) | Wild‐type GBS strain or an isogenic hyper‐virulent, and hyper‐haemolytic GBS strain (GBSΔcovR); 45 days’ gestation | No | Bacterial invasion, fetal (Harrell et al. 2017) |
| Preterm birth (C‐section) | C‐section delivery at 62 days’ gestation; 1 mg kg−1 of betamethasone 24 and 12 h before delivery, neonates received 50 μl of surfactant | No | |
| Preterm birth (induced) | Induced at 62 days’ gestation; aglepristone 10 mg kg−1 24 h prior to and on the morning of delivery, oxytocin 3 IU kg−1 repeated in 30 min intervals until end of labour, betamethasone 1 mg kg−1 48 and 24 h before delivery | No | |
| Induced by RU486 3 mg kg−1 body weight at 55 and 56 days’ gestation | No | Placenta, parturition (Gomez‐Lopez et al. 2015) | |
| Birth asphyxia | Asphyxia induced by clamping the umbilical cord at birth for 5 min | No | Brain, postnatal (Sanchez‐Aparicio et al. 2008) |
| Asphyxia induced by submersing in a water bath (37°C) for 2–4 min at birth | No | Brain, postnatal (Bernert et al. 2003) | |
| Spontaneous fetal growth restriction | Variation in placental size and fetal nutrition due to spontaneous variation in litter size, birth to term | Yes, in litters of 6–7 body weight reduced by 22–38% compared to litters of 2 | Fetal and placental weights, prenatal (Ibsen, 1928) |
| Yes, in litters of 5 body weight reduced by 38% compared to litters of 1 | Growth, body composition and appetite, postnatal (Horton et al. 2016) |
Programming of neurodevelopment
The neurodevelopmental consequences of fetal and perinatal adversity (Table 1) may alter total or regional brain structure or volume, cause white matter injury or delay and/or alter neurogenesis. Progesterone, maintained at high levels in human and guinea pig pregnancy, is a potent neurosteroid as well as an important precursor in the production of other neurotrophic steroids such as allopregnanolone. Intrauterine growth restriction (IUGR) and prenatal stress disrupt allopregnanolone production and result in reduced myelination, astrogliosis, altered GABAA receptor expression and behavioural deficits that are maintained in adolescence (McKendry et al. 2010; Kelleher et al. 2011; Bennett et al. 2013, 2015, 2017; Cumberland et al. 2017b). Similarly, preterm birth results in early loss of exposure to progesterone and its derivatives. The recent development of models of long‐term survival following preterm birth in guinea pigs (Kelleher et al. 2013; Palliser et al. 2015; Shaw et al. 2015, 2016, 2017a) opens a critical area of developmental research in which the consequence of curtailed progesterone exposure and differences in substrate availability in preterm‐delivered offspring compared to fetuses at the same postconceptional age can be explored.
Guinea pigs are a suitable for the investigation of long term behavioural and cognitive deficits following perinatal challenges. Commonly used assessments include the open field arena for quantification of exploration, locomotion, anxiety and hyperactive behaviours and social interactions (Iqbal et al. 2004; Kapoor & Matthews, 2005; Zipser et al. 2014; von Engelhardt et al. 2015; Shaw et al. 2016; Crombie et al. 2017; Cumberland et al. 2017a). The elevated plus maze (Rex et al. 1993; Crombie et al. 2017) and dark/light box (Zipser et al. 2014) are also used for measurements of anxiety. The Y‐maze (Dobson et al. 2012b), modified Biel maze (Dobson et al. 2012b) and forced swim arena (Wicke et al. 2007) have been used in guinea pigs for measurement of behavioural inhibition, depressive states, spatial learning and memory deficits. In addition, the acoustic startle chamber (Rehn et al. 2004; Kapoor & Matthews, 2011) has been characterised for use in assessment of sensorimotor gating and attention deficits and the step tower (Zipser et al. 2014) to assess risk behaviours. Together, these tests provide key indicators of locomotor, emotional and cognitive disorders as well as risk‐taking behaviour, social interaction and memory deficits. These tests can be paired with endocrine profiling using non‐invasive salivary samples, which can readily be obtained from guinea pigs without inducing stress (Kapoor & Matthews, 2005; Kapoor et al. 2008).
Neurodevelopmental outcomes of IUGR in a number of animal species including guinea pigs has been extensively reviewed (Basilious et al. 2015; Hunter et al. 2016). Clinically, infants born preterm exhibit reduced white matter volumes that have been linked to cerebral palsy in early premature infants and altered cognition‐ and emotion‐based disorders such as anxiety and attention deficit hyperactivity disorder (ADHD) in moderate to late preterm infants (Bhutta et al. 2002; Linnet et al. 2006; Novik et al. 2006; Mulder et al. 2009; Johnson et al. 2010; McLean et al. 2011). Guinea pig studies assessing prematurity and perinatal stress exposure have found similarly disrupted maturation of the oligodendrocyte lineage and reduced myelination, as well as dendrite and neurite developmental disruptions. As seen clinically, the deficits in white matter are commonly seen in the hippocampus, cortex, corpus callosum and cerebellum. Interestingly, postnatal studies have linked these disturbances to a number of behavioural deficits in exposed guinea pig offspring including increases in anxiety‐like behaviours in female offspring and ADHD‐like behaviours in male offspring (Bennett et al. 2016; Shaw et al. 2016; Cumberland et al. 2017a). The mechanism contributing to these adverse behavioural consequences remains unclear, but the overall process likely involves premature loss of placental factors that have sex‐dependent trophic effects on late gestation neurodevelopment. These findings suggest that a replacement approach may improve long‐term neurodevelopmental outcome. Current studies are evaluating neuroprotective benefits of replacing key placentally derived steroids, specifically progesterone and its neuroactive metabolite allopregnanolone, which are lost following preterm birth or reduced during times of stress using the guinea pig (Palliser et al. 2015; Crombie et al. 2017; Shaw et al. 2017b).
Programming of the HPA axis and stress‐related behaviours
The effects of excess glucocorticoids (maternal glucocorticoid exposure and maternal stress in pregnancy) on the programming of the HPA axis and stress‐related behaviour has been investigated using the long‐term programming effects of synthetic glucocorticoid (sGC) administration and chronic and acute maternal prenatal stress (for review see Moisiadis & Matthews, 2014a,b). From these studies, it has become clear that prenatal glucocorticoid exposure and maternal stress lead to profound long‐term changes in basal and activated HPA function as well as modified behaviours. The phenotypes are driven by dramatic changes in gene expression in the brain, pituitary and adrenal, which in turn appear to involve complex changes in epigenetic regulation.
The nature of these effects is highly dependent on the timing of exposure, sex and age of offspring, and in females the stage of reproductive cycle at which phenotype is assessed. Prenatal stress at GD50 resulted in increased anxiety behaviour in juvenile male offspring, and reduced ambulatory activity, reduced attention and elevated basal HPA activity in adult male offspring (Kapoor & Matthews, 2005, 2011). In contrast, prenatal stress at GD60 resulted in an increased cortisol response to stress in male adult offspring (Kapoor & Matthews, 2005). Interestingly the behavioural affects appeared to be modulated at least in part by testosterone. In adult female offspring, prenatal stress at GD60, but not GD50, resulted in lower basal cortisol levels (Kapoor & Matthews, 2008). Additional evidence that timing of exposure is critical to outcome is illustrated by the fact that prenatal stress at GD50 decreased spatial learning in adult male offspring, whereas the same stressor delivered at GD60 increased spatial learning (Kapoor et al. 2009). The effects of prenatal stress on programming of HPA function are sensitive to reproductive cycles in females, and the relatively long reproductive cycle in this species allows testing within each phase. Prenatal stress at GD50 or GD60 reduced the cortisol stress response during the oestrous phase, but not during the luteal phase of the cycle, in adult female offspring (Kapoor & Matthews, 2008). Effects of glucocorticoid exposures are age‐ and well as sex‐dependent. Exposure to sGC (betamethasone) during late gestation resulted in decreased cortisol response to a stressor in juvenile male offspring (Owen & Matthews, 2007a). On the other hand, sGC (dexamethasone) exposure resulted in a reduction in basal and stress‐induced cortisol levels in young adult male offspring (Liu et al. 2001). Interestingly, this effect was no longer observed in older males (Banjanin et al. 2004). In adult females, prenatal sGC (betamethasone or dexamethasone) exposure resulted in reduced basal HPA activity only in the luteal phase and increased basal HPA activity only in the oestrous phase of the reproductive cycle (Liu et al. 2001; Dunn et al. 2010). Finally, single versus multiple courses of sGC exposure have different effects on offspring HPA function. A single course of sGC in late gestation led to a reduced cortisol stress response in juvenile males (Dean et al. 2001), but in contrast, exposure to multiple courses of sGC led to decreased stress response in juvenile male offspring (Owen & Matthews, 2007a).
Transgenerational effects of sGC
Programming studies in the guinea pig have been extended to demonstrate transgenerational effects of fetal exposures to sGC and nutrient restriction on HPA function and behaviour, via both maternal and paternal transmission. Prenatal sGC exposure resulted in a blunted cortisol response to stress in male and female F2 offspring of treated mothers (i.e. maternal transmission), as well as altered activity in an open field (Iqbal et al. 2012). In addition, F2 males exhibited increased negative feedback sensitivity to glucocorticoids while females showed decreased feedback sensitivity (Iqbal et al. 2012). These effects are potentially due to the direct effects of sGC exposure on F2 gametes in F0 females, gametic (genetic) selection, or transgenerational transmission by other mechanisms such as epigenetic modifications of DNA methylation. Accumulating evidence points to the latter explanation. In a recent study, prenatal sGC treatment led to altered cortisol responses to stress and behaviours in female and male juvenile F2 and F3 offspring from both parental lines, demonstrating maternal and paternal transgenerational programming to F3 (Moisiadis et al. 2017). Interestingly, the endocrine and behavioural phenotypes were strongest in juvenile females following paternal transmission, strongly implicating transgenerational epigenetic transmission. Also demonstrating transgenerational programming, maternal undernutrition changed guinea pig heart structure and HPA function across two generations (Bertram et al. 2008). F1 male offspring showed elevated blood pressure, as well as increased thickness and mass of the left ventricular wall. These changes in the heart structure were carried over to the F2 male offspring. Maternal undernutrition also increased basal cortisol and altered HPA response to a stress challenge in both F1 and F2 offspring.
Programming of cardiovascular function
Cardiac response to stress
The maturity of the cardiovascular system of the fetal guinea pig is an advantage over other species for studying the effects of in utero stress on fetal heart development and the programming effects that ensue after birth. Studies have identified hypoxia‐induced increased gene/protein expression in inducible nitric oxide synthase (NOS) in fetal guinea pig heart ventricles (Thompson & Dong, 2005; Dong & Thompson, 2006; Thompson et al. 2009; Evans et al. 2012a) and increased expression (Dong & Thompson, 2006) and vasodilator contribution of endothelial NOS in the coronary circulation (Thompson et al. 2000), all of which will programme poor heart health after birth. Prenatal hypoxia increases other signalling factors such as the generation of lipid peroxide products (maldondialdehyde) (Evans et al. 2012a), proinflammatory cytokines (TNFα, IL‐6, IL‐1b) and matrix metalloproteinases (MMP2 and 9) (Oh et al. 2008b) in cardiac left ventricles of the term fetal guinea pig (Fig. 6). This may be mediated by oxidative stress via generation of reactive oxygen species as evidenced by the inhibitory effect of maternal administration of the antioxidant N‐acetylcysteine. Malondialdehyde levels of the fetal guinea pig heart were elevated under conditions of chronic intrauterine hypoxia and reversed to normoxic levels in the presence of N‐acetylcysteine (Evans et al. 2012a; Al‐Hasan et al. 2013). In addition, maternal N‐acetylcysteine reversed the hypoxia‐induced increase in fetal cardiac MMP9 (Evans et al. 2012a) protein levels and the decrease in fetal cardiac mitochondrial cytochrome c oxidase activity (Al‐Hasan et al. 2013). The decreased cytochrome c oxidase activity of hypoxic fetal guinea pig ventricles was sustained in hearts of prenatally hypoxic guinea pig offspring, suggesting mitochondrial programming in offspring hearts (Al‐Hasan et al. 2014).
Figure 6. Immunostaining of matrix metalloproteinase 9 (MMP9) of normoxic (A and C) and 14 day hypoxic (B and D) fetal guinea pig hearts.
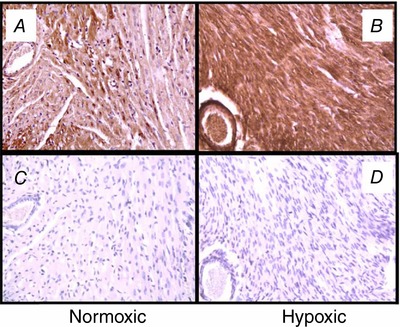
Positive immunostaining (brown stain) for MMP9 was localized in both cardiac tissue and blood vessels. Negative controls were generated in the absence of the primary antibody to MMP9 protein (C and D). Sections were counterstained with haematoxylin QS (Vector Laboratories). Original magnification: ×200. From Oh et al. (2008b) with permission.
Vascular function
Guinea pigs are born with both functional and well‐differentiated adrenergic nerves in the peripheral tissues (O'Donnell & Saar, 1975) comparable to humans (Armati‐Gulson & Burnstock, 1983). This is particularly important since emerging data suggests that ex‐preterm offspring, particularly females, have altered autonomic regulation of the cardiovascular system (Berry et al. 2013; Kim et al. 2014), which may contribute to their increased cardiovascular risk. Comparative studies in humans and guinea pigs have demonstrated similar profiles of vascular transition in both term‐ and preterm‐born newborns. In preterm animals born at GD62, as in preterm humans born prior to 29 weeks’ completed gestation, there is a period of high microvascular flow after birth, associated with central cardiovascular compromise, morbidity and mortality (Stark et al. 2008; Dyson et al. 2012, 2014) providing a model for studying cardiovascular transition at birth in preterm newborns, and the contribution of early microvascular compromise to adult cardiovascular disease in the ex‐preterm adult. Human studies now consistently demonstrate higher blood pressure in ex‐preterms than in controls (Hack et al. 2005), especially in females (Bonamy et al. 2005). Furthermore, this is associated with lower skin capillary density (Bonamy et al. 2007) and retinal microvascular changes (Kistner et al. 2002), which highlights the need for an appropriate animal model to interrogate the vascular mechanisms contributing to increased cardiovascular disease risk in the ex‐preterm.
As in humans, there is increased mean arterial blood pressure in adult rodents including guinea pigs that were growth restricted by experimentally induced reductions in uterine blood flow (Persson & Jansson, 1992; Battista et al. 2002). These changes in blood pressure are also associated with peripheral endothelial dysfunction and vascular remodelling in the aorta early in the neonatal period with progressively worsening impairment throughout life in human studies of IUGR (Yzydorczyk et al. 2017). Similarly, low birth weight adult guinea pigs have increased stiffness in conduit arteries (Thompson et al. 2011b) along with a decreased NOS‐mediated relaxation that is not further impaired by the exposure to an obesogenic diet (Thompson et al. 2014). This is preceded by fetal vascular changes. Aortae from growth‐restricted guinea pig fetuses show a decreased elastic lamina (Thompson et al. 2011b) as well as an increased stiffness, contractile force and media thickness (Canas et al. 2017). These changes are also observed in femoral resistance arteries (Canas et al. 2017). Additionally, these fetal vessels have a decreased NO‐dependent relaxation that is mainly related with an impaired endothelial function (Herrera et al. 2017). This model identifies signs of endothelial dysfunction in aorta, femoral and umbilical arteries after poor growth in utero (Herrera et al. 2016, 2017), findings that can be translated to clinical practice to predict systemic vascular dysfunction in the long term based on umbilical vascular impairment.
Studies in placental and umbilical vessels represent an important source of data for DOHaD that can be difficult to collect, especially in human. Studies in umbilical arteries from normal weight and growth restricted guinea pig fetuses show remarkable similarities with human umbilical arteries (Bruch et al. 1997; Burkhardt et al. 2009; Krause et al. 2013a) in their ex vivo vascular responses and remodelling. These differences between normal weight and growth restricted guinea pig fetuses include an impaired endothelial function (Herrera et al. 2017), reduced media thickness and decreased contractile force (Canas et al. 2017).
Programming of the lung
To date, limited studies have exploited the similarities in the temporal development of the lung between guinea pigs and humans. Guinea pigs delivered ‘late preterm’ (GD65/68; 96% completed gestation) require respiratory support (Berry et al. 2015) and exhibit transient respiratory distress, with evidence of acute lung injury (atelectasis, pulmonary oedema, fibrin deposition and inflammatory cell infiltration) 96 h after birth. This injury is exacerbated when preterm animals are exposed to high oxygen concentration (95% O2), and/or high tidal volume ventilation strategies as frequently experienced by neonates being resuscitated and receiving respiratory support in the NICU (Koshy et al. 2011). Together with similar timing of lung maturation between guinea pigs and humans (Sosenko & Frank, 1987; Kelly et al. 1991a), these similar acute outcomes in guinea pigs suggest that this species may be a suitable model for studying the long‐term consequences of the lung injury that follows preterm birth.
Programming of renal function
As is the case with human development, nephrogenesis is completed in late gestation in the guinea pig. Perinatal insults such as fetal growth restriction and preterm birth disrupt nephrogenesis, leading to altered renal structure and function in later life in both humans (Newsome et al. 2017) and guinea pigs (Briscoe et al. 2004). With the increasing burden of morbidity posed by chronic kidney disease, work in guinea pigs is ideal to assess the mechanisms linking perinatal insult to later morbidity as well as to assess the potential efficacy of therapeutic interventions in early or later life.
Programming of metabolic outcomes
A suitable animal model is needed to investigate the mechanisms underpinning the range of programmed metabolic dysfunction described in humans including obesity, insulin resistance, and perturbed cholesterol and glucose metabolism. Muscle development is extensively prenatal, similar to the human, and thus is vulnerable to developmental programming. Altered development of these metabolic tissues in the guinea pig fetus has been demonstrated in response to a perturbed in utero environment. For example, maternal feed restriction reduces relative weight of the fetal biceps brachii muscle (Dwyer et al. 1995; Kind et al. 2005), and reduces fibre numbers, particularly secondary fibres, in the biceps (Ward & Stickland, 1991; Dwyer & Stickland, 1992; Dwyer et al. 1995). This effect of maternal undernutrition on fetal muscle development differs between muscles, with fibre numbers in soleus muscle, a muscle with more slow‐twitch Type 1 fibres, not affected (Ward & Stickland, 1991; Dwyer & Stickland, 1992). Further postnatally, insulin sensitivity is reduced in low birth weight pups and the percentage of body weight is negatively correlated with birth weight (Davis & Auten, 2010).
Maternal feed restriction also reduces relative weight of fetal liver, but in contrast, increases relative weight of interscapular and retroperitoneal fats in GD60 fetuses (Kind et al. 2005). Adipocyte populations in fetal adipose tissue are also altered with an increased proportion of large lipid locules in the perirenal depot (Nguyen et al. 2010) and increased proportion of unilocular adipocytes in the interscapular depot, suggesting sparing of white adipose tissue at the expense of brown adipose tissue in the fetus of feed restricted mothers (Kind et al. 2005).
Postnatal growth and body composition
A number of studies, with differing prenatal or perinatal insults, have also reported altered postnatal adipose tissue development. Guinea pigs have been proposed as a good model for studying the effects of maternal obesity because the overnutrition results in increase adipose stores and decreasing fertility and litter size (Michel & Bonnet, 2012). Young adult males with low birth weight have increased relative epididymal fat weight, independent of changes in whole body adiposity, but accompanied by adipocyte hypertrophy, increased lipid storage and altered expression of genes involved in lipid metabolism in the visceral epididymal depot (Sarr et al. 2014). Similarly, relative retroperitoneal fat weight is increased in young adult male offspring of feed‐restricted mothers (Kind et al. 2003). Chronic prenatal ethanol exposure throughout gestation decreases birth weight, but increases total, visceral and subcutaneous adipose volumes, assessed by MRI, in young adult guinea pigs (Castaneda‐Gutierrez et al. 2011; Dobson et al. 2012a). A high fat diet during pregnancy and lactation also increases the percentage of body fat in offspring at 2 and 21 days of age, and following weaning onto a high fat diet increases relative weight of retroperitoneal fat in 145‐day‐old offspring (Castaneda‐Gutierrez et al. 2011). Other body fat depots did not differ in these adult offspring of fat‐fed mothers, and these depot‐specific effects of prenatal environment may reflect differences in the extent to which the fat depots undergo hyperplasia before birth in the guinea pig (Castaneda‐Gutierrez et al. 2011).
Changes in adiposity in young adult guinea pigs may also relate, at least in part, to alterations in feed intake. Relative energy intake from weaning to day 60 is higher in low birth weight male guinea pigs (Sarr et al. 2014). Similarly, feed intake is increased during the juvenile period (day 40–60) in IUGR offspring from larger litters. Offspring from larger litters also exhibit increased fractional growth rates in both the neonatal and juvenile periods, suggesting a change in partitioning of nutrients towards growth, and consistent with this, neonatal catch‐up growth predicts increased visceral adiposity in male offspring (Horton et al. 2016). Differences in postnatal growth, with low‐birth‐weight guinea pigs attaining similar weights to their non‐growth retarded counterparts, is also reported in other studies (Dobson et al. 2012a; Sarr et al. 2014; Nevin et al. 2018), including neonatal catch‐up growth (Dobson et al. 2012a; Nevin et al. 2018), which also occurs in humans. Other perinatal compromises also alter postnatal growth trajectories. For example, between term equivalent age and weaning ex‐preterm animals demonstrate increased weight and ponderal index relative to term‐born pups (Berry et al. 2015).
Cholesterol metabolism
Guinea pigs resemble humans in that they carry the majority of their cholesterol in low density lipoprotein (LDL), have a similar moderate level of hepatic cholesterol synthesis and catabolism, have plasma cholesterol ester transfer protein, lecithin:cholesterol acyltransferase and lipoprotein lipase activity, and exhibit similar sex‐based differences in plasma lipoprotein profiles (Fernandez & Volek, 2006; Fernandez, 2008). The guinea pig is also an appropriate model for studying hypercholesterolaemia (Aggarwal et al. 2006) and atherosclerosis (Fernandez & Volek, 2006; Mangathayaru et al. 2009) because of its moderate plasma cholesterol response and normal triglyceride response to a high fat–high cholesterol diet (deOgburn et al. 2012), and is a model for statin‐induced cholesterol lowering and myotoxicity (Madsen et al. 2008). Total plasma cholesterol is higher in male offspring of feed‐restricted mothers both before and after a 6‐week challenge with high dietary cholesterol (Kind et al. 1999). When offspring were divided according to birth size, plasma cholesterol levels did not differ when fed a standard diet, but low‐birth‐weight guinea pigs had higher total and LDL cholesterol levels when challenged with a high cholesterol diet (Kind et al. 1999).
Insulin sensitivity and glucose tolerance
The guinea pig provides a unique model to explore developmental programming of insulin‐regulated glucose metabolism, since its insulin is immunologically distinct from that of other species (Watt, 1985), which allows exogenous and endogenous insulin to be differentiated in assays. Because the insulin receptor of guinea pigs is less divergent from that of other species than the insulin molecule itself (Muggeo et al. 1979), glucose uptake can be stimulated by administering readily available insulin from other species, including recombinant human insulin. Hyperinsulinaemic euglycaemic clamps can be performed repeatedly in guinea pigs, and we have used this approach to show dose‐dependent insulin‐stimulated glucose uptake in this species (Horton et al. 2017). Although sensitivity to human insulin is lower in guinea pig than in humans, maximal glucose‐uptake responses are similar in both species (Horton et al. 2017). Guinea pig models of type 2 diabetes based on high‐fat–high‐carbohydrate feeding have been developed, and exhibit impaired glucose tolerance and compensatory hyperinsulinaemia (Podell et al. 2017). There is some evidence for programming of glucose metabolism by maternal nutrition in guinea pigs, as occurs in humans (Ravelli et al. 1998). Fasting insulin levels are increased and glucose tolerance is impaired in young adult male offspring of moderately feed‐restricted guinea pigs, when compared to offspring of mildly restricted mothers (Kind et al. 2003). When divided according to weight at birth, low‐birth‐weight males exhibit fasting hyperinsulinaemia, suggestive of insulin resistance, but no impairment of glucose tolerance compared to control offspring at this age (Kind et al. 2003). This area is relatively underexplored, however, with few studies having assessed developmental programming of glucose metabolism in the guinea pig, including at later adult ages when earlier insulin resistance may result in development of impaired glucose tolerance. Alterations in pancreatic β‐cell area and hepatic expression of insulin signalling genes occur in adult offspring following chronic prenatal ethanol exposure (Dobson et al. 2012a); however, the programming of insulin sensitivity and its tissue‐specific determinants in the guinea pig are an area requiring further study.
Programming of reproductive function
The physiological similarities in reproductive physiology between guinea pigs and humans make them an ideal experimental model to explore the reproductive consequences of an altered perinatal environment. For instance, postnatal growth is impacted by prenatal exposures, and body weight of non‐pregnant adult females correlates positively with number of corpora lutea (Eckstein & McKeown, 1955) as well as their subsequent litter sizes when mated (Horton et al. 2016); it is therefore likely that reproductive outcomes in females are programmed by many prenatal factors than modify fetal growth. Programming of male fertility and its determinants is a comparatively understudied field. However, some evidence for developmental programming of male reproduction has been reported for Cavia aperea, the ancestor of domestic guinea pigs, Cavia porcellus. In Cavia aperea, males whose mothers were exposed to unstable social environments (fortnightly regrouping with novel animals) throughout pregnancy and lactation had different developmental profiles of testosterone during development than F1 males from control dams (unchanged social group throughout pregnancy and lactation), including higher testosterone in early postnatal life (Siegeler et al. 2013). Other reproductive parameters including testis and accessory gland weights, sperm ploidy, motility and DNA fragmentation in the F1 males as adults at 107 days of age were, however, unchanged (Siegeler et al. 2013).
There is also direct, albeit limited, evidence for transmission of reproductive programming through both female and male lines in Cavia porcellus. Maternal undernutrition in early (days 1–35) or late (days 36–70) pregnancy did not affect age at first oestrous or mating in their F1 daughters, i.e. the generation that was in utero during maternal exposure (Bertram et al. 2008). However, daughters of dams that were nutrient restricted in late pregnancy took twice as long to become pregnant as daughters of unrestricted dams, with no effect of maternal undernutrition in early pregnancy on F1 age at first pregnancy (Bertram et al. 2008). Birth weight of male and female progeny was reduced at both F1 and F2 by late pregnancy undernutrition in the F0 dams (Bertram et al. 2008). Prenatal exposure to repeated maternal courses of the synthetic corticosteroids betamethasone or dexamethasone in the second half of gestation also did not alter the timing of reproductive maturation (first oestrous), nor did these affect cycle length (Dunn et al. 2010). Intriguingly, although betamethasone‐exposed F1 females took about twice as many cycles to conceive as controls, similar to effects of F0 pregnancy undernutrition, neither glucocorticoid altered litter size, gestation length or litter average birth weight in F1 pregnancies (Dunn et al. 2010). F0 repeated glucocorticoid exposure, in this case dexamethasone and not betamethasone, also altered sex balance at birth of the F2 generation, with a higher proportion of females in the F2 litters of F1 dams exposed to dexamethasone in utero (Dunn et al. 2010). In recently reported studies of transgenerational effects in this model, the lack of effect of repeated F0 betamethasone courses on reproductive parameters including age at conception, gestation length, litter size and sex ratio was confirmed for pregnancies in F0, F1 and F2 females in the maternal line (Moisiadis et al. 2017). Intriguingly F2 daughters of F1 sons from sGC‐exposed pregnancies did tend to have longer gestations than F2 daughters of control F1 males (Moisiadis et al. 2017).
Role of epigenetics in DOHaD and use of ‐omics data in guinea pigs
Epigenetics plays a crucial role in programming. Epigenetic mechanisms include DNA methylation/demethylation, histone post‐transcriptional modification, chromatin remodelling and non‐coding RNA. Recently, it has been demonstrated that fetal growth restriction in guinea pig fetuses is associated with changes to the DNA methylation pattern in the nos3 (eNOS) gene promoter (Herrera et al. 2017). This altered DNA methylation pattern occurs in endothelial cells from the aorta, umbilical and femoral arteries. Comparable findings have been reported in human umbilical artery endothelial cells from pregnancies affected by fetal growth restriction (Krause et al. 2013b).
There is accumulating evidence that epigenetic mechanisms are involved in the programming of long‐term endocrine and behavioural outcomes following prenatal glucocorticoid exposure. Studies have examined activation of the glucocorticoid receptor and its binding to glucocorticoid response elements that regulate a large number of genes. These studies have utilized multi‐dimensional omics analyses of mRNA with gene expression array, glucocorticoid receptor binding with chromatin immunoprecipitation and microarray hybridization (ChIP‐on‐chip), and DNA methylation with immunoprecipitation followed by qPCR (mDIP‐qPCR) (Crudo et al. 2012, 2013a,b). The endogenous glucocorticoid surge in late gestation as well as sGC exposure has a substantial impact on the hippocampal transcriptome, glucocorticoid receptor–DNA binding and the DNA methylation landscape in the fetal hippocampus at 24 h and 14 days following the sGC exposure. These data support the hypothesis that glucocorticoid exposure in late gestation plays a significant role in modifying the transcriptional and epigenetic landscapes of the developing fetal and juvenile hippocampus (Crudo et al. 2013a). Transcription factor binding has been mapped at promoters and enhancers associated with active transcription in adult hippocampi. Furthermore, reduced representation bisulfite sequencing and ChIP‐sequencing has been undertaken to examine the DNA methylation status of genomic regions that overlap with RNApolII at transcription start sites in promoters and H3K4me at enhancer regions (Boureau et al. 2018). Based on this map, a candidate gene approach has been utilized to assess DNA methylation levels using DNA capture to measure the effects of sGC during development. Recently, the same group have shown that sGC exposure during late gestation programmed transgenerational gene expression changes in the paraventricular nucleus of the hypothalamus using RNA‐seq (Moisiadis et al. 2017). These alterations included gene networks for type II diabetes, thermoregulation and collagen formation. Taken together, the guinea pig serves as an excellent model not only because it shares similar patterns of brain development with the human but also because it is accessible for the use of transcriptomic, genomic and epigenomic approaches.
Future directions for developing DOHaD interventions using guinea pig models
Pregnant guinea pigs have been used to study the effects of maternal infection on fetal development, such as by cytomegalovirus and listeria, because of their similar susceptibility and clinical outcomes to humans (Wadhwa Desai & Smith, 2017). This commonality between the human and guinea pig immune responses has also been applied to the development of vaccines for tuberculosis (Dey et al. 2009, 2011; Jain et al. 2011). However, compared to mice and rat models of DOHaD, guinea pigs have been relatively underutilised as a preclinical model in which to develop potential therapeutic fetal interventions. The reasons for this are likely to be multifactorial and pragmatic: the benefits of guinea pig models in terms of their long gestational age length, relatively small pup number and DOHaD outcomes are likely to have been underappreciated. Published studies of drug or surgical interventions in guinea pig pregnancy are few in number. Regulatory authorities currently lack familiarity with data from guinea pigs for decisions about novel therapeutics; this lack of familiarity will inevitably influence decisions about the choice of specific animal models (Swanson & David, 2015).
Despite their physiological advantages for DOHaD studies, guinea pigs are rarely used for reproductive toxicology studies of new drugs. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines govern reproductive toxicity studies in women and men (ICH, 2017). They specify that any programme should allow exposure of the novel chemical to all stages of development throughout one complete life cycle: for example, from conception in one generation to conception in the following generation. In practice, a number of overlapping studies are conducted to cover fertility and early embryonic development, pre‐ and postnatal development including lactation and weaning, and embryo–fetal development (Baldwin, 2009). Fertility and pre‐ and postnatal development studies need only be conducted in one mammalian species, which, primarily for logistical reasons, is usually the rat. For embryonic and fetal development two mammalian species are tested: one should be a rodent, often the rat for pragmatic reasons, and the other a non‐rodent, usually the rabbit, where there is a large body of historical data for comparison. Despite this historical precedent, the guinea pig affords a species that has a high degree of commonality in placentation and fetal developmental trajectory to humans; thus, we suggest that the increased housing and care costs of guinea pigs compared to rats are more than offset by their advantages as a superior model for pregnancy‐related drug development studies.
Some of the most challenging and complex areas of clinical research lie in understanding the late effects of adverse pregnancy outcome, especially preterm birth and fetal growth restriction. Robust guinea pig models have been developed that rely on the similarities between the guinea pig and human, across key developmental, neuroendocrine and metabolic characteristics, to mirror the physiological phenotype of human patients. These models are an important tool for advancing our understanding of the mechanisms underpinning the later pathophysiological complications of adverse pregnancy outcome, but will also enable the efficacy and safety of new therapeutics to be developed in a species with huge direct translational potential.
Therapeutic interventions to prevent/correct programming
Early studies investigated the effect of exercise on the adult guinea pig as a tool to evaluate cardiopulmonary function (Yilmaz et al. 2008), plasma and hepatic lipid profiles (Ensign et al. 2002), amino acid incorporation into skeletal muscle (McManus et al. 1975; Rogers et al. 1979) and hepatic glucose production in denervated livers (Wiersma et al. 1995), the latter because of rich liver innervation comparable to humans and in contrast to rat and dog. Treadmill exercise increased ventilation and CO2 output linearly with oxygen uptake, increased lung diffusing capacity with respect to pulmonary blood flow as well as membrane diffusing capacity and pulmonary capillary blood volume (Yilmaz et al. 2008). The functional recruitment of alveolar microvasculature with exercise was similar to that measured in dog and human (Yilmaz et al. 2008). Exercise improved plasma lipid profiles in adult guinea pigs with 33% lower triacylglycerol, 66% higher HDL cholesterol and 31% lower plasma free fatty acid levels compared to rest, similar to the effects on lipid profiles in humans (Ensign et al. 2002). In newborn guinea pigs, treadmill exercise of 1–3 weeks had no effect on lung growth or alveolar multiplication, indicating that the guinea pig alveoli are fully developed at the time of birth (Ross & Thurlbeck, 1992), different from rat or mice whose alveoli are absent at birth (Thurlbeck, 1975). Only two studies using the pregnant guinea pig have reported the effects of maternal exercise on the fetus (Nelson et al. 1983; Smith et al. 1983), although other species such as the sheep have been used (Lotgering et al. 1983). Maternal exercise of pregnant guinea pigs decreases fetal weight, kidney weight, placental weight and placental diffusing capacity (Nelson et al. 1983). The decrease in diffusing capacity is directly related to the maternal surface exchange area of the labyrinth and the total surface area of the placenta (Smith et al. 1983). This suggests that maternal exercise can reduce fetal weight by compromising placental development.
Prenatal interventions aimed at improving DOHaD outcomes have already been successfully evaluated in guinea pig pregnancy. Maternal treatment with the antioxidant N‐acetylcysteine (500 mg kg−1 day−1) restored fetal growth following experimentally induced growth restriction (Herrera et al. 2017). Similarly, maternal sildenafil (50 or 500 μg kg−1 day−1) from mid‐gestation until delivery protected pups against induced asphyxia at birth in a dose‐dependent manner, and the higher sildenafil dose increased fetal pup weight at term (Sanchez‐Aparicio et al. 2008) compared to untreated growth‐restricted pregnancies. Both N‐acetylcysteine and sildenafil are now being considered for the treatment approaches of fetal growth restriction in human pregnancy.
Targeting the uteroplacental circulation can be achieved in guinea pig pregnancy through laparotomy and external application of thermolabile pluronic gel containing therapeutic drugs to exposed uterine arteries and radial arteries (Mehta et al. 2016). Direct injection of the uterine arteries was associated with high fetal loss, while upstream injection of the internal iliac arteries did not target the uteroplacental circulation. Applying an adenoviral vascular endothelial growth factor vectors in combination with pluronic gel led to local transgenic vascular endothelial growth factor protein expression in transduced arteries, altered vascular reactivity and increased fetal growth in global maternal nutrient restriction FGR pregnancies (Swanson et al. 2016). In addition, atrial natriuretic peptide infusion selectively increased blood flow to placentas of FGR fetuses while placental blood flow of normal‐sized fetuses remained unchanged (Jansson, 1992). Many other exciting and novel therapeutic interventions to support cardiometabolic and neurodevelopmental health in preterm and/or growth‐restricted pups are also under evaluation as the awareness of guinea pigs as a translational model for DOHaD research continues to increase (Table 2).
Table 2.
Gaps in our knowledge that can be filled using guinea pig models
| Current uses of guinea pig models in DOHaD | What the guinea pig model can offer in future DOHaD studies |
| DOHaD models of common, clinically important conditions have been established in guinea pigs that result in similar outcomes as in humans | The commonality in physiology between guinea pigs and humans can be exploited much more aggressively in studies of reproductive toxicology, maternal medication exposure and therapeutic fetal interventions |
| Safety and efficacy studies of novel therapeutic interventions have been tested in guinea pigs and their results used to inform the development of human studies | Guinea pigs can enable assessments of long term outcomes, including multi‐ and trans‐generational outcomes as well as a rigorous assessment of the paternal contribution to DOHaD |
Limitations of guinea pigs as a model of DOHaD
Despite the many strengths of guinea pig models in DOHaD outlined in this review, it is important to note that there are some limitations. Guinea pigs are difficult to chronically catheterise and therefore it is difficult to invasively monitor and manipulate their intrauterine environment; such studies are more easily carried out in fetal sheep. Having made this point, elegant studies by Peeters's team used indwelling catheters to show fat uptake by the uterus and the fetus (Peeters et al. 1984, 1986). Molecular biology techniques are less developed than in mice and rats, requiring custom rather than ‘off the shelf’ solutions. It is feasible to design and optimize primers for gene expression studies, but there are instances where there are no available antibodies to measure protein abundance. Not all outcome measures that can be made in rats and mice can be currently made in guinea pigs; however, as more researchers with different interests and expertise enter the area, these experimental tools will be developed as we have seen with gene expression, cardiovascular function and behavioural testing to date. These limitations highlight the need for appropriate selection of animal models to answer each scientific question. The longer gestation length, time to F2 and F3 generations and husbandry requirements needed to maintain a guinea pig colony mean that guinea pig studies will inevitably cost more to run than those in rats or mice. However, guinea pig studies are still considerably cheaper to run than large animal or non‐human primate studies, with significant translational potential as described above to justify these slightly higher costs.
Guinea pigs are multiparous, producing more than one offspring at a birth. Litter‐bearing models of pregnancy or fetal studies can introduce an increased level of complexity to the statistical design of a study (Festing, 2006). With all litter‐bearing species, litter effects can be introduced when females, whole litters or siblings within a litter are assigned to the same group (Festing, 2006). The litter is the experimental unit, not the individual pup (Williams et al. 2017) and thus an adequate number of dams must be used. Additionally, the use of more advanced statistical methods such as restricted (or residual, or reduced) maximum likelihood (REML), mixed effects models and nested analysis can take into account a range of factors like maternal diet, weight, litter size, pup weight and sex within the statistical model (Wainwright et al. 2007). Employing these techniques will reduce intra‐litter variation making for more robust statistical interpretation during analysis of litter‐bearing animal models of pregnancy and development. For example, it would not be appropriate to use more than one pup of each sex at one time point in an analysis without nesting for litter (Lee et al. 2014).
Conclusion
In conclusion, the guinea pig is a versatile species in which the key facets of human pregnancy and fetal development are mirrored both in health and following clinically relevant perturbation. The relatively short life cycle, modest housing and husbandry requirements and docile temperament make it an ideal species to investigate reproductive issues and for the study of DOHaD. The evidence to date supports the notion that more interventional studies can be performed in guinea pigs to develop and test the efficacy of potential therapeutic interventions following high‐risk pregnancy. The guinea pig is also the ideal species to assess the multi‐ and transgenerational effects of both perinatal perturbation and any intervention. These translational data will inform clinical research and speed up the rate at which clinically important perinatal research can innovate and progress to safeguard the health of vulnerable pregnancies and vulnerable babies throughout their life course.
Additional information
Competing interests
The authors have no competing interests.
Author contributions (Fig. 7)
Figure 7. The authors.

Conception or design of the work: J.L.M., K.J.B., J.R.T.D., A.L.D., K.L.G., K.L.K., S.G.M., T.R.H.R., L.P.T., M.J.B. Acquisition or analysis or interpretation of data for the work: J.L.M., K.J.B, J.R.T.D., R.M.D., K.L.G., C.G., E.A.H., J.J.H., B.K., K.L.K., B.J.K., S.G.M., H.K.P., T.R.H.R., B.S.R., A.S., L.P.T., M.J.B. Drafting the work or revising it critically for important intellectual content: J.L.M., K.J.B, J.R.T.D., A.L.D., R.M.D., K.L.G., C.G., E.A.H., J.J.H., B.K., K.L.K., B.J.K., S.G.M., H.K.P., T.R.H.R., B.S.R., A.S., L.P.T., M.J.B. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
J.L.M. was funded by a NHMRC Career Development Fellowship (APP1066916). J.R.T.D. was funded by an Australian Government Research Training Program (RTP) scholarship. A.L.D. is supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre and included research funded by Action Medical Research and Rosetrees Trust. R.M.D. is supported by a University of Otago Health Sciences Career Development Postdoctoral Fellowship. E.A.H. is funded by The National Fund for Scientific and Technological Development (FONDECYT 1151119 and 1181341). B.J.K. is funded by Proyecto Puente P1714 2017, Pontificia Universidad Católica de Chile and The National Fund for Scientific and Technological Development (FONDECYT 1181341). L.P.T. is supported by the National Institutes of Health from the Heart Lung and Blood Institute (NIH HL126859).
Acknowledgements
The authors would like to acknowledge the contributions of our teams of students and early career researchers that have contributed to the work that we do in the area of developmental programming using guinea pig models.
Biography
The authors of this review represent laboratories, including clinicians, scientists, laboratory heads, post‐doctoral fellows and trainees, from around the world that study the developmental origins of health and disease (DOHaD). These groups use a range of animal models, one of which is the guinea pig, to understand why and how changes in the fetal and neonatal environment are associated with an increased risk of non‐communicable disease in adult life. Together, using guinea pigs as a model of human development, this work can answer important mechanistic questions about DOHaD and aid in the design and testing of interventions aimed at reducing the impact of non‐communicable disease on quality and quantity of life around the world.

Edited by: Ole Petersen & Laura Bennet
References
- Abedin N, Ashraf Q, Mishra OP & Delivoria‐Papadopoulos M (2005). Effect of hypoxia on the expression of pro‐ and anti‐apoptotic proteins in neuronal nuclei of the guinea pig fetus during gestation. Brain Res Dev 156, 32–37. [DOI] [PubMed] [Google Scholar]
- Agata N, Kato Y, Tanaka H & Shigenobu K (1994). Differential effects of hypoxia on electrical and mechanical activities of isolated ventricular muscles from fetal and adult guinea‐pigs. Gen Pharmacol 25, 15–18. [DOI] [PubMed] [Google Scholar]
- Agata N, Tanaka H & Shigenobu K (1993). Developmental changes in action potential properties of the guinea‐pig myocardium. Acta Physiol Scand 149, 331–337. [DOI] [PubMed] [Google Scholar]
- Aggarwal D, Freake HC, Soliman GA, Dutta A & Fernandez ML (2006). Validation of using gene expression in mononuclear cells as a marker for hepatic cholesterol metabolism. Lipids Health Dis 5, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Gubory KH, Solari A & Mirman B (2000). Effects of luteectomy on the maintenance of pregnancy, circulating progesterone concentrations and lambing performance in sheep. Reprod Fert Develop 11, 317–322. [DOI] [PubMed] [Google Scholar]
- Al‐Hasan YM, Evans LC, Pinkas GA, Dabkowski ER, Stanley WC & Thompson LP (2013). Chronic hypoxia impairs cytochrome oxidase activity via oxidative stress in selected fetal guinea pig organs. Reprod Sci 20, 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Hasan YM, Pinkas GA & Thompson LP (2014). Prenatal hypoxia reduces mitochondrial protein levels and cytochrome c oxidase activity in offspring guinea pig hearts. Reprod Sci 21, 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews MH, Kostaki A, Setiawan E, McCabe L & Matthews SG (2004). Developmental regulation of 5‐HT1A receptor mRNA in the fetal limbic system: response to antenatal glucocorticoid. Brain Res Dev 149, 39–44. [DOI] [PubMed] [Google Scholar]
- Armati‐Gulson P & Burnstock G (1983). The development of adrenergic innervation in some human foetal blood vessels. J Auton Nerv Syst 7, 111–118. [DOI] [PubMed] [Google Scholar]
- Ashwell M, Purkins L, Cowen T & Day KC (1987). Pre‐ and postnatal development of adipose tissue at four sites in the guinea pig: effect of maternal diet restriction during the second half of pregnancy. Ann Nutr Metab 31, 197–210. [DOI] [PubMed] [Google Scholar]
- Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ & Kinney HC (2001). Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci 21, 1302–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baello S, Iqbal M, Kearney S, Kuthiala S, Bloise E, Gibb W & Matthews SG (2016). Glucocorticoids modify effects of TGF‐β1 on multidrug resistance in the fetal blood‐brain barrier. Growth Factors 34, 33–41. [DOI] [PubMed] [Google Scholar]
- Baldwin J ( 2009). Reproductive and developmental toxicity In International Pharmaceutical Product Registration, 2nd edn, ed. Cartwright AC. & Matthews BR, pp. 429–440. Informa Healthcare, New York. [Google Scholar]
- Banjanin S, Kapoor A & Matthews SG (2004). Prenatal glucocorticoid exposure alters hypothalamic‐pituitary‐adrenal function and blood pressure in mature male guinea pigs. J Physiol 558, 305–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ (2004). The developmental origins of adult disease. J Am Coll Nutr 23, 588S–595S. [DOI] [PubMed] [Google Scholar]
- Barker DJ (2005). The developmental origins of insulin resistance. Horm Res 64(Suppl 3), 2–7. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Bull AR, Osmond C & Simmonds SJ (1990). Fetal and placental size and risk of hypertension in adult life. BMJ 301, 259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Eriksson JG, Forsen T & Osmond C (2002). Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 31, 1235–1239. [DOI] [PubMed] [Google Scholar]
- Barrie SE & Harris P (1977). Myocardial enzyme activities in guinea pigs during development. Am J Physiol 233, H707–H710. [DOI] [PubMed] [Google Scholar]
- Basilious A, Yager J & Fehlings MG (2015). Neurological outcomes of animal models of uterine artery ligation and relevance to human intrauterine growth restriction: a systematic review. Dev Med Child Neurol 57, 420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista MC, Oligny LL, St‐Louis J & Brochu M (2002). Intrauterine growth restriction in rats is associated with hypertension and renal dysfunction in adulthood. Am J Physiol Endocrinol Metab 283, E124–E131. [DOI] [PubMed] [Google Scholar]
- Bazer FW ( 2015). History of maternal recognition of pregnancy. Adv Anat Embryol Cell Biol 216, 5–25. [DOI] [PubMed] [Google Scholar]
- Bennett GA, Palliser HK, Saxby B, Walker DW & Hirst JJ (2013). Effects of prenatal stress on fetal neurodevelopment and responses to maternal neurosteroid treatment in guinea pigs. Dev Neurosci 35, 416–426. [DOI] [PubMed] [Google Scholar]
- Bennett GA, Palliser HK, Shaw JC, Palazzi KL, Walker DW & Hirst JJ (2017). Maternal stress in pregnancy affects myelination and neurosteroid regulatory pathways in the guinea pig cerebellum. Stress 20, 580–588. [DOI] [PubMed] [Google Scholar]
- Bennett GA, Palliser HK, Shaw JC, Walker D & Hirst JJ (2015). Prenatal stress alters hippocampal neuroglia and increases anxiety in childhood. Dev Neurosci 37, 533–545. [DOI] [PubMed] [Google Scholar]
- Bennett GA, Palliser HK, Walker D & Hirst J (2016). Severity and timing: How prenatal stress exposure affects glial developmental, emotional behavioural and plasma neurosteroid responses in guinea pig offspring. Psychoneuroendocrinology 70, 47–57. [DOI] [PubMed] [Google Scholar]
- Bernert G, Hoeger H, Mosgoeller W, Stolzlechner D & Lubec B (2003). Neurodegeneration, neuronal loss, and neurotransmitter changes in the adult guinea pig with perinatal asphyxia. Pediatr Res 54, 523–528. [DOI] [PubMed] [Google Scholar]
- Berry M, Gray C, Wright K, Dyson R & Wright I (2015). Premature guinea pigs: a new paradigm to investigate the late‐effects of preterm birth. J Dev Orig Health Dis 6, 143–148. [DOI] [PubMed] [Google Scholar]
- Berry MJ, Jaquiery AL, Oliver MH, Harding JE & Bloomfield FH (2013). Antenatal corticosteroid exposure at term increases adult adiposity: an experimental study in sheep. Acta Obstet Gynecol Scand 92, 862–865. [DOI] [PubMed] [Google Scholar]
- Bertram C, Khan O, Ohri S, Phillips DI, Matthews SG & Hanson MA (2008). Transgenerational effects of prenatal nutrient restriction on cardiovascular and hypothalamic‐pituitary‐adrenal function. J Physiol 586, 2217–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutta AT, Cleves MA, Casey PH, Cradock MM & Anand KJ (2002). Cognitive and behavioral outcomes of school‐aged children who were born preterm: a meta‐analysis. JAMA 288, 728–737. [DOI] [PubMed] [Google Scholar]
- Bloise E, Ortiga‐Carvalho TM, Reis FM, Lye SJ, Gibb W & Matthews SG (2016). ATP‐binding cassette transporters in reproduction: a new frontier. Hum Reprod Update 22, 164–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blutstein T, Castello MA, Viechweg SS, Hadjimarkou MM, McQuail JA, Holder M, Thompson LP & Mong JA (2013). Differential responses of hippocampal neurons and astrocytes to nicotine and hypoxia in the fetal guinea pig. Neurotox Res 24, 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonamy AK, Bendito A, Martin H, Andolf E, Sedin G & Norman M (2005). Preterm birth contributes to increased vascular resistance and higher blood pressure in adolescent girls. Pediatr Res 58, 845–849. [DOI] [PubMed] [Google Scholar]
- Bonamy AK, Martin H, Jorneskog G & Norman M (2007). Lower skin capillary density, normal endothelial function and higher blood pressure in children born preterm. J Intern Med 262, 635–642. [DOI] [PubMed] [Google Scholar]
- Bookhout CG ( 1946). The development of the guinea pig ovary from sexual differentiation to maturity. Anat Rec 94, 359. [PubMed] [Google Scholar]
- Bossi E & Greenberg RE (1972). Sources of blood glucose in the rat fetus. Pediatr Res 6, 765–772. [DOI] [PubMed] [Google Scholar]
- Boureau L, Constantinof A, Moisiadis V, Matthews S & Szyf M (2018). The DNA methylation landscape of enhancers in the guinea pig hippocampus. Epigenomics (in press; 10.2217/epi-2017-0064). [DOI] [PubMed] [Google Scholar]
- Briscoe TA, Rehn AE, Dieni S, Duncan JR, Wlodek ME, Owens JA & Rees SM (2004). Cardiovascular and renal disease in the adolescent guinea pig after chronic placental insufficiency. Am J Obstet Gynecol 191, 847–855. [DOI] [PubMed] [Google Scholar]
- Bruch JF, Sibony O, Benali K, Challier JC, Blot P & Nessmann C (1997). Computerized microscope morphometry of umbilical vessels from pregnancies with intrauterine growth retardation and abnormal umbilical artery Doppler. Hum Pathol 28, 1139–1145. [DOI] [PubMed] [Google Scholar]
- Buonocore G, Liberatori S, Bini L, Mishra OP, Delivoria‐Papadopoulos M, Pallini V & Bracci R (1999). Hypoxic response of synaptosomal proteins in term guinea pig fetuses. J Neurochem 73, 2139–2148. [PubMed] [Google Scholar]
- Burdge GC, Slater‐Jefferies J, Torrens C, Phillips ES, Hanson MA & Lillycrop KA (2007). Dietary protein restriction of pregnant rats in the F0 generation induces altered methylation of hepatic gene promoters in the adult male offspring in the F1 and F2 generations. Br J Nutr 97, 435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt T, Matter CM, Lohmann C, Cai H, Luscher TF, Zisch AH & Beinder E (2009). Decreased umbilical artery compliance and igf‐I plasma levels in infants with intrauterine growth restriction – implications for fetal programming of hypertension. Placenta 30, 136–141. [DOI] [PubMed] [Google Scholar]
- Canas D, Herrera EA, Garcia‐Herrera C, Celentano D & Krause BJ (2017). Fetal growth restriction induces heterogeneous effects on vascular biomechanical and functional properties in guinea pigs (Cavia porcellus). Front Physiol 8, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carberry AE, Colditz PB & Lingwood BE (2010). Body composition from birth to 4.5 months in infants born to non‐obese women. Pediatr Res 68, 84–88. [DOI] [PubMed] [Google Scholar]
- Carter AM (2007). Animal models of human placentation – a review. Placenta 28(Suppl A), S41–S47. [DOI] [PubMed] [Google Scholar]
- Carter AM, Enders AC, Jones CJ, Mess A, Pfarrer C, Pijnenborg R & Soma H (2006). Comparative placentation and animal models: patterns of trophoblast invasion – a workshop report. Placenta 27(Suppl A), S30–S33. [DOI] [PubMed] [Google Scholar]
- Carter AM, Kingston MJ, Han KK, Mazzuca DM, Nygard K & Han VK (2005). Altered expression of IGFs and IGF‐binding proteins during intrauterine growth restriction in guinea pigs. J Endocrinol 184, 179–189. [DOI] [PubMed] [Google Scholar]
- Castaneda‐Gutierrez E, Pouteau E, Pescia G, Moulin J, Aprikian O & Mace K (2011). The guinea pig as a model for metabolic programming of adiposity. Am J Clin Nutr 94, 1838S–1845S. [DOI] [PubMed] [Google Scholar]
- Challis JR, Heap RB & Illingworth DV (1971). Concentrations of oestrogen and progesterone in the plasma of non‐pregnant, pregnant and lactating guinea‐pigs. J Endocrinol 51, 333–345. [DOI] [PubMed] [Google Scholar]
- Chan SY, Andrews MH, Lingas R, McCabe CJ, Franklyn JA, Kilby MD & Matthews SG (2005). Maternal nutrient deprivation induces sex‐specific changes in thyroid hormone receptor and deiodinase expression in the fetal guinea pig brain. J Physiol 566, 467–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, So K, Kim E, Kim S & Jeon Y (2015). Immunoreactivity of neurogenic factor in the guinea pig brain after prenatal hypoxia. Ann Anat 200, 66–72. [DOI] [PubMed] [Google Scholar]
- Coleman H, Hirst J & Parkington HC (2013). The GABAA excitatory‐toinhibitory switch in the hippocampus of perinatal guinea‐pigs. In 40th Annual Meeting Fetal and Neonatal Physiological Society, September 1st–4th, 2013, Puerto Varas, Chile, Abstract 015.
- Cook MN, Marks GS, Vreman HJ, Nakatsu K, Stevenson DK & Brien JF (1997). Heme oxygenase activity and acute and chronic ethanol exposure in the hippocampus, frontal cerebral cortex, and cerebellum of the near‐term fetal guinea pig. Alcohol 14, 117–124. [DOI] [PubMed] [Google Scholar]
- Crombie GK, Palliser HK, Shaw JC & Hirst JJ (2017). Neurosteroid replacement therapy ameliorates behavioural deficits following perinatal stress in male guinea pig offspring. In 44th Annual Meeting Fetal and Neonatal Physiological Society, Osaka, Japan.
- Crudo A, Petropoulos S, Moisiadis VG, Iqbal M, Kostaki A, Machnes Z, Szyf M & Matthews SG (2012). Prenatal synthetic glucocorticoid treatment changes DNA methylation states in male organ systems: multigenerational effects. Endocrinology 153, 3269–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crudo A, Petropoulos S, Suderman M, Moisiadis VG, Kostaki A, Hallett M, Szyf M & Matthews SG (2013a). Effects of antenatal synthetic glucocorticoid on glucocorticoid receptor binding, DNA methylation, and genome‐wide mRNA levels in the fetal male hippocampus. Endocrinology 154, 4170–4181. [DOI] [PubMed] [Google Scholar]
- Crudo A, Suderman M, Moisiadis VG, Petropoulos S, Kostaki A, Hallett M, Szyf M & Matthews SG (2013b). Glucocorticoid programming of the fetal male hippocampal epigenome. Endocrinology 154, 1168–1180. [DOI] [PubMed] [Google Scholar]
- Csapo AI & Pulkkinen M (1978). Indispensability of the human corpus luteum in the maintenance of early pregnancy. Luteectomy evidence. Obstet Gynecol Surv 33, 69–81. [DOI] [PubMed] [Google Scholar]
- Csapo AI, Puri CP & Tarro S (1981). Relationship between timing of ovariectomy and maintenance of pregnancy in the guinea‐pig. Prostaglandins 22, 131–140. [DOI] [PubMed] [Google Scholar]
- Cumberland AL, Palliser HK, Crombie GK, Walker DW & Hirst JJ (2017a). Increased anxiety‐like phenotype in female guinea pigs following reduced neurosteroid exposure in utero. Int J Dev Neurosci 58, 50–58. [DOI] [PubMed] [Google Scholar]
- Cumberland AL, Palliser HK & Hirst JJ (2014). Increased placental neurosteroidogenic gene expression precedes poor outcome in the preterm guinea pig. J Dev Orig Health Dis 5, 74–78. [DOI] [PubMed] [Google Scholar]
- Cumberland AL, Palliser HK, Rani P, Walker DW & Hirst JJ (2017b). Effects of combined IUGR and prenatal stress on the development of the hippocampus in a fetal guinea pig model. J Dev Orig Health Dis 8, 584–596. [DOI] [PubMed] [Google Scholar]
- Davis JM & Auten RL (2010). Maturation of the antioxidant system and the effects on preterm birth. Semin Fetal Neonatal Med 15, 191–195. [DOI] [PubMed] [Google Scholar]
- Davis SR, Mepham TB & Lock KJ (1979). Relative importance of pre‐partum and post‐partum factors in the control of milk yield in the guinea‐pig. J Dairy Res 46, 613–621. [DOI] [PubMed] [Google Scholar]
- De Champlain J, Malmfors T, Olson L & Sachs C (1970). Ontogenesis of peripheral adrenergic neurons in the rat: pre‐ and postnatal observations. Acta Physiol Scand 80, 276–288. [DOI] [PubMed] [Google Scholar]
- Dean F & Matthews SG (1999). Maternal dexamethasone treatment in late gestation alters glucocorticoid and mineralocorticoid receptor mRNA in the fetal guinea pig brain. Brain Res 846, 253–259. [DOI] [PubMed] [Google Scholar]
- Dean F, Yu C, Lingas RI & Matthews SG (2001). Prenatal glucocorticoid modifies hypothalamo‐pituitary‐adrenal regulation in prepubertal guinea pigs. Neuroendocrinology 73, 194–202. [DOI] [PubMed] [Google Scholar]
- deOgburn R, Leite JO, Ratliff J, Volek JS, McGrane MM & Fernandez ML (2012). Effects of increased dietary cholesterol with carbohydrate restriction on hepatic lipid metabolism in guinea pigs. Comp Med 62, 109–115. [PMC free article] [PubMed] [Google Scholar]
- Detmer A, Gu W & Carter AM (1991). The blood supply to the heart and brain in the growth retarded guinea pig fetus. J Dev Physiol 15, 153–160. [PubMed] [Google Scholar]
- Dey B, Jain R, Gupta UD, Katoch VM, Ramanathan VD & Tyagi AK (2011). A booster vaccine expressing a latency‐associated antigen augments BCG induced immunity and confers enhanced protection against tuberculosis. PLoS One 6, e23360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey B, Jain R, Khera A, Rao V, Dhar N, Gupta UD, Katoch VM, Ramanathan VD & Tyagi AK (2009). Boosting with a DNA vaccine expressing ESAT‐6 (DNAE6) obliterates the protection imparted by recombinant BCG (rBCGE6) against aerosol Mycobacterium tuberculosis infection in guinea pigs. Vaccine 28, 63–70. [DOI] [PubMed] [Google Scholar]
- Dickinson H, Moss TJ, Gatford KL, Moritz KM, Akison L, Fullston T, Hryciw DH, Maloney CA, Morris MJ, Wooldridge AL, Schjenken JE, Robertson SA, Waddell BJ, Mark PJ, Wyrwoll CS, Ellery SJ, Thornburg KL, Muhlhausler BS & Morrison JL (2016). A review of fundamental principles for animal models of DOHaD research: an Australian perspective. J Dev Orig Health Dis 7, 449–472. [DOI] [PubMed] [Google Scholar]
- Dieni S & Rees S (2003). Dendritic morphology is altered in hippocampal neurons following prenatal compromise. J Neurobiol 55, 41–52. [DOI] [PubMed] [Google Scholar]
- Dieni S & Rees S (2005). BDNF and TrkB protein expression is altered in the fetal hippocampus but not cerebellum after chronic prenatal compromise. Exp Neurol 192, 265–273. [DOI] [PubMed] [Google Scholar]
- Dobbing J & Sands J (1970). Growth and development of the brain and spinal cord of the guinea pig. Brain Res 17, 115–123. [DOI] [PubMed] [Google Scholar]
- Dobson CC, Mongillo DL, Brien DC, Stepita R, Poklewska‐Koziell M, Winterborn A, Holloway AC, Brien JF & Reynolds JN (2012a). Chronic prenatal ethanol exposure increases adiposity and disrupts pancreatic morphology in adult guinea pig offspring. Nutr Diabetes 2, e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson CC, Mongillo DL, Poklewska‐Koziell M, Winterborn A, Brien JF & Reynolds JN (2012b). Sensitivity of modified Biel‐maze task, compared with Y‐maze task, to measure spatial learning and memory deficits of ethanol teratogenicity in the guinea pig. Behav Brain Res 233, 162–168. [DOI] [PubMed] [Google Scholar]
- Dong Y & Thompson LP (2006). Differential expression of endothelial nitric oxide synthase in coronary and cardiac tissue in hypoxic fetal guinea pig hearts. J Soc Gynecol Investig 13, 483–490. [DOI] [PubMed] [Google Scholar]
- Dong Y, Yu Z, Sun Y, Zhou H, Stites J, Newell K & Weiner CP (2011). Chronic fetal hypoxia produces selective brain injury associated with altered nitric oxide synthases. Am J Obstet Gynecol 204, 254.e16–254.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn E, Kapoor A, Leen J & Matthews SG (2010). Prenatal synthetic glucocorticoid exposure alters hypothalamic–pituitary–adrenal regulation and pregnancy outcomes in mature female guinea pigs. J Physiol 588, 887–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer CM, Madgwick AJ, Ward SS & Stickland NC (1995). Effect of maternal undernutrition in early gestation on the development of fetal myofibres in the guinea‐pig. Reprod Fertil Dev 7, 1285–1292. [DOI] [PubMed] [Google Scholar]
- Dwyer CM & Stickland NC (1992). Does the anatomical location of a muscle affect the influence of undernutrition on muscle fibre number? J Anat 181, 373–376. [PMC free article] [PubMed] [Google Scholar]
- Dwyer CM & Stickland NC (1994). Supplementation of a restricted maternal diet with protein or carbohydrate alone prevents a reduction in fetal muscle fibre number in the guinea‐pig. Br J Nutr 72, 173–180. [DOI] [PubMed] [Google Scholar]
- Dyson RM, Palliser HK, Kelleher MA, Hirst JJ & Wright IM (2012). The guinea pig as an animal model for studying perinatal changes in microvascular function. Pediatr Res 71, 20–24. [DOI] [PubMed] [Google Scholar]
- Dyson RM, Palliser HK, Lakkundi A, de Waal K, Latter JL, Clifton VL & Wright IM (2014). Early microvascular changes in the preterm neonate: a comparative study of the human and guinea pig. Physiol Rep 2, e12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson RM, Palliser HK, Latter JL, Kelly MA, Chwatko G, Glowacki R & Wright IM (2015). Interactions of the gasotransmitters contribute to microvascular tone (dys)regulation in the preterm neonate. PLoS One 10, e0121621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein P & McKeown T (1955). The influence of maternal age, parity and weight on litter size in the guinea‐pig. J Endocrinol 12, 115–119. [DOI] [PubMed] [Google Scholar]
- Elias AA, Ghaly A, Matushewski B, Regnault TR & Richardson BS (2016). Maternal nutrient restriction in guinea pigs as an animal model for inducing fetal growth restriction. Reprod Sci 23, 219–227. [DOI] [PubMed] [Google Scholar]
- Elias AA, Maki Y, Matushewski B, Nygard K, Regnault TRH & Richardson BS (2017). Maternal nutrient restriction in guinea pigs leads to fetal growth restriction with evidence for chronic hypoxia. Pediatr Res 82, 141–147. [DOI] [PubMed] [Google Scholar]
- Emack J, Kostaki A, Walker CD & Matthews SG (2008). Chronic maternal stress affects growth, behaviour and hypothalamo‐pituitary‐adrenal function in juvenile offspring. Horm Behav 54, 514–520. [DOI] [PubMed] [Google Scholar]
- Emack J & Matthews SG (2011). Effects of chronic maternal stress on hypothalamo‐pituitary‐adrenal (HPA) function and behavior: no reversal by environmental enrichment. Horm Behav 60, 589–598. [DOI] [PubMed] [Google Scholar]
- Ensign WY, McNamara DJ & Fernandez ML (2002). Exercise improves plasma lipid profiles and modifies lipoprotein composition in guinea pigs. J Nutr Biochem 13, 747–753. [DOI] [PubMed] [Google Scholar]
- Evans LC, Liu H, Pinkas GA & Thompson LP (2012a). Chronic hypoxia increases peroxynitrite, MMP9 expression, and collagen accumulation in fetal guinea pig hearts. Pediatr Res 71, 25–31. [DOI] [PubMed] [Google Scholar]
- Evans LC, Liu H & Thompson LP (2012b). Differential effect of intrauterine hypoxia on caspase 3 and DNA fragmentation in fetal guinea pig hearts and brains. Reprod Sci 19, 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez ML & Volek JS (2006). Guinea pigs: a suitable animal model to study lipoprotein metabolism, atherosclerosis and inflammation. Nutr Metab 3, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez ML, Wood RJ (2008). Guinea pigs as models for human cholesterol and lipoprotein metabolism In Sourcebook of Models for Biomedical Research, ed. Conn PM, pp. 201–212. Humana Press, Totowa, NJ, USA. [Google Scholar]
- Festing MF (2006). Design and statistical methods in studies using animal models of development. ILAR J 47, 5–14. [DOI] [PubMed] [Google Scholar]
- Forabosco A & Sforza C (2007). Establishment of ovarian reserve: a quantitative morphometric study of the developing human ovary. Fertil Steril 88, 675–683. [DOI] [PubMed] [Google Scholar]
- Forsdahl A (1977). Are poor living conditions in childhood and adolescence an important risk factor for arteriosclerotic heart disease? Br J Prev Soc Med 31, 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman WF (1972). The intrinsic physiologic properties of the developing heart. Prog Cardiovasc Dis 15, 87–111. [DOI] [PubMed] [Google Scholar]
- Gluckman PD & Hanson MA (2004). Maternal constraint of fetal growth and its consequences. Semin Fetal Neonatal Med 9, 419–425. [DOI] [PubMed] [Google Scholar]
- Go KS, Lingas R, Wheeler MB, Irwin DM & Matthews SG (2001). Decreased CRH mRNA expression in the fetal guinea pig hypothalamus following maternal nutrient restriction. Brain Res 896, 179–182. [DOI] [PubMed] [Google Scholar]
- Gomez‐Lopez N, Tong WC, Arenas‐Hernandez M, Tanaka S, Hajar O, Olson DM, Taggart MJ & Mitchell BF (2015). Chemotactic activity of gestational tissues through late pregnancy, term labor, and RU486‐induced preterm labor in Guinea pigs. Am J Reprod Immunol 73, 341–352. [DOI] [PubMed] [Google Scholar]
- Graham E, Mishra OP & Delivoria‐Papadopoulos M (1993). Brain cell membrane Na+,K+‐ATPase modification following hypoxia in the guinea pig fetus. Neurosci Lett 153, 93–97. [DOI] [PubMed] [Google Scholar]
- Graham E, Mishra OP & Delivoria‐Papadopoulos M (1995). Effect of in utero hypoxia on the ouabain/strophanthidin binding site of the fetal guinea pig brain cell membrane Na+,K+‐ATPase. Neurosci Lett 185, 159–162. [DOI] [PubMed] [Google Scholar]
- Guo R, Hou W, Dong Y, Yu Z, Stites J & Weiner CP (2010). Brain injury caused by chronic fetal hypoxemia is mediated by inflammatory cascade activation. Reprod Sci 17, 540–548. [DOI] [PubMed] [Google Scholar]
- Hack M, Schluchter M, Cartar L & Rahman M (2005). Blood pressure among very low birth weight (<1.5 kg) young adults. Pediatr Res 58, 677–684. [DOI] [PubMed] [Google Scholar]
- Harrell MI, Burnside K, Whidbey C, Vornhagen J, Adams Waldorf KM & Rajagopal L (2017). Exploring the pregnant guinea pig as a model for group B streptococcus intrauterine infection. J Infect Dis Med 2, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Pinkas G, Evans L, Liu H, Al‐Hasan Y & Thompson LP (2012). Protective effect of N‐acetylcysteine on liver damage during chronic intrauterine hypoxia in fetal guinea pig. Reprod Sci 19, 1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heap RB & Deanesly R (1966). Progesterone in systemic blood and placentae of intact and ovariectomized pregnant guinea‐pigs. J Endocrinol 34, 417–423. [DOI] [PubMed] [Google Scholar]
- Herrera EA, Alegria R, Farias M, Diaz‐Lopez F, Hernandez C, Uauy R, Regnault TR, Casanello P & Krause BJ (2016). Assessment of in vivo fetal growth and placental vascular function in a novel intrauterine growth restriction model of progressive uterine artery occlusion in guinea pigs. J Physiol 594, 1553–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera EA, Cifuentes‐Zuniga F, Figueroa E, Villanueva C, Hernandez C, Alegria R, Arroyo‐Jousse V, Penaloza E, Farias M, Uauy R, Casanello P & Krause BJ (2017). N‐Acetylcysteine, a glutathione precursor, reverts vascular dysfunction and endothelial epigenetic programming in intrauterine growth restricted guinea pigs. J Physiol 595, 1077–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hew KW & Keller KA (2003). Postnatal anatomical and functional development of the heart: a species comparison. Birth Defects Res B Dev Reprod Toxicol 68, 309–320. [DOI] [PubMed] [Google Scholar]
- Hewitt AJ, Dobson CC, Brien JF, Wynne‐Edwards KE & Reynolds JN (2014). Chronic ethanol exposure increases the non‐dominant glucocorticoid, corticosterone, in the near‐term pregnant guinea pig. Alcohol 48, 477–481. [DOI] [PubMed] [Google Scholar]
- Hewitt AJ, Knuff AL, Jefkins MJ, Collier CP, Reynolds JN & Brien JF (2011). Chronic ethanol exposure and folic acid supplementation: fetal growth and folate status in the maternal and fetal guinea pig. Reprod Toxicol 31, 500–506. [DOI] [PubMed] [Google Scholar]
- Hewitt AJ, Walker KR, Kobus SM, Poklewska‐Koziell M, Reynolds JN & Brien JF (2010). Differential effects of chronic ethanol exposure on cytochrome P450 2E1 and the hypothalamic‐pituitary‐adrenal axis in the maternal‐fetal unit of the guinea pig. Neurotoxicol Teratol 32, 164–170. [DOI] [PubMed] [Google Scholar]
- Hodyl NA, Stark MJ, Butler M & Clifton VL (2013). Placental P‐glycoprotein is unaffected by timing of antenatal glucocorticoid therapy but reduced in SGA preterm infants. Placenta 34, 325–330. [DOI] [PubMed] [Google Scholar]
- Hoerter JA, Ventura‐Clapier R & Kuznetsov A (1994). Compartmentation of creatine kinases during perinatal development of mammalian heart. Mol Cell Biochem 133–134, 277–286. [DOI] [PubMed] [Google Scholar]
- Horton DM, Saint DA, Owens JA, Gatford KL & Kind KL (2017). Use of the hyperinsulinemic euglycemic clamp to assess insulin sensitivity in guinea pigs: dose response, partitioned glucose metabolism, and species comparisons. Am J Physiol Regul Integr Comp Physiol 313, R19–R28. [DOI] [PubMed] [Google Scholar]
- Horton DM, Saint DA, Owens JA, Kind KL & Gatford KL (2016). Spontaneous intrauterine growth restriction due to increased litter size in the guinea pig programmes postnatal growth, appetite and adult body composition. J Dev Orig Health Dis 7, 548–562. [DOI] [PubMed] [Google Scholar]
- Houin SS, Rozance PJ, Brown LD, Hay WW Jr, Wilkening RB & Thorn SR (2015). Coordinated changes in hepatic amino acid metabolism and endocrine signals support hepatic glucose production during fetal hypoglycemia. Am J Physiol Endocrinol Metab 308, E306–E314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt AN, Kelly FJ & Postle AD (1991). Developmental variation in whole human lung phosphatidylcholine molecular species: a comparison with guinea pig and rat. Early Hum Dev 25, 157–171. [DOI] [PubMed] [Google Scholar]
- Hunter DS, Hazel SJ, Kind KL, Owens JA, Pitcher JB & Gatford KL (2016). Programming the brain: common outcomes and gaps in knowledge from animal studies of IUGR. Physiol Behav 164, 233–248. [DOI] [PubMed] [Google Scholar]
- Ibsen HL (1928). Prenatal growth in guinea‐pigs with special reference to environmental factors affecting weight at birth. J Exp Zool 51, 51–94. [Google Scholar]
- Illingworth DV, Challis JR & Henville A (1971). Plasma levels of progesterone and oestrogen in the pregnant guinea‐pig. J Reprod Fertil 25, 305–306. [DOI] [PubMed] [Google Scholar]
- Iqbal M, Baello S, Javam M, Audette MC, Gibb W & Matthews SG (2016). Regulation of multidrug resistance P‐glycoprotein in the developing blood‐brain barrier: interplay between glucocorticoids and cytokines. J Neuroendocrinol 28, 12360. [DOI] [PubMed] [Google Scholar]
- Iqbal M, Moisiadis VG, Kostaki A & Matthews SG (2012). Transgenerational effects of prenatal synthetic glucocorticoids on hypothalamic‐pituitary‐adrenal function. Endocrinology 153, 3295–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal U, Brien JF, Banjanin S, Andrews MH, Matthews SG & Reynolds JN (2005). Chronic prenatal ethanol exposure alters glucocorticoid signalling in the hippocampus of the postnatal guinea pig. J Neuroendocrinol 17, 600–608. [DOI] [PubMed] [Google Scholar]
- Iqbal U, Brien JF, Kapoor A, Matthews SG & Reynolds JN (2006). Chronic prenatal ethanol exposure increases glucocorticoid‐induced glutamate release in the hippocampus of the near‐term foetal guinea pig. J Neuroendocrinol 18, 826–834. [DOI] [PubMed] [Google Scholar]
- Iqbal U, Dringenberg HC, Brien JF & Reynolds JN (2004). Chronic prenatal ethanol exposure alters hippocampal GABAA receptors and impairs spatial learning in the guinea pig. Behav Brain Res 150, 117–125. [DOI] [PubMed] [Google Scholar]
- Jain R, Dey B, Khera A, Srivastav P, Gupta UD, Katoch VM, Ramanathan VD & Tyagi AK (2011). Over‐expression of superoxide dismutase obliterates the protective effect of BCG against tuberculosis by modulating innate and adaptive immune responses. Vaccine 29, 8118–8125. [DOI] [PubMed] [Google Scholar]
- Jansson T & Persson E (1990). Placental transfer of glucose and amino acids in intrauterine growth retardation: studies with substrate analogs in the awake guinea pig. Pediatr Res 28, 203–208. [DOI] [PubMed] [Google Scholar]
- Jansson T, Thordstein M & Kjellmer I (1986). Placental blood flow and fetal weight following uterine artery ligation. Temporal aspects of intrauterine growth retardation in the guinea pig. Biol Neonate 49, 172–180. [DOI] [PubMed] [Google Scholar]
- Jansson TB (1992). Low‐dose infusion of atrial natriuretic peptide in the conscious guinea pig increases blood flow to the placenta of growth‐retarded fetuses. Am J Obstet Gynecol 166, 213–218. [DOI] [PubMed] [Google Scholar]
- Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D & Marlow N (2010). Psychiatric disorders in extremely preterm children: longitudinal finding at age 11 years in the EPICure study. J Am Acad Child Adolesc Psychiatry 49, 453–463.e1. [PubMed] [Google Scholar]
- Jones CT & Ashton IK (1976). The appearance, properties, and functions of gluconeogenic enzymes in the liver and kidney of the guinea pig during fetal and early neonatal development. Arch Biochem Biophys 174, 506–522. [DOI] [PubMed] [Google Scholar]
- Jones CT, Lafeber HN, Price DA & Parer JT (1987). Studies on the growth of the fetal guinea pig. Effects of reduction in uterine blood flow on the plasma sulphation‐promoting activity and on the concentration of insulin‐like growth factors‐I and ‐II. J Dev Physiol 9, 181–201. [PubMed] [Google Scholar]
- Jones CT, Lafeber HN & Roebuck MM (1984). Studies on the growth of the fetal guinea pig. Changes in plasma hormone concentration during normal and abnormal growth. J Dev Physiol 6, 461–472. [PubMed] [Google Scholar]
- Jones CT & Parer JT (1983). The effect of alterations in placental blood flow on the growth of and nutrient supply to the fetal guinea‐pig. J Physiol 343, 525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CT & Rolph TP (1985). Metabolism during fetal life: a functional assessment of metabolic development. Physiol Rev 65, 357–430. [DOI] [PubMed] [Google Scholar]
- Kalabis GM, Petropoulos S, Gibb W & Matthews SG (2009). Multidrug resistance phosphoglycoprotein (ABCB1) expression in the guinea pig placenta: developmental changes and regulation by betamethasone. Can J Physiol Pharmacol 87, 973–978. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Kostaki A, Janus C & Matthews SG (2009). The effects of prenatal stress on learning in adult offspring is dependent on the timing of the stressor. Behav Brain Res 197, 144–149. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Leen J & Matthews SG (2008). Molecular regulation of the hypothalamic‐pituitary‐adrenal axis in adult male guinea pigs after prenatal stress at different stages of gestation. J Physiol 586, 4317–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A & Matthews SG (2005). Short periods of prenatal stress affect growth, behaviour and hypothalamo‐pituitary‐adrenal axis activity in male guinea pig offspring. J Physiol 566, 967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A & Matthews SG (2008). Prenatal stress modifies behavior and hypothalamic‐pituitary‐adrenal function in female guinea pig offspring: effects of timing of prenatal stress and stage of reproductive cycle. Endocrinology 149, 6406–6415. [DOI] [PubMed] [Google Scholar]
- Kapoor A & Matthews SG (2011). Testosterone is involved in mediating the effects of prenatal stress in male guinea pig offspring. J Physiol 589, 755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsetos CD, Spandou E, Legido A, Taylor ML, Zanelli SA, de Chadarevian JP, Christakos S, Mishra OP & Delivoria‐Papadopoulos M (2001). Acute hypoxia‐induced alterations of calbindin‐D28k immunoreactivity in cerebellar Purkinje cells of the guinea pig fetus at term. J Neuropathol Exp Neurol 60, 470–482. [DOI] [PubMed] [Google Scholar]
- Kaufmann P, Black S & Huppertz B (2003). Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod 69, 1–7. [DOI] [PubMed] [Google Scholar]
- Kelleher MA, Hirst JJ & Palliser HK (2013). Changes in neuroactive steroid concentrations after preterm delivery in the guinea pig. Reprod Sci 20, 1365–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher MA, Palliser HK, Walker DW & Hirst JJ (2011). Sex‐dependent effect of a low neurosteroid environment and intrauterine growth restriction on foetal guinea pig brain development. J Endocrinol 208, 301–309. [DOI] [PubMed] [Google Scholar]
- Kelly FJ, Rickett GM, Hunt AN, Town GI, Holgate ST & Postle TD (1991a). Biochemical maturation of the guinea pig lung and survival following premature delivery. Int J Biochem 23, 467–471. [DOI] [PubMed] [Google Scholar]
- Kelly FJ, Town GI, Phillips GJ, Holgate ST, Roche WR & Postle AD (1991b). The pre‐term guinea‐pig: a model for the study of neonatal lung disease. Clin Sci 81, 439–446. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Esclapez M, Caillard O, Bernard C, Khalilov I, Tyzio R, Hirsch J, Dzhala V, Berger B & Ben‐Ari Y (2001). Early development of neuronal activity in the primate hippocampus in utero. J Neurosci 21, 9770–9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Choi IY, Dong Y, Wang WT, Brooks WM, Weiner CP & Lee P (2015). Chronic fetal hypoxia affects axonal maturation in guinea pigs during development: a longitudinal diffusion tensor imaging and T2 mapping study. J Magn Reson Imaging 42, 658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Finch AM, Lumbers ER, Boyce AC, Gibson KJ, Eiby YA & Lingwood BE (2014). Expression of adrenoceptor subtypes in preterm piglet heart is different to term heart. PLoS One 9, e92167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kind KL, Clifton PM, Grant PA, Owens PC, Sohlstrom A, Roberts CT, Robinson JS & Owens JA (2003). Effect of maternal feed restriction during pregnancy on glucose tolerance in the adult guinea pig. Am J Physiol Regul Integr Comp Physiol 284, R140–R152. [DOI] [PubMed] [Google Scholar]
- Kind KL, Clifton PM, Katsman AI, Tsiounis M, Robinson JS & Owens JA (1999). Restricted fetal growth and the response to dietary cholesterol in the guinea pig. Am J Physiol 277, R1675–R1682. [DOI] [PubMed] [Google Scholar]
- Kind KL, Roberts CT, Sohlstrom AI, Katsman A, Clifton PM, Robinson JS & Owens JA (2005). Chronic maternal feed restriction impairs growth but increases adiposity of the fetal guinea pig. Am J Physiol Regul Integr Comp Physiol 288, R119–R126. [DOI] [PubMed] [Google Scholar]
- Kind KL, Simonetta G, Clifton PM, Robinson JS & Owens JA (2002). Effect of maternal feed restriction on blood pressure in the adult guinea pig. Exp Physiol 87, 469–477. [DOI] [PubMed] [Google Scholar]
- Kirtland J & Pavey DE (1981). Body lipids of guinea pigs exposed to different dietary fats from mid‐gestation to 3 months of age. IV. Effect of food restriction at 3 months on the distribution and cellularity of adipose tissue. Ann Nutr Metab 25, 165–177. [DOI] [PubMed] [Google Scholar]
- Kistner A, Jacobson L, Jacobson SH, Svensson E & Hellstrom A (2002). Low gestational age associated with abnormal retinal vascularization and increased blood pressure in adult women. Pediatr Res 51, 675–680. [DOI] [PubMed] [Google Scholar]
- Konje JC, Howarth ES, Kaufmann P & Taylor DJ (2003). Longitudinal quantification of uterine artery blood volume flow changes during gestation in pregnancies complicated by intrauterine growth restriction. BJOG 110, 301–305. [PubMed] [Google Scholar]
- Koshy S, Beard LL, Kuzenko SR, Li T & Folkesson HG (2011). Lung fluid absorption is induced in preterm guinea pigs ventilated with low tidal volumes. Exp Lung Res 37, 44–56. [DOI] [PubMed] [Google Scholar]
- Krause BJ, Carrasco‐Wong I, Caniuguir A, Carvajal J, Farias M & Casanello P (2013a). Endothelial eNOS/arginase imbalance contributes to vascular dysfunction in IUGR umbilical and placental vessels. Placenta 34, 20–28. [DOI] [PubMed] [Google Scholar]
- Krause BJ, Costello PM, Munoz‐Urrutia E, Lillycrop KA, Hanson MA & Casanello P (2013b). Role of DNA methyltransferase 1 on the altered eNOS expression in human umbilical endothelium from intrauterine growth restricted fetuses. Epigenetics 8, 944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause BJ, Herrera EA, Diaz‐Lopez FA, Farias M, Uauy R & Casanello P (2016). Pre‐gestational overweight in guinea pig sows induces fetal vascular dysfunction and increased rate of large and small fetuses. J Dev Orig Health Dis 7, 237–243. [DOI] [PubMed] [Google Scholar]
- Lafeber HN, Rolph TP & Jones CT (1984). Studies on the growth of the fetal guinea pig. The effects of ligation of the uterine artery on organ growth and development. J Dev Physiol 6, 441–459. [PubMed] [Google Scholar]
- Lampley EC Jr, Mishra OP, Graham E & Delivoria‐Papadopoulos M (1995). Neuroprotective effect of phenytoin against in utero hypoxic brain injury in fetal guinea pigs. Neurosci Lett 186, 192–196. [DOI] [PubMed] [Google Scholar]
- Lee AM, Morrison JL, Botting KJ, Shandala T & Xian CJ (2014). Effects of maternal hypoxia during pregnancy on bone development in offspring: a guinea pig model. Int J Endocrinol 2014, 916918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Mauroy B, James JL, Tawhai MH & Clark AR (2016). A multiscale model of placental oxygen exchange: The effect of villous tree structure on exchange efficiency. J Theor Biol 408, 1–12. [DOI] [PubMed] [Google Scholar]
- Lin Y & Lechner AJ (1990). Ultrastructural analysis of regional type II cell development within fetal and neonatal lungs. Am J Physiol 259, L359–L364. [DOI] [PubMed] [Google Scholar]
- Lingas R, Dean F & Matthews SG (1999). Maternal nutrient restriction (48 h) modifies brain corticosteroid receptor expression and endocrine function in the fetal guinea pig. Brain Res 846, 236–242. [DOI] [PubMed] [Google Scholar]
- Lingas RI & Matthews SG (2001). A short period of maternal nutrient restriction in late gestation modifies pituitary‐adrenal function in adult guinea pig offspring. Neuroendocrinology 73, 302–311. [DOI] [PubMed] [Google Scholar]
- Linnet KM, Wisborg K, Agerbo E, Secher NJ, Thomsen PH & Henriksen TB (2006). Gestational age, birth weight, and the risk of hyperkinetic disorder. Arch Dis Child 91, 655–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Li A & Matthews SG (2001). Maternal glucocorticoid treatment programs HPA regulation in adult offspring: sex‐specific effects. Am J Physiol Endocrinol Metab 280, E729–E739. [DOI] [PubMed] [Google Scholar]
- Loeliger MM, Briscoe T & Rees SM (2008). BDNF increases survival of retinal dopaminergic neurons after prenatal compromise. Invest Ophthalmol Vis Sci 49, 1282–1289. [DOI] [PubMed] [Google Scholar]
- Lotgering FK, Gilbert RD & Longo LD (1983). Exercise responses in pregnant sheep: oxygen consumption, uterine blood flow, and blood volume. J Appl Physiol Respir Environ Exerc Physiol 55, 834–841. [DOI] [PubMed] [Google Scholar]
- Mace K, Shahkhalili Y, Aprikian O & Stan S (2006). Dietary fat and fat types as early determinants of childhood obesity: a reappraisal. Int J Obes 30(Suppl 4), S50–57. [DOI] [PubMed] [Google Scholar]
- Madsen CS, Janovitz E, Zhang R, Nguyen‐Tran V, Ryan CS, Yin X, Monshizadegan H, Chang M, D'Arienzo C, Scheer S, Setters R, Search D, Chen X, Zhuang S, Kunselman L, Peters A, Harrity T, Apedo A, Huang C, Cuff CA, Kowala MC, Blanar MA, Sun CQ, Robl JA & Stein PD (2008). The guinea pig as a preclinical model for demonstrating the efficacy and safety of statins. J Pharmacol Exp Ther 324, 576–586. [DOI] [PubMed] [Google Scholar]
- Mallard C, Loeliger M, Copolov D & Rees S (2000). Reduced number of neurons in the hippocampus and the cerebellum in the postnatal guinea‐pig following intrauterine growth‐restriction. Neuroscience 100, 327–333. [DOI] [PubMed] [Google Scholar]
- Mallard EC, Rehn A, Rees S, Tolcos M & Copolov D (1999). Ventriculomegaly and reduced hippocampal volume following intrauterine growth‐restriction: implications for the aetiology of schizophrenia. Schizophr Res 40, 11–21. [DOI] [PubMed] [Google Scholar]
- Mangathayaru K, Kuruvilla S, Balakrishna K & Venkhatesh J (2009). Modulatory effect of Inula racemosa Hook. f. (Asteraceae) on experimental atherosclerosis in guinea‐pigs. J Pharm Pharmacol 61, 1111–1118. [DOI] [PubMed] [Google Scholar]
- Maulik D, Ashraf QM, Mishra OP & Delivoria‐Papadopoulos M (2002). Effect of hypoxia on calcium influx and calcium/calmodulin‐dependent kinase activity in cortical neuronal nuclei of the guinea pig fetus during development. Am J Obstet Gynecol 186, 658–662. [DOI] [PubMed] [Google Scholar]
- Maulik D, Ashraf QM, Mishra OP & Delivoria‐Papadopoulos M (2008). Activation of p38 mitogen‐activated protein kinase (p38 MAPK), extracellular signal‐regulated kinase (ERK) and c‐jun N‐terminal kinase (JNK) during hypoxia in cerebral cortical nuclei of guinea pig fetus at term: role of nitric oxide. Neurosci Lett 439, 94–99. [DOI] [PubMed] [Google Scholar]
- Maulik D, Mishra OP & Delivoria‐Papadopoulos M (2005). Effect of post‐hypoxic MgSO4 administration in utero on Ca2+‐influx and Ca2+/calmodulin kinase IV activity in cortical neuronal nuclei. Neurosci Lett 386, 127–132. [DOI] [PubMed] [Google Scholar]
- Maulik D, Numagami Y, Ohnishi ST, Mishra OP & Delivoria‐Papadopoulos M (1998). Direct measurement of oxygen free radicals during in utero hypoxia in the fetal guinea pig brain. Brain Res 798, 166–172. [DOI] [PubMed] [Google Scholar]
- Maulik D, Qayyum I, Powell SR, Karantza M, Mishra OP & Delivoria‐Papadopoulos M (2001). Post‐hypoxic magnesium decreases nuclear oxidative damage in the fetal guinea pig brain. Brain Res 890, 130–136. [DOI] [PubMed] [Google Scholar]
- Maulik D, Zanelli S, Numagami Y, Ohnishi ST, Mishra OP & Delivoria‐Papadopoulos M (1999). Oxygen free radical generation during in‐utero hypoxia in the fetal guinea pig brain: the effects of maturity and of magnesium sulfate administration. Brain Res 817, 117–122. [DOI] [PubMed] [Google Scholar]
- McCabe L, Marash D, Li A & Matthews SG (2001). Repeated antenatal glucocorticoid treatment decreases hypothalamic corticotropin releasing hormone mRNA but not corticosteroid receptor mRNA expression in the fetal guinea‐pig brain. J Neuroendocrinol 13, 425–431. [DOI] [PubMed] [Google Scholar]
- McGregor HP, Westcott K & Walker DW (1998). The effect of prenatal exposure to carbon monoxide on breathing and growth of the newborn guinea pig. Pediatr Res 43, 126–131. [DOI] [PubMed] [Google Scholar]
- McIntosh GH, Baghurst KI, Potter BJ & Hetzel BS (1979). Foetal brain development in the sheep. Neuropathol Appl Neurobiol 5, 103–114. [DOI] [PubMed] [Google Scholar]
- McKelvey A, Pateman K, Balchin I, Peebles DM, Rodeck CH & David AL (2017). Total uterine artery blood volume flow rate in nulliparous women is associated with birth weight and gestational age at delivery. Ultrasound Obstet Gynecol 49, 54–60. [DOI] [PubMed] [Google Scholar]
- McKendry AA, Palliser HK, Yates DM, Walker DW & Hirst JJ (2010). The effect of betamethasone treatment on neuroactive steroid synthesis in a foetal guinea pig model of growth restriction. J Neuroendocrinol 22, 166–174. [DOI] [PubMed] [Google Scholar]
- McLean CP, Asnaani A, Litz BT & Hofmann SG (2011). Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res 45, 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus BM, Lamb DR, Judis JJ & Scala J (1975). Skeletal muscle leucine incorporation and testosterone uptake in exercised guinea pigs. Eur J Appl Physiol Occup Physiol 34, 149–156. [DOI] [PubMed] [Google Scholar]
- McPherson NO, Owens JA, Fullston T & Lane M (2015). Preconception diet or exercise intervention in obese fathers normalizes sperm microRNA profile and metabolic syndrome in female offspring. Am J Physiol Endocrinol Metab 308, E805–E821. [DOI] [PubMed] [Google Scholar]
- Mehta V, Ofir K, Swanson A, Kloczko E, Boyd M, Barker H, Avdic‐Belltheus A, Martin J, Zachary I, Peebles D & David AL (2016). Gene targeting to the uteroplacental circulation of pregnant guinea pigs. Reprod Sci 23, 1087–1095. [DOI] [PubMed] [Google Scholar]
- Mess A (2007). The guinea pig placenta: model of placental growth dynamics. Placenta 28, 812–815. [DOI] [PubMed] [Google Scholar]
- Mess A, Zaki N, Kadyrov M, Korr H & Kaufmann P (2007). Caviomorph placentation as a model for trophoblast invasion. Placenta 28, 1234–1238. [DOI] [PubMed] [Google Scholar]
- Michel CL & Bonnet X (2012). Influence of body condition on reproductive output in the guinea pig. J Exp Zool A Ecol Genet Physiol 317, 24–31. [DOI] [PubMed] [Google Scholar]
- Miglino MA, Carter AM, Ambrosio CE, Bonatelli M, De Oliveira MF, Dos Santos Ferraz RH, Rodrigues RF & Santos TC (2004). Vascular organization of the hystricomorph placenta: a comparative study in the agouti, capybara, guinea pig, paca and rock cavy. Placenta 25, 438–448. [DOI] [PubMed] [Google Scholar]
- Mishra OP & Delivoria‐Papadopoulos M (1988a). Anti‐oxidant enzymes in fetal guinea pig brain during development and the effect of maternal hypoxia. Brain Res 470, 173–179. [DOI] [PubMed] [Google Scholar]
- Mishra OP & Delivoria‐Papadopoulos M (1988b). Na+,K+‐ATPase in developing fetal guinea pig brain and the effect of maternal hypoxia. Neurochem Res 13, 765–770. [DOI] [PubMed] [Google Scholar]
- Mishra OP & Delivoria‐Papadopoulos M (1989). Lipid peroxidation in developing fetal guinea pig brain during normoxia and hypoxia. Brain Res Dev Brain Res 45, 129–135. [DOI] [PubMed] [Google Scholar]
- Mishra OP & Delivoria‐Papadopoulos M (1992). NMDA receptor modification in the fetal guinea pig brain during hypoxia. Neurochem Res 17, 1211–1216. [DOI] [PubMed] [Google Scholar]
- Mishra OP, Kubin JA, McGowan JE & Delivoria‐Papadopoulos M (1995). Kainate receptor modification in the fetal guinea pig brain during hypoxia. Neurochem Res 20, 1171–1177. [DOI] [PubMed] [Google Scholar]
- Mishra OP, Wagerle LC & Delivoria‐Papadopoulos M (1988). 5′‐Nucleotidase and adenosine deaminase in developing fetal guinea pig brain and the effect of maternal hypoxia. Neurochem Res 13, 1055–1060. [DOI] [PubMed] [Google Scholar]
- Mitchell BF & Taggart MJ (2009). Are animal models relevant to key aspects of human parturition? Am J Physiol Regul Integr Comp Physiol 297, R525–R545. [DOI] [PubMed] [Google Scholar]
- Moisiadis VG, Constantinof A, Kostaki A, Szyf M & Matthews SG (2017). Prenatal glucocorticoid exposure modifies endocrine function and behaviour for 3 generations following maternal and paternal transmission. Sci Rep 7, 11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisiadis VG & Matthews SG (2014a). Glucocorticoids and fetal programming part 1: Outcomes. Nat Rev Endocrinol 10, 391–402. [DOI] [PubMed] [Google Scholar]
- Moisiadis VG & Matthews SG (2014b). Glucocorticoids and fetal programming part 2: Mechanisms. Nat Rev Endocrinol 10, 403–411. [DOI] [PubMed] [Google Scholar]
- Morrison JL ( 2008). Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clin Exp Pharmacol Physiol 35, 730–743. [DOI] [PubMed] [Google Scholar]
- Muggeo M, Ginsberg BH, Roth J, Neville DM Jr, De Meyts P & Kahn CR (1979). The insulin receptor in vertebrates is functionally more conserved during evolution than insulin itself. Endocrinology 104, 1393–1402. [DOI] [PubMed] [Google Scholar]
- Mulder H, Pitchford NJ, Hagger MS & Marlow N (2009). Development of executive function and attention in preterm children: a systematic review. Dev Neuropsychol 34, 393–421. [DOI] [PubMed] [Google Scholar]
- Murphy JD, Aronovitz MJ & Reid LM (1986). Effects of chronic in utero hypoxia on the pulmonary vasculature of the newborn guinea pig. Pediatr Res 20, 292–295. [DOI] [PubMed] [Google Scholar]
- Murphy VE & Clifton VL (2003). Alterations in human placental 11β‐hydroxysteroid dehydrogenase type 1 and 2 with gestational age and labour. Placenta 24, 739–744. [DOI] [PubMed] [Google Scholar]
- Myers SA, Sparks JW, Makowski EL, Meschia G & Battaglia FC (1982). Relationship between placental blood flow and placental and fetal size in guinea pig. Am J Physiol 243, H404–H409. [DOI] [PubMed] [Google Scholar]
- Nacher J, Palop JJ, Ramirez C, Molowny A & Lopez‐Garcia C (2000). Early histological maturation in the hippocampus of the guinea pig. Brain Behav Evol 56, 38–44. [DOI] [PubMed] [Google Scholar]
- Neel JV ( 1962). Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet 14, 353–362. [PMC free article] [PubMed] [Google Scholar]
- Nelson PS, Gilbert RD & Longo LD (1983). Fetal growth and placental diffusing capacity in guinea pigs following long‐term maternal exercise. J Dev Physiol 5, 1–10. [PubMed] [Google Scholar]
- Nerbonne JM (2016). Molecular basis of functional myocardial potassium channel diversity. Card Electrophysiol Clin 8, 257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin CL, Formosa E, Maki Y, Matushewski B, Regnault TRH & Richardson BS (2018). Maternal nutrient restriction in guinea pigs as an animal model for studying growth restricted offspring with post‐natal catch‐up growth. Am J Physiol Regul Integr Comp Physiol 314, R647–R654. [DOI] [PubMed] [Google Scholar]
- Newsome AD, Davis GK, Ojeda NB & Alexander BT (2017). Complications during pregnancy and fetal development: implications for the occurrence of chronic kidney disease. Expert Rev Cardiovasc Ther 15, 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LT, Muhlhausler BS, Botting KJ & Morrison JL (2010). Maternal undernutrition alters fat cell size distribution, but not lipogenic gene expression, in the visceral fat of the late gestation guinea pig fetus. Placenta 31, 902–909. [DOI] [PubMed] [Google Scholar]
- Nitsos I & Rees S (1990). The effects of intrauterine growth retardation on the development of neuroglia in fetal guinea pigs. An immunohistochemical and an ultrastructural study. Int J Dev Neurosci 8, 233–244. [DOI] [PubMed] [Google Scholar]
- Novik TS, Hervas A, Ralston SJ, Dalsgaard S, Rodrigues Pereira R & Lorenzo MJ (2006). Influence of gender on attention‐deficit/hyperactivity disorder in Europe–ADORE. Eur Child Adolesc Psychiatry 15(Suppl 1), I15–I24. [DOI] [PubMed] [Google Scholar]
- O'Donnell SR & Saar N (1975). Some evidence for the maturity of peripheral adrenergic nerves in new‐born guinea‐pigs. Aust J Exp Biol Med Sci 53, 215–222. [DOI] [PubMed] [Google Scholar]
- Oh C, Dong Y, Harman C, Mighty HE, Kopelman J & Thompson LP (2008a). Chronic hypoxia differentially increases glutathione content and gamma‐glutamyl cysteine synthetase expression in fetal guinea pig organs. Early Hum Dev 84, 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh C, Dong Y, Liu H & Thompson LP (2008b). Intrauterine hypoxia upregulates proinflammatory cytokines and matrix metalloproteinases in fetal guinea pig hearts. Am J Obstet Gynecol 199, 78.e1–78.e6. [DOI] [PubMed] [Google Scholar]
- Olausson H & Sohlstrom A (2003). Effects of food restriction and pregnancy on the expression of insulin‐like growth factors‐I and ‐II in tissues from guinea pigs. J Endocrinol 179, 437–445. [DOI] [PubMed] [Google Scholar]
- Owen D & Matthews SG (2003). Glucocorticoids and sex‐dependent development of brain glucocorticoid and mineralocorticoid receptors. Endocrinology 144, 2775–2784. [DOI] [PubMed] [Google Scholar]
- Owen D & Matthews SG (2007a). Prenatal glucocorticoid exposure alters hypothalamic‐pituitary‐adrenal function in juvenile guinea pigs. J Neuroendocrinol 19, 172–180. [DOI] [PubMed] [Google Scholar]
- Owen D & Matthews SG (2007b). Repeated maternal glucocorticoid treatment affects activity and hippocampal NMDA receptor expression in juvenile guinea pigs. J Physiol 578, 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palliser HK, Kelleher MA, Tolcos M, Walker DW & Hirst JJ (2015). Effect of postnatal progesterone therapy following preterm birth on neurosteroid concentrations and cerebellar myelination in guinea pigs. J Dev Orig Health Dis 6, 350–361. [DOI] [PubMed] [Google Scholar]
- Pavey DE & Widdowson EM (1980). Body lipids of guinea pigs exposed to different dietary fats from mid‐gestation to 3 months of age. V. The fatty acid composition of brain lipids at birth. Nutr Metab 24, 357–366. [DOI] [PubMed] [Google Scholar]
- Peeters LL, Martensson L, van Kreel BK, Saxena PR & Wallenburg HC (1986). Movement of oxygen, glucose, and lactate across the uterus of the awake near‐term guinea pig. Pediatr Res 20, 730–734. [DOI] [PubMed] [Google Scholar]
- Peeters LL, Martensson L, van Kreel BK & Wallenburg HC (1984). Uterine arterial and venous concentrations of glucose, lactate, ketones, free fatty acids, and oxygen in the awake pregnant guinea pig. Pediatr Res 18, 1172–1175. [DOI] [PubMed] [Google Scholar]
- Pembrey ME, Bygren LO, Kaati G, Edvinsson S, Northstone K, Sjostrom M & Golding J (2006). Sex‐specific, male‐line transgenerational responses in humans. Eur J Hum Genet 14, 159–166. [DOI] [PubMed] [Google Scholar]
- Persson E & Jansson T (1992). Low birth weight is associated with elevated adult blood pressure in the chronically catheterized guinea‐pig. Acta Physiol Scand 145, 195–196. [DOI] [PubMed] [Google Scholar]
- Podell BK, Ackart DF, Richardson MA, DiLisio JE, Pulford B & Basaraba RJ (2017). A model of type 2 diabetes in the guinea pig using sequential diet‐induced glucose intolerance and streptozotocin treatment. Dis Models Mech 10, 151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qayyum I, Zubrow AB, Ashraf QM, Kubin J, Delivoria‐Papadopoulos M & Mishra OP (2001). Nitration as a mechanism of Na+,K+‐ATPase modification during hypoxia in the cerebral cortex of the guinea pig fetus. Neurochem Res 26, 1163–1169. [DOI] [PubMed] [Google Scholar]
- Racca AW, Klaiman JM, Pioner JM, Cheng Y, Beck AE, Moussavi‐Harami F, Bamshad MJ & Regnier M (2016). Contractile properties of developing human fetal cardiac muscle. J Physiol 594, 437–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimbach SJ & Thomas AL (1990). Renin and angiotensin converting enzyme concentrations in the fetal and neonatal guinea‐pig. J Physiol 423, 441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah R, Glaser EM & Hirshfield AN (1992). The changing architecture of the neonatal rat ovary during histogenesis. Dev Dyn 194, 177–192. [DOI] [PubMed] [Google Scholar]
- Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN & Bleker OP (1998). Glucose tolerance in adults after prenatal exposure to famine. Lancet 351, 173–177. [DOI] [PubMed] [Google Scholar]
- Razdan B, Kubin J, Mishra OP & Delivoria‐Papadopoulos M (1996). Modification of the glycine (co‐activator) binding site of the N‐methyl‐D‐aspartate receptor in the guinea pig fetus brain during development following hypoxia. Brain Res 733, 15–20. [DOI] [PubMed] [Google Scholar]
- Reddy S, Bibby NJ & Elliott RB (1992). An immunocytochemical study of endocrine cell development in the early fetal guinea pig pancreas. Gen Comp Endocrinol 86, 275–283. [DOI] [PubMed] [Google Scholar]
- Reddy S & Elliott RB (1988). Ontogenic development of peptide hormones in the mammalian fetal pancreas. Experientia 44, 1–9. [DOI] [PubMed] [Google Scholar]
- Rehn AE, Van Den Buuse M, Copolov D, Briscoe T, Lambert G & Rees S (2004). An animal model of chronic placental insufficiency: relevance to neurodevelopmental disorders including schizophrenia. Neuroscience 129, 381–391. [DOI] [PubMed] [Google Scholar]
- Rex A, Marsden CA & Fink H (1993). Effect of diazepam on cortical 5‐HT release and behaviour in the guinea‐pig on exposure to the elevated plus maze. Psychopharmacology 110, 490–496. [DOI] [PubMed] [Google Scholar]
- Richardson B, Harding R & Walker D (2014). Behavioural states in the fetus: Relationship to fetal health and development In Creasy and Resnik's Maternal‐Fetal Medicine: Principles and Practice, 7th edn, ed. Resnik R, Creasy R, Iams JD, Lockwood CJ, Moore TR. & Greene MF. Elsevier Saunders, Philadelphia. [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M & Kaila K (1999). The K+/Cl− co‐transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397, 251–255. [DOI] [PubMed] [Google Scholar]
- Roberts CT, Kind KL, Earl RA, Grant PA, Robinson JS, Sohlstrom A, Owens PC & Owens JA (2002). Circulating insulin‐like growth factor (IGF)‐I and IGF binding proteins‐1 and ‐3 and placental development in the guinea‐pig. Placenta 23, 763–770. [DOI] [PubMed] [Google Scholar]
- Roberts CT, Sohlstrom A, Kind KL, Earl RA, Khong TY, Robinson JS, Owens PC & Owens JA (2001a). Maternal food restriction reduces the exchange surface area and increases the barrier thickness of the placenta in the guinea‐pig. Placenta 22, 177–185. [DOI] [PubMed] [Google Scholar]
- Roberts CT, Sohlstrom A, Kind KL, Grant PA, Earl RA, Robinson JS, Khong TY, Owens PC & Owens JA (2001b). Altered placental structure induced by maternal food restriction in guinea pigs: a role for circulating IGF‐II and IGFBP‐2 in the mother? Placenta 22, S77–82. [DOI] [PubMed] [Google Scholar]
- Rockwell LC, Keyes LE & Moore LG (2000). Chronic hypoxia diminishes pregnancy‐associated DNA synthesis in guinea pig uteroplacental arteries. Placenta 21, 313–319. [DOI] [PubMed] [Google Scholar]
- Rogers PA, Jones GH & Faulkner JA (1979). Protein synthesis in skeletal muscle following acute exhaustive exercise. Muscle Nerve 2, 250–256. [DOI] [PubMed] [Google Scholar]
- Rolph TP, Jones CT & Parry D (1982). Ultrastructural and enzymatic development of fetal guinea pig heart. Am J Physiol 243, H87–H93. [DOI] [PubMed] [Google Scholar]
- Romero NB, Mezmezian M & Fidzianska A (2013). Main steps of skeletal muscle development in the human: morphological analysis and ultrastructural characteristics of developing human muscle. Handb Clin Neurol 113, 1299–1310. [DOI] [PubMed] [Google Scholar]
- Ross KA & Thurlbeck WM (1992). Lung growth in newborn guinea pigs: effects of endurance exercise. Respir Physiol 89, 353–364. [DOI] [PubMed] [Google Scholar]
- Sadava D, Frykman P, Harris E, Majerus D, Mustard J & Bernard B (1992). Development of enzymes of glycolysis and gluconeogenesis in human fetal liver. Biol Neonate 62, 89–95. [DOI] [PubMed] [Google Scholar]
- Saif Z, Dyson RM, Palliser HK, Wright IM, Lu N & Clifton VL (2016). Identification of eight different isoforms of the glucocorticoid receptor in guinea pig placenta: relationship to preterm delivery, sex and betamethasone exposure. PLoS One 11, e0148226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath‐Kumar R, Matthews SG & Yang K (1998). 11β‐Hydroxysteroid dehydrogenase type 2 is the predominant isozyme in the guinea pig placenta: decreases in messenger ribonucleic acid and activity at term. Biol Reprod 59, 1378–1384. [DOI] [PubMed] [Google Scholar]
- Sanchez‐Aparicio P, Mota‐Rojas D, Nava‐Ocampo AA, Trujillo‐Ortega ME, Alfaro‐Rodriguez A, Arch E & Alonso‐Spilsbury M (2008). Effects of sildenafil on the fetal growth of guinea pigs and their ability to survive induced intrapartum asphyxia. Am J Obstet Gynecol 198, 127.e1–127.e6. [DOI] [PubMed] [Google Scholar]
- Santos J, Fonseca E, van Melis J & Miglino MA (2014). Morphometric analysis of fetal development of Cavia porcellus (Linnaeus, 1758) by ultrasonography—pilot study. Theriogenology 81, 896–900. [DOI] [PubMed] [Google Scholar]
- Sarr O, Blake A, Thompson JA, Zhao L, Rabicki K, Walsh JC, Welch I & Regnault TR (2015). The differential effects of low birth weight and western diet consumption upon early life hepatic fibrosis development in guinea pig. J Physiol 594, 1753–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarr O, Thompson JA, Zhao L, Lee TY & Regnault TR (2014). Low birth weight male guinea pig offspring display increased visceral adiposity in early adulthood. PLoS One 9, e98433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaper J, Meiser E & Stammler G (1985). Ultrastructural morphometric analysis of myocardium from dogs, rats, hamsters, mice, and from human hearts. Circ Res 56, 377–391. [DOI] [PubMed] [Google Scholar]
- Scheffen I, Kaufmann P, Philippens L, Leiser R, Geisen C & Mottaghy K (1990). Alterations of the fetal capillary bed in the guinea pig placenta following long‐term hypoxia. Adv Exp Med Biol 277, 779–790. [DOI] [PubMed] [Google Scholar]
- Schopper H, Palme R, Ruf T & Huber S (2011). Chronic stress in pregnant guinea pigs (Cavia aperea f. porcellus) attenuates long‐term stress hormone levels and body weight gain, but not reproductive output. J Comp Physiol B 181, 1089–1100. [DOI] [PubMed] [Google Scholar]
- Schopper H, Palme R, Ruf T & Huber S (2012). Effects of prenatal stress on hypothalamic‐pituitary‐adrenal (HPA) axis function over two generations of guinea pigs (Cavia aperea f. porcellus). Gen Comp Endocrinol 176, 18–27. [DOI] [PubMed] [Google Scholar]
- Sedmak G, Jovanov‐Milosevic N, Puskarjov M, Ulamec M, Kruslin B, Kaila K & Judas M (2016). Developmental expression patterns of KCC2 and functionally associated molecules in the human brain. Cereb Cortex 26, 4574–4589. [DOI] [PubMed] [Google Scholar]
- Setiawan E, Jackson MF, MacDonald JF & Matthews SG (2007). Effects of repeated prenatal glucocorticoid exposure on long‐term potentiation in the juvenile guinea‐pig hippocampus. J Physiol 581, 1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E, Owen D, McCabe L, Kostaki A, Andrews MH & Matthews SG (2004). Glucocorticoids do not alter developmental expression of hippocampal or pituitary steroid receptor coactivator‐1 and ‐2 in the late gestation fetal guinea pig. Endocrinology 145, 3796–3803. [DOI] [PubMed] [Google Scholar]
- Sferruzzi‐Perri AN, Owens JA, Standen P, Taylor RL, Heinemann GK, Robinson JS & Roberts CT (2006). Early treatment of the pregnant guinea pig with IGFs promotes placental transport and nutrient partitioning near term. Am J Physiol Endocrinol Metab 292, E668–E676. [DOI] [PubMed] [Google Scholar]
- Shaw JC, Palliser HK, Dyson RM, Berry MJ & Hirst JJ (2017a). Disruptions to the cerebellar GABAergic system in juvenile guinea pigs following preterm birth. Int J Dev Neurosci 65, 1–10. [DOI] [PubMed] [Google Scholar]
- Shaw JC, Palliser HK, Dyson RM, Berry MJ & Hirst JJ (2017b). Ganaxolone as a therapy to prevent neurological impairment following preterm birth in the guinea pig. In 44th Annual Meeting Fetal and Neonatal Physiological Society, Osaka, Japan.
- Shaw JC, Palliser HK, Dyson RM, Hirst JJ & Berry MJ (2016). Long‐term effects of preterm birth on behavior and neurosteroid sensitivity in the guinea pig. Pediatr Res 80, 275–283. [DOI] [PubMed] [Google Scholar]
- Shaw JC, Palliser HK, Walker DW & Hirst JJ (2015). Preterm birth affects GABAA receptor subunit mRNA levels during the foetal‐to‐neonatal transition in guinea pigs. J Dev Orig Health Dis 6, 250–260. [DOI] [PubMed] [Google Scholar]
- Shea KM, Hewitt AJ, Olmstead MC, Brien JF & Reynolds JN (2012). Maternal ethanol consumption by pregnant guinea pigs causes neurobehavioral deficits and increases ethanol preference in offspring. Behav Pharmacol 23, 105–112. [DOI] [PubMed] [Google Scholar]
- Shiotani M, Harada T, Abe J, Hamada Y & Horii I (2008). Aging‐related changes of QT and RR intervals in conscious guinea pigs. J Pharmacol Toxicol Methods 57, 23–29. [DOI] [PubMed] [Google Scholar]
- Siegeler K, Wistuba J, Damm OS, von Engelhardt N, Sachser N & Kaiser S (2013). Early social instability affects plasma testosterone during adolescence but does not alter reproductive capacity or measures of stress later in life. Physiol Behav 120, 143–149. [DOI] [PubMed] [Google Scholar]
- Smith AD, Gilbert RD, Lammers RJ & Longo LD (1983). Placental exchange area in guinea pigs following long‐term maternal exercise: a stereological analysis. J Dev Physiol 5, 11–21. [PubMed] [Google Scholar]
- Smith R, Wickings EJ, Bowman ME, Belleoud A, Dubreuil G, Davies JJ & Madsen G (1999). Corticotropin‐releasing hormone in chimpanzee and gorilla pregnancies. J Clin Endocrinol Metab 84, 2820–2825. [DOI] [PubMed] [Google Scholar]
- So K, Chung Y, Lee H, Kim E & Jeon Y (2013). The effect of chronic prenatal hypoxia on the development of mature neurons in the cerebellum. J Neurodev Disord 5, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohlstrom A, Katsman A, Kind KL, Roberts CT, Owens PC, Robinson JS & Owens JA (1998). Food restriction alters pregnancy‐associated changes in IGF and IGFBP in the guinea pig. Am J Physiol 274, E410–E416. [DOI] [PubMed] [Google Scholar]
- Soo PS, Hiscock J, Botting KJ, Roberts CT, Davey AK & Morrison JL (2012). Maternal undernutrition reduces P‐glycoprotein in guinea pig placenta and developing brain in late gestation. Reprod Toxicol 33, 374–381. [DOI] [PubMed] [Google Scholar]
- Sosenko IR & Frank L (1987). Lung development in the fetal guinea pig: surfactant, morphology, and premature viability. Pediatr Res 21, 427–431. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, Concha H, Hassan M, Ryden M, Frisen J & Arner P (2008). Dynamics of fat cell turnover in humans. Nature 453, 783–787. [DOI] [PubMed] [Google Scholar]
- Stark MJ, Clifton VL & Wright IM (2008). Sex‐specific differences in peripheral microvascular blood flow in preterm infants. Pediatr Res 63, 415–419. [DOI] [PubMed] [Google Scholar]
- Swanson AM & David AL (2015). Animal models of fetal growth restriction: Considerations for translational medicine. Placenta 36, 623–630. [DOI] [PubMed] [Google Scholar]
- Swanson AM, Mehta V, Ofir K, Rowe M, Rossi C, Ginsberg Y, Griffin H, Barker H, White T, Boyd M & David AL (2017). The use of ultrasound to assess fetal growth in a guinea pig model of fetal growth restriction. Lab Anim 51, 181–190. [DOI] [PubMed] [Google Scholar]
- Swanson AM, Rossi CA, Ofir K, Mehta V, Boyd M, Barker H, Ledwozyw A, Vaughan O, Martin J, Zachary I, Sebire N, Peebles DM & David AL (2016). Maternal therapy with Ad.VEGF‐A165 increases fetal weight at term in a guinea‐pig model of fetal growth restriction. Hum Gene Ther 27, 997–1007. [DOI] [PubMed] [Google Scholar]
- Swifka J, Weiss J, Addicks K, Eckel J & Rosen P (2008). Epicardial fat from guinea pig: a model to study the paracrine network of interactions between epicardial fat and myocardium? Cardiovasc Drugs Ther 22, 107–114. [DOI] [PubMed] [Google Scholar]
- Szeto HH & Hinman DJ (1985). Prenatal development of sleep‐wake patterns in sheep. Sleep 8, 347–355. [DOI] [PubMed] [Google Scholar]
- Szeto HH, Vo TD, Dwyer G, Dogramajian ME, Cox MJ & Senger G (1985). The ontogeny of fetal lamb electrocortical activity: a power spectral analysis. Am J Obstet Gynecol 153, 462–466. [DOI] [PubMed] [Google Scholar]
- Thomas CR & Lowy C (1983). Placental transfer of free fatty acids: factors affecting transfer across the guinea‐pig placenta. J Dev Physiol 5, 323–332. [PubMed] [Google Scholar]
- Thompson JA, Gros R, Richardson BS, Piorkowska K & Regnault TR (2011a). Central stiffening in adulthood linked to aberrant aortic remodeling under suboptimal intrauterine conditions. Am J Physiol Regul Integr Comp Physiol 301, R1731–R1737. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Sarr O, Piorkowska K, Gros R & Regnault TR (2014). Low birth weight followed by postnatal over‐nutrition in the guinea pig exposes a predominant player in the development of vascular dysfunction. J Physiol 592, 5429–5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LP, Aguan K, Pinkas G & Weiner CP (2000). Chronic hypoxia increases the NO contribution of acetylcholine vasodilation of the fetal guinea pig heart. Am J Physiol Regul Integr Comp Physiol 279, R1813–R1820. [DOI] [PubMed] [Google Scholar]
- Thompson L, Dong Y & Evans L (2009). Chronic hypoxia increases inducible NOS‐derived nitric oxide in fetal guinea pig hearts. Pediatr Res 65, 188–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LP & Dong Y (2005). Chronic hypoxia decreases endothelial nitric oxide synthase protein expression in fetal guinea pig hearts. J Soc Gynecol Investig 12, 388–395. [DOI] [PubMed] [Google Scholar]
- Thompson LP, Liu H, Evans L & Mong JA (2011b). Prenatal nicotine increases matrix metalloproteinase 2 (MMP‐2) expression in fetal guinea pig hearts. Reprod Sci 18, 1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LP, Pence L, Pinkas G, Song H & Telugu BP (2016). Placental hypoxia during early pregnancy causes maternal hypertension and placental insufficiency in the hypoxic guinea pig model. Biol Reprod 95, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordstein M & Kjellmer I (1988). Cerebral tolerance of hypoxia in growth‐retarded and appropriately grown newborn guinea pigs. Pediatr Res 24, 633–638. [DOI] [PubMed] [Google Scholar]
- Thornburg KL, O'Tierney PF & Louey S (2010). Review: The placenta is a programming agent for cardiovascular disease. Placenta 31(Suppl), S54–S59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlbeck WM (1975). Lung growth and alveolar multiplication. Pathobiol Annu 5, 1–34. [PubMed] [Google Scholar]
- Tolcos M, Bateman E, O'Dowd R, Markwick R, Vrijsen K, Rehn A & Rees S (2011). Intrauterine growth restriction affects the maturation of myelin. Exp Neurol 232, 53–65. [DOI] [PubMed] [Google Scholar]
- Tolcos M, Markwick R, O'Dowd R, Martin V, Turnley A & Rees S (2015). Intrauterine growth restriction: effects on neural precursor cell proliferation and angiogenesis in the foetal subventricular zone. Dev Neurosci 37, 453–463. [DOI] [PubMed] [Google Scholar]
- Tolcos M & Rees S (1997). Chronic placental insufficiency in the fetal guinea pig affects neurochemical and neuroglial development but not neuronal numbers in the brainstem: a new method for combined stereology and immunohistochemistry. J Comp Neurol 379, 99–112. [DOI] [PubMed] [Google Scholar]
- Turan S, Aberdeen GW & Thompson LP (2017). Chronic hypoxia alters maternal uterine and fetal hemodynamics in the full‐term pregnant guinea pig. Am J Physiol Regul Integr Comp Physiol 313, R330–R339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AJ & Trudinger BJ (2000). Ultrasound measurement of biparietal diameter and umbilical artery blood flow in the normal fetal guinea pig. Comp Med 50, 379–384. [PubMed] [Google Scholar]
- Turner AJ & Trudinger BJ (2009). A modification of the uterine artery restriction technique in the guinea pig fetus produces asymmetrical ultrasound growth. Placenta 30, 236–240. [DOI] [PubMed] [Google Scholar]
- Umans JG, Cox MJ, Hinman DJ, Dogramajian ME, Senger G & Szeto HH (1985). The development of electrocortical activity in the fetal and neonatal guinea pig. Am J Obstet Gynecol 153, 467–471. [DOI] [PubMed] [Google Scholar]
- ICH (2017). Detection of Toxicity to Reproduction for Human Pharmaceuticals S5(R3). International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), Geneva. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S5/S5-R3EWG_Step2_Guideline_2017_0705.pdf. [Google Scholar]
- Vibert YM, Ashraf QM, Mishra OP & Delivoria‐Papadopoulos M (2008). Mechanism of Ca2+‐influx and Ca2+/calmodulin‐dependent protein kinase IV activity during in utero hypoxia in cerebral cortical neuronal nuclei of the guinea pig fetus at term. Neurosci Lett 440, 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt N, Kowalski GJ & Guenther A (2015). The maternal social environment shapes offspring growth, physiology, and behavioural phenotype in guinea pigs. Front Zool 12(Suppl 1), S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa Desai R & Smith MA (2017). Pregnancy‐related listeriosis. Birth Defects Res 109, 324–335. [DOI] [PubMed] [Google Scholar]
- Wainwright PE, Leatherdale ST & Dubin JA (2007). Advantages of mixed effects models over traditional ANOVA models in developmental studies: a worked example in a mouse model of fetal alcohol syndrome. Dev Psychobiol 49, 664–674. [DOI] [PubMed] [Google Scholar]
- Wang WT, Lee P, Dong Y, Yeh HW, Kim J, Weiner CP, Brooks WM & Choi IY (2016). In vivo neurochemical characterization of developing guinea pigs and the effect of chronic fetal hypoxia. Neurochem Res 41, 1831–1843. [DOI] [PubMed] [Google Scholar]
- Ward SS & Stickland NC (1991). Why are slow and fast muscles differentially affected during prenatal undernutrition? Muscle Nerve 14, 259–267. [DOI] [PubMed] [Google Scholar]
- Watt VM (1985). Sequence and evolution of guinea pig preproinsulin DNA. J Biol Chem 260, 10926–10929. [PubMed] [Google Scholar]
- Welling LW, Evan AP, Gattone VH 2nd, Rollins S, Saunders R, Kaskel FJ & Spitzer A (1989). Correlation of structure and function in developing proximal tubule of guinea pig. Am J Physiol 256, F13–F17. [DOI] [PubMed] [Google Scholar]
- Wicke KM, Rex A, Jongen‐Relo A, Groth I & Gross G (2007). The guinea pig forced swim test as a new behavioral despair model to characterize potential antidepressants. Psychopharmacology 195, 95–102. [DOI] [PubMed] [Google Scholar]
- Wiersma MM, Vissing J, Mikines KJ, Steffens AB & Galbo H (1995). Effect of liver denervation on glucose production during running in guinea pigs. Am J Physiol 268,R72–R77. [DOI] [PubMed] [Google Scholar]
- Wilkening RB & Meschia G (1992). Current topic: Comparative physiology of placental oxygen transport. Placenta 13, 1–15. [DOI] [PubMed] [Google Scholar]
- Williams DR, Carlsson R & Burkner PC (2017). Between‐litter variation in developmental studies of hormones and behavior: inflated false positives and diminished power. Front Neuroendocrinol 47, 154–166. [DOI] [PubMed] [Google Scholar]
- Yilmaz C, Dane DM & Hsia CC (2008). Assessing recruitment of lung diffusing capacity in exercising guinea pigs with a rebreathing technique. J Appl Physiol (1985) 105, 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Hao S, Yang B, Zhao Y, Zhang W & Yang J (2016). Mild maternal iron deficiency anemia induces hearing impairment associated with reduction of ribbon synapse density and dysregulation of VGLUT3, myosin VIIa, and prestin expression in young guinea pigs. Neurotox Res 29, 594–604. [DOI] [PubMed] [Google Scholar]
- Yzydorczyk C, Armengaud JB, Peyter AC, Chehade H, Cachat F, Juvet C, Siddeek B, Simoncini S, Sabatier F, Dignat‐George F, Mitanchez D & Simeoni U (2017). Endothelial dysfunction in individuals born after fetal growth restriction: cardiovascular and renal consequences and preventive approaches. J Dev Orig Health Dis 8, 448–464. [DOI] [PubMed] [Google Scholar]
- Zanelli SA, Numagami Y, McGowan JE, Mishra OP & Delivoria‐Papadopoulos M (1999). NMDA receptor‐mediated calcium influx in cerebral cortical synaptosomes of the hypoxic guinea pig fetus. Neurochem Res 24, 437–446. [DOI] [PubMed] [Google Scholar]
- Zanelli SA, Spandou E, Mishra OP & Delivoria‐Papadopoulos M (2005). Hypoxia modifies nuclear calcium uptake pathways in the cerebral cortex of the guinea‐pig fetus. Neuroscience 130, 949–955. [DOI] [PubMed] [Google Scholar]
- Zipser B, Schleking A, Kaiser S & Sachser N (2014). Effects of domestication on biobehavioural profiles: a comparison of domestic guinea pigs and wild cavies from early to late adolescence. Front Zool 11, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]


