Abstract
Caesarean section and instrumental delivery rates are increasing in many parts of the world for a range of cultural and medical reasons, with limited consideration as to how ‘mode of delivery’ may impact on childhood and long‐term health. However, babies born particularly by pre‐labour caesarean section appear to have a subtly different physiology from those born by normal vaginal delivery, with both acute and chronic complications such as respiratory and cardio‐metabolic morbidities being apparent. It has been hypothesized that inherent mechanisms within the process of labour and vaginal delivery, far from being a passive mechanical process by which the fetus and placenta are expelled from the birth canal, may trigger certain protective developmental processes permissive for normal immunological and physiological development of the fetus postnatally. Traditionally the primary candidate mechanism has been the hormonal surges or stress response associated with labour and vaginal delivery, but there is increasing awareness that transfer of the maternal microbiome to the infant during parturition. Transgenerational transmission of disease traits through epigenetics are also likely to be important. Interventions such as probiotics, neonatal gut seeding and different approaches to clinical care have potential to influence parturition physiology and improve outcomes for infants.
Keywords: pregnancy, caesarean section, infant, health, microbiome, immunity, lifecourse, vaginal delivery, stress hormones, epigenetics
Introduction
Given the fundamental nature of parturition, there is relatively limited understanding of how the route by which a baby is born can have prolonged physiological consequences for infants. The process of normal parturition (labour) may take many hours, and a neonate can be exposed to a range of mechanical forces, periods of transient hypoxia, oxidative stress and stress hormone surges that potentially influence physiology and impact the life‐long health of the baby. However, the mechanisms responsible for transmitting these physiological stimuli have yet to be fully elucidated.
Now, more than ever, a greater understanding of how mode of delivery (and onset, progression and duration of labour) can impact on normal development and long‐term health outcomes for the baby is critical given the increasing caesarean section (CS) rate worldwide.
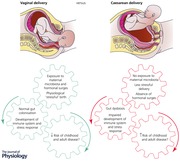
A growing number of studies indicate that babies born by pre‐labour (elective) CS appear to have a subtly different physiology from those born by normal vaginal delivery (VD), with both acute and chronic complications such as respiratory and other morbidities being apparent (Hyde & Modi, 2012; Taylor, 2015; Dahlen et al. 2016). It has been hypothesized that labour and VD, far from being mere passive mechanical processes by which the fetus and placenta are expelled from the uterus, provide important, permissive developmental cues required to trigger immunological and physiological maturation of the fetus in preparation for postnatal life.
This review examines the most recent epidemiological and experimental evidence linking mode of delivery and future outcomes in infants and discusses the three main mechanisms that have been proposed to explain why mode of delivery, spontaneous and/or induced/augmented vaginal delivery versus CS, may affect neonatal development. These are (1) exposure to different levels of physical stress and stress hormone surges during delivery, (2) aberrant microbial colonization of the infant intestinal tract, and (3) epigenetic modification of gene expression. The scientific evidence in support of (or contraindicating) clinical interventions such as bacterial ‘seeding’ of the neonatal gut microbiome via vaginal swabs and probiotic supplementation is also considered. Finally, we will discuss potential clinical care factors that could optimize physiological processes during parturition.
What is the evidence that mode of delivery impacts on infant health and immune system?
Approximately 1 in 5 women around the world will give birth via CS, and there have been repeated reports of increasing CS rates over time (Betrán et al. 2016; Miller et al. 2016). CS rates in England are now approximately 28%, and even higher in the USA (32%) and Brazil (51.9%) (Martin et al. 2015; Miller et al. 2016; Nakamura‐Pereira et al. 2016; NHS Digital, 2017). This rise predominantly parallels the rapid increases in maternal obesity, macrosomia and pregnancy complications such as pre‐eclampsia, and a concomitant increase in elective CS. A rise in CS rates does not necessarily correlate with improved outcomes (Miller et al. 2016). Increased maternal requests for CS can be due to a range of social and cultural pressures; fear of pain; the belief that CS is safer for the baby; convenience for health professionals, mothers and family; fear of medical litigation; and lower tolerance to any complications or outcomes other than the perfect baby.
In the UK, there is consensus that elective CS should only be offered in the context of providing women with balanced information of the risks and benefits (UK National Institute for Health and Care Excellence, 2011). However, the World Health Organisation has stated that that there is no evidence to suggest a mother or child would benefit from performing a CS when not medically indicated (World Health Organisation, 2015) and recommend further research into the effects of CS on outcomes such as maternal and perinatal morbidity, paediatric outcomes, and psychological and social well‐being.
Strong epidemiological evidence supports the concept that CS is associated with both short and long‐term health effects via altered immune system and metabolism (Hyde & Modi, 2012; Taylor, 2015; Dahlen et al. 2016). Infants delivered via CS are more likely to develop respiratory and immune disorders such as asthma, atopy, juvenile arthritis and inflammatory bowel disease (Bager et al. 2008; Negele et al. 2004; Sevelsted et al. 2015). Interestingly, the effects of CS can vary between elective pre‐labour CS and emergency CS when a woman is in established labour; this provides a natural control for differences in some neonatal exposures. In a register‐based cohort study of 750,000 children born in Denmark (1997–2013), risk of lower respiratory infection and asthma is higher in elective CS than emergency CS (Kristensen & Henriksen, 2016). The Prediction of Allergy in Taiwanese Children (PATCH) study, a prospective birth cohort of 579 children, reported that CS was associated with an increased incidence of ‘wheeze’ and reduced lung function at 12 months of age (Liao et al. 2017). Furthermore, these infants had impaired neonatal production of pro‐inflammatory cytokines in isolated mononuclear cells linking mode of delivery with development of the neonatal immune system (Liao et al. 2017; Chu et al. 2017b). The longer term effects of CS on wheeze and allergy have been explored in a metanalysis; there was an increased odds ratio for asthma up to 12 years of age, with less conclusive results for wheeze and allergy/atopy (Keag et al. 2018).
Predisposition to immune disorders may be due to altered immune maturation and reduced exposure to the maternal microbiota (see below) in infants delivered via CS (Daniel et al. 2008; Cho & Norman, 2013; Thysen et al. 2015; Treviño‐Garza et al. 2016). In an animal model of CS, Daniel et al. (2008) found that piglets born by CS had increased basal serum levels of tumour necrosis factor α at 2 weeks of age, while interferon γ was lower. Some variations in immune function in infants delivered via CS rectify themselves over time, with basal serum levels of adrenocorticotrophin and cortisol being elevated at birth in piglets born via CS but not differing from VD piglets at 2 weeks. Thus, long‐term alterations in immune maturation in CS infants should be examined.
Higher rates of overweight and obesity have been observed in individuals born via CS (Pluymen et al. 2016; Yuan et al. 2016; Keag et al. 2018). These effects can extend into adulthood, increasing the risk of developing metabolic syndrome and cardiovascular disease (Hyde & Modi, 2012; Darmasseelane et al. 2014; Magne et al. 2017). Data from a large US cohort, the ‘Growing Up Today Study’ (GUTS, 22,068 individuals aged 9–14 years and 20–28 years) found, after adjusting for confounding factors, that any CS was associated with 15% increase risk of obesity, while an elective CS was associated with an ever higher (30%) increased risk (Yuan et al. 2016). CS may also be an independent risk factor for adult hypertension (Fernandes et al. 2015).
CS can also impact on neurological outcomes including schizophrenia, autism spectrum disorders (ASD), altered longer term dopamine responses and behaviour (Boksa & El‐Khodor, 2003; Curran et al. 2015b; Hultman et al. 2002; Rutayisire et al. 2017). The concept stemmed from an interesting series of animal studies on CS (± anoxia exposure post‐birth) (Boksa & El‐Khodor, 2003) and has promoted an ongoing debate as to whether childhood emotional and behavioural problems may be increased in children delivered by CS. However, whilst associations have been identified between CS and ASD, attention deficit hyperactivity disorder (ADHD) (Curran et al. 2015b) and behavioural problems (Silva et al. 2014), other studies using similar methodologies could not replicate the findings (Khalaf et al. 2015; Curran et al. 2016a, b ). Curran and colleagues, following a systematic review and meta‐analysis of the literature (Curran et al. 2015b), undertook analyses of sibling pairs discordant for ASD and ADHD and revised their conclusions. Whilst children from the Swedish Medical Birth Register born by CS were approximately 20% more likely to be diagnosed as having ASD, the association did not persist when employing sibling controls, implying that the association was due to familial confounding by genetic and/or environmental factors (Curran et al. 2015a). Similarly, when investigating mode of delivery and ADHD in sibling pairs, CS was associated with a small increased risk of ADHD; however, among siblings the association only remained for emergency CS and not elective. If this were a causal effect, the association would be expected to persist for both types of CS (Curran et al. 2016b).
Potentially, there may also be associations with altered childhood outcomes with more mechanical (vacuum and forceps) modes of delivery and pharmacological interventions as well as exposure to antibiotics in the intrapartum period, but this requires more research. Indeed, the Epigenetic Impact of Childbirth (EPIIC) hypothesis proposes that interventions in labour such as exposure to synthetic oxytocin during labour induction and augmentation could influence neonatal health through mediation of epigenetic changes and there are some interesting data from animal studies (Dahlen et al. 2013; Boksa et al. 2015) suggesting that maternal oxytocin administration may have multiple acute effects on central nervous system metabolic responses to anoxia at birth involving oxidative stress and inflammation which can affect the molecular epigenetic machinery.
Differences in the physiological impact of vaginal versus caesarean section delivery
Interruption of the normal parturition physiological pathway in CS
There are clearly differences in the timing and physical experience of vaginal delivery versus elective CS, but the question is what are they and why do they matter? The first consideration is that neonates delivered prior to labour onset, even at term gestations (i.e. elective CS), have not been exposed to the full gamut of hormonal and inflammatory signals associated with parturition. Depending on the gestational age at which elective sections are performed this could (1) reduce exposure to physiologically increasing concentrations of hormones such as oestrogen and cortisol, (2) diminish the effects of functional progesterone withdrawal and (3) lead to suboptimal preparation for neonatal transition (e.g. maturation of fetal lung, fluid absorption mechanisms and exposure to surfactant proteins). The latter, in part contributes to the association between CS and childhood lung function (Ramachandrappa & Jain, 2008). However, in many epidemiological studies, gestational age at time of elective CS has not always been controlled for, or indeed distinction made between, elective versus emergency CS and prior exposure to induction/augmentation agents such as prostaglandins and oxytocin.
There is accumulating evidence that labour influences hormone concentrations at birth. Umbilical venous plasma prolactin levels are significantly lower in pre‐labour CS neonates (Heasman et al. 1997). Plasma concentration of thyroxine and triiodothyronine and cortisol concentration are also lower after pre‐labour CS compared to labour, with higher mean umbilical plasma thyroid‐stimulating hormone (TSH) concentration (Bird et al. 1996). Labour reduces plasma TSH concentrations at birth in association with a surge in cortisol and appears to be a developmental stimulus in the subsequent surge in triiodothyronine (T3) that occurs postpartum after vaginal birth (Bird et al. 1996). The consequences of an absent T3 surge at birth is not entirely clear, but T3 has important maturational effects in the fetus, and in congenitally hypothyroid rats, early postnatal (P7) but not late (P21) T4 treatment improves learning and memory performance (Reid et al. 2007) supporting a neurotrophic role of thyroid hormones during certain critical developmental windows.
Umbilical cord concentrations of adrenaline, noradrenaline and cortisol are also significantly lower in babies born by CS compared with VD (Elbay et al. 2016; Vogl et al. 2006), which may result in impaired development of the hypothalamic–pituitary–adrenal (HPA) axis and abnormal reactivity to stress (Taylor et al. 2000). The absence of stress hormones in the cord blood, may cause maladaptation of the HPA axis and may also explain why babies delivered by elective and emergency CS have lower systolic blood pressure on the first day of life. This may also be due to the use of anaesthetics during surgery, but clearly these early haemodynamic changes may have implications for the plasticity of neonatal cardiovascular and autonomic nervous system development (Sedaghat et al. 2008). Maladaptations made during birth may establish physiological set points for a developmental trajectory for future cardiovascular dysfunction and disease. Heart rate variability analysis from electrocardiogram traces would allow assessment of the relative contribution of the sympathetic and parasympathetic components of the autonomic nervous system in the regulation of blood pressure, for which sympathetic tone is a biomarker for subsequent cardiovascular disease. The contribution of anaesthetic regimens employed during CS is not easily dissected, although well designed animal studies may provide proof of principle and invaluable mechanistic insight in this regard.
Physical forces and stress responses to labour versus CS
A second consideration are the physical forces a fetus experiences during labour. Early labour and established labour can manifest for many hours (and even days). This exposure to intermittent contractile forces and intermittent hypoxia and oxidative stress (due to rhythmic spontaneous contractions) as well as other hormonal responses to maternal stress and pain have significant potential to influence physiological development of the neonate (Lagercrantz, 2016). The physical forces exerted through the second stage are also important – in response to strong propulsive contractions generated by the uterine fundus, the fetus is squeezed through the cervical opening and upwards through the pelvis and out through the vaginal canal. A fetus born via CS experiences very different forces and pressures and is delivered far faster than a vaginal birth. Indeed, delivery seems to be a necessary stress, which acts as a cue for the development of various systems in the infant.
The physiological importance of the process of delivery is also exemplified in foals presenting with neonatal equine maladjustment syndrome, or ‘dummy foal’ syndrome. This condition, associated with rapid assisted delivery, is characterized by low awareness, abnormal suckling and inability to bond with the mare. It is thought that this is caused by the absence of major physiological milestones during delivery (Aleman et al. 2017). Levels of inhibitory neurosteroids are elevated in these foals after birth, keeping them in a reduced state of consciousness (Aleman et al. 2017). The ‘Madigan squeeze’ technique, used to treat these foals, involves compressions of the thorax for 20 min, mimicking compression of the fetus in the birth canal during normal delivery. Foals that receive this treatment recover faster than those that receive medication only (Aleman et al. 2017).
The mode of delivery can also affect steroid concentrations in neonates. McCallie et al. (2017) measured the natural course of nine neurosteroids over the first 2 days of life in 39 full‐term neonates. The postnatal decline in progesterone, epipregnanolone and pregnanolone sulfate was more significant in infants born by VD compared to pre‐labour CS (McCallie et al. 2017). As this reaction is similar to that of foals with neonatal equine maladjustment syndrome, the authors suggest that this may be due to a lack of labour rather than the delivery mode itself. The speed at which elective CS is carried out, and the lack of the physical forces of labour, may skip vital steps needed to prepare the fetus for extrauterine transition. Lack of ‘vaginal squeeze’ had been investigated in terms of poor neonatal lung clearance at birth (Jain & Eaton, 2006), but the consensus now holds that reduced ENaC channel expression/function, secondary to lower levels of corticosteroid (or stimulatory catecholamines) at birth is a more plausible explanation, although these mechanisms need not be mutually exclusive.
Yektaei‐Karin and colleagues (2007) speculated that the reduced cortisol surge, measured in cord blood, associated with CS (Gitau et al. 2001) may underpin differences in immune function observed with VD versus CS. The detected rise in IL‐8 and neutrophils and monocytes in normal labour was suppressed in CS infants and exaggerated in assisted delivery. They proposed that the setting of the HPA axis at birth is a promoter of an alarm response and a surge of neuroendocrine immuno‐modulating factors. Suppression of antimicrobial defences in the new‐born delivered by CS could have profound implications for immune function development in the neonate.
The reduced exposure to the physical factors and stress hormones in CS compared with VD also results in a significantly increased infant response to stress/pain 2 h after birth (Chiș et al. 2017), indicative of altered programming of the HPA axis. Whether this difference was directly linked to stress of delivery or type of anaesthesia could not be determined, but the altered stress responses seems to remain into childhood as children born via CS are more likely to suffer from anxiety, depression and sleep disturbances (Kelmanson, 2013).
Animal models of CS and ‘stress’
Animal models are needed to fully elucidate the complex mechanisms implied by the observational human studies described above. There are still many gaps in the literature especially for longer term cardiovascular outcomes. In terms of neurodevelopment, it has been found in rats that CS can produce long‐term changes in central nervous system dopamine signalling, compared to VD (Boksa & Zhang, 2008). Similarly, rat pups born by CS demonstrate long‐term reciprocal changes in dopamine levels and metabolism in the nucleus accumbens and prefrontal cortex (El‐Khodor & Boksa, 1997). Adrenaline administration to rats at birth following CS prevents these long‐term neurological changes as well as the observed increase in tyrosine hydroxylase activity in response to a stress challenge in adulthood (Boksa & Zhang, 2008).
Another rodent model of mode of delivery employed CS delivery in guillotined rat dams, to dissect the influence of isoflurane/N2O anaesthetic effects and global birth anoxia on brain biochemistry in neonatal rats compared with controls (Vaillancourt et al. 1999). A short period of global anoxia just before birth, increased blood and brain lactate levels, decreased brain ATP and increased systemic , presumably due to ventilatory responses to hypoxaemia and hypercapnia (Vaillancourt et al. 1999). Animal studies also indicate how hypoxia at birth can contribute to long‐term changes in brain chemistry and behaviour (Berger et al. 2000; Venerosi et al. 2006).
Overall, the lack of exposure to physical forces of labour and associated stress hormones appears likely to have an influential effect on neonatal outcomes. Longer term follow‐up will determine the persistence of these phenotypic changes into childhood and beyond.
Impaired microbial colonization of the infant intestinal tract
Mode of delivery can determine the bacterial populations that colonize the infant microbiota in the first day of life (Dominguez‐Bello et al. 2010; Bäckhed et al. 2015; Azad et al. 2016; Bokulich et al. 2016; Yassour et al. 2016; Sakwinska et al. 2017; Chu et al. 2017a). Common practices in the delivery theatre, including intravenous prophylactic antibiotics up to 1 h before surgery, chlorohexidine alcohol preparation of the skin, hair removal, and removal of maternal stool contamination in the birthing area, impact the bacterial exposure of the infant (Azad et al. 2016; Martinez et al. 2017), as well as CS effectively bypassing normal microbial seeding by maternal vaginal and intestinal flora.
Exposure to the vaginal microbiota appears to be important for initial colonization of infant skin and gut. Infants born naturally via vaginal birth possess undifferentiated bacterial communities on their skin, oral and nasopharyngeal cavities, and gut that resemble the maternal vaginal microbiota. In contrast, CS infants have an initial microbiota more closely related to the maternal skin microbiota (Dominguez‐Bello et al. 2010; Jakobsson et al. 2014). Importantly, CS infants have lower levels of the protective Bifidobacterium longum subspecies infantis (B. infantis) at just 3 days of age (Biasucci et al. 2010). More recent evidence indicates that exposure to maternal rectal bacteria during VD may be more important than the vaginal microbiome in the vertical transmission and colonization (‘seeding’) of the infant gut (Makino et al. 2013; Sakwinska et al. 2017). Indeed, it is more plausible that infant gut colonization should be driven by transfer of maternal intestinal microbiota rather than vaginal bacteria given the very different environments these species needed to thrive. There are also data suggesting microbial transfer from breast milk (Pannaraj et al. 2017). These routes of transmission still require more investigation as although Sakwinska and colleagues (2017) reported a greater similarity between paired maternal rectal and infant stool microbiota, it was minimal in early infancy and only showed a strong resemblance 4–6 month after birth. They concluded that physiological conditions such as immune system maturation, which could also be affected by mode of delivery, could be important. It was also not discussed whether all VD babies were exposed to maternal stool during birth.
Clinical confounders such as onset of lactogenesis can be affected by labour interventions and are not always considered by studies linking mode of delivery and the gut microbiome. Breastfeeding can be challenging for women who have had a CS. Duration of breast feeding can be curtailed and there can be an initial physiological delay in the onset of lactogenesis (Hobbs et al. 2016), both situations that could have a direct impact on microbiota development, i.e. bifidobacterial colonization is dependent on the presence of human milk oligosaccharides in the breast milk, (Underwood et al. 2015). Decreased numbers of intestinal Bifidobacteria have clinical relevance as this type of intestinal dysbiosis early in life predisposes to inflammation (Sirilun et al. 2015; Walker, 2017) and numerous studies have observed positive associations with bifidobacterial strains and normal neonatal gut microbiota (Guo et al. 2015; Patole et al. 2016; Stewart et al. 2016, 2017).
Similarly, augmentation of labour with oxytocin, maternal obesity and increased maternal age can impact on establishment of breastfeeding and breast‐feeding duration. The link between mode of delivery and gut microbiome in infants, 6 weeks post‐birth, was recently refuted in a small cohort of 81 women (Chu et al. 2017a). In contrast to others, this study attempted to control for several fixed effects including intrapartum antibiotics, breastfeeding practices, gestational weight gain, maternal gestational diabetes or pre‐pregnancy BMI, and gestational age at the time of delivery. However, the sample size was relatively small and significance was borderline.
The causal link between dysbiosis at birth/early neonatal period and future human health needs more supporting evidence. The mechanistic pathways have been examined in some detail in animal models (see below, Fig. 1) and strengthen the argument for a causal relationship between gut dysbiosis and poor adult health, but animal studies that also simultaneously assess the impact of mode of deliver would complement these data.
Figure 1.
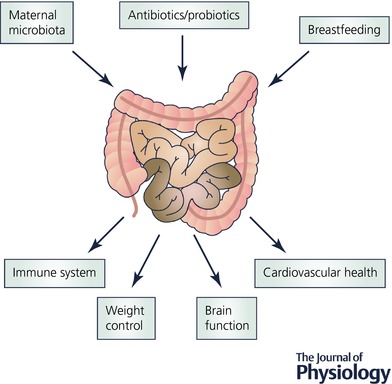
Exposures that contribute to gut colonization and how this impacts on health
Impact of infant gut colonization at birth: evidence from germ‐free mice
The consequences of different microbiota colonizing the infant gut can be directly investigated using germ‐free (GF) animal models, i.e. animals lacking microbial colonization/microbiota. ‘Colonization dysbiosis’ appears to occur during critical windows of microbiome development and can result in long‐term immunological abnormalities. Chung et al. (2012) found that host‐specific bacterial species were critical for immune maturation in GF mice. Colonization of the gut with murine microbiota at birth normalized the immune phenotype in GF mice to that of wild‐type mice. Similarly, GF mice exhibit airway hyper‐responsiveness (Herbst et al. 2011) that can be prevented by appropriate microbiota colonization during the first 2 weeks of life. This is linked to the formation of ‘airway hyper‐responsive protective’ Helios‐Treg cells that are microbiota‐dependent within 2 weeks of birth. Further studies would be invaluable to help determine both the microbial components conferring this immune tolerance, their ecological origin, and their human microbiota counterparts.
Altered gut microbiota after CS can impact risk for overweight and obesity, possibly related to the energy harvesting ability of the microbiota. Short chain fatty acids, produced by gut microbiota via anaerobic fermentation, are an energy source for colonic epithelial cells and activate G‐coupled protein receptors, such as free fatty acid receptor 3 (FFAR3 or Gpr41), which can modulate energy harvest. Experimentally, GF Gpr41−/− mice show increased weight gain on standard diet compared to wild‐type (Samuel et al. 2008), which could be prevented by specific gut bacterial inoculation. Importantly, Martinez et al. (2017) compared weight gain and gut microbiota in mice born by CS (n = 34) to vaginally delivered control mice (n = 35) and found that the bacterial taxa associated with VD conferred a lean phenotype.
Gut dysbiosis has also been linked to the development of hypertension (Adnan et al. 2017; Li et al. 2017). Li et al. (2017) found that the gut microbiota of pre‐hypertensive and hypertensive individuals was enriched in Prevotella, which favours the development of a pro‐inflammatory environment. This low‐grade inflammation, triggered by lipopolysaccharide in Gram‐negative bacteria, is thought to be responsible for the pathophysiology of hypertension in these subjects. The authors also demonstrated that hypertension could be transferred to GF mice after a faecal transplant from a human hypertensive donor (Li et al. 2017).
While the relation between the gut peptides and central nervous system has long been recognized, recent evidence from animal studies indicates that the gut microbiota itself may also influence brain function and development (Farmer et al. 2014). Sudo et al. (2004) found that GF mice had reduced expression of cortical and hippocampal brain‐derived neurotrophic factor, a protein involved in neuronal growth and survival, low levels of which has been linked with anxiety‐like conditions (Sudo et al. 2004; Bercik et al. 2011; Farmer et al. 2014).
Overall these germ‐free models highlight a clear mechanistic pathway from dysbiosis to a range of adverse outcomes, but the specific contribution of mode of delivery as causal requires further elucidation.
Effect of perinatal antibiotics
The increase in prophylactic antibiotic use during surgery may impact development of infant microbiota (Azad et al. 2016; Martinez et al. 2017). Intrapartum antibiotics can lead to increased abundance of Bacteroides and Enterobacteria and reduction of Bacteroidetes in the neonatal gut (Li et al. 2017). Others have also observed an altered gut composition in infants who had been exposed to antibiotics, with reduced diversity and richness of the gut microbiota immediately after birth and impaired growth of Clostridiales and Ruminococcus up to 9 months of age (Bokulich et al. 2016).
Mode of birth and epigenetic modification of gene expression
Epigenetics offers a molecular mechanism and theoretical construct whereby parturition can impact on gene expression, potentially in a stable and heritable manner, to programme phenotypic traits. DNA methylation is the most commonly studied of the epigenetic mechanisms, and in general terms, hypermethylation can effectively silence or attenuate gene expression and hypomethylation ensure genes remain ‘switched on’.
According to the emerging EPIIC hypothesis, ‘eustress’, a healthy stress experienced in normal delivery, is thought to be responsible for priming the fetus for optimal responses in extrauterine life. The stress triggers epigenetic modification of certain genes linked to immune responses, weight control and specific tumour suppressor genes. ‘Dys‐stress’, on the other hand, is a stress response that is either depressed below or elevated above a physiological eustress norm (Dahlen et al. 2013) proposed to affect normal epigenetic processes.
However, it is not yet clear how the events during parturition (use of synthetic oxytocin, antibiotics and CS) would affect the epigenetic remodelling and subsequent health of the mother and offspring. Behavioural exposures, e.g. stress, could activate signalling pathways in the brain, which through classical membrane receptor signalling cascades can target histone acetyltransferase, for example (Gabory et al. 2009). It is also likely that intrapartum antibiotics and anaesthetic drugs used during CS may indirectly lead to epigenetic changes through altering the composition of the commensal organisms in both mother and baby.
There are limited studies of mode of delivery and epigenetic changes and those that exist present contradictory findings. One reported that global DNA methylation in white blood cells obtained from cord blood was elevated in newborns born by CS without labour compared with those born by vaginal delivery with labour (Schlinzig et al. 2009), but another found no evidence of global methylation changes associated with CS versus VD (Virani et al. 2012) despite a larger sample size. Methodological differences in methylation assay and adjustment for maternal factors may account for the disparity between these two studies. In a third study, of cord blood samples from babies born via VD (n = 37) and CS (n = 27), higher levels of global methylation was observed in DNA from CD34+ cells of CS infants (Almgren et al. 2014). Thus, it is plausible that epigenetic effects of birth have implications in development of the immune system.
Experimental studies employing neonatal stress paradigms have also demonstrated epigenetic alterations in DNA methylation of glucocorticoid receptors in the adult hippocampus associated with increased stress reactivity in later life (Weaver et al. 2004). Furthermore, exposure to synthetic oxytocin, used to induce and augment labour, has been shown to influence feeding cues and can lead to difficulties with breastfeeding by an unknown mechanism (Bell et al. 2013; Marín Gabriel et al. 2015). Dahlen et al. (2013) suggest that these effects of synthetic oxytocin are modulated by epigenetic remodelling in both mother and infant. The functional correlates of any ‘parturition’‐associated global methylation status in cord blood and persistence into childhood and beyond requires further follow‐up.
More numerous and larger human studies are needed to assess the impact of mode of delivery on epigenetic mechanisms and long‐term biological implications. Developing appropriate animal models would help in this regard.
What can be done to mitigate the effects of CS?
Optimization of childbirth
Ideally, the negative impact of mode of delivery could be avoided by promoting normal physiological processes during childbirth in an appropriate and safe environment. Assessment, care planning, health education and promotion of normal processes could set the stage for strengthening the woman's capabilities in preparation for labour, birth and motherhood (Renfrew et al. 2014). Helping women understand the physiological value of awaiting spontaneous labour and to prepare to cope with the rigors of labour can foster eustress and support the interconnection of the complex biological and chemical processes to benefit the mother and newborn. Several processes can be supported by clinical care givers in this regard including the following. (i) Minimization of fear and anxiety: most mammals will only give birth in situations where they feel safe and this has implications for human childbirth. Feeling safe may account for robust evidence from Cochrane Reviews on the effectiveness of midwife‐led continuity of care, alternative birth settings and continuous support in labour on increased spontaneous vaginal birth (Hodnett et al. 2012; Sandall et al. 2016; Bohren et al. 2017). Reciprocal knowledge and trust in the woman–provider relationship may be one of many factors to improved physiological labour functioning and avoidance of CS. (ii) Organizational procedures could reduce unnecessary CS through implementation of appropriate guidelines (Khunpradit et al. 2011; National Institute for Health and Care Excellence, 2017). A study of 2400 US mothers’ experience of childbirth found that women felt pressured to have certain interventions such as labour induction (25%), primary CS (28%) and repeat CS (22%) (Declercq et al. 2013). Expectant management and avoiding interventions unless clinically indicated can limit the use of synthetic oxytocin, averting uterine hyperstimulation, increased pain, and disruption of the maternal and fetal/newborn regulation that promotes breastfeeding (prolactin), attachment and maternal mood postpartum (Buckley, 2015). All interventions must be evaluated from a ‘first, do not harm’ perspective. Simply because a CS is a relatively safe procedure, potential long‐term consequences cannot be ignored (Kennedy et al. 2016).
However, there will always be critical medical indications for assisted delivery and CS, currently approximately 10%, but increasing with advancing maternal age and the obesity epidemic. Thus, efforts should be made to keep CS as physiological as possible to evoke normal developmental cues. One such method of maintaining the physiological integrity of normal delivery could be to mimic the surge of hormones associated with VD. As identified above, infants delivered by CS can have an increased risk of respiratory complications because of reduced exposure to cortisol during delivery (Jain & Dudell, 2006). Promising results from randomized trials showed that administration of antenatal steroids to mothers delivering full‐term infants by elective CS improved neonatal respiratory outcomes (Stutchfield, 2005). Furthermore, a systematic review, including three randomized controlled trials of full‐term infants delivered by elective CS, found that administering antenatal steroids 48 h prior to delivery reduced the risk of respiratory distress syndrome and transient tachypnoea, and reduced the length of stay in the neonatal care unit (Saccone & Berghella, 2016). However, the use of corticosteroids is controversial and would have to be very carefully reviewed before being implemented on a wide scale.
An alternative method of mimicking the effects of normal delivery that may be more acceptable is ensuring skin to skin contact at delivery. Skin to skin contact, resembling the Madigan squeeze technique, was administered to 39 full‐term neonates born via CS and VD during the first 2 days of life; a dose–response relationship was observed and a reduction in the levels of inhibitory neurosteroids (McCallie et al. 2017). Although this effect was only significant for pregnanolone in all deliveries, plus progesterone in infants delivered via VD, the authors suggested that a different response may be observed in critically ill or preterm infants (McCallie et al. 2017).
Vaginal seeding
Vaginal seeding is a relatively new practice, but is increasing in popularity. This technique involves a sterile gauze being incubated inside the vagina and applied to the neonate's skin to inoculate with the maternal bacteria it would have otherwise been exposed to during a vaginal delivery (Dominguez‐Bello et al. 2016). However, there is limited evidence on safety of this practice and its efficacy in improving infant health in the long term (Cunnington et al. 2016; Dominguez‐Bello et al. 2016). One important consideration when carrying out this technique is the risk of infections, particularly Streptococcus B (Cunnington et al. 2016). However, as discussed above, the limited evidence indicating that the vaginal microbiota are responsible for seeding the gut indicates that facilitation of maternal faecal seeding or probiotic intervention should also be considered.
Breastfeeding
Breastfeeding contributes to colonization of the gut and strongly influences the seeding of the gut, particularly in those born by CS (Hill et al. 2017). Human milk oligosaccharides are the third most abundant component of breastmilk and support B. infantis growth (Underwood et al. 2015), which is particularly important for infants born via CS due to their low abundance of Bifidobacterium (Frese et al. 2017). Infant formula, which has been found to alter the normal development of gut microbiota in both VD infants and those delivered via CS (Yasmin et al. 2017), does not contain these essential human milk oligosaccharides. The INFANTMET cohort examined the evolution of gut microbiota in full‐term and preterm infants born by CS and VD and found that at 4 weeks old the gut microbiota of breastfed infants born via CS begins to resemble that of an infant delivered vaginally, with an increased abundance of Actinobacteria (Hill et al. 2017), and this resemblance strengthened from weeks 4 to 8 with an increase in Bacteroidetes in CS infants. This highlights the importance of breastfeeding in establishing a ‘normal’ gut microbiota in infants born via CS. As women who deliver via CS have increased rates of breastfeeding problems and early cessation, particularly those who have an elective CS, one pathway to optimizing the health of the infant would be to consistently provide women delivering via CS with more support to breastfeed.
Supplementation of probiotics
Given the role the gut microbiota plays in the development of the immune system, reversing dysbiosis could potentially improve long‐term outcome for CS infants, especially if undertaken in parallel with breast feeding.
Animal studies have demonstrated the potential use of probiotic supplementation of neonates to improve immune function (Underwood et al. 2014; Lewis et al. 2017). In human neonates, supplementation of the probiotic B. infantis (strain EVC001, IMPRINT Study) for 21 days appears to be tolerated in breastfed babies. Supplementation significantly increased the level of B. infantis in infant faecal samples compared to the control (Smilowitz et al. 2017), an effect that persisted for more than 30 days after supplementation. B. infantis supplementation also led to an increase in faecal acetate and lactate, decreases in faecal pH and bacterial endotoxin and fewer stools, all indications of improved microbiome (Frese et al. 2017). Whilst mode of delivery was not the focus of this study, babies from CS deliveries generally had less B. infantis than VD babies and this was reversed by probiotic treatment. A larger study of CS infants including child health follow‐up would be useful.
Conclusions
There is a growing body of evidence that mode of delivery, particularly pre‐labour CS, can have long‐term health consequences for the infant. There is still a need to experimentally establish a direct causal pathway from mode of delivery via microbiome alterations to long‐term health issues, but observational evidence is convincing. Increased efforts to reduce CS rates per se as well implementing care pathways that optimize parturition physiology are required. There seems promise in pursing interventions that promote neonatal gut health and breastfeeding. The impact of epigenetic changes imposed by mode of delivery is an interesting concept, but requires more corroborative experimental evidence.
Additional information
Competing interests
None of the authors has any conflict of interest. However, R.T. and P.T. have received grant funding from commercial companies involved in probiotic and prebiotic supplementation in infants and animal models, respectively.
Author contributions
R.M.T., P.T. and N.M.K. conceived the structure of the review and all authors significantly contributed to writing and reviewing the final manuscript. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Acknowledgements
The authors would like to thank Caitlin Daniels for help with illustrations. J.S. is supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South London at King's College Hospital NHS Foundation Trust. The views expressed are those of the author[s] and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Biographies
Rachel Tribe and Paul Taylor (pictured above) are physiologists who undertake translational research focused on improving outcome for pregnant women and their children. Senior academics in the Department of Women and Children's Health, King's College London, they both run thriving research groups and publish widely on pregnancy complications and the impact of pregnancy on life‐long health.

Niamh Kelly has recently joined Rachel Tribe's team to study breastfeeding, probiotics and infant health.
Doug Rees is a PhD student in Paul Taylor's team studying the gut microbiome in obese pregnancy.
Jane Sandall (King's College London) and Holly Kennedy (Yale School of Nursing) have academic interests in birth outcomes, epigenetic changes during labour and clinical care factors to optimize birth.
Edited by: Ole Petersen & Laura Bennet
References
- Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM & Durgan DJ (2017). Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics 49, 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman M, Weich KM & Madigan JE (2017). Survey of veterinarians using a novel physical compression squeeze procedure in the management of neonatal maladjustment syndrome in foals. Animals 7, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almgren M, Schlinzig T, Gomez‐Cabrero D, Gunnar A, Sundin M, Johansson S, Norman M & Ekström TJ (2014). Cesarean delivery and hematopoietic stem cell epigenetics in the newborn infant: implications for future health? Am J Obstet Gynecol 211, 502.e1–502.e8. [DOI] [PubMed] [Google Scholar]
- Azad M, Konya T, Persaud R, Guttman D, Chari R, Field C, Sears M, Mandhane P, Turvey S, Subbarao P, Becker A, Scott J & Kozyrskyj A (2016). Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG 123, 983–993. [DOI] [PubMed] [Google Scholar]
- Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva‐Datchary P, Li Y, Xia Y, Xie H, Zhong H, Khan MT, Zhang J, Li J, Xiao L, Al‐Aama J, Zhang D, Lee YS, Kotowska D, Colding C, Tremaroli V, Yin Y, Bergman S, Xu X, Madsen L, Kristiansen K, Dahlgren J & Wang J (2015). Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17, 690–703. [DOI] [PubMed] [Google Scholar]
- Bager P, Wohlfahrt J & Westergaard T (2008). Caesarean delivery and risk of atopy and allergic disesase: meta‐analyses. Clin Exp Allergy 38, 634–642. [DOI] [PubMed] [Google Scholar]
- Bell AF, White‐Traut R & Rankin K (2013). Fetal exposure to synthetic oxytocin and the relationship with prefeeding cues within one hour postbirth. Early Hum Dev 89, 137–143. [DOI] [PubMed] [Google Scholar]
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, MacRi J, McCoy KD, Verdu EF & Collins SM (2011). The intestinal microbiota affect central levels of brain‐derived neurotropic factor and behavior in mice. Gastroenterology 141, 599–609.e3. [DOI] [PubMed] [Google Scholar]
- Berger N, Vaillancourt C & Boksa P (2000). Interactive effects of anoxia and general anesthesia during birth on the degree of CNS and systemic hypoxia produced in neonatal rats. Exp Brain Res 131, 524–531. [DOI] [PubMed] [Google Scholar]
- Betrán AP, Ye J, Moller A‐B, Zhang J, Gülmezoglu AM & Torloni MR (2016). The increasing trend in caesarean section rates: global, regional and national estimates: 1990–2014. PLoS One 11, e0148343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasucci G, Rubini M, Riboni S, Morelli L, Bessi E & Retetangos C (2010). Mode of delivery affects the bacterial community in the newborn gut. Early Hum Dev 86, 13–15. [DOI] [PubMed] [Google Scholar]
- Bird JA, Spencer JA, Mould T & Symonds ME (1996). Endocrine and metabolic adaptation following caesarean section or vaginal delivery. Arch Dis Child Fetal Neonatal Ed 74, F132–F134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohren MA, Hofmeyr GJ, Sakala C, Fukuzawa RK & Cuthbert A (2017). Continuous support for women during childbirth. Cochrane Database Syst Rev 7, CD003766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksa P & El‐Khodor BF (2003). Birth insult interacts with stress at adulthood to alter dopaminergic function in animal models: possible implications for schizophrenia and other disorders. Neurosci Biobehav Rev 27, 91–101. [DOI] [PubMed] [Google Scholar]
- Boksa P & Zhang Y (2008). Epinephrine administration at birth prevents long‐term changes in dopaminergic parameters caused by Cesarean section birth in the rat. Psychopharmacology (Berl) 200, 381–391. [DOI] [PubMed] [Google Scholar]
- Boksa P, Zhang Y & Nouel D (2015). Maternal oxytocin administration before birth influences the effects of birth anoxia on the neonatal rat brain. Neurochem Res 40, 1631–1643. [DOI] [PubMed] [Google Scholar]
- Bokulich NA, Chung J, Battaglia T, Henderson N, Jay M, Li H, D Lieber A, Wu F, Perez‐Perez GI, Chen Y, Schweizer W, Zheng X, Contreras M, Dominguez‐Bello MG & Blaser MJ (2016). Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med 8, 343ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley SJ (2015). Hormonal Physiology of Childbearing: Evidence and Implications for Women, Babies, and Maternity Care Childbirth Connection. National Partnership for Women & Families, Washington DC, http://www.nationalpartnership.org/research-library/maternal-health/hormonal-physiology-of-childbearing.pdf [Accessed 18 December 2017]. [Google Scholar]
- Chiș A, Vulturar R, Andreica S, Prodan A & Miu AC (2017). Behavioral and cortisol responses to stress in newborn infants: effects of mode of delivery. Psychoneuroendocrinology 86, 203–208. [DOI] [PubMed] [Google Scholar]
- Cho CE & Norman M (2013). Cesarean section and development of the immune system in the offspring. Am J Obstet Gynecol 208, 249–254. [DOI] [PubMed] [Google Scholar]
- Chu DM, Ma J, Prince AL, Antony KM, Seferovic MD & Aagaard KM (2017a). Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med 23, 314–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S, Chen Q, Chen Y, Bao Y, Wu M & Zhang J (2017b). Cesarean section without medical indication and risk of childhood asthma, and attenuation by breastfeeding. PLoS One 12, e0184920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, Reading NC, Villablanca EJ, Wang S, Mora JR, Umesaki Y, Mathis D, Benoist C, Relman DA & Kasper DL (2012). Gut immune maturation depends on colonization with a host‐specific microbiota. Cell 149, 1578–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnington AJ, Sim K, Deierl A, Kroll JS, Brannigan E & Darby J (2016). “Vaginal seeding” of infants born by caesarean section. BMJ 352, i227. [DOI] [PubMed] [Google Scholar]
- Curran EA, Cryan JF, Kenny LC, Dinan TG, Kearney PM & Khashan AS (2016a). Obstetrical mode of delivery and childhood behavior and psychological development in a british cohort. J Autism Dev Disord 46, 603–614. [DOI] [PubMed] [Google Scholar]
- Curran EA, Dalman C, Kearney PM, Kenny LC, Cryan JF, Dinan TG & Khashan AS (2015a). Association between obstetric mode of delivery and autism spectrum disorder. JAMA Psychiatry 72, 935. [DOI] [PubMed] [Google Scholar]
- Curran EA, Khashan AS, Dalman C, Kenny LC, Cryan JF, Dinan TG & Kearney PM (2016b). Obstetric mode of delivery and attention‐deficit/hyperactivity disorder: a sibling‐matched study. Int J Epidemiol 532–542. [DOI] [PubMed] [Google Scholar]
- Curran EA, O'Neill SM, Cryan JF, Kenny LC, Dinan TG, Khashan AS & Kearney PM (2015b). Research Review: Birth by caesarean section and development of autism spectrum disorder and attention‐deficit/hyperactivity disorder: A systematic review and meta‐analysis. J Child Psychol Psychiatry Allied Discip 56, 500–508. [DOI] [PubMed] [Google Scholar]
- Dahlen HG, Downe S, Wright ML, Kennedy HP & Taylor JY (2016). Childbirth and consequent atopic disease: emerging evidence on epigenetic effects based on the hygiene and EPIIC hypotheses. BMC Pregnancy Childbirth 16, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlen HG, Kennedy HP, Anderson CM, Bell AF, Clark A, Foureur M, Ohm JE, Shearman AM, Taylor JY, Wright ML & Downe S (2013). The EPIIC hypothesis: intrapartum effects on the neonatal epigenome and consequent health outcomes. Med Hypotheses 80, 656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JA, Carroll JA, Keisler DH & Kojima CJ (2008). Evaluation of immune system function in neonatal pigs born vaginally or by Cesarean section. Domest Anim Endocrinol 35, 81–87. [DOI] [PubMed] [Google Scholar]
- Darmasseelane K, Hyde MJ, Santhakumaran S, Gale C & Modi N (2014). Mode of delivery and offspring body mass index, overweight and obesity in adult life: a systematic review and meta‐analysis. PLoS One 9, e87896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declercq ER, Sakala C, Corry MP, Applebaum S & Herrlich A (2013). Listening to Mothers III: Pregnancy and Birth. Childbirth Connection, New Yok, http://transform.childbirthconnection.org/wp-content/uploads/2013/06/LTM-III_Pregnancy-and-Birth.pdf. [Google Scholar]
- Dominguez‐Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N & Knight R (2010). Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA 107, 11971–11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez‐Bello MG, De Jesus‐Laboy KM, Shen N, Cox LM, Amir A, Gonzalez A, Bokulich NA, Song SJ, Hoashi M, Rivera‐Vinas JI, Mendez K, Knight R & Clemente JC (2016). Partial restoration of the microbiota of cesarean‐born infants via vaginal microbial transfer. Nat Med 22, 250–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbay A, Celik U, Celik B, Ozer OF, Kilic G, Akkan JCU, Bayraktar BT & Kaymak NZ (2016). Intraocular pressure in infants and its association with hormonal changes with vaginal birth versus cesarean section. Int Ophthalmol 36, 855–860. [DOI] [PubMed] [Google Scholar]
- El‐Khodor BF & Boksa P (1997). Long‐term reciprocal changes in dopamine levels in prefrontal cortex versus nucleus accumbens in rats born by caesarean section compared to vaginal birth. Exp Neurol 145, 118–129. [DOI] [PubMed] [Google Scholar]
- Farmer AD, Randall HA & Aziz Q (2014). It's a gut feeling: How the gut microbiota affects the state of mind. J Physiol 592, 2981–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes M, Ferraro A, Cardosa VC, Goldani M, Moura de Silva AA, Bettiol H & Baribieri M ( 2015). Cesarean section as an independent risk factor for hypertension among young adults. J Dev Orig Health Dis 6, S29. [Google Scholar]
- Frese SA, Hutton AA, Contreras LN, Shaw CA, Palumbo MC, Casaburi G, Xu G, Davis JCC, Lebrilla CB, Henrick BM, Freeman SL, Barile D, German JB, Mills DA, Smilowitz JT, Underwood MA & Krajmalnik‐Brown R (2017). Persistence of supplemented Bifidobacterium longum subsp. infantis EVC001 in breastfed infants. mSphere 2, e00341–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabory A, Attig L & Junien C (2009). Sexual dimorphism in environmental epigenetic programming. Mol Cell Endocrinol 304, 8–18. [DOI] [PubMed] [Google Scholar]
- Gitau R, Menson E, Pickles V, Fisk NM, Glover V & MacLachlan N (2001). Umbilical cortisol levels as an indicator of the fetal stress response to assisted vaginal delivery. Eur J Obstet Gynecol Reprod Biol 98, 14–17. [DOI] [PubMed] [Google Scholar]
- Guo S, Guo Y, Ergun A, Lu L, Walker WA & Ganguli K (2015). Secreted metabolites of Bifidobacterium infantis and Lactobacillus acidophilus protect immature human enterocytes from IL‐1β‐induced inflammation: a transcription profiling analysis. PLoS One 10, e0124549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman L, Spencer JA & Symonds ME (1997). Plasma prolactin concentrations after caesarean section or vaginal delivery. Arch Dis Child Fetal Neonatal Ed 77, 237–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst T, Sichelstiel A, Schär C, Yadava K, Bürki K, Cahenzli J, McCoy K, Marsland BJ & Harris NL (2011). Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am J Respir Crit Care Med 184, 198–205. [DOI] [PubMed] [Google Scholar]
- Hill CJ, Lynch DB, Murphy K, Ulaszewska M, Jeffery IB, O'Shea CA, Watkins C, Dempsey E, Mattivi F, Tuohy K, Ross RP, Ryan CA, O’ Toole PW & Stanton C (2017). Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome 5, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs AJ, Mannion CA, Mcdonald SW, Brockway M & Tough SC (2016). The impact of caesarean section on breastfeeding initiation, duration and difficulties in the first four months postpartum. BMC Pregnancy Childbirth 16, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodnett ED, Downe S & Walsh D (2012). Alternative versus conventional institutional settings for birth. Cochrane Database Syst Rev, CD000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman CM, Sparén P & Cnattingius S (2002). Perinatal risk factors for infantile autism. Epidemiology 13, 417–423. [DOI] [PubMed] [Google Scholar]
- Hyde MJ & Modi N (2012). The long‐term effects of birth by caesarean section: The case for a randomised controlled trial. Early Hum Dev 88, 943–949. [DOI] [PubMed] [Google Scholar]
- Jain L & Dudell GG (2006). Respiratory transition in infants delivered by cesarean section. Semin Perinatol 30, 296–304. [DOI] [PubMed] [Google Scholar]
- Jain L & Eaton DC (2006). Physiology of fetal lung fluid clearance and the effect of labor. Semin Perinatol 30, 34–43. [DOI] [PubMed] [Google Scholar]
- Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, Björkstén B, Engstrand L & Andersson AF (2014). Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by Caesarean section. Gut 63, 559–566. [DOI] [PubMed] [Google Scholar]
- Keag OE, Norman JE & Stock SJ (2018). Long‐term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: Systematic review and meta‐analysis. PLoS Med 15, e1002494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelmanson IA (2013). Emotional and behavioural features of preschool children born by Caesarean deliveries at maternal request. Eur J Dev Psychol 10, 676–690. [Google Scholar]
- Kennedy HP, Yoshida S, Costello A, Declercq E, Dias MA, Duff E, Gherissi A, Kaufman K, McConville F, McFadden A, Michel‐Schuldt M, Moyo NT, Schuiling K, Speciale AM & Renfrew MJ (2016). Asking different questions: research priorities to improve the quality of care for every woman, every child. Lancet Glob Heal 4, e777–e779. [DOI] [PubMed] [Google Scholar]
- Khalaf SY Al, O'Neill SM, O'Keeffe LM, Henriksen TB, Kenny LC, Cryan JF & Khashan AS (2015). The impact of obstetric mode of delivery on childhood behavior. Soc Psychiatry Psychiatr Epidemiol 50, 1557–1567. [DOI] [PubMed] [Google Scholar]
- Khunpradit S, Tavender E, Lumbiganon P, Laopaiboon M, Wasiak J & Gruen RL (2011). Non‐clinical interventions for reducing unnecessary caesarean section. Cochrane Database Syst Rev CD005528. [DOI] [PubMed] [Google Scholar]
- Kristensen K & Henriksen L (2016). Cesarean section and disease associated with immune function. J Allergy Clin Immunol 137, 587–590. [DOI] [PubMed] [Google Scholar]
- Lagercrantz H (2016). The good stress of being born. Acta Paediatr Int J Paediatr 105, 1413–1416. [DOI] [PubMed] [Google Scholar]
- Lewis MC, Merrifield CA, Berger B, Cloarec O, Duncker S, Mercenier A, Nicholson JK, Holmes E & Bailey M (2017). Early intervention with Bifidobacterium lactis NCC2818 modulates the host‐microbe interface independent of the sustained changes induced by the neonatal environment. Sci Rep 7, 5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B, Zhang W, Weldon R, Auguste K, Yang L, Liu X, Chen L, Yang X, Zhu B & Cai J (2017). Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 5, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S‐L, Tsai M‐H, Yao T‐C, Hua M‐C, Yeh K‐W, Chiu C‐Y, Su K‐W, Huang S‐Y, Kao C‐C, Lai S‐H & Huang J‐L (2017). Caesarean section is associated with reduced perinatal cytokine response, increased risk of bacterial colonization in the airway, and infantile wheezing. Sci Rep 7, 9053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magne F, Puchi Silva A, Carvajal B & Gotteland M (2017). The elevated rate of cesarean section and its contribution to non‐communicable chronic diseases in Latin America: the growing involvement of the microbiota. Front Pediatr 5, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino H, Kushiro A, Ishikawa E, Kubota H, Gawad A, Sakai T, Oishi K, Martin R, Ben‐Amor K, Knol J & Tanaka R (2013). Mother‐to‐infant transmission of intestinal bifidobacterial strains has an impact on the early development of vaginally delivered infant's microbiota. PLoS One 8, e78331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín Gabriel MA, Olza Fernández I, Malalana Martínez AM, González Armengod C, Costarelli V, Millán Santos I, Fernández‐Cañadas Morillo A, Pérez Riveiro P, López Sánchez F & García Murillo L (2015). Intrapartum synthetic oxytocin reduce the expression of primitive reflexes associated with breastfeeding. Breastfeed Med 10, 209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Osterman MJK, Curtin SC & Mathews TJ (2015). National vital statistics reports births: final data for 2013. Natl Vital Stat Reports 64, 1–104. [PubMed] [Google Scholar]
- Martinez KA, Devlin JC, Lacher CR, Yin Y, Cai Y, Wang J & Dominguez‐Bello MG (2017). Increased weight gain by C‐section: functional significance of the primordial microbiome. Sci Adv 3, eaao1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallie K, Gaikwad N, Cuadrado C, Aleman M, Madigan J, Stevenson D & Bhutani V (2017). Skin‐to‐skin contact after birth and the natural course of neurosteroid levels in healthy term newborns. J Perinatol 37, 591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Abalos E, Chamillard M, Ciapponi A, Colaci D, Comandé D, Diaz V, Geller S, Hanson C, Langer A, Manuelli V, Millar K, Morhason‐Bello I, Castro CP, Pileggi VN, Robinson N, Skaer M, Souza JP, Vogel JP & Althabe F (2016). Beyond too little, too late and too much, too soon: a pathway towards evidence‐based, respectful maternity care worldwide. Lancet 388, 2176–2192. [DOI] [PubMed] [Google Scholar]
- Nakamura‐Pereira M, do Carmo Leal M, Esteves‐Pereira AP, Domingues RMSM, Torres JA, Dias MAB & Moreira ME (2016). Use of Robson classification to assess cesarean section rate in Brazil: the role of source of payment for childbirth. Reprod Health 13, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence (2017). Intrapartum Care for Healthy Women and Babies https://www.nice.org.uk/guidance/cg190/ifp/chapter/Care-of-women-and-their-babies-during-labour-and-birth [Accessed 18 December 2017].
- Negele K, Heinrich J, Borte M, Von Berg A, Schaaf B, Lehmann I, Wichmann HE & Bolte G (2004). Mode of delivery and development of atopic disease during the first 2 years of life. Pediatr Allergy Immunol 15, 48–54. [DOI] [PubMed] [Google Scholar]
- NHS Digital (2017). NHS Maternity Statistics, England 2016–17 https://digital.nhs.uk/catalogue/PUB30137 [Accessed 17 November 2017].
- Pannaraj PS, Li F, Cerini C, Bender JM, Yang S, Rollie A, Adisetiyo H, Zabih S, Lincez PJ, Bittinger K, Bailey A, Bushman FD, Sleasman JW & Aldrovandi GM (2017). Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr 171, 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patole SK, Rao SC, Keil AD, Nathan EA, Doherty DA & Simmer KN (2016). Benefits of Bifidobacterium breve M‐16 V supplementation in preterm neonates – a retrospective cohort study. PLoS One 11, e0150775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluymen LPM, Smit HA, Wijga AH, Gehring U, De Jongste JC & Van Rossem L (2016). Cesarean delivery, overweight throughout childhood, and blood pressure in adolescence. J Pediatr 179, 111–117.e3. [DOI] [PubMed] [Google Scholar]
- Ramachandrappa A & Jain L (2008). Elective cesarean section: its impact on neonatal respiratory outcome. Clin Perinatol 35, 373–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RE, Kim E‐M, Page D, O'Mara SM & O'Hare E (2007). Thyroxine replacement in an animal model of congenital hypothyroidism. Physiol Behav 91, 299–303. [DOI] [PubMed] [Google Scholar]
- Renfrew MJ, McFadden A, Bastos MH, Campbell J, Channon AA, Cheung NF, Silva DRAD, Downe S, Kennedy HP, Malata A, McCormick F, Wick L & Declercq E (2014). Midwifery and quality care: findings from a new evidence‐informed framework for maternal and newborn care. Lancet 384, 1129–1145. [DOI] [PubMed] [Google Scholar]
- Rutayisire E, Wu X, Huang K, Tao S, Chen Y & Tao F (2017). Childhood emotional and behavior problems and their associations with cesarean delivery. Rev Bras Psiquiatr, 10.1590/1516-4446-2016-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone G & Berghella V (2016). Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta‐analysis of randomized controlled trials. BMJ 355, i5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakwinska O, Foata F, Berger B, Brüssow H, Combremont S, Mercenier A, Dogra S, Soh S‐E, Yen JCK, Heong GYS, Lee YS, Yap F, Meaney MJ, Chong Y‐S, Godfrey KM & Holbrook JD (2017). Does the maternal vaginal microbiota play a role in seeding the microbiota of neonatal gut and nose? Benef Microbes 8, 763–778. [DOI] [PubMed] [Google Scholar]
- Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M & Gordon JI (2008). Effects of the gut microbiota on host adiposity are modulated by the short‐chain fatty‐acid binding G protein‐coupled receptor, Gpr41. Proc Natl Acad Sci USA 105, 16767–16772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandall J, Soltani H, Gates S, Shennan A & Devane D (2016). Midwife‐led continuity models versus other models of care for childbearing women. Cochrane Database Syst Rev, CD004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinzig T, Johansson S, Gunnar A, Ekström T & Norman M (2009). Epigenetic modulation at birth – altered DNA‐methylation in white blood cells after Caesarean section. Acta Paediatr 98, 1096–1099. [DOI] [PubMed] [Google Scholar]
- Sedaghat N, Ellwood D, Shadbolt B, Kecskes Z, Falk MC, Brussel T & Kent AL (2008). The effect of mode of delivery and anaesthesia on neonatal blood pressure. Aust New Zeal J Obstet Gynaecol 48, 172–178. [DOI] [PubMed] [Google Scholar]
- Sevelsted A, Stokholm J, Bonnelykke K & Bisgaard H (2015). Cesarean section and chronic immune disorders. Pediatrics 135, 92–98. [DOI] [PubMed] [Google Scholar]
- Silva D, Colvin L, Hagemann E & Bower C (2014). Environmental risk factors by gender associated with attention‐deficit/hyperactivity disorder. Pediatrics 133, 14–22. [DOI] [PubMed] [Google Scholar]
- Sirilun S, Takahashi H, Boonyaritichaikij S, Chaiyasut C, Lertruangpanya P, Koga Y & Mikami K (2015). Impact of maternal bifidobacteria and the mode of delivery on Bifidobacterium microbiota in infants. Benef Microbes 6, 767–774. [DOI] [PubMed] [Google Scholar]
- Smilowitz JT, Moya J, Breck MA, Cook C, Fineberg A, Angkustsiri K & Underwood MA (2017). Safety and tolerability of Bifidobacterium longum subspecies infantis EVC001 supplementation in healthy term breastfed infants: a phase I clinical trial. BMC Pediatr 17, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CJ, Embleton ND, Marrs ECL, Smith DP, Fofanova T, Nelson A, Skeath T, Perry JD, Petrosino JF, Berrington JE & Cummings SP (2017). Longitudinal development of the gut microbiome and metabolome in preterm neonates with late onset sepsis and healthy controls. Microbiome 5, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CJ, Embleton ND, Marrs ECL, Smith DP, Nelson A, Abdulkadir B, Skeath T, Petrosino JF, Perry JD, Berrington JE & Cummings SP (2016). Temporal bacterial and metabolic development of the preterm gut reveals specific signatures in health and disease. Microbiome 4, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutchfield P (2005). Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. BMJ 331, 662–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu X‐N, Kubo C & Koga Y (2004). Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J Physiol 5581, 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Fisk NM & Glover V (2000). Mode of delivery and subsequent stress response. Lancet 355, 120. [DOI] [PubMed] [Google Scholar]
- Taylor PD (2015). Bugs and stress “on top of genetics”: Can the way we are born affect our health? Midwifery 31, 341–344. [DOI] [PubMed] [Google Scholar]
- Thysen AH, Larsen JM, Rasmussen MA, Stokholm J, Bønnelykke K, Bisgaard H & Brix S (2015). Prelabor cesarean section bypasses natural immune cell maturation. J Allergy Clin Immunol 136, 1123–1125e6. [DOI] [PubMed] [Google Scholar]
- Treviño‐Garza C, Villarreal‐Martínez L, Estrada‐Zúñiga CM, Leal‐Treviño M, Rodríguez‐Balderrama I, Nieto‐Sanjuanero A, Cárdenas‐Del Castillo B, Montes‐Tapia FF & de la O‐Cavazos M (2016). Leptin, IL‐6 and TNF‐α levels in umbilical cord blood of healthy term newborns in relation to mode of delivery. J Obstet Gynaecol (Lahore) 36, 719–721. [DOI] [PubMed] [Google Scholar]
- UK National Institute for Health and Care Excellence (2011). Caesarean Section Clinical Guideline CG132. Available at: https://www.nice.org.uk/guidance/cg132. [PubMed]
- Underwood MA, Arriola J, Gerber CW, Kaveti A, Kalanetra KM, Kananurak A, Bevins CL, Mills DA & Dvorak B (2014). Bifidobacterium longum subsp. infantis in experimental necrotizing enterocolitis: alterations in inflammation, innate immune response, and the microbiota. Pediatr Res 76, 326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood MA, German JB, Lebrilla CB & Mills DA (2015). Bifidobacterium longum subspecies infantis: champion colonizer of the infant gut. Pediatr Res 77, 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt C, Berger N & Boksa P (1999). Effects of vaginal birth versus caesarean section birth with general anesthesia on blood gases and brain energy metabolism in neonatal rats. Exp Neurol 160, 142–150. [DOI] [PubMed] [Google Scholar]
- Venerosi A, Cutuli D, Chiarotti F & Calamandrei G (2006). C‐section birth per se or followed by acute global asphyxia altered emotional behaviour in neonate and adult rats. Behav Brain Res 168, 56–63. [DOI] [PubMed] [Google Scholar]
- Virani S, Dolinoy DC, Halubai S, Jones TR, Domino SE, Rozek LS, Nahar MS & Padmanabhan V (2012). Delivery type not associated with global methylation at birth. Clin Epigenetics 4, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl SE, Worda C, Egarter C, Bieglmayer C, Szekeres T, Huber J & Husslein P (2006). Mode of delivery is associated with maternal and fetal endocrine stress response. BJOG 113, 441–445. [DOI] [PubMed] [Google Scholar]
- Walker WA (2017). The importance of appropriate initial bacterial colonization of the intestine in newborn, child, and adult health. Pediatr Res 82, 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver ICG, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M & Meaney MJ (2004). Epigenetic programming by maternal behavior. Nat Neurosci 7, 847–854. [DOI] [PubMed] [Google Scholar]
- World Health Organisation (2015). WHO Statement on caesarean section rates. Reprod Health Matters 23, 149–150. [DOI] [PubMed] [Google Scholar]
- Yasmin F, Tun HM, Konya TB, Guttman DS, Chari RS, Field CJ, Becker AB, Mandhane PJ, Turvey SE, Subbarao P, Sears MR; CHILD Study Investigators , Scott JA, Dinu I & Kozyrskyj AL (2017). Cesarean section, formula feeding, and infant antibiotic exposure: separate and combined impacts on gut microbial changes in later infancy. Front Pediatr 5, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassour M, Vatanen T, Siljander H, Hämäläinen A‐M, Härkönen T, Ryhänen SJ, Franzosa EA, Vlamakis H, Huttenhower C, Gevers D, Lander ES, Knip M, Xavier RJ & Xavier RJ (2016). Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med 8, 343ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yektaei‐Karin E, Moshfegh A, Lundahl J, Berggren V, Hansson LO, Marchini G (2007). The stress of birth enhances in vitro spontaneous and IL‐8‐induced neutrophil chemotaxis in the human newborn. Pediatr Allergy Immunol 18, 643–651. [DOI] [PubMed] [Google Scholar]
- Yuan C, Gaskins AJ, Blaine AI, Zhang C, Gillman MW, Missmer SA, Field AE & Chavarro JE (2016). Association between cesarean birth and risk of obesity in offspring in childhood, adolescence, and early adulthood. JAMA Pediatr 170, e162385. [DOI] [PMC free article] [PubMed] [Google Scholar]


