Abstract
Key points
Fetal growth restriction increases the risk of fetal and neonatal mortality and morbidity, and contributes to increased risk of chronic disease later in life.
Intra‐amniotic insulin‐like growth factor‐1 (IGF1) treatment of the growth‐restricted ovine fetus improves fetal growth, but postnatal effects are unknown.
Here we report that intra‐amniotic IGF1 treatment of the growth‐restricted ovine fetus alters size at birth and mechanisms of early postnatal growth in a sex‐specific manner.
We also show that maternal plasma C‐type natriuretic peptide (CNP) products are related to fetal oxygenation and size at birth, and hence may be useful for non‐invasive monitoring of fetal growth restriction.
Intrauterine IGF1 treatment in late gestation is a potentially clinically relevant intervention that may ameliorate the postnatal complications of fetal growth restriction.
Abstract
Placental insufficiency‐mediated fetal growth restriction (FGR) is associated with altered postnatal growth and metabolism, which are, in turn, associated with increased risk of adult disease. Intra‐amniotic insulin‐like growth factor‐1 (IGF1) treatment of ovine FGR increases growth rate in late gestation, but the effects on postnatal growth and metabolism are unknown. We investigated the effects of intra‐amniotic IGF1 administration to ovine fetuses with uteroplacental embolisation‐induced FGR on phenotypical and physiological characteristics in the 2 weeks after birth. We measured early postnatal growth velocity, amino‐terminal propeptide of C‐type natriuretic peptide (NTproCNP), body composition, tissue‐specific mRNA expression, and milk intake in singleton lambs treated weekly with 360 μg intra‐amniotic IGF1 (FGRI; n = 13 females, 19 males) or saline (FGRS; n = 18 females, 12 males) during gestation, and in controls (CON; n = 15 females, 22 males). There was a strong positive correlation between maternal NTproCNP and fetal oxygenation, and size at birth in FGR lambs. FGR lambs were ∼20% lighter at birth and demonstrated accelerated postnatal growth velocity. IGF1 treatment did not alter perinatal mortality, partially abrogated the reduction in newborn size in females, but not males, and reduced accelerated growth in both sexes. IGF1‐mediated upregulation of somatotrophic genes in males during the early postnatal period could suggest that treatment effects are associated with delayed axis maturation, whilst treatment outcomes in females may rely on the reprogramming of nutrient‐dependent mechanisms of growth. These data suggest that the growth‐restricted fetus is responsive to intra‐amniotic intervention with IGF1, and that sex‐specific somatotrophic effects persist in the early postnatal period.
Keywords: fetal growth restriction, insulin‐like growth factor‐1, somatotrophic maturation, intrauterine intervention, growth and metabolism, amino‐terminal propeptide of C‐type natriuretic peptide
Key points
Fetal growth restriction increases the risk of fetal and neonatal mortality and morbidity, and contributes to increased risk of chronic disease later in life.
Intra‐amniotic insulin‐like growth factor‐1 (IGF1) treatment of the growth‐restricted ovine fetus improves fetal growth, but postnatal effects are unknown.
Here we report that intra‐amniotic IGF1 treatment of the growth‐restricted ovine fetus alters size at birth and mechanisms of early postnatal growth in a sex‐specific manner.
We also show that maternal plasma C‐type natriuretic peptide (CNP) products are related to fetal oxygenation and size at birth, and hence may be useful for non‐invasive monitoring of fetal growth restriction.
Intrauterine IGF1 treatment in late gestation is a potentially clinically relevant intervention that may ameliorate the postnatal complications of fetal growth restriction.
Introduction
Fetal growth restriction (FGR) increases morbidity and mortality and necessitates intensive antenatal surveillance and specialist management to optimise the timing of birth (Alberry & Soothill, 2007). Although constrained fetal growth in the face of nutritional deprivation is immediately beneficial for fetal survival (Patel & Kalhan, 1992; Diderholm et al. 2007), compromised metabolic regulation (Chiesa et al. 2008; Diderholm, 2009) and growth (Modi et al. 2006) persist postnatally. The growth‐restricted neonate is smaller and thinner than a normally‐grown baby, with reduced lean (Padoan et al. 2004) and fat mass (Verkauskiene et al. 2007), and greater risk of adverse perinatal outcomes (Bernstein et al. 2000; Unterscheider et al. 2013). Although vasodilatory hormones, such as C type natriuretic peptide (CNP), have been suggested to play an important role in maintaining placental function and fetal growth in compromised pregnancy (Prickett et al. 2007; McNeill et al. 2009; Reid et al. 2014; Espiner et al. 2015), non‐invasive fetal monitoring of late gestation compromised pregnancy is limited. There are no therapies that have been shown to improve fetal growth during compromised pregnancy in the clinical setting. Prenatal management focuses on regular assessment to balance the risk of intrauterine death with the adverse effects of preterm delivery (Baschat et al. 2001; Hecher et al. 2001; The GRIT Study Group, 2004).
Management of the growth‐restricted neonate focuses on mitigation of morbidity in the perinatal period, such as hypoglycaemia and hypothermia, and appropriate post‐natal growth. Small size at birth and thinness followed by accelerated postnatal growth velocity, termed “catch‐up” growth, is associated with increased visceral adiposity (Singhal et al. 2010) and increased leptin concentrations in childhood (Beltrand et al. 2009). It has been proposed that infant nutrition should be managed to prevent postnatal compensatory growth acceleration in order to reduce the future risk of obesity (Chomtho et al. 2008). However, this may contribute to postnatal growth restriction and compromise developmental outcomes (Sakurai et al. 2008). Thus, an intrauterine intervention that improves fetal growth may present an opportunity to improve perinatal and long‐term outcomes by reducing the adverse effects associated with both FGR and abnormal postnatal growth patterns.
Previous ovine FGR research has been integral to the assessment of prenatal interventions to improve in utero growth (Spiroski et al. 2016). A potential therapy that has been studied extensively through maternal (Liu et al. 1994), fetal (Jensen et al. 1999), and amniotic routes (Bloomfield et al. 2002a, b; Darp et al. 2010) is insulin‐like growth factor‐1 (IGF1). IGF1 is widely expressed in the developing fetus (Han et al. 1988) and is implicated in the regulation of placental function and fetal growth (Owens, 1991; Gluckman, 1997). Fetal IGF1 is regulated predominantly by fetal nutritional supply (Bloomfield et al. 2013), whereas maturation of the somatotrophic axis and endocrine activation occur around parturition (Gluckman et al. 1983). Intra‐amniotic IGF1 treatment of the FGR ovine fetus has been shown to increase fetal growth rate in late gestation (Eremia et al. 2007), even when given in physiological concentrations at weekly intervals (Wali et al. 2012). However, the effects of this brief prenatal intervention on phenotypic, physiologic, and molecular outcomes after birth are unknown. Thus, we investigated the effect of intra‐amniotic IGF1‐treatment of the growth‐restricted fetus on perinatal mortality and on neonatal growth, including assessment of key somatotrophic gene expression in liver and muscle, body composition, metabolism, and also on concentrations of glucocorticoid and thyroid hormones, key to maturational changes associated with birth transition. We predicted that FGR would reduce size at birth and accelerate postnatal growth, and that intra‐amniotic IGF1 infusion would abrogate these changes.
Methods
Ethical approval
All experimental procedures were approved by the University of Auckland Animal Ethics Committee (AEC/02/2008/R628 and AEC/03/2011/R874) and conducted in accordance with these approvals. Reporting of this experiment conforms with The Journal of Physiology’s Principles and Standards for Reporting Animal Experiments (Grundy, 2015) and with the ARRIVE Guidelines (Kilkenny et al. 2010).
Generation of experimental groups
The primary outcome for the experiment was glucose tolerance at 18 months of age (young adulthood); these data will be reported elsewhere and this manuscript reports fetal and neonatal outcomes following intra‐amniotic IGF1 treatment. Based on previous studies of postnatal endocrinology in young adult sheep, we calculated that to detect a change in mean glucose area under the curve in a glucose tolerance test of 15% from 712 (SD 99) mmol·min−1·L−1, with 80% power and a 2‐tailed significance level of 0.05, 12 successful studies at 18 months were required per experimental group. Accounting for perinatal and post‐perinatal losses and exclusions due to technical difficulties, we estimated that we needed to generate 102 pregnant ewes.
Multiparous Romney ewes purchased from Landcorp Farming Limited (Wellington, New Zealand) were bred on site with Poll Dorset rams, and scanned ∼40 and ∼70 days after mating to confirm pregnancy and fetal number. Singleton‐carrying ewes were acclimatised to a feedlot with 12‐hour controlled photoperiods, free access to water and a complete pelleted feed diet (Oliver et al. 2005). Ewes were allocated using a random number generator to control (CON) or placental embolisation‐induced fetal growth restriction (FGR) initially, with a second randomisation of FGR ewes once embolization was complete to either intra‐amniotic vehicle (saline, FGRS), or IGF1 (FGRI) to reduce potential bias.
Ewes randomised to FGR underwent surgery between 97 and 100 days’ gestational age (dGA). After an overnight fast with free access to water, surgery was conducted under isoflurane anaesthesia (Medsource, Linyi, Shangdong, China). Both fetal femoral arteries were catheterised (Murotsuki et al. 1995). Maternal uterine arteries were catheterised bilaterally and the catheters advanced into the primary artery supplying each horn (Jensen et al. 1999) and an amniotic catheter was placed in the amniotic sac. Because CON fetuses did not undergo surgery, fetal data are presented for growth‐restricted groups only. Fetal blood gas, plasma hormone and metabolite concentrations in chronically‐catheterised fetal CON sheep have been published previously from similar preparations (Bloomfield et al. 2002a; de Boo et al. 2008; Wali et al. 2012).
FGR was induced by twice‐daily bilateral uterine artery embolisation with 20–50 μm Superose® 12 polymerised polysaccharide microspheres (GE Healthcare, Auckland, NZ) over a 5‐day period (103–107 dGA), titrated against fetal blood gas and metabolite measures as previously described (Bloomfield et al. 2002b).
FGR fetuses were randomly allocated to receive either 360 μg (100 μg·mL−1) human recombinant IGF1 (FGRI; Genetech, San Francisco, California, United States) or an equivalent volume of sterile saline (FGRS) once‐weekly as previously described (Wali et al. 2012), commencing at 107 dGA with the last treatment at 135 dGA. Following the final intra‐amniotic injection, the flank wound was thoroughly cleaned, catheters occluded and tucked subcutaneously, the wound closed and the ewes given 3.0 mL intramuscular Duplocillin (Schering‐Plough Animal Health Ltd., Upper Hut, NZ).
Lambs were born vaginally after spontaneous labour, and remnants of protruding catheters were removed after birth. If within 1 h of birth the lamb was unable to latch and feed, the ewe was hand‐milked and the lamb fed manually, repeated as necessary for up to 2 h after birth or until the lamb displayed successful feeding behaviour.
If euthanasia was recommended by the attending veterinarian, sheep were euthanised with 100–120 mg·kg−1 sodium pentobarbitone (300 mg·mL−1, Provet, Auckland, NZ) administered intravenously.
Blood sampling, hormone and metabolite analyses
Fetal whole blood was sampled 1–2 days post‐surgery to monitor blood gases and at 102 dGA to establish basal measures. Basal partial pressures of carbon dioxide (, mmHg) and oxygen (, mmHg), and pH were measured on an i‐STAT® (Abbott Point of Care, Inc., Princeton, New Jersey, USA) with CG3+ or CG4+ i‐STAT® cartridges. Glucose (mmol·L−1) and lactate (mmol·L−1) were measured on an YSI 2300 SELECT™ Biochemistry Analyser (Yellow Springs Instruments, Queensland, AU).
Two hours post‐delivery the lamb was removed from the ewe but kept within line‐of‐sight and hearing of the ewe. Blood samples were collected by venepuncture, centrifuged at 3,220 g, 4°C for 10 min and the resultant plasma stored at −80°C until analysis. Blood sampling using the same method was repeated weekly.
Hormone and metabolite analyses
11‐deoxycortisol (S), cortisol (F) and cortisone (E) concentrations were analysed with high‐performance liquid chromatography‐tandem mass spectrometry (HPLC‐MS) as described previously (Rumball et al. 2008). Mean inter‐ and intra‐assay coefficients of variation (CVs) were 5.1% and 10.7%, respectively, for 11‐deoxycortisol, 5.9% and 6.5% for cortisol, and 4.5% and 7.0% for cortisone.
˪‐3,3′,5‐triiodothyronine (T3) and thyroxine (T4) were analysed on an Elecsys 2010 (Hitachi High‐Technologies Corporation, Tokyo, Japan). Mean inter‐ and intra‐assay CVs were 1.1% and 1.3%, respectively, for T3, 3.1% and 1.7% for T4.
Metabolite concentrations were measured on a Hitachi 902 autoanalyser (Hitachi High‐Technologies Corporation, Tokyo, Japan). Mean inter‐ and intra‐assay CVs were 5.1% and 3.7%, respectively, for lactate; 3.4% and 2.4% for non‐esterified fatty acids (NEFA); 4.3% and 3.8% for urea, and mean intra‐assay CV for glucose was 1.6%.
Insulin was measured by radioimmunoassay (RIA) with ovine insulin (Sigma‐Aldrich, St. Louis, Missouri, USA) as the standard (Oliver et al. 1993). The minimum level of detection was 0.03 ng·mL−1. Mean inter‐ and intra‐assay CVs were 8.6% and 11.7%, respectively. IGF1 was measured by an IGFBP‐blocked RIA (Blum & Breier, 1994). The minimum level of detection was 0.03 ng·mL−1. Mean inter‐ and intra‐assay CVs were 3.6 and 11.4%, respectively. Amino‐terminal propeptide of C‐type natriuretic peptide (NTproCNP) was measured by RIA as described previously (Prickett et al. 2001, 2004) using primary rabbit antiserum (J39) raised against human NTproCNP (1‐15). The inter‐ and intra‐assay CVs were 6.5% and 5.0%, respectively at 33 pmol·L−1.
Milk intake during the second week after birth was measured using the deuterium oxide (D2O) dilution technique as described previously (Jaquiery et al. 2011).
Measurement of growth and body composition
Lamb weight, crown‐rump length (CRL), chest and abdominal girth (ABDO), forelimb (FL), hindlimb (HL) and hock‐to‐toe length (HT), and biparietal diameter (BPD) were measured at birth, on the third day after birth and at days 7 and 14.
Relative weight per unit body length was expressed as body mass index (BMI, kg·CRL−2), G index (GI, kg·CRL−1.5) (Gootwine, 2013), and ponderal index (PI, kg·CRL−3) (Rohrer, 1921). Growth velocity was calculated using an exponential method (Patel et al. 2005):
Where W1 represents WT in kg at D1 and Wn represent weight on any given day after D1, Dn. Growth velocity of linear measures were calculated in the same manner.
At one week of age, dual X‐ray absorptiometry (DXA) scans were conducted with a Norland, XR‐800 (Cooper Surgical Ltd, Fort Atkinson, WI, USA) followed by percutaneous liver and muscle biopsies under intravenous sedation induced with 5.0 mg·kg−1 ketamine (Parnell Co. Ltd, Auckland, NZ) and 0.25 mg·kg−1 diazepam (Ceva Animal Health Ltd., Auckland, NZ), titrated as needed to maintain a stable plane of anaesthesia.
DXA scans were undertaken at a spatial resolution of 3.0 × 3.0 mm. The area analysed was defined proximo‐distally as the area of the trunk from the thoracic inlet along the curve of the spine through the base of the tail and around the rump through the pelvic limb just distal to the tibial plateau, along the curve of the abdomen and the anterior margin of the breast. Fat mass, lean mass and bone mineral content were calculated using Norland software (Cooper Surgical Ltd, Fort Atkinson, WI, USA) and are expressed in absolute grams and relative to total bodyweight.
Muscle and liver biopsies
Following the DXA procedure a percutaneous biopsy of the right lobe of the liver, and of the vastus lateralis were collected using aseptic techniques. The liver biopsy was performed from a posterior approach using a 10‐gauge trocar advancing through the diaphragm and into the liver. The lamb was placed in left lateral recumbency, with the neck supported in line with the spine. The biopsy site, located at the 4th intercostal space posteriorly, was infiltrated subcutaneously with 2% lignocaine hydrochloride (AstraZeneca, Auckland, NZ) and the biopsy needle advanced approximately 1.5 cm into the thorax, orienting ventrocaudally at a 30° angle from the spine (pointing towards the umbilicus) and 20° from the external plane of the trunk. The needle was advanced until the diaphragm was punctured, then rotated towards the point of the sternum and advanced approximately 2.0 cm into the liver at a 45–60° angle from the spine and 30° from the external plane of the trunk until a granular‐textured tissue was identified. The trocar was removed and a 5.0 mL sterile syringe was fitted to the cannula. Negative pressure was developed by withdrawing the syringe approximately 2.5 mL whilst advancing the cannula 0.5–1.0 cm. The negative pressure was maintained while the apparatus was withdrawn. The incised skin was immediately closed with Superglue™ to avoid pneumothorax and sprayed with TetraVet (Bomac Laboratories Ltd., Auckland, NZ). The muscle biopsy site was infiltrated subcutaneously with 2% lignocaine hydrochloride (AstraZeneca, Auckland, NZ). Vastus lateralis tissue was collected and the skin was closed and treated as described previously. Samples were snap frozen and stored at −80°C until analysis. The lamb was given 1.0 mL intramuscular Duplocillin and monitored for adverse events. The lamb was returned to the ewe once capable of independent ambulation and following return of feed‐seeking behaviours.
Quantitative PCR
A TRIzol®‐based extraction protocol was utilised to isolate mRNA from a ∼20 mg tissue sample (Kimura et al. 2004; Triant & Whitehead, 2009). We were unable to isolate protein from the same sample to a high enough quality to perform Western blot analyses. Briefly, snap‐frozen tissue was ground with a liquid nitrogen pre‐cooled stainless steel mortar and pestle and scooped into a 1.5 mL Eppendorf tube kept on dry ice. 1.0 mL TRIzol® (Life Technologies, Auckland, NZ) was added and the solution vortexed to mix. Samples were sonicated at 35%, 2‐cycle for 15 s and stored on ice. Sonication was repeated until sample was fully solubilised. Samples were centrifuged at 4°C, 10 621 g for 10 min and fatty layers discarded.
200 μL chloroform (Sigma‐Aldrich, St. Louis, Missouri, USA) per mL TRIzol® was added and the solution vortexed to mix. Samples were incubated for 5 min at room temperature, and centrifuged at 4°C, 17 949 g for 15 min. The aqueous phase was aspirated and transferred to a fresh nuclease‐free Eppendorf tube.
Cold isopropanol (500 μL, Sigma‐Aldrich, St. Louis, Missouri, USA) was added to the aqueous phase and the solution was incubated 30–40 min at −20°C. The precipitate was centrifuged at 4°C, 17 949 g for 15 min and the supernatant discarded. The pellet was washed with 1.0 mL 75% ethanol (Sigma‐Aldrich, St. Louis, Missouri, USA) and centrifuged at 4°C, 17 949 g for 5 min. The wash step was repeated and the pellet semi‐dried for 10 min at room temperature. The pellet was dissolved on ice in 10–20 μL nuclease‐free water (Life Technologies, Auckland, NZ) and stored overnight at −80°C.
During protocol development mRNA integrity and purity were confirmed with a 1.5% agarose (Life Technologies, Auckland, NZ) RNA denaturation gel and NanoDrop ND‐1000 spectrophotometer (3.1.2 NanoDrop Software, BioLab Ltd., Auckland, NZ). Due to the small tissue sample size, mRNA template isolation was limited. Thus, to ensure sufficient mRNA to complete the study in its entirety, mRNA purity was assessed by NanoDrop, and a standard DNase I gDNA elimination step was conducted on all samples prior to reverse transcription. Total mRNA concentration was quantified with a NanoDrop. Samples were of acceptable purity if 260/280 absorbance was >1.9 and 260/230 absorbance was >1.6.
mRNA template concentration for each primer/probe set was optimised for each tissue using samples from a CON male lamb. 1.5 μg mRNA was incubated with 4 U RNase‐free DNase I (Life Technologies, Auckland, NZ) for 15 min at room temperature to eliminate potential gDNA contamination. To halt DNA digestion, 1.0 μL 25 mmol EDTA was added and samples were incubated at 65°C for 10 min. Complementary DNA (cDNA) was synthesised in 20 μL reaction volumes with SuperScript® VILO™ cDNA synthesis kits (Life Technology, Auckland, NZ s). 1x VILO™ reaction mix, 1x SuperScript® III enzyme mix containing SuperScript® III reverse transcriptase and RNaseOUT™ recombinant ribonuclease inhibitor were added to 1.5 μg DNase‐treated mRNA. Reverse transcription PCR (RT‐PCR) was run on an Eppendorf Mastercycler (Eppendorf AG, Hamburg, Germany) under the following conditions: 25°C for 10 min, 42°C for 60 min, 85°C for 5 min and cooled to 4°C. Complementary DNA (cDNA) was stored at −80°C until qPCR analysis.
Transcript abundance was determined by singleplex amplification in 384‐well plates in triplicate by qPCR, including intra‐ and inter‐plate, positive and negative controls. 10.0 μL reaction volumes with 5.0 μL TaqMan Master Mix (Applied Biosystems, Foster City, California, USA), 2.0 μL cDNA template, 900 nmol forward and reverse primers and 200 nmol probe (Applied Biosystems) were assayed on an ABI 7900HT sequence detector (Applied Biosystems) under the following conditions: 50°C for 2 min and 95°C for 10 min, 40 cycle‐repeats of 95°C for 15 s and 60°C for 1 min. FAM fluorescent reporter dye was bound to all TaqMan probes except 18S (VIC reporter) at the 3′ end, and a molecular‐groove binding non‐fluorescence quencher (MGBNFQ) was bound at the 5′ end.
Ovine target genes involved in the somatotrophic axis and nutrient uptake were analysed: insulin‐like growth factor binding protein acid labile subunit (IGFALS), IGF1, IGF2, IGF1 receptor (IGF1R), growth hormone receptor (GHR), IGF binding protein‐1 (IGFBP1) and ‐3 (IGFBP3), glucose transporters SLC2A2 and SLC2A4, mammalian target of rapamycin (MTOR) and glucocorticoid receptor (NR3C1), (Table 1). The stability of seven potential housekeeping genes were determined for each tissue: 18S ribosomal protein 1 (18S); beta actin (ACTB); peptidylprolyl isomerase A (PPIA); glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH); hypoxanthine phosphoribosyltransferase 1 (HPRT1); ribosomal protein L 19 (RPL19); tyrosine 3‐monooxygenase/tryptophan 5‐monooxygenase activation protein, and zeta polypeptide (YWHAZ) (Table 1).
Table 1.
Target genes analysed by qPCR
| Gene accession ID | Primer/probe | Sequences (5′–3′) | T m (°C) | Location (bp) | Size (bp) |
|---|---|---|---|---|---|
| IGFALS | F | GCG TCA AGG CCA ATG TCT TC | 59 | 1178–1197 | 63 |
| XM_004021110.1 | R | GGT TGT GGT CCA GGT AGA G | 59 | 1222–1240 | |
| P | 6FAM‐TCA AGC TGC CCA AGC T‐MGBNFQ | 70 | 1199–1214 | ||
| IGF1 | F | CTT CCG GAG CTG TGA TCT GA | 58 | 389–408 | 73 |
| NM_001009774.3 | R | TGA GCG GGC CGA CTT G | 59 | 446–461 | |
| P | 6FAM‐CTG TGC GCC TCT CAA G‐MGBNFQ | 68 | 425–440 | ||
| IGF2 | F | CGA GGC ATC CAG CGA TTA G | 58 | 623–641 | 62 |
| NM_001009311.1 | R | TAG ATG GTG TCA CTT GGC AGA ATT | 59 | 661–684 | |
| P | 6FAM‐AGT GAG CCA AAG TGT C‐MGBNFQ | 69 | 643–658 | ||
| IGF1R | F | TCT AAC TTT GTC TTT GCA AGA ACC A | 58 | 73–97 | 64 |
| AY162434.1 | R | TCA CTG GCC CAG GAA TGT C | 58 | 118–136 | |
| P | 6FAM‐CCT GCA GAA GGA GCA G‐MGBNFQ | 69 | 100–115 | ||
| GHR | F | CAT AAA GCC TGG AGG AAA CCA T | 58 | 119–140 | 59 |
| NM_001009323.2 | R | CCG GCA GAC AAT CCT GAA A | 58 | 159–177 | |
| P | 6FAM‐CGA AAA TCC AGC CTC T‐MGBNFQ | 68 | 142–157 | ||
| IGFBP1 | F | GCC AAA CTG CAA CAA GAA TGG | 59 | 210–221 | 52 |
| NM_001145177.1 | R | TCC GTC CAG CGA AGT CTC A | 59 | 242–261 | |
| P | 6FAM‐TTC TAT CAC AGC AAA CAG T‐MGBNFQ | 69 | 223–241 | ||
| IGFBP3 | F | GCC AGC GCT ACA AGG TTG AC | 59 | 59–78 | 68 |
| EU616622.1 | R | CTT GGA CTC GGA GGA GAA GTT CT | 59 | 104–126 | |
| P | 6FAM‐ACG AGT CTC AGA GCA C‐MGBNFQ | 69 | 80–95 | ||
| SLC2A2 | F | GAA GAA TCA AAG CCC TGT TGG T | 60 | 258–279 | 70 |
| AJ318925.1 | R | TTC GAA AAC CCC ATC AAG AGA | 58 | 307–327 | |
| P | 6FAM‐CAA ACA TTC TTT CAT TAG TTG GA‐MGBNFQ | 69 | 282–304 | ||
| SLC2A4 | F | AAC CCA GCA CAG AAC TGG AGT AC | 58 | 71–93 | 60 |
| AB005283.1 | R | CCT GTG TGG ACC CTC AGT CA | 58 | 110–130 | |
| P | 6FAM‐AGG GCC GGA TGA GA‐MGBNFQ | 68 | 96–109 | ||
| MTOR | F | CTG CAC GTC AGC ACC ATC A | 59 | 3772–3790 | 80 |
| NM_001145455.1 | R | AGC CAT TCC AAC CAA TCA TCT T | 58 | 3830–3851 | |
| P | 6FAM‐CCT CCA AAA GGC C‐MGBNFQ | 69 | 3792–3804 | ||
| NR3C1 | F | GGG CCA ACA TAA TTG GCA ATA A | 60 | 509–531 | 84 |
| NM_001114186.1 | R | CCC AGA GGT ACT CAC ACC ATG A | 59 | 570–592 | |
| P | 6FAM‐ATG TCT GCC ATT TCT‐MGBNFQ | 69 | 532–547 | ||
| RNA18S | Eukaryotic 18S rRNA 6VIC/MGBNFQ | ||||
| ACTB | F | ACC AGT TCG CCA TGG ATG ATG | 61 | 76–96 | 53 |
| NM_001009784.1 | R | CCG GAG CCG TTG TCA AC | 58 | 113–128 | |
| P | 6FAM‐ACG AGC GCA GCA ATA T‐MGBNFQ | 97–112 | |||
| GAPDH | F | GGG CTG CTT TTA ATA CTG GCA AA | 60 | 91–114 | 81 |
| NM_001190390.1 | R | CAT GTA GAC CAT GTA GTG AAG GTC AA | 61 | 146–171 | |
| P | 6FAM‐CAT CGT TGC CAT CAA TG‐MGBNFQ | 120–136 | |||
| HPRT1 | F | AGG TGT TTA TTC CTC ATG GAC TAA TTA TGG | 61 | 1–30 | 75 |
| JN811683.1 | R | CAC CCA TCT CCT TCA TCA CAT CTC | 61 | 52–75 | |
| P | 6FAM‐ACA GGA CCG AAC GAC TG‐MGBNFQ | 31–47 | |||
| PPIA | F | GTA CTG GTG GCA AGT CCA TCT | 60 | 105–125 | 72 |
| JX534530.1 | R | CAG GAC CTG TAT GCT TCA GAA TGA | 60 | 153–176 | |
| P | 6FAM‐ATG GCG AGA AAT TTG‐MGBNFQ | 126–140 | |||
| RPL19 | F | CAA AAA CAA GCG GAT TCT CAT G | 58 | 340–361 | 65 |
| AY158223.1 | R | GCT TCT TGC GAG CCT TGT CT | 58 | 385–404 | |
| P | 6FAM‐AAC ATA TCC ACA AGC TGA A‐MGBNFQ | 363–381 | |||
| YWHAZ | F | GAG GGT CGT CTC CAG TAT TGA G | 60 | 25–46 | 67 |
| NM_001267887.1 | R | TTC TCG AGC CAT CTG CTG TTT T | 61 | 70–91 | |
| P | 6FAM‐CAG CAC CTT CCG TCT TT‐MGBNFQ | 49–65 |
Sequences for forward (F) and reverse (R) primers; fluorescent reporter dye (FAM, VIC) bound TaqMan probes with molecular‐groove binding non‐fluorescence quencher (MGBNFQ). Melting temperature (T m) and base pairs (bp) noted.
GenBank ovine sequences were analysed for target genes with Primer Express Software (Applied Biosystems). Minor groove binder primer and probe locations were determined, and sequences were BLAST searched to confirm target‐specific oligonucleotide sequences. PCR amplicons were verified as previously described (Bansal et al. 2015). Hepatic cDNA was undiluted for IGF1R, and SLC2A4, diluted 5‐fold for MTOR, 10‐fold for IGFBP1, IGFBP3, and NR3C1, 25‐fold for GHR, 100‐fold for IGFALS, IGF1, IGF2, SLC2A2, HPRT1, and YWHAZ, and 1000‐fold for GAPDH. Skeletal muscle cDNA was undiluted for NR3C1, diluted 5‐fold for MTOR, 10‐fold for IGF1R, GHR, IGFBP3, SLC2A4, and HPRT1, 100‐fold for IGF1, IGF2, and PPIA, and 1000‐fold for ACTB. Amplification efficiencies for all target genes and potential housekeeping genes were calculated from the slopes of the standard curves run on cDNA from the tissues for optimisation (Table 2). Housekeeping gene stability of expression across a random selection of samples was assessed mathematically by assessing the coefficient of variation and standard deviation of a panel of available genes.
Table 2.
qPCR validation
| Gene | Standard curve dilution (fold) | Regression equation | r2 | Efficiency |
|---|---|---|---|---|
| Vastus lateralis | ||||
| GHR | 10 | y = −3.3289x + 24.37 | 0.9877 | 2.00 |
| IGF1 | 10 | y = −3.4452x + 19.718 | 0.9979 | 1.95 |
| IGF2 | 10 | y = −3.4162x + 17.996 | 0.9951 | 1.96 |
| IGF1R | 10 | y = −3.1689x + 24.118 | 0.9968 | 2.03 |
| IGFBP3 | 10 | y = −3.7169x + 23.946 | 0.9967 | 1.86 |
| SLC2A4 | 10 | y = −3.126 × 22.529 | 0.9987 | 2.05 |
| MTOR | 5 | y = −3.5147 + 23.11 | 0.9997 | 1.93 |
| NR3C1 | 10 | y = −3.1675x + 24.181 | 0.9927 | 2.07 |
| ACTB | 10 | y = −3.6882x + 18.27 | 0.9968 | 1.87 |
| HRPT1 | 10 | y = −3.4986x + 23.159 | 0.9978 | 1.93 |
| PPIA | 10 | y = −3.0689x + 20.22 | 0.9889 | 2.12 |
| Liver | ||||
| GHR | 5 | y = −3.3357x + 19.696 | 0.9907 | 1.99 |
| IGF1 | 10 | y = −3.2571 + 20.527 | 0.9995 | 2.03 |
| IGF2 | 10 | y = −3.1495x + 19.883 | 0.9987 | 2.08 |
| IGF1R | 10 | y = −3.2151x + 26.03 | 0.9931 | 2.05 |
| IGFBP1 | 10 | y = −3.0762x + 25.996 | 0.9802 | 2.11 |
| IGFBP3 | 10 | y = −3.4713x + 21.13 | 0.987 | 1.94 |
| IGFALS | 10 | y = −3.135x + 22.746 | 0.9988 | 2.08 |
| SLC2A2 | 10 | y = −3.0713x + 21.828 | 0.9983 | 2.12 |
| MTOR | 5 | y = −3.1686x + 24.506 | 0.9973 | 2.07 |
| NR3C1 | 10 | y = −3.0665x + 24.463 | 0.9967 | 2.12 |
| HRPT1 | 10 | y = −2.9729x + 21.684 | 0.9817 | 2.17 |
| GAPDH | 10 | y = −3.454x + 19.719 | 0.9846 | 1.99 |
| YWHAZ | 10 | y = −3.1492x + 22.65 | 0.9996 | 2.08 |
The geometric mean (GEO) of the three most stable housekeeping genes across the experimental groups were calculated for each tissue (vastus lateralis: ACTB, HRPT1 and PPIA; liver: GAPDH, HRPT1 and YWHAZ) (Pfaffl et al. 2004). The threshold cycle (Ct) for target gene (TG) mRNA expression levels were calculated relative to the housekeeping geometric mean with a mathematical model which accounts for variation in transcript amplification efficiency (Pfaffl, 2001). The standard error of the mean (SEM) was calculated for each group and a 99% confidence interval (CI) was used for statistical analysis of qPCR results, where b is the experimental group compared with either CON or FGRS (a):
Results are reported as fold‐change with 99% CI relative to either Control (FGRS and FGRI) or FGRS (FGRI).
Statistics
Data were analysed in JMP 12 (SAS Institute Inc., Cary, North Carolina, USA). Distribution was verified with the Shapiro‐Wilk test. Non‐parametric data were log transformed to approximate a normal distribution where necessary. The effects of FGR and intra‐amniotic treatment of FGR with IGF1 were analysed by t test, factorial or repeated measures analysis of variance (ANOVA and RM ANOVA, respectively) by sex with breeding year as a random effect. RM ANOVA was used to examine longitudinal changes over time. Breeding year was included in the analyses to adjust for the effect on outcomes. Significance was set at P < 0.05 unless otherwise stated. Tukey's post hoc testing was conducted where appropriate. Data are presented as the mean ± SEM.
Results
One hundred and ten singleton sheep were generated for the purposes of this experiment over two consecutive breeding years (Fig. 1).
Figure 1. Characteristics of experimental groups and animal use.
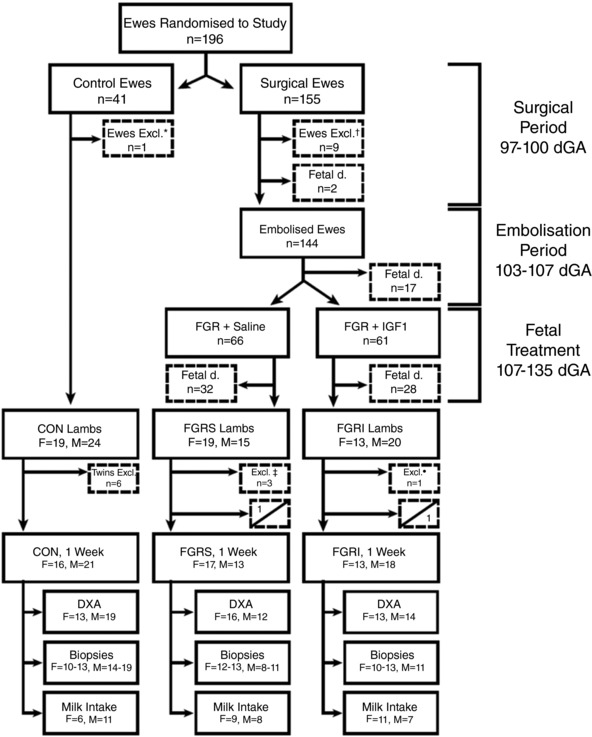
Animals utilised during the project according to experimental group and sex. Dashed boxes report exclusions, deaths and killing. Split boxes reporting postnatal deaths indicate the number of females (top left) and males (bottom right) for each experimental group: control (CON), saline‐treated fetal growth restricted (FGRS), and insulin‐like growth factor 1‐treated fetal growth restricted (FGRI). Dual X‐ray absorptiometry (DXA), biopsies, and milk intake were conducted in all groups. Circumstances constituting exclusion from the project and/or killing were: *pregnancy toxaemia, †mammary abscess n = 5, aspiration of rumen contents prior to intubation n = 1, twin pregnancy n = 2 and intrauterine infection n = 1; ‡twin lambs n = 2, intestinal atresia n = 1; °preterm (<136 dGA) birth n = 1.
Fetal metabolic status and survival
Fetal hock‐to‐toe at the time of surgery was not different between FGRS and FGRI groups (both 9.9 ± 0.1 cm). Placental embolisation increased the partial pressure of carbon dioxide () (P = 0.02, Fig. 2 A) and decreased the partial pressure of oxygen (, P < 0.0001, Fig. 2 B), pH (P < 0.0001, Fig. 2 C) and arterial blood glucose concentration (P = 0.05, Fig. 2 D), but did not alter arterial blood lactate concentrations (P = 0.1, Fig. 2 E). Fetal blood gas and metabolite concentrations did not differ between FGR groups. Fifty‐seven FGR fetuses died prior to delivery. Fetal mortality (P = 0.2) and gestational age at death, defined as the date of abortion, did not differ between FGRS and FGRI fetuses (P = 0.7) nor between sexes (P = 0.9). Postnatal mortality also was not different between FGR groups (FGRS, n = 3; FGRI, n = 3), and did not occur in control lambs (Table 3).
Figure 2. Fetal whole blood measures and maternal mean plasma N‐terminal pro‐C‐type natriuretic peptide.
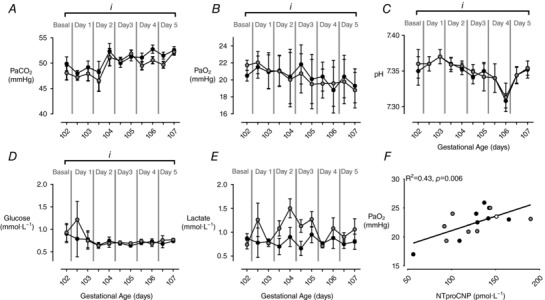
Data are means ± SEM. Whole blood measures of partial pressure of carbon dioxide (, mmHg; A); partial pressure of oxygen (, mmHg; B); pH (C); glucose (mmol·L−1; D); and) lactate (mmol·L−1; E) in FGRS (grey, n = 20–34) and FGRI (black, n = 20–32) fetuses; and the correlation between mean maternal N‐terminal pro‐C‐type natriuretic peptide (NTproCNP, pmol·L−1) during the embolisation period (103–107 dGA) and fetal in growth‐restricted FGR (white, n = 2), FGRS (grey, n = 7), and FGRI (black, n = 7) fetuses (F). Symbols denote significant differences over time (RM ANOVA: i, P < 0.05).
Table 3.
Animal losses during experimental procedures
| CON | FGR | FGRS | FGRI | |
|---|---|---|---|---|
| Prenatal losses | ||||
| Experimentally induced | — | 12 | 26 | 21 |
| Catheter tangles | — | 0 | 3 | 0 |
| Congenital defect | — | 3● | 2● | 3● |
| Intrauterine infection | — | 2 | 0 | 2 |
| Stillbirth | — | 0 | 1 | 2 |
| Early postnatal losses | ||||
| Experimental complications | 0 | — | 1‡ | 1‡, 1¥ |
| Illness | 0 | — | 1† | 1† |
| Congenital defect | 0 | — | 1* | 0 |
Congenital defects were observed during the prenatal (●, brachycephaly) and postnatal (*, intestinal atresia) experimental period in fetal growth restricted (FGR) lambs prior to randomisation to experimental groups, saline‐treated fetal growth restricted (FGRS), and insulin‐like growth factor 1‐treated fetal growth restricted (FGRI) lambs. Control (CON) lambs did not undergo instrumentation. Early postnatal losses include respiratory distress and/or congenital heart failure secondary to pulmonary aneurysm (†), and experimental complications (‡, liver biopsy; ¥, anaesthesia).
Plasma NTproCNP concentration
Maternal mean NTproCNP was not different (P = 0.9) during the embolisation period (102–106 dGA) between ewes subsequently allocated FGRS and FGRI groups (105.6 ± 27.8 vs. 104.3 ± 27.8 pmol·L−1). There was a significant positive correlation between maternal mean NTproCNP and fetal during the embolisation period (R2 = 0.43, P = 0.006, Fig. 2 F). There was a positive correlation between mean maternal plasma NTproCNP concentration during the embolisation period and birthweight (R2 = 0.64, P = 0.0005), CRL (R2 = 0.52, P = 0.004) and HT length (R2 = 0.57, P = 0.002) in growth‐restricted lambs (Fig. 3). There were no differences amongst groups in plasma NTproCNP in lambs at birth (CON, 96.5 ± 5.2; FGRS, 95.4 ± 6.7; FGRI, 86.8 ± 4.8 pmol·L−1), day 7 (CON, 68.6 ± 4.7; FGRS, 78.3 ± 5.2; FGRI, 70.2 ± 4.3 pmol·L−1), or day 14 (CON, 63.5 ± 2.9; FGRS, 69.1 ± 3.4; FGRI, 67.6 ± 2.9 pmol·L−1).
Figure 3. Maternal mean plasma N‐terminal pro‐C‐type natriuretic peptide and offspring size at birth.
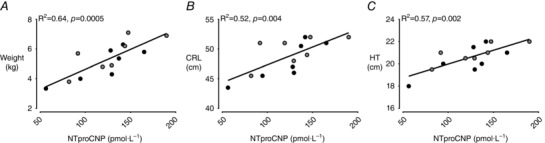
Data are means ± SEM. The correlation between mean maternal N‐terminal pro‐C‐type natriuretic peptide (NTproCNP) during the embolisation period (103–107 dGA) and birthweight (A), crown–rump length (B), and hock–toe length (C) in FGRS (grey, n = 7), and FGRI (black, n = 7) lambs.
Size at birth and neonatal growth
Birth characteristics
Gestational age at birth in surviving fetuses (Table 4) was not different amongst groups (P = 0.2, Female; P = 0.4, Male). FGRS, but not FGRI, female lambs were smaller than CON at birth with reduced birthweight, birthweight Z‐score, abdominal circumference, BMI and GI (all, P < 0.05, Table 4); both FGRS and FGRI female lambs had shorter hindlimb length than CON. Males in both growth‐restricted groups were lighter than CON (P = 0.005) with FGRS lambs having shorter forelimb length than CON (P = 0.03) and FGRI lambs having reduced GI (P = 0.02) and lesser chest circumference and hindlimb length than CON (P = 0.01 and P = 0.001, respectively) (Table 4).
Table 4.
Characteristics of experimental groups at birth
| Female | Male | P value for treatment effect | ||||||
|---|---|---|---|---|---|---|---|---|
| CON | FGRS | FGRI | CON | FGRS | FGRI | |||
| n = 15 | n = 18 | n = 13 | n = 22 | n = 12 | n = 19 | Female | Male | |
| GA at birth (days) | 147 ± 1 | 147 ± 1 | 149 ± 1 | 148 ± 1 | 148 ± 1 | 147 ± 1 | ns | ns |
| BW (kg) | 5.7 ± 0.3 | 4.6 ± 0.3 | 5.1 ± 0.3 | 6.4 ± 0.2 | 5.4 ± 0.2 | 5.4 ± 0.2 | 0.02* | 0.005*† |
| BW Z‐Score | 0.3 ± 0.2 | −0.4 ± 0.2 | −0.1 ± 0.2 | 0.8 ± 0.1 | 0.2 ± 0.2 | 0.1 ± 0.2 | 0.02* | 0.005*† |
| CRL (cm) | 51.0 ± 0.8 | 48.5 ± 0.7 | 49.8 ± 0.7 | 52.3 ± 0.6 | 50.0 ± 0.7 | 49.0 ± 1.0 | ns | ns |
| Chest (cm) | 39.5 ± 0.6 | 37.4 ± 0.5 | 38.2 ± 0.6 | 41.2 ± 0.4 | 39.2 ± 0.6 | 38.3 ± 0.6 | ns | 0.01† |
| Abdomen (cm) | 40.7 ± 1.0 | 36.9 ± 0.9 | 39.0 ± 1.2 | 41.8 ± 0.6 | 40.3 ± 0.8 | 39.2 ± 0.8 | 0.01* | ns |
| Hock–toe (cm) | 21.1 ± 0.3 | 19.9 ± 0.3 | 20.2 ± 0.3 | 21.7 ± 0.2 | 20.8 ± 0.3 | 21.0 ± 03 | ns | 0.06 |
| Hindlimb (cm) | 39.5 ± 0.6 | 37.4 ± 0.5 | 37.3 ± 0.9 | 41.4 ± 0.3 | 39.3 ± 0.5 | 38.5 ± 0.2 | 0.03*† | 0.001† |
| Forelimb (cm) | 34.1 ± 0.5 | 32.5 ± 0.5 | 33.1 ± 0.5 | 35.5 ± 0.4 | 33.7 ± 0.5 | 33.9 ± 0.5 | ns | 0.03* |
| BPD (cm) | 6.4 ± 0.1 | 6.2 ± 0.1 | 6.2 ± 0.1 | 6.6 ± 0.04 | 6.5 ± 0.06 | 6.4 ± 0.03 | ns | ns |
| BMI (kg CRL−2) | 22.0 ± 0.9 | 19.5 ± 0.9 | 20.7 ± 1.0 | 23.6 ± 0.8 | 21.6 ± 0.9 | 22.01 ± 0.9 | 0.02* | ns |
| GI (kg CRL−1.5) | 15.7 ± 0.7 | 13.6 ± 0.6 | 14.6 ± 0.7 | 17.0 ± 0.5 | 15.5 ± 0.6 | 15.3 ± 0.6 | 0.01* | 0.02† |
| PI (kg CRL−3) | 43.6 ± 1.9 | 40.1 ± 1.8 | 41.9 ± 2.0 | 45.6 ± 1.8 | 43.1 ± 2.2 | 44.6 ± 2.0 | ns | ns |
Data are means ± SEM. Gestational age (GA), birthweight (BW), crown–rump length (CRL), chest circumference (Chest), abdominal circumference (Abdomen), hock–toe length, hindlimb length, forelimb length, biparietal diameter (BPD), body mass index (BMI), g index (GI), and ponderal index (PI) at birth. Symbols denote significant differences between experimental groups on post hoc testing: *CON vs. FGRS; †CON vs. FGRI; ns, not significant.
Neonatal growth velocity
In female FGRS, but not FGRI, lambs, growth velocity of weight (P = 0.009) and of CRL (P = 0.002) in week 2 were greater than in CON lambs (Table 5). Despite this, female FGRS lambs remained lighter with smaller CRL, hindlimb and hock‐to‐toe length than CON from birth to one week whereas CON and FGRI females were not different in size (Fig. 4). In males, GV of weight was greater in FGRS than in CON in the first week after birth (P = 0.04), but was greater in FGRI than CON in the second week (P = 0.02) (Table 5). In contrast to females, male FGRI lambs were lighter with reduced crown‐rump and hindlimb length, chest circumference and biparietal diameter than CON, whilst there was no difference between FGRS and CON males (Fig. 5).
Table 5.
Growth velocity in the first 2 weeks after birth
| Female | Male | P value for treatment effect | ||||||
|---|---|---|---|---|---|---|---|---|
| CON | FGRS | FGRI | CON | FGRS | FGRI | |||
| n = 15 | n = 16 | n = 13 | n = 22 | n = 12 | n = 17 | Female | Male | |
| GV0‐1 WT | 53.6 ± 3.4 | 62.1 ± 3.2 | 55.6 ± 4.0 | 52.6 ± 2.0 | 62.4 ± 2.4 | 58.3 ± 3.1 | 0.08 | 0.04* |
| GV0‐1 CRL | 2.0 ± 0.2 | 1.6 ± 0.2 | 1.9 ± 0.2 | 1.7 ± 0.2 | 1.6 ± 0.2 | 1.7 ± 0.2 | ns | ns |
| GV1‐2 WT | 41.2 ± 3.4 | 49.3 ± 2.2 | 44.2 ± 2.5 | 40.4 ± 1.4 | 41.7 ± 1.8 | 45.6 ± 1.7 | 0.009* | 0.02† |
| GV1‐2 CRL | 1.2 ± 0.2 | 1.9 ± 0.2 | 1.5 ± 0.2 | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.1 | 0.002* | ns |
Growth velocity (GV) in the first (GV0‐1) and second (GV1‐2) week after birth for body weight (WT; g kg−1 day−1) and crown–rump length (CRL; cm m−1 day−1). Data are means ± SEM. Symbols denote significant differences amongst experimental groups on post hoc testing: *CON vs. FGRS; †CON vs. FGRI; ns, not significant.
Figure 4. Early postnatal growth in females.
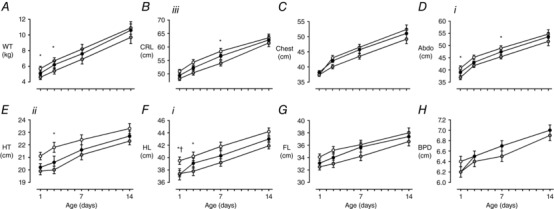
Data are means ± SEM. A, bodyweight (WT); B, crown–rump length (CRL); C, chest circumference; D, abdominal circumference (Abdo); E, hock–toe length (HT); F, hindlimb length (HL); G, forelimb length (FL); H, biparietal diameter (BPD). CON (white, n = 15), FGRS (grey, n = 16–18), FGRI (black, n = 13). Roman numerals denote the significant difference between experimental groups (RM ANOVA: i P < 0.05; ii P < 0.01; iiitime × experimental group interaction P < 0.05), symbols refer to post hoc P values for differences between experimental groups at each time point (ANOVA: CON vs. FGRS: * P < 0.05; CON vs. FGRI: † P < 0.05).
Figure 5. Early postnatal growth in males.
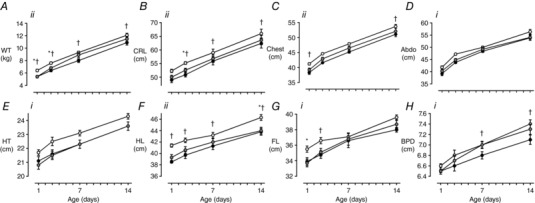
Data are means ± SEM. A, bodyweight (WT); B, crown–rump length (CRL); C, chest circumference; D, abdominal circumference (Abdo); E, hock—toe length (HT); F, hindlimb length (HL); G, forelimb length (FL); H, biparietal diameter (BPD). CON (white, n = 21–22), FGRS (grey, n = 13), FGRI (black, n = 18‐19). Symbols denote significant difference between experimental groups (RM ANOVA: i P < 0.05; ii P < 0.01), and refer to post hoc P values for differences between experimental groups at each time point (ANOVA: CON vs. FGRS: * P < 0.05; CON vs. FGRI: † P < 0.05).
Early postnatal body composition
FGRS and FGRI females had reduced bone mineral density (P < 0.01 for both) compared with CON; FGRS lambs also had reduced bone mineral content compared with CON (P = 0.04) (Table 6). There were no significant differences in body composition measures between groups in males. Fat mass at one week of age was below the limit of detection in lambs from all groups (Table 6).
Table 6.
Body composition at one week of age
| Female | Male | P value for treatment effect | ||||||
|---|---|---|---|---|---|---|---|---|
| CON | FGRS | FGRI | CON | FGRS | FGRI | |||
| n = 12 | n = 15 | n = 13 | n = 20 | n = 11 | n = 14 | Female | Male | |
| BMC (g) | 96 ± 5 | 78 ± 4 | 83 ± 4 | 104 ± 4 | 92 ± 6 | 91 ± 5 | 0.04* | ns |
| BMD (g cm−2) | 0.53 ± 0.02 | 0.45 ± 0.01 | 0.46 ± 0.01 | 0.53 ± 0.01 | 0.49 ± 0.02 | 0.50 ± 0.02 | <0.01*† | ns |
| LM (kg) | 6.3 ± 0.3 | 5.5 ± 0.2 | 5.7 ± 0.3 | 7.0 ± 0.3 | 6.1 ± 0.4 | 6.2 ± 0.3 | 0.08 | 0.06 |
Bone mineral content (BMC), bone mineral density (BMD) and lean mass (LM) measured by dual X‐ray absorptiometry. Data are means ± SEM. Symbols denote significant differences amongst experimental groups on post hoc testing: *CON vs. FGRS; †CON vs. FGRI; ns, not significant.
Neonatal plasma hormone and metabolite concentrations
FGRS, but not FGRI, females had lower plasma IGF1 concentrations at birth than CON; there were no differences amongst groups in males (Fig. 6). There was a positive correlation between plasma IGF1 concentration and birthweight in FGRS and FGRI lambs (females: P = 0.007 and P = 0.001; males P = 0.002 and P = 0.06 respectively). This relationship was not present in CON lambs of either sex (Fig. 6). The correlation between plasma IGF1 concentration and birthweight was not different between male and female lambs (P = 0.055).
Figure 6. Plasma IGF1 characteristics at birth.
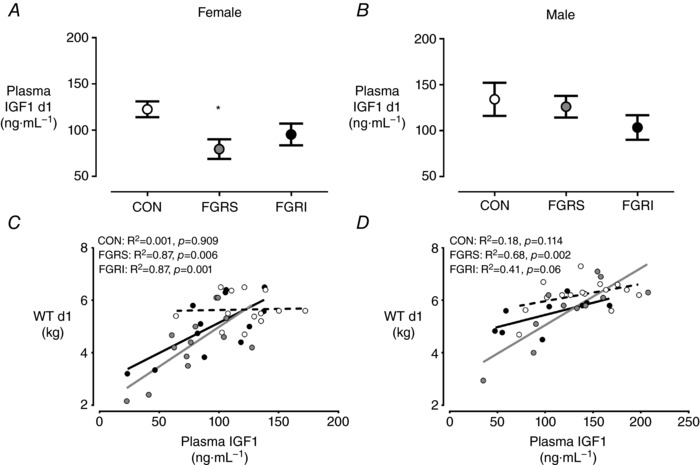
Plasma concentrations of IGF1 in females (A) and males (B), and their correlation with weight at birth (C and D, respectively). Female CON (white) n = 13, FGRS (grey) n = 13, FGRI (black) n = 12, and male CON (white) n = 15, FGRS (grey) n = 11 and FGRI (black) n = 9 lambs. Data are means ± SEM. Symbols refer to post hoc P values for differences between experimental groups (ANOVA: CON vs. FGRS: * P < 0.05).
Plasma concentrations of cortisol, cortisone and the 11‐deoxycortisol, the cortisol to cortisone ratio, the cortisol:11‐deoxycortisol ratio, and the cortisol to weight ratio were not different amongst groups in either sex (Table 7).
Table 7.
Plasma corticosteroids and thyroid hormones in the first week after birth
| Female | Male | P values for treatment effect | ||||||
|---|---|---|---|---|---|---|---|---|
| CON | FGRS | FGRI | CON | FGRS | FGRI | |||
| n = 11–15 | n = 10–13 | n = 10–12 | n = 12—16 | n = 10‐11 | n = 9–10 | Female | Male | |
| Day 1 | ||||||||
| Cortisol (ng mL−1) | 70.1 ± 9.9 | 59.5 ± 9.5 | 52.5 ± 9.5 | 72.0 ± 25.8 | 64.3 ± 22.8 | 80.5 ± 24.2 | ns | ns |
| Cortisone (ng mL−1) | 20.0 ± 5.6 | 22.2 ± 5.8 | 15.8 ± 5.8 | 20.1 ± 7.0 | 16.8 ± 6.8 | 21.7 ± 7.0 | ns | ns |
| ln 11‐deoxycortisol | −0.8 ± 0.2 | −1.2 ± 0.2 | −1.1 ± 0.2 | −0.8 ± 0.6 | −1.0 ± 0.3 | −0.6 ± 0.4 | ns | ns |
| ln cortisol:cortisone ratio | 1.4 ± 0.2 | 1.5 ± 0.2 | 1.4 ± 0.2 | 1.4 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.1 | ns | ns |
| Cortisol:11‐deoxycortisol ratio | 155 ± 42 | 183 ± 45 | 123 ± 45 | 187 ± 16 | 144 ± 29 | 170 ± 33 | ns | ns |
| T3 (pg mL−1) | 17.5 ± 1.7 | 14.0 ± 1.9 | 14.7 ± 2.2 | 18.0 ± 2.9 | 13.3 ± 2.9 | 11.9 ± 3.0 | ns | 0.04† |
| T4 (pmol mL−1) | 73.0 ± 4.5 | 49.7 ± 5.8 | 44.4 ± 7.5 | 59.3 ± 1.06 | 56.1 ± 10.4 | 49.8 ± 10.6 | <0.01*† | ns |
| ln T3:T4 tatio | −1.4 ± 0.1 | −1.3 ± 0.1 | −1.2 ± 0.1 | −1.2 ± 0.1 | −1.4 ± 0.1 | −1.5 ± 0.1 | ns | ns |
| Day 7 | ||||||||
| Cortisol (ng mL−1) | 31.2 ± 8.8 | 39.2 ± 9.9 | 22.4 ± 9.8 | 25.5 ± 4.6 | 48.4 ± 8.3 | 31.7 ± 9.4 | ns | ns |
| Cortisone (ng mL−1) | 8.3 ± 1.3 | 8.1 ± 1.5 | 5.5 ± 1.5 | 8.2 ± 0.7 | 10.8 ± 1.3 | 7.4 ± 1.5 | ns | ns |
| ln 11‐deoxycortisol | −1.3 ± 0.5 | −0.9 ± 1.2 | −1.6 ± 1.3 | −1.3 ± 0.4 | −0.7 ± 0.4 | −0.8 ± 0.5 | ns | ns |
| ln cortisol:cortisone ratio | 1.6 ± 0.2 | 1.8 ± 0.2 | 1.9 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.9 ± 0.1 | ns | ns |
| Cortisol:11‐deoxycortisol ratio | 98.5 ± 16.9 | 76.0 ± 19.4 | 80.6 ± 19.2 | 73.4 ± 15.7 | 76.7 ± 14.0 | 65.1 ± 15.4 | ns | ns |
| T3 (pg mL−1) | 16.0 ± 0.6 | 16.7 ± 0.7 | 16.1 ± 0.7 | 15.7 ± 1.3 | 15.6 ± 1.2 | 14.5 ± 1.3 | ns | ns |
| T4 (pmol mL−1) | 45.2 ± 1.1 | 44.0 ± 1.3 | 36.8 ± 1.2 | 41.2 ± 2.6 | 39.5 ± 2.4 | 36.3 ± 2.6 | <0.0001*‡ | ns |
| ln T3:T4 ratio | −1.5 ± 0.1 | −1.1 ± 0.1 | −1.1 ± 0.1 | −1.2 ± 0.1 | −1.2 ± 0.1 | −1.2 ± 0.1 | ns | ns |
Absolute plasma corticosteroids and thyroid hormones, natural log transformation (ln) of plasma corticosteroids and thyroid hormones and ratios at birth (Day 1) and 7 days after birth. l‐3,3′,5‐triiodothyronine (T3), thyroxine (T4). Data are means ± SEM. Symbols denote significant differences amongst experimental groups upon post hoc testing: *CON vs. FGRS; †CON vs. FGRI; ‡FGRS vs. FGRI; ns, not significant.
Plasma T4 concentrations were lower at birth in both FGRS and FGRI females compared with CON females (P < 0.01) and remained lower at one week after birth in FGRI females compared with both CON and FGRS lambs (P < 0.0001). Plasma T3 concentrations were lower in male FGRI lambs compared with CON at birth, but not different at one week. There were no differences amongst groups in either sex in the T3:T4 ratio (Table 7).
Plasma glucose and insulin concentrations, and the glucose: insulin ratio over the first two weeks after birth were not different amongst groups in either sex (Figs 7 and 8). Female, but not male, FGRS lambs had a greater increase in plasma insulin from birth to day seven compared with CON (P = 0.04) (Fig. 7).
Figure 7. Plasma hormone and metabolite concentrations over the first 2 weeks after birth in females.
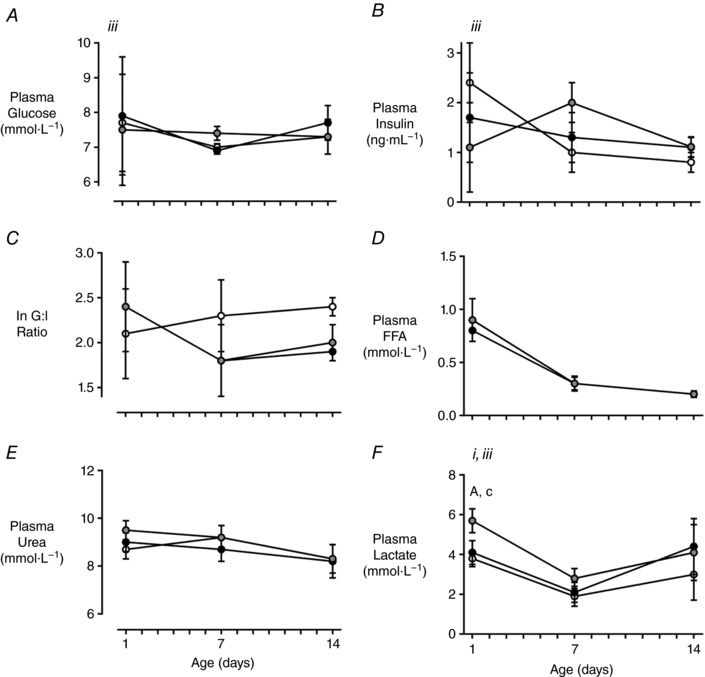
Plasma concentrations of glucose (A) insulin (B), non‐esterified fatty acids (NEFA; F), urea (G) and lactate (H), and the change in insulin over the first (C) and second (D) weeks, and the natural log transformation (ln) of the glucose to insulin (G:I) ratio in the first 2 weeks after birth (E). CON (white) n = 14, FGRS (grey) n = 11–14 and FGRI (black) n = 13 lambs. Data are means ± SEM. Roman numerals denote the significant difference between experimental groups (RM ANOVA: i P < 0.05, iiitime × experimental group interaction P < 0.05). Symbols denote post hoc P values for differences between experimental groups at each time point (ANOVA: CON vs. FGRS: * P < 0.05; CON vs. FGRI: † P < 0.05; ‡ P < 0.05 FGRS vs. FGRI).
Figure 8. Plasma hormone and metabolite concentrations over the first 2 weeks after birth in males.
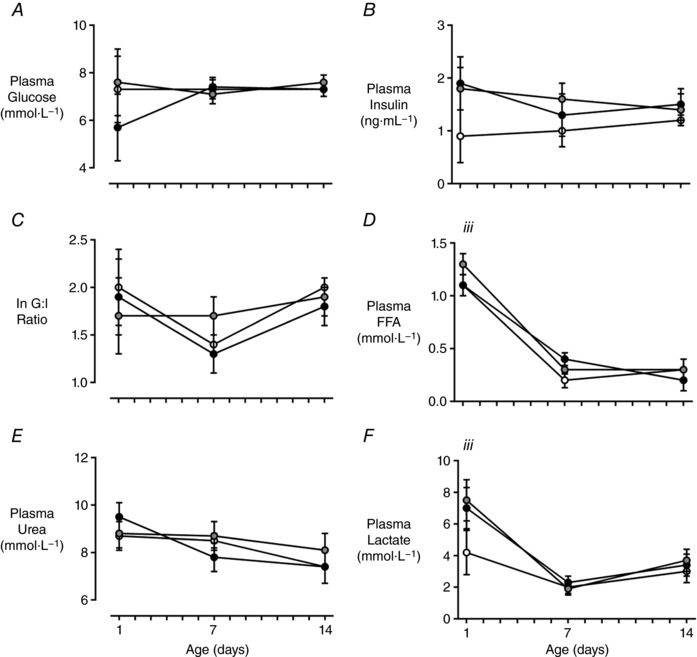
Plasma concentrations of glucose (A), insulin (B), non‐esterified fatty acids (NEFA; F), urea (G) and lactate (H), and the change in insulin over the first (C) and second (D) weeks, and the natural log transformation (ln) of the glucose to insulin (G:I) ratio in the first 2 weeks after birth (E). CON (white) n = 16, FGRS (grey) n = 11 and FGRI (black) n = 10–11 lambs. Data are means ± SEM. Roman numerals denote significant difference between experimental groups (RM ANOVA: iiitime × experimental group interaction P < 0.05). Symbols denote post hoc P values for differences between experimental groups at each time point (ANOVA: CON vs. FGRS: * P < 0.05; CON vs. FGRI: † P < 0.05).
Female FGRS lambs had greater plasma lactate concentrations at birth than both CON and FGRI groups (P = 0.009 and P = 0.04, respectively, Fig. 7), but these were comparable amongst groups by day seven (time*experimental group interaction, P < 0.05). Plasma lactate concentrations were not significantly different amongst groups at any time point in males, although there was a time*experimental group interaction (P < 0.05) reflecting a greater drop in concentration from birth to day 7 in the FGR groups than in CON (Fig. 8). Plasma NEFA and urea concentrations were not different amongst groups (Figs 7 and 8).
Neonatal milk intake
Milk intake in the second week after birth was approximately 20% greater in FGRI females compared with CON (P = 0.03) and FGRS (P = 0.1) (172 ± 7, 142 ± 9 and 147 ± 9 ml·kg−1·d−1, respectively). There was no difference in milk intake amongst groups in males (CON, 148 ± 5; FGRS, 148 ± 5; FGRI, 151 ± 5 ml·kg−1·d−1).
Skeletal muscle mRNA expression
In female FGRS, but not FGRI lambs, skeletal muscle mRNA levels were increased for GHR and SLC2A4 and decreased for IGF2 and IGF1R compared to CON. mRNA levels in FGRI compared with FGRS lambs were increased for IGF1R and decreased for GHR, IGF1, and MTOR (Table 8).
Table 8.
Skeletal muscle and hepatic mRNA expression 1 week after birth
| Female | Male | |||||
|---|---|---|---|---|---|---|
| FGRS vs. CON | FGRI vs. CON | FGRI vs. FGRS | FGRS vs. CON | FGRI vs. CON | FGRI vs. FGRS | |
| Vastus lateralis | ||||||
| Somatotrophic axis | ||||||
| GHR | 1.39 (1.17–1.64)* | 0.82 (0.26–2.57) | 0.59 (0.46–0.75)* | 0.99 (0.89–1.10) | 1.37 (1.20–1.57)* | 1.38 (1.13–1.69)* |
| IGF1 | 1.20 (0.92–1.57) | 0.54 (0.16–1.81) | 0.45 (0.42–0.48)* | 1.14 (1.01–1.30)* | 0.91 (0.67–1.24) | 0.80 (0.55–1.14) |
| IGF2 | 0.79 (0.73–0.86)* | 0.74 (0.19–2.87) | 0.93 (0.61–1.41) | 0.71 (0.47–1.06) | 0.66 (0.41–1.01) | 0.93 (0.58–1.47) |
| IGF1R | 0.88 (0.87–0.90)* | 1.02 (0.28–3.69) | 1.16 (1.09–1.23)* | 0.86 (0.85–0.87)* | 0.68 (0.66–0.70)* | 0.79 (0.78–0.81)* |
| IGFBP3 | 1.15 (0.91–1.45) | 0.74 (0.18–3.02) | 0.64 (0.05–8.00) | 3.37 (1.64–6.93)* | 1.20 (0.83–1.74) | 0.36 (0.14–0.88)* |
| Nutrient uptake | ||||||
| SLC2A4 | 1.23 (1.05–1.44)* | 0.93 (0.38–2.23) | 0.75 (0.56–1.01) | 0.68 (0.57–0.81)* | 0.25 (0.17–0.37)* | 0.37 (0.29–0.46)* |
| MTOR | 1.50 (0.93–2.40) | 0.64 (0.31–1.32) | 0.43 (0.24–0.78)* | 1.18 (0.69–2.00) | 0.31 (0.18–0.51)* | 0.26 (0.15–0.44)* |
| Glucocorticoid receptor | ||||||
| NR3C1 | 0.95 (0.82–1.11) | 0.96 (0.30–3.04) | 1.01 (0.51–1.97) | 0.81 (0.50–1.32) | 0.47 (0.25–0.88)* | 0.58 (0.30–1.12) |
| Liver | ||||||
| Somatotrophic axis | ||||||
| GHR | 1.28 (0.93–1.74) | 1.27 (0.99–1.62) | 0.99 (0.88–1.13) | 0.96 (0.74–1.23) | 0.55 (0.28–1.10) | 0.58 (0.30–1.10) |
| IGF1 | 1.84 (1.45–2.34)* | 1.55 (1.33–1.80)* | 0.84 (0.77–0.91)* | 1.24 (1.08–1.42)* | 0.67 (0.48–0.94)* | 0.55 (0.37–0.81)* |
| IGF2 | 1.00 (0.94–1.07) | 1.08 (0.97–1.21) | 1.08 (1.05–1.11)* | 0.97 (0.89–1.05) | 1.61 (1.34–1.93)* | 1.67 (1.31–2.12)* |
| IGF1R | 0.35 (0.32–0.37)* | 0.51 (0.33–0.80* | 1.48 (1.22–1.80)* | 0.94 (0.89–1.00) | 1.21 (1.19–1.24)* | 1.29 (1.28–1.30)* |
| IGFBP1 | 0.71 (0.44–1.17) | 0.48 (0.27–0.86)* | 0.67 (0.34–1.32) | 4.42 (1.90–10.28)* | 2.84 (1.50–5.39)* | 0.64 (0.41–1.00) |
| IGFBP3 | 1.30 (1.15–1.47)* | 1.56 (1.12–2.16)* | 1.20 (1.19–1.20)* | 1.98 (1.27–3.10)* | 1.97 (1.46–2.66)* | 0.99 (0.91–1.08) |
| IGFALS | 0.95 (0.90–0.99)* | 0.99 (0.66–1.50) | 1.05 (1.04–1.06)* | 0.68 (0.56–0.83)* | 1.25 (1.18–1.31)* | 1.83 (1.75–1.91)* |
| Nutrient uptake | ||||||
| SLC2A2 | 2.43 (1.43–4.13)* | 2.50 (2.23–2.80)* | 1.03 (1.03–1.03)* | 0.97 (0.83–1.12) | 1.47 (1.32–1.65)* | 1.53 (1.42–1.64)* |
| MTOR | 0.58 (0.46–0.68)* | 0.76 (0.63–0.92)* | 1.31 (1.28–1.34)* | 0.98 (0.79–1.21) | 0.95 (0.83–1.09) | 0.98 (0.79–1.21) |
| Glucocorticoid receptor | ||||||
| NR3C1 | 0.48 (0.39–0.59)* | 0.63 (0.38–1.03) | 1.30 (1.10–1.53)* | 0.71 (0.62–0.82)* | 0.93 (0.83–1.04) | 1.31 (1.31–1.31)* |
Skeletal muscle (Female: CON, n = 7; FGRS, n = 6; FGRI n = 4; Male: CON, n = 8; FGRS, n = 5–7; FGRI n = 5‐8) and liver (Female: CON, n = 7; FGRS, n = 8; FGRI n = 8; Male: CON, n = 8; FGRS, n = 8; FGRI n = 8) mRNA expression in lambs 1 week after birth. Data are normalised to the geometric mean of 3 HK genes, and are reported as fold change (99% CI). Where 99% CIs do not include 1.0 (*), mRNA expression is statistically different from CON (FGRS and FGRI) or FGRS (FGRI) at the 1% level.
In male FGRS lambs, skeletal muscle mRNA levels were increased for IGF1 and IGFBP3 compared with CON. mRNA levels in both FGRS and FGRI lambs were decreased for IGF1R and SLC2A4 compared with CON. In FGRI lambs mRNA levels were increased for GHR and decreased for IGF1R, MTOR, and SLC2A4 compared to both CON and FGRS and also decreased for NR3C1 compared with FGRS (Table 8).
Hepatic mRNA expression
In females, hepatic mRNA levels of IGF1, IGFBP3, and SLC2A2 were increased in both FGRS and FGRI compared with CON lambs. FGRI lambs had decreased mRNA levels of IGF1 but increased mRNA levels of IGF1R, IGF2, IGFALS, IGFBP3, MTOR, NR3C1, and SLC2A2 compared with FGRS (Table 8).
In males, hepatic mRNA levels of IGFBP1, and IGFBP3 were increased in both FGRS and FGRI compared with CON. FGRS lambs also had increased IGF1, and decreased IGFALS and NR3C1 mRNA levels compared with CON. FGRI lambs had increased IGF1R, IGF2, IGFALS, and SLC2A2 mRNA levels compared with both CON and FGRS (Table 8).
Discussion
This study demonstrates that intra‐amniotic IGF1 treatment of fetuses with FGR induced by placental embolisation, which we previously have reported increases late gestation fetal growth (Wali et al. 2012), does not alter perinatal morbidity or mortality. Although, in contrast to previous studies (Wali et al. 2012), fetal lactate concentrations during embolisation were not statistically increased, there was a trend towards higher concentrations in embolised fetuses. As the lambs in this study were to be born naturally and followed postnatally, the extent of embolisation had to be judged both to ensure longer fetal survival to term (compared with termination in late gestation in previous studies) and survival of labour. Thus, it is likely that the more modest trend in elevated lactate concentrations reflects less severe embolisation. Similarly, we did not measure daily intrauterine growth as in previous studies as live birth with growth catheters in situ was not deemed feasible. Birthweight of FGR lambs treated with vehicle was significantly less than controls in both males and females; however, only in females did IGF1‐treatment increase birthweight to be similar to controls. We have not found this sexually dimorphic effect in previous studies, although this experiment was intentionally powered to detect effects by sex, in contrast to the earlier fetal studies.
Consistent with our hypothesis and the effect of IGF1 treatment on intra‐uterine growth in females, postnatal growth velocity over the first two weeks after birth was similar in female FGRI lambs and controls, in contrast to the accelerated postnatal growth seen in FGRS female lambs. The pattern of postnatal growth in male lambs is intriguing, with accelerated growth velocity in the first week in FGRS but not FGRI, but the reverse pattern in week 2, despite similar birthweights. Accelerated postnatal growth in the first 2–3 weeks after birth has been reported in studies of ovine FGR induced by carunclectomy (De Blasio et al. 2007; Duffield et al. 2009). Although the patterns of growth in those studies were not reported to be different between males and females (although males grew faster), of note is the fact that growth rate in week one in males was associated with concentrations of non‐esterified fatty acids, whereas no such relationship existed in females (Duffield et al. 2009) further supporting the concept that growth is regulated differently in males and females. Body composition analysis by DXA demonstrated decreased lean mass in female FGRS, but not FGRI, lambs and decreased bone mineral density in both groups of FGR female lambs. Birthweight in male and female FGR lambs in both IGF1 and vehicle treated groups was moderately to strongly correlated with plasma IGF1 concentrations shortly after birth, consistent with both the nutritional regulation of fetal IGF1 concentrations and the key role IGF1 plays in fetal growth.
The pattern of mRNA expression in key genes in the somatotrophic axis may help provide further insight into these findings. In the FGRS females, mRNA levels of GHR in muscle and of IGF1 and IGFBP3 in liver were significantly greater than in controls and FGRI lambs, which also had lower levels of IGF1 mRNA in muscle than FGRS. In contrast, IGF1R mRNA levels in FGRI were greater than in FGRS. In the fetal liver, expression levels of somatotrophic and metabolic genes are altered by changes in nutrition and oxygenation (Gentili et al. 2009; Thorn et al. 2012), to the extent that expression can vary in the two hepatic lobes which receive differing levels of nutrition and oxygen from the umbilical and portal circulations (Darp et al. 2010). We speculate that intra‐uterine administration of physiological doses of IGF1 impacted upon the fetal somatotrophic response to FGR, possibly due to a tissue‐specific delay in somatotrophic maturation, which persisted beyond the period of birth transition into the postnatal period.
In females, both FGR groups had reduced plasma T4, which persisted at postnatal day 7. Although this is consistent with other studies of FGR lambs utilising placental restriction (De Blasio et al. 2006), FGR females did not have reduced plasma T3 concentrations, which could suggest a growth restriction‐mediated reduction in capacity for thyroid hormone secretion that is not resolved by IGF1 treatment. Appropriate thyroid function is necessary to mediate somatotrophic axis maturation around parturition in the liver and skeletal muscle (Forhead et al. 2000, 2002). Thus, reduced plasma T3 at birth in FGRI males could delay the somatotrophic switch from fetal to postnatal GH‐dependent growth. In addition to reduced plasma T3 at birth, FGRI males grew faster in the second week after birth. Upregulation of hepatic somatotrophic (IGF2, IGF1R, IGFBP1, IGFBP3, and IGFALS) and metabolic (SLC2A2) mRNA expression in FGRI compared with CON males, with similar changes compared with FGRS at postnatal day 7, could indicate that T3 is the predominant factor affecting hepatic somatotrophic maturation in males. As FGRI females and males had similar changes in hepatic mRNA expression compared to FGRS without disparity in plasma T3 concentration, this could suggest that somatotrophic maturation and nutrient sequestration in females in the early postnatal period is reliant on other mechanisms.
Independent of alterations in plasma corticosteroids, FGRI males had a significant reduction in plasma T3 compared with CON lambs at birth. The pre‐partum rise in plasma T3 is associated with hepatic upregulation of gluconeogenic gene expression (Forhead et al. 2003) and increased hepatic glyconeogenesis (Forhead et al. 2009). Thus, reduced plasma T3 in FGRI males could suggest compromised gluco‐ and glyco‐neogenic capacity at birth. This is supported by plasma glucose concentrations within the first 2 h after birth in FGRI males that were 2.0 mmol·L−1 lower than in both CON and FGRS groups, although this finding was not statistically significant. Interestingly, FGRS females had a significantly greater plasma lactate concentration at birth than both control and IGF1‐treated females, which could suggest reduced metabolic flexibility. Assessment of hepatic molecular gluco‐ and glyco‐neogenic gene expression was not feasible due to the small quantity of tissues collected.
Despite accelerated postnatal growth velocity in female FGRS lambs compared with CON in the first two weeks after birth, and increased mRNA levels of hepatic IGF1 and SLC2A2 and skeletal muscle GHR SLC2A4, lean mass remained reduced at postnatal day 7. During this postnatal period of rapid weight gain, fat deposition is greatest (Ricordeau et al. 1961), and is due to increased peripheral insulin sensitivity (Gardner et al. 2005) and activation of adipogenic and lipogenic pathways (Graugnard et al. 2009; Isganaitis et al. 2009). Interestingly, FGRI females had greater milk intake than controls, but this was not associated with differential skeletal muscle mRNA expression or accelerated growth velocity. As FGRI females did not display a greater glucose to insulin ratio, a proxy measure for basal insulin sensitivity (Green et al. 2011), which could suggest greater reliance on non‐insulin dependent nutrient uptake, and altered mechanisms of neonatal growth compared with saline‐treated lambs.
The novel finding of a relationship between maternal NTproCNP, which derives from the placenta in sheep (McNeill et al. 2011), and fetal oxygenation and size at birth reported in this study is significant. Due to rapid degradation of CNP at source, plasma NTproCNP, which is biologically inactive and physiologically stable, is the best marker for CNP secretion. In sheep, the CNP signalling pathway is greatly upregulated in the uterine arterial endothelium where it is thought to mediate, in part, the massive increase in uterine blood flow in late gestation (Itoh et al. 1998). Previous experimental studies have shown that both acute and chronic fetal nutrient restriction, and increasing litter size, increase maternal plasma CNP and NTproCNP (Prickett et al. 2007; McNeill et al. 2009; Madhavan et al. 2017) indicating fetal‐placental regulation of CNP concentrations in the maternal circulation that reflect fetal status. These findings are supported by studies in women that report higher CNP and NTproCNP concentrations in maternal plasma from mid‐pregnancy in women who later develop complications associated with a precarious fetal nutrient supply, such as pre‐eclampsia, hypertension and growth restriction (Reid et al. 2014; Espiner et al. 2015). The current work extends these findings in suggesting that the maintenance of higher fetal during isolated placental insufficiency induced through placental embolisation could be mediated, at least in part, by a CNP‐evoked adaptive vasodilator response. Although this study was not designed to elucidate the underlying mechanisms linking fetal with maternal CNP, the potential clinical utility of maternal plasma NTproCNP in non‐invasive fetal testing for the monitoring or prediction of late‐gestation placental insufficiency warrants investigation. Conceivably, positive findings could open up the possibility of therapeutic use of exogenous CNP, which has already been approved for use in humans (ClinicalTrials.gov.NCT055157), in settings of maternal vascular complications.
This is the first study to describe the postnatal effects of an intra‐amniotic intervention on endocrine, metabolic and somatotrophic maturation associated with alterations in growth velocity and body composition in the early postnatal period. These physiological and molecular adaptations during the early postnatal period following IGF1 treatment suggest differential mechanisms for the abrogation of early postnatal catch‐up growth in a sex‐specific manner. In FGRS males, catch‐up growth occurred in the first week following birth, and in females in the second week; IGF1 delayed accelerated growth velocity in males only. Together, these data suggest that, rather than relying on postnatal interventions initiated long after phenotypic and physiologic adaptation to fetal deprivation, intrauterine intervention provides an opportunity to alter fetal growth potential, influence postnatal growth, and potentially abrogate FGR‐associated disease development prior to the development of postnatal complications. Whether the observed changes in mRNA expression in IGF1‐treated lambs are advantageous in abrogating growth restriction‐mediated alterations in somatotrophic sensitivity remains unclear. However, we acknowledge that discrete and limited tissue‐specific measurements should be interpreted with care. Future time‐course studies could help determine the timing and magnitude of tissue‐specific alterations in the somatotrophic axis in response to intra‐amniotic IGF1 treatment, and identify the appropriate sex‐specific timing of intervention.
Perspectives and significance
This is the first study to show that a brief intra‐amniotic intervention alters the postnatal growth of the growth‐restricted fetus and may, therefore, have clinical utility. Discordance in treatment effects between the sexes could be associated with compensatory mechanisms in response to treatment, or differential mechanisms associated with physiological maturation and, thus, capacity for postnatal growth. Elucidation of longitudinal changes would provide insight into the postnatal implications of this brief prenatal intervention.
Additional information
Competing interests
None declared.
Author contributions
This work was conducted at The Liggins Institute, Ngapouri, and The Liggins Institute, Auckland, New Zealand. Conceived and designed experiments: F.H.B., J.E.H., M.H.O., A.M.S. Acquired, analysed and interpreted data: A.M.S., F.H.B., M.H.O., T.P., E.E. Drafted manuscript: A.M.S., F.H.B. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by the Health Research Council of New Zealand (08/088A and 09/095) and Gravida: National Centre for Growth and Development. A. M. Spiroski was supported by a Gravida Doctoral Scholarship and a Liggins Institute Doctoral Scholarship.
Present address
A. M. Spiroski: Experimental Medicine and Immunotherapeutics, Department of Medicine, University of Cambridge, UK.
Translational perspectives
Fetal growth restriction (FGR) is a significant pregnancy complication for which there currently is no treatment. Babies with FGR frequently are born early and small, with increased perinatal mortality and morbidity. They then commonly show accelerated growth soon after birth, and this is associated with increased risks of later metabolic disease. Previous animal studies have shown that physiological doses of insulin‐like growth factor (IGF) 1, a key fetal growth factor, administered into amniotic fluid are swallowed by the fetus and increase fetal growth. However, it is not known whether this treatment alters perinatal mortality or postnatal growth and metabolism. We hypothesised that intra‐amniotic IGF1 given to the FGR ovine fetus would mitigate the accelerated postnatal growth without adverse effects on perinatal mortality and morbidity. We found that this approach appears safe, with no increase in perinatal mortality or morbidity. Furthermore, there were sex‐specific effects on postnatal growth that were not related to milk intake but were associated with changes in expression of key genes in the somatotrophic axis. We also report that maternal concentrations of N‐terminal pro‐C‐type natriuretic peptide (NTproCNP), a placental hormone, correlate with fetal arterial partial pressures of oxygen, suggesting that this may have potential as a novel non‐invasive maternal test of fetal well‐being. These findings suggest that intrauterine treatment of FGR may be feasible. However, data on long‐term effects on growth, body composition, metabolic profile are needed before clinical application. The potential utility of NTproCNP as a marker of fetal oxygenation merits further investigation.
Acknowledgments
We would like to thank the staff at The Liggins Institute, Ngapouri for their excellence in animal care, and the LiFePATH Group at The Liggins Institute, University of Auckland, for their assistance with this work.
Edited by: Laura Bennet & Janna Morrison
Linked articles This article is highlighted in a Perspectives article by Wood. To read this article, visit http://doi.org/10.1113/JP275612.
This is an Editor's Choice article from the 1 December 2018 issue.
References
- Alberry M & Soothill P (2007). Management of fetal growth restriction. Arch Dis Child Fetal Neonatal Ed 92, F62–F67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A, Bloomfield FH, Connor KL, Dragunow M, Thorstensen EB, Oliver MH, Sloboda DM, Harding JE & Alsweiler JM (2015). Glucocorticoid‐induced preterm birth and neonatal hyperglycemia alter ovine β‐cell development. Endocrinology 156, 3763–3776. [DOI] [PubMed] [Google Scholar]
- Baschat AA, Gembruch U & Harman C (2001). The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet Gynecol 18, 571–577. [DOI] [PubMed] [Google Scholar]
- Beltrand J, Nicolescu R, Kaguelidou F, Verkauskiene R, Sibony O, Chevenne D, Claris O & Lévy‐Marchal C (2009). Catch‐up growth following fetal growth restriction promotes rapid restoration of fat mass but without metabolic consequences at one year of age. PLoS ONE 4, e5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein IM, Horbar JD, Badger GJ, Ohlsson A & Golan A (2000). Morbidity and mortality among very‐low‐birth‐weight neonates with intrauterine growth restriction. Am J Obstet Gynecol 182, 198–206. [DOI] [PubMed] [Google Scholar]
- Bloomfield FH, Bauer MK, Van Zijl PL, Gluckman PD & Harding JE (2002a). Amniotic IGF‐I supplements improve gut growth but reduce circulating IGF‐I in growth‐restricted fetal sheep. Am J Physiol Endocrinol Metab 282, E259–E269. [DOI] [PubMed] [Google Scholar]
- Bloomfield FH, Spiroski A‐M & Harding JE (2013). Fetal growth factors and fetal nutrition. Semin Fetal Neonatal Med 18, 118–123. [DOI] [PubMed] [Google Scholar]
- Bloomfield FH, van Zijl PL, Bauer MK & Harding JE (2002b). Effects of intrauterine growth restriction and intraamniotic insulin‐like growth factor‐I treatment on blood and amniotic fluid concentrations and on fetal gut uptake of amino acids in late‐gestation ovine fetuses. J Pediatr Gastroenterol Nutr 35, 287–297. [DOI] [PubMed] [Google Scholar]
- Chiesa C, Osborn JF, Haass C, Natale F, Spinelli M, Scapillati E, Spinelli A & Pacifico L (2008). Ghrelin, leptin, IGF‐1, IGFBP‐3, and insulin concentrations at birth: Is there a relationship with fetal growth and neonatal anthropometry? Clin Chem 54, 550–558. [DOI] [PubMed] [Google Scholar]
- Chomtho S, Wells JC, Williams JE, Davies PS, Lucas A & Fewtrell MS (2008). Infant growth and later body composition: evidence from the 4‐component model. Am J Clin Nutr 87, 1776–1784. [DOI] [PubMed] [Google Scholar]
- Darp R, de Boo H, Phua H, Oliver M, Derraik J, Harding J & Bloomfield F (2010). Differential regulation of igf1 and igf1r mRNA levels in the two hepatic lobes following intrauterine growth restriction and its treatment with intra‐amniotic insulin‐like growth factor‐1 in ovine fetuses. Reprod Fertil Dev 22, 1188–1197. [DOI] [PubMed] [Google Scholar]
- De Blasio MJ, Gatford KL, Robinson JS & Owens JA (2006). Placental restriction alters circulating thyroid hormone in the young lamb postnatally. Am J Physiol Regul Integr Comp Physiol 291, R1016–R1024. [DOI] [PubMed] [Google Scholar]
- De Blasio MJ, Gatford KL, Robinson JS & Owens JA (2007). Placental restriction of fetal growth reduces size at birth and alters postnatal growth, feeding activity, and adiposity in the young lamb. Am J Physiol Regul Integr Comp Physiol 292, R875–R886. [DOI] [PubMed] [Google Scholar]
- de Boo HA, Eremia SC, Bloomfield FH, Oliver MH & Harding JE (2008). Treatment of intrauterine growth restriction with maternal growth hormone supplementation in sheep. Am J Obstet Gynecol 199, 559.e1‐9. [DOI] [PubMed] [Google Scholar]
- Diderholm B (2009). Perinatal energy metabolism with reference to IUGR & SGA: studies in pregnant women & newborn infants. Indian J Med Res 130, 612–617. [PubMed] [Google Scholar]
- Diderholm B, Ewald U, Ahlsson F & Gustafsson J (2007). Energy substrate production in infants born small for gestational age. Acta Pædiatr 96, 29–34. [DOI] [PubMed] [Google Scholar]
- Duffield JA, Vuocolo T, Tellam R, McFarlane JR, Kauter KG, Muhlhausler BS & McMillen IC (2009). Intrauterine growth restriction and the sex specific programming of leptin and peroxisome proliferator‐activated receptor α (PPARα) mRNA expression in visceral fat in the lamb. Pediatr Res 66, 59–65. [DOI] [PubMed] [Google Scholar]
- Eremia SC, de Boo HA, Bloomfield FH, Oliver MH & Harding JE (2007). Fetal and amniotic insulin‐like growth factor‐I supplements improve growth rate in intrauterine growth restriction fetal sheep. Endocrinology 148, 2963–2972. [DOI] [PubMed] [Google Scholar]
- Espiner EA, Prickett TCR, Taylor RS, Reid RA & McCowan LM (2015). Effects of pre‐eclampsia and fetal growth restriction on C‐type natriuretic peptide. BJOG 122, 1236–1243. [DOI] [PubMed] [Google Scholar]
- Forhead AJ, Cutts S, Matthews PA & Fowden AL (2009). Role of thyroid hormones in the developmental control of tissue glycogen in fetal sheep near term. Exp Physiol 94, 1079–1087. [DOI] [PubMed] [Google Scholar]
- Forhead AJ, Li J, Gilmour RS, Dauncey MJ & Fowden AL (2002). Thyroid hormones and the mRNA of the GH receptor and IGFs in skeletal muscle of fetal sheep. Am J Physiol Endocrinol Metab 282, E80–E86. [DOI] [PubMed] [Google Scholar]
- Forhead AJ, Li J, Saunders JC, Dauncey MJ, Gilmour RS & Fowden AL (2000). Control of ovine hepatic growth hormone receptor and insulin‐like growth factor I by thyroid hormones in utero. Am J Physiol Endocrinol Metab 278, E1166–E1174. [DOI] [PubMed] [Google Scholar]
- Forhead AJ, Poore KR, Mapstone J & Fowden AL (2003). Developmental regulation of hepatic and renal gluconeogenic enzymes by thyroid hormones in fetal sheep during late gestation. J Physiol 548, 941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner DS, Tingey K, Van Bon BWM, Ozanne SE, Wilson V, Dandrea J, Keisler DH, Stephenson T & Symonds ME (2005). Programming of glucose‐insulin metabolism in adult sheep after maternal undernutrition. Am J Physiol Regul Integr Comp Physiol 289, R947–R954. [DOI] [PubMed] [Google Scholar]
- Gentili S, Morrison JL & McMillen IC (2009). Intrauterine growth restriction and differential patterns of hepatic growth and expression of IGF1, PCK2, and HSDL1 mRNA in the sheep fetus in late gestation. Biol Reprod 80, 1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD (1997). Endocrine and nutritional regulation of prenatal growth. Acta Paediatr Suppl 423, 153–158. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Butler JH & Elliott TB (1983). The ontogeny of somatotropic binding sites in ovine hepatic membranes. Endocrinology 112, 1607–1612. [DOI] [PubMed] [Google Scholar]
- Gootwine E (2013). Meta‐analysis of morphometric parameters of late‐gestation fetal sheep developed under natural and artificial constraints. J Anim Sci 91, 111–119. [DOI] [PubMed] [Google Scholar]
- Graugnard D, Piantoni P, Bionaz M, Berger L, Faulkner D & Loor J (2009). Adipogenic and energy metabolism gene networks in longissimus lumborum during rapid post‐weaning growth in Angus and Angus × Simmental cattle fed high‐starch or low‐starch diets. BMC Genomics 10, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AS, Macko AR, Rozance PJ, Yates DT, Chen X, Hay WW & Limesand SW (2011). Characterization of glucose‐insulin responsiveness and impact of fetal number and sex difference on insulin response in the sheep fetus. Am J Physiol Endocrinol Metab 300, E817–E823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments. J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han V, Lund P, Lee D & D'ercole J (1988). Expression of somatomedin/insulin‐like growth factor messenger ribonucleic acids in the human fetus: Identification, characterization, and tissue distribution. J Clin Endocrinol Metab 66, 422–429. [DOI] [PubMed] [Google Scholar]
- Hecher K, Bilardo C & Stigter R (2001). Monitoring of fetuses with intrauterine growth restriction: a longitudinal study. Ultrasound Obstet Gynecol 18, 564–570. [DOI] [PubMed] [Google Scholar]
- Isganaitis E, Jimenez‐Chillaron J, Woo M, Chow A, DeCoste J, Vokes M, Liu M, Kasif S, Zavacki A‐M, Leshan RL, Myers MG & Patti M‐E (2009). Accelerated postnatal growth increases lipogenic gene expression and adipocyte size in low‐birth weight mice. Diabetes 58, 1192–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Bird IM, Nakao K & Magness RR (1998). Pregnancy increases soluble and particulate guanylate cyclases and decreases the clearance receptor of natriuretic peptides in ovine uterine, but not systemic, arteries. Endocrinology 139, 3329–3341. [DOI] [PubMed] [Google Scholar]
- Jaquiery AL, Oliver MH, Bloomfield FH & Harding JE (2011). Periconceptional events perturb postnatal growth regulation in sheep. Pediatr Res 70, 261–266. [DOI] [PubMed] [Google Scholar]
- Jensen EC, Harding JE, Bauer MK & Gluckman PD (1999). Metabolic effects of IGF‐I in the growth retarded fetal sheep. J Endocrinol 161, 485–494. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M & Altman DG (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br J Pharmacol 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Jindo T, Narita T, Naruse K, Kobayashi D, Shin‐I T, Kitagawa T, Sakaguchi T, Mitani H, Shima A, Kohara Y & Takeda H (2004). Large‐scale isolation of ESTs from medaka embryos and its application to medaka developmental genetics. Mech Dev 121, 915–932. [DOI] [PubMed] [Google Scholar]
- Liu L, Harding JE, Evans PC & Gluckman PD (1994). Maternal insulin‐like growth factor‐I infusion alters feto‐placental carbohydrate and protein metabolism in pregnant sheep. Endocrinology 135, 895–900. [DOI] [PubMed] [Google Scholar]
- McNeill BA, Barrell GK, Wellby M, Prickett TCR, Yandle TG & Espiner EA (2009). C‐Type natriuretic peptide forms in pregnancy: maternal plasma profiles during ovine gestation correlate with placental and fetal maturation. Endocrinology 150, 4777–4783. [DOI] [PubMed] [Google Scholar]
- McNeill BA, Barrell GK, Wooding FB, Prickett TC, Espiner EA (2011). The trophoblast binucleate cell is the source of maternal circulating C‐type natriuretic peptide during ovine pregnancy. Placenta 32, 645–650. [DOI] [PubMed] [Google Scholar]
- Madhavan S, Prickett TCR, Espiner EA & Barrell GK (2017). Nutrient restriction in early ovine pregnancy stimulates C‐type natriuretic peptide production. Reprod Fertil Dev 29, 575–584. [DOI] [PubMed] [Google Scholar]
- Modi N, Thomas E, Harrington T, Uthaya S, Dore C & Bell J (2006). Determinants of adiposity during preweaning postnatal growth in appropriately grown and growth‐restricted term infants. Pediatr Res 60, 345–348. [DOI] [PubMed] [Google Scholar]
- Murotsuki J, Challins JRG, Johnston L & Gagnon R (1995). Increased fetal plasma prostaglandin E2 concentrations during fetal placental embolization in pregnant sheep. Am J Obstet Gynecol 173, 30–35. [DOI] [PubMed] [Google Scholar]
- Oliver M, Harding JE, Breier B, Evans PC & Gluckman PD (1993). Glucose but not mixed amino acid infusion regulates plasma insulin‐like growth factor‐I concentrations in fetal sheep. Pediatr Res 34, 62–65. [DOI] [PubMed] [Google Scholar]
- Oliver MH, Hawkins P & Harding JE (2005). Periconceptional undernutrition alters growth trajectory and metabolic and endocrine responses to fasting in late‐gestation fetal sheep. Pediatr Res 57, 591–598. [DOI] [PubMed] [Google Scholar]
- Owens JA (1991). Endocrine and substrate control of fetal growth. Placental and maternal influences and insulin‐like growth factors. Reprod Fertil Dev 3, 501–517. [DOI] [PubMed] [Google Scholar]
- Padoan A, Rigano S, Ferrazzi E, Beaty BL, Battaglia FC & Galan HL (2004). Differences in fat and lean mass proportions in normal and growth‐restricted fetuses. Am J Obstet Gynecol 191, 1459–1464. [DOI] [PubMed] [Google Scholar]
- Patel AL, Engstrom JL, Meier PP & Kimura RE (2005). Accuracy of methods for calculating postnatal growth velocity for extremely low birth weight infants. Pediatrics 116, 1466–1473. [DOI] [PubMed] [Google Scholar]
- Patel D & Kalhan S (1992). Glycerol metabolism and triglyceride‐fatty acid cycling in the human newborn: effect of maternal diabetes and intrauterine growth retardation. Pediatr Res 31, 52–58. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW (2001). A new mathematical model for relative quantification in real‐time RT–PCR. Nucleic Acids Res 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW, Tichopad A, Prgomet C & Neuvians TP (2004). Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper – Excel‐based tool using pair‐wise correlations. Biotechnol Lett 26, 509–515. [DOI] [PubMed] [Google Scholar]
- Prickett T, Yandle T, Nicholls M, Espiner E & Richards A (2001). Identification of amino‐terminal pro‐C‐type natriuretic peptide in human plasma. Biochem Biophys Res Comm 286, 513–517. [DOI] [PubMed] [Google Scholar]
- Prickett TC, Kaaja RJ, Nicholls MG, Espiner EA, Richards AM & Yandle TG (2004). N‐terminal pro‐C‐type natriuretic peptide, but not C‐type natriuretic peptide, is greatly elevated in the fetal circulation. Clin Sci 106, 535–540. [DOI] [PubMed] [Google Scholar]
- Prickett TCR, Rumball CWH, Buckley AJ, Bloomfield FH, Yandle TG, Harding JE & Espiner EA (2007). C‐Type natriuretic peptide forms in the ovine fetal and maternal circulations: evidence for independent regulation and reciprocal response to undernutrition. Endocrinology 148, 4015–4022. [DOI] [PubMed] [Google Scholar]
- Reid RA, Prickett TCR, Pullar BE, Darlow BA, Gullam JE & Espiner EA (2014). C‐Type natriuretic peptide in complicated pregnancy: increased secretion precedes adverse events. J Clin Endocrinol Metab 99, 1470–1478. [DOI] [PubMed] [Google Scholar]
- Ricordeau G, Boccard R, Damiani C & Van Willigen A (1961). Relations entre la quantité de lait consommé par les agneaux et leur croissance In Annales de Zootechnie, pp. 113–125. EDP Sciences, France. [Google Scholar]
- Rohrer F (1921). Der Index der Korperfulle als Mass des Ernahrungszustandes. MMW Munch Med Wochenschr 68, 580–582. [Google Scholar]
- Rumball CWH, Oliver MH, Thorstensen EB, Jaquiery AL, Husted SM, Harding JE & Bloomfield FH (2008). Effects of twinning and periconceptional undernutrition on late‐gestation hypothalamic‐pituitary‐adrenal axis function in ovine pregnancy. Endocrinology 149, 1163–1172. [DOI] [PubMed] [Google Scholar]
- Sakurai M, Itabashi K, Sato Y, Hibino S & Mizuno K (2008). Extrauterine growth restriction in preterm infants of gestational age ≤32 weeks. Pediatr Int 50, 70–75. [DOI] [PubMed] [Google Scholar]
- Singhal A, Kennedy K, Lanigan J, Fewtrell M, Cole TJ, Stephenson T, Elias‐Jones A, Weaver LT, Ibhanesebhor S, MacDonald PD, Bindels J & Lucas A (2010). Nutrition in infancy and long‐term risk of obesity: evidence from 2 randomized controlled trials. Am J Clin Nutr 92, 1133–1144. [DOI] [PubMed] [Google Scholar]
- Spiroski A‐M, Oliver MH, Harding JE & Bloomfield FH (2016). Intrauterine intervention for the treatment of fetal growth restriction. Curr Pediatr Rev 12, 168–178. [DOI] [PubMed] [Google Scholar]
- The GRIT Study Group (2004). Infant wellbeing at 2 years of age in the Growth Restriction Intervention Trial (GRIT): multicentred randomised controlled trial. Lancet 364, 513–520. [DOI] [PubMed] [Google Scholar]
- Thorn SR, Sekar SM, Lavezzi JR, O'Meara MC, Brown LD, Hay WW & Rozance PJ (2012). A physiological increase in insulin suppresses gluconeogenic gene activation in fetal sheep with sustained hypoglycemia. Am J Physiol Regul Integr Comp Physiol 303, R861–R869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triant DA & Whitehead A (2009). Simultaneous extraction of high‐quality RNA and DNA from small tissue samples. J Hered 100, 246–250. [DOI] [PubMed] [Google Scholar]
- Unterscheider J, Daly S, Geary MP, Kennelly MM, McAuliffe FM, O'Donoghue K, Hunter A, Morrison JJ, Burke G, Dicker P, Tully EC & Malone FD (2013). Optimizing the definition of intrauterine growth restriction: the multicenter prospective PORTO Study. Am J Obstet Gynecol 208, 290.e291–290.e296. [DOI] [PubMed] [Google Scholar]
- Verkauskiene R, Beltrand J, Claris O, Chevenne D, Deghmoun S, Dorgeret S, Alison M, Gaucherand P, Sibony O & Levy‐Marchal C (2007). Impact of fetal growth restriction on body composition and hormonal status at birth in infants of small and appropriate weight for gestational age. Eur J Endocrinol 157, 605–612. [DOI] [PubMed] [Google Scholar]
- Wali JA, de Boo HA, Derraik JGB, Phua HH, Oliver MH, Bloomfield FH & Harding JE (2012). Weekly intra‐amniotic IGF‐1 treatment increases growth of growth‐restricted ovine fetuses and up‐regulates placental amino acid transporters. PLoS ONE 7, e37899. [DOI] [PMC free article] [PubMed] [Google Scholar]


