Abstract
Key points
Perinatal hypoxia causes pulmonary hypertension in neonates, including humans. However, in species adapted to hypoxia, such as the llama, there is protection against pulmonary hypertension.
Nitric oxide (NO) is a vasodilatator with an established role in the cardiopulmonary system of many species, but its function in the hypoxic pulmonary vasoconstrictor response in the newborn llama is unknown. Therefore, we studied the role of NO in the cardiopulmonary responses to acute hypoxia in high‐ and lowland newborn llamas.
We show that high‐ compared to lowland newborn llamas have a reduced pulmonary vasoconstrictor response to acute hypoxia. Protection against excessive pulmonary vasoconstriction in the highland llama is mediated via enhancement of NO pathways, including increased MYPT1 and reduced ROCK expression as well as Ca2+ desensitization.
Blunting of pulmonary hypertensive responses to hypoxia through enhanced NO pathways may be an adaptive mechanism to withstand life at high altitude in the newborn llama.
Abstract
Llamas are born in the Alto Andino with protection against pulmonary hypertension. The physiology underlying protection against pulmonary vasoconstrictor responses to acute hypoxia in highland species is unknown. We determined the role of nitric oxide (NO) in the cardiopulmonary responses to acute hypoxia in high‐ and lowland newborn llamas. The cardiopulmonary function of newborn llamas born at low (580 m) or high altitude (3600 m) was studied under acute hypoxia, with and without NO blockade. In pulmonary arteries, we measured the reactivity to potassium and sodium nitroprusside (SNP), and in lung we determined the content of cGMP and the expression of the NO‐related proteins: BKCa, PDE5, PSer92‐PDE5, PKG‐1, ROCK1 and 2, MYPT1, PSer695‐MYPT1, PThr696‐MYPT1, MLC20 and PSer19‐MLC20. Pulmonary vascular remodelling was evaluated by morphometry and based on α‐actin expression. High‐ compared to lowland newborn llamas showed lower in vivo pulmonary arterial pressor responses to acute hypoxia. This protection involved enhanced NO function, as NO blockade reverted the effect and the pulmonary arterial dilatator response to SNP was significantly enhanced in highland neonates. The pulmonary expression of ROCK2 and the phosphorylation of MLC20 were lower in high‐altitude llamas. Conversely, MYPT1 was up‐regulated whilst PSer695‐MYPT1 and PThr695‐MYPT1 did not change. Enhanced NO‐dependent mechanisms were insufficient to prevent pulmonary arterial remodelling. Combined, the data strongly support that in the highland newborn llama reduced ROCK, increased MYPT1 expression and Ca2+ desensitization in pulmonary tissue allow an enhanced NO biology to limit hypoxic pulmonary constrictor responses. Blunting of hypoxic pulmonary hypertensive responses may be an adaptive mechanism to life at high altitude.
Keywords: hypoxia, high altitude, pulmonary circulation, nitric oxide, vasodilatation
Key points
Perinatal hypoxia causes pulmonary hypertension in neonates, including humans. However, in species adapted to hypoxia, such as the llama, there is protection against pulmonary hypertension.
Nitric oxide (NO) is a vasodilatator with an established role in the cardiopulmonary system of many species, but its function in the hypoxic pulmonary vasoconstrictor response in the newborn llama is unknown. Therefore, we studied the role of NO in the cardiopulmonary responses to acute hypoxia in high‐ and lowland newborn llamas.
We show that high‐ compared to lowland newborn llamas have a reduced pulmonary vasoconstrictor response to acute hypoxia. Protection against excessive pulmonary vasoconstriction in the highland llama is mediated via enhancement of NO pathways, including increased MYPT1 and reduced ROCK expression as well as Ca2+ desensitization.
Blunting of pulmonary hypertensive responses to hypoxia through enhanced NO pathways may be an adaptive mechanism to withstand life at high altitude in the newborn llama.
Introduction
Nitric oxide (NO) plays a key role in contributing to the mechanisms mediating the pulmonary vasodilatation that occurs during the fetal to neonatal transition at birth (Abman, 1999, 2007; Gao & Raj, 2010). NO produced by endothelial nitric oxide synthase (eNOS) diffuses through to pulmonary arterial smooth muscle cells (PASMCs) to initiate a cGMP protein kinase G (PKG‐1)‐dependent cascade, which promotes smooth muscle relaxation via mechanisms including calcium desensitization and hyperpolarization (Gao & Raj, 2011; Jernigan & Resta, 2014; Gai et al. 2015). These dilatatory effects of NO on the pulmonary vasculature are particularly important when the fetal to neonatal transition takes place at high altitude where there is a low atmospheric, alveolar and pulmonary arterial . Despite protective effects of NO‐mediated pulmonary vasodilatation, it is established that high‐altitude hypobaric hypoxia promotes persistent pulmonary hypertension of the newborn in humans (Gao & Raj, 2011) and other animals (Herrera et al. 2007) from sea level ancestry. This is different in the llama, a species thought to have lived in the Alto Andino for at least two million years (Webb, 1978; Stanley et al. 1994), allowing the selection of genes to promote greater cardiopulmonary protective mechanisms to withstand hypoxia.
Several studies in the llama during the fetal, newborn and adult period support the idea that this species is genetically adapted to the hypobaric hypoxia of life at high altitude. Among such adaptations, fetal llamas have lower basal cardiac output and organ blood flows, and greater peripheral vasoconstrictor responses to acute stress (Giussani et al. 1999; Llanos et al. 2007). Arterial blood from newborn llamas shows higher saturation of haemoglobin with oxygen compared with highland newborn sheep, suggesting a greater affinity for oxygen in their haemoglobin (Moraga et al 1996). Further, in contrast to sheep, newborn or adult llamas at high altitude do not show an increase in haemoglobin concentration in their blood (Llanos et al. 2007; Herrera et al. 2008a) and we have previously reported that neonatal llamas at high altitude show normotensive values for pulmonary arterial pressure during basal conditions (Herrera et al. 2008a).
At sea level, NO is not only important in protecting the pulmonary circulation during basal conditions but also in response to acute episodes of hypoxic stress. In newborn animals the vasodilatatory actions of NO also protect against exaggerated pulmonary arterial constrictor responses during the period of oxygen deprivation (Kylhammar & Radegran, 2017). However, the effects on cardiopulmonary function of acute hypoxia superimposed on high‐altitude hypobaric hypoxia in the newborn llama and any protective role of NO under these stimulated conditions is unknown. Therefore, adopting an integrative approach combining studies in vivo with experiments at the isolated organ and molecular levels, we determined the role of NO in the cardiopulmonary responses to an acute episode of hypoxia in high‐ and lowland newborn llamas. We tested the hypothesis that the pulmonary arterial hypertensive response to acute hypoxia in highland relative to lowland newborn llamas is diminished by enhanced NO‐dependent mechanisms. The programme of work determined: (1) in vivo pulmonary and systemic arterial blood pressure, during basal conditions and during an episode of acute hypoxia with and without in vivo NO blockade; (2) constrictor and dilatator reactivity in isolated pulmonary arterial resistance vessels; (3) morphological remodelling of small pulmonary arteries; and (4) establishing in vitro in lung tissue the expression of proteins and small molecules involved in vasodilatator mechanisms downstream from NO, including cGMP, large‐conductance calcium‐dependent potassium channels (BKCa), phosphodiesterase 5 (PDE5), phospho‐serine92‐phosphodiesterase5 (Pser92‐PDE5), cGMP‐dependent protein kinase (PKG‐1), Rho‐associated kinase 1 and 2 (ROCK1 and 2), myosin phosphatase target subunit 1 (MYPT1), phospho‐serine695‐myosin phosphatase target subunit 1 (PSer695‐MYPT1), phospho‐threonine696‐myosin phosphatase target subunit 1 (PThr696‐MYPT1), myosin light chain (MLC20) and phospho‐serine 19 myosin light chain (PSer19‐MLC20).
Methods
All animal care, procedures and experimentation were conducted in accordance with The Guide for the Care and Use of Laboratory Animals of the National Research Council and the UK Animals (Scientific Procedures) Act 1986. All procedures were approved by the Ethical Review Committee of the Faculty of Medicine (Protocols CBA#040 and CBA0232), University of Chile. After in vivo experimentation, instrumented animals were submitted to a new anaesthetic protocol to remove the catheters. Daily postoperative care was implemented until full recovery and animals were returned to the flock. These were not used in further experiments. The authors understand the ethical principles under which the journal operates and declare that the present work complies with these policies (Grundy, 2015).
Animals
Seven lowland llamas (LALL age = 8.9 ± 1.2 days, weight = 13.8 ± 0.9 kg), born and raised at the Estación Experimental Germán Grove Silva, Universidad de Chile, Santiago, 580 m above sea level and six highland llamas (HALL age = 9.5 ± 0.7 days, weight = 10.1 ± 0.2 kg), born and raised at Estación Experimental Putre, International Centre for Andean Studies (INCAS), Universidad de Chile, 3600 m above sea level were used in this study. The newborn animals and their mothers were housed in an open yard with access to food and water ad libitum and the newborn animals were familiarized with the laboratory conditions for 3 days prior to catheterization. Tissues for ex vivo small artery experiments and in vitro procedures were obtained from a separate group of non‐instrumented newborn LALL (n = 6) and HALL (n = 7) at equivalent ages.
In vivo experiments
Surgical preparation of newborn llamas
Surgery was carried out at 9–10 days old. The animals were pre‐medicated with atropine (0.04 mg kg−1 i.m., atropine sulphate, Laboratorio Chile, Santiago, Chile) and anaesthetized with ketamine (ketamine, 10 mg kg−1 i.m., Ketostop, Drag Pharma‐Invectec, Santiago, Chile) and diazepam (0.1–0.5 mg kg−1 i.m., Laboratorio Biosano, Santiago, Chile) with additional local infiltration of lidocaine (2% lidocaine hydrochloride, Dimecaína, Laboratorio Beta, Santiago, Chile) in the incision area. Polyvinyl catheters (0.8 mm i.d.) were placed in the descending aorta and inferior vena cava via femoral vessels. The polyvinyl catheters were exteriorized subcutaneously through the flank and kept in a pouch sewn onto the skin. A Swan‐Ganz catheter (Edwards Swan‐Ganz 7 French, Baxter Healthcare, Irvine, CA, USA) was placed into the pulmonary artery through the right external jugular vein, exteriorized and located in a pouch around the neck of the animal. All vascular catheters were filled with heparinized solution (500 IU heparin ml−1 0.9% NaCl) and plugged with a copper pin. Ampicillin (10 mg kg−1 i.m.; Ampicilina, Laboratorio Best‐Pharma, Santiago, Chile) and gentamycin (4 mg kg−1 i.m.; Gentamicina Sulfato, Laboratorio Biosano) were administered every 12 h while the animals were instrumented. Sodium metamizole (0.1 mg kg−1 i.m.) was given immediately postoperatively and for a further 3 days. The experiments commenced 3 days after surgery.
Cardiopulmonary recording during acute hypoxic challenge
LALL and HALL were subjected to experiments based on a 3 h protocol divided into three periods: 1 h of baseline, 1 h of hypoxia and 1 h of recovery. After the first hour when the animal breathed environmental air (baseline), a loosely tied transparent polyethylene bag was placed over the animal's head into which a controlled mixture of air, N2 and CO2 was passed at ca. 15 l min−1. Hypoxia (9%O2 and 2–3% CO2 in N2) was induced to reduce the to ca. 30 mmHg, without altering (Giussani et al. 1999; Herrera et al. 2008b, 2010; Parrau et al. 2013; López et al. 2016; Castillo‐Galán et al. 2016). After the hour of isocapnic hypoxia the animal was returned to breathing atmospheric air for a further 60 min (recovery). During the first experiment, the llamas received an infusion of a solution of 0.9% NaCl (control vehicle treatment). The following day, N G‐nitro‐l‐arginine methyl ester (l‐NAME, Sigma Chemical Co., St Louis, MO, USA; 20 mg kg−1 bolus plus infusion at 0.5 mg kg−1 min−1 dissolved in 0.9% NaCl) was administered to inhibit in vivo NOS activity (Rees et al. 1990; Zou et al. 1998, Sanhueza et al. 2005). Infusions were given via the inferior vena cava, starting 15 min before hypoxia, until the end of the hypoxic challenge. Arterial blood samples (0.3 ml) were taken in heparinized syringes from the animals at 15 and 45 min of baseline, at intervals of 15 min during the hypoxic hour, and at 15 and 45 min during the recovery period. Arterial pH, , (ABL 555, Blood gas Monitor, Radiometer, Copenhagen, Denmark; measurements corrected at 38.5°C), percentage saturation of haemoglobin and haemoglobin concentration were measured (OSM3 Haemoximeter, Radiometer). Systemic and pulmonary arterial pressure (PAP) were measured continuously (Statham P23 transducers BB‐db, Hato Rey, Puerto Rico) and recorded using a data acquisition system connected to a PC. Heart rate, and mean systemic (mSAP) and pulmonary (mPAP) arterial pressures were calculated from this recording.
Cardiac output was determined as before by thermodilution as the average of three measurements after injection of 5 ml 0.9% NaCl chilled to 0 °C (Baxter COM‐2 cardiac output computer, Irvine, CA, USA; Herrera et al. 2007). Pulmonary (PVR) and systemic vascular resistance (SVR) were calculated using the following equations:
Blood oxygen content (O2cont), was calculated using the following equation:
Wire myography
Non‐instrumented newborn llamas were killed via an overdose of sodium thiopentone 100 mg kg−1 i.v. (Tiopental; Laboratorio Biosano) at 9–10 days old. The right lung was isolated and immediately immersed in ice‐cold saline. Under a dissecting microscope (Nikon #102, 40×), the pulmonary vascular tree was dissected, and fifth to sixth generation vessels (counting from the pulmonary artery trunk) were carefully isolated, cleaned and cut into ∼2.0 mm segments (Herrera et al. 2007, 2008b; Parrau et al. 2013; Torres et al. 2015). The vessel segments were mounted onto a wire myography chamber (410M, Dual Wire Myograph, Danish Myo Technologies, Aarhus, Denmark), filled with Krebs‐Ringer‐Bicarbonate (KRB) solution containing (in mM) NaCl 118.5, NaHCO3 25, KCl 4.7, KH2PO4 1.2, MgSO4 1.2, CaCl2 2.5 and glucose 5.5 at a pH of 7.4, at 37°C and aerated with 95% O2/5% CO2. Each artery was stretched to its individual optimal lumen diameter, determined as the diameter at which it developed the strongest contractile response to 125 mm K+, as previously described (Villamor et al. 2002; Herrera et al. 2007). Once the optimal diameter was established, the vessels were placed under resting conditions for 30 min. Concentration–response curves to contractile stimulation by K+ (4.75–125 mm K+) were obtained by equimolar replacement of NaCl by KCl in the KRB solution. Concentration–response curves to the relaxant agent sodium nitroprusside (SNP) (10−10–10−3 m) were constructed in vessels pre‐contracted with 125 mm K+. Two minutes were allowed between each dose. Furthermore, 30 min of resting conditions was allowed between each experimental protocol. Dose–response curves were analysed in terms of sensitivity and maximal response by fitting experimental data to a Boltzmann equation and by a non‐linear dose–response equation for KCl and SNP, respectively (Prism 5.0, GraphPad Software, La Jolla, CA, USA). Responses were expressed in terms of net tension for K+ (force divided by length of the arterial segment in N m–1) or in relation to the K+‐induced maximal constriction (%KMAX). Sensitivity was calculated as pD 2, where pD 2 = −log[EC50], EC50 being the concentration at which 50% of the maximal response was obtained (Herrera et al. 2007).
cGMP determination
Lung tissue was homogenized in five volumes of 5% trichloroacetic acid followed by water‐saturated diethyl ether extraction. The resulting lung lysate was assayed for cGMP content with a commercial ELISA kit (Cyclic GMP ELISA kit, Cayman Chemical 581021, Ann Harbor, MI, USA).
Immunoblot
Lung lysates were prepared in RIPA buffer and submitted to immunoblot analysis with specific anti‐BKCa (monoclonal, Neuromab, Davis, CA, USA, clone L6/60), anti‐PKG‐1 (polyclonal, Enzo LifeSciences, Plymouth Meeting, PA, USA), anti‐PSer92‐PDE5 (polyclonal, FabGennnix, Frisco, TX, USA), anti‐ROCK2 and anti‐PDE5 (monoclonal, BD Transduction Labs), anti‐ROCK1 and anti‐MYPT1 (monoclonal, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti‐PSer695MYPT1 (Polyclonal, Santa Cruz Biotechnology), anti‐PThr696MYPT1 (polyclonal, Upstate, Lake Placid, NY, USA), anti‐MLC20 (monoclonal, Santa Cruz Biotechnology) and anti‐PSer19‐MLC20 (polyclonal, Cell Signaling, Danvers, MA, USA), anti‐α‐actin and anti‐β‐actin antibodies (monoclonal, Sigma). The intensities of the bands corresponding to the immunoblot experiments were quantified by densitometry, using Scion Image Software (Scion Image Beta 4.02 Win; Scion Image Corporation, Fredrick, MD, USA).
Histology of pulmonary arteries
The left lung was isolated and perfused with 4% paraformaldehyde. A piece was isolated and further fixed in 4% formaldehyde for 24 h at 4°C, washed in PBS and embedded in paraffin. Van Gieson staining was performed on 10 μm slices. Images of parenchymal arterioles were acquired using a binocular microscope (Olympus BX‐41) coupled to a digital camera (Qimaging GO3) and a computer equipped with ImagePro 6.3 software for determination of smooth muscle vascular area. The area of vascular smooth muscle was calculated as follows: smooth muscle cell (SMC) area (%) = external muscle area − internal area/external muscle area × 100, where the external muscle area and the internal area are the external and internal boundaries of the tunica media, respectively (Minamino et al. 2001; Herrera et al. 2008a). At least five arteries (100–300 μm) per lung were chosen and the average of five measurements from each artery was recorded.
Statistical analysis
All values are expressed as means ± SEM. For in vivo experiments, the hypoxic episode was divided into early hypoxia (EH, the first 15 min) and late hypoxia (LH, the last 45 min; see Giussani et al. 1999). Moreover, the area under the curve of the PAP response during acute hypoxia was calculated. Statistical analyses were performed using a two‐way analysis of variance (ANOVA) followed by the Newman–Keuls post hoc test. For ex vivo and in vitro experiments, differences between mean values were assessed by Student's t test for unpaired data or the Mann–Whitney test, where appropriate. For all comparisons, statistical significance was accepted at P < 0.05 (Glantz & Slinker, 2001).
Results
Acid–base status and blood gases
Control experiment
Values for pH were similar between experimental groups during all experimental periods (Table 1). In contrast, and were lower in HALL relative to LALL during basal, basal + NaCl and recovery stages. In both HALL and LALL, decreased to similar values during early and late hypoxia compared to basal and recovery periods (Table 1). We calculated the alveolar difference (data not shown) and did not find any physiological shunt across the pulmonary circulation in HALL. Values for Hb saturation with O2 also decreased in LALL and HALL during early and late hypoxia when compared to corresponding basal and recovery periods. However, O2 content decreased significantly only in LALL during superimposed hypoxia.
Table 1.
Blood gases in lowland (LALL) and highland (HALL) newborn llamas infused with 0.9% NaCl (control)
| B | B + I | EH + I | LH + I | R | ||
|---|---|---|---|---|---|---|
| pHa | LALL (n = 7) | 7.44 ± 0.01 | 7.46 ± 0.01 | 7.46 ± 0.01 | 7.45 ± 0.01 | 7.48 ± 0.02 |
| HALL (n = 6) | 7.46 ± 0.01 | 7.45 ± 0.02 | 7.45 ± 0.01 | 7.45 ± 0.01 | 7.48 ± 0.01 | |
| (mmHg) | LALL (n = 7) | 91 ± 2 | 90 ± 2 | 30 ± 2* | 34 ± 3* | 96 ± 6 |
| HALL (n = 6) | 49 ± 2† | 45 ± 3† | 34 ± 2* | 33 ± 2* | 54 ± 2† | |
| (mmHg) | LALL (n = 7) | 39 ± 2 | 38 ± 2 | 36 ± 2 | 39 ± 2 | 35 ± 2 |
| HALL (n = 6) | 31 ± 2† | 30 ± 4† | 29 ± 3† | 30 ± 2† | 27 ± 2† | |
| Sat Hb (%) | LALL (n = 7) | 97 ± 2 | 96 ± 2 | 68 ± 3* | 69 ± 5* | 95 ± 3 |
| HALL (n = 6) | 92 ± 11 | 88 ± 6 | 78 ± 4* | 75 ± 4* | 94 ± 1 | |
| [Hb] (g dl–1) | LALL (n = 7) | 9.8 ± 0.4 | 10.0 ± 0.4 | 11.6 ± 0.7* | 11.4 ± 0.7* | 9.9 ± 0.4 |
| HALL (n = 6) | 8.6 ± 0.9 | 9.4 ± 2.0 | 9.1 ± 1.5† | 9.1 ± 1.3† | 8.4 ± 0.7 | |
| O2 cont (ml dl–1) | LALL (n = 7) | 12.0 ± 0.8 | 12.8 ± 0.4 | 10.4 ± 0.6* | 10.4 ± 0.6* | 12.6 ± 0.5 |
| HALL (n = 6) | 9.1 ± 1.8† | 11.2 ± 1.4† | 9.6 ± 1.1† | 9.4 ± 1.0† | 10.6 ± 0.9† |
Arterial blood gases and acid–base status in control lowland (LALL) and highland (HALL) newborn llamas. Values are means + SEM. B, basal; B + I, basal + 0.9%NaCl infusion; EH + I, early hypoxia+0.9%NaCl infusion; LH + I, late hypoxia+0.9% NaCl infusion; R, recovery. Significant differences (P < 0.05, ANOVA + Student–Newman–Keuls test): * vs. basal; †LALL vs. HALL. pHa, arterial pH; , arterial O2 partial pressure; , arterial CO2 partial pressure; % sat Hb, percentage saturation of haemoglobin with oxygen; [Hb], haemoglobin concentration; O2 cont, oxygen content.
l‐NAME experiment
During l‐NAME administration, changes in the values for blood gases and pH were equivalent to those measured in the control protocol for both HALL and LALL groups (Table 2).
Table 2.
Blood gases in lowland (LALL) and highland (HALL) newborn llamas infused with l‐NAME
| B | B + I | EH + I | LH + I | R | ||
|---|---|---|---|---|---|---|
| pHa | LALL (n = 6) | 7.45 ± 0.01 | 7.44 ± 0.01 | 7.44 ± 0.01 | 7.43 ± 0.01 | 7.47 ± 0.01 |
| HALL (n = 6) | 7.45 ± 0.02 | 7.43 ± 0.02 | 7.41 ± 0.01 | 7.42 ± 0.01 | 7.45 ± 0.01 | |
| (mmHg) | LALL (n = 6) | 94 ± 3 | 88 ± 3 | 29 ± 2* | 31 ± 1* | 94 ± 5 |
| HALL (n = 6) | 52 ± 4† | 46 ± 5† | 31 ± 1* | 33 ± 2 * | 54 ± 3† | |
| (mmHg) | LALL (n = 6) | 37 ± 1 | 36 ± 2 | 37 ± 2 | 37 ± 2 | 34 ± 2 |
| HALL (n = 6) | 32 ± 2† | 31 ± 4† | 34 ± 2† | 32 ± 2† | 29 ± 2† | |
| Sat Hb (%) | LALL (n = 6) | 97 ± 1 | 96 ± 2 | 65 ± 4* | 68 ± 3* | 94 ± 4 |
| HALL (n = 6) | 92 ± 2 | 86 ± 6 | 71 ± 3* | 72 ± 3* | 92 ± 1 | |
| [Hb] (g dl–1) | LALL (n = 6) | 9.9 ± 0.4 | 10.3 ± 0.4 | 11.8 ± 0.6* | 11.5 ± 0.6* | 10.3 ± 0.5 |
| HALL (n = 6) | 8.7 ± 0.7 | 8.8 ± 1.6 | 9.3 ± 1.2† | 8.9 ± 1.2† | 8.3 ± 0.4 | |
| O2 cont (ml dl–1) | LALL (n = 6) | 12.8 ± 0.5 | 13.1 ± 0.5 | 10.3 ± 0.6 | 10.6 ± 0.5 | 12.9 ± 0.5 |
| HALL (n = 6) | 10.7 ± 0.9† | 10.2 ± 1.3† | 9.0 ± 1.0† | 8.8 ± 1.0† | 10.3 ± 0.6† |
Arterial blood gases and acid‐base status in l‐NAME‐treated lowland (LALL) and highland (HALL) newborn llamas. Values are means + SEM. B, basal; B + I, basal + l‐NAME infusion; EH + I, early hypoxia+ l‐NAME infusion; LH + I, late hypoxia+ l‐NAME infusion; R, recovery. Significant differences (P < 0.05, ANOVA + Student–Newman–Keuls test): * vs. basal; †LALL vs. HALL. pHa, arterial pH; , arterial O2 partial pressure; , arterial CO2 partial pressure; Sat Hb, percentage saturation of haemoglobin with oxygen; Hb, haemoglobin concentration; O2 cont, oxygen content.
In vivo pulmonary circulation responses
Control experiment
LALL and HALL had similar values for mPAP during basal conditions (Fig. 1 A). Both LALL and HALL responded to acute hypoxia with a biphasic elevation in mPAP, showing a greater increase during the first 15 min, followed by less marked but still significant pulmonary hypertension during the remaining 45 min of acute hypoxia (Fig. 1 A). Importantly, during early hypoxia, HALL showed a diminished rise in mPAP relative to LALL (Fig. 1 A). The area under the curve during the acute hypoxic episode was also significantly smaller in HALL compared with LALL (Fig. 1 A, inset). During the recovery period, mPAP returned to basal values in both groups of animals (Fig. 1 A). Cardiac output remained unchanged from baseline during all experimental periods in both LALL and HALL groups (Fig. 2 A). Therefore, values for PVR showed a similar response pattern to values for mPAP in both LALL and HALL animals. However, values for PVR did not differ significantly between LALL and HALL groups during acute hypoxia (Fig. 2 B).
Figure 1. Mean pulmonary arterial pressure (mPAP).
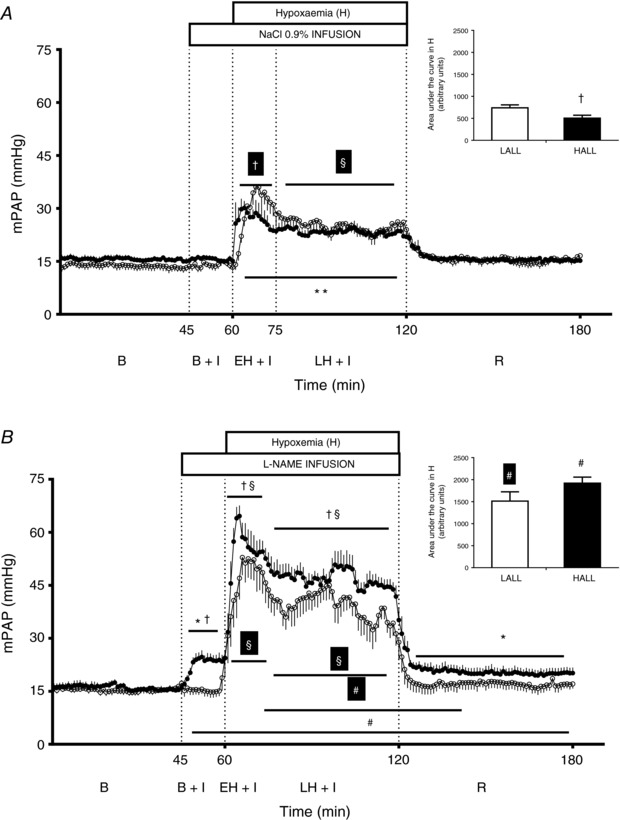
Groups are low‐altitude (LALL) and high‐altitude (HALL) newborn llamas infused with NaCl 0.9% (Control) or l‐NAME. Continuous recording of mPAP and area under the curve during acute early and late hypoxia (inset) in (A) NaCl‐infused and (B) l‐NAME‐infused LALL (open symbols) and HALL (filled symbols). B, basal; B + I, basal + 0.9% NaCl infusion; EH + I, early hypoxia + 0.9% NaCl infusion; LH + I, late hypoxia + 0.9% NaCl infusion; R, recovery. Significant differences (P < 0.05, ANOVA + Student–Newman–Keuls test): * vs. basal; ** vs. B, B + I, R; § vs. all in the same group; †LALL vs. HALL; #NaCl vs. l‐NAME. Repeated symbols either in black or white refer to within‐group comparisons for HALL and LALL, respectively.
Figure 2. Cardiopulmonary variables.
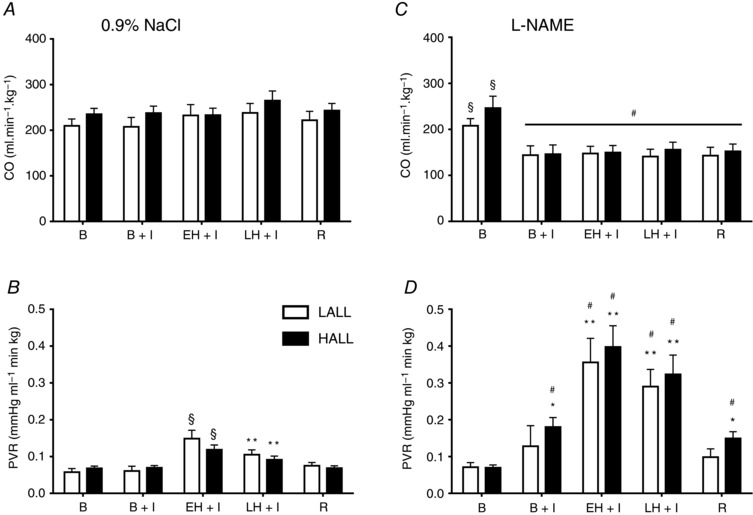
Groups are low‐altitude (LALL) and high‐altitude (HALL) newborn llamas infused with NaCl 0.9% (Control) or l‐NAME, basally and during a superimposed episode of acute hypoxia. Cardiac output (CO) in LALL (open symbols) and HALL (filled symbols) infused with (A) NaCl and (C) l‐NAME and pulmonary vascular resistance (PVR) in LALL and HALL infused with (B) NaCl and (D) l‐NAME. Values are means + SEM. B, basal; B + I, basal + l‐NAME infusion; EH + I, early hypoxia + l‐NAME infusion; LH + I, late hypoxia + l‐NAME infusion; R, recovery. Significant differences P < 0.05 (ANOVA + Student–Newman–Keuls test): * vs. basal; ** vs. B, B+I, R; § vs. all in the same group; †LALL vs. HALL; #NaCl vs. l‐NAME.
l‐NAME experiment
Infusion with l‐NAME during the basal period increased mPAP in HALL but not in LALL (Fig. 1 B). During acute hypoxia, treatment with l‐NAME led to a greater increase in mPAP in HALL compared with LALL, and in both groups the increase in mPAP was greater than the increase measured during saline infusion in the control experiment (Fig. 1 A and B). During l‐NAME infusion, there was a similar fall in CO in both LALL and HALL groups (Fig. 2 B), which remained significantly lower throughout all experimental periods compared to saline infusion during the control experiment (Fig. 2 C). In LALL animals, compared to saline infusion in the control experiment, values for PVR were significantly greater during early and late hypoxia with l‐NAME treatment. In contrast, in HALL animals, compared to saline infusion in the control experiment, values for PVR were significantly greater during baseline, hypoxia and recovery periods with l‐NAME treatment (Fig. 2 D).
In vivo systemic cardiovascular responses
Control experiment
Basal HR was not different and increased similarly during early and late hypoxia in both LALL and HALL animals during saline infusion (Fig. 3 A). In contrast, values for mSAP and SVR remained unchanged from baseline during acute hypoxia in LALL and HALL animals during saline infusion (Fig. 3 B and C).
Figure 3. Cardiovascular variables.
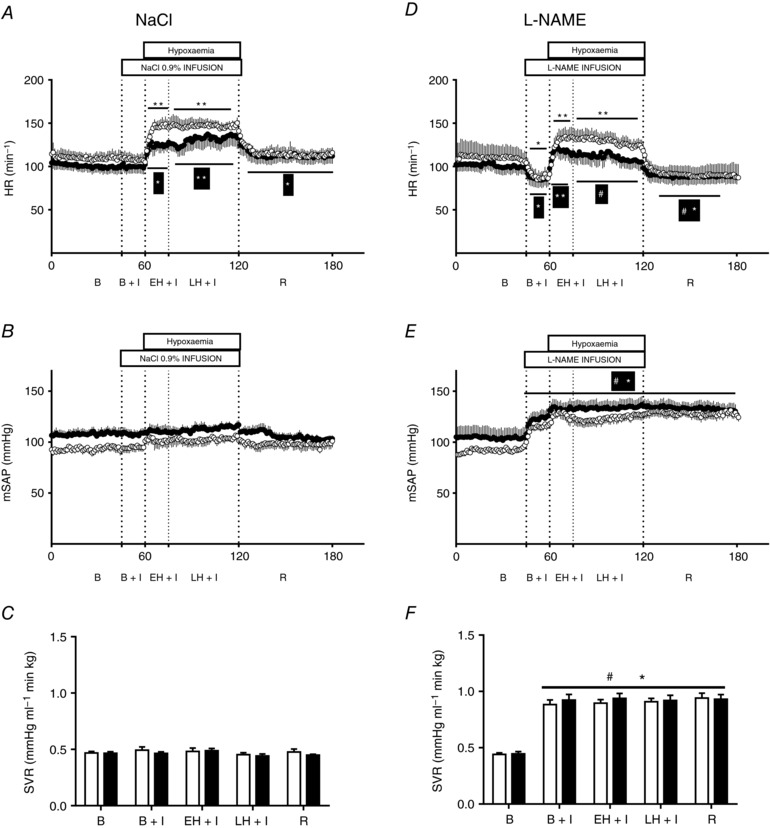
Groups are low‐altitude (LALL) and high‐altitude (HALL) newborn llamas infused with NaCl 0.9% (Control) or l‐NAME. Heart rate (HR) in LALL (open symbols) and HALL (filled symbols) infused with (A) NaCl or (D) l‐NAME; mean systemic arterial pressure (mSAP) in LALL and HALL infused with (B) NaCl or (E) l‐NAME; and systemic vascular resistance (SVR) in LALL and HALL infused with (C) NaCl or (F) l‐NAME. Values are means + SEM. B, basal; B + I, basal + l‐NAME infusion; EH + I, early hypoxia + l‐NAME infusion; LH + I, late hypoxia + l‐NAME infusion; R, recovery. Significant differences (P < 0.05, ANOVA + Student–Newman–Keuls test): * vs. basal; ** vs. B, B+I, R; § vs. all in the same group; †LALL vs. HALL; #NaCl vs. l‐NAME.
l‐NAME experiment
Infusion with l‐NAME reduced HR under basal conditions, but it did not prevent its increase during early or late hypoxia in both LALL and HALL animals (Fig. 3 D). Infusion with l‐NAME also increased values for mSAP and SVR to similar extents during all experimental treatments in both LALL and HALL animals, compared to saline infusion during the control experiment (Fig. 3 E and F).
Vascular reactivity of pulmonary resistance arteries
The concentration–response curve to K+ based on isolated segments of pulmonary arteries showed that HALL had a significantly greater maximal contractile response (KMAX), but similar sensitivity (EC50) compared with LALL (Fig. 4 A and B). By contrast, the concentration–response curve to SNP showed that isolated pulmonary arteries from HALL compared with LALL had a significantly greater maximal relaxant response and greater sensitivity (pD 2) to the NO donor (Fig. 5 A and B).
Figure 4. Contractile capacity in small pulmonary arteries from lowland (LALL) and highland (HALL) newborn llamas.
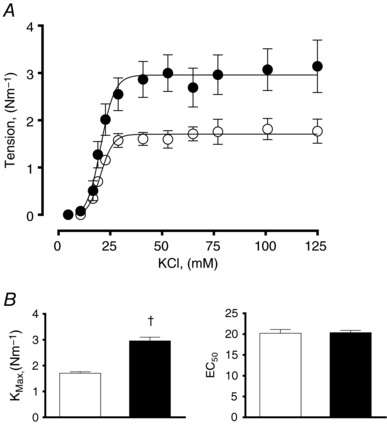
A, concentration–contraction curve to potassium; B, maximal contraction (K max) and sensitivity (EC50) to potassium. Open symbols, LALL; closed symbols, HALL. Values are means ± SEM. Significant differences (P < 0.05, unpaired t test): †LALL vs. HALL.
Figure 5. NO‐dependent relaxation in small pulmonary arteries from lowland (LALL) and highland (HALL) newborn llamas.
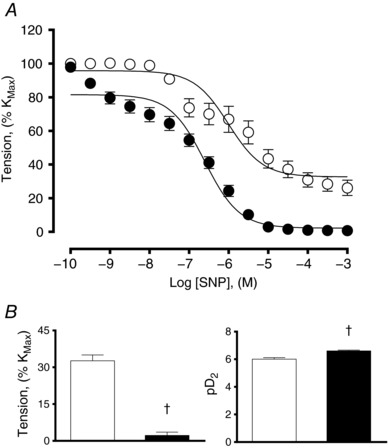
A, concentration–response curve to sodium nitroprusside (SNP); B, minimal tension and sensitivity (pD 2) to SNP. Open symbols, LALL; closed symbols, HALL. Values are means ± SEM. Significant differences (P < 0.05, unpaired t test): †LALL vs. HALL.
Pulmonary cGMP content
The pulmonary cGMP content was similar for LALL (13.3 ± 1.9 pmol g−1 wet tissue) and HALL (13.9 ± 1.7 pmol g−1 wet tissue) (Fig. 6).
Figure 6. Pulmonary content of cGMP in lowland (LALL) and highland (HALL) newborn llamas.
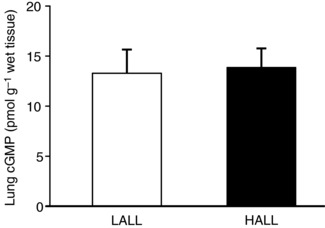
Open bars, LALL; closed bars, HALL. Values are means ± SEM.
Pulmonary expression of proteins downstream of NO signalling
The pulmonary expression of phosphodiesterase‐5 (PDE5/β‐actin = 0.26 ± 0.09 for LALL vs. 0.25 ± 0.04 for HALL), its phosphoserine more active form PSer92‐PDE5 (PSer92‐PDE5/β‐actin = 0.59 ± 0.21 for LALL vs. 0.42 ± 0.05 for HALL), protein kinase G (PKG‐1/β‐actin = 0.85 ± 0.09 for LALL vs. 0.79 ± 0.07 for HALL), BKCa channels (BKCa/β‐actin = 0.45 ± 0.12 for LALL vs. 0.61 ± 0.11 for HALL) and Rho‐associated kinase1 ROCK1 (ROCK1/β‐actin = 0.67 ± 0.14 for LALL vs.0.67 ± 0.04 for HALL) did not differ between LALL and HALL groups (Fig. 7). In contrast, the expression of Rho‐associated kinase 2 was down‐regulated in HALL (ROCK2/β‐actin = 1.65 ± 0.48 for LALL vs.0.94 ± 0.05 for HALL, P < 0.05) (Fig. 7).
Figure 7. Pulmonary expression of BKCa, PDE5, PSer92‐PDE5, PKG‐1, ROCK1 and ROCK2 proteins in lowland (LALL) and highland (HALL) newborn llamas.
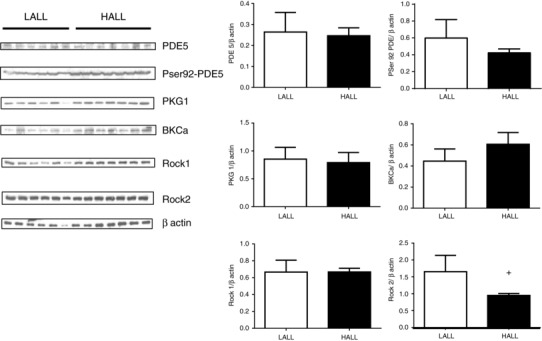
Open bars, LALL; closed bars, HALL. Values are means ± SEM. Significant differences (P < 0.05, Mann–Whitney test): †LALL vs. HALL.
Pulmonary expression and phosphorylation of the regulatory subunit of the myosin light chain phosphatase and myosin light chain
The pulmonary expression of total regulatory subunit of the myosin light chain phosphatase, also known as myosin phosphatase target 1 (MYPT1), was increased in highland compared to lowland newborn llamas (MYPT1/β‐actin = 0.98 ± 0.22 for LALL vs. 1.63 ± 0.11 for HALL, P < 0.05). In contrast, neither the expression of its phosphoserine and more active form (PSer695‐MYPT1/β‐actin = 0.79 ± 0.22 for LALL vs. 0.65 ± 0.07 for HALL) nor the expression of its phosphothreonine and less active form (PThr696‐MYPT1/β‐actin: 1.291 ± 0.48 for LALL vs. 2.04 ± 0.25 for HALL) changed between the two groups (Fig. 8). The expression of total myosin light chain (MLC20) increased in highland compared to lowland newborn llamas (MLC20/β‐actin: 0.68 ± 0.16 for LALL vs. 1.17 ± 0.11 for HALL, P < 0.05), but its phosphoserine and more contractile form (PSer19‐MLC20/β‐actin) was down‐regulated in highland compared to lowland newborn llamas (1.56 ± 0.32 for LALL vs. 0.89 ± 0.13 for HALL, P < 0.05) (Fig. 8).
Figure 8. Pulmonary expression of total MYPT1, PSer695‐MYPT1, PThr696‐MYPT1, MLC20 and PSer19‐MLC20 proteins in lowland (LALL) and highland (HALL) newborn llamas.
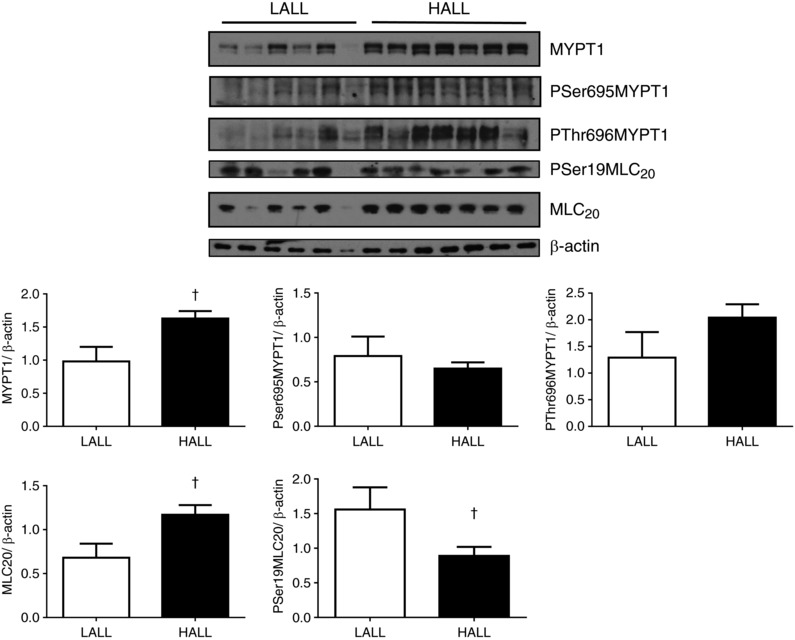
Open bars, LALL; closed bars, HALL. Values are means ± SEM. Significant differences (P < 0.05, Mann–Whitney test): †LALL vs. HALL.
Pulmonary α‐actin expression and morphometric analysis of pulmonary arteries
The pulmonary artery expression of α‐actin was similar between LALL and HALL groups (α‐actin/β‐actin = 1.37 ± 0.33 for LALL vs. 1.34 ± 0.14 for HALL) (Fig. 9 A). However, the medial layer area of small pulmonary arteries isolated from HALL was significantly greater compared to small pulmonary arteries isolated from LALL animals (medial layer area = 27.00 ± 1.62 for LALL vs. 44.70 ± 0.93 for HALL, P < 0.05) (Fig. 9 B).
Figure 9. Pulmonary α‐actin expression and medial layer area of pulmonary arteries in lowland (LALL) and highland (HALL) newborn llamas.
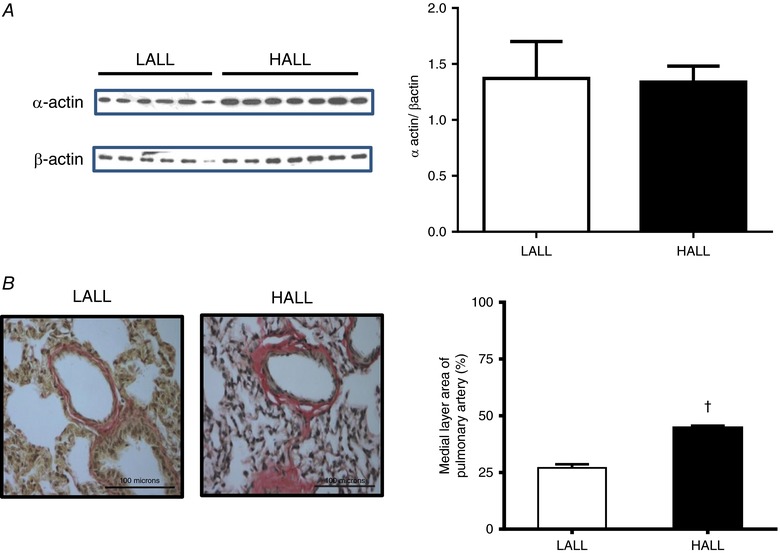
A, immunoblot determination of α‐actin and β‐actin; B, van Gieson staining and medial layer area of pulmonary arteries; open bars, LALL; closed bars, HALL. Values are means ± SEM. Significant differences (P < 0.05, unpaired t test): †LALL vs. HALL. [Color figure can be viewed at http://wileyonlinelibrary.com]
Discussion
In this study, we determined the role of NO in the cardiopulmonary responses to an episode of acute hypoxia in high‐ and lowland neonatal llamas. Adopting an integrative approach, combining functional studies in vivo and in isolated vessels with cellular and molecular techniques, we tested the hypothesis that the pulmonary arterial hypertensive response to acute hypoxia in highland relative to lowland newborn llamas is diminished by enhanced NO‐dependent mechanisms. The data support the hypothesis tested and show that highland compared with lowland newborn llamas have a significantly lower pulmonary arterial pressor response to acute hypoxia, despite similar cardiac output and systemic arterial blood pressure during the challenge. The mechanism of the pulmonary vascular protection to superimposed acute hypoxia in highland animals is mediated in part via enhanced NO, as in vivo treatment with l‐NAME led to a greater increase in mean arterial pulmonary pressure and the ex vivo relaxant response to SNP in isolated pulmonary arterial segments was greater in highland compared to lowland newborn llamas. However, the pulmonary expression of proteins involved in dilatator mechanisms downstream from NO, including the large‐conductance calcium‐dependent potassium channels (BKCa), phosphodiesterase 5 (PDE5) and its phosphoserine activated fraction (PSer92‐PDE5), as well as cGMP‐dependent protein kinase (PKG‐1) were not different between the groups. Consequently, the pulmonary cGMP content of lowland and highland llamas and the expression of the phosphoserine‐ and phosphothreonine‐myosin phosphatase target 1 subunit (PSer695MYPT1 and PThr696MYPT1) were also similar. In contrast, the expression of total myosin phosphatase target 1 (MYPT1) was increased, whilst Rho‐associated kinase 2 (ROCK2) and phospho‐serine 19 myosin light chain (PSer19‐MLC20) were decreased in isolated lung tissue. Combined, these data suggest that blunted pulmonary tissue Rho‐kinase and Ca2+ desensitization may encompass adaptive mechanisms allowing NO‐dependent vasodilatator actions in the highland llama. NO‐dependent mechanisms mediating protective pulmonary vasoactive responses were insufficient to prevent pulmonary arterial remodelling, as evidenced by a greater pulmonary medial layer area and a greater pulmonary arterial constrictor response to potassium in highland compared to lowland newborn llamas.
Previous studies have suggested a role for NO‐dependent mechanisms in conferring protection against basal pulmonary arterial hypertension in humans and animals adapted to chronic hypoxia. Beall et al. (2001) reported that exhalation of NO by chronically hypoxic populations of adult Tibetan residents living at 4200 m and of Bolivian Aymara residents living at 3900 m is unexpectedly increased compared with a reference sample of low‐altitude dwellers. Therefore, increasing concentrations of pulmonary NO in two geographically distant high‐altitude populations is in keeping with its adaptive role in offsetting adverse responses to chronic hypoxia. The same group of investigators measured higher exhaled NO associated with higher basal pulmonary blood flow in young adult Tibetan residents relative to lowland controls (Hoit et al. 2005). They suggested that NO in the lung may play a key beneficial role in allowing human highland populations to compensate for environmental chronic hypoxia with greater basal pulmonary blood flow and oxygen delivery without the need and, thereby, the adverse consequences of higher basal PAP (Hoit et al. 2005). Similarly, in adult yaks (Ishizaki et al. 2005) and in highland newborn llamas and sheep (Herrera et al. 2008a), NO blockade elevated basal PAP. Furthermore, a recent study of Tibetan babies reported an association between being born at high altitude and increased exhaled NO during the first week of life (Wu et al. 2016). In the present study, despite highland relative to lowland newborn llamas being chronically hypoxic with lower arterial values, highland neonates not only showed normal values for pulmonary arterial blood pressure during basal conditions, but also a significantly blunted pulmonary hypertensive response to superimposed acute hypoxia; these effects were reversed following in vivo blockade of NO with l‐NAME. In addition, isolated pulmonary vessels of highland newborn llamas had a markedly greater dilatator response to the NO donor SNP. Therefore, combined in vivo and in vitro data in the present study are consistent with up‐regulation of pulmonary NO in protecting against pulmonary hypertensive responses to superimposed acute hypoxia in newborns from species adapted to the chronic hypoxia of life at high altitude, such as the llama.
Additional molecular data in the present study show that the enhanced action of NO in the pulmonary vasculature of high‐ relative to lowland llama newborns cannot be attributed to a greater pulmonary protein expression of BKCa or PDE5. We also measured the expression of phosphorylated PDE5 at serine92 because phosphorylation at serine 92 activates PDE5 (Rybalkin et al. 2002). We observed a similar expression of PSer92‐PDE5 and a similar content of cGMP in lungs from high‐ and lowland llamas. By contrast, the expression of PKG‐1 was also the same in both groups. We did not measure PKG enzyme activity, nor did we evaluate the vasodilatation of llama pulmonary arteries in response to PKG activation, but taken together, our data suggest that an enhanced nitrergic tone in HALL may rely on a mechanism downstream of PKG‐1. In species that develop pulmonary hypertension, exposure to chronic hypoxia blunts the vasodilatator response of pulmonary arteries in response to PKG. For instance, despite the increase in PKG activity and protein content observed in pulmonary vessels from adult rats submitted to chronic hypoxia, the vasodilatation in response to activation of PKG‐1 with 8Br‐cGMP was decreased (Jernigan et al. 2003). By contrast, the expression of PKG‐1 and its target MYPT1 is decreased in fetal ovine pulmonary artery myocytes exposed to low oxygenation in culture, reducing the interaction between PKG‐1 and MYPT1, and favouring the interaction with ROCK1/2 and MYPT1. These findings suggest that Ca2+ sensitization may be a mechanism underlying the pulmonary artery constrictor hyper‐reactivity when these species are exposed to hypoxia (Singh et al. 2011). In the present work, we observed that the amount of MYPT1 phosphorylated either in serine 695, accounting for a more active phosphatase, or in threonine 696, accounting for a less active phosphatase (Jernigan & Resta, 2014), did not change under chronic hypoxia. However, the expression of total MYPT1 was increased, whilst the expression of ROCK2 was decreased in HALL. Moreover, despite the expression of MLC20 being greater in HALL than in LALL, the expression of PSer19‐MLC20 accounting for a more contractile state was lower in HALL than in LALL. The parallel increase in MYPT1 may be a mechanism to counteract the increase in MLC20 expression under chronic hypoxia. Taken together, these data suggest that blunted Rho‐kinase, higher phosphatase and Ca2+ desensitization may include mechanisms contributing to protect the newborn llama from excessive increases in pulmonary artery pressure and pulmonary vascular resistance during chronic hypoxia. Blunted pulmonary Rho‐kinase function has also been measured in other species tolerant to hypoxia such as the yak, and this has been proposed as a mechanism to circumvent excessive hypoxic pulmonary vasoconstriction (Ishizaki et al. 2015). Alternative pathways mediating dilatation downstream of NO include activation of Kir2.1 or Trek‐1 potassium channels (Schubert et al. 2004; Dallas et al. 2008).
It is also possible that the enhanced contribution of NO measured in the pulmonary vasculature of the high‐ relative to lowland newborn llama in the present study may be due to a decrease in NO metabolism rather than enhanced NO production and/or NO mechanisms of action. Several groups have now reported that developmental hypoxia increases oxidative stress (reviewed by Giussani & Davidge, 2013; Giussani et al. 2012, 2014; Herrera et al. 2014) and that increased generation of reactive oxygen species during hypoxia may decrease the bioavailability of NO, promoting an enhanced vasoconstrictor oxidant tone (Thakor et al. 2010a, b; Herrera et al. 2012; Kane et al. 2012, 2014). Conversely, there is evidence that humans and animals adapted to chronic hypoxia have increased expression of antioxidant defences. For instance, it is reported that highland pregnancy increased catalase and SOD activity to a greater extent in Andean relative to European women (Julian et al. 2012). The mole rat, a species adapted to the chronic hypoxia of life underground, has an increased tissue expression of catalase, superoxide dismutase, glutathione peroxidase and heme‐oxigenase 1, markedly enhancing its endogenous antioxidant capacity (Schülke et al. 2012; Lewis et al. 2013). Enhanced antioxidant defences will quench the superoxide anion, relieving the sequestration of NO and thereby increasing its bioavailability. Certainly, there is evidence that antioxidant treatment can shift the vascular oxidant tone ratio towards vasodilatation in the placental and fetal circulation during normoxia or hypoxia (Thakor et al. 2010a,b; Kane et al. 2012, 2014; Herrera et al. 2012, 2014; Giussani et al. 2012; Richter et al. 2012). Similarly, antioxidant treatment with melatonin, or supplementation with tetrahydrobiopterin, reduces mean pulmonary arterial blood pressure and increases pulmonary NO bioavailability in newborn sheep and adult rats, respectively (Francis et al. 2014; Torres et al. 2015).
After inhibition of NOS we found a substantial increase in mPAP during hypoxia, in both LALL and HALL. In addition, NOS blockade induced an ∼8 mmHg rise in mPAP under basal conditions in HALL but not in LALL. Together, these results not only indicate an important role for NO in modulating pulmonary perfusion pressure either during acute or chronic hypoxia in both the high‐ or low‐altitude llama neonate, but they also suggest an essential nitrergic contribution to the basal pulmonary artery tone in the high‐altitude llama neonate. eNOS activity and expression, as well as soluble guanylate cyclase (sGC) expression are similar in LALL and HALL. In contrast, haemoxygenase – carbon monoxide (HO‐CO) signalling is up‐regulated in HALL, suggesting that the HO‐CO system protects HALL from hypoxic pulmonary hypertension (Herrera et al. 2008a). The decreased ROCK2 expression, together with the increased MYPT1 expression observed in the present work, suggest blunted Rho‐kinase function and decreased Ca2+ sensitization, changes that may represent additional adaptive features of high‐altitude adaptation in the llama.
In the present study, the myography data derived from isolated small pulmonary arteries show a markedly higher maximal contraction to K+ in high‐ relative to lowland newborn llamas. This is consistent with increased contractile capacity and with greater muscularization of pulmonary arteries from highland newborn llamas. This is also in keeping with the morphometry data reported in the present study, which showed a thickening of the pulmonary artery medial wall in high‐ relative to lowland newborn llamas, matching the increased expression of total pulmonary MLC20. However, these data differ from changes reported in a previous study in high‐altitude adult South American camelids that showed low PAP together with thinning of pulmonary arterial walls (Harris et al. 1982). These reported differences are probably due to an effect of age in the llamas studied (adult vs. newborn) and in the heterogeneity of the South American species investigated (only llama vs. a mix of llama, guanaco and alpaca). In chronically hypoxic pulmonary hypertensive rats, prolonged inhalation of NO reduces the pathological thickening of the pulmonary artery walls (Kouyoumdjian et al. 1994). Therefore, the enhanced pulmonary NO in highland newborn llamas may still convey protection against pulmonary artery remodelling by adulthood in these animals, as in the study reported by Harris et al. (1982). Conversely, we cannot rule out a hypoxic up‐regulation of voltage‐dependent Ca2+ channels to explain the greater K+ tension in pulmonary arteries of HALL relative to LALL, as already described for chronically hypoxic mice (Wan et al. 2013).
A final point of interest reported in the present study relates to data on haemoglobin concentration in arterial blood. High‐ relative to lowland newborn llamas had lower basal values but similar or even slightly lower haemoglobin concentration in arterial blood. A lower haemoglobin concentration reduces blood viscosity and peripheral vascular resistance (Gilbert‐Kawai et al. 2014). Therefore, the data may represent an adaptive blunted haematopoietic response to the chronic hypoxia of life at high altitude in highland newborn llamas. A blunted haematopoietic response in the highland newborn llama could be related to mutations in the EPAS1 gene that codes for HIF‐2α protein, as described in high‐altitude Tibetans (Beall et al. 2010). Camelids are known to compensate for lower haemoglobin concentrations and reduced oxygen‐carrying capacity by other means, including higher affinity for oxygen in their haemoglobin, smaller elliptical red blood cells that increase the exchange surface area and highly efficient extraction of oxygen in tissue (Moraga et al. 1996, Benavides et al. 1989; Ostojic et al. 2002). Another adaptation in the highland newborn llama may be lower values for , without the development of alkalosis. The latter suggests greater capacity to eliminate blood bicarbonate through urinary excretion in high‐ relative to lowland newborn llamas. Accordingly, chronic hypoxia in rodents reduces the abundance of bicarbonate transporters, such as NBCe1, NBCn1, NDCBE and NBCn2 (Parker & Boron, 2013). It is possible that the ability to preserve, or even to increase the expression of these transporters may be an adaptive strategy of the newborn llama to withstand hypoxia whilst avoiding blood bicarbonate accumulation relative to .
In summary, we show that high‐ relative to lowland newborn llamas have a blunted pulmonary hypertensive response to superimposed acute hypoxia and that the mechanisms involved include enhanced NO function in the pulmonary vasculature without any changes in BKCa, PDE5 and PKG‐1. Blunting of the pulmonary pressor response to acute hypoxia may represent an adaptive response to life at high altitude in the newborn high‐ relative to lowland llama. However, this adaptation is insufficient to prevent pulmonary artery remodelling at least in the neonatal period.
Additional information
In memoriam
This paper is dedicated to our dear friend and colleague Professor Julian T. (Bill) Parer who passed away on 3 August 2016.
Acknowledgements
We are grateful for the technical assistance of Carlos Brito, Gabino Llusco and Mario Morales. This work was supported by the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) grant nos. 1010636, 1050479, 1080663, 1120605, 1130424, 1140647 and 1151119, and Vicerrectoría de Investigación y Desarrollo, Universidad de Chile (VID‐Enlace, ENL023f16). Dino Giussani is Professor of Cardiovascular Developmental Physiology & Medicine at the Department of Physiology Development & Neuroscience at the University of Cambridge, Professorial Fellow and Director of Studies in Medicine at Gonville & Caius College, a Lister Institute Fellow and a Royal Society Wolfson Research Merit Award Holder.
Biographies
Roberto V. Reyes is Associate Professor at Pathophysiology Program, ICBM, Faculty of Medicine, University of Chile.

Marcela Díaz is Associate Professor at Department for the Women and Newborn Health Promotion, Faculty of Medicine, University of Chile.
Germán Ebensperger is Assistant Professor at Pathophysiology Program, ICBM, Faculty of Medicine, University of Chile.
Emilio A. Herrera is Associate Professor at Pathophysiology Program, ICBM, Faculty of Medicine, University of Chile.
Sebastián A. Quezada is Biotechnology Engineer, MSc, Graduate student at Pathophysiology Program, ICBM, Faculty of Medicine, University of Chile.
Ismael Hernandez is at Pathophysiology Program, ICBM, Faculty of Medicine, University of Chile.
Emilia M. Sanhueza is Associate Professor at Pathophysiology Program, ICBM, Faculty of Medicine, University of Chile.
Julian T. Parer was Full Professor at Department of Obstetrics, Gynecology and Reproductive Sciences, University of California San Francisco (died August 2016).
Dino A. Giussani is Full Professor at Department of Physiology, Development and Neuroscience, University of Cambridge, UK.
Aníbal J. Llanos is Full Professor at Pathophysiology Program, ICBM, Faculty of Medicine, University of Chile.
Edited by: Harold Schultz & Frank Powell
Contributor Information
Roberto V. Reyes, Email: vicreyes@med.uchile.cl, Email: virreyc@gmail.com.
Aníbal J. Llanos, Email: allanos@med.uchile.cl
References
- Abman SH (1999). Abnormal vasoreactivity in the pathophysiology of persistent pulmonary hypertension of the newborn. Pediatr Rev 20, e103–e109. [PubMed] [Google Scholar]
- Abman SH (2007). Recent advances in the pathogenesis and treatment of persistent pulmonary hypertension of the newborn. Neonatology 91, 283–290. [DOI] [PubMed] [Google Scholar]
- Beall CM, Laskowski D, Strohl KP, Soria R, Villena M, Vargas E, Alarcon AM, Gonzales C & Erzurum SC (2001). Pulmonary nitric oxide in mountain dwellers. Nature 414, 411–412. [DOI] [PubMed] [Google Scholar]
- Beall CM, Cavalleri GL, Deng L, Elston RC, Gao Y, Knight J, Li C, Li JC, Liang Y, McCormack M, Montgomery HE, Pan H, Robbins PA, Shianna KV, Tam SC, Tsering N, Veeramah KR, Wang W, Wangdui P, Weale ME, Xu Y, Xu Z, Yang L, Zaman MJ, Zeng C, Zhang L, Zhang X, Zhaxi P & Zheng YT (2010). Natural selection on EPAS1 (HIF2α) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci USA 107, 11459–11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides C, Pérez R, Espinoza M, Cabello G, Riquelme R, Parer JT & Llanos AJ (1989). Cardiorespiratory functions in the fetal llama. Respir Physiol 75, 327–334. [DOI] [PubMed] [Google Scholar]
- Castillo‐Galán S, Quezada S, Moraga F, Ebensperger G, Herrera EA, Beñaldo F, Hernandez I, Ebensperger R, Ramirez S, Llanos AJ & Reyes RV (2016). 2‐Aminoethyldiphenylborinate modifies the pulmonary circulation in pulmonary hypertensive newborn lambs with partial gestation at high altitude. Am J Physiol Lung Cell Mol Physiol 311, L788–L799. [DOI] [PubMed] [Google Scholar]
- Dallas ML, Scragg JL & Peers C (2008). Modulation of hTREK‐1 by carbon monoxide. Neuroreport 19, 354–358. [DOI] [PubMed] [Google Scholar]
- Ebensperger G, Ebensperger R, Herrera EA, Riquelme RA, Sanhueza E, Lesage F, Marengo JJ, Tejo RI, Llanos AJ & Reyes RV (2005). Fetal brain hypometabolism during prolonged hypoxaemia in the llama. J Physiol 567, 963–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis BN, Hale A, Channon KM, Wilkins MR & Zhao L (2014). Effects of tetrahydrobiopterin oral treatment in hypoxia‐induced pulmonary hypertension in rat. Pulm Circ 4, 462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gai XY, Wei YH, Zhang W, Wuren TN, Wang YP, Li ZQ, Liu S, Ma L, Lu DX, Zhou Y & Ge RL (2015). Echinacoside induces rat pulmonary artery vasorelaxation by opening the NO‐cGMP‐PKG‐BKCa channels and reducing intracellular Ca2+ levels. Acta Pharmacol Sin 36, 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y & Raj JU (2010). Regulation of the pulmonary circulation in the fetus and newborn. Physiol Rev 90, 1291–1335. [DOI] [PubMed] [Google Scholar]
- Gao Y & Raj JU (2011). Hypoxic pulmonary hypertension of the newborn. Compr Physiol 1, 61–79. [DOI] [PubMed] [Google Scholar]
- Gilbert‐Kawai ET, Milledge JS, Grocott MPW & Martin DS (2014). King of the mountains: Tibetan and sherpa physiological adaptations for life at high altitude. Physiology 29, 388–402. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Riquelme RA, Sanhueza EM, Hanson MA, Blanco CE & Llanos AJ (1999). Adrenergeic and vassopressinergic contributions to the cardiovascular response to acute hypoxaemia in the llama fetus. J Physiol 515, 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA, Camm EJ, Niu Y, Richter H, Blanco CE, Gottschalk R, Blake EZ, Horder KA, Thakor AS, Hansell JA, Kane AD, Wooding FBP, Cross CM & Herrera EA (2012). Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress. PLoS One 7, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA & Davidge ST (2013). Developmental programming of cardiovascular disease by prenatal hypoxia. J Dev Orig Health Dis 4, 328–337. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Niu Y, Herrera EA, Richter HG, Camm EJ, Thakor AS, Kane AD, Hansell JA, Brain KL, Skeffington KL, Itani N, Wooding FBP, Cross CM & Allison AJ (2014). Heart disease link to fetal hypoxia and oxidative stress. Adv Exp Med Biol 814, 77–87. [DOI] [PubMed] [Google Scholar]
- Glantz SA & Slinker BK (2001). Primer of Applied Regression and Analysis of Variance (2nd edn), Chapter 9: Repeated measures. pp. 418–507. McGraw‐Hill, New York. [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology . J Physiol 543, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P, Heath D, Smith P, Williams DR, Ramirez A, Krüger H & Jones DM (1982). Pulmonary circulation of the llama at high and low altitudes. Thorax 37, 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera EA, Pulgar VM, Riquelme RA, Sanhueza EM, Reyes VR, Ebensperger G, Parer JT, Valdez EA, Giussani DA, Blanco CE, Hanson MA & Llanos AJ (2007). High altitude chronic hypoxia during gestation and after birth modifies cardiovascular responses in newborn sheep. Am J Physiol Regul Integr Comp Physiol 292, R2234–R2240. [DOI] [PubMed] [Google Scholar]
- Herrera EA, Reyes RV, Giussani DA, Riquelme RA, Sanhueza EM, Ebensperger G, Casanello P, Méndez N, Ebensperger R, Sepúlveda‐Kattan E, Pulgar VM, Cabello G, Blanco CE, Hanson MA, Parer JT & Llanos AJ (2008a). Carbon monoxide: a novel pulmonary artery vasodilator in neonatal llamas of the Andean altiplano. Cardiovasc Res 77, 197–201. [DOI] [PubMed] [Google Scholar]
- Herrera EA, Ebensperger G, Krause BJ, Riquelme RA, Reyes VR, Capetillo M, Gonzalez S, Parer JT & Llanos AJ (2008b). Sildenafil reverses hypoxic pulmonary hypertension in lowland and highland newborn sheep. Pediatr Res 63, 169–175. [DOI] [PubMed] [Google Scholar]
- Herrera EA, Riquelme RA, Ebensperger G, Reyes RV, Ulloa CE, Cabello G, Krause BJ, Parer JT, Giussani DA & Llanos AJ (2010). Long‐term exposure to high‐altitude chronic hypoxia during gestation induces neonatal pulmonary hypertension at sea level. Am J Physiol Regul Integr Comp Physiol 299, R1676–R1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera EA, Kane AD, Hansell JA, Thakor AS, Allison BJ, Niu Y & Giussani DA (2012). A role for xanthine oxidase in the control of fetal cardiovascular function in late gestation sheep. J Physiol 590, 1825–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera EA, Krause B, Ebensperger G, Reyes RV, Casanello P, Parra‐Cordero M & Llanos AJ (2014). The placental pursuit for an adequate oxidant balance between the mother and the fetus. Front Pharmacol 5, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoit BD, Dalton ND, Erzurum SC, Laskowski D, Strohl KP & Beall CM (2005). Nitric oxide and cardiopulmonary hemodynamics in Tibetan highlanders. J Appl Physiol 99, 1796–1801. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Koizumi T, Ruan Z, Wang Z, Chen Q & Sakai A (2005). Nitric oxide inhibitor altitude‐dependently elevates pulmonary arterial pressure in high‐altitude adapted yaks. Respir Physiol Neurobiol 146, 225–230. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Mizuno S, Sakai A, Matsukawa S, Kojonazarov B, Zamirbek B, Umeda Y, Morikawa M, Anzai M, Ishizuka T & Aldashev A (2015). Blunted activation of Rho‐kinase in yak pulmonary circulation. Biomed Res Int 10.1155/2015/720250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan NL, Walker BR & Resta TC (2003). Pulmonary PKG‐1 is upregulated following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 285, L634–L642. [DOI] [PubMed] [Google Scholar]
- Jernigan NL & Resta TC (2014). Calcium homeostasis and sensitization in pulmonary arterial smooth muscle. Microcirculation 21, 259–271. [DOI] [PubMed] [Google Scholar]
- Julian CG, Vargas E, Browne VA, Wilson MJ, Bigham AW, Rodriguez C, McCord JM & Moore LG (2012). Potential role for elevated maternal enzymatic antioxidant status in Andean protection against altitude‐associated SGA. J Matern Fetal Neonatal Med 25, 1233–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane AD, Herrera EA, Hansell JA, Giussani DA (2012). Statin treatment depresses the fetal defence to acute hypoxia via increasing nitric oxide bioavailability. J Physiol 590, 323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane AD, Hansell JA, Herrera EA, Allison BJ, Niu Y, Brain KL, Kaandorp JJ, Derks JB & Giussani DA (2014). Xanthine oxidase and the fetal cardiovascular defence to hypoxia in late gestation ovine pregnancy. J Physiol 592, 475–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouyoumdjian C, Adnot S, Levame M, Eddahibi S, Bousbaa H & Raffestin B (1994). Continuous inhalation of nitric oxide protects against development of pulmonary hypertension in chronically hypoxic rats. J Clin Invest 94, 578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kylhammar D & Radegran G (2017). The principal pathways involved in the in vivo modulation of hypoxic pulmonary vasoconstriction, pulmonary arterial remodelling and pulmonary hypertension. Acta Physiol (Oxf) 219, 728–756. [DOI] [PubMed] [Google Scholar]
- Lewis KN, Andziak B, Yang T & Buffenstein R (2013). The naked mole‐rat response to oxidative stress: just deal with it. Antioxid Redox Signal 19,1388‐1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llanos AJ, Riquelme RA, Herrera EA, Ebensperger G, Krause B, Reyes RV, Sanhueza EM, Pulgar VM, Behn C, Cabello G, Parer JT, Giussani DA, Blanco CE & Hanson MA (2007). Evolving in thin air‐lessons from the llama fetus in the altiplano. Respir Physiol Neurobiol 158, 298–306. [DOI] [PubMed] [Google Scholar]
- Lopez NC, Ebensperger G, Herrera EA, Reyes RV, Calaf G, Cabello G, Moraga FA, Beñaldo FA, Diaz M, Parer JT & Llanos AJ (2016). Role of the RHOA/ROCK pathway in high‐altitude associated neonatal pulmonary hypertension in lambs. Am J Physiol Regul Integr Comp Physiol 310, R1053–R1063. [DOI] [PubMed] [Google Scholar]
- Minamino T, Christou H, Hsieh C‐M, Liu Y, Dhawan V Abraham NG, Perella MA, Mitsialis SA & Kourembanas S (2001). Targeted expression of heme oxygenase‐1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci USA 98, 8798–8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraga, F , Monge C, Riquelme R & Llanos AJ (1996). Fetal and maternal blood oxygen affinity: a comparative study in llamas and sheep. Comp Biochem Physiol 115A, 111–115. [DOI] [PubMed] [Google Scholar]
- Ostojic H, Cifuentes V & Monge C (2002). Hemoglobin affinity in Andean rodents. Biol Res 35, 27–30. [DOI] [PubMed] [Google Scholar]
- Parker MD & Boron WF (2013). The divergence, actions, roles, and relatives of sodium‐coupled bicarbonate transporters. Physiol Rev 93, 803–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrau D, Ebensperger G, Herrera EA, Moraga F, Riquelme RA, Ulloa CE, Rojas RT, Silva P, Hernandez I, Ferrada J, Diaz M, Parer JT, Cabello G, Llanos AJ & Reyes RV (2013). Store‐operated channels in the pulmonary circulation of high‐ and low‐altitude neonatal lambs. Am J Physiol Lung Cell Mol Physiol 304, L540–548. [DOI] [PubMed] [Google Scholar]
- Rees DD, Palmer RM, Schulz R, Hodson HF & Moncada S (1990). Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo . Br J Pharmacol 101, 746–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter HG, Camm EJ, Modi BN, Naeem F, Cross CM, Cindrova‐Davies T, Spasic‐Boskovic O, Dunster C, Mudway IS, Kelly FJ, Burton GJ, Poston L & Giussani DA (2012). Ascorbate prevents placental oxidative stress and enhances birth weight in hypoxic pregnancy in rats. J Physiol 590: 1377–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybalkin SD, Rybalkina IG, Feil R, Hofmann F & Beavo JA (2002). Regulation of cGMP‐specific phosphodiestyerase (PDE5) phosphorylation in smooth muscle cells. J Biol Chem 277, 3310–3317. [DOI] [PubMed] [Google Scholar]
- Sanhueza EM, Riquelme RA, Herrera EA, Giussani DA, Blanco CE, Hanson MA & Llanos AJ (2005). Vasodilator tone in the llama fetus: the role of nitric oxide during normoxemia and hypoxemia. Am J Physiol Regul Integr Comp Physiol 289, R776–R783. [DOI] [PubMed] [Google Scholar]
- Schülke S, Dreidax D, Malik A, Burmester T, Nevo E, Band M, Avivi A & Hankeln T (2012). Living with stress: regulation of antioxidant defence genes in the subterranean, hypoxia‐tolerant mole rat, Spalax. Gene 500, 199–206. [DOI] [PubMed] [Google Scholar]
- Schubert R, Krien U, Wulfsen I, Schiemann D, Lehmann G, Ulfig N, Veh RW, Schwarz JR & Gago H (2004). Nitric oxide donor sodium nitroprusside dilates rat small arteries by activation of inward rectifier potassium channels. Hypertension 43, 891–896. [DOI] [PubMed] [Google Scholar]
- Singh DK, Sarkar J, Raghavan A, Reddy SP & Raj JU (2011). Hypoxia modulates the expression of leucine zipper‐positive MYPT1 and its interaction with protein kinase G and Rho kinases in pulmonary arterial smooth muscle cells. Pulm Circ 1, 487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley HF, Kadwell M & Wheeler JC (1994). Molecular evolution of the family Camelidae: a mitochondrial DNA study. Proc Biol Sci 256, 1–6. [DOI] [PubMed] [Google Scholar]
- Thakor AS, Herrera EA, Serón‐Ferré M & Giussani D (2010a). Melatonin and vitamin C increase umbilical blood flow via nitric oxide‐dependent mechanisms. J Pineal Res 49, 399–406. [DOI] [PubMed] [Google Scholar]
- Thakor AS, Richter HG, Kane AD, Dunster C, Kelly FJ, Poston L & Giussani DA (2010b). Redox modulation of the fetal cardiovascular defence to hypoxaemia. J Physiol 588, 4235–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres F, González‐Candia A, Montt C, Ebensperger G, Chubretovic M, Serón‐Ferré M, Reyes RV, Llanos AJ & Herrera EA (2015). Melatonin reduces oxidative stress and improves vascular function in pulmonary hypertensive newborn sheep. J Pineal Res 58, 362–373. [DOI] [PubMed] [Google Scholar]
- Villamor E, Ruijtenbeek K, Pulgar V, De Mey JG & Blanco CE (2002). Vascular reactivity in intrapulmonary arteries of chicken embryos during transition to ex ovo life. Am J Physiol 282, R917–R927. [DOI] [PubMed] [Google Scholar]
- Wan J, Yamamura A, Zimnicka AM, Voirot G, Smith KA, Tang H, Ayon RJ, Choudhury MSR, Ko EA, Wang J, Wang C, Makino A & Yuan JX‐J (2013). Chronic hypoxia selectively enhances L‐ and T‐type voltage‐dependent Ca2+ channel activity in pulmonary artery by upregulating Ca1.2 and Ca3.2. Am J Physiol Lung Cell Mol Physiol 305, L154–L164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SD (1978). A history of savanna vertebrates in the New World: part II South America and the Great Interchange. Ann Rev Ecol Syst 9, 393–426. [Google Scholar]
- Wu P, Shan M, Liang K, Yue H, Qian L & Sun B (2016). Exhaled nitric oxide is associated with postnatal adaptation to hypoxia in Tibetan and non‐Tibetan newborn infants. Acta Paediatrica 105, 475–482. [DOI] [PubMed] [Google Scholar]
- Zou LB, Yamada K, Tanaka T, Kameyama T & Nabeshima T (1998). Nitric oxide synthase inhibitors impair reference memory formation in a radial arm maze task in rats. Neuropharmacology 37, 323–330. [DOI] [PubMed] [Google Scholar]


