Abstract
This review covers our current knowledge of the causes of perinatal brain injury leading to cerebral palsy‐like outcomes, and argues that much of this brain damage is preventable. We review the experimental evidence that there are treatments that can be safely administered to women in late pregnancy that decrease the likelihood and extent of perinatal brain damage that occurs because of acute and severe hypoxia that arises during some births, and the additional impact of chronic fetal hypoxia, infection, inflammation, growth restriction and preterm birth. We discuss the types of interventions required to ameliorate or even prevent apoptotic and necrotic cell death, and the vulnerability of all the major cell types in the brain (neurons, astrocytes, oligodendrocytes, microglia, cerebral vasculature) to hypoxia/ischaemia, and whether a pan‐protective treatment given to the mother before birth is a realistic prospect.
Keywords: perinatal brain damage, cerebral palsy, Oxidative stress, neuroprotection, prophylaxis
Introduction
The incidence of brain damage arising from ‘oxygen starvation’ and infection at birth, or from preterm birth, is about 5‐fold greater in low‐income than in high‐income countries (Liu et al. 2016). Even in ‘first world’ countries where women have access to the best medical care, the unpredictable nature of fetal hypoxia, fetal growth restriction (FGR), preterm birth and hypoxic/asphyxic events during fetal–neonatal transition presents a unique set of challenges to clinicians. There are few if any in utero sentinel events in fetal life that indicate either that damage to the brain is imminent, or that it might have already occurred and is becoming progressively worse, and which finally manifest as cerebral palsy (CP). However, there is much evidence to suggest that brain injury – especially with FGR – occurs in utero rather than after birth as shown by fetal MRI and the presence of established injury at birth (Dubois et al. 2008; Ramenghi et al. 2011; Businelli et al. 2015; Miller et al. 2016). Preterm birth is an additional and important risk factor for CP, often associated with the form of white matter damage known as periventricular leukomalacia (Back, 2006; Volpe, 2009). Prematurity leads to a spectrum of neurological deficits, including persistent neuromotor abnormalities (commonly spastic diplegia), cognitive and planning deficits, and problems of sensorimotor integration that lead to functional impairments in learning, academic difficulties and problems with social–emotional adjustment.
The challenge
The challenge for obstetricians and neonatologists is to devise strategies that protect the immature brain before birth, i.e. by finding an effective prophylactic treatment that can be used antenatally, or immediately after birth. We recognize that to deliver a treatment via the maternal compartment treats not only the mother but also the placenta, an organ with a high metabolic rate and a source of oxygen/nitrogen free radicals and cytotoxic metabolites that can compromise organ function in the fetus (Myatt & Cui, 2004; Burton et al. 2016). In this review, we discuss the proposal that antenatal and very early post‐birth treatments are available that can dramatically decrease, and even eliminate, the brain damage that arises when the fetus becomes chronically deprived of oxygen due to placental insufficiency, is infected, or faces the serious physiological challenge of preterm birth. While it is expected such treatments would be delivered only to ‘at risk’ patients (i.e. where fetal compromise is already present, or considered highly likely to arise), an important consideration is whether the proposed treatments are also benign in the absence of the extreme conditions discussed above that cause perinatal brain damage. This is important, because it addresses the globally significant problem of intrapartum and postpartum brain damage present in underdeveloped and developing countries where clinical resources are limited, where pregnant women may receive little care, and where the global burden of CP is the greatest. Clearly, the approach we would like to recommend must be suitable for use in any birth setting and not just tertiary level hospital care. It should also be inexpensive and simple to use, and yet based on the emerging mechanistic physiology of the inflammation, oxidative stress and neurotoxicity widely agreed to induce altered brain development and damage in infants.
What approach should we take?
The recognition that head cooling (hypothermia) commenced within 6 h of birth is effective in reducing the severity of brain injury following hypoxic–ischaemic encephalopathy (HIE) in term infants (Edwards et al. 2010) shows that the pathogenic processes leading to injury can be interrupted, providing a strong impetus to examine further options targeting the pathways likely to be involved in injury to the young brain. However, a protective strategy must prevent or correct the pathophysiology that causes tissue injury, whether in the brain or elsewhere. This is particularly challenging in perinatal HIE because, as pointed out above, the pathways that lead to cellular degeneration in the immature brain are certainly multifactorial, and include glutamate‐mediated excitotoxicity, oxidative stress, inflammation, inhibition of progenitor cell differentiation and maturation, demise of mitochondrial function, and induction of cell‐death signalling pathways (Ferriero, 2004). Magnesium sulphate has a rapid neuroprotective effect, and can be given to mothers shortly before delivery. The evidence is clear that this protects against gross motor dysfunction, reduces the risk of CP in infants born preterm, and is not associated with adverse long‐term maternal or neonatal outcomes (Doyle et al. 2009; Crowther et al. 2017). Notwithstanding that there is now general agreement on the dose, timing and indications for using magnesium sulphate (see the Royal College of Obstetricians and Gynaecologists (RCOG) Scientific Advisory Committee Opinion Paper no. 29, 2011), its use only when labour is imminent or already present may create time‐critical situations that conflict with other demands of obstetric care. Furthermore, the risk of adverse effects associated with this medication (albeit, low) mandates that its use will remain largely confined to tertiary level medical care settings. Thus, a requirement that we are keen to address is finding a prophylaxis that can be administered safely in a very wide range of settings. It is our opinion that current treatments do not address the problem of preventing, limiting or repairing pre‐existing fetal brain injury.
Timely identification of the unborn ‘at risk’ baby remains a major problem in obstetric practice. This is almost always done indirectly – e.g. from an abnormal heart rate or absence of fetal movements – and it is never known with confidence if a fetus is already hypoxic, is in danger of becoming hypoxic, or perhaps is chronically hypoxic but satisfactorily adapted to this low oxygen condition. Much of the adaptive response to hypoxia (and to infection and inflammation) is cardiovascular in nature (Giussani, 2016), which is critical in ensuring the fetal heart and brain are supplied with increased blood flow despite the subnormal oxygen content of fetal blood. Hence, any proposed treatment given prophylactically to pregnant women should not interfere with these critical physiological defences, and any treatment is likely to be given in the presence of co‐morbidities such as chorioamnionitis, poor placental function and FGR. Finally, such treatments should not affect the major transformation of the fetal circulation at birth, when the placental circulation is lost and pulmonary blood flow must increase rapidly.
In addition to magnesium sulphate (discussed further below), a number of antenatally applied treatments that aim to prevent brain damage arising from chronic and/or intrapartum hypoxia have been proposed, and include the use of ascorbic acid, tetrahydrobiopterin, phenobarbital, N‐acetylcysteine (NAC), xenon and argon (Bel & Groenendaal, 2016). However, these have either provided disappointing results (ascorbic acid, xenon), or require specialized ventilatory equipment (xenon, argon), or revealed unfavourable safety profiles (NAC), or, while showing promise (e.g. allopurinol, melatonin, argon), currently lack full clinical evaluation. NAC administration is known to reduce sequelae such as perinatal brain injury in offspring, but nevertheless it has limitations when given systemically (Buhimschi et al. 2003; Lee et al. 2005; Beloosesky et al. 2012). Nanoparticle‐based drug delivery systems, such as dendrimer‐based NAC (DNAC) offer many advantages compared to the free drugs, including improved efficacy and reduce side effects such as nausea, vomiting, stomatitis and fever (Kurtoglu et al. 2009; Kannan et al. 2014; Lei et al. 2017). Systemic administration of DNAC prenatally and postnatally in rodents targets activated microglia in the injured pup's brain and prevents perinatal neurobehavioral outcomes (Kannan et al. 2012; Burd et al. 2014). Targeted drug delivery using nanoparticles thus represents a new way forward, providing that careful pre‐clinical experiments have produced evidence for the critical role of the pathway in the genesis of perinatal brain injury.
We will discuss the injurious pathways that contribute to perinatal brain injury with the intention of drawing out the common pathways that are amenable to prophylaxis, but three introductory comments are offered. Firstly, perinatal brain injury is clearly multifactorial, whether the birth is preterm or at term (Volpe, 2009). Secondly, nearly all the medications in use or proposed for protecting the perinatal brain have short half‐lives (minutes to a few hours), and when given during labour or immediately after birth may not provide benefits over the critical hours and days of early postnatal life. Finally, the focus on neuroprotection has obscured the importance of other systemic sequelae of hypoxia, metabolic acidosis and/or inflammation on the fetus and neonate. It is in a minority of cases (perhaps <15%) that the brain exhibits dysfunction after hypoxia–ischaemia at birth; injury to the lungs, myocardium, kidney and gut can have devastating effects on neonatal homeostasis (Hankins et al. 2002; Antonucci et al. 2014; Singh & Sengar, 2016). Low oxygen may not be the direct or only cause of neonatal HIE – it may develop secondary to renal, hepatic and cardiac dysfunction following birth asphyxia – and therefore a therapy that minimizes tissue injury globally may have advantages over one that is specifically targeted to neuronal processes and mechanisms.
Dealing with infection and inflammation
Intrauterine infection
Chorioamnionitis, caused by intrauterine infection and/or inflammation, is an important risk factor for perinatal brain injury and neurodevelopmental disability (Dammann & Leviton, 1997, 2014; Strunk et al. 2014). Intrauterine infections often involve the genital Ureaplasma species (e.g. U. urealyticum and U. parvum), which can be isolated from amniotic fluid, cord blood, the respiratory tract and cerebrospinal fluid of infants born prematurely (Goldenberg et al. 2008). Extensive evidence has established a link between infection with Ureaplasma spp. and the development of perinatal lung disease (Viscardi & Kallapur, 2015). However, the specific role of these species in perinatal brain injury has been less clear. Importantly, serum Ureaplasma‐positive infants have a 2.3‐fold increased risk of intraventricular haemorrhage (Morency & Bujold, 2007). Ureaplasma spp. have also been isolated from cases of neonatal meningitis, particularly in infants born prematurely (reviewed by Glaser & Speer, 2015). Experimental studies with rodents and sheep have provided evidence for the effects of Ureaplasma infection on the perinatal brain, including microglial activation (Normann et al. 2009; Gussenhoven et al. 2017). More recently, non‐human primate models that have shown a causal link between intrauterine Ureaplasma infection and preterm birth (Novy et al. 2009) have been employed to examine effects of infection and prematurity on the developing brain (Kelleher et al. 2017).
As to treatment of infection, the antenatal use of antibiotics to prevent early labour, prolong gestation and improve immediate neonatal outcomes remains controversial and the subject of vigorous debate (McDonald et al. 2007; Morency & Bujold, 2007; Swadpanich et al. 2008). Conflicting outcomes of published studies may be attributed to potential confounders and variations embedded in the study designs, which differ substantially (Stetzer & Mercer, 2000; King & Flenady, 2002; Waites et al. 2009). For example, a study that has gained a great deal of attention is the 7‐year follow‐up of the ORACLE II trial, which suggests an increased (albeit small) risk of adverse neurological impairment following prescription of erythromycin during pregnancy (Kenyon et al. 2008). This trial highlights the importance of ensuring that antenatal treatments do not cause long‐term postnatal harm. However, evidence from ovine and primate studies (Grigsby et al. 2012) also suggest that with the appropriate safeguards – such as correct patient selection, early diagnosis of infection and appropriate choice, route and timing of antimicrobial treatment – treatments for infection that delay or prevent preterm labour may also provide immediate benefit by reducing preterm neonatal morbidity with the potential for improved long‐term outcomes as well.
In addition, the use of the newer macrolide azithromycin and other related agents has proven more effective in animal studies at treating Ureaplasma infections than erythromycin, potentially due to intrinsic anti‐inflammatory actions and pharmacokinetics that allow it to accumulate in target tissues and amniotic fluid (Ramsey et al. 2003; Dando et al. 2010; Acosta et al. 2014; Keelan et al. 2014; Kemp et al. 2014). Azithromycin eradicates U. parvum from the amniotic cavity and fetal tissues in the rhesus monkey (Grigsby et al. 2012) and fetal sheep (Miura et al. 2014). This maternal treatment targets inflammatory signalling, as shown by decreased levels of pro‐inflammatory cytokines in amniotic fluid, prolongs gestation and reduces fetal lung injury (Grigsby et al. 2012). The effect of these treatments on the fetal brain is an important and ongoing research question. Employing relevant pre‐clinical models that allow functional outcomes to be studied (Kelleher et al. 2017) will be essential to establishing the long‐term safety and efficacy of antimicrobial treatment of prematurity of infectious aetiology. As we consider the need for personalized medicine so must we consider unique approaches for the different micro‐organisms and viruses that have been implicated in the pathophysiology of premature birth, requiring precise selection of antimicrobial agents, potential adjuvant therapies with anti‐inflammatories (Keelan, 2011) and therefore the need for development of rapid diagnostic tests and identification of biomarkers for early detection.
Is it plausible to administer a macrolide antibiotic during pregnancy not only to delay preterm birth, but also reduce the severity of fetal sequelae to intra‐amniotic infection? Recent data on functional neurodevelopmental indices suggests that it is, with preliminary observations suggesting no long‐term neurobehavioral and cognitive deficits following in utero treatment of intra‐amniotic U. parvum infection with azithromycin (Grigsby et al. 2012). This therapeutic approach may reduce CNS compromise and injury that sometimes follows prolonged intra‐amniotic exposure to U. parvum, which has been shown to include cognitive, sensorimotor and attention deficits in preterm rhesus infants (Kelleher et al. 2017).
While clinical resistance to the use of antenatal antibiotics remains, the direct treatment of fetal inflammation and infection is an approach that needs to be considered. In utero infection necessarily involves the placenta, and both placental and fetal inflammation involves dysregulation of key pro‐inflammatory cytokines such as IL‐1 and IL‐6, inappropriate anti‐inflammatory and regulatory responses (e.g. IL‐4, IL‐10) in the fetal brain (Lei et al. 2015) and up‐regulation of the tryptophan–kynurenine pathway with production of cytotoxic quinolate metabolites that potentially damage the fetal brain (Manuelpillai et al. 2005). While the use of single cytokine antagonists such as the interleukin‐1 (IL‐1) receptor antagonist kineret has shown some promise as an immunomodulatory therapy in animal models of maternal inflammation (Rosenzweig et al. 2014), it may not easily translate to an in utero therapy.
Immunomodulation in utero using mesenchymal stem cells
Mesenchymal stem cells (MSCs) are an attractive therapeutic option because of their inherent low immunogenicity, potential to treat graft‐versus‐host disease (Newman et al. 2009; Herrero & Perez‐Simon, 2010; Han et al. 2012) and their suitability for cross‐species experiments (Karussis et al. 2008; Hoogduijn et al. 2010; Soleymaninejadian et al. 2012). MSCs have been shown to promote postnatal neurological recovery from perinatal stroke and hypoxia–ischaemia (Phillips et al. 2013; Verina et al. 2013), and their promise as a maternal cell therapy to ameliorate perinatal morbidity follows from their inherent immunomodulatory property; e.g. they provoke increased production of the anti‐inflammatory cytokine IL‐10 (Nemeth et al. 2009). MSCs have been used in US Food and Drug Administration (FDA)‐approved clinical trials for myocardial infarction, stroke, limb ischaemia, graft‐versus‐host disease, autoimmune disorders and, by virtue of their capacity to regulate inflammation, MSCs decrease apoptosis, promote endogenous neuronal growth and encourage the formation of synaptic connections in the brain damaged by ischaemia and other interventions (Ohtaki et al. 2008; Titomanlio et al. 2011; Dalous et al. 2012).
MSCs probably act, in part, via Toll‐like receptor 4 (TLR4) pathways (Lombardo et al. 2009; Wang et al. 2012; Guijarro‐Munoz et al. 2014). In pregnant mice and rabbits, exposure to the TLR4 ligand lipopolysaccharide (LPS) induces intrauterine inflammation (Burd et al. 2012), and well‐defined phenotypes of preterm birth including fetal neuroinflammation, fetal neuronal injury and death as well as short‐ and long‐term grey and white matter damage, and neurological sequelae in the offspring (Burd et al. 2010; Dada et al. 2014). In a study of LPS‐induced intrauterine inflammation in mice, maternal MSC administration increased levels of the anti‐inflammatory cytokine IL‐10 (maternal serum) and the Th2 anti‐inflammatory cytokine IL‐4 (placenta) but decreased the pro‐inflammatory cytokine IL‐6 in the fetal brain in response to intrauterine inflammation (Lei et al. 2015). This study provides the proof‐of‐principle evidence that maternal MSC administration can alleviate fetal brain injury, specifically in the cortex via decreased microglial activation, with the translational effect that the maternal MSC treatment improved the neurobehavioral performance of the pups after birth (Lei et al. 2015).
Dealing with hypoxia and oxidative stress
Tissue hypoxia
A primary manifestation of hypoxia–ischaemia in all organs is derangement of the mitochondrial electron transport chain (ETC), disruption to oxidative phosphorylation, and the generation of reactive oxygen (ROS) and reactive nitrogen (RNS) species. This results in rapid depletion of intracellular energy (in particular, ATP), damage to nuclear and mitochondrial DNA, and acute induction of inflammatory and apoptotic cascades, followed for many days by waves of cell death, ultimately resulting in permanent injury in multiple organs (Saikumar & Venkatachalam, 2003; Sun et al. 2008). The hydroxyl radical (•OH), generated largely in mitochondria, is one of the most toxic of all the ROS and should be considered a key target for therapeutic intervention (Murphy, 2009; Miller et al. 2012).
When cells become hypoxic, mitochondria eventually are unable to maintain adequate levels of oxidative phosphorylation, and along with functional demise they become susceptible to structural damage (Murphy, 2009). As a result of the failure of ATP‐dependent processes and the loss of function of plasma membrane ionic pumps, the intracellular accumulation of calcium ions causes osmotic oedema, cell swelling, opening of the mitochondrial membrane transition pore (mMTP), and release of mitochondrial proteins (including cytochrome c) into the cytosol, which then initiates nuclear processes that start the apoptotic cascade. Partial loss of mitochondria places increased demands on surviving mitochondria, and many studies have shown that the increased metabolic activity of mitochondria results in further production of reactive oxygen species (ROS), including •OH. Mitochondria are also highly susceptible to ROS attack due, in part, to alterations of intra‐mitochondrial proteins, lipids and nucleic acids that then further compromise mitochondrial function. Thus, a vicious cycle of ROS formation and mitochondrial dysfunction is initiated (Murphy, 2009).
Oxidative stress
Normal human pregnancy is a state prone to oxidative stress compared to non‐pregnancy, predominantly due to a high metabolic rate of the placenta (Miller et al. 2012), placing demand on antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidase to prevent the accumulation of ROS. The ability of maternal and placental antioxidant defences to mitigate ROS is obviously important for normal placental function, and therefore for normal fetal growth, but pregnancies complicated by placental insufficiency and FGR are associated with decreased activity of placental SOD and glutathione peroxidase (Wiktor & Kankofer, 1998; Wiktor et al. 2000). Furthermore, there is clear evidence of increased oxidative stress in maternal, placental and fetal tissues (Myatt & Cui, 2004). For example, 8‐hydroxy‐2′‐deoxyguanosine, generated by oxidative breakdown of DNA, is significantly elevated in maternal urine (Scholl & Stein, 2001) and the placenta (Takagi et al. 2004; Wiktor et al. 2004) in FGR pregnancies. In pregnant sheep oxidative stress arising from placental insufficiency is a significant contributor to abnormal fetal brain development and neurobehavioural deficits in the lambs (Miller et al. 2007, 2014). The asymmetric FGR induced in fetal sheep by the surgical technique of single umbilical artery ligation at ∼0.7 gestation causes not only chronic fetal hypoxia and hypoglycaemia (Supramaniam et al. 2006; Miller et al. 2009), but also significant up‐regulation of the lipid peroxidation product 4‐hydroxynonenal in the brain (Miller et al. 2014). This is an important observation because the fetal/neonatal brain has low endogenous antioxidant defences (Mishra & Delivoria‐Papadopoulos, 1999; Back, 2006). Furthermore, this lipid peroxidation causes breakdown of myelin proteins and leads to axonal damage (Miller et al. 2014).
Thus it is pertinent to ask, can this oxidative damage be prevented or ameliorated antenatally? Therapies that support mitochondrial respiration under conditions of hypoxia and high levels of oxidative stress could potentially be important, but what are they?
Creatine and tissue hypoxia
Creatine is an amino acid derivative obtained from an omnivorous diet that includes meat, fish and dairy, and it is also synthesized endogenously from the amino acids arginine, glycine and methionine (Wallimann et al. 1992; Wyss & Kaddurah‐Daouk, 2000). Creatine drives the creatine kinase circuit, a phosphogen pathway responsible for the regeneration of ATP from ADP in an oxygen‐independent manner. Once in the cell, a proportion of creatine is rapidly phosphorylated, and phosphocreatine acts as a phosphate donor to produce ATP from ADP. The rephosphorylation of ADP by PCr via the reversible chemical reaction driven by creatine kinase (CK) utilizes a proton [H+], giving the creatine/phosphocreatine reaction the ability to prevent the cytosol of the cell from becoming acidic, particularly under hypoxic conditions (Wallimann et al. 1992). Oral creatine supplementation in rats attenuated ROS in neural tissue, including accumulation of superoxide anion radical O2 −, hydrogen peroxide (H2O2) and •OH, and by amounts similar to those affected by conventional antioxidants (Matthews et al. 1998). In skeletal muscle, creatine had no effect on the redox‐driven expression of antioxidant enzymes, supporting the hypothesis that creatine acts as a ROS scavenger in its own right (Guimaraes‐Ferreira et al. 2012). Creatine has been shown to protect mitochondrial DNA (mtDNA) from damage associated with hypoxia and oxidative stress by stabilization of cellular pH and mitochondrial membrane potential (Guidi et al. 2008), which appears to be dose‐dependent, with intracellular concentrations of creatine at 3 mm being ineffective, but 10 mm protecting mtDNA for up to 24 h after a hypoxic insult (Sestili et al. 2006).
The ability of creatine to maintain ATP turnover, acid–base balance and mitochondrial function, together with its antioxidant, vasodilator and anti‐excitotoxic properties (Gualano et al. 2012), make it a candidate for the treatment of the ischaemic–reperfusion injury that occurs in neonates who develop HIE. Indeed, in brain slices prepared from immature mouse and fetal guinea pigs, increased creatine in the external medium sustained ATP turnover and reduced neuronal cell injury when the slices were exposed to hypoxia (Wilken et al. 1998; Berger et al. 2004). These results support the concept that antenatally administered creatine may act to protect the neonatal brain from HIE induced by intrapartum asphyxia (Ireland et al. 2011). However, the animal experiments showing increased survival of offspring of creatine‐fed dams after birth asphyxia also suggest that prenatal creatine loading is protective for other major fetal organs (Ireland et al. 2008), as shown by reduction of damage to the diaphragm muscle, axial skeletal muscles and kidneys following birth asphyxia (Cannata et al. 2010; Ellery et al. 2013, 2017; LaRosa et al. 2016b). A particular benefit in relation to CP is not only the decrease in severity of brain damage, but also the protection of skeletal muscle fibres and improved resistance to fatigue in the diaphragm (LaRosa et al. 2016a). Neonatal acute kidney injury, which has a high prevalence in neonates that have experienced intrapartum hypoxia and is a co‐morbidity associated with poor neurological outcomes due to the acid–base and electrolyte imbalance (Perlman et al. 1989), is also prevented by antenatal creatine treatment (Ellery et al. 2013). This body of work has led to a Cochrane Review on creatine supplementation for women in pregnancy for neuroprotection of the fetus (Dickinson et al. 2014), and is the basis of a proposed randomized control trial of maternal creatine supplementation for neuroprotection of the fetus, but it remains that we still know little about creatine biosynthesis and homeostasis in human pregnancy. A pre‐clinical study in the non‐human primate (macaque) has demonstrated some benefits to the fetus of antenatal maternal creatine loading (M. Kelleher, P. Grigsby, S. Ellery, D. Walker and L. Sherman, unpublished observations), and this study has not identified any unwanted side effects of creatine treatment in the mother.
Allopurinol
Allopurinol has clear neuro‐ and cardiovascular protective effects for the fetus and neonate, and the ease of it use, and its rapid onset of action and clearance are reasons to persist with evaluation of it as a treatment to be used at the delivery of high‐risk obstetric patients. Allopurinol is an inhibitor of xanthine oxidase, and its administration reduces ROS production by preventing the conversion of hypoxanthine to uric acid. It also has the effect of scavenging •OH by chelating non‐protein‐bound iron, although this effect is seen only at the higher doses. The experimental data supporting the neuroprotective benefit of allopurinol is compelling. For example, allopurinol given to pregnant sheep prevented neuronal loss in the fetal brain in the event of hypoxia–ischaemia (Saugstad, 1996; Kaandorp et al. 2014), and maternal allopurinol administration also improved fetal cardiac function and allowed these fetuses to maintain umbilical blood flow during the period of ischaemia–reperfusion (Derks et al. 2010). Improved cardiac function due to allopurinol is also known from paediatric cardiac surgery (Clancy et al. 2001), and its intrapartum use is therefore likely to be beneficial for the fetus undergoing hypoxic stress at birth.
Over 25 years ago, it was shown that allopurinol treatment of newborn preterm babies reduced mortality associated with respiratory distress syndrome, an effect possibly mediated by a decrease in the production of superoxide radicals. The recently published ALLO‐Trial in humans examined the administration of allopurinol to pregnant women at term when acute severe intrauterine fetal hypoxia during labour was suspected (Kaandorp et al. 2015). The ALLO‐Trial aimed to reduce HIE after birth by muting the burden of early oxidative stress in the fetal brain during or after hypoxia. Although the ALLO trial showed there was a slight reduction in the serum values of S‐100B and neuroketal (markers of neuronal damage), this was only significant for girls and not for boys, thereby suggesting that pathways of apoptosis and neuroprotection are more sensitive to treatment in females. In addition, the fetuses included in the ALLO trial only had a relatively mild hypoxia, consistent with the fact that current fetal monitoring has a very low sensitivity for identifying the really hypoxic fetus. Although the results from the ALLO trial were inconclusive, a major grant was awarded by the European Union for the ALBINO trial, in which allopurinol is to be administered to asphyxiated newborns in combination with hypothermia, with the aim of providing new data on neonatal outcomes, and possibly, on longer‐term cardiovascular and neurological outcomes in childhood. The outcome of this trial will hopefully give more evidence about the efficacy of allopurinol as a protective agent for perinatal HIE.
Melatonin
Melatonin is known for regulating circadian rhythms, but it is also a very efficient antioxidant, acting both as a scavenger of oxygen free radicals (including the highly destructive hydroxyl radical) and by up‐regulation of endogenous antioxidant processes. Melatonin readily crosses the placenta and fetal blood–brain barrier (Miller et al. 2005), and is safe for human pregnancy (Tamura et al. 2008), making it very suitable as an in utero antioxidant therapy. The neuroprotective potential of antenatal melatonin for FGR has been shown by treating pregnant sheep carrying a growth restricted fetus, with continuous i.v. infusion of melatonin commencing soon after the onset of placental insufficiency and for the remainder of the pregnancy, a duration of up to 30 days. Melatonin was very effective in reducing the hypomyelination and axonal damage in FGR sheep, and it also improved motor and cognitive function of growth‐restricted lambs after birth (Miller et al. 2014). These neuroprotective actions of melatonin have been attributed to its antioxidant ability, but antenatal melatonin also improved fetal oxygen saturation and fetal cardiac function (Miller et al. 2014; Tare et al. 2014), which may be the result of increased uteroplacental blood flow (Thakor et al. 2010). These pre‐clinical results suggest that melatonin is an effective antenatal treatment for reducing brain injury associated with FGR. In a small clinical trial of women with moderate to severe FGR, antenatal melatonin reduced the serum markers of placental oxidative stress (Miller et al. 2014). Thus, there is justification for considering melatonin as a neuroprotective therapy whenever moderate to severe human FGR is detected antenatally.
Magnesium sulphate
Considerable examination of multiple trials of MgSO4 leads to the conclusion that it is highly effective in preventing the brain damage that may arise with preterm birth (Shepherd et al. 2016; Crowther et al. 2017), and it is now standard practice in many Western countries. The overall effect is modest, with the ‘need to treat’ of about 1 in 46, but this is nevertheless a valuable outcome in terms of health economics. Careful consideration about dose and dosing regime has led to avoidance of the risk of magnesium toxicity in the mother, and the worries about neonatal respiratory depression not being confirmed (Johnson et al. 2012). Provided that neonatal serum magnesium concentrations are <4.5 mEq dl−1, MgSO4 has powerful neuroprotective effects (Narasimhulu et al. 2017). Neonatal (and presumably, fetal) serum concentrations correlate closely with the total maternal dose and duration of therapy, and serum concentrations between 2.5 and 4.5 mEq dl−1 are optimal for neuroprotection, whereas concentrations >4.5 mEq dl−1 may be associated with periventricular leukomalacia (Narasimhulu et al. 2017). Of note, this study also identified increased risk of grade 3–4 intraventricular haemorrhage in neonates with serum concentrations <2.5 mEq dl−1, suggesting there are some pregnancies that would benefit from maternal magnesium supplementation. However, measurement of serum magnesium in the mother is not always practical when preterm birth is imminent, nor is the obtaining of an early sample from the neonate.
While it is likely that the use of MgSO4 will be restricted to preterm birth because the imminence of delivery means that the duration of exposure to this cation is limited, the clinical success of MgSO4 has obscured other issues about its mechanisms of action, and concerns about its use at later stages of pregnancy, particularly at term. For example, it is unlikely that magnesium concentrations in the CNS increase to the levels that fully block the Ca2+ channel on the NMDA receptor as shown to be necessary in vitro (Galinsky et al. 2014), and therefore other mechanisms of action that lead to the apparent ‘neuroprotection’ need to be considered, e.g. vasodilatation (Altura et al. 1987), anti‐inflammation (Sugimoto et al. 2012) and/or the confounding effect of spontaneous hypothermia arising from the use of MgSO4 (Zhu et al. 2004). These considerations have importance beyond the argument of whether MgSO4 should be restricted to impending preterm birth, because it may be that increased Mg2+ ion in the fetal and neonatal circulation in increasing peripheral vascular conductance has protective effects for the renal and mesenteric circulations, thereby decreasing the impact of peripheral inflammation that arises from post‐asphyxial ischaemia on cerebral tissue (Galinsky et al. 2016).
Dealing with preterm birth and fetal growth restriction
As mentioned above, FGR is a major risk factor for poor neurodevelopmental outcomes into childhood, including cerebral palsy (Miller et al. 2016), and this is even greater if they have experienced ‘oxygen starvation’ at birth, have been exposed to intrauterine infection or inflammation, or are born preterm (McIntyre et al. 2013). FGR is primarily caused by chronic placental insufficiency, which exposes the fetus to long‐term and progressive hypoxia and hypoglycaemia, particularly in the second and third trimesters as fetal demand for nutrients outstrips placental capacity. At the same time (24–32 weeks’ human gestation), the brain is beginning the critical process of white matter development (Volpe, 2009). Accordingly, it is not surprising that white matter brain injury is a common neuropathological finding in FGR and preterm infants, and is the principal aetiology associated with CP.
Animal studies show that perturbation of white matter development in the FGR brain is often the result of impaired maturation of oligodendrocytes, in addition to astrogliosis and microgliosis (Segovia et al. 2008; Tolcos et al. 2011; Reid et al. 2012; Miller et al. 2014; Castillo‐Melendez et al. 2015). Oligodendrocytes mature according to a well‐defined sequence in which four stages of oligodendrocytes development have been defined, as shown in Fig. 1. Oligodendrocyte precursor cell (OPCs) are highly proliferative, and mature oligodendrocytes develop in sequential waves from multi‐potent progenitor cells found in restricted proliferative zones during fetal and early postnatal life. However, critically ill preterm neonates are highly susceptible to white matter injury due, in part, to poor and incomplete myelination, and at least one mechanism behind this is a failure of OPCs to mature into myelin‐forming oligodendrocytes (Segovia et al. 2008; Buser et al. 2012). In number, the pre‐oligodendrocytes and OPCs predominate in the human brain at 23–32 weeks of gestation prior to the onset of myelination proper, and it is the vulnerability of the immature oligodendrocytes to hypoxia and inflammation in particular that causes the delay, or even blockage, of the myelination process in the preterm and FGR brain. Preventing the accumulation of ROS provides significant benefits in terms of limiting the advance of brain injury and, as discussed above in relation to melatonin, this not only decreases the markers of oxidative stress such as lipid peroxidation and DNA/RNA fragmentation, but also promotes myelination when given to the FGR fetus.
Figure 1. Oligodendrocyte progenitor cells (OPCs) undergo distinct changes as they mature into mature, myelinating oligodendrocytes.
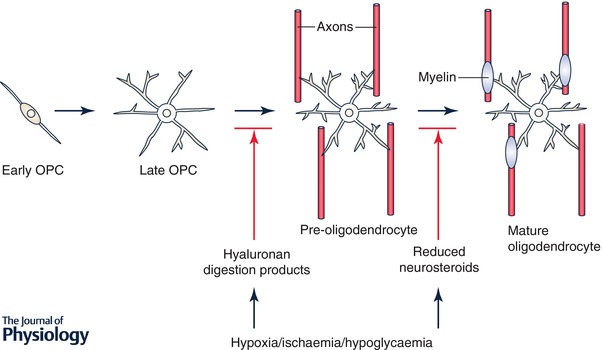
Different signals in the injured perinatal nervous system (e.g. hyaluronan, changes in neurosteroids) influence maturation at different stages.
In specifically addressing the impact of FGR on white matter, we discuss three further approaches based on interactions with pathways known to be critical to the maturation of oligodendrocytes.
Hyaluronic acid and myelination
Myelination failure is consistently associated with astrogliosis, accumulation of the glycosaminoglycan hyaluronan (HA) and elevated expression of the transmembrane HA receptor, CD44 (Buser et al. 2012). HA is synthesized predominantly by reactive astrocytes as a high molecular mass (>1 MDa) molecule. It accumulates in demyelinating lesions from patients with multiple sclerosis, in mice with the inflammatory demyelinating disease experimental autoimmune encephalomyelitis (EAE) and in traumatic spinal cord injuries (Back et al. 2005; Struve et al. 2005; Sherman et al. 2015), coincident with elevated expression of CD44. Overexpression of CD44 by OPCs leads to HA accumulation and dysmyelination (Tuohy et al. 2004; Back et al. 2005), while the addition of HA to lysolecithin‐demyelinated corpus callosum lesions inhibits remyelination by promoting the accumulation of OPCs that fail to become myelin basic protein‐positive (MBP+) oligodendrocytes. The administration of HA to OPC cultures reversibly inhibits their maturation.
These findings supported the hypothesis that high molecular mass HA itself prevents remyelination by inhibiting OPC maturation, and that the interaction of HA with oligodendrocytes is a potential therapeutic approach for myelination failure (Back et al. 2005). However, OPCs express multiple hyaluronidases (Sloane et al. 2010; Preston et al. 2013), some of which are transiently induced in white matter lesions following preterm ischaemic white matter injury (Hagen et al. 2014), and in reactive astrocytes and OPCs in demyelinating EAE and MS patient lesions (Preston et al. 2013). Elevated expression of hyaluronidases by OPCs blocks OPC maturation. Furthermore, digestion products of HA are sufficient to inhibit OPC maturation and remyelination (Preston et al. 2013). Pharmacological inhibition of hyaluronidase activity is sufficient to promote OPC maturation and remyelination (Sloane et al. 2010; Preston et al. 2013) and increased conduction velocities through demyelinating lesions (Preston et al. 2013).
Together, these findings support a model in which preterm white matter injury results in increased astrogliosis, accumulation of high molecular mass HA and the concomitant induction of hyaluronidase activity. The resulting HA digestion products then act to inhibit OPC maturation and prevent or delay myelination. This effect is mediated by TLR4 expressed by OPCs (Srivastava et al. 2018). Nonetheless, the finding that a hyaluronidase inhibitor promotes OPC maturation and functional remyelination in these clinically relevant models of white matter injury (Sloane et al. 2010; Preston et al. 2013) suggests that blocking the activities of specific hyaluronidases could be an effective means of promoting myelination in neonates that experience preterm or perinatal white matter injury.
Thyroid hormone signalling as a postnatal therapy for myelination deficits in FGR
Thyroid hormones (T3, triiodothyronine; T4, thyroxine) are integral to brain development and, in particular, for oligodendrocyte differentiation, maturation (Barres et al. 1994), expression of myelin‐specific genes (Younes‐Rapozo et al. 2006) and eventually myelin production by mature oligodendrocytes (Barres et al. 1994; Younes‐Rapozo et al. 2006). In the brain, the pro‐hormone T4 is de‐iodinated to T3, which then binds to nuclear thyroid hormone receptors TRα and TRβ to regulate the expression of key myelin protein genes such as MBP. However, for thyroid hormone to reach the nuclear TRα/β receptors, T3 and T4 must first be transported across the cell membrane by a thyroid hormone (monocarboxylate) transporter such as MCT8 or MCT10. Children with a congenital MCT8 mutation show severe and persistent myelination delay (Gika et al. 2010; Lopez‐Espindola et al. 2014), despite the presence of high serum T3 (Gika et al. 2010).
Quantification of MCT8 (Chan et al. 2014) and all isoforms of the thyroid hormone receptor (Kilby et al. 2000) by immunostaining shows that both are significantly reduced in post‐mortem brain tissue from FGR babies compared to babies appropriately grown for gestational age. Given that thyroid hormone can only be effective if functional and sufficient MCT8 is present, T3 or T4 therapy is a relatively ineffective treatment for the FGR brain. For example, thyroid hormone supplementation has limited success in improving white matter injury in animal models of perinatal hypoxia–ischaemia (Hung et al. 2013), or in preterm infants with intraventricular haemorrhage (Vose et al. 2013). Nor does thyroid hormone treatment improve growth or neurological outcomes in children born preterm (Chowdhry et al. 1984), nor does it always promote myelin recovery in rodent models of inflammation‐induced white matter injury (Schang et al. 2014). The ineffectiveness of thyroid hormone treatment in these cases is understandable if the cellular uptake of thyroid hormone is impaired. The MCT8 deficit found in the brains of FGR babies at post‐mortem is also replicated in at least one small animal model of induced FGR (rat), giving sufficient evidence to warrant further investigation of the limited ability of endogenous thyroid hormone to initiate the transcription of myelin genes to produce myelin proteins in oligodendrocytes, accounting for the impaired maturation and delay in myelination reported in FGR (Olivier et al. 2005; Tolcos et al. 2011; Reid et al. 2012). Thus, as discussed in detail elsewhere (Tolcos et al. 2017), there is a need to identify candidate compounds that mimic the ability of T3 to promote oligodendrocyte maturation and myelination, but which do not require MCT8 for cellular uptake, and such compounds could have a major therapeutic impact in the treatment of white matter injury in general, but in the neonate in particular.
Neurosteroids
The placenta produces large amounts of progesterone during pregnancy, much of which is metabolized to precursors for the production of neurosteroids in the fetal brain. The 5α‐reduced metabolites of progesterone, including allopregnanolone, have neuroprotective effects because of agonist actions at the GABAA receptor (Nguyen et al. 2003; Hirst et al. 2008, 2014). Allopregnanolone levels are higher in the fetal brain than at any other time of life, and decline markedly at birth whether this occurs at term or preterm (Kelleher et al. 2013). Because these neurosteroids also have growth stimulating, ‘trophic’ effects on GABA networks (Hirst et al. 2014), preterm birth carries with it not only the loss of a steroid‐mediated neuroprotection, but also the loss of an essential neuro‐hormone that promotes brain development in utero. It has been suggested that the reduced development of GABAergic pathways associated with fetal hypoxia, FGR, infection and even maternal psychosocial stress (Mitchell et al. 2008; Elgen et al. 2015) arises not only from direct effects on GABAergic networks but also from disruption of the supply of essential neurosteroid precursors from the placenta. In the case of preterm birth, the early loss of these precursors of placental origin compounds all of these effects.
Neurosteroids promote myelination by stimulating maturation of pre‐oligodendrocytes, and upregulating myelin production by mature oligodendrocytes (Gago et al. 2001, 2004). For example, in pregnant guinea pigs the 5α‐reductase inhibitor finasteride was used to reduce allopregnanolone levels over the last 8 days of gestation. This treatment caused a marked reduction in myelination in the CA1 region of the hippocampus and cerebellum at term (Cumberland et al. 2017). Thus, we have proposed that loss of neurosteroids is one of the reasons that preterm birth is so disruptive for brain development, and as a consequence, replacement of neurosteroids after preterm birth should ameliorate the long‐term harm caused by neurosteroid deficiency in the period from the inappropriately early birth to term‐age equivalence (Hirst et al. 2014). Indeed, it can be shown that treating prematurely delivered guinea pig neonates with progesterone from birth until term equivalent age markedly increases allopregnanolone and progesterone concentrations in plasma and the brain at term equivalent age (Palliser et al. 2015). However, plasma cortisol levels were also markedly increased by the progesterone treatment (Palliser et al. 2015), and as cortisol may have negative effects on development, this finding suggests treatment with neurosteroid analogues less likely to raise cortisol might be a more sensible approach. It was previously shown that the allopregnanolone analogue alfaxalone is neuroprotective in the fetus, but its short half‐life renders it unsuitable as a neonatal therapy (Yawno et al. 2009). In contrast, ganaxolone is a synthetic, GABAA receptor agonist neurosteroid that has a longer half‐life and is thus likely to be effective in replacing the loss of brain neurosteroids following preterm birth. An alternative approach may be the stimulation of the mitochondrial translocator protein (TSPO), which is located in the inner mitochondrial membrane and mediates the metabolism of cholesterol to pregnenolone. The TSPO ligand emapunil (XBD173) increases pregnenolone synthesis and thus provides increased substrate for the downstream neurosteroids such as allopregnanolone (Rupprecht et al. 2010). Treatment with this agent during the period between preterm delivery and normal term may raise steroid synthesis in the neonatal brain and replace neurosteroid‐induced stimulation of myelination that is disrupted with the premature loss of placental support for neurosteroid synthesis. In preliminary studies we found have that emapunil treatment of neonates that had been exposed to stress in the perinatal period reversed stress‐induces changes in GABAA receptor pathways (Crombie et al. 2017).
Conclusions
We have discussed prospects for antenatal and very early post‐birth treatments that could dramatically decrease, and perhaps even eliminate, the brain damage arising from acute fetal hypoxia and infection at term, or from chronic fetal hypoxia due to placental insufficiency and intrauterine infection leading to FGR, or from preterm birth. The manipulation of neurosteroids, thyroid status and hyaluronidases may each provide options for treatments that prevent devastating injury to white matter in the perinatal brain.
Several of the experimental approaches we discuss have been evaluated in non‐human primates and shown to be effective (macrolide antibiotics, creatine). And many of the potential therapies have been evaluated with respect to their effectiveness in ameliorating or even preventing the onset of abnormal postnatal behaviours (melatonin, creatine, neurosteroids), showing that early treatment can have enduring and curative effects.
It should also be noted that the treatments we have discussed are either inexpensive dietary interventions (e.g. creatine), or simple treatments that effectively decrease oxidative stress (e.g. allopurinol, melatonin). This illustrates the suitability of these approaches for use in environments other than tertiary level hospital care – for example, underdeveloped and developing countries – where the burden of intrapartum and postpartum brain damage and cerebral palsy is the greatest. In developed countries CP is a defined set of phenotypes that has provoked substantial investigation into cost–benefit analyses in the health system, and engendered significant investment into the treatment of complications arising from CP (i.e. learning disabilities, vision, hearing and speech impairments). In underdeveloped countries CP does not attract this support, and the need for the approach we outline here is of even greater importance. In these settings, the priority may be on treatments that deal with damage that has already occurred, such as with neurosteroids, hyaluronidase inhibitors, or analogues of thyroid hormone as discussed above. Thus, while the burden of perinatal brain injury remains high in all medical settings, and the priorities will differ between the ‘have’ and ‘have not’ economies, on the basis of new understandings of the mechanistic pathways that lead to perinatal brain damage, there has been excellent progress towards finding targeted therapies that could even prove the impossible to be possible – preventing cerebral palsy.
Additional information
Competing interests
All authors declare there are no competing interests.
Author contributions
This review and opinion piece is based on presentations given by each of the authors at a symposium at the 5th International Conference of Cerebral Palsy, held in Stockholm, 1–4 June 2016. S.E. and D.W. contributed the section on creatine; M.K., P.G. and I.B. contributed the section Infection and inflammation; J.D. and S.L.M. contributed the section on hypoxia and oxidative stress; J.J.H. and D.W. contributed the section on neurosteroids; M.T. and L.S. contributed the section on oligodendrocytes and thyroid hormone. All authors contributed to the overall structure of the review, and were involved in all revisions of the manuscript. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Acknowledgements
The authors acknowledge their various funding agencies and institutions for support in the primary studies that provide the material that is drawn from their primary publications that is discussed in this review.
Biography
Top row: Stacey Ellery, Meredith Kelleher, Peta Grigsby, Irina Burd and Jan B Derks. Bottom row: Jon J Hirst, Suzie Miller, Larry S Sherman, Mary Tolcos and David W. Walker. This review arose from papers presented by the authors at a minisymposium entitled ‘Prevention of Cerebral Palsy and Childhood Disability – Is the Impossible Possible?’, presented at the 5th International Conference on Cerebral Palsy and other Childhood‐onset Disabilities in Stockholm, 1–4 June 2016. Considerable discussion took place from then until the date of publication to which all of the authors contributed. Rather than undertaking a formal systematic review of the existing literature in this important area of perinatal research, we have opted to present the ideas arising from our own biomedical research projects in order to emphasize the prospects for further development of these ideas into clinical practice. In this regard, all authors contributed equally to this work.

Edited by: Ole Petersen & David Wyllie
References
- Acosta EP, Grigsby PL, Larson KB, James AM, Long MC, Duffy LB, Waites KB & Novy MJ (2014). Transplacental transfer of Azithromycin and its use for eradicating intra‐amniotic ureaplasma infection in a primate model. J Infect Dis 209, 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altura B, Altura B, Carella A, Gebrewold A, Murakawa T & Nishio A (1987). Mg2+–Ca2+ interaction in contractility of vascular smooth muscle: Mg2+ versus organic calcium channel blockers on myogenic tone and agonist‐induced responsiveness of blood vessels. Can J Physiol Pharmacol 65, 729–745. [DOI] [PubMed] [Google Scholar]
- Antonucci R, Porcella A & Pilloni MD (2014). Perinatal asphyxia in the term newborn. J Pediatr Neonat Individual Med 3, e030269. [Google Scholar]
- Back SA ( 2006). Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment Retard Dev Disabil Res Rev 12, 129–140. [DOI] [PubMed] [Google Scholar]
- Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, Luo NL, Banine F, Liu Y, Chang A, Trapp BD, Bebo BF Jr, Rao MS & Sherman LS (2005). Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med 11, 966–972. [DOI] [PubMed] [Google Scholar]
- Barres BA, Lazar MA & Raff MC (1994). A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development 120, 1097–1108. [DOI] [PubMed] [Google Scholar]
- Bel F & Groenendaal F (2016). Drugs for neuroprotection after birth asphyxia: Pharmacologic adjuncts to hypothermia. Semin Perinatol 40, 152–159. [DOI] [PubMed] [Google Scholar]
- Beloosesky R, Weiner Z, Ginsberg Y & Ross MG (2012). Maternal N‐acetyl‐cysteine (NAC) protects the rat fetal brain from inflammatory cytokine responses to lipopolysaccharide (LPS). J Matern Fetal Neonatal Med 25, 1324–1328. [DOI] [PubMed] [Google Scholar]
- Berger R, Middelanis J, Vaihinger H‐M, Mies G, Wilken B & Jensen A (2004). Creatine protects the immature brain from hypoxic‐ischemic injury. J Soc Gynecol Investig 11, 9–15. [DOI] [PubMed] [Google Scholar]
- Buhimschi IA, Buhimschi CS & Weiner CP (2003). Protective effect of N‐acetylcysteine against fetal death and preterm labor induced by maternal inflammation. Am J Obstet Gynecol 188, 203–208. [DOI] [PubMed] [Google Scholar]
- Burd I, Balakrishnan B & Kannan S (2012). Models of fetal brain injury, intrauterine inflammation, and preterm birth. Am J Reprod Immunol 67, 287–294. [DOI] [PubMed] [Google Scholar]
- Burd I, Bentz AI, Chai J, Gonzalez J, Monnerie H, Le Roux PD, Cohen AS, Yudkoff M & Elovitz MA (2010). Inflammation‐induced preterm birth alters neuronal morphology in the mouse fetal brain. J Neurosci Res 88, 1872–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd I, Zhang F, Dada T, Mishra MK, Borbiev T, Lesniak WG, Baghlaf H, Kannan S & Kannan RM (2014). Fetal uptake of intra‐amniotically delivered dendrimers in a mouse model of intrauterine inflammation and preterm birth. Nanomed Nanotechnol Biol Med 10, 1343–1351. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Fowden AL & Thornburg KL (2016). Placental origins of chronic disease. Physiol Rev 96, 1509–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser JR, Maire J, Riddle A, Gong X, Nguyen T, Nelson K, Luo NL, Ren J, Struve J, Sherman LS, Miller SP, Chau V, Hendson G, Ballabh P, Grafe MR & Back SA (2012). Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol 71, 93–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Businelli C, de Wit C, Visser GH & Pistorius LR (2015). Ultrasound evaluation of cortical brain development in fetuses with intrauterine growth restriction. J Matern Fetal Neonatal Med 28, 1302–1307. [DOI] [PubMed] [Google Scholar]
- Cannata DJ, Ireland Z, Dickinson H, Snow RJ, Russell AP, West JM & Walker DW (2010). Maternal creatine supplementation from mid‐pregnancy protects the diaphragm of the newborn spiny mouse from intrapartum hypoxia‐induced damage. Pediatr Res 68, 393–398. [DOI] [PubMed] [Google Scholar]
- Castillo‐Melendez M, Yawno T, Allison BJ, Jenkin G, Wallace EM & Miller SL (2015). Cerebrovascular adaptations to chronic hypoxia in the growth restricted lamb. Int J Dev Neurosci 45, 55–65. [DOI] [PubMed] [Google Scholar]
- Chan SY, Hancox LA, Martin‐Santos A, Loubiere LS, Walter MN, Gonzalez AM, Cox PM, Logan A, McCabe CJ, Franklyn JA & Kilby MD (2014). MCT8 expression in human fetal cerebral cortex is reduced in severe intrauterine growth restriction. J Endocrinol 220, 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhry P, Scanlon JW, Auerbach R & Abbassi V (1984). Results of controlled double‐blind study of thyroid replacement in very low‐birth‐weight premature infants with hypothyroxinemia. Pediatrics 73, 301–305. [PubMed] [Google Scholar]
- Clancy RR, McGaurn SA, Goin JE, Hirtz DG, Norwood WI, Gaynor JW, Jacobs ML, Wernovsky G, Mahle WT, Murphy JD, Nicolson SC, Steven JM & Spray TL (2001). Allopurinol neurocardiac protection trial in infants undergoing heart surgery using deep hypothermic circulatory arrest. Pediatrics 108, 61–70. [DOI] [PubMed] [Google Scholar]
- Crombie G PH, J Shaw JC, Rani P Walker DW and JHirst JJ (2017). Neurosteroid replacement therapy ameliorates behavioural and GABAergic deficits following perinatal stress in male guinea pig offspring. Meeting of the Australian Society for Medical Research, Newcastle 2017, Abstract: P6.
- Crowther CA, Middleton PF, Voysey M, Askie L, Duley L, Pryde PG, Marret S, Doyle LW & Group A (2017). Assessing the neuroprotective benefits for babies of antenatal magnesium sulphate: An individual participant data meta‐analysis. PLoS Med 14, e1002398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumberland AL, Palliser HK, Walker DW & Hirst JJ (2017). Cerebellar changes in guinea pig offspring following suppression of neurosteroid synthesis during late gestation. Cerebellum 16, 306–313. [DOI] [PubMed] [Google Scholar]
- Dada T, Rosenzweig JM, Al Shammary M, Firdaus W, Al Rebh S, Borbiev T, Tekes A, Zhang J, Alqahtani E, Mori S, Pletnikov MV, Johnston MV & Burd I (2014). Mouse model of intrauterine inflammation: sex‐specific differences in long‐term neurologic and immune sequelae. Brain Behav Immun 38, 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalous J, Larghero J & Baud O (2012). Transplantation of umbilical cord‐derived mesenchymal stem cells as a novel strategy to protect the central nervous system: technical aspects, preclinical studies, and clinical perspectives. Pediatr Res 71, 482–490. [DOI] [PubMed] [Google Scholar]
- Dammann O & Leviton A (1997). Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res 42, 1–8. [DOI] [PubMed] [Google Scholar]
- Dammann O & Leviton A (2014). Intermittent or sustained systemic inflammation and the preterm brain. Pediatr Res 75, 376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dando SJ, Nitsos I, Newnham JP, Jobe AH, Moss TJ & Knox CL (2010). Maternal administration of erythromycin fails to eradicate intrauterine ureaplasma infection in an ovine model. Biol Reprod 83, 616–622. [DOI] [PubMed] [Google Scholar]
- Derks JB, Oudijk MA, Torrance HL, Rademaker CM, Benders MJ, Rosen KG, Cindrova‐Davies T, Thakor AS, Visser GH, Burton GJ, van Bel F & Giussani DA (2010). Allopurinol reduces oxidative stress in the ovine fetal cardiovascular system after repeated episodes of ischemia‐reperfusion. Pediatr Res 68, 374–380. [DOI] [PubMed] [Google Scholar]
- Dickinson H, Bain E, Wilkinson D, Middleton P, Crowther CA & Walker DW (2014). Creatine for women in pregnancy for neuroprotection of the fetus. Cochrane Database Syst Rev (12), CD010846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle LW, Crowther CA, Middleton P, Marret S & Rouse D (2009). Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev (1), CD004661. [DOI] [PubMed] [Google Scholar]
- Dubois J, Benders M, Borradori‐Tolsa C, Cachia A, Lazeyras F, Ha‐Vinh Leuchter R, Sizonenko SV, Warfield SK, Mangin JF & Huppi PS (2008). Primary cortical folding in the human newborn: an early marker of later functional development. Brain 131, 2028–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, Strohm B, Thoresen M, Whitelaw A & Azzopardi D (2010). Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta‐analysis of trial data. BMJ 340, c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgen SK, Sommerfelt K, Leversen KT & Markestad T (2015). Minor neurodevelopmental impairments are associated with increased occurrence of ADHD symptoms in children born extremely preterm. Eur Child Adolesc Psychiatry 24, 463–470. [DOI] [PubMed] [Google Scholar]
- Ellery SJ, Ireland Z, Kett MM, Snow R, Walker DW & Dickinson H (2013). Creatine pretreatment prevents birth asphyxia‐induced injury of the newborn spiny mouse kidney. Pediatr Res 73, 201–208. [DOI] [PubMed] [Google Scholar]
- Ellery SJ, LaRosa DA, Cullen‐McEwen LA, Brown RD, Snow RJ, Walker DW, Kett MM & Dickinson H (2017). Renal dysfunction in early adulthood following birth asphyxia in male spiny mice, and its amelioration by maternal creatine supplementation during pregnancy. Pediatr Res 81, 646–653. [DOI] [PubMed] [Google Scholar]
- Ferriero DM ( 2004). Neonatal brain injury. N Engl J Med 351, 1985–1995. [DOI] [PubMed] [Google Scholar]
- Gago N, Akwa Y, Sananes N, Guennoun R, Baulieu EE, El‐Etr M & Schumacher M (2001). Progesterone and the oligodendroglial lineage: stage‐dependent biosynthesis and metabolism. Glia 36, 295–308. [DOI] [PubMed] [Google Scholar]
- Gago N, El‐Etr M, Sananes N, Cadepond F, Samuel D, Avellana‐Adalid V, Baron‐Van Evercooren A & Schumacher M (2004). 3α,5α‐Tetrahydroprogesterone (allopregnanolone) and γ‐aminobutyric acid: autocrine/paracrine interactions in the control of neonatal PSA‐NCAM+ progenitor proliferation. J Neurosci Res 78, 770–783. [DOI] [PubMed] [Google Scholar]
- Galinsky R, Bennet L, Groenendaal F, Lear CA, Tan S, Van Bel F, Juul SE, Robertson NJ, Mallard C & Gunn AJ (2014). Magnesium is not consistently neuroprotective for perinatal hypoxia‐ischemia in term‐equivalent models in preclinical studies: a systematic review. Dev Neurosci 36, 73–82. [DOI] [PubMed] [Google Scholar]
- Galinsky R, Davidson JO, Drury PP, Wassink G, Lear CA, den Heuij LG, Gunn AJ & Bennet L (2016). Magnesium sulphate and cardiovascular and cerebrovascular adaptations to asphyxia in preterm fetal sheep. J Physiol 594, 1281–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gika AD, Siddiqui A, Hulse AJ, Edward S, Fallon P, McEntagart ME, Jan W, Josifova D, Lerman‐Sagie T, Drummond J, Thompson E, Refetoff S, Bonnemann CG & Jungbluth H (2010). White matter abnormalities and dystonic motor disorder associated with mutations in the SLC16A2 gene. Dev Med Child Neurol 52, 475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA (2016). The fetal brain sparing response to hypoxia: physiological mechanisms. J Physiol 594, 1215–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser K & Speer CP (2015). Neonatal CNS infection and inflammation caused by Ureaplasma species: rare or relevant? Expert Rev Anti Infect Ther 13, 233–248. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Andrews WW, Goepfert AR, Faye‐Petersen O, Cliver SP, Carlo WA & Hauth JC (2008). The Alabama Preterm Birth Study: umbilical cord blood Ureaplasma urealyticum and Mycoplasma hominis cultures in very preterm newborn infants. Am J Obstet Gynecol 198, 43.e1–43.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigsby PL, Novy MJ, Sadowsky DW, Morgan TK, Long M, Acosta E, Duffy LB & Waites KB (2012). Maternal azithromycin therapy for Ureaplasma intraamniotic infection delays preterm delivery and reduces fetal lung injury in a primate model. Am J Obstet Gynecol 207, 475.e1–475.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualano B, Roschel H, Lancha‐Jr AH, Brightbill CE & Rawson ES (2012). In sickness and in health: the widespread application of creatine supplementation. Amino Acids 43, 519–529. [DOI] [PubMed] [Google Scholar]
- Guidi C, Potenza L, Sestili P, Martinelli C, Guescini M, Stocchi L, Zeppa S, Polidori E, Annibalini G & Stocchi V (2008). Differential effect of creatine on oxidatively‐injured mitochondrial and nuclear DNA. Biochim Biophys Acta 1780, 16–26. [DOI] [PubMed] [Google Scholar]
- Guijarro‐Munoz I, Compte M, Alvarez‐Cienfuegos A, Alvarez‐Vallina L & Sanz L (2014). Lipopolysaccharide activates Toll‐like receptor 4 (TLR4)‐mediated NF‐κB signaling pathway and proinflammatory response in human pericytes. J Biol Chem 289, 2457–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes‐Ferreira L, Pinheiro CH, Gerlinger‐Romero F, Vitzel KF, Nachbar RT, Curi R & Nunes MT (2012). Short‐term creatine supplementation decreases reactive oxygen species content with no changes in expression and activity of antioxidant enzymes in skeletal muscle. Eur J Appl Physiol 112, 3905–3911. [DOI] [PubMed] [Google Scholar]
- Gussenhoven R, Ophelders D, Kemp MW, Payne MS, Spiller OB, Beeton ML, Stock SJ, Cillero‐Pastor B, Barre FPY, Heeren RMA, Kessels L, Stevens B, Rutten BP, Kallapur SG, Jobe AH, Kramer BW & Wolfs T (2017). The paradoxical effects of chronic intra‐amniotic Ureaplasma parvum exposure on ovine fetal brain development. Dev Neurosci 39, 472–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen MW, Riddle A, McClendon E, Gong X, Shaver D, Srivastava T, Dean JM, Bai JZ, Fowke TM, Gunn AJ, Jones DF, Sherman LS, Grafe MR, Hohimer AR & Back SA (2014). Role of recurrent hypoxia‐ischemia in preterm white matter injury severity. PLoS One 9, e112800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Jing Y, Zhang S, Liu Y, Shi Y & Wei L (2012). The role of immunosuppression of mesenchymal stem cells in tissue repair and tumor growth. Cell Biosci 2, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins GDV, Koen S, Gei AF, Lopez SM, Van Hook JW & Anderson GD (2002). Neonatal organ system injury in acute birth asphyxia sufficient to result in neonatal encephalopathy. Obstet Gynecol 99, 688. [DOI] [PubMed] [Google Scholar]
- Herrero C & Perez‐Simon JA (2010). Immunomodulatory effect of mesenchymal stem cells. Braz J Med Biol Res 43, 425–430. [DOI] [PubMed] [Google Scholar]
- Hirst JJ, Kelleher MA, Walker DW & Palliser HK (2014). Neuroactive steroids in pregnancy: key regulatory and protective roles in the foetal brain. J Steroid Biochem Mol Biol 139, 144–153. [DOI] [PubMed] [Google Scholar]
- Hirst JJ, Palliser HK, Yates DM, Yawno T & Walker DW (2008). Neurosteroids in the fetus and neonate: potential protective role in compromised pregnancies. Neurochem Int 52, 602–610. [DOI] [PubMed] [Google Scholar]
- Hoogduijn MJ, Popp F, Verbeek R, Masoodi M, Nicolaou A, Baan C & Dahlke MH (2010). The immunomodulatory properties of mesenchymal stem cells and their use for immunotherapy. Int Immunopharmacol 10, 1496–1500. [DOI] [PubMed] [Google Scholar]
- Hung PL, Huang CC, Huang HM, Tu DG & Chang YC (2013). Thyroxin treatment protects against white matter injury in the immature brain via brain‐derived neurotrophic factor. Stroke 44, 2275–2283. [DOI] [PubMed] [Google Scholar]
- Ireland Z, Castillo‐Melendez M, Dickinson H, Snow R & Walker D (2011). A maternal diet supplemented with creatine from mid‐pregnancy protects the newborn spiny mouse brain from birth hypoxia. Neuroscience 194, 372–379. [DOI] [PubMed] [Google Scholar]
- Ireland Z, Dickinson H, Snow R & Walker D (2008). Maternal creatine: does it reach the fetus and improve survival after an acute hypoxic episode in the spiny mouse (Acomys cahirinus)? Am J Obstet Gynecol 198, 431–436. [DOI] [PubMed] [Google Scholar]
- Johnson LH, Mapp DC, Rouse DJ, Spong CY, Mercer BM, Leveno KJ, Varner MW, Iams JD, Sorokin Y & Ramin SM (2012). Association of cord blood magnesium concentration and neonatal resuscitation. J Pediatr 160, 573–577.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaandorp JJ, Benders MJ, Schuit E, Rademaker CM, Oudijk MA, Porath MM, Oetomo SB, Wouters MG, van Elburg RM, Franssen MT, Bos AF, de Haan TR, Boon J, de Boer IP, Rijnders RJ, Jacobs CJ, Scheepers LH, Gavilanes DA, Bloemenkamp KW, Rijken M, van Meir CA, von Lindern JS, Huisjes AJ, Bakker SC, Mol BW, Visser GH, Van Bel F & Derks JB (2015). Maternal allopurinol administration during suspected fetal hypoxia: a novel neuroprotective intervention? A multicentre randomised placebo controlled trial. Arch Dis Child Fetal Neonatal Ed 100, F216–F223. [DOI] [PubMed] [Google Scholar]
- Kaandorp JJ, Derks JB, Oudijk MA, Torrance HL, Harmsen MG, Nikkels PG, van Bel F, Visser GH & Giussani DA (2014). Antenatal allopurinol reduces hippocampal brain damage after acute birth asphyxia in late gestation fetal sheep. Reprod Sci 21, 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan R, Nance E, Kannan S & Tomalia DA (2014). Emerging concepts in dendrimer‐based nanomedicine: from design principles to clinical applications. J Intern Med 276, 579–617. [DOI] [PubMed] [Google Scholar]
- Kannan S, Dai H, Navath RS, Balakrishnan B, Jyoti A, Janisse J, Romero R & Kannan RM (2012). Dendrimer‐based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci Transl Med 4, 130ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karussis D, Kassis I, Kurkalli BG & Slavin S (2008). Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): a proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. J Neurol Sci 265, 131–135. [DOI] [PubMed] [Google Scholar]
- Keelan JA ( 2011). Pharmacological inhibition of inflammatory pathways for the prevention of preterm birth. J Reprod Immunol 88, 176–184. [DOI] [PubMed] [Google Scholar]
- Keelan JA, Kemp MW, Payne MS, Johnson D, Stock SJ, Saito M, Fernandes P & Newnham JP (2014). Maternal administration of solithromycin, a new, potent, broad‐spectrum fluoroketolide antibiotic, achieves fetal and intra‐amniotic antimicrobial protection in a pregnant sheep model. Antimicrob Agents Chemother 58, 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher MA, Hirst JJ & Palliser HK (2013). Changes in neuroactive steroid concentrations after preterm delivery in the guinea pig. Reprod Sci 20, 1365–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher MA, Liu Z, Wang X, Kroenke CD, Houser LA, Dozier BL, Martin LD, Waites KB, McEvoy C & Schelonka RL (2017). Beyond the uterine environment: a nonhuman primate model to investigate maternal‐fetal and neonatal outcomes following chronic intrauterine infection. Pediatr Res 82, 244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp MW, Miura Y, Payne MS, Watts R, Megharaj S, Jobe AH, Kallapur SG, Saito M, Spiller OB, Keelan JA & Newnham JP (2014). Repeated maternal intramuscular or intraamniotic erythromycin incompletely resolves intrauterine Ureaplasma parvum infection in a sheep model of pregnancy. Am J Obstet Gynecol 211, 134.e1–134.e9. [DOI] [PubMed] [Google Scholar]
- Kenyon S, Pike K, Jones DR, Brocklehurst P, Marlow N, Salt A & Taylor DJ (2008). Childhood outcomes after prescription of antibiotics to pregnant women with spontaneous preterm labour: 7‐year follow‐up of the ORACLE II trial. Lancet 372, 1319–1327. [DOI] [PubMed] [Google Scholar]
- Kilby MD, Gittoes N, McCabe C, Verhaeg J & Franklyn JA (2000). Expression of thyroid receptor isoforms in the human fetal central nervous system and the effects of intrauterine growth restriction. Clin Endocrinol (Oxf) 53, 469–477. [DOI] [PubMed] [Google Scholar]
- King J & Flenady V (2002). Prophylactic antibiotics for inhibiting preterm labour with intact membranes. Cochrane Database Syst Rev (4), CD000246. [DOI] [PubMed] [Google Scholar]
- Kurtoglu YE, Navath RS, Wang B, Kannan S, Romero R & Kannan RM (2009). Poly (amidoamine) dendrimer–drug conjugates with disulfide linkages for intracellular drug delivery. Biomaterials 30, 2112–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa DA, Ellery SJ, Parkington HC, Snow RJ, Walker DW & Dickinson H (2016a). Maternal creatine supplementation during pregnancy prevents long‐term changes in diaphragm muscle structure and function after birth asphyxia. PLoS One 11, e0149840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa DA, Ellery SJ, Snow RJ, Walker DW & Dickinson H (2016b). Maternal creatine supplementation during pregnancy prevents acute and long‐term deficits in skeletal muscle after birth asphyxia: a study of structure and function of hind limb muscle in the spiny mouse. Pediatr Res 80, 852–860. [DOI] [PubMed] [Google Scholar]
- Lee CC, MacKay JA, Fréchet JM & Szoka FC (2005). Designing dendrimers for biological applications. Nat Biotechnol 23, 1517. [DOI] [PubMed] [Google Scholar]
- Lei J, Firdaus W, Rosenzweig JM, Alrebh S, Bakhshwin A, Borbiev T, Fatemi A, Blakemore K, Johnston MV & Burd I (2015). Murine model: maternal administration of stem cells for prevention of prematurity. Am J Obstet Gynecol 212, 639.e1–639.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei J, Rosenzweig JM, Mishra MK, Alshehri W, Brancusi F, McLane M, Almalki A, Bahabry R, Arif H & Rozzah R (2017). Maternal dendrimer‐based therapy for inflammation‐induced preterm birth and perinatal brain injury. Sci Rep 7, 6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, Lawn JE, Cousens S, Mathers C & Black RE (2016). Global, regional, and national causes of under‐5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet North Am Ed 388, 3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo E, DelaRosa O, Mancheno‐Corvo P, Menta R, Ramirez C & Buscher D (2009). Toll‐like receptor‐mediated signaling in human adipose‐derived stem cells: implications for immunogenicity and immunosuppressive potential. Tissue Eng Part A 15, 1579–1589. [DOI] [PubMed] [Google Scholar]
- Lopez‐Espindola D, Morales‐Bastos C, Grijota‐Martinez C, Liao XH, Lev D, Sugo E, Verge CF, Refetoff S, Bernal J & Guadano‐Ferraz A (2014). Mutations of the thyroid hormone transporter MCT8 cause prenatal brain damage and persistent hypomyelination. J Clin Endocrinol Metab 99, E2799–E2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelpillai U, Ligam P, Smythe G, Wallace EM, Hirst J & Walker DW (2005). Identification of kynurenine pathway enzyme mRNAs and metabolites in human placenta: up‐regulation by inflammatory stimuli and with clinical infection. Am J Obstet Gynecol 192, 280–288. [DOI] [PubMed] [Google Scholar]
- Matthews RT, Yang L, Jenkins BG, Ferrante RJ, Rosen BR, Kaddurah‐Daouk R & Beal MF (1998). Neuroprotective effects of creatine and cyclocreatine in animal models of Huntington's disease. J Neurosci 18, 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald HM, Brocklehurst P & Gordon A (2007). Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev (1), CD000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre S, Blair E, Badawi N, Keogh J & Nelson KB (2013). Antecedents of cerebral palsy and perinatal death in term and late preterm singletons. Obstet Gynecol 122, 869–877. [DOI] [PubMed] [Google Scholar]
- Miller SL, Chai M, Loose J, Castillo‐Melendez M, Walker DW, Jenkin G & Wallace EM (2007). The effects of maternal betamethasone administration on the intrauterine growth‐restricted fetus. Endocrinology 148, 1288–1295. [DOI] [PubMed] [Google Scholar]
- Miller SL, Huppi PS & Mallard C (2016). The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J Physiol 594, 807–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Supramaniam VG, Jenkin G, Walker DW & Wallace EM (2009). Cardiovascular responses to maternal betamethasone administration in the intrauterine growth‐restricted ovine fetus. Am J Obstet Gynecol 201, 613.e1–613.e8. [DOI] [PubMed] [Google Scholar]
- Miller SL, Wallace EM & Walker DW (2012). Antioxidant therapies: a potential role in perinatal medicine. Neuroendocrinology 96, 13–23. [DOI] [PubMed] [Google Scholar]
- Miller SL, Yan EB, Castillo‐Melendez M, Jenkin G & Walker DW (2005). Melatonin provides neuroprotection in the late‐gestation fetal sheep brain in response to umbilical cord occlusion. Dev Neurosci 27, 200–210. [DOI] [PubMed] [Google Scholar]
- Miller SL, Yawno T, Alers NO, Castillo‐Melendez M, Supramaniam VG, Vanzyl N, Sabaretnam T, Loose JM, Drummond GR, Walker DW, Jenkin G & Wallace EM (2014). Antenatal antioxidant treatment with melatonin to decrease newborn neurodevelopmental deficits and brain injury caused by fetal growth restriction. J Pineal Res 56, 283–294. [DOI] [PubMed] [Google Scholar]
- Mishra OP & Delivoria‐Papadopoulos M (1999). Cellular mechanisms of hypoxic injury in the developing brain. Brain Res Bull 48, 233–238. [DOI] [PubMed] [Google Scholar]
- Mitchell EA, Herd MB, Gunn BG, Lambert JJ & Belelli D (2008). Neurosteroid modulation of GABAA receptors: molecular determinants and significance in health and disease. Neurochem Int 52, 588–595. [DOI] [PubMed] [Google Scholar]
- Miura Y, Payne MS, Keelan JA, Noe A, Carter S, Watts R, Spiller OB, Jobe AH, Kallapur SG, Saito M, Stock SJ, Newnham JP & Kemp MW (2014). Maternal intravenous treatment with either azithromycin or solithromycin clears Ureaplasma parvum from the amniotic fluid in an ovine model of intrauterine infection. Antimicrob Agents Chemother 58, 5413–5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morency AM & Bujold E (2007). The effect of second‐trimester antibiotic therapy on the rate of preterm birth. J Obstet Gynaecol Can 29, 35–44. [DOI] [PubMed] [Google Scholar]
- Murphy MP ( 2009). How mitochondria produce reactive oxygen species. Biochem J 417, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myatt L & Cui X (2004). Oxidative stress in the placenta. Histochem Cell Biol 122, 369–382. [DOI] [PubMed] [Google Scholar]
- Narasimhulu D, Brown A, Egbert N, Rojas M, Haberman S, Bhutada A, Minkoff H & Rastogi S (2017). Maternal magnesium therapy, neonatal serum magnesium concentration and immediate neonatal outcomes. J Perinatol 37, 1297. [DOI] [PubMed] [Google Scholar]
- Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA & Mezey E (2009). Bone marrow stromal cells attenuate sepsis via prostaglandin E2‐dependent reprogramming of host macrophages to increase their interleukin‐10 production. Nat Med 15, 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman RE, Yoo D, LeRoux MA & Danilkovitch‐Miagkova A (2009). Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets 8, 110–123. [DOI] [PubMed] [Google Scholar]
- Nguyen PN, Billiards SS, Walker DW & Hirst JJ (2003). Changes in 5α‐pregnane steroids and neurosteroidogenic enzyme expression in the perinatal sheep. Pediatr Res 53, 956–964. [DOI] [PubMed] [Google Scholar]
- Normann E, Lacaze‐Masmonteil T, Eaton F, Schwendimann L, Gressens P & Thebaud B (2009). A novel mouse model of Ureaplasma‐induced perinatal inflammation: effects on lung and brain injury. Pediatr Res 65, 430–436. [DOI] [PubMed] [Google Scholar]
- Novy MJ, Duffy L, Axthelm MK, Sadowsky DW, Witkin SS, Gravett MG, Cassell GH & Waites KB (2009). Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci 16, 56–70. [DOI] [PubMed] [Google Scholar]
- Ohtaki H, Ylostalo JH, Foraker JE, Robinson AP, Reger RL, Shioda S & Prockop DJ (2008). Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci U S A 105, 14638–14643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier P, Baud O, Evrard P, Gressens P & Verney C (2005). Prenatal ischemia and white matter damage in rats. J Neuropathol Exp Neurol 64, 998–1006. [DOI] [PubMed] [Google Scholar]
- Palliser HK, Kelleher MA, Tolcos M, Walker DW & Hirst JJ (2015). Effect of postnatal progesterone therapy following preterm birth on neurosteroid concentrations and cerebellar myelination in guinea pigs. J Dev Orig Health Dis 6, 350–361. [DOI] [PubMed] [Google Scholar]
- Perlman JM, Tack ED, Martin T, Shackelford G & Amon E (1989). Acute systemic organ injury in term infants after asphyxia. Am J Dis Child 143, 617–620. [DOI] [PubMed] [Google Scholar]
- Phillips AW, Johnston MV & Fatemi A (2013). The potential for cell‐based therapy in perinatal brain injuries. Transl Stroke Res 4, 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston M, Gong X, Su W, Matsumoto SG, Banine F, Winkler C, Foster S, Xing R, Struve J, Dean J, Baggenstoss B, Weigel PH, Montine TJ, Back SA & Sherman LS (2013). Digestion products of the PH20 hyaluronidase inhibit remyelination. Ann Neurol 73, 266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramenghi LA, Martinelli A, De Carli A, Brusati V, Mandia L, Fumagalli M, Triulzi F, Mosca F & Cetin I (2011). Cerebral maturation in IUGR and appropriate for gestational age preterm babies. Reprod Sci 18, 469–475. [DOI] [PubMed] [Google Scholar]
- Ramsey PS, Vaules MB, Vasdev GM, Andrews WW & Ramin KD (2003). Maternal and transplacental pharmacokinetics of azithromycin. Am J Obstet Gynecol 188, 714–718. [DOI] [PubMed] [Google Scholar]
- Reid MV, Murray KA, Marsh ED, Golden JA, Simmons RA & Grinspan JB (2012). Delayed myelination in an intrauterine growth retardation model is mediated by oxidative stress upregulating bone morphogenetic protein 4. J Neuropathol Exp Neurol 71, 640–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig JM, Lei J & Burd I (2014). Interleukin‐1 receptor blockade in perinatal brain injury. Front Pediatr 2, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, Groyer G, Adams D & Schumacher M (2010). Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov 9, 971–988. [DOI] [PubMed] [Google Scholar]
- Saikumar P & Venkatachalam MA (2003). Role of apoptosis in hypoxic/ischemic damage in the kidney. Semin Nephrol 23, 511–521. [DOI] [PubMed] [Google Scholar]
- Saugstad OD ( 1996). Role of xanthine oxidase and its inhibitor in hypoxia: reoxygenation injury. Pediatrics 98, 103–107. [PubMed] [Google Scholar]
- Schang AL, Van Steenwinckel J, Chevenne D, Alkmark M, Hagberg H, Gressens P & Fleiss B (2014). Failure of thyroid hormone treatment to prevent inflammation‐induced white matter injury in the immature brain. Brain Behav Immun 37, 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl TO & Stein TP (2001). Oxidant damage to DNA and pregnancy outcome. J Matern Fetal Med 10, 182–185. [DOI] [PubMed] [Google Scholar]
- Segovia KN, McClure M, Moravec M, Luo NL, Wan Y, Gong X, Riddle A, Craig A, Struve J & Sherman LS (2008). Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol 63, 520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestili P, Martinelli C, Bravi G, Piccoli G, Curci R, Battistelli M, Falcieri E, Agostini D, Gioacchini AM & Stocchi V (2006). Creatine supplementation affords cytoprotection in oxidatively injured cultured mammalian cells via direct antioxidant activity. Free Radic Biol Med 40, 837–849. [DOI] [PubMed] [Google Scholar]
- Shepherd E, Middleton P, Makrides M, McIntyre SJ, Badawi N & Crowther CA (2016). Antenatal and intrapartum interventions for preventing cerebral palsy: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev (8), CD012077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman LS, Matsumoto S, Su W, Srivastava T & Back SA (2015). Hyaluronan synthesis, catabolism, and signaling in neurodegenerative diseases. Int J Cell Biol 2015, 368584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KS & Sengar G (2016). A study of multiorgan dysfunction in asphyxiated neonates. In J Contemp Pediatr 3, 625–630. [Google Scholar]
- Sloane JA, Batt C, Ma Y, Harris ZM, Trapp B & Vartanian T (2010). Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc Natl Acad Sci U S A 107, 11555–11560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleymaninejadian E, Pramanik K & Samadian E (2012). Immunomodulatory properties of mesenchymal stem cells: cytokines and factors. Am J Reprod Immunol 67, 1–8. [DOI] [PubMed] [Google Scholar]
- Srivastava T, Diba P, Dean JM, Banine F, Shaver D, Hagen M, Gong X, Su W, Emery B, Marks DL, Harris EN, Baggenstoss B, Weigel PH, Sherman LS & Back SA (2018). A TLR/AKT/FoxO3 immune tolerance‐like pathway disrupts the repair capacity of oligodendrocyte progenitors. J Clin Invest 128, 2025–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetzer BP & Mercer BM (2000). Antibiotics and preterm labor. Clin Obstet Gynecol 43, 809–817. [DOI] [PubMed] [Google Scholar]
- Strunk T, Inder T, Wang X, Burgner D, Mallard C & Levy O (2014). Infection‐induced inflammation and cerebral injury in preterm infants. Lancet Infect Dis 14, 751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struve J, Maher PC, Li YQ, Kinney S, Fehlings MG, Kuntz Ct & Sherman LS (2005). Disruption of the hyaluronan‐based extracellular matrix in spinal cord promotes astrocyte proliferation. Glia 52, 16–24. [DOI] [PubMed] [Google Scholar]
- Sugimoto J, Romani AM, Valentin‐Torres AM, Luciano AA, Kitchen CMR, Funderburg N, Mesiano S & Bernstein HB (2012). Magnesium decreases inflammatory cytokine production: a novel innate immunomodulatory mechanism. J Immunol 188, 6338–6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Zhang X, Ito K, Li Y, Montgomery RA, Tachibana S & Williams GM (2008). Amelioration of oxidative mitochondrial DNA damage and deletion after renal ischemic injury by the KATP channel opener diazoxide. Am J Physiol Renal Physiol 294, F491–F498. [DOI] [PubMed] [Google Scholar]
- Supramaniam VG, Jenkin G, Loose J, Wallace EM & Miller SL (2006). Chronic fetal hypoxia increases activin A concentrations in the late‐pregnant sheep. BJOG 113, 102–109. [DOI] [PubMed] [Google Scholar]
- Swadpanich U, Lumbiganon P, Prasertcharoensook W & Laopaiboon M (2008). Antenatal lower genital tract infection screening and treatment programs for preventing preterm delivery. Cochrane Database Syst Rev (2), CD006178. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Nikaido T, Toki T, Kita N, Kanai M, Ashida T, Ohira S & Konishi I (2004). Levels of oxidative stress and redox‐related molecules in the placenta in preeclampsia and fetal growth restriction. Virchows Arch 444, 49–55. [DOI] [PubMed] [Google Scholar]
- Tamura H, Nakamura Y, Terron MP, Flores LJ, Manchester LC, Tan DX, Sugino N & Reiter RJ (2008). Melatonin and pregnancy in the human. Reprod Toxicol 25, 291–303. [DOI] [PubMed] [Google Scholar]
- Tare M, Parkington HC, Wallace EM, Sutherland AE, Lim R, Yawno T, Coleman HA, Jenkin G & Miller SL (2014). Maternal melatonin administration mitigates coronary stiffness and endothelial dysfunction, and improves heart resilience to insult in growth restricted lambs. J Physiol 592, 2695–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakor AS, Herrera EA, Seron‐Ferre M & Giussani DA (2010). Melatonin and vitamin C increase umbilical blood flow via nitric oxide‐dependent mechanisms. J Pineal Res 49, 399–406. [DOI] [PubMed] [Google Scholar]
- Titomanlio L, Kavelaars A, Dalous J, Mani S, El Ghouzzi V, Heijnen C, Baud O & Gressens P (2011). Stem cell therapy for neonatal brain injury: perspectives and challenges. Ann Neurol 70, 698–712. [DOI] [PubMed] [Google Scholar]
- Tolcos M, Bateman E, O'Dowd R, Markwick R, Vrijsen K, Rehn A & Rees S (2011). Intrauterine growth restriction affects the maturation of myelin. Exp Neurol 232, 53–65. [DOI] [PubMed] [Google Scholar]
- Tolcos M, Petratos S, Hirst JJ, Wong F, Spencer SJ, Azhan A, Emery B & Walker DW (2017). Blocked, delayed, or obstructed: what causes poor white matter development in intrauterine growth restricted infants? Prog Neurobiol 154, 62–77. [DOI] [PubMed] [Google Scholar]
- Tuohy TM, Wallingford N, Liu Y, Chan FH, Rizvi T, Xing R, Bebo B, Rao MS & Sherman LS (2004). CD44 overexpression by oligodendrocytes: a novel mouse model of inflammation‐independent demyelination and dysmyelination. Glia 47, 335–345. [DOI] [PubMed] [Google Scholar]
- Verina T, Fatemi A, Johnston MV & Comi AM (2013). Pluripotent possibilities: human umbilical cord blood cell treatment after neonatal brain injury. Pediatr Neurol 48, 346–354. [DOI] [PubMed] [Google Scholar]
- Viscardi RM & Kallapur SG (2015). Role of Ureaplasma respiratory tract colonization in bronchopulmonary dysplasia pathogenesis: current concepts and update. Clin Perinatol 42, 719–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ (2009). Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 8, 110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vose LR, Vinukonda G, Jo S, Miry O, Diamond D, Korumilli R, Arshad A, Zia MT, Hu F, Kayton RJ, La Gamma EF, Bansal R, Bianco AC & Ballabh P (2013). Treatment with thyroxine restores myelination and clinical recovery after intraventricular hemorrhage. J Neurosci 33, 17232–17246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites KB, Schelonka RL, Xiao L, Grigsby PL & Novy MJ (2009). Congenital and opportunistic infections: Ureaplasma species and Mycoplasma hominis . Semin Fetal Neonatal Med 14, 190–199. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Wyss M, Brdiczka D, Nicolay K & Eppenberger HM (1992). Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem J 281, 21–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Cheng Q, Li L, Wang J, Xia L, Xu X & Sun Z (2012). Toll‐like receptors 2 and 4 mediate the capacity of mesenchymal stromal cells to support the proliferation and differentiation of CD34+ cells. Exp Cell Res 318, 196–206. [DOI] [PubMed] [Google Scholar]
- Wiktor H & Kankofer M (1998). Superoxide dismutase activity in normal and preeclamptic placentas. Ginekol Pol 69, 915–918. [PubMed] [Google Scholar]
- Wiktor H, Kankofer M, Schmerold I, Dadak A, Lopucki M & Niedermuller H (2004). Oxidative DNA damage in placentas from normal and pre‐eclamptic pregnancies. Virchows Arch 445, 74–78. [DOI] [PubMed] [Google Scholar]
- Wiktor H, Kankofer M, Zrubek H & Kotarski J (2000). [Glutathione peroxidase activity in normal and preeclamptic placentas]. Ginekol Pol 71, 799–803. [PubMed] [Google Scholar]
- Wilken B, Ramirez J, Probst I, Richter D & Hanefeld F (1998). Creatine protects the central respiratory network of mammals under anoxic conditions. Pediatr Res 43, 8–14. [DOI] [PubMed] [Google Scholar]
- Wyss M & Kaddurah‐Daouk R (2000). Creatine and creatinine metabolism. Physiol Rev 80, 1107–1213. [DOI] [PubMed] [Google Scholar]
- Yawno T, Hirst JJ, Castillo‐Melendez M & Walker DW (2009). Role of neurosteroids in regulating cell death and proliferation in the late gestation fetal brain. Neuroscience 163, 838–847. [DOI] [PubMed] [Google Scholar]
- Younes‐Rapozo V, Berendonk J, Savignon T, Manhaes AC & Barradas PC (2006). Thyroid hormone deficiency changes the distribution of oligodendrocyte/myelin markers during oligodendroglial differentiation in vitro. Int J Dev Neurosci 24, 445–453. [DOI] [PubMed] [Google Scholar]
- Zhu H, Meloni BP, Moore SR, Majda BT & Knuckey NW (2004). Intravenous administration of magnesium is only neuroprotective following transient global ischemia when present with post‐ischemic mild hypothermia. Brain Res 1014, 53–60. [DOI] [PubMed] [Google Scholar]


