Abstract
Brain injury in intrauterine growth restricted (IUGR) infants is a major contributing factor to morbidity and mortality worldwide. Adverse outcomes range from mild learning difficulties, to attention difficulties, neurobehavioral issues, cerebral palsy, epilepsy, and other cognitive and psychiatric disorders. While the use of medication to ameliorate neurological deficits in IUGR neonates has been identified as warranting urgent research for several years, few trials have been reported. This review summarises clinical trials focusing on brain protection in the IUGR newborn as well as therapeutic interventions trialled in animal models of IUGR. Therapeutically targeting mechanisms of brain injury in the IUGR neonate is fundamental to improving long‐term neurodevelopmental outcomes. Inflammation is a key mechanism in neonatal brain injury; and therefore an appealing target. Ibuprofen, an anti‐inflammatory drug currently used in the preterm neonate, may be a potential therapeutic candidate to treat brain injury in the IUGR neonate. To better understand the potential of ibuprofen and other therapeutic agents to be neuroprotective in the IUGR neonate, long‐term follow‐up information of neurodevelopmental outcomes must be studied. Where agents such as ibuprofen are shown to be effective, have a good safety profile and are relatively inexpensive, they can be widely adopted and lead to improved outcomes.
Keywords: inflammation, ibuprofen, growth retardation
Introduction
Intrauterine growth restriction (IUGR) is a condition where a fetus fails to achieve appropriate growth due to a suboptimal environment and is a major cause of perinatal mortality and morbidity (Regev et al. 2003; Laws et al. 2006). IUGR and small for gestational age (SGA) have been used interchangeably in medical literature; however, small differences exist between the two conditions. Small for gestational age (SGA) only considers weight at birth and is defined as a birth weight below the 10th percentile. While birth weight is taken into account, IUGR is further defined by physiological determinants and neonatal features of malnutrition and in utero growth retardation (Sharma et al. 2016). The most common cause of IUGR is placental insufficiency with restricted blood flow to the placenta resulting in an inadequate supply of nutrients and oxygen to support normal growth of the fetus (Sharma et al. 2016). Growth of the fetus in a chronic hypoxic environment affects the development of numerous organ systems including the fetal brain which is particularly vulnerable (Miller et al. 2016). Factors associated with abnormal brain development in the IUGR fetus/newborn include preterm birth, timing of placental insufficiency and whether the brain growth measured as head circumference has been compromised, onset and subsequent severity of fetal compromise, fetal cerebrovascular response and the redistribution of blood flow, and other co‐existing pregnancy complications.
Both structural and functional brain deficits are observed in IUGR neonates (reviewed in Miller et al. 2016). Clinical imaging studies of IUGR infants demonstrate alterations in grey matter and white matter volume and structure (Tolsa et al. 2004; Esteban et al. 2010; Padilla et al. 2015). Furthermore, these structural changes are still evident at 1 year of age and are associated with significant developmental disabilities (Tolsa et al. 2004; Esteban et al. 2010). Adverse neurological outcomes associated with IUGR range from mild learning difficulties, to attention difficulties, neurobehavioral issues, cerebral palsy (CP), altered sensory organ function, epilepsy, and other cognitive and psychiatric disorders (Jarvis et al. 2003; Ozanne et al. 2004; Geva et al. 2006; Freire et al. 2015). Key developmental processes are likely to underpin brain damage in IUGR infants offering the potential for targeted treatments to improve neurodevelopmental outcomes.
Brain injury in IUGR
Mechanisms of brain injury in the IUGR neonate are complex and not well understood. The IUGR fetal brain is occasionally referred to as hypoxic–ischaemic (HI) (Rees et al. 2011) but the brain may not be globally ischaemic as a differential distribution of cerebral blood flow occurs to regions of the brain over time (Hernandez‐Andrade et al. 2008). However the IUGR fetus is relatively hypoxic due to placental insufficiency, which may compromise cerebral blood perfusion and delivery of oxygen to the brain (Rees et al. 2011). These fluctuations initiate a cascade of biochemical and cellular events that result in cellular injury. Several mechanisms mediate cellular injury in the IUGR brain, including excitotoxicity, oxidative stress, blood–brain barrier (BBB) disruption, necrotic and apoptotic degeneration and neuroinflammation and its further pathways to injury (Rees et al. 2011; Miller et al. 2016). Animal studies demonstrate these events occur in utero (Rees et al. 2011; Castillo‐Melendez et al. 2015; Miller et al. 2016; Alves de Alencar Rocha et al. 2017) and evolve throughout gestation, with some recent studies demonstrating the injurious mechanisms continuing into the newborn/adolescent period (Olivier et al. 2005, 2007; Black et al. 2015; Pham et al. 2015). Altered growth processes in the brain may also have occurred in utero over some days/weeks. Growth is an energy consuming process and when brain oxygenation becomes compromised growth processes within the brain could be altered including neurogenesis, synaptogenesis and myelination. Although these alterations may occur in utero, they may be amenable to postnatal therapeutic interventions. Therefore it is critical to ascertain which mechanisms continue following birth, and for how long.
Inflammation in the IUGR brain
Neuroinflammation is emerging as one of the key mechanisms that mediates abnormal brain development in IUGR neonates (Wixey et al. 2017). Neuroinflammation encompasses a number of processes and is characterised by increased numbers of activated microglia, elevated levels of proinflammatory cytokines (such as interleukin‐1β and tumour necrosis factor‐α), decreased production of anti‐inflammatory cytokines, and astrogliosis (Cai et al. 2006; Kremlev et al. 2007; Carty et al. 2008; Leonardo et al. 2008; Huang et al. 2009; Wixey et al. 2009, 2011). Recent animal studies demonstrate increased activated microglia and astrogliosis in the IUGR brain, indicative of an inflammatory response (Olivier et al. 2005, 2007; Guo et al. 2010; Tolcos et al. 2011; Black et al. 2015; Pham et al. 2015; Wixey et al. 2017). There are also indications from these studies that neuroinflammation may be associated with neuronal and white matter injury in the IUGR brain (Olivier et al. 2007; Guo et al. 2010; Pham et al. 2015). Severe inflammation (microglial activation and astrogliosis) in the brain is evident and closely aligns with a delay in myelination and white matter lesions in rat models of IUGR (Olivier et al. 2007; Pham et al. 2015). In guinea‐pig models of IUGR, increases in interleukin‐1β (IL‐1β) and tumour necrosis factor‐α (TNF‐α) in the fetal brain correlate with worsening brain injury (Guo et al. 2010).
While there is evidence of a direct inflammatory response in the brain of IUGR neonates, the role of systemic inflammation in mediating brain injury is also gaining attention. In a recent human study, systemic inflammation, as demonstrated by elevated concentrations of proinflammatory cytokines in blood, is associated with abnormal neurodevelopment and poorer neurological outcomes in IUGR neonates (McElrath et al. 2010). Under normal conditions the brain is protected from harmful systemic toxins by the BBB. Alterations in BBB composition have been reported in IUGR neonates (Castillo‐Melendez et al. 2015, 2017). Decreased BBB integrity may result in an increased infiltration of systemic inflammatory mediators into the brain, further exacerbating the neuroinflammatory response. Likewise, BBB breakdown may facilitate the passage of brain‐derived inflammatory cells into the blood resulting in heightened systemic responses.
It is unclear whether neuroinflammation is a direct cause of brain injury in the IUGR fetus/newborn or is a secondary response to cellular injury. Whilst much of the focus is on the detrimental effects of proinflammatory cytokines, targeting the activation of anti‐inflammatory cytokines may play a role in protecting the brain. Examination of both pro‐ and anti‐inflammatory cytokine responses in the IUGR newborn is important, as both pathways could be exploited to minimise injury to the brain.
Detection of brain injury in the growth restricted newborn
A number of tools currently available may predict long‐term neurodevelopmental outcomes in IUGR infants following birth (see Malhotra et al. 2013 for further details). Gross measures such as head circumference are a good predictor of outcome, with small head circumference correlating with poor neurodevelopmental outcome (Gale et al. 2006). Advanced neuroimaging provides the opportunity to identify and monitor the progression of brain injury in IUGR newborns. The use of non‐invasive in vivo techniques, such as magnetic resonance imaging (MRI) and electroencephalography (EEG) hold promise in characterising structural and functional aspects of myelination and connectivity in relation to injury progression, neuroplasticity and repair. Diffusion based MRI techniques such as diffusion kurtosis imaging (DKI) and neurite orientation dispersion and density imaging (NODDI) will enable more detailed and specific evaluation of white matter microstructure and integrity as well as cortical complexity.
Analysis of umbilical vein blood using nuclear magnetic resonance spectroscopy (MRS) has shown metabolite changes in IUGR infants that may be indicative of brain injury (Sanz‐Cortes et al. 2013). The presence of proteins such as S‐100β (from astrocytes) or neuron‐specific enolase (NSE; from neurons) are considered as evidence that these cells have been damaged, and the proteins are released into the blood through increased permeability of the BBB. Elevated cord blood levels of S100β and NSE show a relationship with neonatal complications such as mortality and necrotising enterocolitis (NEC) in IUGR newborns (Velipasaoglu et al. 2015). While promising, the search for reliable blood biomarkers of brain injury remains ongoing.
Brain injury that follows IUGR is a major cause of morbidity and mortality worldwide but despite this, pharmacological interventions have been insufficiently studied. Antenatal treatment is likely to be beneficial, but the majority of IUGR babies are first diagnosed at or around birth (Sovio et al. 2015). The developing brain exhibits plasticity and the potential for regeneration (neurogenesis) following injury. Studies in rodents have shown neurogenesis occurs after stressors or injuries such as seizures, stroke or HI (Kadam et al. 2008; Yoo et al. 2008; Scharfman & McCloskey, 2009). Even though neurogenesis may be preserved or even increased after an insult, the immature brain has limited capacity to fully regenerate alone (i.e. without treatment) after injury (Donega et al. 2013). Postnatal treatments have been shown to induce neuronal regeneration in neonatal HI models (reviewed in Donega et al. 2013); therefore it is important to detect growth restriction early to maximise the chance an intervention will have to prevent long‐term adverse neurological outcomes.
Treatments in clinical trials for the IUGR fetus and newborn
The importance of timing of delivery, mode of delivery, avoidance of additional HI insult during labour, and minimizing fetal/neonatal inflammation is crucial to the health of the IUGR infant, as all of these factors could exacerbate brain injury. Management options for the IUGR infant are limited to postnatal strategies to reduce neurological deficits and include the Newborn Individualised Developmental and Assessment Program (NIDCAP) (Als et al. 2011, 2012; McAnulty et al. 2017), but evidence of efficacy is not strong. Other management options are limited. No treatments are currently available to reduce brain injury in the IUGR fetus or newborn, but a number of clinical trials examining the protective effects of therapeutic interventions are underway.
Clinical trials
Most of the current clinical trials are focused towards the improvement in overall growth of IUGR babies. Primary outcomes for clinical trials examining administration of sildenafil citrate, heparin, L‐arginine, omega 3, aspirin and diet modification are an increase in fetal weight and/or healthy survival; however, very few of these studies have investigated the effects of these interventions on brain outcomes. Of the few clinical trials to examine neurological outcomes most are as secondary outcome measures.
Antenatal treatments
The STRIDER studies are multiple international clinical trials of sildenafil citrate administration to pregnant mothers in order to improve uteroplacental blood flow. Recent studies have identified that sildenafil citrate results in vasodilatation of small myometrial vessels with associated improvements in amniotic fluid index, uterine and umbilical artery Doppler patterns and fetal weight. A study of 100 pregnant women administered sildenafil citrate showed improvement in perinatal outcome and no long‐term adverse effects on the mother or baby as followed up at 3 years (Premalatha et al. 2016). The majority of STRIDER clinical trials do not examine neurological outcomes; with the exception of two studies currently recruiting. A STRIDER trial (NCT02277132) recruiting through Universiteit van Amsterdam aims to examine age adequate performance on the 2 year Bayley Scale of Infant and Toddler Development (BSITD‐III). Another STRIDER trial recruiting through University of British Columbia (NCT02442492) will examine intact survival without evidence of severe central nervous system injury by either ultrasound or MRI. However, modifications to these trials (or potentially cessation) may be imminent with the recently published outcomes from a STRIDER study in the United Kingdom reporting that sildenafil treatment did not improve survival nor reduce short‐term neonatal morbidity; the authors do not recommend prescription of sildenafil for treatment of IUGR (Sharp et al. 2018).
The EVERREST Project (doEs Vascular endothelial growth factor gene therapy safEly impRove outcome in seveRe Early‐onset fetal growth reSTriction?) (NCT02097667; currently recruiting) is a multicentre clinical trial investigating a treatment designed to increase fetal growth in severe early onset fetal growth restriction. Using an adenoviral vector, maternal vascular endothelial growth factor (VEGF) factor therapy will be delivered to the pregnant mother's uterine arteries to increase uterine artery blood flow and thus fetal growth. Primary outcomes include growth and neurodevelopment at 2 years of age (Spencer et al. 2017). The detailed neurodevelopmental follow‐up plan includes: Prechtl video assessment at 3 and 6 months, Hammersmith assessment at 12 and 24 months, BSITD‐III at 12 and 24 months, Gross Motor Function Classification Systems (GMFCS) and Manual Ability Classification System (MACS) at 24 months.
A clinical trial titled ‘Melatonin to prevent brain injury in unborn growth restricted babies’ (NCT01695070; completed) came about from favourable results from animal studies (Miller et al. 2014). Even though the running title of the clinical trial mentions the prevention of brain injury, the main aim of the study was the examination of oxidative stress (from umbilical artery). However, one of the secondary outcome measures was to determine abnormal neurological assessment, though specific details are lacking on the exact measures. In the published study protocol for this clinical trial (Alers et al. 2013), the authors aim for long‐term follow‐up using neurodevelopmental examination, questionnaires and MRI, but note this will depend on funding.
Postnatal treatments
A multicentre randomised controlled trial (RCT) (NCT02999945; not yet recruiting) examining nutritional management with enhanced nutrients given fortified breastmilk or formula for 3 months to infants who are SGA or IUGR is aiming to evaluate the difference in metabolic profiles of these groups. Data from discharge to 3 months will include long‐term neurodevelopmental outcomes using the BSITD‐III at 24 months, body composition, metabolic programming, metabolomics and the microbiome.
Erythropoietin (Epo) has been trialled as a neuroprotectant in extremely low birth weight infants (ELBW, ≤ 1000 g birth weight) (Juul et al. 2008; Neubauer et al. 2010) (these studies have not yet stratified for IUGR). One study found Epo did not cause excess morbidity or mortality in ELBW infants (Juul et al. 2008) and the authors aim to proceed with clinical trials. Only a limited number of ELBW infants have been followed up for long‐term neurodevelopmental outcomes but to date there is no indication that Epo has a neuroprotective effect or improves neurodevelopmental outcomes at 18–22 months’ corrected age (Ohls et al. 2004; Bierer et al. 2006). In contrast, a study of 200 ELBW children assessed at 10–13 years of age with an intelligence quotient score demonstrated a neuroprotective effect of Epo treatment administered in the first week of life (Neubauer et al. 2010). The variable dosage and timing of Epo treatment may result in these discrepant results. While neuroprotective effects may be apparent, studies in preterm infants demonstrate adverse outcomes of Epo administration such as an increase in rate of retinopathy of prematurity (ROP) (Ohlsson & Aher, 2012). This Cochrane review concluded from the limited clinical benefits and the increase in ROP that Epo administration is not recommended to preterm or LBW newborns (Ohlsson & Aher, 2012).
Treatments in animal models of IUGR
The use of animal studies allows for detailed examination of neurological outcomes following both maternal antenatal treatment (Liu et al. 2011, 2013, 2012; Roman et al. 2013; Miller et al. 2014; Liu et al. 2015a, b ; Castillo‐Melendez et al. 2017; Wang et al. 2017), as well as postnatal therapies (Mazur et al. 2013; Pham et al. 2015) (Fig. 1). The direct effect of several treatments, including those already in clinical trials on neurodevelopment have been studied in detail in IUGR animal models with encouraging results.
Figure 1.
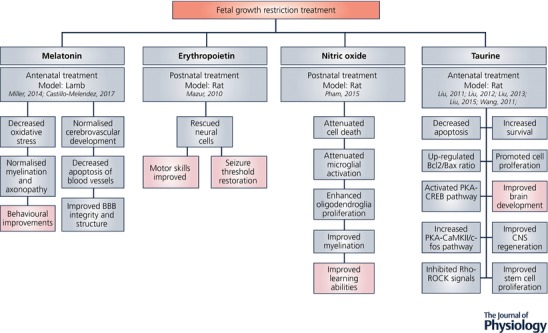
Mechanisms and behavioural outcomes of treatments trialled in IUGR animal models
Pham et al. (2015) demonstrated in antenatal hypoxia‐induced IUGR rats that 7 days of low dose (5 ppm) inhaled nitric oxide during the first postnatal week significantly attenuated cell death and microglial activation and improved myelination and cognitive function (Pham et al. 2015). Inhaled nitric oxide was associated with upregulation of P27kip1, which initiates oligodendrocyte differentiation.
Epo is known to exert anti‐inflammatory effects in the neonatal brain (Feng, 2006) and to reduce inflammatory cytokine production in term and preterm infants (Strunk et al. 2008). In the neonatal rat placental insufficiency model, Epo was administered postnatally at three different dosages: low (500 IU kg−1 for 1 day), moderate (1000 IU kg−1 for 3 days), and high (2000 IU kg−1 for 5 days). Only the high‐dose regimen was used for adult neurological outcomes. Although inflammatory markers were not directly examined in this study, adult rats showed enhanced oligodendrocyte and neuronal survival, as well as histological improvement (Mazur et al. 2013). However, as mentioned previously, proceeding to clinical trials should be considered with caution due to potential adverse effects in preterm infants treated with Epo.
Melatonin is an anti‐oxidant which also has anti‐inflammatory properties. Melatonin is effective in reducing maternal LPS‐induced neonatal brain inflammation and related brain injury (Carloni et al. 2016). Preclinical studies of maternal antenatal melatonin administration (intravenous infusion of 0.1 mg kg day−1 from 0.7 gestation until term) in pregnant sheep demonstrated an amelioration of oxidative stress, recovery of disrupted white matter tracts and axonal damage in newborn lambs 24 h after delivery (Miller et al. 2014). In the same model melatonin administration reduced BBB disruption by protecting the perivascular cells which are important for maintenance and stability of the neurovascular unit (Castillo‐Melendez et al. 2017). This study also showed a disruption to astrocyte endfeet attachment in the white matter of the IUGR lambs, which was normalised with melatonin administration.
Taurine may be a potential neuroprotectant in the IUGR animal model. Taurine is one of the most abundant amino acids in the brain and plays a role in oxidative stress; however, its mechanism of neuroprotectant action is unclear (Ripps & Shen, 2012). Maternal antenatal taurine administration (300 mg kg day−1 from conception until day of birth) improved IUGR fetal brain development in the newborn rat pup, as demonstrated by increasing brain weight, reducing neuronal apoptosis and improving brain ultrastructure (Liu et al. 2011, 2013). Maternal antenatal taurine administration also promotes cell proliferation and activation of neurotrophic factors and improves neural stem cell proliferation in the newborn IUGR rat (Liu et al. 2012, 2015a, b ; Wang et al. 2017). Glial fibrillary acidic protein (GFAP)‐immunoreactive cells were found to be increased in the IUGR animals with further increases in the taurine‐treated groups (Liu et al. 2011). The authors speculated that as astrocytes perform an important protective role during brain development, the increased counts of GFAP‐immunoreactive cells may have been a compensatory beneficial mechanism that was further enhanced by taurine treatment. However, the morphology of the astrocytes was not examined. Reactive astrocytes, which are identified by short thickened processes, release detrimental proinflammatory cytokines, and thus increased counts may in fact exacerbate neuronal injury.
Even though maternal taurine treatment demonstrated favourable brain outcomes in animal studies (Liu et al. 2011, 2013, 2012, 2015a, b ; Wang et al. 2017), several clinical trials of taurine supplementation have failed to show any positive effect on neurological outcome in LBW infants (Verner et al. 2007). The trials of postnatal supplementation of taurine that have shown no benefit compared with the positive effects in animal studies of antenatal supplementation may be due to differences in route of administration or sensitivity of the brain at different stages in development.
Only one of the aforementioned treatments has progressed to clinical trials – melatonin. As melatonin exerts anti‐inflammatory effects on the neonatal brain, investigating other treatment options targeting inflammatory mechanisms could provide appealing alternatives.
Ibuprofen
Ibuprofen is an anti‐inflammatory drug that is currently safely used in the preterm neonate; it is therefore a potential therapeutic candidate to treat brain injury in the IUGR neonate. Ibuprofen is a non‐steroidal anti‐inflammatory drug (NSAID) that is used widely for its anti‐inflammatory, anti‐pyretic and analgesic properties. The molecular basis for its therapeutic action is its ability to inhibit cyclooxygenase (COX) activity and thereby block the production of prostaglandins, which themselves are neuroinflammatory mediators (Lehmann et al. 1997; Boje et al. 2003) that have been found to be elevated in IUGR infants (McElrath et al. 2010). Ibuprofen is a lipophilic compound that readily crosses the BBB following systemic administration (Parepally et al. 2006; Kokki et al. 2007). Side‐effects of ibuprofen administration to the newborn are rare, but include pulmonary hypotension, displacement of bilirubin, transient renal effects and gastrointestinal problems (Gournay et al. 2002; Tatli et al. 2004; Desfrere et al. 2005; Ambat et al. 2008). These complications particularly occur when ibuprofen is administered in high doses.
Ibuprofen and cerebral haemodynamics in the preterm brain
Ibuprofen has been shown to be safe and well tolerated in the preterm human neonate and is widely used for treatment of patent ductus arteriosus (PDA) (Ohlsson et al. 2015); with fewer adverse side‐effects than the previous therapy (indomethacin) (Chemtob et al. 1991; Varvarigou et al. 1996; Patel et al. 2000; Ohlsson et al. 2015; Kalani et al. 2016). Ibuprofen is administered in relatively low doses in the preterm neonate for PDA, typically 5–20 mg kg day−1 (Ohlsson et al. 2015). A prospective study of 96 preterm neonates showed ibuprofen administration was associated with a reduction of intraventricular haemorrhage (IVH) without any significant side‐effects like renal dysfunction, gastrointestinal bleeding or NEC (Kalani et al. 2016). One study examined cerebral haemodynamics in preterm infants with PDA and showed that ibuprofen enhanced autoregulation of cerebral blood flow, cerebral blood volume and cerebral oxygen delivery (Patel et al. 2000). Abnormalities of cerebral perfusion play an important role in the development of cerebral injury in newborn infants. Therefore ibuprofen administration, which had no adverse effects on cerebral haemodynamics in preterm babies, may represent a favourable drug choice. Studies in newborn piglets show ibuprofen administration enhances cerebral blood flow autoregulation (Chemtob et al. 1990), and protects retinal function following neonatal asphyxia (Chemtob et al. 1993). Stabilising cerebral blood flow can have many positive consequences on the IUGR neonatal brain such as stabilising cerebral oxygenation; as potentially harmful fluctuations in cerebral oxygenation can result in cellular injury.
Effects of ibuprofen on neuroinflammation in the newborn hypoxic animal model
Although the potential neuroprotective effects of ibuprofen administration have not been examined in animal models of IUGR, there is evidence from neonatal animal models of acute HI. However, we note acute HI insults at birth and chronic hypoxia in IUGR fetuses resulting in brain injury are different insults which could involve different mechanistic pathways. It is becoming increasingly apparent that systemic ibuprofen inhibits central neuroinflammation and has neuroprotective effects in animal models of neonatal HI (Carty et al. 2011; Wixey et al. 2012). Ibuprofen treatment attenuates HI‐induced increases in COX‐2 expression and proinflammatory cytokine levels in the neonatal HI rat brain (Wixey et al. 2012). Ibuprofen administration concurrently protects the white matter and neurons in this preterm rat model of HI injury (Carty et al. 2011; Wixey et al. 2012). The author postulates this is due to ibuprofen's ability to inhibit COX‐2 and downstream neuroinflammatory mediators such as prostaglandins and proinflammatory cytokines (Wixey et al. 2012). However behavioural tests were lacking in these studies. It would be beneficial to assess whether ibuprofen administration can improve neurobehavioural deficits. Though, there is an emerging role for COX‐2 inhibitors to improve behavioural and cognitive functions following neonatal HI and adult ischaemic brain injury in animal models (Candelario‐Jalil et al. 2004, 2005; Fathali et al. 2010). Ibuprofen may be a favourable candidate to reduce brain injury in the IUGR neonatal brain.
Better understanding of when neuronal and white matter injury occurs would assist with determining therapeutic interventional timing. Do we have to treat in utero or is there the opportunity to treat postnatally? Preclinical IUGR piglet studies demonstrate that brain injury occurs late in gestation and continues postnatally (Kalanjati et al. 2017). The spontaneous/naturally occurring IUGR piglet shows neuronal and white matter injury from 104 days of gestation (term = 115 days) to postnatal day 7, with no significant white matter or neuronal injury detected at 100 days of gestation. As neuroinflammation may play a major role in the progression of this brain injury, this raises the opportunity to reduce the ongoing neuronal and white matter injury by therapeutically targeting inflammation in the IUGR newborn.
Ibuprofen in clinical trials in newborns–neurological outcomes
Very few clinical trials have investigated neurological outcomes in IUGR, preterm or LBW infants following ibuprofen treatment. A study by Schmid (NCT01428180) completed in April 2016 examined PDA treatment (ibuprofen, indomethacin and ligation) effects on cerebral tissue oxygenation, but no study results have been reported. Another interesting RCT (NCT01630278) is currently recruiting for early ibuprofen treatment for PDA in premature infants with 2 year survival without cerebral palsy as a primary endpoint. A 2015 Cochrane review of 33 studies compared the effectiveness of ibuprofen administration methods and other treatment regimens for PDA, but noted the paucity of long‐term neurodevelopmental follow‐up data is of concern (Patel et al. 2000; Ohlsson et al. 2015; Cohen et al. 2017). There have only been a handful of studies reporting 18–24 months follow‐up data for ibuprofen treatment for PDA (see Table 1). However, only one of these studies reported neurodevelopmental outcomes in ibuprofen‐treated infants vs. no treatment (Bourgoin et al. 2016). In this retrospective study, ibuprofen treatment did not improve ‘non‐optimal outcome’ and there was no difference in mean developmental quotient.
Table 1.
Ibuprofen administration to newborns with patent ductus arteriosus – neurodevelopmental outcomes
| Treatment comparison | Number of participants | Study type | Time frame | Neurodevelopmental outcomes | Other outcomes |
|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
However, a recent retrospective cohort study shows a potential neuroprotective effect of ibuprofen in extremely preterm infants (EPT) (Padilla et al. 2015). This study compared MRI brain morphology in EPT infants at term and term‐born infants. While all EPT infants have significant global reduction in cortical and subcortical grey matter, the EPT infants treated with ibuprofen for PDA had preserved brain volumes compared to those who had not received ibuprofen.
Conclusion
The life‐long neurodevelopmental disabilities that occur in some IUGR infants place considerable burden on the individual, their family, the health system and societal resources. Clinical trials are underway to test interventions to improve outcomes in the IUGR newborn. It is vitally important to incorporate brain outcomes into the studies; as protecting the brain is critical to improve neurodevelopmental outcomes. The recently announced Cosgrove project (Core Outcome Set for GROwth restriction: developing Endpoints) does aim to identify both a core outcome set to be measured in future studies on pregnancies complicated by fetal growth restriction and to improve reporting on meaningful neurodevelopmental outcomes. At the moment, different studies into the prevention or treatment of fetal growth restriction measure different outcomes in different ways, which makes comparison or structured analysis problematic.
The development of therapies in fetal IUGR and neonatal brain injury has been identified as warranting urgent research for several years, but few trials have been reported. To better understand the potential of ibuprofen and other agents to be neuroprotective in the IUGR neonate, long‐term follow‐up information of neurodevelopmental outcomes must be studied. Where agents such as ibuprofen are shown to be effective, have a good safety profile and are relatively inexpensive, they can be widely adopted and lead to improved outcomes.
Additional information
Competing interests
None of the authors have any conflicts of interest.
Funding
S.T.B. is supported by Lion's Medical Research Fellowship. J.A.W. is supported by Royal Brisbane and Women's Hospital Research Grant.
Biographies
Julie Wixey is a Postdoctoral Researcher at the Perinatal Research Centre, The University of Queensland Centre for Clinical Research under the supervision of Professor Paul Colditz. She received her PhD from The University of Queensland in 2013. Her research focuses on the role of inflammation in perinatal brain injury; such as intrauterine growth restriction, hypoxia–ischaemia and preterm birth. Her goal is to develop neuroprotective therapies to reduce brain injury in these babies.

Kirat Chand is an Early Career Researcher who received his PhD from The University of Queensland in 2015. He joined the Perinatal Research Centre as a Postdoctoral Researcher in 2016 under the supervision of Dr Tracey Bjӧrkman. His research focuses on perinatal brain injury, with a particular interest in the role of neuroinflammation and its contribution in progression of injury in the newborn brain.
Edited by: Ole Petersen & Laura Bennet
References
- Alers NO, Jenkin G, Miller SL & Wallace EM (2013). Antenatal melatonin as an antioxidant in human pregnancies complicated by fetal growth restriction – a phase I pilot clinical trial: study protocol. BMJ Open 3, e004141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Als H, Duffy FH, McAnulty G, Butler SC, Lightbody L, Kosta S, Weisenfeld NI, Robertson R, Parad RB, Ringer SA, Blickman JG, Zurakowski D & Warfield SK (2012). NIDCAP improves brain function and structure in preterm infants with severe intrauterine growth restriction. J Perinatol 32, 797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Als H, Duffy FH, McAnulty GB, Fischer CB, Kosta S, Butler SC, Parad RB, Blickman JG, Zurakowski D & Ringer SA (2011). Is the Newborn Individualized Developmental Care and Assessment Program (NIDCAP) effective for preterm infants with intrauterine growth restriction? J Perinatol 31, 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves de Alencar Rocha AK, Allison BJ, Yawno T, Polglase GR, Sutherland AE, Malhotra A, Jenkin G, Castillo‐Melendez M & Miller SL (2017). Early‐ versus late‐onset fetal growth restriction differentially affects the development of the fetal sheep brain. Dev Neurosci 39, 141–155. [DOI] [PubMed] [Google Scholar]
- Ambat MT, Ostrea EM Jr & Aranda JV (2008). Effect of ibuprofen L‐lysinate on bilirubin binding to albumin as measured by saturation index and horseradish peroxidase assays. J Perinatol 28, 287–290. [DOI] [PubMed] [Google Scholar]
- Bierer R, Peceny MC, Hartenberger CH & Ohls RK (2006). Erythropoietin concentrations and neurodevelopmental outcome in preterm infants. Pediatrics 118, e635–640. [DOI] [PubMed] [Google Scholar]
- Black AM, Armstrong EA, Scott O, Juurlink BJ & Yager JY (2015). Broccoli sprout supplementation during pregnancy prevents brain injury in the newborn rat following placental insufficiency. Behav Brain Res 291, 289–298. [DOI] [PubMed] [Google Scholar]
- Boje KM, Jaworowicz D Jr & Raybon JJ (2003). Neuroinflammatory role of prostaglandins during experimental meningitis: evidence suggestive of an in vivo relationship between nitric oxide and prostaglandins. J Pharmacol Exp Ther 304, 319–325. [DOI] [PubMed] [Google Scholar]
- Bourgoin L, Cipierre C, Hauet Q, Basset H, Gournay V, Roze JC, Flamant C & Gascoin G (2016). Neurodevelopmental outcome at 2 years of age according to patent ductus arteriosus management in very preterm infants. Neonatology 109, 139–146. [DOI] [PubMed] [Google Scholar]
- Cai Z, Lin S, Fan L‐W, Pang Y & Rhodes PG (2006). Minocycline alleviates hypoxic‐ischemic injury to developing oligodendrocytes in the neonatal rat brain. Neuroscience 137, 425–435. [DOI] [PubMed] [Google Scholar]
- Candelario‐Jalil E, González‐Falcón A, García‐Cabrera M, León OS & Fiebich BL (2004). Wide therapeutic time window for nimesulide neuroprotection in a model of transient focal cerebral ischemia in the rat. Brain Res 1007, 98–108. [DOI] [PubMed] [Google Scholar]
- Candelario‐Jalil E, Mhadu NH, González‐Falcón A, García‐Cabrera M, Muñoz E, León O & Fiebich BL (2005). Effects of the cyclooxygenase‐2 inhibitor nimesulide on cerebral infarction and neurological deficits induced by permanent middle cerebral artery occlusion in the rat. J Neuroinflammation 2, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carloni S, Favrais G, Saliba E, Albertini MC, Chalon S, Longini M, Gressens P, Buonocore G & Balduini W (2016). Melatonin modulates neonatal brain inflammation through endoplasmic reticulum stress, autophagy, and miR‐34a/silent information regulator 1 pathway. J Pineal Res 61, 370–380. [DOI] [PubMed] [Google Scholar]
- Carty ML, Wixey JA, Colditz PB & Buller KM (2008). Post‐hypoxia‐ischemia minocycline treatment attenuates neuroinflammation and white matter injury in the neonatal rat; a comparison of two different dose regimens. Int J Dev Neurosci 26, 477–485. [DOI] [PubMed] [Google Scholar]
- Carty ML, Wixey JA, Reinebrant HE, Gobe G, Colditz PB & Buller KM (2011). Ibuprofen inhibits neuroinflammation and attenuates white matter damage following hypoxia–ischemia in the immature rodent brain. Brain Res 1402, 9–19. [DOI] [PubMed] [Google Scholar]
- Castillo‐Melendez M, Yawno T, Allison BJ, Jenkin G, Wallace EM & Miller SL (2015). Cerebrovascular adaptations to chronic hypoxia in the growth restricted lamb. Int J Dev Neurosci 45, 55–65. [DOI] [PubMed] [Google Scholar]
- Castillo‐Melendez M, Yawno T, Sutherland A, Jenkin G, Wallace EM & Miller SL (2017). Effects of antenatal melatonin treatment on the cerebral vasculature in an ovine model of fetal growth restriction. Dev Neurosci 39, 323–337. [DOI] [PubMed] [Google Scholar]
- Chemtob S, Beharry K, Barna T, Varma DR & Aranda JV (1991). Differences in the effects in the newborn piglet of various nonsteroidal antiinflammatory drugs on cerebral blood flow but not on cerebrovascular prostaglandins. Pediatr Res 30, 106–111. [DOI] [PubMed] [Google Scholar]
- Chemtob S, Beharry K, Rex J, Varma DR & Aranda JV (1990). Prostanoids determine the range of cerebral blood flow autoregulation of newborn piglets. Stroke 21, 777–784. [DOI] [PubMed] [Google Scholar]
- Chemtob S, Roy MS, Abran D, Fernandez H & Varma DR (1993). Prevention of postasphyxial increase in lipid peroxides and retinal function deterioration in the newborn pig by inhibition of cyclooxygenase activity and free radical generation. Pediatr Res 33, 336–340. [DOI] [PubMed] [Google Scholar]
- Cohen E, Dix L, Baerts W, Alderliesten T, Lemmers P & van Bel F (2017). Reduction in cerebral oxygenation due to patent ductus arteriosus is pronounced in small‐for‐gestational‐age neonates. Neonatology 111, 126–132. [DOI] [PubMed] [Google Scholar]
- Desfrere L, Zohar S, Morville P, Brunhes A, Chevret S, Pons G, Moriette G, Rey E & Treluyer JM (2005). Dose‐finding study of ibuprofen in patent ductus arteriosus using the continual reassessment method. J Clin Pharm Ther 30, 121–132. [DOI] [PubMed] [Google Scholar]
- Donega V, van Velthoven CT, Nijboer CH, Kavelaars A & Heijnen CJ (2013). The endogenous regenerative capacity of the damaged newborn brain: boosting neurogenesis with mesenchymal stem cell treatment. J Cereb Blood Flow Metab 33, 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban FJ, Padilla N, Sanz‐Cortes M, de Miras JR, Bargallo N, Villoslada P & Gratacos E (2010). Fractal‐dimension analysis detects cerebral changes in preterm infants with and without intrauterine growth restriction. Neuroimage 53, 1225–1232. [DOI] [PubMed] [Google Scholar]
- Fathali N, Ostrowski RP, Lekic T, Jadhav V, Tong W, Tang J & Zhang JH (2010). Cyclooxygenase‐2 inhibition provides lasting protection against neonatal hypoxic‐ischemic brain injury. Crit Care Med 38, 572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q ( 2006). Beyond erythropoiesis: the anti‐inflammatory effects of erythropoietin. Cardiovasc Res 71, 615–617. [DOI] [PubMed] [Google Scholar]
- Freire G, Shevell M & Oskoui M (2015). Cerebral palsy: phenotypes and risk factors in term singletons born small for gestational age. Eur J Paediatr Neurol 19, 218–225. [DOI] [PubMed] [Google Scholar]
- Gale CR, O'Callaghan FJ, Bredow M, Martyn CN & Avon Longitudinal Study of Parents and Children Study Team (2006). The influence of head growth in fetal life, infancy, and childhood on intelligence at the ages of 4 and 8 years. Pediatrics 118, 1486–1492. [DOI] [PubMed] [Google Scholar]
- Geva R, Eshel R, Leitner Y, Valevski AF & Harel S (2006). Neuropsychological outcome of children with intrauterine growth restriction: a 9‐year prospective study. Pediatrics 118, 91–100. [DOI] [PubMed] [Google Scholar]
- Gournay V, Savagner C, Thiriez G, Kuster A & Roze JC (2002). Pulmonary hypertension after ibuprofen prophylaxis in very preterm infants. Lancet 359, 1486–1488. [DOI] [PubMed] [Google Scholar]
- Guo R, Hou W, Dong Y, Yu Z, Stites J & Weiner CP (2010). Brain injury caused by chronic fetal hypoxemia is mediated by inflammatory cascade activation. Reprod Sci 17, 540–548. [DOI] [PubMed] [Google Scholar]
- Hernandez‐Andrade E, Figueroa‐Diesel H, Jansson T, Rangel‐Nava H & Gratacos E (2008). Changes in regional fetal cerebral blood flow perfusion in relation to hemodynamic deterioration in severely growth‐restricted fetuses. Ultrasound Obstet Gynecol 32, 71–76. [DOI] [PubMed] [Google Scholar]
- Huang Z, Liu J, Cheung PY & Chen C (2009). Long‐term cognitive impairment and myelination deficiency in a rat model of perinatal hypoxic‐ischemic brain injury. Brain Res 1301, 100–109. [DOI] [PubMed] [Google Scholar]
- Jarvis S, Glinianaia SV, Torrioli MG, Platt MJ, Miceli M, Jouk PS, Johnson A, Hutton J, Hemming K, Hagberg G, Dolk H, Chalmers J & Surveillance of Cerebral Palsy in Europe (SCPE) collaboration of European Cerebral Palsy Registers (2003). Cerebral palsy and intrauterine growth in single births: European collaborative study. Lancet 362, 1106–1111. [DOI] [PubMed] [Google Scholar]
- Juul SE, McPherson RJ, Bauer LA, Ledbetter KJ, Gleason CA & Mayock DE (2008). A phase I/II trial of high‐dose erythropoietin in extremely low birth weight infants: pharmacokinetics and safety. Pediatrics 122, 383–391. [DOI] [PubMed] [Google Scholar]
- Kadam SD, Mulholland JD, McDonald JW & Comi AM (2008). Neurogenesis and neuronal commitment following ischemia in a new mouse model for neonatal stroke. Brain Res 1208, 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani M, Shariat M, Khalesi N, Farahani Z & Ahmadi S (2016). A comparison of early ibuprofen and indomethacin administration to prevent intraventricular hemorrhage among preterm infants. Acta Med Iran 54, 788–792. [PubMed] [Google Scholar]
- Kalanjati VP, Wixey JA, Miller SM, Colditz PB & Bjorkman ST (2017). GABAA receptor expression and white matter disruption in intrauterine growth restricted piglets. Int J Dev Neurosci 59, 1–9. [DOI] [PubMed] [Google Scholar]
- Kokki H, Kumpulainen E, Lehtonen M, Laisalmi M, Heikkinen M, Savolainen J & Rautio J (2007). Cerebrospinal fluid distribution of ibuprofen after intravenous administration in children. Pediatrics 120, e1002–1008. [DOI] [PubMed] [Google Scholar]
- Kremlev SG, Roberts RL & Palmer C (2007). Minocycline modulates chemokine receptors but not interleukin‐10 mRNA expression in hypoxic‐ischemic neonatal rat brain. J Neurosci Res 85, 2450–2459. [DOI] [PubMed] [Google Scholar]
- Laws PJ, Grayson N & Sullivan EA (2006). Australia's Mothers and Babies 2004. Perinatal Statistics Series No. 18, AIHW Cat. No. PER 34. AIHW National Perinatal Statistics, Sydney. [Google Scholar]
- Lehmann JM, Lenhard JM, Oliver BB, Ringold GM & Kliewer SA (1997). Peroxisome proliferator‐activated receptors alpha and gamma are activated by indomethacin and other non‐steroidal anti‐inflammatory drugs. J Biol Chem 272, 3406–3410. [DOI] [PubMed] [Google Scholar]
- Leonardo CC, Eakin AK, Ajmo JM, Collier LA, Pennypacker KR, Strongin AY & Gottschall PE (2008). Delayed administration of a matrix metalloproteinase inhibitor limits progressive brain injury after hypoxia‐ischemia in the neonatal rat. J Neuroinflammation 5, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Liu Y, Liu J & Ma LY (2015a). Antenatal taurine improves intrauterine growth‐restricted fetal rat brain development which is associated with increasing the activity of PKA‐CaMKII/c‐fos signal pathway. Neuropediatrics 46, 299–306. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu L & Chen H (2011). Antenatal taurine supplementation for improving brain ultrastructure in fetal rats with intrauterine growth restriction. Neuroscience 181, 265–270. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu Y, Wang XF, Chen H & Yang N (2013). Antenatal taurine supplementation improves cerebral neurogenesis in fetal rats with intrauterine growth restriction through the PKA‐CREB signal pathway. Nutr Neurosci 16, 282–287. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu L, Wang XF, Teng HY & Yang N (2012). Antenatal supplementation of taurine for protection of fetal rat brain with intrauterine growth restriction from injury by reducing neuronal apoptosis. Neuropediatrics 43, 258–263. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang HW, Liu F & Wang XF (2015b). Antenatal taurine improves neuronal regeneration in fetal rats with intrauterine growth restriction by inhibiting the Rho‐ROCK signal pathway. Metab Brain Dis 30, 67–73. [DOI] [PubMed] [Google Scholar]
- McAnulty G, Duffy FH, Kosta S, Weisenfeld NI, Warfield SK, Butler SC, Alidoost M, Bernstein JH, Robertson R, Zurakowski D & Als H (2013). School‐age effects of the newborn individualized developmental care and assessment program for preterm infants with intrauterine growth restriction: preliminary findings. BMC Pediatr 13, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElrath TF, Allred EN, Van Marter L, Fichorova RN, Leviton A & ELGAN Study Investigators (2013). Perinatal systemic inflammatory responses of growth‐restricted preterm newborns. Acta Paediatr 102, e439–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra A, Ditchfield M, Fahey MC, Castillo‐Melendez M, Allison BJ, Polglase GR, Wallace EM, Hodges R, Jenkin G & Miller SL (2017). Detection and assessment of brain injury in the growth‐restricted fetus and neonate. Pediatr Res 82, 184–193. [DOI] [PubMed] [Google Scholar]
- Mazur M, Miller RH & Robinson S (2010). Postnatal erythropoietin treatment mitigates neural cell loss after systemic prenatal hypoxic‐ischemic injury. J Neurosurg Pediatr 6, 206–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Huppi PS & Mallard C (2016). The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J Physiol 594, 807–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Yawno T, Alers NO, Castillo‐Melendez M, Supramaniam VG, VanZyl N, Sabaretnam T, Loose JM, Drummond GR, Walker DW, Jenkin G & Wallace EM (2014). Antenatal antioxidant treatment with melatonin to decrease newborn neurodevelopmental deficits and brain injury caused by fetal growth restriction. J Pineal Res 56, 283–294. [DOI] [PubMed] [Google Scholar]
- Neubauer AP, Voss W, Wachtendorf M & Jungmann T (2010). Erythropoietin improves neurodevelopmental outcome of extremely preterm infants. Ann Neurol 67, 657–666. [DOI] [PubMed] [Google Scholar]
- Ohls RK, Ehrenkranz RA, Das A, Dusick AM, Yolton K, Romano E, Delaney‐Black V, Papile LA, Simon NP, Steichen JJ, Lee KG & National Institute of Child Health and Human Development Neonatal Research Network (2004). Neurodevelopmental outcome and growth at 18 to 22 months' corrected age in extremely low birth weight infants treated with early erythropoietin and iron. Pediatrics 114, 1287–1291. [DOI] [PubMed] [Google Scholar]
- Ohlsson A & Aher SM (2012). Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev CD004863. [DOI] [PubMed] [Google Scholar]
- Ohlsson A, Walia R & Shah SS (2015). Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database Syst Rev CD003481. [DOI] [PubMed] [Google Scholar]
- Olivier P, Baud O, Bouslama M, Evrard P, Gressens P & Verney C (2007). Moderate growth restriction: deleterious and protective effects on white matter damage. Neurobiol Dis 26, 253–263. [DOI] [PubMed] [Google Scholar]
- Olivier P, Baud O, Evrard P, Gressens P & Verney C (2005). Prenatal ischemia and white matter damage in rats. J Neuropathol Exp Neurol 64, 998–1006. [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Fernandez‐Twinn D & Hales CN (2004). Fetal growth and adult diseases. Semin Perinatol 28, 81–87. [DOI] [PubMed] [Google Scholar]
- Padilla N, Alexandrou G, Blennow M, Lagercrantz H & Aden U (2015). Brain growth gains and losses in extremely preterm infants at term. Cereb Cortex 25, 1897–1905. [DOI] [PubMed] [Google Scholar]
- Parepally JM, Mandula H & Smith QR (2006). Brain uptake of nonsteroidal anti‐inflammatory drugs: ibuprofen, flurbiprofen, and indomethacin. Pharm Res 23, 873–881. [DOI] [PubMed] [Google Scholar]
- Patel J, Roberts I, Azzopardi D, Hamilton P & Edwards AD (2000). Randomized double‐blind controlled trial comparing the effects of ibuprofen with indomethacin on cerebral hemodynamics in preterm infants with patent ductus arteriosus. Pediatr Res 47, 36–42. [DOI] [PubMed] [Google Scholar]
- Pham H, Duy AP, Pansiot J, Bollen B, Gallego J, Charriaut‐Marlangue C & Baud O (2015). Impact of inhaled nitric oxide on white matter damage in growth‐restricted neonatal rats. Pediatr Res 77, 563–569. [DOI] [PubMed] [Google Scholar]
- Premalatha HL, Raghupathi KMS, Srinivas DNB, Vankatesh & Laxmi K (2016). Study of effect of sildenafil citrate in pregnant women with intrauterine growth restriction/oligohydramnios. Int J Reprod Contracept Obstet Gynecol 5, 3094–3097. [Google Scholar]
- Rees S, Harding R & Walker D (2011). The biological basis of injury and neuroprotection in the fetal and neonatal brain. Int J Dev Neurosci 29, 551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev RH, Lusky A, Dolfin T, Litmanovitz I, Arnon S, Reichman B & Israel Neonatal N (2003). Excess mortality and morbidity among small‐for‐gestational‐age premature infants: a population‐based study. J Pediatr 143, 186–191. [DOI] [PubMed] [Google Scholar]
- Ripps H & Shen W (2012). Review: taurine: a “very essential” amino acid. Mol Vis 18, 2673–2686. [PMC free article] [PubMed] [Google Scholar]
- Roman A, Desai N, Rochelson B, Gupta M, Solanki M, Xue X, Chatterjee PK & Metz CN (2013). Maternal magnesium supplementation reduces intrauterine growth restriction and suppresses inflammation in a rat model. Am J Obstet Gynecol 208, 383 e381–387. [DOI] [PubMed] [Google Scholar]
- Sanz‐Cortes M, Carbajo RJ, Crispi F, Figueras F, Pineda‐Lucena A & Gratacos E (2013). Metabolomic profile of umbilical cord blood plasma from early and late intrauterine growth restricted (IUGR) neonates with and without signs of brain vasodilation. PLoS One 8, e80121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE & McCloskey DP (2009). Postnatal neurogenesis as a therapeutic target in temporal lobe epilepsy. Epilepsy Res 85, 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D, Shastri S & Sharma P (2016). Intrauterine growth restriction: antenatal and postnatal aspects. Clin Med Insights Pediatr 10, 67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp A, Cornforth C, Jackson R, Harrold J, Turner MA, Kenny LC, Baker PN, Johnstone ED, Khalil A, Dedelszen P & Papageorghiou AT (2018). Maternal sildenafil for severe fetal growth restriction (STRIDER): a multicentre, randomised, placebo‐controlled, double‐blind trial. Lancet Child Adolesc Health 2, 93–102. [DOI] [PubMed] [Google Scholar]
- Sovio U, White IR, Dacey A, Pasupathy D & Smith GCS (2015). Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the Pregnancy Outcome Prediction (POP) study: a prospective cohort study. Lancet 386, 2089–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer R, Ambler G, Brodszki J, Diemert A, Figueras F, Gratacos E, Hansson SR, Hecher K, Huertas‐Ceballos A, Marlow N, Marsal K, Morsing E, Peebles D, Rossi C, Sebire NJ, Timms JF, David AL & EVERREST Consortium (2017). EVERREST prospective study: a 6‐year prospective study to define the clinical and biological characteristics of pregnancies affected by severe early onset fetal growth restriction. BMC Pregnancy Childbirth 17, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunk T, Hartel C, Temming P, Matzke N, Zimmer J & Schultz C (2008). Erythropoietin inhibits cytokine production of neonatal and adult leukocytes. Acta Paediatr 97, 16–20. [DOI] [PubMed] [Google Scholar]
- Tatli MM, Kumral A, Duman N, Demir K, Gurcu O & Ozkan H (2004). Spontaneous intestinal perforation after oral ibuprofen treatment of patent ductus arteriosus in two very‐low‐birthweight infants. Acta Paediatr 93, 999–1001. [DOI] [PubMed] [Google Scholar]
- Tolcos M, Bateman E, O'Dowd R, Markwick R, Vrijsen K, Rehn A & Rees S (2011). Intrauterine growth restriction affects the maturation of myelin. Exp Neurol 232, 53–65. [DOI] [PubMed] [Google Scholar]
- Tolsa CB, Zimine S, Warfield SK, Freschi M, Sancho Rossignol A, Lazeyras F, Hanquinet S, Pfizenmaier M & Huppi PS (2004). Early alteration of structural and functional brain development in premature infants born with intrauterine growth restriction. Pediatr Res 56, 132–138. [DOI] [PubMed] [Google Scholar]
- Varvarigou A, Bardin CL, Beharry K, Chemtob S, Papageorgiou A & Aranda JV (1996). Early ibuprofen administration to prevent patent ductus arteriosus in premature newborn infants. JAMA 275, 539–544. [PubMed] [Google Scholar]
- Velipasaoglu M, Yurdakok M, Ozyuncu O, Portakal O & Deren O (2015). Neural injury markers to predict neonatal complications in intrauterine growth restriction. J Obstet Gynaecol 35, 555–560. [DOI] [PubMed] [Google Scholar]
- Verner A, Craig S & McGuire W (2007). Effect of taurine supplementation on growth and development in preterm or low birth weight infants. Cochrane Database Syst Rev CD006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li XW, Liu J & Fu W (2017). Antenatal taurine supplementation in fetal rats with growth restriction improves neural stem cell proliferation by inhibiting the activities of Rho family factors. J Matern Fetal Neonatal Med 31, 1–8. [DOI] [PubMed] [Google Scholar]
- Wixey JA, Chand KK, Colditz PB & Bjorkman ST (2017). Neuroinflammation in intrauterine growth restriction. Placenta 54, 117–124. [DOI] [PubMed] [Google Scholar]
- Wixey JA, Reinebrant HE & Buller KM (2011). Inhibition of neuroinflammation prevents injury to the serotonergic network after hypoxia‐ischemia in the immature rat brain. J Neuropathol Exp Neurol 70, 23–35. [DOI] [PubMed] [Google Scholar]
- Wixey JA, Reinebrant HE & Buller KM (2012). Post‐insult ibuprofen treatment attenuates damage to the serotonergic system after hypoxia‐ischemia in the immature rat brain. J Neuropathol Exp Neurol 71, 1137–1148. [DOI] [PubMed] [Google Scholar]
- Wixey JA, Reinebrant HE, Carty ML & Buller KM (2009). Delayed P2X4R expression after hypoxia‐ischemia is associated with microglia in the immature rat brain. J Neuroimmunol 212, 35–43. [DOI] [PubMed] [Google Scholar]
- Yoo SW, Kim SS, Lee SY, Lee HS, Kim HS, Lee YD & Suh‐Kim H (2008). Mesenchymal stem cells promote proliferation of endogenous neural stem cells and survival of newborn cells in a rat stroke model. Exp Mol Med 40, 387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]


