Abstract
Key points
Preterm birth occurs when the heart muscle is immature and ill‐prepared for the changes in heart and lung function at birth.
MRI imaging studies show differences in the growth and function of the heart of young adults born preterm, with the effects more pronounced in the right ventricle.
The findings of this study, conducted in sheep, showed that following moderate preterm birth the right ventricular wall was thinner in adulthood, with a reduction in the number and size of the heart muscle cells; in addition, there was impaired blood flow in the main artery leading from the right ventricle to the lungs.
The findings indicate that being born only a few weeks early adversely affects the cellular structure of the right ventricle and blood flow to the lungs in adulthood.
The reduced number of heart muscle cells has the potential to deleteriously affect right ventricular growth potential and function.
Abstract
Preterm birth prematurely exposes the immature heart to the haemodynamic transition at birth, which has the potential to induce abnormal cardiac remodelling. Magnetic resonance imaging studies in young adults born preterm have shown abnormalities in the gross structure of the ventricles (particularly the right ventricle; RV), but the cellular basis of these alterations is unknown. The aim of this study, conducted in sheep, was to determine the effect of moderate preterm birth on RV cellular structure and function in early adulthood. Male singleton lambs were delivered moderately preterm (132 ± 1 days; n = 7) or at term (147 ± 1 days; n = 7). At 14.5 months of age, intra‐arterial blood pressure and heart rate were measured. Pulmonary artery diameter and peak systolic blood flow were determined using ultrasound imaging, and RV stroke volume and output calculated. Cardiomyocyte number, size, nuclearity and levels of cardiac fibrosis were subsequently assessed in perfusion‐fixed hearts using image analysis and stereological methods. Blood pressure (systolic, diastolic and mean), heart rate, levels of myocardial fibrosis and RV stroke volume and output were not different between groups. There was, however, a significant reduction in RV wall thickness in preterm sheep, and this was accompanied by a significant reduction in peak systolic blood flow in the pulmonary artery and in RV cardiomyocyte number. Cellular changes in the RV wall and reduced pulmonary artery blood flow following preterm birth have the potential to adversely affect cardiac and respiratory haemodynamics, especially when the cardiovascular system is physiologically or pathologically challenged.
Keywords: cardiomyocyte, preterm birth, heart development, right ventricle
Key points
Preterm birth occurs when the heart muscle is immature and ill‐prepared for the changes in heart and lung function at birth.
MRI imaging studies show differences in the growth and function of the heart of young adults born preterm, with the effects more pronounced in the right ventricle.
The findings of this study, conducted in sheep, showed that following moderate preterm birth the right ventricular wall was thinner in adulthood, with a reduction in the number and size of the heart muscle cells; in addition, there was impaired blood flow in the main artery leading from the right ventricle to the lungs.
The findings indicate that being born only a few weeks early adversely affects the cellular structure of the right ventricle and blood flow to the lungs in adulthood.
The reduced number of heart muscle cells has the potential to deleteriously affect right ventricular growth potential and function.
Introduction
Preterm birth (birth preceding 37 completed weeks of gestation) has a global incidence of 9–13% (Beck et al. 2010; Blencowe et al. 2012), with the majority of these births occurring moderately preterm or near‐term (between 32 and 36 weeks of gestation) (Goldenberg et al. 2008). At the time of preterm birth, many key organs are structurally and functionally immature. As a result, preterm birth can lead to functional and/or structural adaptations in organ systems of the newborn that facilitate survival, but may increase vulnerability to dysfunction and disease (Hofman et al. 2004; Moster et al. 2008; Sutherland et al. 2014; Bensley et al. 2016).
Structural adaptations induced by preterm birth are particularly relevant to the heart (Lewandowski & Leeson, 2014; Bensley et al. 2016), as cardiomyocytes normally undergo maturation and differentiation during the third trimester in preparation for the haemodynamic transition that necessarily occurs at birth (Rudolph, 2000; Huttenbach et al. 2001; Sedmera & Thompson, 2011). When birth occurs before term, the myocardium, containing immature and incompletely differentiated cardiomyocytes, is not functionally or structurally prepared for the circulatory demands initiated by the loss of the placenta and the onset of air breathing (increase in systemic arterial pressure and heart rate; Louey et al. 2000; De Matteo et al. 2016), or for the changing demands required of the right ventricle (RV) and left ventricle (LV) (Hooper et al. 2015; Bensley et al. 2016). As a result, preterm birth can lead to cellular changes in the developing myocardium in the neonatal period, as demonstrated in lambs at 9 weeks post term‐equivalent age that were born moderately preterm (Bensley et al. 2010); these effects can potentially persist into adulthood, but whether this is the case is currently unknown. Indeed, in human infants, echocardiography imaging studies from birth until 3 months of age have shown a disproportionate increase in both LV and RV mass in preterm infants born moderately to late preterm (Aye et al. 2017). Furthermore, magnetic resonance imaging (MRI) of the hearts of young adults born preterm show alterations in the overall macroscopic structure of the heart, and some impairment of function (Lewandowski et al. 2013a,b). These effects were most pronounced in the right ventricle, with reduced ventricular lumen size, increased ventricle mass, and reduced ejection fraction (Lewandowski et al. 2013b). Changes in the microscopic cellular structure of the myocardium are likely to be the underlying cause of these functional and macroscopic structural changes in the adult heart. Following preterm birth there are often cardiorespiratory complications (including pulmonary hypertension; Kinsella et al. 2006), even in those born late preterm (Consortium on Safe Labor et al. 2010), and this may account for the greater impact of preterm birth on the RV.
Therefore, the aim of the present study was to determine the effect of moderate preterm birth on the function and cellular structure of the RV in early adulthood. Our study was performed using an established ovine model of moderately preterm birth (De Matteo et al. 2010; Nguyen et al. 2016). The sheep is an excellent species in which to examine the effect of preterm birth on the structure of the heart (Bensley et al. 2010; Nguyen et al. 2016), as the cardiomyocytes follow a similar prenatal and postnatal growth trajectory to that of the human heart (Burrell et al. 2003; Jonker et al. 2007, 2015). We hypothesised that moderate preterm birth would detrimentally affect the growth of the RV, resulting in persistent maladaptive remodelling of the myocardium. We chose to specifically study males, given that males are considered to be developmentally delayed and thus more vulnerable to preterm birth (Ishak et al. 2012; Altman et al. 2013; De Matteo et al. 2016). Indeed, we have previously shown that preterm‐born adult sheep, when challenged, have an increased blood pressure response compared to term‐born sheep, with the males (but not females) exhibiting a dampened baroreflex response (Allison et al. 2018). It was therefore considered likely that any alterations in structural remodelling would be more apparent in the RV of males born preterm than in females.
Methods
Ethical approval
All experiments were approved by the Monash University Animal Ethics Committee (approval MMCA‐2011/01). Experiments were conducted in accordance with the Australian National Health and Medical Research Council's code of practice for the care and handling of animals for scientific purposes, and conform with the principles and regulations described by Grundy (2015).
Animal studies
Fourteen Border Leicester × White Suffolk male singleton lambs housed at Monash University Animal Services were used in this study; a detailed description of the animal model has been previously published (Nguyen et al. 2016). Briefly, ewes of known mating dates were obtained from Monash Gippsland Animal Services and randomly assigned to deliver at term (147 ± 1 days gestation; n = 7) or moderately preterm (132 ± 1 days, ∼0.9 of gestation; n = 7). During pregnancy, the ewes were housed in paddocks at Monash Gippsland (Churchill, VIC, Australia) and allowed to feed ad libitum. In the week prior to delivery, the ewes were moved to individual indoor pens and monitored closely by staff. To induce preterm birth, ewes were administered epostane (20 mg in 2 mL 100% ethanol, i.v.; Sanofi‐Synthelabo; Macquarie Park, NSW, Australia) at approximately 43 h before expected preterm delivery. Ewes assigned to deliver at term were administered the epostane at approximately 24 h before delivery. Prior to preterm delivery, two doses of betamethasone (both 11.4 mg, i.m.; Celestone Soluspan, Schering‐Plough; North Ryde, NSW, Australia) were administered; the first at 48 h before expected delivery (approximately 5 h before the epostane injection), and the second 24 h later. Lambs at this gestation rarely survive without antenatal steroid administration due to lung immaturity (De Matteo et al. 2010). Ewes assigned to deliver at term were not administered betamethasone as they are exposed to a natural cortisol surge prior to delivery (Fowden et al. 1996). Male term (n = 7) and male preterm (n = 7) lambs were delivered vaginally and were weaned at 12 weeks of age. After weaning lambs were maintained in outdoor paddocks where they fed on natural grass pastures ad libitum until adulthood. Three to four weeks prior to necropsy (at 14.5 months ± 2 weeks of age) the offspring were transported to Monash Medical Centre Animal Facility (in Clayton, VIC, Australia), where they were maintained in individual indoor pens and fed 800–1000 g of lucerne hay/chaff twice daily; water was available ad libitum.
Assessment of heart rate and blood pressure
Two weeks prior to necropsy, term and preterm sheep were aseptically instrumented with femoral arterial catheters and a flow probe (Allison et al. 2018). The arterial catheters were used for the measurement of heart rate and systemic arterial blood pressure; the flow probe was used in another study (in a larger cohort that included both males and females) to assess peripheral blood flow (Allison et al. 2018). Sheep were sedated with 20 mL of sodium thiopentone (50 mg mL−1, Jurox; Rutherford, NSW, Australia), then intubated and anaesthetised by inhalation of 1–5% isofluorane in 70% O2:30% medical air (Bomac, Animal Health; Hornsby, NSW, Australia). Catheters were inserted into the femoral artery and vein of the right hindlimb, and flushed with sterile heparinised saline (50 IU mL−1). A 4 mm vascular flow probe (Transonic Systems; Ithaca, NY, USA) was then placed around the left femoral artery. For 72 h following surgery, sheep received analgesic medication via a transdermal fentanyl patch (75 μg h−1; Janssen Cilag; North Ryde, NSW, Australia), and antibiotics were administered intravenously for 4 days (500 mg Engemycin (100 mg mL−1; Coopers Animal Health, Bendigo East, VIC, Australia) and 500 mg Ampicillin (200 mg mL−1), Aspen Pharmacare Australia Pty Ltd; St Leonards, NSW, Australia)).
Femoral catheters and the flow probe were connected to pressure transducers (DTX Plus, Becton Dickinson; Singapore), and arterial blood pressure and heart rate were continuously recorded (Powerlab, ADinstruments; Castle Hill, NSW, Australia). Continuous recordings of systolic, diastolic and mean arterial pressure and heart rate were made over a 1 h period on day 5 following surgery, and average values calculated.
Ultrasound assessment of the main pulmonary artery and RV output
On the day of necropsy, sheep were sedated and then anaesthetized with 5% isofluorane in oxygen (Bomac, Animal Health; Hornsby, NSW, Australia) delivered via a positive pressure ventilator. Two‐dimensional ultrasound B‐mode imaging (Philips ATL 5000 SonoCT ultrasound system) was used to visualise the proximal main pulmonary artery in the left parasternal short axis view (Rudski et al. 2010) and the internal diameter was measured using electronic calipers. Main pulmonary artery peak systolic blood flow velocity was assessed using spectral Doppler imaging, with an average measurement determined from spectral traces over at least 4 consecutive cardiac cycles. In addition, the average area under the curve of 4 spectral Doppler traces, the velocity–time integral (VTI), was calculated using GraphPad Prism (version 7.01, GraphPad Software Inc.; La Jolla, CA, USA). RV stroke volume was subsequently determined using the formula (Quinones et al. 2002):
RV output was calculated according to the formula:
RV sampling and processing
Immediately after the ultrasound assessments, the sheep were humanely killed via an intravenous lethal dose of pentobarbitone (120 mg kg−1; Virbac Australia P/L, Milperra, NSW, Australia). At necropsy, the hearts were excised and perfusion‐fixed with 10% buffered formalin. The fixed hearts were cleared of connective tissue and weighed. The ventricles were sliced and the RV sampled using a smooth fractionator approach (Stacy et al. 2009). The RV samples were embedded in glycolmethacrylate, serially sectioned at 20 μm, and every 10th section collected and stained with haematoxylin (Amber Scientific; Midvale, WA, Australia). Additional pieces of RV were embedded in paraffin and sectioned at 5 μm, for subsequent immunohistochemical and histological analyses. All analyses of the myocardium were performed while blinded to the experimental group.
Right ventricular wall volume and thickness
Prior to sampling, an orthogonal grid was superimposed over the ventricular slices. The number of grid points that overlaid the RV wall tissue was counted, and RV wall volume subsequently determined using the Cavalieri principle (Lim et al. 2010). The thickness of the RV free wall was also measured.
Immunohistochemistry
Cardiomyocyte cell membranes were immunohistochemically labelled using wheat germ agglutinin (WGA) and an antibody for laminin, in both longitudinal and transverse sections (Fig. 1). Initially, heat‐mediated antigen retrieval was performed on de‐waxed 5 μm sections using a Dako Target Retrieval Solution (Dako; Glostrup, Denmark) for 30 min at 98°C, followed by H2O2 and serum‐free blocking medium (Dako). The sections were then incubated with rabbit anti‐laminin antibody (1:40, ab11575, Abcam; Cambridge, MA, USA) for 1 h at room temperature, followed by Dako EnVision+ System horseradish peroxidase (HRP)‐conjugated anti‐rabbit secondary antibody (Dako) for 1 h at room temperature. HRP‐conjugated WGA (1:40, L7017, Sigma‐Aldrich; Castle Hill, NSW, Australia) was then applied for 1 h at room temperature, followed by 3,3′‐diaminobenzidine (DAB, Dako) for 10 min to visualise antibody and WGA binding, and slides were counterstained with haematoxylin.
Figure 1. Representative photomicrographs of cardiomyocytes from an adult sheep heart.
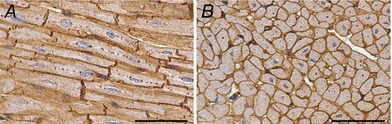
A, longitudinal orientation. B, transverse orientation. Cell boundaries stained with laminin and wheat germ agglutinin (brown) and nuclei stained with haematoxylin (blue). Scale bars = 50 μm.
Cardiomyocyte number, nuclearity and size
The total number of cardiomyocyte nuclei in the RV was estimated in haematoxylin‐stained glycolmethacrylate sections using an optical disector fractionator approach, as previously described (Stacy et al. 2009; Bensley et al. 2010). This procedure utilised an Olympus BX51 microscope fitted with a motorised stage (Olympus; Tokyo, Japan) and a 100× Olympus ApoE oil‐immersion lens. Using C.A.S.T software (Computer Aided Stereological Toolbox, v.2.1.4, Olympus; Alberstand, Denmark), sections were sampled (beginning at a random point at the top of the tissue) at a step length of 2000 μm in the x‐ and y‐axes. An unbiased counting frame (544.5 μm2) was superimposed over each field of view. Nuclei that came into focus within the counting frame, and/or touched the inclusion lines, were counted. Nuclei were only counted if they were observed within the middle 10 μm depth (z‐axis) of the 20 μm thick section. Cardiomyocyte nuclei were discernible from those of other cell types (such as fibroblasts and endothelial cells) by their characteristic elongated oval‐shaped appearance and light purple stain (Stacy et al. 2009).
The total number of cardiomyocyte nuclei (CmN) within the RV of each sheep heart was calculated according to the formula:
F 1 is the first sampling fraction using the smooth fractionator (every 3rd piece of tissue was sampled); F 2 is the second sampling fraction also using the smooth fractionator (after the slices were cut into smaller pieces using a razor blade device, every 9–10th piece was selected to be embedded); F 3 is the sampled fraction of the RV tissue that was embedded in glycolmethacrylate blocks (every 8th section was selected for analysis); F 4 relates to the proportion of the section that was assessed (step length x‐axis (2000 μm) × step length y‐axis (2000 μm)/area of counting frame (544.5 μm2)); F 5 is the fraction of the depth of the section in which nuclei were counted (10 μm/20 μm); and Q − is the number of cardiomyocyte nuclei counted.
Total cardiomyocyte number was subsequently determined by adjusting for cardiomyocyte nuclearity (number of nuclei per cell). Cardiomyocyte nuclearity was assessed in tissue sections immuno‐stained with anti‐laminin and WGA as described above (to visualize the cell boundaries) and haematoxylin (to stain the nuclei) (Fig. 1 A and B). Sections were viewed at 40× magnification and were uniformly and systematically sampled. Only cardiomyocytes oriented longitudinally with a completely visible cell boundary were examined (Fig. 1 A). The number of nuclei per cardiomyocyte was recorded and the proportion of mono‐, bi‐ and multi‐nucleated cardiomyocytes subsequently determined. Approximately 250 cardiomyocytes per RV were analysed.
Concomitant with the nuclearity analyses, the cell boundaries of the longitudinally oriented cardiomyocytes were traced, and the longitudinal area of the cardiomyocytes measured using Image J software (v.6.2, National Institutes of Health; MD, USA); the average cell area per heart was then determined. Cross‐sectional area of cardiomyocytes was also measured in tissue sections where the cardiomyocytes were oriented in cross‐section (Fig. 1 B); only cardiomyocytes where the nucleus was in the plane of view were measured.
Interstitial fibrosis
The percentage of interstitial fibrosis within the RV myocardium was analysed in sections stained with Picrosirius Red (Bensley et al. 2010; Goh et al. 2011). The sections were systematically sampled (8–10 regions per section) using C.A.S.T (Computer Aided Stereological Toolbox, v.2.1.4, Olympus) software, and the percentage of collagen within the interstitium measured using Image‐Pro Plus software (v.6.2, Media Cybernetics; WA, USA).
Statistical analysis
Statistical analyses were performed using GraphPad Prism (v.6.0 for Windows, GraphPad Software). Data were checked for normality and then differences between groups were assessed using a two‐tailed Student's t test or a two‐way analysis of variance and Bonferroni post hoc test (where appropriate). Data are presented as the mean ± standard deviation (SD). Statistical significance was accepted at P < 0.05.
Results
Arterial pressure, heart rate, and functional assessments of the pulmonary artery and right ventricle
As shown in Table 1, at 14.5 months of age there was no significant difference in systemic arterial pressure (systolic, diastolic or mean) between the sheep born preterm and those born at term. There was considerable variability in heart rate within groups, which may reflect increased stress in some animals at the time of measurement; however, there was no difference in heart rate between the groups and the variability was evident in both term and preterm groups (Table 1). Although there was no significant difference in main pulmonary artery diameter between the preterm and term‐born sheep, the peak systolic blood flow was significantly lower in the main pulmonary artery of the preterm sheep (Table 1). Overall, there was considerable variability in the stroke volume of the RV within both the preterm and term groups, with no detectable differences in stroke volume, relative stroke volume or total RV output between groups (Table 1).
Table 1.
Systemic arterial pressures, heart rate, and functional assessments of the pulmonary artery and right ventricle in term and preterm‐born 14.5‐month‐old male sheep
| Term | Preterm | |
|---|---|---|
| Systolic arterial pressure (mmHg) | 119.7 ± 5.9 | 113.3 ± 23.0 |
| (110–125) | (94–151) | |
| Diastolic arterial pressure (mmHg) | 63.2 ± 7.5 | 67.8 ± 13.1 |
| (54–76) | (48–85) | |
| Mean arterial pressure (mmHg) | 88.2 ± 5.7 | 89.5 ± 11.8 |
| (82–96) | (78–111) | |
| Heart rate (beats min−1) | 88.2 ± 29.2 | 90.2 ± 15.0 |
| (60–136) | (75–119) | |
| Main pulmonary artery diameter (cm) | 1.91 ± 0.4 | 1.74 ± 0.3 |
| (1.4–2.4) | (1.4–2.1) | |
| Main pulmonary artery peak systolic flow (cm s−1) | 86.8 ± 10.8 | 73.6 ± 6.2* |
| (76.2–107.3) | (63.1–83.2) | |
| RV stroke volume (mL) | 63.8 ± 27.8 | 48.4 ± 17.9 |
| (32.3–94.3) | (24.9–79.1) | |
| RV relative stroke volume (mL kg−1) | 1.01 ± 0.4 | 0.90 ± 0.5 |
| (0.57–1.48) | (0.41–1.86) | |
| RV output (L min−1) | 5.1 ± 2.3 | 4.1 ± 1.7 |
| (2.0–7.3) | (2.1–7.0) |
Data shown as mean ± SD (range). * P = 0.02 versus term group.
Body weight and heart weight
Body weight was not significantly different between groups at 14.5 months of age (Fig. 2 A). At necropsy, the hearts of preterm sheep were significantly lighter (by 13%) than hearts of those born at term (Fig. 2 B). Heart weight relative to body weight was not significantly different between groups (Fig. 2 C).
Figure 2. Body and heart weights.
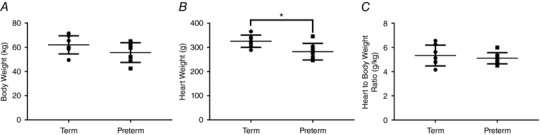
Necropsy weight (A), heart weight (B), and heart weight to body weight ratio (C) of 14.5‐month‐old male sheep born at term (147 days gestation) or preterm (132 days gestation). * P < 0.05.
Right ventricular wall volume and thickness
RV wall thickness was significantly lower in the sheep born preterm compared to term (Fig. 3 A). Similarly, there was a strong trend (P = 0.050) for a lower RV wall volume in the sheep born preterm compared to those born at term (Fig. 3 B).
Figure 3. RV wall thickness.

Right ventricular wall thickness (A) and wall volume (B) of 14.5‐month‐old male sheep born at term (147 days gestation) or preterm (132 days gestation). * P < 0.05.
Cardiomyocyte nuclearity, number and size
The majority of cardiomyocytes (≥86%) in the adult RV (both groups) were binucleated, with the remainder mononucleated (Fig. 4 A); no tri‐ or tetra‐nucleated cardiomyocytes were observed. There were significant differences, however, in cardiomyocyte nuclearity between groups; the proportion of binucleated cardiomyocytes within the RV of sheep born at term was significantly greater compared to those born preterm, whereas the preterm‐born sheep had a significantly greater proportion of mononucleated cardiomyocytes (Fig. 4 A).
Figure 4. Cardiomyocyte nuclearity and number.

The percentage of mononucleated and binucleated cardiomyocytes (A) and cardiomyocyte number (B) in the right ventricle of 14.5‐month‐old male sheep born at term (147 days gestation) or preterm (132 days gestation). Data in A analysed by two‐way ANOVA with the factors nuclearity (P Nuclearity), preterm birth (P Preterm), and their interaction (P NxP). * P < 0.05, *** P < 0.001 (Bonferroni post hoc test).
The total number of cardiomyocytes in the RV was significantly lower (by 17%) in preterm‐born sheep (2.06 ± 0.42 × 109) than in sheep born at term (2.48 ± 0.29 × 109; P = 0.046) (Fig. 4 B).
The cross‐sectional area of RV cardiomyocytes was variable in all sheep, ranging from 122 to 243 μm2 in the term‐born sheep and 144 to 228 μm2 in those born preterm, with no significant difference between groups (Fig. 5 A). The average area of the cardiomyocytes when viewed in the long axis, however, was significantly greater in sheep born at term (1786 ± 9.9 μm2) compared to those born preterm (1685 ± 15.1 μm2; P < 0.0001). Similarly, when assessed according to nuclearity, the cardiomyocytes of preterm‐born sheep were significantly smaller overall than those of the term‐born sheep (P = 0.03; Fig. 5 B); binucleated cardiomyocytes were on average 15.1% larger than the mononucleated cardiomyocytes (Fig. 5 B).
Figure 5. Cardiomyocyte size.

Cardiomyocyte cross‐sectional area (A) and longitudinal area according to nuclei number (B) in the right ventricle of 14.5‐month‐old male sheep born at term (147 days gestation) or preterm (132 days gestation). Data in B analysed by two‐way ANOVA with the factors nuclearity (P Nuclearity), preterm birth (P Preterm), and their interaction (P NxP).
Myocardial interstitial fibrosis
Representative images of myocardial interstitial fibrosis in each group are shown in Fig. 6. Overall, there was no significant difference in the percentage of fibrosis within the myocardium of the RV between the two groups (Fig. 6 A).
Figure 6. Ventricular fibrosis.

A, the percentage of collagen in the right ventricle of 14.5‐month‐old male sheep born at term (147 days gestation) or preterm (132 days gestation). B and C, representative images of Picrosirius Red stained myocardial sections from term (B) and preterm (C) animals, with collagen shown in red and myocardium in yellow. Scale bars = 100 μm.
Discussion
Human preterm birth is associated with high neonatal mortality and morbidity, with males more vulnerable than females (Elsmen et al. 2004; Zisk et al. 2011). It is now emerging that the adverse health consequences following preterm birth can extend into postnatal life and may be lifelong, even in infants born close to term (Teune et al. 2011; Gill & Boyle, 2017); the findings of this study add to this growing body of evidence.
Our findings clearly show that there are cellular changes in the myocardium of the RV of the adult heart following moderately preterm birth. By early adulthood there was a significant reduction in the thickness of the wall of the RV in male sheep born preterm, with a concomitant reduction in cardiomyocyte number and longitudinal area, and in the proportion of binucleated cardiomyocytes. Furthermore, there was a significant reduction in peak systolic blood flow velocity in the pulmonary artery, which probably reflects a reduced contractile capacity of a thinner RV wall. The reduced number of cardiomyocytes within the wall of the RV has the potential to adversely affect the functional and adaptive capabilities of the RV, especially if it is challenged during adulthood (for example, by pulmonary hypertension or obesity). Already, the reduced peak systolic blood flow in the main pulmonary artery, in the absence of no changes in arterial diameter, suggests that the contractile force generated by the RV is less in the preterm sheep. Alternatively, it may indicate downstream increased pulmonary vascular resistance, which is possible given that preterm birth (including late preterm birth) is often associated with impaired postnatal respiratory function (Cock et al. 2005; O'Reilly et al. 2013; Bolton et al. 2015; Kotecha et al. 2016). The absence of RV hypertrophy and no evidence of cardiomyocyte hypertrophy or cardiac fibrosis, however, does not support this concept. There also remains the possibility that the impact of preterm birth on the RV wall has in some way affected the myocardium so that it is unable to mount a growth response. To explore these possibilities further, in future studies it would be beneficial to measure both pulmonary arterial pressure and pulmonary vascular resistance.
Interestingly, our findings are contrary to the MRI studies in young human adults born preterm, where a significant increase in RV mass was reported compared to young adults born at term (Lewandowski et al. 2013b). In that study (Lewandowski et al. 2013b), however, the increase in RV mass was shown to be greatest in those born extremely preterm and only a mild elevation was apparent in those born moderately preterm; this occurred with an overall 2.7% increase in RV mass per 1 week reduction in gestational age at birth. In addition, the authors observed a significant increase in systemic arterial pressure in the young adults born preterm (Lewandowski et al. 2013a,b), whereas in our study there were no differences in arterial pressure in the preterm and term sheep in early adulthood. Indeed, gross structural differences may not manifest in adulthood until the heart is challenged, with systemic and aortic systolic arterial pressure shown by Lewandowski et al. (2013b) to be significantly correlated with RV mass; LV mass has also been shown to be disproportionately increased in response to high systemic arterial pressure in adults born preterm (Lewandowski et al. 2013a). However, a recent study by Aye et al. (2017) does not support this notion, as they demonstrated that both LV and RV hypertrophy were already apparent in preterm infants at 3 months of age, yet diastolic blood pressure was significantly reduced and systolic blood pressure was not different compared to term‐born infants. Interestingly, and in contrast to these findings, in our previous studies in sheep we did not detect LV or RV hypertrophy in moderately preterm lambs at 9 weeks post term‐equivalent age, and there were no significant differences in systolic, diastolic or mean arterial pressure when compared to term‐born lambs (Bensley et al. 2010). Further studies are therefore required to elucidate the apparent species differences. Indeed, there may be factors in the care of the human infants (for example, ventilation, oxygen exposure and postnatal steroid treatment) that directly influence the growth of the heart and this warrants further investigation.
Our findings of a 17% reduction in cardiomyocyte number in the RV of adult sheep born preterm is of concern, given that once adulthood is reached there is negligible formation of cardiomyocytes (Mollova et al. 2013). The reduction in cardiomyocyte number directly reflects the reduced RV wall thickness and volume in the adult preterm sheep and hence, may be attributed to the overall attenuated growth of the preterm sheep up until adulthood (as reported by Nguyen et al. 2016). If this is the case, it is expected that females may not be affected to the same extent as males, given that in larger cohorts, measured over multiple time points, we have shown a persistent long‐term attenuation of body weight and stature in the male adult sheep born preterm, in comparison to the females where there was a gradual catch‐up in growth (Nguyen et al. 2016). Furthermore, we have previously shown in this cohort of animals that there are sex‐specific effects of preterm birth on the autonomic control of cardiovascular function (Allison et al. 2018). In humans, long‐term attenuation of body growth often occurs following preterm birth (Batista et al. 2012; Roberts et al. 2013; Derraik et al. 2017). Whether similar reductions in cardiomyocyte number occurs in the RV of the adult human heart when there is a persistent attenuation in body growth is yet to be determined, and may be dependent on whether RV hypertrophy has developed.
An early postnatal impact of preterm birth on the growth of cardiomyocytes may be a potential explanation for the reduced complement of cardiomyocytes in the RV of the adult sheep born preterm; however, previous studies in our laboratory do not support this concept (Bensley et al. 2010). Using an ovine model of moderate preterm birth similar to that used in the present study (with the exception that we used a lower dose of maternal antenatal corticosteroid) it was shown that there was no difference in the number of cardiomyocytes in the RV or LV of preterm lambs at 9 weeks post term‐equivalent age when compared to term lambs (Bensley et al. 2010). In light of these findings, it appears unlikely that preterm delivery per se has caused the attenuation in cardiomyocyte number in the RV of the preterm sheep in the present study. Alternatively, the attenuation in cardiomyocyte number may have been mediated by the higher dose of antenatal steroids used in the present study (11.4 mg at 48 h and 24 h prior to delivery, as used clinically (Antenatal Corticosteroid Clinical Practice Guidelines Panel, 2015)) compared to the earlier study of Bensley et al. (2010), where a single antenatal dose of 3.7–5 mg was administered. Indeed, it is well established that antenatal corticosteroid exposure (which mimics the natural cortisol rise in late gestation) leads to accelerated maturation of the fetal lung (Roberts & Dalziel, 2006), and there is experimental evidence to show that this also occurs in the kidneys (Stonestreet et al. 1983; Gubhaju et al. 2009). Notably, studies have shown changes in fetal haemodynamics with antenatal betamethasone, leading to increases in pulmonary blood flow (and presumably RV output) prior to delivery in the unborn fetal lamb, thus reflecting the changes that are normally seen in the early postnatal period (Crossley et al. 2009). Therefore, it is conceivable that the dose of antenatal steroids administered to the ewes in the present study may have led to early cessation of proliferation and concomitant accelerated maturation of the cardiomyocytes.
Generally, in the sheep heart, binucleated cardiomyocytes are considered to be mature and differentiated, whereas mononucleated cardiomyocytes are thought to be immature and relatively undifferentiated (Jonker et al. 2007). Hence, significant increases in the proportion of mononucleated cardiomyocytes and concomitant decreases in the proportion of binucleated cardiomyocytes in the preterm RV suggest that there may be an increased reserve of immature undifferentiated cardiomyocytes in the adult preterm RV, which may still be capable of division. Similar findings in preterm lambs, however, do not support this idea. In our previous study, the majority of the mononucleated cardiomyocytes were shown to be polyploid, whereby DNA replication had occurred without karyokinesis (Bensley et al. 2010). Whether this is the case in our adult preterm sheep has yet to be elucidated. Indeed, polypoidy is a common phenomenon in the human heart and is associated with stress of cardiomyocytes (Adler & Friedburg, 1986; Brodsky et al. 1994; Anatskaya & Vinogradov, 2004).
Conclusions
Although moderately preterm infants rarely exhibit neonatal complications after birth, the findings of this study add to the mounting body of evidence that late preterm birth can lead to long‐term differences in organ growth and function. Our findings thus emphasise that subjects born moderately or late preterm (although only a few weeks premature) should not be considered as having the same postnatal development in major organ systems as in term‐born individuals. The structural and cellular changes in the RV that we observed could deleteriously affect long‐term cardiac function and are a likely mediator of the reduced pulmonary artery blood flow that we observed. Additionally, the reduced complement of cardiomyocytes in the RV of the adult preterm heart has the potential to adversely impact the adaptive and functional capabilities of the RV when physiologically or pathologically challenged.
Additional information
Competing interests
The authors have no conflicts of interest to declare.
Author contributions
All animal deliveries and long‐term housing were conducted at Monash University Animal Services, Churchill, Victoria, Australia. Experimental analyses prior to termination were performed at the Hudson Institute, Clayton, Victoria, Australia, and tissue analyses were performed at the Department of Anatomy and Developmental Biology, Monash University, Clayton, Victoria, Australia. M.J.B., G.R.P., P.L., R.H. and R.D.M. contributed to the conception and design of the work. M.M.M., V.B.N., P.L., M.R.S., J.G.B., I.N., B.J.A., R.H., R.D.M., M.S., G.R.P. and M.J.B. contributed to the acquisition, analysis, or interpretation of data for this work. M.M.M., P.L., M.R.S., R.H., R.D.M., G.R.P. and M.J.B. contributed to drafting the work, and all authors contributed to revising it critically for important intellectual content. All authors approve the final version of the manuscript, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; all persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by National Health and Medical Research Council of Australia (NHMRC) grant number 1011354, an NHMRC and National Heart Foundation Research Fellowship [G.R.P.:1105526] and the Victorian Government's Operational Infrastructure Support Program. V.B.N. was the recipient of a Monash University Department of Anatomy and Developmental Biology postgraduate scholarship, and M.R.S. was the recipient of a NHMRC CJ Martin Early Career Fellowship.
Acknowledgements
We thank the staff of the Monash University Histology Platform, especially Sue Connell, Julie Hickey, Camilla Cohen, Jelena Kezic and Stefania Tombs, for their technical assistance.
Edited by: Laura Bennet & Bruce Smaill
Linked articles This article is highlighted in a Perspectives article by Mohamedet al. To read this article, visit http://doi.org/10.1113/JP276067.
References
- Adler CP & Friedburg H (1986). Myocardial DNA content, ploidy level and cell number in geriatric hearts: post‐mortem examinations of human myocardium in old age. J Mol Cell Cardiol 18, 39–53. [DOI] [PubMed] [Google Scholar]
- Allison BJ, Nguyen V, Yiallourou SR, Nitsos I, Black MJ & Polglase GR (2018). The effect of sex and prematurity on the cardiovascular baroreflex response in sheep. Exp Physiol 103, 9–18. [DOI] [PubMed] [Google Scholar]
- Altman M, Vanpee M, Cnattingius S & Norman M (2013). Risk factors for acute respiratory morbidity in moderately preterm infants. Paediatr Perinat Epidemiol 27, 172–181. [DOI] [PubMed] [Google Scholar]
- Anatskaya OV & Vinogradov AE (2004). Paradoxical relationship between protein content and nucleolar activity in mammalian cardiomyocytes. Genome 47, 565–578. [DOI] [PubMed] [Google Scholar]
- Antenatal Corticosteroid Clinical Practice Guidelines Panel (2015). Antenatal Corticosteroids Given to Women Prior to Birth to Improve Fetal, Infant, Child and Adult Health: Clinical Practice Guidelines. Liggins Institute, The University of Auckland, Auckland, New Zealand. [Google Scholar]
- Aye CYL, Lewandowski AJ, Lamata P, Upton R, Davis E, Ohuma EO, Kenworthy Y, Boardman H, Wopperer S, Packham A, Adwani S, McCormick K, Papageorghiou AT & Leeson P (2017). Disproportionate cardiac hypertrophy during early postnatal development in infants born preterm. Pediatr Res 82, 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista RF, Silva AA, Barbieri MA, Simoes VM & Bettiol H (2012). Factors associated with height catch‐up and catch‐down growth among schoolchildren. PLoS One 7, e32903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, Rubens C, Menon R & Van Look PF (2010). The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ 88, 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensley JG, De Matteo R, Harding R & Black MJ (2016). The effects of preterm birth and its antecedents on the cardiovascular system. Acta Obstet Gynecol Scand 95, 652–663. [DOI] [PubMed] [Google Scholar]
- Bensley JG, Stacy VK, De Matteo R, Harding R & Black MJ (2010). Cardiac remodelling as a result of pre‐term birth: implications for future cardiovascular disease. Eur Heart J 31, 2058–2066. [DOI] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L & Lawn JE (2012). National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379, 2162–2172. [DOI] [PubMed] [Google Scholar]
- Bolton CE, Bush A, Hurst JR, Kotecha S & McGarvey L (2015). Lung consequences in adults born prematurely. Thorax 70, 574–580. [DOI] [PubMed] [Google Scholar]
- Brodsky V, Sarkisov DS, Arefyeva AM, Panova NW & Gvasava IG (1994). Polyploidy in cardiac myocytes of normal and hypertrophic human hearts; range of values. Virchows Arch 424, 429–435. [DOI] [PubMed] [Google Scholar]
- Burrell JH, Boyn AM, Kumarasamy V, Hsieh A, Head SI & Lumbers ER (2003). Growth and maturation of cardiac myocytes in fetal sheep in the second half of gestation. Anat Rec A Discov Mol Cell Evol Biol 274, 952–961. [DOI] [PubMed] [Google Scholar]
- Cock M, Hanna M, Sozo F, Wallace M, Yawno T, Suzuki K, Maritz G, Hooper S & Harding R (2005). Pulmonary function and structure following mild preterm birth in lambs. Pediatr Pulmonol 40, 336–348. [DOI] [PubMed] [Google Scholar]
- Consortium on Safe Labor , Hibbard JU, Wilkins I, Sun L, Gregory K, Haberman S, Hoffman M, Kominiarek MA, Reddy U, Bailit J, Branch DW, Burkman R, Gonzalez Quintero VH, Hatjis CG, Landy H, Ramirez M, VanVeldhuisen P, Troendle J & Zhang J (2010). Respiratory morbidity in late preterm births. JAMA 304, 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley KJ, Morley CJ, Allison BJ, Davis PG, Polglase GR, Wallace MJ, Zahra VA & Hooper SB (2009). Antenatal corticosteroids increase fetal, but not postnatal, pulmonary blood flow in sheep. Pediatr Res 66, 283–288. [DOI] [PubMed] [Google Scholar]
- De Matteo R, Blasch N, Stokes V, Davis P & Harding R (2010). Induced preterm birth in sheep: a suitable model for studying the developmental effects of moderately preterm birth. Reprod Sci 17, 724–733. [DOI] [PubMed] [Google Scholar]
- De Matteo R, Ishak N, Hanita T, Harding R & Sozo F (2016). Respiratory adaptation and surfactant composition of unanesthetized male and female lambs differ for up to 8 h after preterm birth. Pediatr Res 79, 13–21. [DOI] [PubMed] [Google Scholar]
- Derraik JG, Lundgren M, Cutfield WS & Ahlsson F (2017). Association between preterm birth and lower adult height in women. Am J Epidemiol 185, 48–53. [DOI] [PubMed] [Google Scholar]
- Elsmen E, Hansen Pupp I & Hellstrom‐Westas L (2004). Preterm male infants need more initial respiratory and circulatory support than female infants. Acta Paediatr 93, 529–533. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Szemere J, Hughes P, Gilmour RS & Forhead AJ (1996). The effects of cortisol on the growth rate of the sheep fetus during late gestation. J Endocrinol 151, 97–105. [DOI] [PubMed] [Google Scholar]
- Gill JV & Boyle EM (2017). Outcomes of infants born near term. Arch Dis Child 102, 194–198. [DOI] [PubMed] [Google Scholar]
- Goh JM, Bensley JG, Kenna K, Sozo F, Bocking AD, Brien J, Walker D, Harding R & Black MJ (2011). Alcohol exposure during late gestation adversely affects myocardial development with implications for postnatal cardiac function. Am J Physiol Heart Circ Physiol 300, H645–H651. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD & Romero R (2008). Epidemiology and causes of preterm birth. Lancet 371, 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D ( 2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology . J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubhaju L, Sutherland MR, Yoder BA, Zulli A, Bertram JF & Black MJ (2009). Is nephrogenesis affected by preterm birth? Studies in a non‐human primate model. Am J Physiol Ren Physiol 297, F1668–F1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman PL, Regan F, Jackson WE, Jefferies C, Knight DB, Robinson EM & Cutfield WS (2004). Premature birth and later insulin resistance. N Engl J Med 351, 2179–2186. [DOI] [PubMed] [Google Scholar]
- Hooper SB, Te Pas AB, Lang J, van Vonderen JJ, Roehr CC, Kluckow M, Gill AW, Wallace EM & Polglase GR (2015). Cardiovascular transition at birth: a physiological sequence. Pediatr Res 77, 608–614. [DOI] [PubMed] [Google Scholar]
- Huttenbach Y, Ostrowski ML, Thaller D & Kim HS (2001). Cell proliferation in the growing human heart: MIB‐1 immunostaining in preterm and term infants at autopsy. Cardiovasc Pathol 10, 119–123. [DOI] [PubMed] [Google Scholar]
- Ishak N, Hanita T, Sozo F, Maritz G, Harding R & De Matteo R (2012). Sex differences in cardiorespiratory transition and surfactant composition following preterm birth in sheep. Am J Physiol Regul Integr Comp Physiol 303, R778–R789. [DOI] [PubMed] [Google Scholar]
- Jonker SS, Louey S, Giraud GD, Thornburg KL & Faber JJ (2015). Timing of cardiomyocyte growth, maturation, and attrition in perinatal sheep. FASEB J 29, 4346–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker SS, Zhang L, Louey S, Giraud GD, Thornburg KL & Faber JJ (2007). Myocyte enlargement, differentiation, and proliferation kinetics in the fetal sheep heart. J Appl Physiol 102, 1130–1142. [DOI] [PubMed] [Google Scholar]
- Kinsella JP, Greenough A & Abman SH (2006). Bronchopulmonary dysplasia. Lancet 367, 1421–1431. [DOI] [PubMed] [Google Scholar]
- Kotecha SJ, Gallacher DJ & Kotecha S (2016). The respiratory consequences of early‐term birth and delivery by Caesarean sections. Paediatr Respir Rev 19, 49–55. [DOI] [PubMed] [Google Scholar]
- Lewandowski AJ, Augustine D, Lamata P, Davis EF, Lazdam M, Francis J, McCormick K, Wilkinson AR, Singhal A, Lucas A, Smith NP, Neubauer S & Leeson P (2013a). Preterm heart in adult life: cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation 127, 197–206. [DOI] [PubMed] [Google Scholar]
- Lewandowski AJ, Bradlow WM, Augustine D, Davis EF, Francis J, Singhal A, Lucas A, Neubauer S, McCormick K & Leeson P (2013b). Right ventricular systolic dysfunction in young adults born preterm. Circulation 128, 713–720. [DOI] [PubMed] [Google Scholar]
- Lewandowski AJ & Leeson P (2014). Preeclampsia, prematurity and cardiovascular health in adult life. Early Hum Dev 90, 725–729. [DOI] [PubMed] [Google Scholar]
- Lim K, Zimanyi MA & Black MJ (2010). Effect of maternal protein restriction during pregnancy and lactation on the number of cardiomyocytes in the postproliferative weanling rat heart. Anat Rec 293, 431–437. [DOI] [PubMed] [Google Scholar]
- Louey S, Cock ML, Stevenson KM & Harding R (2000). Placental insufficiency and fetal growth restriction lead to postnatal hypotension and altered postnatal growth in sheep. Pediatr Res 48, 808–814. [DOI] [PubMed] [Google Scholar]
- Mollova M, Bersell K, Walsh S, Savla J, Das LT, Park SY, Silberstein LE, Dos Remedios CG, Graham D, Colan S & Kuhn B (2013). Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Nat Acad Sci USA 110, 1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moster D, Lie RT & Markestad T (2008). Long‐term medical and social consequences of preterm birth. N Engl J Med 359, 262–273. [DOI] [PubMed] [Google Scholar]
- Nguyen VB, De Matteo R, Harding R, Stefanidis A, Polglase GR & Black MJ (2016). Experimentally induced preterm birth in sheep following a clinical course of antenatal betamethasone: effects on growth and long‐term survival. Reprod Sci 24, 1203–1213. [DOI] [PubMed] [Google Scholar]
- O'Reilly M, Sozo F & Harding R (2013). Impact of preterm birth and bronchopulmonary dysplasia on the developing lung: long‐term consequences for respiratory health. Clin Exp Pharmacol Physiol 40, 765–773. [DOI] [PubMed] [Google Scholar]
- Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA & Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography (2002). Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr 15, 167–184. [DOI] [PubMed] [Google Scholar]
- Roberts D & Dalziel S (2006). Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 3, CD004454. [DOI] [PubMed] [Google Scholar]
- Roberts G, Cheong J, Opie G, Carse E, Davis N, Duff J, Lee KJ, Doyle L & Victorian Infant Collaborative Study Group (2013). Growth of extremely preterm survivors from birth to 18 years of age compared with term controls. Pediatrics 131, e439–445. [DOI] [PubMed] [Google Scholar]
- Rudolph AM (2000). Myocardial growth before and after birth: clinical implications. Acta Paediatr 89, 129–133. [DOI] [PubMed] [Google Scholar]
- Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK & Schiller NB (2010). Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography: endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23, 685–713. [DOI] [PubMed] [Google Scholar]
- Sedmera D & Thompson RP (2011). Myocyte proliferation in the developing heart. Dev Dyn 240, 1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy V, De Matteo R, Brew N, Sozo F, Probyn ME, Harding R & Black MJ (2009). The influence of naturally occurring differences in birthweight on ventricular cardiomyocyte number in sheep. Anat Rec 292, 29–37. [DOI] [PubMed] [Google Scholar]
- Stonestreet BS, Hansen NB, Laptook AR & Oh W (1983). Glucocorticoid accelerates renal functional maturation in fetal lambs. Early Hum Dev 8, 331–341. [DOI] [PubMed] [Google Scholar]
- Sutherland M, Ryan D, Black MJ & Kent AL (2014). Long‐term renal consequences of preterm birth. Clin Perinatol 41, 561–573. [DOI] [PubMed] [Google Scholar]
- Teune MJ, Bakhuizen S, Gyamfi Bannerman C, Opmeer BC, van Kaam AH, van Wassenaer AG, Morris JM & Mol BW (2011). A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol 205, 374e1–374e9. [DOI] [PubMed] [Google Scholar]
- Zisk JL, Genen LH, Kirkby S, Webb D, Greenspan J & Dysart K (2011). Do premature female infants really do better than their male counterparts? Am J Perinatol 28, 241–246. [DOI] [PubMed] [Google Scholar]


