Abstract
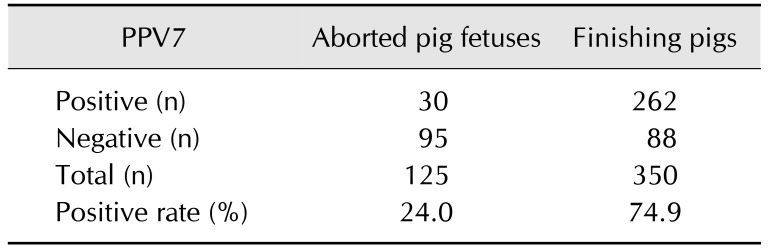
Porcine parvovirus 7 (PPV7) was first detected in Korean pig farms in 2017. The detection rate of PPV7 DNA was 24.0% (30/125) in aborted pig fetuses and 74.9% (262/350) in finishing pigs, suggesting that PPV7 has circulated among Korean domestic pig farms. Phylogenetic analysis based on capsid protein amino acid sequences demonstrated that the nine isolated Korean strains (PPV-KA1-3 and PPV-KF1-6) were closely related to the previously reported USA and Chinese PPV7 strains. In addition, the Korean strains exhibit genetic diversity with both insertion and deletion mutations. This study contributes to the understanding of the molecular epidemiology of PPV7 in Korea.
Keywords: Korea, aborted fetus, detection, pig farm, porcine parvovirus 7
To date, seven genotypes of porcine parvoviruses (PPV) have been identified in pig populations, and those PPV have been taxonomically divided into four genera based on amino acid similarity in the non-structural protein 1 (NS1): PPV1 in Protoparvovirus; PPV2 and PPV3 in Tetraparvovirus; PPV4, PPV5, and PPV6 in Copiparvovirus; and PPV7 in Chapparvovirus [4,5,8]. Although PPV1 is a well-known infectious agent that causes reproductive failure in swineherds worldwide, the clinical significance of the other genotypes of PPV infections remains incompletely described. PPV7 is the most recently identified PPV genotype and was first identified by performing mutagenomic sequencing of samples from healthy adult pigs in the USA in 2016 [4]. It was also detected from serum samples obtained from two Chinese pig farms and had a relatively higher prevalence rate (32.8%) than that observed in the US (8.6%) [8], suggesting that PPV7 commonly circulates in pig herds in both countries. However, the clinical presentations of PPV7 infection and the distribution of PPV7 in the global pig population remain to be determined. Despite growing concerns about PPV7, knowledge about the distribution of this virus in the Republic of Korea is limited. The aim of this study was to provide the first detection and genetic characterization of PPV7 from aborted pig fetuses and finishing pigs in Korean domestic pig farms.
A total of 125 aborted pig fetuses and 350 lung tissues from finishing pigs were collected from commercial pig farms located in four provinces of the Republic of Korea and were submitted to the Viral Disease Division of the Animal and Plant Quarantine Agency for diagnosis of reproductive and respiratory disorders in 2017 (Table 1). The fetuses and the lung tissues of the finishing pigs were homogenized with phosphate-buffered saline (pH 7.2) and stored at −80℃ until use. Total DNA was extracted from the homogenized samples by using the DNeasy mini kit (Qiagen, Germany) according to the manufacturer's instructions. The PPV7 DNA was amplified with primers targeting the capsid protein (VP) gene of PPV7 (PPV7-3434-F and PPV7-3654-R) by using the Hotstart PCR premix (Bioneer, Korea) as previously described [8]. The expected 241 base pair (bp) amplicons for PPV7 were confirmed. To further characterize Korean PPV7 strains, the replicase (REP) or VP genes of PPV7 were amplified using four sets of primers (PPV7-380-F and PPV7-1336-R, PPV7-1270-F and PPV7-2262-R, PPV7-2158-F and PPV7-3203-R, or PPV7-3022-F and PPV7-4033-R) as previously described [8]. The amplicons were ligated into the pDrive vector (Qiagen) and sent to a commercial sequencing company (Macrogen, Korea) for sequencing of the REP or VP gene. The sequences were assembled using the SeqMan program in Lasergene 12.0 software (DNASTAR, USA) and aligned by using by Clustal Omega (EMBL-EBI, UK) with other PPV sequences downloaded from GenBank. Phylogenetic trees were inferred from the amino acid sequences of the VP by applying the maximum-likelihood method and using the Le and Gascuel model with gamma-distributed rate variation and frequency of each amino acid (LG + F + I) as implemented in MEGA v6.06 [6]. Support for the individual nodes was determined by using 1,000 bootstrap replicates [1,6].
Table 1. Porcine parvovirus 7 (PPV7) DNA detection rates in aborted pig fetuses and finishing pigs.
PPV7 DNA was detected in 30 of the 125 tested fetal samples and in 262 of the 350 tested finishing pig samples (Table 1). The prevalence of PPV7 in the aborted pig fetal samples (24.0%, 30/125) was higher than the prevalence observed in the USA (8.6%) but lower than that detected in China (32.8%). In addition, the prevalence of PPV7 in the finishing pig lung tissue samples (74.9%, 262/350) was higher than the prevalences observed in the USA (8.6%) and China (32.8%) [4,8]. These results suggest that PPV7 commonly circulates in Korean pig farms and that PPV7 infection may be associated with reproductive failure in breeding pigs. In this regard, additional studies, such as virus isolation and artificial infection with virus isolates are needed to elucidate the pathogenesis and clinical presentation of PPV7 in pigs.
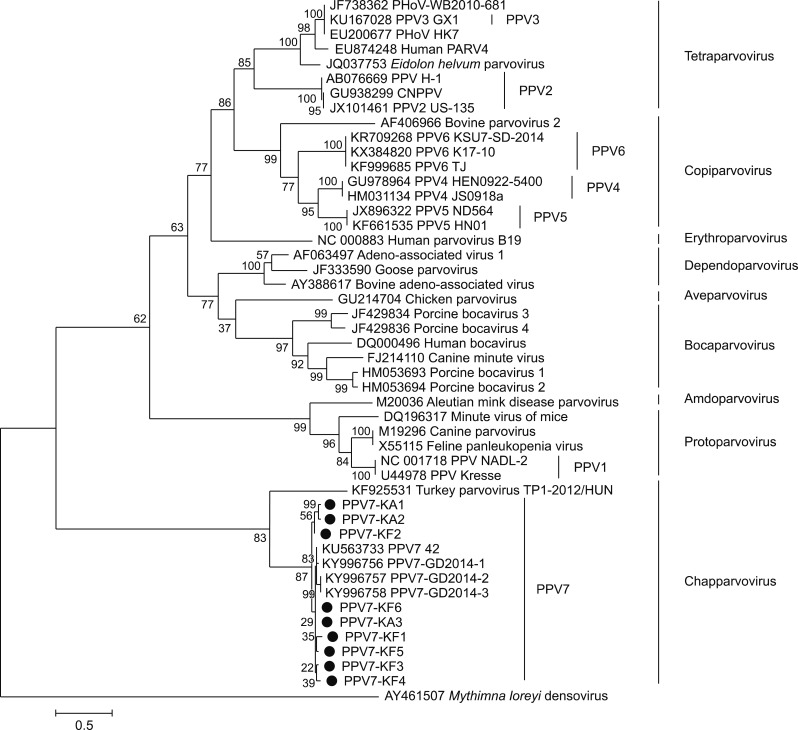
To further characterize the Korean PPV7, VP genes of three aborted pig fetus strains (designated as PPV7-KA1-3) and REP and VP genes of six finishing pig strains (designated as PPV7-KF1–6) were analyzed and deposited in GenBank under accession numbers MH293507 (PPV7-KA1), MH293508 (PPV7-KA2), MH293509 (PPV7-KA3), MH422962 (PPV7-KF1), MH422963 (PPV7-KF2), MH422964 (PPV7-KF3), MH422965 (PPV7-KF4), MH422966 (PPV7-KF5), and MH422967 (PPV7-KF6). The VP genes of PPV7-KA3, -KF2, and -KF6 were 1410 bp in nucleotide length, which was consistent with a previous report describing the USA PPV7 42 strain (GenBank accession No. KU563733) and the Chinese GD 2014-1 (GenBank accession No. KY996756); it was 9 bp longer than Chinese GD 2014-2 (GenBank accession No. KY996757) and GD 2014-3 (GenBank accession No. KY996758). In contrast, the nucleotide length of the other four isolates (PPV7-KA1, -KA2, and PPV7-KF1, -KF4) were 1425 bp, which is longer than that observed in the USA and Chinese strains due to the successive insertion of five amino acids (181–185 amino acids). In addition, the nucleotide length of the remaining two isolates (PPV7-KF3 and PPV7-KF5) were 1404 bp and 1392 bp, respectively, which are shorter than that observed in the USA strain due to the deletion of two amino acids (140–141 amino acids) or to two and four amino acid deletions (140–141 and 147–150 amino acids) [4,8]. Based on the sequence analysis of the VP genes, nine Korean PPV7 strains share the following nucleotide and amino acid level identities: 88.1% to 97.9% and 88.2% to 99.5% with each other, 89.8% to 98.2% and 90.5% to 99.3% with the USA PPV7 42 strain, and 87.5% to 97.8% and 85.2% to 98.2% with the Chinese PPV7 strains, respectively [4,8]. The conserved calcium-binding loop (YXGXG) motif was identified in the VP of the Korean PPV7 isolates. However, the catalytic residues (HDXXY) of the putative secretory phospholipase A2 were missing due to a single amino acid mutation at amino acid 304 (Y to N) in the VP of PPV7 strains such as the USA and Chinese strains [7,8]. Phylogenetic analysis based on VP amino acid sequences demonstrated that the nine Korean strains were closely related to previously reported USA and Chinese PPV7 strains, and they clustered in the Chapparvovirus group together with turkey parvovirus (GenBank accession No. KR925531) (Fig. 1), which is consistent with a previously performed phylogenetic analysis based on the NS protein [4,8]. In addition, the Korean PPV7 strains have various mutations when compared to USA and Chinese strains (Fig. 1). These results indicate that Korean PPV7 has more genetic diversity than USA and Chinese PPV7.
Fig. 1. Phylogenetic tree constructed based on the capsid protein (VP) sequences. Phylogenetic tree of VP sequences was derived from 39 Parvovirinae genomes. The tree was inferred from amino acid sequences of the VP by applying the maximum-likelihood method using the LG + F + I model with 1,000 bootstrap resampling iterations. The Korean porcine parvovirus 7 (PPV7) strains identified in this study are represented by black circles.
PPV1 was reported to be prevalent in Korean pig farms and is continuously surveyed for due to significant economic costs it has had on the swine industry [3]. Additionally, PPV2 was recently (2016) isolated from pigs in Korea [2]. However, PPV7 infection has not yet been reported in the Republic of Korea. To the best our knowledge, this is the first report of PPV7 detection in aborted pig fetuses and finishing pigs in the Republic of Korea. The results of this study contribute to the understanding of the molecular epidemiology of PPV7, but further studies using PPV7 isolates will be needed to elucidate the pathogenesis of this virus in pigs.
Acknowledgments
This study was supported by the Research of Animal and Plant Quarantine Agency (project No. M-1543083-2018-20-01) and Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, and Forestry (IPET) through Golden Seed Project (213010-05-2-CG600), funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA), Ministry of Oceans and Fisheries (MOF), Rural Development Administration (RDA) and Korea Forest Services (KFS).
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Le SQ, Gascuel O. An improved general amino acid replacement matrix. Mol Biol Evol. 2008;25:1307–1320. doi: 10.1093/molbev/msn067. [DOI] [PubMed] [Google Scholar]
- 2.Lee JY, Kim EJ, Cho IS, Lee KK, Shin YK. Complete genome sequences of porcine parvovirus 2 isolated from swine in the Republic of Korea. Genome Announc. 2017;5:e01738-16. doi: 10.1128/genomeA.01738-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh WT, Kim RY, Nguyen VG, Chung HC, Park BK. Perspectives on the evolution of porcine parvovirus. Viruses. 2017;9:pii: E196. doi: 10.3390/v9080196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palinski RM, Mitra N, Hause BM. Discovery of a novel Parvovirinae virus, porcine parvovirus 7, by metagenomic sequencing of porcine rectal swabs. Virus Genes. 2016;52:564–567. doi: 10.1007/s11262-016-1322-1. [DOI] [PubMed] [Google Scholar]
- 5.Streck AF, Canal CW, Truyen U. Molecular epidemiology and evolution of porcine parvoviruses. Infect Genet Evol. 2015;36:300–306. doi: 10.1016/j.meegid.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao CT, Giménez-Lirola LG, Jiang YH, Halbur PG, Opriessnig T. Characterization of a novel porcine parvovirus tentatively designated PPV5. PLoS One. 2013;8:e65312. doi: 10.1371/journal.pone.0065312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xing X, Zhou H, Tong L, Chen Y, Sun Y, Wang H, Zhang G. First identification of porcine parvovirus 7 in China. Arch Virol. 2018;163:209–213. doi: 10.1007/s00705-017-3585-9. [DOI] [PubMed] [Google Scholar]