Abstract
Adjuvants potentiate and direct the type of immunity elicited during vaccination. However, there is a shortage of adjuvants that elicit robust type-1 immunity required for the control of intracellular pathogens, including viruses. RNA derived from Sendai virus defective viral genomes (DVGs) stimulates RIG-I-like receptor signaling leading to type-1 immunity during infection. Here, we investigated whether a 268nt DVG-derived oligonucleotide (DDO) functions as a strong type-1 immunity-inducing adjuvant during vaccination against influenza virus. We show that DDO induces robust IgG2c antibody production when used in an inactivated influenza A virus (IAV) vaccine. Additionally, DDO induces Th1 and CD8+ T-cell responses able to protect against heterosubtypic IAV challenge. Interestingly, DDO synergized with AddaVax and skewed the immune response towards type-1 immunity. The adjuvancy of DDO alone and in synergy with AddaVax was heavily dependent on type I interferon signaling. Our data support a critical role for type I interferon in the induction of type-1 immune responses during vaccination and demonstrate that DDO is a type-1 immunity orienting vaccine adjuvant that can be used alone or in synergy with other adjuvants.
Keywords: Defective viral genomes, Influenza vaccine, Adjuvant, Type-1 immunity
1. Introduction
Subunit and inactivated vaccines are ideal for vaccine development because they do not revert to virulence and are unlikely to cause disease in immunocompromised individuals. Unfortunately, inactivated and subunit vaccines lack the danger signals required to induce robust adaptive immunity. Thus, adjuvants that boost and shape the immune response towards vaccinated antigens are added to improve vaccine efficacy.
Currently, there are no licensed vaccine adjuvants that induce robust type-1 immunity [1–4]. Type-1 immune responses are important for the control of viruses and other intracellular pathogens and are characterized by the generation of cytotoxic CD8+ T-cells, Th1 CD4+ T-cells, and antibodies of the isotypes IgG2b/c [5,6]. Induction of type-1 immunity is also critical for pathogens where induction of other types of immunity leads to enhanced pathogenesis. For example, infants with type-2 immunity-primed lungs suffer from increased morbidity when infected with respiratory pathogens, such as respiratory syncytial virus (RSV) or rhinovirus [7–9].
Alum, the oldest and most widely used vaccine adjuvant, induces robust type-2 immunity. While effective against extracellular pathogens, type-2 immunity does not protect against most intracellular pathogens [10]. In addition to Alum, the TLR4 ligand monophosphoryl lipid A (MPL) absorbed onto Alum, named adjuvant system04 (AS04) [11], and oil-in-water emulsions, such as MF59, are approved for use in human vaccines [10]. These can induce protective antibodies and mild Th1 responses, but no approved vaccines use these adjuvants to induce protective CD8+ T-cell responses [10].
Many type-1 immunity-inducing adjuvant candidates rely on pathogen associated molecular patterns (PAMPs) recognized by pattern recognition receptors including Toll-like receptors (TLRs) and RIG-I-like receptors (RLRs) [12,13]. The use of natural PAMPs more closely mimics the immune responses obtained during infections, such as the induction of type I interferon (IFN). In addition to MPL used in AS04, synthetic PAMPs have been examined as potential type-1 immunity inducing adjuvants. These include the viral RNA mimic polyI:C, which failed to induce strong type-1 immune responses without toxicity [14], and CpG, which is used in a combination adjuvant [15]. Notably, emulsions provide an ideal platform for adjuvant synergy and the combinatorial effects of using multiple adjuvants in a single vaccine can be exploited to generate optimized adjuvants.
We previously identified a Sendai virus PAMP originating from a copy-back defective viral genome (DVG) naturally generated during viral replication [16] and characterized the immunostimulatory RNA motif responsible for recognition by RLRs [17]. Subcutaneous injection of a synthetic version of this 546nt DVG (DVG-546) resulted in a local and distinct cytokine profile from polyI:C [18]. Additionally, DVG-546 promoted the accumulation of DCs in draining lymph nodes of mice. Mice vaccinated with DVG-546 and inactivated RSV developed type-1 immunity-associated antibody responses [18]. Additional work indicated an important role for RLRs for the induction of protective immunity in DVG-546-adjuvanted vaccines [19]. These studies suggest that derivatives of this molecule are valuable candidates for type-1 immunity inducing adjuvants.
Here we report a 268nt DVG-derived oligonucleotide (DDO) with enhanced immunostimulatory capabilities in vitro [17]. We demonstrate its ability to induce protective type-1 humoral and cellular immune responses during immunization with whole inactivated influenza A virus (inIAV) or a HA-subunit vaccine. Additionally, we show that DDO synergizes with AddaVax (the research version of MF59) to induce potent type-1 polarized immune responses and that both humoral and Th1 responses elicited by vaccines adjuvanted with DDO rely on type I IFN.
2. Methods
2.1. Ethics statement
Studies in mice were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animal of the National Institute of Health. The protocol (804691) was approved by the Institutional Animal Care and Use Committee, University of Pennsylvania Animal Welfare Assurance Number A3079-01.
2.2. Mice and viruses
C57BL/6 mice (6–8 weeks old) were obtained from Jackson Laboratory. Ifnar1−/− mice [20] were a kind donation of Dr. Thomas Moran (Icahn School of Medicine at Mount Sinai) and were used with gender and age matched C57BL/6 mice (Jackson Laboratory bred in house). All experiments were performed in male and female mice. Influenza A/X-31 H3N2 (IAV X-31) and A/California/7/2009 H1N1 with D225G HA mutation (IAV-Cal/09-D225G) that allows the wild-type A/California/7/2009 virus to grow in eggs [21] were used as challenge strains. All strains of IAV were grown in 10 day-old embryonated chicken eggs (Charles River Laboratory) at 30,000 medium tissue culture infectious dose (TCID50) at 37 °C. Allantoic fluid from infected eggs was collected 40 h later.
2.3. Vaccine formulation
Inactivated IAV (inIAV) vaccine: Influenza A/Puerto Rico/8/1934 H1N1 (IAV PR/8) was harvested from allantoic fluid of 10 day old embryonated eggs and purified through a 35% sucrose cushion. Virus was inactivated with UV light (254 nm at 6-inch distance) for 40 min. Inactivation was confirmed by the inability of the virus to replicate in MDCK cells (Madin-Darby canine kidney cells, gift from Dr. Scott Hensley, University of Pennsylvania) in the presence of 2 mg/ml trypsin. The inIAV vaccine had a total protein concentration of 989 μg/ml, HA titer of 10240U/ml at a 1:100 dilution and an endotoxin level of <1.2 EU/ml. Recombinant IAV-HA protein from IAV-Cal/09-D225G used as a subunit vaccine was obtained from BEI Resource (NR-13691). DDO is a 268nt non-coding, replication-incompetent ssRNA sequence that contains the immunostimulatory DVG motif identified previously [17,18]. DDO was produced, stored, and used as previously described [17]. Purity and integrity of DDO were confirmed using an Agilent Bioanalyzer 2100 and had an OD260/280 ratio of 2.16, an OD260/230 ratio of 2.3, and endotoxin level below 0.1EU/ml/300ug.
2.4. Mouse immunization and challenge
For immunization, mice were anesthetized with isoflurane and injected intramuscularly (i.m.) into the thigh with 10 μg inIAV vaccine or 1 μg recombinant IAV-HA protein diluted in PBS adjuvanted with 5 μg DDO, AddaVax (InVivogen) at 50% v/v, or Alum (Alhydrogel 2%, InvivoGen) at 50% v/v at final volume of 50 μl per dose. Mice were primed and boosted 14 days later with the same vaccine formulation. In some experiments, mice were challenged intranasally with 103.5 TCID50 of IAV X-31 (heterosubyptic challenge) or 2 × 104 TCID50 of IAV-Cal/09-D225G 21 days after boost. All mice were weighed daily post-challenge. Lung tissue was harvested 4 or 10 days post-challenge for viral load quantification or histology.
2.5. Viral load quantification
IAV titration was performed by limiting dilution in MDCK cells as previously described [18]. For quantifying IAV-NP transcripts in lung homogenate, 1–2 μg of RNA isolated by TRIzol (Invitrogen) was reversed transcribed using high capacity RNA to cDNA reagents (Applied Biosystem). qPCR assays were performed using SYBR Green PCR Master Mix (Applied Biosystem) in a Viia7 Applied Biosystem Lightcycler. Primers used in the assay were: Gapdh for-5′-ctcccactcttccaccttcg-3′ and rev-5′-ccaccaccctgttgctgtag-3′ and IAV-NP for-5′-cagcctaatcagaccaaatg-3′ and rev-5′-tacctgcttctcagtt caag-3′.
2.6. Quantification of influenza-specific serum antibodies
Sera from immunized mice were analyzed for anti-IAV total IgG, IgG1, and IgG2c antibodies on day 14 post-boost using ELISA to evaluate peak antibody response [18]. Briefly, sera pre-diluted (1:100, 1:1000, and 1:10,000) were added in triplicate to ELISA plates (Immulon, 4 HBX Extra High Binding) coated with 5 μg/ml purified IAV or 2 μg/ml IAV HA protein (BEI Resource, #NR-13691) followed by HRP-conjugated anti-mouse IgG, IgG1, or IgG2c (Southern Biotech) and ABTS substrate (Roche).
2.7. Flow cytometry
Single-cell suspensions of spleen and inguinal lymph node were prepared and stained with fluorochrome-labeled antibodies as previously described [22]. LIVE/DEAD Fixable Near-IR Dead stain was obtained from Invitrogen. Fixable Viability Dye eFluor506, monoclonal antibody specific for mouse CD8 (clone 53-6.7), IFNγ (clone XMG1.2), and TNFα (clone MP6-XT22) were obtained from eBio-science. Monoclonal antibodies specific for mouse CD3 (clone 17A2), CD4 (clone GK1.5), CD11a (clone H11578), CD44 (clone IM7) and MHC-II (clone M5/114.15.2) were obtained from Biolegend. Monoclonal antibodies specific for mouse CD8 (clone 53-6.7) were obtained from BD BioSciences. IAV-specific tetramers: H-2Db tetramers bearing NP336-374 (ASNENMETM) and I-Ab tetramers bearing NP311-325 (QVYSLIRPNENPAHK) were obtained from NIH Tetramer Core Facility at Emory University. Samples were acquired on a LSRFortessa (BD Bioscience) cytometer and analyzed using the FlowJo Software (TreeStar). We restimulated splenocytes using PMA (0.1 mg·mL) and Ionomycin (1mg/ml) for 4 h at 37 °C.
2.8. Lung pathology
On day 10 post heterosubtypic challenge, the left lobe of the lung was inflated and fixed with 1 mL of 10% buffered formalin solution. Fixed lungs were paraffin-embedded and sectioned using standard procedures. Lung sections were cleared of paraffin and Periodic acid–Schiff (PAS) stained. Lung sections were blinded and small airways were scored. Epithelial metaplasia severity was scored according to the following scale: 0, no metaplasia; 1, mild (2 layers); 2, moderate (3 layers); 3, severe (>4 layers). Percent of airways experiencing epithelial metaplasia were scored: 0, no airways; 1, <25% of airways; 2, 26–50%; 3, 51–75%; 4, >75%. Goblet cell hyperplasia severity was scored: 0, none; 1, <10 PAS+ cells/airway; 2,10–20 cells; 3, >20 cells. Percent of airways experiencing hyperplasia were scored; 0, no airways; 1, <10%; 2, 10–20%; 3, 20–40%; 4, >40%.
2.9. Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 for Mac (GraphPad Software). RT-qPCR and flow cytometry data were analyzed by one-way ANOVA followed by Bonferroni’s multiple comparison post hoc test. Weight-loss and ELISA data were analyzed by two-way ANOVA followed by Bonferroni’s multiple comparison post hoc test.
3. Results
3.1. DDO is a thermostable small RNA with strong immunostimulatory activity
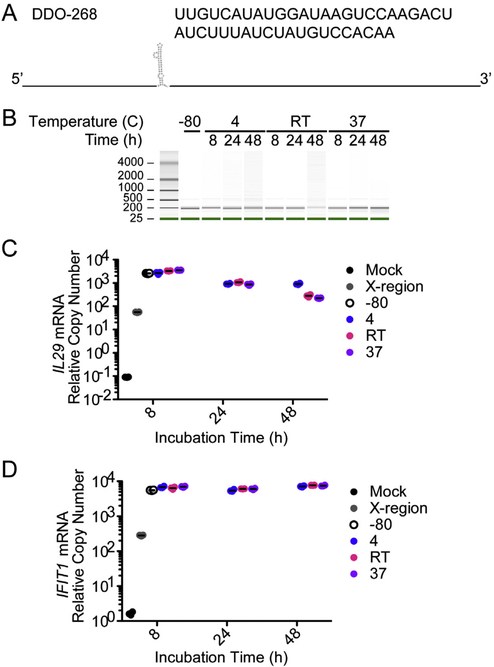
DDO containing a previously described motif responsible for its immunostimulatory activity (Fig. 1A) are stable at −80 °C for more than a year [17]. To test the stability of DDO across multiple vaccination-relevant temperatures, DDO were stored at 4 °C, room temperature (26 °C/RT), or 37 °C for up to 48 h and analyzed for degradation using a Bioanalyzer. DDO showed no appreciable degradation at any of the conditions tested (Fig. 1B). To test for immunostimulatory activity, RNA stored at each condition was transfected into cells and cells were examined by RT-qPCR for the expression of IL29 (also known as IFNλ) and the IFN-stimulated gene IFIT1, two early indicators of antiviral activity. After 8 h of incubation at different temperatures, DDO was as immunostimulatory as DDO stored at −80 °C. After 24 h, while there was no visible degradation, IL29 expression slightly decreased in all 3 conditions, but DDO remained more immunostimulatory than the HCV X-region [17], a similarly structured RNA lacking immunostimulatory capabilities (Fig. 1C). Interestingly, the levels of IFIT1 remained constant across all DDO conditions and time points indicating that the slight decline in primary IFN production observed did not impact downstream gene expression (Fig. 1D).
Fig. 1.
DDO-268 is a thermostable small RNA with strong immunostimulatory activity. (A) DDO is a 268 nucleotide single-stranded RNA with an immunostimulatory motif shown in its secondary structure together with its sequence. DDO was incubated at 4 °C, room temperature (26 °C/RT), or 37 °C for 8 h, 24 h, or 48 h. (B) Integrity of the RNA was analyzed using electrophoretic analysis on a Bioanalyzer. (C and D) Expression of IL29 (C) and IFIT1 (D) mRNA measured by RT-qPCR from A549 cells transfected for 6 h with 4.15pmo DDO used in (B). The experiment was repeated 3 times with a single representative repeat shown here. Data are expressed as copy numbers relative to the housekeeping gene GAPDH, value represent mean ± SEM from triplicate technical repeats.
3.2. DDO promotes an IgG2c-biased antibody response and T-cells associated with type-1 immunity
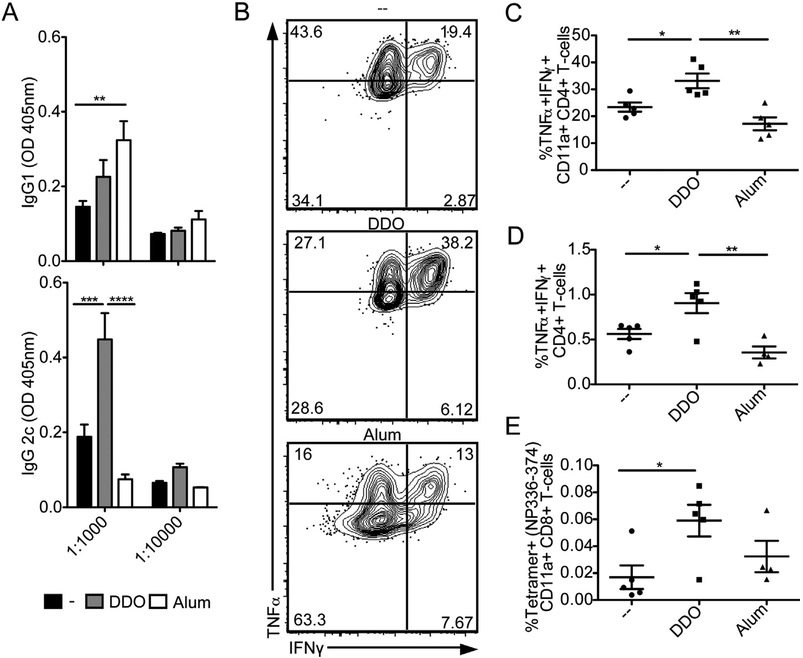
To assess whether DDO-adjuvanted vaccines enhance adaptive immunity against vaccinated antigens, we evaluated the antibody response generated against IAV 14 days after boost immunization with inIAV alone or adjuvanted with DDO or Alum. While Alum induced a type-2 immunity-associated IgG1 response, DDO significantly enhanced the type-1 immunity-associated IgG2c response (Fig. 2A).
Fig. 2.
DDO promotes type-1 immunity. C57BL/6 mice immunized on day 0 and day 14 with 10 μg of UV-inactivated IAV PR/8 (inIAV) alone or adjuvanted with 5 μg DDO or with 50% v/v Alum. (A) H1N1-IAV specific IgG1 and IgG2c antibodies in sera of mice on day 14 post-boost. Data correspond to the mean ± SEM of ELISA at 1:1000 and 1:10000 dilution point from the sera of vaccinated mice (n = 5/group). **p < 0.01, ***p < 0.001, ****p < 0.0001 by two-way ANOVA with Bonferroni’s multiple comparison post-hoc test. (B-E) Antigen-experienced cells in the spleen and draining lymph nodes were examined on day 7 post-boost using flow cytometry. (B) Representative flow cytometry plots for IFNγ+ TNFα+, CD11a+CD4+ T-cells from the spleens of vaccinated mice. IFNγ+ TNFα+ were identified by gating on live, singlets, CD3+, CD4+CD8−, CD11a+ cells. (C) Percentage of IFNγ+ TNFα+, CD4+ T-cells in the spleens of individual mice in each vaccination group after PMA/Ionomycin restimulation. (D) Percentage of IFNγ+ TNFα+, CD4+ T-cells in the draining lymph nodes of individual mice in each vaccination group after PMA/Ionomycin restimulation. (E) Percentage CD11a+Tetramer+ (NP336-374) CD8+ T-cells from the spleens of individual mice in each vaccination group. Percentage of was quantified by intracellular cytokine staining after gating on live, singlets, CD3+, CD4−CD8+, CD11a+. Data correspond to individual mice with mean ± SEM (n = 5/group). *p < 0.05, **p < 0.01 by one-way ANOVA with Bonferroni’s multiple comparison test.
To assess how DDO influence the T-cell response, spleens and draining (inguinal) lymph nodes were harvested 7 days postboost immunization. Mice that received DDO-inIAV had significantly more TNFα+ IFNγ+ Th1 cells in the spleen (Fig. 2B and C) and draining lymph node (Fig. 2D) than mice immunized with Alum-inIAV or inIAV alone. Additionally, DDO-inIAV-vaccinated mice had significantly more IAV-specific CD8+ T-cells (Fig. 2E). Together, these results demonstrate that DDO induces type-1 immunity when used as a vaccine adjuvant.
3.3. Immunization with DDO adjuvant induces long-term CD8+ T-cell protection against heterosubtypic challenge
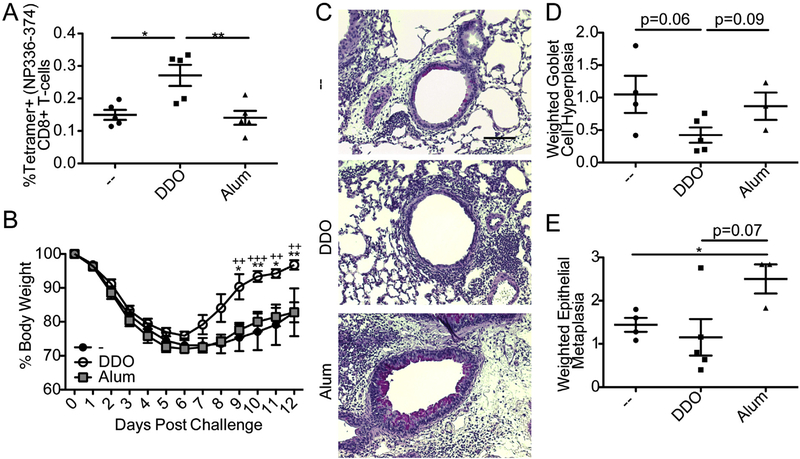
We next evaluated the longevity and effectiveness of CD8+ T-cell responses induced by DDO. Mice immunized with DDO-inIAV had significantly more IAV-specific CD8+ T-cells on day 46 post-boost than mice immunized with Alum-inIAV or inIAV alone (Fig. 3A). To better understand the effector capabilities of the CD8+ T-cells induced by DDO-adjuvanted vaccines, mice were challenged with heterosubtypic IAV differing only in external hemagglutinin (H) and neuraminidase (N) proteins. As internal proteins are not targeted for antibody neutralization, heterosubtypic challenge after immunization heavily relies on CD8+ T-cells for clearance and recovery. Mice immunized with inIAV PR/8 (H1N1) were challenged 21 days after-boost with IAV X-31 (H3N2). Mice immunized with DDO-inIAV recovered more quickly than mice immunized with Alum-inIAV or inIAV alone (Fig. 3B).
Fig. 3.
DDO promotes long-term CD8+ T-cell memory and heterosubtypic immunity to IAV after prime-boost immunization. (A) Percentage of IAV-tetramer specific CD8+ T-cells in the spleens of mice on day 46 post-boost with 10 μg of inIAV alone or adjuvanted with 5 μg DDO or 50% v/v Alum. Tetramer+CD44+ positive cells were identified after gating on singlets, live, CD3+CD4−CD8+ cells. Data correspond to individual mice with mean ± SEM (n = 5/group). *p < 0.05, **p < 0.01 by one-way ANOVA with Bonferroni’s multiple comparison test. (B-E) Mice were challenged with 103.5 TCID50 IAV-X31 (H3N2) intranasally on day 21 after boost immunization. (B) Weight loss. Data correspond to mean ± SEM of each group (n = 5/group). *p < 0.05, **p < 0.01, ***p < 0.001 by two-way ANOVA with Bonferroni’s multiple comparison test. * compares DDO to controls (−). + compares DDO to Alum. (C) Representative images of paraffin-fixed PAS stained lungs on day 10 post-infection. Magenta staining indicates mucus positive cells. Scale bar = 100 μm. (D) Weighted score of goblet cell hyperplasia calculated by multiplying the score for amount of airways affected by the intensity score and divided by 100. (E) Weighed score of epithelial cell metaplasia scored as in (D). Statistics by T test between each individual group. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
To characterize the pathology induced upon infection in the absence of neutralizing antibodies, lungs were harvested 10 days post-heterosubtypic challenge and PAS stained. Magenta PAS staining indicates mucus and mucus-containing cells such as goblet cells, a characteristic of type-2 immunity associated pathology. Both inIAV and Alum-inIAV-vaccinated mice showed stronger PAS staining and goblet cell hyperplasia compared to DDO-inIAV (Fig. 3C and D). Additionally, Alum-treated mice had airway thickening and increased epithelial cell metaplasia (Fig. 3C and E). Together, these results indicate that DDO adjuvants induce long lasting protective CD8+ T-cell responses without type-2 immunity-associated pathology.
3.4. DDO synergizes with AddaVax to skew immunity towards protective type-1 immune responses upon subunit vaccination
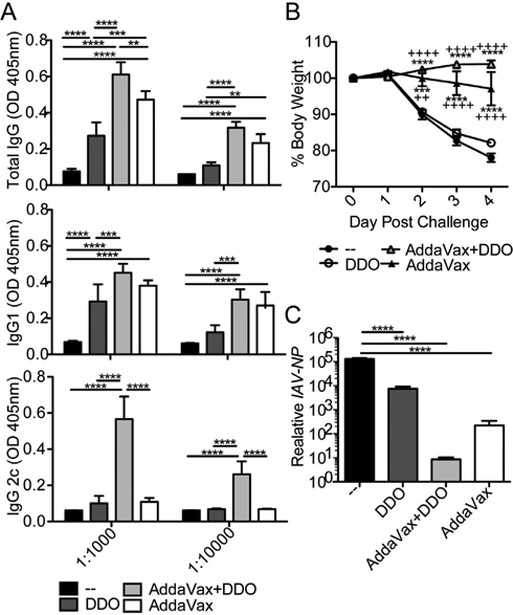
AddaVax, the research version of MF59, induces a mixed type-1 and type-2 immune response. As adjuvant synergy is a promising approach to improve and shape immune responses after vaccination [3], we next tested whether DDO and AddaVax synergized. AddaVax + DDO-adjuvanted immunizations boosted the IgG response above those of mice immunized with either adjuvants or HA alone. More importantly, in a subunit vaccine, DDO significantly increased IgG2c antibody induction when combined with AddaVax (Fig. 4A). While both AddaVax-HA and AddaVax + DDO-HA protected from significant weight loss (Fig. 4B), AddaVax + DDO-HA better protected mice from viral replication measured by the expression of viral NP RNA in lung homogenates on day 4 post-challenge (Fig. 4C). Together, these results show that Addax and DDO synergize to induce potent protective humoral immunity characterized by IgG2c antibodies upon subunit IAV vaccination.
Fig. 4.
DDO and AddaVax synergize to induce protective antibodies of the isotype IgG2c to a protein (HA) vaccine. (A) IAV H1N1-HA specific IgG, IgG1, and IgG2c antibodies in the sera of C57BL/6 mice two weeks after a second i.m. immunization with 1 μg HA protein alone (−) or adjuvanted with 50% v/v AddaVax, 50% v/v AddaVax plus 5 μg DDO (AddaVax + DDO), or 5ug DDO. Data correspond to the mean ± SEM of ELISA at 1:1000 and 1:10000 dilution point from the sera of and vaccinated mice (n = 5/group)., **p < 0.01, ***p < 0.001, ****p < 0.0001 by two-way ANOVA with Bonferroni’s multiple comparison test. (B-C) Mice were challenged with 2 × 104 TCID50 of IAV-Cal/09-D225G three weeks after immunization. (B) Weight loss. **p < 0.01, ***p < 0.001 and ****p < 0.0001 by two-way ANOVA with Bonferroni’s multiple comparison test. * compare to controls (−), + compare to DDO. (C) Expression of IAV-NP RNA Relative to Gapdh mRNA in lung homogenate of the mice 4 days after challenge was analyzed by RT-qPCR Data correspond to mean ± SEM of individual mice (n = 5/group). ****p < 0.0001 by one-way ANOVA with Bonferroni’s multiple comparison test.
3.5. DDO relies on type I IFN to induce humoral and cellular immunity
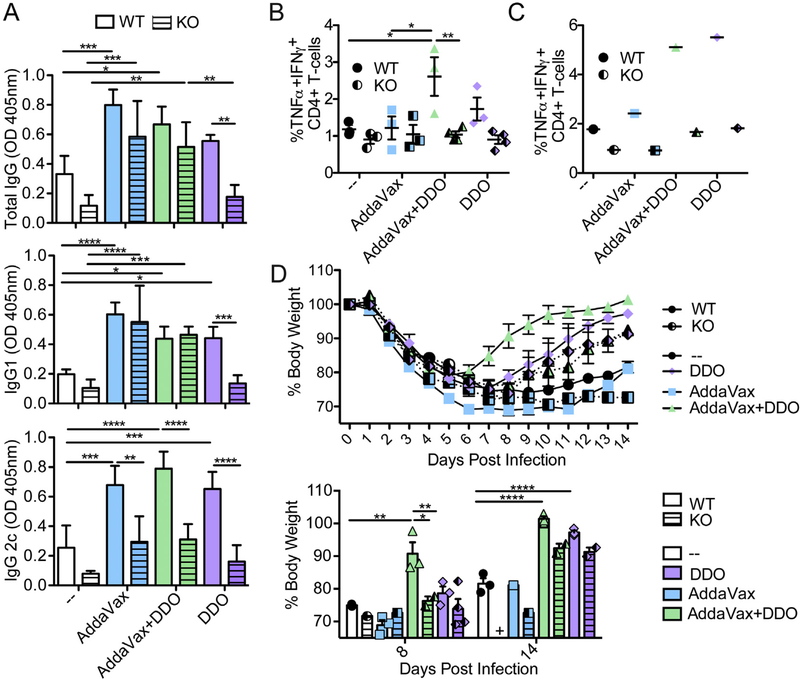
DDO potently induce type I IFN [16–18]. To begin to understand the mechanism of action of DDO adjuvants, we immunized wild-type (WT) or Ifnar1−/− (KO) mice with inIAV alone or adjuvanted with DDO, AddaVax, or AddaVax + DDO. On day 14 post-boost immunization, DDO-inIAV immunized mice required type I IFN for IgG1 and IgG2c antibody production (Fig. 5A). Interestingly, all other vaccine formulations required type I IFN only for IgG2c induction. These results indicate that IFN is critical to induce an IgG2c response across adjuvant formulations and for DDO-induced antibody responses in general.
Fig. 5.
DDO adjuvancy depends on type I IFN signaling. Wild-type (WT) and Ifnar1−/−(KO) C57BL/6 mice were immunized i.m. at day 0 and 14 with 10 μg of inIAV alone (−) or adjuvanted with 50% v/v AddaVax, 5 μg DDO, or 50% v/v AddaVax plus 5 μg of DDO (AddaVax + DDO). (A) IAV specific IgG, IgG1, and IgG2c antibodies in the sera of WT (solid bars) and KO (striped bars) mice two weeks after boost immunization. Data correspond to the mean ± SEM of ELISA at a 1:1000 dilution point from the sera of vaccinated mice (n = 3–4/group). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 by two-way ANOVA with Bonferroni’s multiple comparison test. (B) Percentage of IFNγ+ TNFα+, CD4+ T-cells in spleens was quantified by intracellular cytokine staining after gating on live, singlets, CD3+, CD4+CD8−, PMA/Ionomycin restimulated cells. Data correspond to individual mice with mean ± SEM (n = 3–4/group) *p < 0.05, **p < 0.01 by one-way ANOVA with Bonferroni’s multiple comparison test. (C) Percentage of IFNγ+ TNFα+, CD4+ T-cells in inguinal lymph nodes was quantified as in (B). Data correspond to pooled 3–4 lymph nodes. (D) Mice were challenged with 103.5 TCID50IAV-X31 (H3N2) intranasally on day 21 after boost immunization. Weight loss for WT mice (solid line and symbol) and KO mice (dashed line and half black symbol) is shown. Bottom panel: weight loss on days 8 and 14 post-heterosubtypic challenge of WT (solid bars and symbols) and KO (striped bars and half black symbols) from data in top panel. Data correspond to the mean ± SEM and show individual mice. + indicates no mice survived to time point. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 by two-way ANOVA with Bonferroni’s multiple comparison test.
To explore the role of type I IFN in the induction of Th1 cells, we vaccinated mice as described above and harvested spleens and draining lymph nodes on day 7 post-boost. Consistent with the dependence on type I IFN for antibody induction, TNFα+IFNγ+ Th1 cells in the spleens (Fig. 5B) and lymph nodes (Fig. 5C) required type I IFN when adjuvanted in the presence of DDO.
To test whether type I IFN is required for protection against heterosubtypic infection, mice from Fig. 5A were challenged with heterosubtypic IAV (H3N2) on day 21 post-boost. WT mice vaccinated with AddaVax + DDO-inIAV recovered more rapidly than AddaVax-inIAV and DDO-inIAV immunized mice (Fig. 5D), suggesting that DDO synergized with AddaVax to improve heterosubtypic protection. This synergy relied significantly on type I IFN, while immunization in the presence of DDO alone was less dependent on type I IFN to promote recovery from heterosubtypic challenge (Fig. 5D bottom panel, days 8 and 14 post-challenge). Together, these results indicate that type I IFN is crucial to induce protective cellular immunity upon AddaVax + DDO-adjuvanted vaccinations.
4. Discussion
The lack of type-1 immunity-inducing adjuvants has hindered the development of non-replicative vaccines against intracellular pathogens. Here we show that DDO is an excellent candidate for further development as a type-1 immunity-inducing adjuvant. DDO is a stable, homogenous, and replication-incompetent 268nt-long RNA oligonucleotide. DDO is created using in vitro transcription, a process amenable to mass production. This RNA adjuvant is safe in mice [18] and induces the hallmarks of type-1 immune responses: Th1 cells, CD8+ T-cells, and IgG2c antibodies.
A new strategy in the vaccination field is to combine adjuvants to boost and skew immune responses [10]. AddaVax, the research equivalent of the licensed oil-in-water emulsion adjuvant MF59, induces a mixed type-1/type-2 immune response [2,3,23]. We combined AddaVax and DDO to take advantage of the type-1 skewing properties of DDO to achieve a strong, protective type-1 response in a model IAV vaccine. Using a subunit vaccination approach (HA protein), we show that DDO + AddaVax synergize to induced more robust immune responses than each individual adjuvant alone. This synergy resulted in skewing of the humoral response towards IgG2c and better protection from challenge. In an inactivated virus vaccination approach, AddaVax + DDO synergy protected from heterosubtypic challenge and increased T-cell responses better than DDO alone. Interestingly, the synergy appears to use different mechanisms for whole virus and protein, which could be due to intrinsic adjuvancy from inIAV as it still contains RNA genomes that could act as a PAMP.
DDO efficacy relies heavily on type I IFN for Th1 induction and humoral responses. Despite this, protection from heterosubtypic challenge did not require type I IFN signaling. In contrast, the enhanced heterosubtypic protection by AddaVax + DDO synergy relied on type I IFN. The different requirement for type I IFN signaling further supports the hypothesis of a different mechanism of action for DDO alone and in combination with AddaVax. It is possible that AddaVax + DDO combinations may result in enhanced and distinct local and/or systemic inflammation. Potentially, Adda-Vax could deliver DDO to different cells or lead to different intracellular localization compared to DDO alone. Secondary effects of DDO used in combination with AddaVax should be carefully evaluated.
Type I IFN strongly promotes type-1 immunity during viral infections by enhancing DC maturation and trafficking, positively regulating effector and memory Th1 CD4+ T-cell generation, supporting CD8+ T-cell priming, and instructing isotype switching to IgG2c [24–26]. Interestingly, the few available T-cell-inducing vaccine adjuvants rely on the cytokines IL-12 or IL-27 rather than IFN [27]. However, the experimental adjuvant chitosan, which promotes cellular immunity through the DNA sensor cGAS-STING, also relies on IFN signaling [28]. Investigation on how type I IFN is being induced, sensed, and what cells produce it is important to understand the mechanisms of DDO adjuvancy. A better understanding of the requirements of a type-1 immunity inducing vaccine adjuvant could lead to more informed design of vaccines for intracellular pathogens.
In summary, the antibody isotype induction, T-cell cytokine production, and protection from heterosubtypic challenge demonstrated here with DDO-adjuvanted vaccines illustrate the ability of this molecule to induce type-1 immunity and its potential for future development.
Acknowledgements
The authors wish to thank: Dr. Scott Hensley (University of Pennsylvania) for advising on virus challenge for IAV-Cal/09-D225G. We acknowledge the NIH Tetramer Core Facility (contract HHSN272201300006C) for provision of the IAV-NP tetramers. This work was supported by the US National Institutes of Health National Institute of Allergy and Infectious Diseases (NIH R01 AI083284 to C.B.L).
Footnotes
Conflicts of interest
A patent has been filed for DDO adjuvant by C.B.L. and the University of Pennsylvania
References
- [1].Subbarao K, Joseph T. Scientific barriers to developing vaccines against avian influenza viruses. Nat RevImmunol 2007;7(4):267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity 2010;33(4):492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bergmann-Leitner E, Leitner W. Adjuvants in the driver’s seat: how magnitude, type, fine specificity and longevity of immune responses are driven by distinct classes of immune potentiators. Vaccines 2014;2(2):252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Farrar W Anthrax: virulence and vaccines. Ann Intern Med 1994;121 (5):379–80. [DOI] [PubMed] [Google Scholar]
- [5].Seder RA, Hill AVS. Vaccines against intracellular infections requiring cellular immunity. Nature 2000;406(6797):793–8. [DOI] [PubMed] [Google Scholar]
- [6].Mckee AS, Macleod MK, Kappler JW, Marrack P. Immune mechanisms of protection: can adjuvants rise to the challenge? BMC biology 2010;8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Perez GF, Pancham K, Huseni S, et al. Rhinovirus-induced airway cytokines and respiratory morbidity in severely premature children. Pediatr Allergy Immunol 2015;26(2):145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Message SD, Laza-Stanca V, Mallia P, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci USA 2008;105(36):13562–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Becker Y Respiratory syncytial virus (RSV) evades the human adaptive immune system by skewing the Th1/Th2 cytokine balance toward increased levels of Th2 cytokines and IgE, markers of allergy-a review. Virus Genes 2006;33(2):235–52. [DOI] [PubMed] [Google Scholar]
- [10].Mckee AS, Marrack P. Old and new adjuvants. Curr Opin Immunol 2017;47:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Didierlaurent AM, Morel S, Lockman L, et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol 2009;183 (10):6186. [DOI] [PubMed] [Google Scholar]
- [12].Akira S Innate immunity and adjuvants. Philos Trans Roy Soc Lond Ser B Biol Sci 2011;366(1579):2748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pashine A, Valiante NM, Ulmer JB. Targeting the innate immune response with improved vaccine adjuvants. Nat Med 2005;11(4 Suppl):S63–68. [DOI] [PubMed] [Google Scholar]
- [14].Caskey M, Lefebvre F, Filali-Mouhim A, et al. Synthetic double-stranded RNA induces innate immune responses similar to a live viral vaccine in humans. J Exp Med 2011;208(12):2357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Foulds KE, Wu CY, Seder RA. Th1 memory: implications for vaccine development. Immunol Rev 2006;211:58–66. [DOI] [PubMed] [Google Scholar]
- [16].Tapia K, Kim W-K, Sun Y, et al. Defective viral genomes arising in vivo provide critical danger signals for the triggering of lung antiviral immunity. PLOS Pathogens 2013;9(10):e1003703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xu J, Mercado-López X, Grier JT, et al. Identification of a natural viral RNA motif that optimizes sensing of viral RNA by RIG-I. mBio 2015;6(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mercado-Lopez X, Cotter CR, Kim WK, et al. Highly immunostimulatory RNA derived from a Sendai virus defective viral genome. Vaccine 2013;31 (48):5713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Martinez-Gil L, Goff PH, Hai R, Garcia-Sastre A, Shaw ML, Palese P. A Sendai virus-derived RNA agonist of RIG-I as a virus vaccine adjuvant. J Virol 2013; 87(3):1290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Muller U, Steinhoff U, Reis LF, et al. Functional role of type I and type II interferons in antiviral defense Science (New York, NY: ) 1994;264 (5167):1918–21. [DOI] [PubMed] [Google Scholar]
- [21].Linderman SL, Chambers BS, Zost SJ, et al. Potential antigenic explanation for atypical H1N1 infections among middle-aged adults during the 2013–2014 influenza season. Proc Natl Acad Sci 2014;111(44):15798–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Muallem G, Wagage S, Sun Y, et al. IL-27 limits type 2 immunopathology following parainfluenza virus infection. PLOS Pathogens 2017;13(1): e1006173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Van Aalst S, Ludwig IS, Van Kooten PJ, Van Der Zee R, Van Eden W, Broere F. Dynamics of APC recruitment at the site of injection following injection of vaccine adjuvants. Vaccine 2017;35(12):1622–9. [DOI] [PubMed] [Google Scholar]
- [24].Crouse J, Kalinke U, Oxenius A Regulation of antiviral T cell responses by type I interferons. Nat RevImmunol 2015;15(4):231–42. [DOI] [PubMed] [Google Scholar]
- [25].Huber JP, Farrar JD Regulation of effector and memory T-cell functions by type I interferon. Immunology 2011;132(4):466–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Longhi MP, Trumpfheller C, Idoyaga J, et al. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med 2009;206(7):1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pennock ND, Kedl JD, Kedl RM. T cell vaccinology: beyond the reflection of infectious responses. Trends Immunol 2016;37(3):170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Carroll EC, Jin L, Mori A, et al. The vaccine adjuvant chitosan promotes cellular immunity via DNA sensor cGAS-STING-dependent induction of type I interferons. Immunity 2016;44(3):597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]