Abstract
Preclinical and clinical evidence suggests that mesenchymal stem cells (MSCs) may be beneficial in treating both acute myocardial infarction (AMI) and ischemic heart failure (IHF). However, the safety profile and efficacy of MSC therapy is not well‐known. We conducted a systematic review of clinical trials that evaluated the safety or efficacy of MSCs for AMI or IHF. Embase, PubMed/Medline, and Cochrane Central Register of Controlled Trials were searched from inception to September 27, 2017. Studies that examined the use of MSCs administered to adults with AMI or IHF were eligible. The Cochrane risk of bias tool was used to assess bias of included studies. The primary outcome was safety assessed by adverse events and the secondary outcome was efficacy which was assessed by mortality and left ventricular ejection fraction (LVEF). A total of 668 citations were reviewed and 23 studies met eligibility criteria. Of these, 11 studies evaluated AMI and 12 studies evaluated IHF. There was no association between MSCs and acute adverse events. There was a significant improvement in overall LVEF in patients who received MSCs (SMD 0.73, 95% CI 0.24–1.21). No significant difference in mortality was noted (Peto OR 0.68, 95% CI 0.38–1.22). Results from our systematic review suggest that MSC therapy for ischemic heart disease appears to be safe. There is a need for a well‐designed adequately powered randomized control trial (with rigorous adverse event reporting and evaluations of cardiac function) to further establish a clear risk‐benefit profile of MSCs. Stem Cells Translational Medicine 2018;7:857–866
Keywords: Stem cells, Myocardial infarction, Myocardial ischemia, Systematic review
Significance Statement.
This study provides a comprehensive evaluation of the safety and efficacy of mesenchymal stem cell therapy for acute myocardial infarction and ischemic heart disease. Results from this systematic review suggest that this cellular therapy may be safe. A well‐designed, adequately powered randomized controlled trial with rigorous adverse event reporting and comprehensive assessment of cardiac function is warranted. This will help establish a definitive risk‐benefit profile of mesenchymal stromal cell therapy for ischemic heart disease.
Introduction
Despite major advances in the management of ischemic heart disease, it remains a leading cause of morbidity and mortality worldwide 1. There has been interest in applying cellular therapy to restore cardiac function in ischemic heart disease. One cell type of interest is mesenchymal stem cells (MSCs, adult stem cells), which are a heterogenous population of pericytes that contribute to vascular homeostasis. When MSCs are expanded ex vivo and administered as a therapy, they act via a myriad of paracrine pathways to suppress inflammation and promote organ protection 2, 3, 4. Moreover, MSCs can improve energetics by transferring mitochondria 5, 6. MSCs are potent modulators of the immune system that suppress white blood cells and trigger anti‐inflammatory responses; this may be especially effective in pro‐inflammatory diseases 7, 8.
Previous highly cited clinical systematic reviews have suggested that cell therapy has no efficacy for ischemic heart disease 9, 10. Of note, the results of these reviews have been driven predominantly by large clinical trials using autologous bone marrow mononuclear cells. Given that preclinical meta‐analysis has suggested that MSCs may be more efficacious than other cell types in treating models of ischemic heart disease 11, a methodologically rigorous clinical systematic review focused on MSC therapy is needed. In addition, potential questions surrounding the safety of cellular therapy in this vulnerable population still remain (e.g., embolic phenomena) 12. Therefore, the aim of this systematic review is to evaluate the safety and efficacy of MSC therapies in patients with ischemic heart disease, specifically acute myocardial infarction (AMI) and ischemic heart failure (IHF). This review will help inform future clinical trials of MSC therapy for ischemic heart disease.
Methods
The protocol for this review was prospectively registered with the PROSPERO database of systematic reviews (https://www.crd.york.ac.uk/PROSPERO/). The protocol was prepared according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) protocol checklist, and the final report was prepared in accordance with the PRISMA checklist (Supporting Information Appendix 10) 13.
Search Strategy
Search strategies were developed in consultation with an information specialist (Supporting Information Appendix 1), and underwent a Peer Review of Electronic Search Strategy 14 (Supporting Information Appendix 2). Searches were performed in the following databases: Embase, PubMed/Medline, and Cochrane Central Register of Controlled Trials, from inception to September 27, 2017. http://clinicaltrials.gov was searched for recently completed trials. We did not impose any language restrictions on our search. Reference lists of relevant reviews were searched for eligible articles.
Eligibility Criteria
We included interventional (controlled and noncontrolled, randomized and nonrandomized) and observational (cohort with contemporary or historical comparison group) studies that examined the safety and/or efficacy of MSCs administered to adults with AMI or IHF. Patients with cardiac diseases of nonischemic etiology were excluded, as well as nonadult participants and preclinical studies. MSCs were defined using the criteria outlined by the International Society for Cellular Therapy 15 as a general guide: (1) adherence to plastic, (2) specific antigen expression profile (i.e., CD105 (+), CD45 (−), HLA‐DR (−), etc.), and (3) in vitro differentiation potential to osteoblasts, adipocytes, and chondroblasts. We excluded studies which treated patients with differentiated MSCs, other cell types or therapies, and MSCs engineered to alter the expression of particular genes (other than those used for imaging purposes). Depending on the study type, the patients receiving MSCs were compared to those receiving standard treatment for the management of AMI and IHF or placebo.
Outcome Measures
The primary outcome of our study was safety, which was assessed based on the frequency of adverse events (AE). AEs were grouped according to the timing of the event (i.e., acute events occurring <24 hours and delayed events occurring >24 hours post treatment) and the organ system affected (i.e., cardiovascular, pulmonary, gastrointestinal, renal, and neurological). Our secondary outcomes focused on the efficacy of MSC therapy evaluated using mortality and changes in left ventricular ejection fraction (LVEF). LVEF at the latest outcome window reported was used for analysis. In several studies cardiac function was measured by more than one modality; however, we only included one modality per study for the analysis of the LVEF outcome. The modality was chosen a priori based on a hierarchy of LVEF modalities, established by expert opinion of cardiologists on our review team (PJD, DJS), from most to least accurate: magnetic resonance imaging (MRI), echocardiogram with contrast, regular echocardiogram and radionuclide angiography scan, sestamibi scan, and cardiac catheterization. A number of tertiary outcomes that were measured at the latest follow‐up were assessed. These outcomes included health‐related quality of life (QoL), performance status (i.e., New York Heart Association Functional Classification), and other indices of cardiac function, including wall motion score, end‐systolic and diastolic volume, stroke volume index, myocardial perfusion, and major adverse cardiac events (MACE).
Study Selection Process
After collating citations identified by our literature search, all duplicate studies were removed. Titles and abstracts were screened for inclusion by four independent reviewers (J.Z., Y.D., S.M., and J.M.). Titles and abstracts deemed potentially relevant were recorded and the full text articles were obtained. Two independent reviewers screened the full articles to assess final eligibility, with disagreements settled by consultation with a senior team member (M.M.L.) to achieve consensus. The study selection process was documented and reported using a flow diagram as recommended by the PRISMA (Figure 1).
Figure 1.
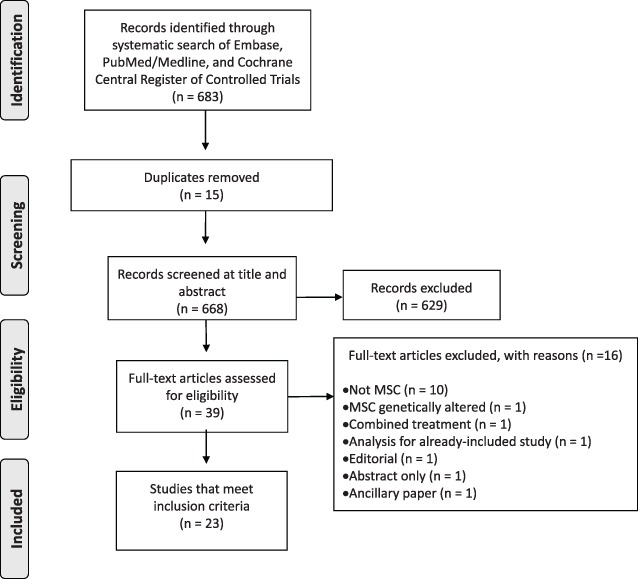
The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram of the study selection process.
Data Collection
Data abstraction was performed by four independent reviewers (J.Z., Y.D., S.M., and J.M.) using standardized forms created in DistillerSR (Evidence Partners, Ottawa, ON). Reviewers discussed discrepancies and if consensus could not be reached, a senior team member (M.M.L.) made a final decision. Each reviewer independently documented publication characteristics (i.e., year of publication, journal, and corresponding author), study populations (i.e., eligibility criteria, age, gender, and comorbidities), intervention details (i.e., type, route, condition, timing, dose, and volume), study designs (methods, setting, sample size, and number of centers), and clinical endpoints (i.e., AEs and efficacy outcomes). In the case of missing or unclear data for the primary or secondary outcome measures, an attempt was made to contact the primary study author for clarification.
Risk of Bias
All studies that met inclusion criteria were assessed for risk of bias according to the Cochrane Risk of Bias Tool for randomized control trials in duplicate by independent reviewers (J.Z., Y.D., S.M., and J.M.) 16, 17. Publication bias was evaluated with funnel plots.
Statistical Analysis
Studies were pooled using Comprehensive Meta‐Analysis (version 3; Biostat Inc., USA). For dichotomous safety and efficacy outcomes, fixed‐effects Peto odds ratios were calculated and presented with accompanying 95% confidence intervals 18. Peto odds ratios were used due to the expected rarity of events. This method allows for this inclusion of continuity corrections of 0.5 for all zero cells across outcomes, allowing us to estimate odds ratios for studies reporting no events in a treatment arm. For continuous outcomes, standardized mean differences were calculated using a random effects inverse‐variance model and presented with accompanying 95% confidence intervals. Standardized mean differences were used due to the variety of measurement methods used for the outcomes of interest (i.e., LVEF, left ventricular end systolic volume [LVESV], left ventricular end diastolic volume [LVEDV]). A post hoc analysis was also done using a weighted mean difference. Results reported at the latest outcome window were used for analysis. Studies which did not provide data in a format suitable for inclusion in a meta‐analysis (i.e., no control group) were analyzed descriptively.
Statistical heterogeneity was assessed using the I 2 statistic, or the Cochrane Q test, depending on the analysis method. An I 2 value of >50% was judged as representing important heterogeneity requiring further exploration. For the Cochrane Q test, a p value of <.10 was deemed to indicate substantial heterogeneity. Publication bias was assessed via funnel plots. We also conducted exploratory subgroup analyses to determine whether the efficacy of the MSCs varied by disease type (AMI vs. IHF), study design (randomized control trial [RCT] vs. non‐RCT), MSC source (bone marrow or umbilical cord), MSC route of administration, immunocompatability of MSCs (allogeneic vs. autologous origin), and timing of MSC administration.
Results
The literature search identified 668 unique citations. Abstract and full‐text screening identified 23 studies (1,148 patients) to be included for data extraction (Figure 1). Reasons for full‐text study exclusion are presented in Figure 1.
Study Characteristics
Of the 23 included studies, 11 evaluated AMI (n = 509 patients) 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 and 12 evaluated IHF (n = 639 patients) 12, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40 (Table 1). Of these studies, eight were RCTs that evaluated AMI (n = 429 patients) 19, 20, 21, 23, 24, 27, 29 and five were RCTs that evaluated IHF (n = 472 patients) 30, 34, 36, 37, 39, 40. The number of evaluated patients who received MSCs ranged from 9 to 58 patients for the AMI studies and from 6 to 107 patients for the IHF studies. Follow‐up duration ranged from 6 to 60 months. Of the studies evaluating AMI, seven studies specified safety 20, 22, 23, 25, 26, 28, 29 and four studies specified efficacy 19, 21, 24, 27 as their primary outcome. Secondary outcomes assessed included safety 21, 24 or efficacy 20, 22, 23, 24, 26. Of the IHF studies, four studies evaluated safety 31, 34, 35, four studies evaluated efficacy 30, 34, 37, 40, and four studies evaluated both 12, 36, 38, 39 as the primary outcome. Secondary outcomes assessed included efficacy 31, 32, 34, 35 or both efficacy and safety 37.
Table 1.
Study characteristics
| Author (year) | Study type | Patients included(n analyzed) | Patients evaluated (n [% male]) | Follow‐up duration(months) | |
|---|---|---|---|---|---|
| T | C | ||||
| Acute myocardial infarction | |||||
| Chen (2004) (China) | RCT | 69 | 34 (94) | 35 (97) | 6 |
| Chullikana (2015) (India) | RCT | 20 (safety) 19 (efficacy) |
10 (100) | 10 (80) | 24 |
| Gao (2013) (China) | RCT | 43 | 21 (100) | 22 (86) | 24 |
| Gao (2015) (China) | RCT | 115 | 58 (95) | 58 (88) | 18 |
| Hare (2009) (USA) | RCT | 53 | 34 (82) | 19 (79) | 6 |
| Houtgraaf (2012) (Netherlands) | RCT | 13 | 9 (69) | 4 (31) | 6 |
| Lee (2014) (South Korea) | RCT | 58 | 30 (90) | 28 (89) | 6 |
| Musialek (2015) (Poland) | Single‐arm | 10 | 10 (50) | 12 | |
| Rodrigo (2013) (Netherlands) | Historical control | 54 | 9 (78) | 45 (78) | 60 |
| Wang (2014) (China) | RCT | 58 | 28 (68) | 30 (53) | 6 |
| Yang (2010) (China) | Single‐arm | 16 | 16 (NR) | 6 | |
| Ischemic heart failure | |||||
| Bartunek (2017) (Belgium) | RCT | 271 | 107 (89) | 136 (90) | 10 |
| Chen (2006) (China) | NRCT | 45 | 22 (88) | 23 (92) | 12 |
| Guijarro (2016) (France) | Single‐arm | 10 | 10 (90) | 12 | |
| Hare (2012) (USA) | Non‐controlled | 30 | 15 (87) | 15 (87) | 13 |
| Heldman (2014) (USA) | RCT | 59 | 19 (95) | 11 (91) | 12 |
| Karantalis (2014) (USA) | Single‐arm | 6 | 6 (100) | 18 | |
| Li (2015) (China) | Single‐arm | 15 | 15 (60) | 24 | |
| Mathiasen (2013) (Denmark) | Single‐arm | 31 | 31 (84) | 36 | |
| Mathiasen (2015) (Denmark) | RCT | 59 | 40 (90) | 20 (70) | 6 |
| Viswanathan (2010) (India) | NRCT | 30 | 15 (100) | 15 (93) | 6 |
| Wang (2006) (China) | RCT | 24 | 12 (75) | 12 (67) | 10 |
| Zhao (2015) (China) | RCT | 59 | 30 (80) | 29 (66) | 6 |
Abbreviations: C, control group; NR, not reported; NRCT, non‐randomized controlled trial; RCT, randomized controlled trial; T, treatment group.
Intervention Characteristics
Of the 11 studies evaluating AMI, 7 studies used autologous MSCs 19, 21, 24, 26, 27, 28, 29 and 4 studies used allogeneic MSCs 20, 22, 23, 25 (Table 2). Of the 12 studies evaluating IHF, 9 studies used autologous MSCs 12, 30, 31, 34, 36, 37, 38, 39 and 3 studies used allogeneic MSCs 32, 35, 40. The majority of studies (18/23) used bone‐marrow derived MSCs 12, 19, 20, 21, 23, 24, 26, 27, 28, 30, 31, 32, 34, 36, 37, 38, 39, 41 and the remaining used either MSCs that were umbilical cord‐derived (4/23) 22, 25, 35, 40 or adipose tissue‐derived (1/23) 29. Comparisons consisted of standard treatment (5/23) 12, 21, 24, 38, 40, saline (4/23) 19, 27, 37, 39, heparinized saline (1/23) 22, historical control (1/23) 26, vehicle placebo (3/23) 23, 29, 34, sham procedure (1/23) 30, plasmalyte A (1/23) 20, or the study did not include a comparison group (5/23) (Table 2) 25, 28, 31, 35, 36. One study compared allogeneic MSCs to autologous MSCs and did not use a control group 32. The number of allogeneic donors were reported in four studies 20, 22, 23, 32, while three studies 25, 35, 40 did not report this information.
Table 2.
Intervention characteristics
| Author (year) | Source of MSCs | Comparison | MSCs | Timing | Dosage and volume |
|---|---|---|---|---|---|
| Route, condition | |||||
| Acute myocardial infarction | |||||
| Chen (2004) | Autologous | Saline | IC, Fresh | 18 ± 0.5 days post‐PCI | 8–10 × 109,1 dose – NR |
| Chullikana (2015) | Allogeneic | Plasmalyte A | IV, Fresh from cryopreserved | 2 days post‐PCI | 2 million cells/kg, 1 dose – 0.5 mL/kg |
| Gao (2013) | Autologous | Standard treatment | IC, Fresh | Post‐PCI Mean 17 ± 1 days |
3 ± 0.5 × 1061 dose – 10 mL |
| Gao (2015) | Allogeneic | Heparanized saline | IC, Fresh | Within 5–7 days of PCI | 6 × 106, 1 dose – 10 mL |
| Hare (2009) | Allogeneic | Placebo | IV, NR | Patients randomized 1–10 days post AMI | 0.5, 1.6, 5 × 106
3 dose escalation cohorts ‐ NR |
| Houtgraaf (2012) | Autologous | Placebo | IC, Fresh | 24 hours post‐PCI | 17.4 ± 4.1 × 106
1 dose ‐ NR |
| Lee (2014) | Autologous | Standard treatment | IC, Fresh | BM aspiration done 4 ± 2 days post‐admission; culture took 25.0 ± 2 days | 7 ± 1 × 107
1 dose ‐ NR |
| Musialek (2015) | Allogeneic | No comparison | IC, Fresh from cyropreserved | Within 5–7 days of PCI | 30 × 106
1 dose – 30 mL |
| Rodrigo (2013) | Autologous | Historical control | IM, Fresh | 21 ± 3 days post‐MI/PCI | 31 ± 2 × 106
1 dose – 5 mL |
| Wang (2014) | Autologous | Saline | IC, Fresh | 14 days post‐PCI | 2 × 108
1 dose – 2 mL |
| Yang (2010) | Autologous | No comparison | IC, Fresh | NR | 1 ± 2 × 107
1 dose ‐ NR |
| Ischemic heart failure | |||||
| Bartunek (2017) | Autologous | Sham procedure | IM, Frozen | NR | NR 1 dose – 0.5 mL |
| Chen (2006) | Autologous | Standard treatment | IC, Fresh | BM aspiration done 8 days post‐PCI; culture for 7 days | 5 × 106, 1 dose – NR |
| Guijarro (2016) | Autologous | No comparison | IV, Fresh | NR | 40 × 106
1 dose – 2‐3 mL |
| Hare (2012) | Allogeneic | Autologous | EC, NR | NR | 0.2, 1, 2 × 108
3 dose cohorts – 5.0 mL each |
| Heldman (2014) | Autologous | Placebo | EC, Fresh from cyropreserved | During cardiac catheterization. Mean time since first MI is 10 ± 10 years. | 2 × 108
1 dose – 5.0 mL |
| Karantalis (2014) | Autologous | Placebo | EP, Fresh from cyropreserved | Post‐CABG; mean time between last MI and study enrollment was 675 days | 2 × 107 or 2 × 108
1 dose – 5.0 mL |
| Li (2015) | Allogeneic | No comparison | IC, Fresh | NR | 3, 4, or 5 × 106
1 dose – NR |
| Mathiasen (2013) | Autologous | No comparison | IM, unclear | NR | Mean 22 × 106
1 dose ‐ NR |
| Mathiasen (2015) | Autologous | Saline | IM, Fresh | NR | Mean 78 ± 68 × 106
1 dose – 2–3 mL |
| Viswanathan (2010) | Autologous | Standard treatment | EP, Fresh from cryopreserved | During CABG; BM culture took 3–4 weeks | 3 × 106 to 26 × 106
1 dose – 1 mL |
| Wang (2006) | Autologous | Saline | IC, Fresh | NR | NR 1 dose – 30 mL |
| Zhao (2015) | Allogeneic | Standard treatment | IC, unclear | NR | NR 1 dose – 20 mL |
Dose is provided as reported by the study or the mean ± SD number of cells delivered to patients. Abbreviations: C, control groups; EC, endocardial; EP, epicardial; FBS, Fetal Bovine Serum; HSA, Human Serum Albumin; IC, intracoronary; IM, intramyocardial; IV, intravenous; MSCs, mesenchymal stromal cells; NR, not reported; NRCT, non‐randomized controlled trial; PCI, percutaneous coronary intervention; RCT, randomized controlled trial; T, treatment group.
The routes of MSC administration were intracoronary (12/23) 12, 19, 21, 22, 24, 25, 27, 28, 29, 35, 39, 40, intravenous (3/23) 20, 23, 31, and intramyocardial (4/23) 26, 30, 36, 37. Studies evaluating IHF also included endocardial (2/23) 32, 33 and epicardial (2/23) 34, 38 routes of administration. Timing of MSCs administration for studies evaluating treatment for AMI ranged from 24 hours to 27 days after percutaneous coronary intervention (PCI) for AMI studies or during/after coronary artery bypass grafting (CABG) in IHF studies 34, 38. MSC doses ranged from 1 dose of 2 × 106 cells/kg to 10 × 109 cells/kg and 3 × 106 cell/kg to 2 × 108 cells/kg for AMI and IHF studies, respectively. Details on cell manufacturing and characterization are reported in Supporting Information Appendix 3.
Primary Outcome—Safety
Acute Adverse Events (<24 hours)
In AMI and IHF studies, acute cardiac AEs were not significantly different between MSC (7/292) and comparison (0/128) groups (Peto OR 3.20, 95% CI 0.70–14.61) (Table 3). Specific acute AEs that occurred in the MSC group included arrhythmias (n = 3 events), MI (n = 2 events), vessel obstruction during procedure (n = 1 event), and pericardial effusion (n = 1 event). Treatment attributable acute AEs (i.e., events deemed by study investigators to be caused by MSC therapy) were rarely reported and are outlined in Supporting Information Appendix 5, Table 5a.
Table 3.
Acute (<24 hours) and delayed (≥24 hours) adverse events
| Treatment (MSC) | Control | Peto OR (95% CI) | |||
|---|---|---|---|---|---|
| Events | Total | Events | Total | <1 favors MSC | |
| Acute adverse event (n) | |||||
| Fever (3) | 0 | 42 | 0 | 12 | 0.22 (0.00–23.09) |
| Respiratory (3) | 3 | 66 | 0 | 33 | 2.67 (0.32–21.82) |
| Cardiac (11) | 7 | 292 | 0 | 128 | 3.20 (0.70–14.61) |
| Neurological (2) | 1 | 19 | 0 | 19 | 2.79 (0.17–46.26) |
| Hematological (5) | 0 | 85 | 0 | 12 | 0.04 (0.00–13.08) |
| GI (1) | 0 | 30 | – | – | n/a |
| Renal | – | – | – | – | n/a |
| Infection (3) | 2 | 30 | 0 | 10 | 1.68 (0.12–23.50) |
| Allergic reaction (4) | 0 | 68 | 0 | 12 | 0.08 (0.00–16.55) |
| Local complication (5) | 0 | 93 | 0 | 29 | 0.25 (0.00–23.80) |
| Other (4) | 2 | 109 | 0 | 58 | 2.25 (0.21–24.55) |
| Delayed adverse event (n) | |||||
| Fever (5) | 7 | 68 | 3 | 27 | 0.92 (0.22–3.85) |
| Respiratory (6) | 10 | 96 | 11 | 61 | 0.53 (0.21–1.33) |
| Cardiac (18) | 95 | 503 | 58 | 394 | 1.35 (0.94–1.93) |
| Arrhythmia | 33 | 377 | 17 | 237 | 1.24 (0.68–2.28) |
| CHF | 17 | 150 | 6 | 85 | 1.68 (0.64–4.45) |
| MI | 5 | 278 | 2 | 245 | 2.23 (0.43–11.57) |
| Biomarker | 0 | 15 | – | – | ‐ |
| Other | 40 | 402 | 33 | 321 | 0.96 (0.59–1.57) |
| Neurological (9) | 17 | 303 | 4 | 259 | 3.79 (1.26–11.41)* |
| Hematological (9) | 5 | 116 | 2 | 79 | 1.73 (0.33–9.17) |
| GI (5) | 21 | 99 | 9 | 50 | 1.22 (0.52–2.84) |
| Renal (4) | 7 | 80 | 1 | 36 | 2.52 (0.54–11.80) |
| Infection (7) | 23 | 203 | 11 | 118 | 1.24 (0.59–2.58) |
| Malignancy/cancer (8) | 1 | 235 | 1 | 147 | 0.61 (0.04–10.68) |
| Other (12) | 39 | 74 | 18 | 29 | 0.68 (0.28–1.64) |
| Administration site reaction (3) | 18 | 74 | 14 | 29 | 0.57 (0.25–1.33) |
| Metabolic (2) | 3 | 40 | 1 | 10 | 0.72 (0.06–9.00) |
| Muscoskeletal (2) | 6 | 40 | 1 | 10 | 1.50 (0.21–10.85) |
| Skin (2) | 1 | 40 | 1 | 10 | 0.15 (0.00–4.89) |
| Vascular (2) | 4 | 40 | 1 | 10 | 1.00 (0.10–9.84) |
| Eye (1) | 1 | 30 | – | – | n/a |
| Surgical procedures (1) | 1 | 30 | – | – | n/a |
| Injury, poisoning (1) | 3 | 30 | – | – | n/a |
| Immune system (1) | 2 | 34 | 0 | 19 | 2.42 (0.22–26.61) |
*p < .05; –, not reported; (), number of studies.
Delayed Adverse Events (≥24 hours)
All‐cause delayed AEs included fever, respiratory, cardiac, hematological, gastrointestinal, renal, infections (only one study specified type of infection [respiratory 22]), malignancy/cancer, and other events (Table 3). Other delayed AEs reported included events associated with administration site reaction, metabolic, musculoskeletal, skin, vascular, eye, surgical procedures, and the immune system (Table 3). There was a statistically significant difference in neurological events between MSC (17/303) and comparison (4/259) groups (Peto OR 3.79, 95% CI 1.26–11.41). Details on the type and severity of neurological AEs were not reported. Treatment attributable delayed AEs occurred infrequently and are outlined in Supporting Information Appendix 5, Table 5b.
Secondary Outcomes—Efficacy
Mortality
A total of four AMI studies (36%, n = 236 patients) 20, 21, 22, 27 and six IHF studies (50%, n = 498 patients) 12, 30, 34, 37, 39, 40 reported on mortality. There was no overall significant difference in the risk of mortality between MSC and control groups (Peto OR 0.68, 95% CI 0.38–1.22). In our subgroup analysis by disease type, there was no significant difference in the AMI studies (Peto OR 0.53, 95% CI 0.10–2.64) or the IHF studies (Peto OR 0.71, 95% CI 0.38–1.32) (Figure 2).
Figure 2.
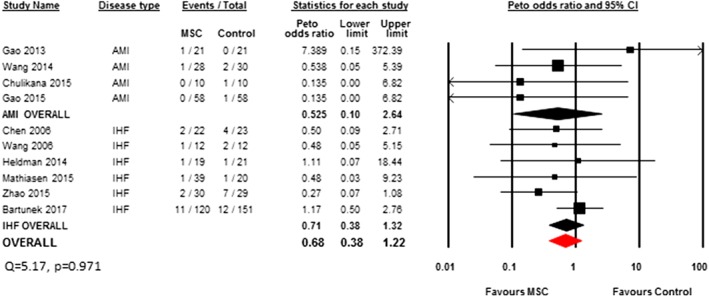
Peto odds ratio (95% CI) and pooled estimates for mortality.
Left Ventricular Ejection Fraction
A total of 10 AMI studies (n = 473 patients) 19, 20, 21, 22, 23, 24, 26, 27, 28, 29 and 5 IHF studies (n = 216 patients) 12, 34, 37, 39, 40 reported data on LVEF that could be included in the meta‐analysis. Patients treated with MSCs had a significantly increased LVEF compared to control (SMD 0.72, 95% CI 0.24–1.21, I 2 = 88%) (Figure 3). Measurement methods of LVEF are included in Supporting Information Appendix 4. In a post hoc analysis using weighted mean difference, patients treated with MSCs had a 4% increase in ejection fraction compared to control (WMD 4.25, 95% CI 1.37–7.13, I 2 = 90%) (Supporting Information Appendix 6, Figure 6f).
Figure 3.
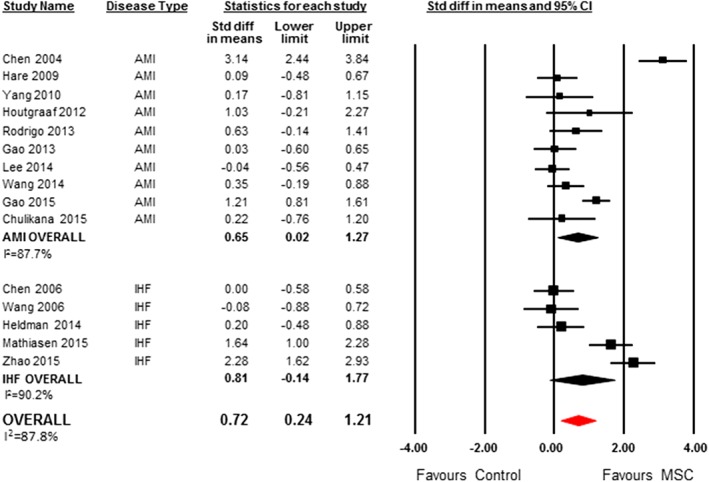
SMD (95% CI) and pooled estimates for left ventricular ejection fraction.
LVEF Subgroup Analyses
In our planned exploratory subgroup analysis by disease type, there was a significant increase in LVEF in patients treated with MSCs for AMI compared to control (SMD 0.65; 95% CI 0.02–1.27, I 2 = 88%), but no difference for IHF patients (SMD 0.81, 95% CI −0.14 to 1.77, I 2 = 90%) (Figure 3). The positive significant increase associated with MSC therapy remained after extreme values from one study were removed 19 (SMD 0.40, 95% CI 0.03–0.77, I 2 = 67%).
LVEF increased significantly for allogeneic MSCs (SMD 0.98, 95% CI 0.18–1.79, I 2 = 86%); however, no significant difference in LVEF was observed in patients who had been administered autologous MSCs (SMD 0.60, 95% CI −0.02 to 1.22, I 2 = 89%) (Supporting Information Appendix 6, Figure 6a). MSCs from an umbilical cord source increased LVEF compared to control treatment (SMD 1.71, 95% CI 0.67–2.76, I 2 = 87%) (Supporting Information Appendix 6, Figure 6b). MSCs from either adipose tissue (SMD 1.03, 95% CI −0.21 to 2.27) or bone marrow (SMD 0.53, 95% CI 0.00–1.05, I 2 = 86%) did not increase LVEF compared to control.
With regard to administration route, MSCs increased LVEF when administered by intracoronary injection (SMD 0.81, 95% CI 0.14–1.47, I 2 = 91%) and intramyocardial injection (SMD 1.64, 95% CI 1.00–2.28). There was no statistically significant difference in LVEF when MSCs were given either epicardially (SMD 0.20, 95% CI −0.48 to 0.88) or intravenously (SMD 0.27, 95% CI −0.15 to 0.69, I 2 = 0%) (Supporting Information Appendix 6, Figure 6c). There was a significant increase in LVEF reported in RCTs between MSC and control (SMD 0.84, 95% CI 0.26–1.41, I 2 = 89.6%), and no significant difference among non‐RCTs (SMD 0.22, 95% CI −0.20 to 0.64, I 2 = 0%) (Supporting Information Appendix 6, Figure 6d).
There was no significant difference in the increase in LVEF whether MSCs were administered 1–10 days (SMD 0.55, 95% CI −0.03 to 1.13, I 2 = 71%) or more than 10 days (SMD 0.67, 95% CI −0.22 to 1.56, I 2 = 92%) after an AMI (Supporting Information Appendix 6, Figure 6e).
LVEF in Studies Not Suitable for Meta‐Analysis
There was one study evaluating AMI that did not provide data suitable for a meta‐analysis 29. This study reported a 4% improvement (52.1%–56.1%) in global LVEF in MSC‐treated patients compared to the placebo group which is deteriorated by 1.7% (52.0%–50.3%) at 6 month follow‐up 29. There were five studies evaluating IHF that did not provide data suitable for meta‐analysis. One RCT showed a significant improvement in LVEF 30 while another did not 32. One non‐RCT showed a significant improvement in LVEF 38. Two single‐arm studies reported a significant improvement in LVEF from baseline to follow‐up 31, 34.
Tertiary Outcomes
Quality of Life
No studies evaluating AMI reported on QoL outcomes. Three RCTs evaluating IHF reported no significant differences in QoL between MSCs and comparator. One RCT with two treatment arms but no control group 32 did not report a significant difference between treatment groups (autologous vs. allogeneic), but did report a significant improvement post‐treatment versus baseline. The mean reduction in Minnesota Living with Heart Failure Questionnaire scores among both groups at 12 months was 7.6 (p = .02) 32. Two single arm studies reported a significant improvement post‐treatment 35, 36, while one single arm study did not show a significant improvement in QoL 31.
Performance Status
New York Heart Association Functional Classification: Two studies evaluating IHF evaluated New York Heart Association Functional Classification (Supporting Information Appendix 7, Figure 7a) 12, 37. There was no difference in performance status between the MSC group and control (SMD −1.01, 95% CI −2.46 to 0.45, I 2 = 91%). Eight studies were not included in the meta‐analysis. Of these, three evaluated AMI patients, one single arm study showed a significant improvement in performance status 28, while one RCT and single‐arm study 37 did not. Of five studies evaluating IHF, three studies showed a significant improvement in performance status 12, 31, 35, while two studies did not 32, 34.
Walk test: Five studies reported the 6‐minute walk test (Supporting Information Appendix 7, Figure 7b) 23, 33, 37, 39, 40. Overall, there was a significant improvement in the 6‐minute walk test between the MSC group and control for both AMI and IHF (SMD 1.16, 95% CI 0.26–2.06, I 2 = 89%). There was no significant difference in the 6‐minute walk test for AMI (SMD 0.25, 95% CI −0.31 to 0.81). For IHF studies, a significant difference was observed (SMD 1.41, 95% CI 0.31–2.52, I 2 = 90%). One single arm study 31 evaluating IHF reported a significant improvement in the 6‐minute walk test and one RCT 32 did not.
Other tertiary outcomes of LVEDV, LVESV, wall motion, myocardial perfusion, and MACEs are presented in Supporting Information Appendix 8a–e. Measurement methods are included in Supporting Information Appendix 4.
Risk of Bias Assessment
No study fulfilled all seven criteria for low risk of bias (Figure 4). Four studies met six of seven risk of bias criteria 20, 30, 34, 37. Nine studies described randomization procedures with a low risk of bias 20, 21, 22, 23, 30, 32, 34, 37, 39. Allocation concealment was performed in five studies with low risk of bias 20, 23, 32, 34, 37. Double‐blinding procedures were described in five studies with low risk of bias 20, 22, 30, 34, 37. One study was at high risk for incomplete outcome data reporting 34 and one study was at high risk for selective reporting 29. Seven studies were deemed to be at a high risk of other biases 8, 20, 23, 24, 26, 33, 37. Visually apparent asymmetry was present in the funnel plot of mortality (Supporting Information Appendix 9, Figure 9a) and LVEF (Supporting Information Appendix 9, Figure 9b) which might indicate that small studies produced bias in the random‐effect model.
Figure 4.

Risk of bias assessment.
Discussion
Our systematic review evaluated the safety and efficacy of MSC therapy for IHF and AMI. Although the majority of studies reported AEs, the definitions and descriptions of these events were highly variable. Available evidence suggested that MSCs appeared to be largely safe in the studies that compared MSCs to a control group. The summary effect measures were marked by considerable heterogeneity; however, it appeared that MSCs improved LVEF but had no effect on mortality in the small number of studies conducted to date. Other outcomes of interest were rarely reported, limiting our conclusions.
We focused our review on carefully detailing AEs in this vulnerable population. Meta‐analysis of the included studies with a control group did not detect a significant association between overall acute AEs and MSC administration. However, there was a significant increased risk for delayed neurological AEs with MSC therapy compared to control groups. The types of neurological AEs were not reported, limiting interpretation of this finding; however, it is possible that this could represent the consequences of microembolization within the cerebral microcirculation. Despite this effect, it should be noted that MSCs have demonstrated efficacy as therapy for serious neurological conditions, such as ischemic stroke 42. The variable detail reported in AEs highlights the importance for future studies to completely document these elements (e.g., using recommended reporting guidelines such as the CONSORT harms extension) 43. This will allow for a more precise profile of MSC related safety to be developed.
Patients receiving MSCs for AMI demonstrated an improvement in LVEF. Our sensitivity analyses demonstrated that even after the removal of the Chen et al. study 19, the effect of MSCs on LVEF remained. This improvement in LVEF for patients receiving MSCs is consistent with previous analyses of MSC therapy after PCIs 44, 45 and in stark contrast to previous highly cited systematic reviews of cell therapy for ischemic heart disease which demonstrated no effect 9, 10. These latter reviews focused only on autologous cells and the results were driven by large trials of bone marrow mononuclear cells. Our review suggests that further optimization and evaluation of MSC therapy may be warranted in ischemic heart disease. Our review provides some suggestions for future trials, including the potentially increased efficacy of umbilical cord derived MSCs versus other sources, as well as potential increased efficacy of intracoronary or intramyocardial routes of administration versus other routes.
Also of potential relevance when considering future trials is the putative mechanism whereby MSCs are thought to act, which largely involves anti‐inflammatory effects. This is reflected by its efficacy in acute graft‐versus‐host‐disease 46, currently the only approved indication for MSC therapy. Thus, it is perhaps not surprising that MSCs had little effect in a chronic condition such as IHF which has a much lower level of inflammatory burden. Further supporting this anti‐inflammatory indication is the single trial which administered MSCs immediately after AMI and demonstrated significant improvements in LVEF 29, as well as preclinical studies of MSCs that have demonstrated efficacy in very acute, largely perioperative and inflammatory settings 11.
Our study was able to identify several knowledge gaps. QoL and performance status were inconsistently reported and often lacked control data which limited our conclusions. The other tertiary outcomes (LVEDV, LVESV, wall motion, myocardial perfusion, and MACEs) were also infrequently reported. Future studies should include reporting of validated QoL assessments as well as detailed cardiac function reporting. Our systematic review also identified several issues with the MSC administration and cell product used in the included studies. For example, the criteria by Dominici et al. that defines MSCs were inconsistently reported. In addition, no study described assessments of cell potency prior to administration. We recommend that future studies enhance reporting and characterization of cell products used.
Our systematic review has limitations. First, there were a limited number of clinical MSC studies included, each with small sample size. Second, the majority of RCTs included in our analyses were deemed to be at a high risk of bias for several domains. Despite these limitations, we provide a comprehensive summary of MSC therapy for IHF and AMI, based on all available contemporary evidence.
Conclusion
Our study provides a comprehensive evaluation of the safety and efficacy of MSCs therapy for AMI and IHF. We did not identify any significant safety signals except delayed neurological events, which were poorly defined. Results from our systematic review suggest that MSC therapy may be safe. A well‐designed adequately powered RCT with rigorous AE reporting and comprehensive assessment of cardiac function is warranted. This will help establish a definitive risk‐benefit profile of MSC therapy for ischemic heart disease.
Author Contributions
M.L.: concept and design, data analysis and interpretation, manuscript writing, final approval of manuscript; S.M.: collection and assembly of data, data analysis and interpretation, manuscript writing; J.Z.: collection and assembly of data; R.(Y.Y.)D.: collection and assembly of data; J.M.: collection and assembly of data, data analysis and interpretation, manuscript writing; L.M.: concept and design, manuscript writing; P.J.D.: concept and design, manuscript writing; D.J.S.: concept and design, manuscript writing, final approval of manuscript; D.M.: concept and design, manuscript writing; D.I.M.: concept and design, manuscript writing. D.A.F.: Concept and design, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
P.J.D. reported advisory role for Boehringer Ingelheim and research funding from Abbott Diagnostics, Boehringer Ingelheim, Philips Healthcare, Roche Diagnostics, and Stryker. All other authors indicated no potential conflicts of interest.
Supporting information
Supporting Information: Appendix S1
Acknowledgments
M.M.L. is supported by The Ottawa Hospital Anesthesia Alternate Funds Association and the Scholarship Protected Time Program, Department of Anesthesiology and Pain Medicine, Ottawa.
References
- 1. Fihn SD, Blankenship JC, Alexander KP et al. ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation 2014;2014:2018. [DOI] [PubMed] [Google Scholar]
- 2. dos Santos CC, Murthy S, Hu P et al. Network analysis of transcriptional responses induced by mesenchymal stem cell treatment of experimental sepsis. Am J Pathol 2012;181:1681–1692. [DOI] [PubMed] [Google Scholar]
- 3. Mei SH, Haitsma JJ, Dos Santos CC et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med 2010;182:1047–1057. [DOI] [PubMed] [Google Scholar]
- 4. Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res 2011;109:923–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jackson MV, Morrison TJ, Doherty DF et al. Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells 2016;34:2210–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Islam MN, Das SR, Emin MT et al. Mitochondrial transfer from bone‐marrow‐derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med 2012;18:759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol 2009;217:318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res 2015;116:1413–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fisher SA, Zhang H, Doree C et al. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev 2015;9:CD006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fisher SA, Doree C, Mathur A et al. Stem cell therapy for chronic ischaemic heart disease and congestive heart failure.Cochrane Database Syst Rev 2016;12:CD007888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van der Spoel TI, Jansen of Lorkeers SJ, Agostoni P et al. Human relevance of pre‐clinical studies in stem cell therapy: systematic review and meta‐analysis of large animal models of ischaemic heart disease. Cardiovasc Res 2011;91:649–658. [DOI] [PubMed] [Google Scholar]
- 12. Chen S, Liu Z, Tian N et al. Intracoronary transplantation of autologous bone marrow mesenchymal stem cells for ischemic cardiomyopathy due to isolated chronic occluded left anterior descending artery. J Invasive Cardiol 2006;18:552–556. [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McGowan J, Sampson M, Salzwedel DM et al. PRESS Peer Review of Electronic Search Strategies: 2015 guideline statement. J Clin Epidemiol 2016;75:40–46. [DOI] [PubMed] [Google Scholar]
- 15. Dominici M, Le Blanc K, Mueller I et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 16. Higgins JP, Altman DG, Gotzsche PC et al. Cochrane Bias Methods G and Cochrane Statistical Methods G. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. 2009. [Google Scholar]
- 18. Brockhaus AC, Grouven U, Bender R. Performance of the Peto odds ratio compared to the usual odds ratio estimator in the case of rare events. Biom J 2016;58:1428–1444. [DOI] [PubMed] [Google Scholar]
- 19. Chen SL, Fang WW, Qian J et al. Improvement of cardiac function after transplantation of autologous bone marrow mesenchymal stem cells in patients with acute myocardial infarction. Chin Med J (Engl) 2004;117:1443–1448. [PubMed] [Google Scholar]
- 20. Chullikana A, Majumdar AS, Gottipamula S et al. Randomized, double‐blind, phase I/II study of intravenous allogeneic mesenchymal stromal cells in acute myocardial infarction. Cytotherapy 2015;17:250–261. [DOI] [PubMed] [Google Scholar]
- 21. Gao LR, Pei XT, Ding QA et al. A critical challenge: dosage‐related efficacy and acute complication intracoronary injection of autologous bone marrow mesenchymal stem cells in acute myocardial infarction. Int J Cardiol 2013;168:3191–3099. [DOI] [PubMed] [Google Scholar]
- 22. Gao LR, Chen Y, Zhang NK et al. Intracoronary infusion of Wharton's jelly‐derived mesenchymal stem cells in acute myocardial infarction: double‐blind, randomized controlled trial. BMC Med 2015;13:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hare JM, Traverse JH, Henry TD et al. A randomized, double‐blind, placebo‐controlled, dose‐escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol 2009;54:2277–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee JW, Lee SH, Youn YJ et al. A randomized, open‐label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. J Korean Med Sci 2014;29:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Musialek P, Mazurek A, Jarocha D et al. Myocardial regeneration strategy using Wharton's jelly mesenchymal stem cells as an off‐the‐shelf 'unlimited' therapeutic agent: results from the Acute Myocardial Infarction First‐in‐Man Study. Postepy Kardiol Inter 2015;11:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rodrigo SF, van Ramshorst J, Hoogslag GE et al. Intramyocardial injection of autologous bone marrow‐derived ex vivo expanded mesenchymal stem cells in acute myocardial infarction patients is feasible and safe up to 5 years of follow‐up. J Cardiovasc Transl Res 2013;6:816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang X, Xi WC, Wang F. The beneficial effects of intracoronary autologous bone marrow stem cell transfer as an adjunct to percutaneous coronary intervention in patients with acute myocardial infarction. Biotechnol Lett 2014;36:2163–2168. [DOI] [PubMed] [Google Scholar]
- 28. Yang Z, Zhang F, Ma W et al. A novel approach to transplanting bone marrow stem cells to repair human myocardial infarction: delivery via a noninfarct‐relative artery. Cardiovasc Ther 2010;28:380–385. [DOI] [PubMed] [Google Scholar]
- 29. Houtgraaf JH, den Dekker WK, van Dalen BM et al. First experience in humans using adipose tissue‐derived regenerative cells in the treatment of patients with ST‐segment elevation myocardial infarction. J Am Coll Cardiol 2012;59:539–540. [DOI] [PubMed] [Google Scholar]
- 30. Bartunek J, Terzic A, Davison BA et al. Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham‐controlled CHART‐1 clinical trial. Eur Heart J 2017;38:648–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guijarro D, Lebrin M, Lairez O et al. Intramyocardial transplantation of mesenchymal stromal cells for chronic myocardial ischemia and impaired left ventricular function: results of the MESAMI 1 pilot trial. Int J Cardiol 2016;209:258–265. [DOI] [PubMed] [Google Scholar]
- 32. Hare JM, Fishman JE, Gerstenblith G et al. Comparison of allogeneic vs autologous bone marrow‐derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA 2012;308:2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Heldman AW, DL DF, Fishman JE et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC‐HFT randomized trial. JAMA 2014;311:62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karantalis V, DL DF, Gerstenblith G et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: the Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ Res 2014;114:1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li X, Hu YD, Guo Y et al. Safety and efficacy of intracoronary human umbilical cord‐derived mesenchymal stem cell treatment for very old patients with coronary chronic total occlusion. Curr Pharm Des 2015;21:1426–1432. [DOI] [PubMed] [Google Scholar]
- 36. Mathiasen AB, Haack‐Sorensen M, Jorgensen E et al. Autotransplantation of mesenchymal stromal cells from bone‐marrow to heart in patients with severe stable coronary artery disease and refractory angina‐‐final 3‐year follow‐up. Int J Cardiol 2013;170:246–251. [DOI] [PubMed] [Google Scholar]
- 37. Mathiasen AB, Qayyum AA, Jorgensen E et al. Bone marrow‐derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo‐controlled trial (MSC‐HF trial). Eur Heart J 2015;36:1744–1753. [DOI] [PubMed] [Google Scholar]
- 38. Viswanathan C, Davidson Y, Cooper K et al. Tansplantation of autologous bone marrow derived mesenchymal stem cells trans‐epicardially in patients undergoing coronary bypass surgery. Indian Heart J 2010;62:43–48. [PubMed] [Google Scholar]
- 39. Wang JA, Xie XJ, He H et al. A prospective, randomized, controlled trial of autologous mesenchymal stem cells transplantation for dilated cardiomyopathy. Zhonghua Xin Xue Guan Bing Za Zhi 2006;34:107–110. [PubMed] [Google Scholar]
- 40. Zhao XF, Xu Y, Zhu ZY et al. Clinical observation of umbilical cord mesenchymal stem cell treatment of severe systolic heart failure. Genet Mol Res 2015;14:3010–3017. [DOI] [PubMed] [Google Scholar]
- 41. Ausset S, Auroy Y, Lambert E et al. Cardiac troponin I release after hip surgery correlates with poor long‐term cardiac outcome. Eur J Anaesthesiol 2008;25:158–164. [DOI] [PubMed] [Google Scholar]
- 42. Nagpal A, Choy FC, Howell S et al. Safety and effectiveness of stem cell therapies in early‐phase clinical trials in stroke: a systematic review and meta‐analysis. Stem Cell Res Ther 2017;8:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ioannidis JP, Evans SJ, Gotzsche PC et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med 2004;141:781–788. [DOI] [PubMed] [Google Scholar]
- 44. Wu HDD, Cao H et al. Efficacy and safety of mesenchymal stromal cells on left ventricular function after acute myocardial infarction: a meta‐analysis of randomized controlled trials. Int J Clin Exp Med 2017;10:5871–5882. [Google Scholar]
- 45. Wang Z, Wang L, Su X et al. Rational transplant timing and dose of mesenchymal stromal cells in patients with acute myocardial infarction: a meta‐analysis of randomized controlled trials. Stem Cell Res Ther 2017;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen X, Wang C, Yin J et al. Efficacy of mesenchymal stem cell therapy for steroid‐refractory acute graft‐versus‐host disease following allogeneic hematopoietic stem cell transplantation: a systematic review and meta‐analysis. PLoS One 2015;10:e0136991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information: Appendix S1


