Summary
Cell‐based therapies have gained interest as a potential treatment method in cardiovascular disease in the past two decades, peripheral artery disease amongst others. Initial pre‐clinical and small pilot clinical studies showed promising effects of cell therapy in peripheral artery disease and chronic limb‐threatening ischemia in particular. However, these promising results were not corroborated in larger high quality blinded randomized trials. This has led to a shift of the field towards more sophisticated cell products, especially mesenchymal stromal cells. Mesenchymal stromal cells have some important benefits, making these cells ideal for regenerative medicine, e.g., potential for allogeneic application, loss of disease‐mediated cell dysfunction, reduced production costs, off‐the‐shelf availability. Future high quality and large clinical studies have to prove the efficacy of mesenchymal stromal cells in the treatment of peripheral artery disease. Stem Cells Translational Medicine 2018;7:842–846
Significance Statement.
Chronic limb‐threatening ischemia is the most severe form of peripheral artery disease, and a considerable number of patients with this condition are not eligible for conventional treatment strategies. Therapies that aim at neovascularization might provide an escape for these patients. Initial clinical studies for first generation cell therapies were quite disappointing; however, more sophisticated and better defined cell therapies—mesenchymal stromal cells in particular—seem promising. This article describes the future perspectives and challenges of cell therapy in limb ischemia.
Introduction
Peripheral artery disease arises from atherosclerosis of major arteries, with a predilection for the lower limbs. In its most severe manifestation, occlusion of limb arteries reaches a point where metabolic demands of the tissue can no longer be met; this stage is termed chronic limb‐threatening ischemia (CLTI) or critical limb ischemia (CLI). CLTI poses a great unmet need for novel treatments, as 20%–40% of the CLTI patients are not eligible for conventional revascularization, ultimately leading to amputation, associated with an immense medical and socio‐economic burden 1, 2, 3. No‐option status in these patients is due to extensive and diffuse, often infrapopliteal, atherosclerotic lesions, comorbidity, and/or lack of a suitable bypass graft 4, 5. Novel approaches that target neovascularization provide a potential solution for these no‐option patients. Cell‐based therapies seem the most promising 6, although initial enthusiasm has abated after negative results in the first generation of progenitor cell trials. Here we will provide a concise review on the available evidence on and future directions for cell therapy in CLTI. In that context we will also briefly address literature regarding cell therapy in myocardial infarction (MI) because objectives in both MI and CLTI trials are to enhance revascularization through cell therapy.
Cell Therapy for CLTI—The Present State of Affairs
The rationale behind using progenitor cell therapy as a treatment for ischemic cardiovascular disease was motivated by the discovery that human blood contains progenitor cells that home to ischemic tissues 7 and augment angiogenesis 8. Relatively soon thereafter, a first generation of progenitor cell trials have been conducted using bone marrow mononuclear cells (BM‐MNCs), a direct BM isolate which contains a variety of different cell‐types, mostly from the hematopoietic line. The primary hypothesis was that BM, as the reservoir of hematopoietic stem cells, also contains endothelial progenitor cells (EPCs) 9. These putative EPCs were initially thought to promote angiogenesis through the formation of new vessels 7 as they actively homed to ischemic areas after injection. Early, uncontrolled clinical studies using BM‐MNCs were promising, but placebo‐controlled trials gave conflicting results. A large, double‐blind, placebo‐controlled randomized trial by our group, the JUVENTAS trial, showed no treatment effects of BM‐MNC administration over placebo 10, which was corroborated by a meta‐analysis 11. Similar results were obtained with BM‐MNC therapy for other indications such as MI, where an aggregated study comprising over a 1,000 patients that were treated with BM‐MNCs for MI failed to find a consistent positive effect 12.
Advancing insight into the biology of progenitor cells has in parallel, revealed that the mechanisms of effect involved in progenitor cell‐therapy are different and more complex than initially thought. The use of cell surface markers to identify EPCs has been shown to be prone to isolation artifacts 13, 14, and several ontologically distinct cell populations display EPC markers. Furthermore it has been shown that BM‐derived cells do not stably incorporate into newly formed vessels and only play an auxiliary role in neovascularization 15. True vasculogenic ability has only been demonstrated for a single cell‐type, designated the endothelial colony forming cell (ECFC) 16, which cannot be obtained from BM. While the auxiliary angiogenic effects of BM‐MNCs have been demonstrated very consistently in animal models, it is likely that they only occur with BM isolates from comparatively young subjects without comorbidities 17, 18. This restricts successful application of BM‐MNCs in MI to specific subsets 19 of patients, and likely severely limits it in CLTI.
Collectively, these observations have precipitated a switch away from undefined raw cell isolates such as BM‐MNCs, toward better‐defined cell therapy products 20. Whereas in the therapy for MI, the focus has shifted away from angiogenic cell therapy to cardiomyocyte regeneration 21, in CLTI the primary objective remains to augment angiogenesis.
Angiogenesis can be induced via different cell‐based strategies (Fig. 1) by supplying endothelial‐like cells, such as ECFCs directly, which will spontaneously organize into new vessels that integrate with the existing vasculature. Alternatively angiogenesis can be promoted indirectly by cells that secrete factors that remodel the extracellular matrix and recruit resident endothelial cells. In this category are circulating endothelial cells, which are of monocyte/myeloid origin, and may potentially act as bridging monocytes in angiogenesis 22. Alternatively there are mesenchymal stromal cells (MSCs), which are of pericyte origin 23 and secrete a host of paracrine angiogenic and immunomodulatory factors 24. However, it is debated that pericytes have the multilineage potential in vivo, which characterize MSCs in vitro and that the observed plasticity of MSCs result from manipulation ex vivo 25. But irrespective of this it is likely that there are synergistic effects of combining these approaches, using a combination of ECFCs and a supportive cell‐type 26, 27. At present, however, translation to a clinical product has proven difficult as ex vivo expansion of the above‐mentioned cells requires specialty cell culture additives that are difficult and costly to obtain for clinical grade production 28. For this reason only MSCs, have advanced to a second generation of clinical trials, as they can be relatively easily obtained from different tissues such as BM, placenta and adipose tissue and reproducibly expanded ex vivo (see Table 1 for overview).
Figure 1.
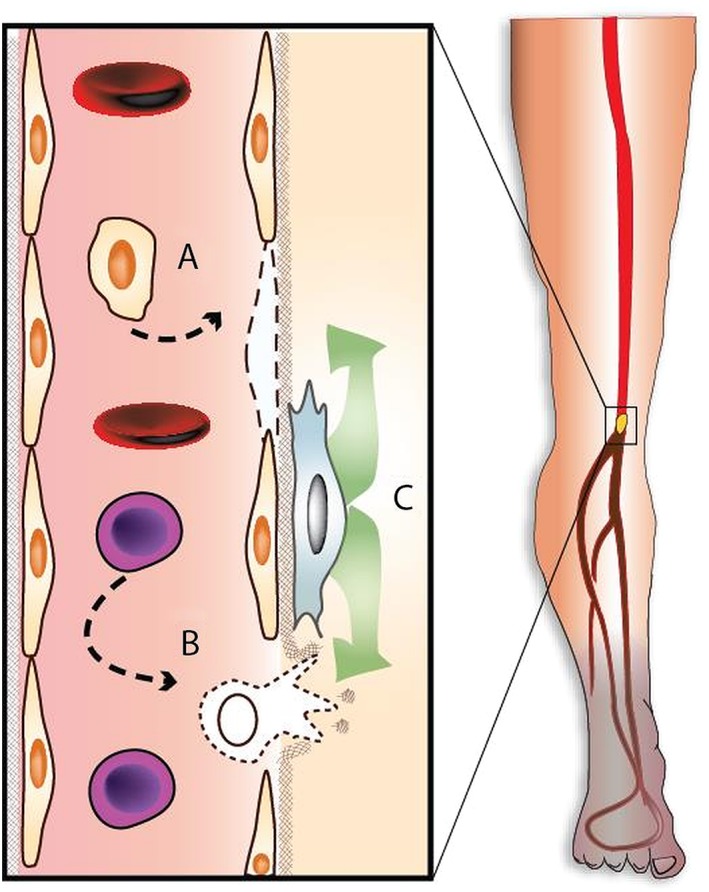
Different potential modes of action of cell therapy. (A): Direct angiogenesis through introduction of endothelial‐like cells that will form new capillaries through vasculogenesis and fill endothelial defects. (B): Indirect angiogenesis through introduction of monocyte‐like cells, that will remodel the extracellular matrix and will recruit and guide new endothelial sprouts. (C): Indirect angiogenesis through paracrine effects, including modulation of monocyte differentiation and recruitment of endothelial cells.
Table 1.
Overview of MSC trials in CLTI
| Author | Year | n | Design | Injection sites | Total dose and source |
|---|---|---|---|---|---|
| Kim et al. 29 | 2006 | 4 | No control group | NM | 1 × 106 allogeneic HLA matched UCB‐MSCs |
| Dash et al. 30 | 2009 | 24 | Open label; control group (1:1 randomization) | NM | 45–60 × 106 autologous BM‐MSCs |
| Lu et al. 31 | 2011 | 41 | Double blind study; randomly assigned treatment per leg; one leg treated with normal saline, the other treated with MNC or MSC | 20 | 9.3 × 108 autologous BM‐MSCs |
| Gupta et al. 32 | 2013 | 20 | Placebo controlled; double blind | 40–60 | 2 × 106 allogeneic BM‐MSCs per kilogram body weight |
| Gupta et al. 33 | 2016 | 90 | Nonrandomized; low dose, high dose or standard care | 40–60 | (1 or 2) × 106 allogeneic BM‐MSCs per kilogram body weight |
Abbreviations: BM, bone marrow; HLA, human leukocyte antigens; MSCs, mesenchymal stromal cells; NM, not mentioned; UCB, umbilical cord blood.
MSC Therapy for CLTI
While MNC have proven to effectively enhance angiogenesis and neovascularization in preclinical studies, it has been suggested that the pro‐angiogenic effect of MSCs is superior compared to MNCs in preclinical studies 34, 35. In vitro and vivo studies have demonstrated that MSC can home to injured tissue and secrete beneficial factors that suppress inflammation and improve angiogenesis via paracrine pathways 36. Several small exploratory clinical trials showed positive effects of MSCs in the treatment of CLTI compared to standard of care or placebo 30, 32, 37. Clinical studies that directly compare BM‐MSCs with MNCs for the treatment of CLTI are scarce. Only one study directly compared the two strategies in 41 diabetic CLTI patients, suggesting that MSC might be better tolerated and more effectively enhance perfusion and ulcer healing compared to MNC 31. The disappointing results of clinical trials on MNC in CLTI, the promising effects of MSCs and several practical benefits of MSCs, in particular the potential for allogeneic application, have led to increased interest of MSCs as potential option for cell therapy in CLTI A similar switch is also observed for studies in cardiac disease 38.
MSCs, rather uniquely among transplanted cell grafts, are only minimally immunogenic 38 and display strong immunomodulatory properties 39. This makes allogeneic application possible, at least in a single administration, as it is still unclear whether rejection occurs upon repeated administration 38. At the present state of knowledge, allogeneic administration of MSCs is the most promising route to clinical application with the advantage of providing an off‐the‐shelf available product. An allogeneic product significantly reduces the burden on the patient, as patients will not have to undergo a (BM) harvesting procedure. Whereas in BM‐MNCs it has been shown that patient‐derived cell isolates show decreased pro‐angiogenic effects 18, this does not necessarily apply to MSCs 40. In a previous study we did not observe reduced angiogenic potency in CLTI MSC isolates in a murine hind limb ischemia model 40, 41. In a clinical trial comparing efficacy of autologous versus allogeneic MSCs in non‐ischemic dilated cardiomyopathy, however, allogeneic MSCs had a more favorable side‐effect profile and a trend toward greater improvement in ejection fraction. Additionally the occurrence of serious adverse events was substantially lower in allogeneic then autologous MSCs; 28% versus 64%, respectively 38. Furthermore, an advantage of allogeneic MSC therapy is that the (angiogenic) potency of the cell isolate can be tested in advance. Demonstrable potency will likely be of importance in the quality control of cell therapy products for clinical regulation 42. As a single MSC isolate generally is sufficient to treat dozens of patients, a priori batch testing can conceivable improve clinical outcomes, provided that validated assays are developed 42, 43. Last, allogeneic MSC administration is significantly less costly. Costs for expansion and quality testing of a BM isolate are high, which in autologous application were the per‐patient cost, but which can be split over multiple patients in allogeneic application 44.
Current Challenges and Future Directions
Allogeneic application makes MSC‐therapy interesting for commercial parties, as a defined cell product can be comparatively easily patented and produced by in‐house companies, without the complications of harvesting donor material for each patient. Fifteen percentage of all clinical trials worldwide involving cell therapy are industry‐sponsored and the vast majority of the remainder by leading academic centers. Additionally facilities and logistics involved in the development of cell therapy products are becoming more available and less expensive due to increased administration as standard of care or as investigational novel treatment in other diseases 45. However, development of evidence‐based accepted approaches remains challenging, due to high developmental costs, regulatory hurdles, and batch‐per‐batch product variation. Some of these factors may be less relevant for non‐cellular cell‐based therapies, such as exosome‐based therapies, which could make commercialization less difficult 46. Another important CLTI‐specific limiting factor is that, historically, the design of high‐quality studies for the treatment of CLTI has proven notoriously difficult 47. Improved clinical management and technical advances in revascularization approaches, endovascular interventions in particular, have led to a near doubling of 1‐year amputation‐free survival for CLTI patients since the first trials with BM‐MNCs. Therefore larger and better‐designed trials are required to determine the potential added value of novel therapies in CLTI 48, 49. In the light of these considerations, small phase I/II 31, 32 or pragmatically designed studies 33 have provided valuable first indications about potential efficacy of MSCs in CLTI. However, at no point can they substitute evidence from placebo‐controlled double‐blind randomized trials. Public demand for cell‐based therapies has been such, that smaller commercial parties are offering cell treatments in the absence of evidence—positive or negative—potentially putting patients at risk 50, leading to public discussions with respect to ethical issues regarding regenerative medicine approaches 51.
We therefore would encourage increased openness and standardization, both in the use of the investigational cell product and trial design. Convincing evidence for efficacy of MSC therapy will only come from well‐designed randomized controlled trials using hard and clinically relevant outcomes, which would be ideally related with future imaging methods to evaluate collateralization and neovascularization. It seems increasingly unlikely that single investigative centers will achieve sufficient statistical power to show efficacy. International collaborative efforts and data sharing are necessary to push the field forward and maintain scientific integrity.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1. Norgren L, Hiatt WR, Dormandy JA et al. Inter‐society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 2007;45:S5–S67. [DOI] [PubMed] [Google Scholar]
- 2. Barshes NR, Chamber JD, Cohen J et al. Cost‐effectiveness in the contemporary management of critical limb ischemia with tissue loss. J Vasc Surg 2012. Oct;56:1015–1024. [DOI] [PubMed] [Google Scholar]
- 3. Farber A, Eberhardt RT. The current state of critical limb ischemia: A systematic review. JAMA Surg 2016;151:1070–1077. [DOI] [PubMed] [Google Scholar]
- 4. TASC Steering Committee , Jaff MR, White CJ et al. An update on methods for revascularization and expansion of the TASC lesion classification to include below‐the‐knee arteries: A supplement to the inter‐society consensus for the management of peripheral arterial disease (TASC II). J Endovasc Ther 2015;22:663–677. [DOI] [PubMed] [Google Scholar]
- 5. Setacci C, de Donato G, Teraa M et al. Chapter IV: Treatment of critical limb ischaemia. Eur J Vasc Endovasc Surg 2011;42:S43–S59. [DOI] [PubMed] [Google Scholar]
- 6. Cooke JP, Losordo DW. Modulating the vascular response to limb ischemia: Angiogenic and cell therapies. Circ Res 2015;116:1561–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Asahara T, Murohara T, Sullivan A et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–967. [DOI] [PubMed] [Google Scholar]
- 8. Kalka C, Masuda H, Takahashi T et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA 2000;97:3422–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Asahara T, Masuda H, Takahashi T et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 1999;85:221–228. [DOI] [PubMed] [Google Scholar]
- 10. Teraa M, Sprengers RW, Schutgens REG et al. Effect of repetitive intra‐arterial infusion of bone marrow mononuclear cells in patients with no‐option limb ischemia: The randomized, double‐blind, placebo‐controlled Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intra‐arterial Supplementation (JUVENTAS) trial. Circulation 2015;131:851–860. [DOI] [PubMed] [Google Scholar]
- 11. Peeters Weem SMO, Teraa M, de Borst GJ et al. Bone marrow derived cell therapy in critical limb ischemia: A meta‐analysis of randomized placebo controlled trials. Eur J Vasc Endovasc Surg 2015;50:775–783. [DOI] [PubMed] [Google Scholar]
- 12. Gyöngyösi M, Wojakowski W, Lemarchand P et al. Meta‐Analysis of Cell‐based CaRdiac stUdiEs (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ Res 2015;116:1346–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prokopi M, Pula G, Mayr U et al. Proteomic analysis reveals presence of platelet microparticles in endothelial progenitor cell cultures. Blood 2009;114:723–732. [DOI] [PubMed] [Google Scholar]
- 14. Gremmels H, Fledderus JO, van Balkom BWM et al. Transcriptome analysis in endothelial progenitor cell biology. Antioxid Redox Signal 2011;15:1029–1042. [DOI] [PubMed] [Google Scholar]
- 15. Ziegelhoeffer T, Fernandez B, Kostin S et al. Bone marrow‐derived cells do not incorporate into the adult growing vasculature. Circ Res 2004;94:230–238. Epub 2003 Dec 4. [DOI] [PubMed] [Google Scholar]
- 16. Yoder MC. Blood cell progenitors: insights into the properties of stem cells. Anat Rec A Discov Mol Cell Evol Biol 2004;276:66–74. [DOI] [PubMed] [Google Scholar]
- 17. Heeschen C, Lehmann R, Honold J et al. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation 2004;109:1615–1622. [DOI] [PubMed] [Google Scholar]
- 18. Dimmeler S, Leri A. Aging and disease as modifiers of efficacy of cell therapy. Circ Res 2008;102:1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zwetsloot PP, Gremmels H, Assmus B et al. Responder definition in clinical stem cell trials in cardiology: Will the real responder please stand up? Circ Res 2016. Aug 5;119:514–518. [DOI] [PubMed] [Google Scholar]
- 20. Chavakis E, Koyanagi M, Dimmeler S. Enhancing the outcome of cell therapy for cardiac repair: Progress from bench to bedside and back. Circulation 2010;121:325–335. [DOI] [PubMed] [Google Scholar]
- 21. Li TS, Cheng K, Malliaras K et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere‐derived cells. J Am Coll Cardiol 2012;59:942–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Urbich C, Heeschen C, Aicher A et al. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation 2003;108:2511–2516. Epub 2003 Oct 27. [DOI] [PubMed] [Google Scholar]
- 23. Crisan M, Deasy B, Gavina M et al. Purification and long‐term culture of multipotent progenitor cells affiliated with the walls of human blood vessels: Myoendothelial cells and pericytes. Methods Cell Biol 2008;86:295–309. [DOI] [PubMed] [Google Scholar]
- 24. Caplan AI, Correa D. The MSC: An injury drugstore. Cell Stem Cell 2011;9:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guimaraes‐Camboa N, Cattaneo P, Sun Y et al. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell 2017;20:345–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yoon CH, Hur J, Park KW et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation 2005;112:1618–1627. [DOI] [PubMed] [Google Scholar]
- 27. Schwarz TM, Leicht SF, Radic T et al. Vascular incorporation of endothelial colony‐forming cells is essential for functional recovery of murine ischemic tissue following cell therapy. Arterioscler Thromb Vasc Biol 2012;32:e13–e21. [DOI] [PubMed] [Google Scholar]
- 28. Zeisberger SM, Zoller S, Riegel M et al. Optimization of the culturing conditions of human umbilical cord blood‐derived endothelial colony‐forming cells under xeno‐free conditions applying a transcriptomic approach. Genes Cells 2010;15:671–687. [DOI] [PubMed] [Google Scholar]
- 29. Kim SW, Han H, Chae GT et al. Successful stem cell therapy using umbilical cord blood‐derived multipotent stem cells for Buerger's disease and ischemic limb disease animal model. Stem Cells 2006;24:1620–1626. [DOI] [PubMed] [Google Scholar]
- 30. Dash NR, Dash SN, Routray P et al. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow‐derived mesenchymal stem cells. Rejuvenation Res 2009;12:359–366. [DOI] [PubMed] [Google Scholar]
- 31. Lu D, Chen B, Liang Z et al. Comparison of bone marrow mesenchymal stem cells with bone marrow‐derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double‐blind, randomized, controlled trial. Diabetes Res Clin Pract 2011;92:26–36. [DOI] [PubMed] [Google Scholar]
- 32. Gupta PK, Chullikana A, Parakh R et al. A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. J Transl Med 2013;11:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gupta PK, Krishna M, Chullikana A et al. Administration of adult human bone marrow‐derived, cultured, pooled, allogeneic mesenchymal stromal cells in critical limb ischemia due to Buerger's disease: Phase II Study report suggests clinical efficacy. Stem Cells Translational Medicine 2016;6:689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arminan A, Gandia C, Garcia‐Verdugo JM et al. Cardiac transcription factors driven lineage‐specification of adult stem cells. J Cardiovasc Transl Res 2010;3:61–65. [DOI] [PubMed] [Google Scholar]
- 35. Iwase T, Nagaya N, Fujii T et al. Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovasc Res 2005;66:543–551. [DOI] [PubMed] [Google Scholar]
- 36. Kinnaird T, Stabile E, Burnett MS et al. Marrow‐derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res 2004;94:678–685. Epub 2004 Jan 22. [DOI] [PubMed] [Google Scholar]
- 37. Gupta PK, Krishna M, Chullikana A et al. Administration of adult human bone marrow‐derived, cultured, pooled, allogeneic mesenchymal stromal cells in critical limb ischemia due to Buerger's disease: Phase II Study report suggests clinical efficacy. Stem Cells Translational Medicine 2017;6:689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hare JM, DiFede DL, Rieger AC et al. Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON‐DCM trial. J Am Coll Cardiol 2017;69:526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Le Blanc K, Tammik C, Rosendahl K et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematology 2003;31:890–896. [DOI] [PubMed] [Google Scholar]
- 40. Gremmels H, Fledderus JO, Teraa M et al. Mesenchymal stromal cells for the treatment of critical limb ischemia: context and perspective. Stem Cell Res Ther 2013;4:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gremmels H, Teraa M, Quax PH et al. Neovascularization capacity of mesenchymal stromal cells from critical limb ischemia patients is equivalent to healthy controls. Mol Ther 2014;22:1960–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.European Medicines Agency. Committee for Advanced Therapies (CAT). Reflection paper on stem cell‐based medicinal products. EMA/CAT/571134/2009. 2011 Jan 14. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/02/WC500101692.pdf
- 43. Wijnand JGJ, Teraa M, Gremmels H et al. Rationale and design of the SAIL trial for intramuscular injection of allogeneic mesenchymal stromal cells in no‐option critical limb ischemia. J Vasc Surg 2018;67:656–661. 10.1016/j.jvs.2017.09.026 Epub 2017 Dec 11. [DOI] [PubMed] [Google Scholar]
- 44. Abou‐El‐Enein M, Bauer G, Medcalf N et al. Putting a price tag on novel autologous cellular therapies. Cytotherapy 2016;18:1056–1061. [DOI] [PubMed] [Google Scholar]
- 45. Clarke D, Stanton J, Powers D et al. Managing particulates in cell therapy: Guidance for best practice. Cytotherapy 2016;18:1063–1076. [DOI] [PubMed] [Google Scholar]
- 46. Rosca AM, Rayia DM, Tutuianu R. Emerging role of stem cells – Derived exosomes as valuable tools for cardiovascular therapy. Curr Stem Cell Ther 2017;12:134–138. [DOI] [PubMed] [Google Scholar]
- 47. Teraa M, Conte MS, Moll FL et al. Critical limb ischemia: Current trends and future directions. J Am Heart Assoc 2016;5:e002938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Benoit E, O'Donnell TF Jr, Kitsios GD et al. Improved amputation‐free survival in unreconstructable critical limb ischemia and its implications for clinical trial design and quality measurement. J Vasc Surg 2012;55:781–789. [DOI] [PubMed] [Google Scholar]
- 49. Iafrati MD, Hallett JW, Geils G et al. Early results and lessons learned from a multicenter, randomized, double‐blind trial of bone marrow aspirate concentrate in critical limb ischemia. J Vasc Surg 2011;54:1650–1658. [DOI] [PubMed] [Google Scholar]
- 50. Taylor PL, Barker RA, Blume KG et al. Patients beware: Commercialized stem cell treatments on the web. Cell Stem Cell 2010;7:43–49. [DOI] [PubMed] [Google Scholar]
- 51. Niemansburg SL, Teraa M, Hesam H et al. Stem cell trials for cardiovascular medicine: ethical rationale. Tissue Eng Part A 2014. Oct;20:2567–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]


