Abstract
The striking rise of methicillin-resistant Staphylococcus aureus (MRSA) infections has become a serious threat to public health worldwide. In an effort to search for new anti-MRSA agents from natural products, a bioassay-guided phytochemical study was conducted on the semi-mangrove plant Myoporum bontioides A. Gray, which led to the isolation of two new sesquiterpene alkaloids (1 and 2) and six known furanosesquiterpenes (3–8). Their structures were elucidated on the basis of extensive analysis of their 1D, 2D NMR and mass spectroscopic data. These two new alkaloids (1 and 2) displayed potent anti-MRSA activity with MIC value of 6.25 μg/mL. This is the first report of sesquiterpene alkaloids from the plants of Myoporum genus and their anti-MRSA activity.
Keywords: Myoporum bontioides, methicillin-resistant Staphylococcus aureus (MRSA), sesquiterpene alkaloids
1. Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) infections have become a global threat to public health [1,2,3]. MRSA is responsible for several intractable infections in human being including skin and soft tissue infections, septicemia, endocarditis, pneumonia, enteritis, meningitis, osteomyelitis as well as toxic shock syndrome [4,5]. MRSA infections worldwide have increased rapidly from 1–5% in the mid-1980s to 60–70% today since MRSA was first discovered by British scientist Jevons in 1961 [6]. At present, MRSA infection has surpassed hepatitis B and AIDS, ranking the first among the three most intractable infectious diseases throughout the world [7]. In a response to antimicrobial stress, almost all clinical MRSA isolates produce β-lactamase and a penicillin-binding protein with low affinity for β-lactam antibiotics [8,9]. Although a variety of non-β-lactam antibiotics such as vancomycin, teicoplanin, linezolid, and daptomycin had been recommended for the treatment of MRSA infections [10,11,12,13], a series of drawbacks including slow bactericidal activity, low tissue penetration, and increasing reports of resistance were described and greatly restricted their utility [13,14,15,16,17,18,19]. Therefore, there is an urgent need to discover alternative anti-MRSA candidates with novel structure scaffold and mechanism of action for the treatment of infections arising from MRSA.
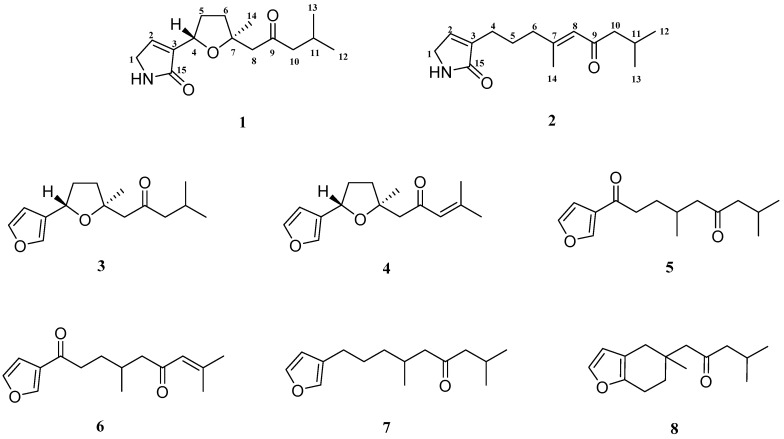
One strategy to develop new anti-MRSA agents is to search for anti-MRSA substances or lead compounds from natural products, which has been proven to be effective in the field of new drug development [20]. During our ongoing investigation to search for novel antibiotics from traditional Chinese medicinal plants, Myoporum bontioides A. Gray was found to possess anti-MRSA activity. M. bontioides, belonging to the genus Myoporum in the family Myoporaceae, is a semi-mangrove plant distributed mainly in China, Japan, Australia, New Zealand, Mauritius, and the Hawaiian Islands [21,22]. It grows above the tide lines by the sea and adapts to saline-alkali sand and rocky land, which plays an important role in wind-breaking and sand-fixation, as well as greening the environment [23]. In China, M. bontioides has been used as a folk medicine for antidermatosis, antipyretic, and antipsychotic [24,25,26,27]. Previous phytochemical studies have revealed some structurally diverse chemicals from this plant, including sesquiterpenoids, iridoids, monoterpenes, phenylethanoids, and flavonoids, some of which showed important bioactivities [28,29,30,31,32,33]. Our previous experiment showed that the extract of M. bontioides possessed anti-MRSA activity. With the aim to find out the potential anti-MRSA substances from M. bontioides, we carried out a bioassay-guided phytochemical study on the semi-mangrove plant M. bontioides, which led to the isolation of two new sesquiterpene alkaloids (1 and 2) and six known furanosesquiterpenes (3–8) (Figure 1). Their structures were elucidated on the basis of extensive spectroscopic analysis. Herein, we report the isolation and structure elucidation of these compounds, as well as their anti-MRSA activity.
Figure 1.
Chemical structures of compounds 1–8.
2. Results and Discussion
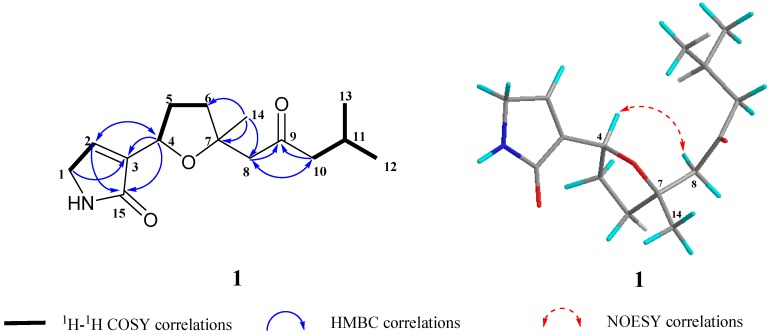
Compound 1 was obtained as a colorless oil with a molecular formula of C15H23NO3 as determined on the basis of HR-EI-MS data, m/z 265.1660 ([M]+), which required five degrees of unsaturation. The 1H NMR spectrum (Table 1) showed the signals of three methyls at δH 0.90 (3H, d, J = 6.5 Hz), 0.91 (3H, d, J = 6.5 Hz) and 1.27 (3H, s), an oxymethine at δH 4.78 (1H, t, J = 7.3 Hz), and an olefinic methine at δH 5.97 (1H, t, J = 1.5 Hz). The 13C NMR spectrum (Table 1), coupled with HSQC analysis, exhibited the signals of fifteen carbons in total, comprising three methyls, five methylenes, three methines, and four quaternary carbons including one oxygenated quaternary carbon at δC 82.6 (C-7), two carbonyl carbons [δC 174.9 (C-15) and 209.2 (C-9)], and an olefinic carbon at δC 163.8 (C-3). Detailed analysis of the NMR data indicated that compound 1 was similar to (–)-epingaione [34,35], a known furanosesquiterpene which was also obtained in this study as compound 3. The main difference was that the signals of the furan group at C-4 in 3 were absent in 1. Instead, proton and carbon signals of an α,β-unsaturated butyrolactam moiety in 1 were exhibited. These findings led us to establish the structure of 1 as shown in Figure 1. This assignment was in accordance with the molecular formula of 1 (C15H23NO3) and well supported by the 2D NMR spectroscopic data. The heteronuclear multiple bond correlations (HMBC) (Figure 2) from δH 4.78 (H-4) to 121.1 (C-2), 163.8 (C-3), and 174.9 (C-15), from δH 4.00 (H-1) to 121.1 (C-2), 163.8 (C-3), and 174.9 (C-15), from δH 5.97 (H-2) to 47.7 (C-1), 163.8 (C-3), 76.1 (C-4), and 174.9 (C-15), combining with the 1H–1H COSY correlation between δH 4.00 (H-1) and 5.97 (H-2) (Figure 2), confirmed the presence of the α,β-unsaturated butyrolactam moiety. The observation of significant NOE correlation of H-4/H-8 (Figure 2) and the absence of NOE correlation of H-4/CH3-14 in the NOESY spectrum further supported the β-orientation of H-4 and α-orientation of CH3-14. Consequently, the structure of compound 1 was elucidated as shown in Figure 1, trivially named as myoporumine A.
Table 1.
1H (600 MHz) and 13C (150 MHz) NMR data of compounds 1 and 2 in CDCl3.
| H/C | 1 | 2 | ||
|---|---|---|---|---|
| δH (mult, J in Hz) | δ C | δH (mult, J in Hz) | δ C | |
| 1 | 4.00 (brs) | 47.7 (CH2) | 3.94 (d, 1.6) | 47.8 (CH2) |
| 2 | 5.97 (t, 1.5) | 121.1 (CH) | 6.94 (t, 1.5) | 140.6 (CH) |
| 3 | 163.8 (C) | 139.7 (C) | ||
| 4 | 4.78 (t, 7.3) | 76.1 (CH) | 2.26 (m) | 25.9 (CH2) |
| 5 | 1.88 (m) 2.24 (m) |
32.5 (CH2) | 1.77 (m) | 26.8 (CH2) |
| 6 | 1.94 (m) 2.09 (m) |
37.2 (CH2) | 2.23 (m) | 41.6 (CH2) |
| 7 | 82.6 (C) | 159.8 (C) | ||
| 8 | 2.67 (q, 15.3) | 53.4 (CH2) | 6.19 (q, 1.1) | 124.9 (CH) |
| 9 | 209.2 (C) | 203.7 (C) | ||
| 10 | 2.32 (dd, 6.9, 2.5) | 53.7 (CH2) | 2.31 (d, 7.0) | 54.3 (CH2) |
| 11 | 2.13 (m) | 24.5 (CH) | 1.71 (m) | 26.4 (CH) |
| 12 | 0.90 (d, 6.5) | 22.7 (CH3) | 0.92 (d, 6.6) | 22.9 (CH3) |
| 13 | 0.91 (d, 6.5) | 22.7 (CH3) | 0.92 (d, 6.6) | 22.9 (CH3) |
| 14 | 1.27 (s) | 27.6 (CH3) | 2.12 (d, 1.3) | 19.4 (CH3) |
| 15 | 174.9 (C) | 176.8 (C) | ||
Figure 2.
Key 1H–1H COSY, HMBC and NOESY correlations of compound 1.
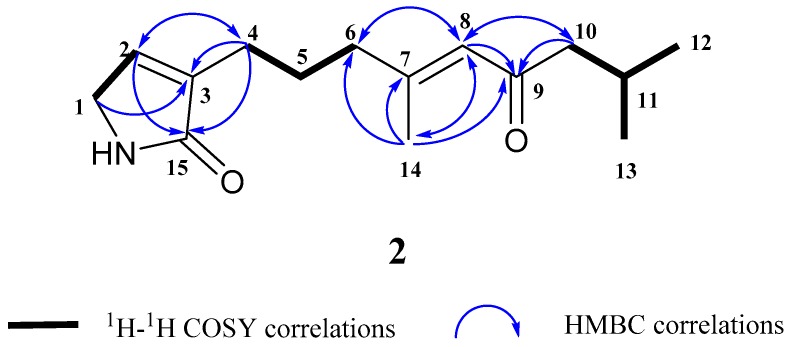
Compound 2, obtained as a colorless oil, was deduced to have the molecular formula C15H23NO2 by the HR-EI-MS data, m/z 249.1720 ([M]+), which required five degrees of unsaturation. Its 1H and 13C NMR spectra (Table 1), in combination with HSQC analysis, indicated three methyls, five methylenes, three methines and four quaternary carbons [including two carbonyl carbons at δC 176.8 (C-15) and 203.7 (C-9), two olefinic carbons at 139.7 (C-3) and 159.8 (C-7). A detailed comparison of the NMR data (Table 1) revealed that compound 2 closely resembled 1 with the main differences of the absence of the oxygen bridge between C-4 and C-7, and the presence of the double bond between C-7 and C-8. This deduction was in accordance with the molecular formula of 2 (C15H23NO2) and well supported by the 2D NMR spectroscopic data, including HSQC, 1H−1H COSY, and HMBC data. The 1H–1H COSY correlations of H-1/H-2, H-4/H-5, H-5/H-6, H-10/H-11, H-11/H-12, and H-11/H-13, together with the HMBC correlations from H-4 to C-2, C-3, and C-15, from H-14 to C-6, C-7, C-8, and C-9, and from H-8 to C-6, C-7, C-9, C-10, and C-14, supported the above deduction (see Figure 3). Hence, the structure of compound 2 was determined as shown in Figure 1, trivially named as myoporumine B.
Figure 3.
Key 1H–1H COSY and HMBC correlations of compound 2.
The six known compounds (3–8) were identified as (−)-epingaione (3) [34,35], (–)-dehydroepingaione (4) [36], myoporone (5) [37], dehydromyoporone (6) [37], 9-(3-furanyl)-2,6-dimethyl-4-nonanone (7) [38] and dihydrocrassifolone (8) [39] respectively, by comparing their spectroscopic data with those reported in the literature.
All the isolated compounds (1–8) were evaluated for their anti-MRSA activity using the microdilution method as we described previously [40]. As shown in Table 2, these two new alkaloids (1 and 2) displayed potent anti-MRSA activity with MIC value of 6.25 μg/mL.
Table 2.
In vitro anti-MRSA activity of the Fraction F4 and compounds 1–8.
| Sample | MIC (μg/mL) | Sample | MIC (μg/mL) |
|---|---|---|---|
| Fraction F4 | 25 | 5 | 50 |
| 1 | 6.25 | 6 | 50 |
| 2 | 6.25 | 7 | >100 |
| 3 | 25 | 8 | >100 |
| 4 | 25 | Vancomycin | 0.78 |
3. Experimental Section
3.1. General Experimental Procedures
1D and 2D Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker DRX-500 NMR spectrometer (Bruker Biospin Gmbh, Rheistetten, Germany). High-resolution (HR) EI–MS was obtained on a Waters AutoSpec Premier P776 mass spectrometer (Waters, Milford, MA, USA). UV spectra were acquired on a Perkin-Elmer Lambda 650 UV–vis spectrometer (Perkin-Elmer, Inc., Waltham, MA, USA). Optical rotations were measured on a Perkin-Elmer Model 341 polarimeter (Perkin-Elmer, Inc., Waltham, MA, USA). Column chromatography (CC) was performed with silica gel (80–100 mesh, Qingdao Haiyang Chemical Co., Qingdao, China), Sephadex LH-20 (Pharmacia Fine Chemical Co. Ltd., Uppsala, Sweden). Preparative HPLC was performed with an HPLC system equipped with a Shimadzu LC-6AD pump and a Shimadzu RID-10A refractive index detector using a Shim-pack PRC-ODS C-18 column (5 μm, 20 × 250 mm). Thin-layer chromatography (TLC) was conducted on precoated silica gel plates (HSGF254, Yantai Jiangyou Silica Gel Development Co. Ltd., Yantai, China) and spot detection was performed by spraying 10% H2SO4 in ethanol, followed by heating. Analytical grade chloroform, methanol, petroleum ether, acetone, n-hexane, and ethyl acetate were purchased from Tianjin Fuyu Fine Chemical Industry Co. (Tianjin, China).
3.2. Plant Material
Leaves of M. bontioides were collected from the Leizhou Peninsula, Guangdong province, China in September 2010, and identified by Prof. Bingtao Li from South China Agricultural University. A voucher specimen (No. 20100915) was deposited in the College of Materials and Energy, South China Agricultural University.
3.3. Extraction and Isolation
The air-dried leaves of M. bontioides (12 kg) were powdered and extracted by supercritical CO2 extraction technology at 15 MPa and 30 °C for 15 min yield a supercritical CO2 extract (116 g). The crude extract was subjected to silica gel column chromatography, eluted with petroleum ether/acetone (from 100:0 to 0:100, v/v), to afford fractions F1–F6 after pooling according to their TLC profiles. According to the result of activity screening, Fraction F4 (2.02 g) showed the most potent anti-MRSA activity with MIC value of 25 μg/mL. Then it was subjected to silica gel column chromatography with the elution of chloroform/methanol (from 100:1 to 100:10) to provide subfractions F4-1–F4-4. Subfraction F4-1 was further chromatographed over a silica gel column eluting with n-hexane/ethyl acetate (20:1 and 15:1) to afford compounds 5 (15 mg), 6 (10 mg), and 7 (4 mg). Subfraction F4-2 was separated by Sephadex LH-20 column chromatography eluted with acetone to give compounds 3 (6 mg) and 4 (4 mg). Subfraction F4-3 was recrystallized to give compound 8 (5 mg). Subfraction F4-4 was applied on Sephadex LH-20 column chromatography with the elution of chloroform/methanol (1:4, v/v) to give F4-4-1 and F4-4-2. F4-4-1 was further purified by preparative HPLC with a Shim-pack PRC-ODS C-18 column (5 μm, 20 × 250 mm) using 40% methanol in water (v/v) as mobile phase at the flow rate of 8 mL/min to obtain compound 1 (4 mg, tR 65 min). F4-4-2 was further purified by preparative HPLC using 25% acetonitrile in water as mobile phase at the flow rate of 10 mL/min to yield compound 2 (3 mg, tR 58 min).
Myoporumine A (1): colorless oil; [α–14.5 (c 0.20, CHCl3); UV (CHCl3) λmax nm (log ε) 255 (3.37); HR-EI-MS: m/z 265.1660 [M]+ (calcd 265.1678, C15H23NO3); 1H NMR and 13C NMR data, see Table 1. The NMR and HREIMS spectra, see Supplementary Materials.
Myoporumine B (2): colorless oil; UV (CHCl3) λmax nm (log ε) 230 (3.21), 255 (3.47); HR-EI-MS: m/z 249.1720 [M]+ (calcd 249.1723, C15H23NO2); 1H NMR and 13C NMR data, see Table 1. The NMR and HREIMS spectra, see Supplementary Materials.
3.4. Anti-MRSA Assay
The anti-MRSA activity of compounds 1–8 was evaluated by the microdilution method as we described previously [40]. The MRSA strain (No. 11646) was provided by State Key Laboratory Respiratory Disease, Guangzhou Institute of Respiratory Disease (Guangzhou, China), which was resistant to methicillin and sensitive to vancomycin. Resazurin was used as a visible indicator in the assay. The count of bacterial suspension was adjusted to 1 × 105 CFU/mL with MHB. Test samples were diluted with the medium (DMSO) by the two-fold dilution method. The final concentrations of each sample in the wells were 100, 50, 25, 12.5, 6.25, 3.12, 1.56, and 0.78 μg/mL. Vancomycin was used as a positive control. Finally, the plates were incubated at 37 °C for 5–6 h until the color of negative control wells (which contained DMSO instead of the test sample) change to pink. The lowest concentration for each test sample at which color change occurred was recorded as the minimal inhibitory concentration (MIC). MIC values of test samples were displayed in Table 2.
4. Conclusions
In summary, two new sesquiterpene alkaloids (1 and 2) and six known furanosesquiterpenes (3–8) were isolated from the semi-mangrove plant M. bontioides. Their structures were elucidated on the basis of extensive analysis of their 1D, 2D NMR and mass spectroscopic data. These two new alkaloids 1 and 2 showed potent anti-MRSA activity, suggesting that they could be worthy of consideration for the development and research of anti-MRSA agents. This is the first report of sesquiterpene alkaloids from the plants of Myoporum genus and their anti-MRSA activity.
Supplementary Materials
The following are available online at http://www.mdpi.com/1660-3397/16/11/438/s1, Figures S1–S13: The NMR and HREIMS spectra of compounds 1 and 2.
Author Contributions
L.-M.D. and L.-L.H. performed the isolation, structural elucidation of the chemicals; H.D. and Q.-L.X. contributed to the extraction and separation; J.-K.O. and X.-C.J. contributed to the bioassay experiments; L.-M.D. wrote the paper; W.-X.G. and J.-W.T. designed the study, supervised the whole experiment and revised the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (31470422 and 31500291), and the Natural Science Foundation of Guangdong Province (2014A030313742 and 2018A030310199).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Brown E.D., Wright G.D. Antibacterial drug discovery in the resistance era. Nature. 2016;529:336–343. doi: 10.1038/nature17042. [DOI] [PubMed] [Google Scholar]
- 2.Hal S.J.V., Jensen S.O., Vaska V.L., Espedido B.A., Paterson D.L., Gosbell I.B. Predictors of mortality in Staphylococcus aureus bacteremia. Clin. Microbiol. Rev. 2012;25:362–386. doi: 10.1128/CMR.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boucher H.W., Talbot G.H., Bradley J.S., Edwards J.E., Gilbert D., Rice L.B., Scheld M., Spellberg B. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 4.Li B.L., Ni S.S., Mao F., Chen F.F., Liu Y.F., Wei H.W., Chen W.H., Zhu J., Lan L.F., Li J. Novel terminal bipheny-based diapophytoene desaturases (CrtN) inhibitors as anti-MRSA/VISR/LRSA agents with reduced hERG activity. J. Med. Chem. 2018;61:224–250. doi: 10.1021/acs.jmedchem.7b01300. [DOI] [PubMed] [Google Scholar]
- 5.Koyama N., Inokoshi J., Tomoda H. Anti-infectious agents against MRSA. Molecules. 2013;18:204–224. doi: 10.3390/molecules18010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taubes G. The bacteria fight back. Science. 2008;321:356–361. doi: 10.1126/science.321.5887.356. [DOI] [PubMed] [Google Scholar]
- 7.Yang C.S., Liu W.E. Advances on mechanism of drug-resistance to MRSA and test method of molecular biology. Chin. J. Nosocomiol. 2007;17:356–358. [Google Scholar]
- 8.GarcãA-Castellanos R., MallorquãFernã G., Marrero A., Potempa J., Coll M., Gomis-RuTh F.X. On the transcriptional regulation of methicillin resistance: MecI repressor in complex with its operator. J. Biol. Chem. 2004;279:17888–17896. doi: 10.1074/jbc.M313123200. [DOI] [PubMed] [Google Scholar]
- 9.Mun S.H., Kim S.B., Kong R., Choi J.G., Kim Y.C., Shin D.W., Kang O.H., Kwon D.Y. Curcumin reverse methicillin resistance in Satphylococcus aureus. Molecules. 2014;19:18283–18295. doi: 10.3390/molecules191118283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong S.Y., Davis J.S., Eichenberger E., Holland T.L., Fowler V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015;28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bal A., David M., Garau J., Gottlieb T., Mazzei T., Scaglione F., Tattevin P., Gould I. Future trends in the treatment of methicillin-resistant Staphylococcus aureus (MRSA) infection: An in-depth review of newer antibiotics active against an enduring pathogen. J. Glob. Antimicrob. Resist. 2017;10:295–303. doi: 10.1016/j.jgar.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Vaneperen A.S., Segreti J. Empirical therapy in methicillin-resistant Staphylococcus aureus infections: An up-to-date approach. J. Infect. Chemother. 2016;22:351–359. doi: 10.1016/j.jiac.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Liu C., Bayer A., Cosgrove S.E., Daum R.S., Fridkin S.K., Gorwitz R.J., Kaplan S.L., Archmer A.W.K., Levine D.P., Murray B.E. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. J. Infect. Dis. 2011;22:S178. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 14.Ernst C.M., Peschel A. Broad-spectrum antimicrobial peptide resistance by MprF-mediated aminoacylation and flipping of phospholipids. Mol. Microbiol. 2011;80:290–299. doi: 10.1111/j.1365-2958.2011.07576.x. [DOI] [PubMed] [Google Scholar]
- 15.Hassoun A., Linden P.K., Friedman B. Incidence, prevalence, and management of MRSA bacteremia across patient populationsda review of recent developments in MRSA management and treatment. Crit. Care. 2017;21:211. doi: 10.1186/s13054-017-1801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han J.H., Edelstein P.H., Lautenbach E. Reduced vancomycin susceptibility and Staphylococcal cassette chromosome mec (SCCmec) type distribution in methicillin-resistant Staphylococcus aureus bacteraemia. J. Antimicrob. Chemoth. 2012;67:2346–2349. doi: 10.1093/jac/dks255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore C.L., Osakikiyan P., Haque N.Z., Perri M.B., Donabedian S., Zervos M.J. Daptomycin versus vancomycin for bloodstream infections due to methicillinresistant Staphylococcus aureus with a high vancomycin minimum inhibitory concentration: A case-control study. Clin. Infect. Dis. 2012;54:51–58. doi: 10.1093/cid/cir764. [DOI] [PubMed] [Google Scholar]
- 18.Li B., Pai R., Di M., Aiello D., Barnes M.H., Butler M.M., Tashjian T.F., Peet N.P., Bowlin T.L., Moir D.T. Coumarin-based inhibitors of Bacillus anthracis and Staphylococcus aureus replicative DNA helicase: Chemical optimization, biological evaluation, and antibacterial activities. J. Med. Chem. 2012;55:10896–10908. doi: 10.1021/jm300922h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moise P.A., Amodio-Groton M., Rashid M., Lamp K.C., Hoffman-Roberts H.L., Sakoulas G., Yoon M.J., Schweitzer S., Rastogi A. Multicenter evaluation of the clinical outcomes of daptomycin with and without concomitant β-lactams in patients with Staphylococcus aureus bacteremia and mild to moderate renal impairment. Antimicrob. Agents Chemother. 2013;57:1192–1200. doi: 10.1128/AAC.02192-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 21.Hatusima S. Flora of Ryukyus, Added and Corrected. The Biological Society of Okinawa; Naha, Japan: 1975. p. 565. [Google Scholar]
- 22.Li H.L. Flora of Taiwan. Editorial Committee of the Flora of Taiwan; Taipei, Taiwan: 1998. Myoporaceae; pp. 731–732. [Google Scholar]
- 23.Sun J., Xu H.M., Zhou R.C., Fan Q., Meng K.K., Zan Q.J., Chen S.F., Liao W.B. Genetic diversity and population structure of Myoporum bontioides, (Myoporaceae) in China revealed by AFLP analysis. Aquat. Bot. 2017;138:1–7. doi: 10.1016/j.aquabot.2017.01.006. [DOI] [Google Scholar]
- 24.Wang Q.G., Ma C.L., Zhai J.J. Furanoeudesmane-B, a new eudesmane sesquiterpenoid from Myoporum bontioides. Acta. Crystallogr. 2000;56:e569. [Google Scholar]
- 25.Kanemoto M., Matsunami K., Otsuka H., Shinzato T., Ishigaki C., Takeda Y. Chlorine-containing iridoid and iridoid glucoside, and other glucosides from leaves of Myoporum bontioides. Phytochemistry. 2008;69:2517–2522. doi: 10.1016/j.phytochem.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Huang L.L., Li J.W., Ni C.L., Gu W.X., Li C.Y. Isolation, crystal structure and inhibitory activity against Magnaporthe grisea of (2R,3R)-3,5,7-trihydroxyflavanone 3-acetate from Myoporum bontioides A. Gray. Chin. J. Struct. Chem. 2011;30:1298–1304. [Google Scholar]
- 27.Weng J.R., Bai L.Y., Chiu C.F., Lin W.Y., Chiu C.F., Chen Y.C., Chao S.W., Feng C.H. A flavone constituent from Myoporum bontioides induces M-phase cell cycle arrest of MCF-7 breast cancer cells. Molecules. 2017;22:472. doi: 10.3390/molecules22030472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He Y.B., Gu W.X., Pang X.F. Bioactivity of several flavonoids against Plutella xylostella (L.) Chin. J. Trop. Agric. 2003;23:19–25. [Google Scholar]
- 29.Li X.Z., Li C.Y., Gu W.X., Wu Z.X. Isolation, identification and bioassay of myoporone from the volatile oil of Myoporum bontioides. Guangdong Chem. Ind. 2010;37:9–10. [Google Scholar]
- 30.Li X.Z., Li C.Y., Wu L.X., Yang F.B., Gu W.X. Chemical constituents from leaves of Myoporum bontioides. Chin. Tradit. Herb. Drug. 2011;42:2204–2207. [Google Scholar]
- 31.Dai H., Huang L.L., Guo Y.H., Gu W.X. Flavonoids from leaves of Myoporum bontioides. J. Trop. Subtrop. Bot. 2013;21:266–272. [Google Scholar]
- 32.Ye H.J., Dai H., Wu L.X., Guo Y.H., Gu W.X. Chemical constituents from leaves of Myoporum bontioides and their bacteriostatic activities. J. Trop. Subtrop. Bot. 2014;22:307–313. [Google Scholar]
- 33.Ma W.X., Guo Y.H., Lu T., Cheng Q.E., Gu W.X. Discussion on technique for purification of Myoporum bontioides A. Gray flavonoid by AB-8 macroporous resin. Guangdong Agric. Sci. 2014;41:97–100. [Google Scholar]
- 34.Chinnock R.J., Ghisalberti E.L., Jefferies P.R. (−)-Epingaione from Bontia daphnoides. Phytochemistry. 1987;26:1202–1203. doi: 10.1016/S0031-9422(00)82381-X. [DOI] [Google Scholar]
- 35.Deng Y.C., Yang Z., Yu Y.Z., Bi X.L. Inhibitory activity against plant pathogenic fungi of extracts from Myoporum bontioides A. Gray and indentification of active ingredients. Pest Manag. Sci. 2008;64:203–207. doi: 10.1002/ps.1491. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton W.D., Park R.J., Perry G.J., Sutherland M.D. Terpenoid chemistry. XXI. (–)-epingaione, (–)-dehydrongaione, (–)-dehydroepingaione, and (–)-deisopropylngaione, toxic furanoid sesquiterpenoid ketones from Myoporum deserti. Aust. J. Chem. 1973;26:375–387. doi: 10.1071/CH9730375. [DOI] [Google Scholar]
- 37.Blackburne I.D., Park R.J., Sutherland M.D. Terpenoid chemistry. XX. Myoporone and dehydromyoporone, toxic furanoid ketones from Myoporum and Eremophila species. Aust. J. Chem. 1972;25:1787–1796. doi: 10.1071/CH9721787. [DOI] [Google Scholar]
- 38.Jakupovic J., Ganzer U., Pritschow P., Lehmann L., Bohlmann F., King R.M. Sesquiterpene lactones and other constituents from Ursinia, species. Phytochemistry. 1992;31:863–880. doi: 10.1016/0031-9422(92)80030-I. [DOI] [Google Scholar]
- 39.Menut C., Cabalion P., Hnawia E., Agnaniet H., Waikedre J., Fruchieret A. Two new furanosesquiterpenes from Myoporum crassifolium from New Caledonia. Flavour Fragr. J. 2005;20:621–625. doi: 10.1002/ffj.1509. [DOI] [Google Scholar]
- 40.Dong L.M., Zhang M., Xu Q.L., Zhang Q., Luo B., Luo Q.W., Liu W.B., Tan J.W. Two new thymol derivatives from the roots of Ageratina adenophora. Molecules. 2017;22:592. doi: 10.3390/molecules22040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.