Abstract
The effects of allulose and two probiotic species on diet-induced obese (DIO) mice were investigated. Lactobacillus sakei LS03 (109 cfu/day) and Leuconostoc kimchii GJ2 (109 cfu/day) were used as probiotics, and allulose (AL) as a prebiotic. The synergistic effect of prebiotics and probiotics in improving obesity was evaluated. Orally fed Lactobacillus sakei LS03 (LS) or Leuconostoc kimchii GJ2 (GJ), significantly decreased hepatic triglyceride (TG) and fatty acid (FA) compared to the high-fat diet (HFD) control. AL markedly decreased visceral adiposity and pro-inflammatory adipokines (leptin and resistin) and cytokines (IL-6 and IL-1β) as well as hepatic TG and FA. In addition, AL exerted synergic effects with probiotics (LS and/or GJ) on the reduction of visceral white adipose tissue (WAT), associated with a decreased leptin: adiponectin ratio. There was no significant differences between the AL-SL and AL group, allulose and GJ combination (AL-GJ) was more effective than allulose in improving dyslipidemia, and decreasing WAT weight and hepatic FA, suggesting allulose may act as a favorable prebiotic for GJ supplement than LS. Combination of allulose with LS and GJ supplementation (AL-LSGJ) was the most effective for improving obesity related complications among the synbiotics groups containing allulose. In conclusion, this study demonstrated that the synbiotic mixture with allulose was more effective in suppressing diet-induced obese (DIO) and its complications via the regulation of lipid metabolism, than the probiotics or allulose alone, suggesting allulose may act as a prebiotic for the two probiotics tested in the study. This new synbiotic mixture with allulose may help ameliorate the deleterious effects of diet-induced obesity and contribute to the growth of the food industry.
Keywords: d-allulose, prebiotics, obesity, body fat reduction
1. Introduction
Prevalence of obesity is considered one of the most important health problems worldwide. Obesity is characterized by an abnormal excess of white adipose tissue, which is a major risk factor for developing diabetes, dyslipidemia, and liver steatosis [1]. Combinations of probiotics and prebiotics, defined as synbiotics, are consumed in diverse forms including yogurt, cheese, and several types of fermented food [2]. Reportedly, synbiotics may enhance the prevention and treatment of dyslipidemia, nonalcoholic fatty liver disease, and obesity [3,4,5]. Therefore, the development of new synbiotics may contribute to the growth of the food industry and enhance the nutrition and health of consumers [6].
In this study, Lactobacillus sakei LS03 and Leuconostoc kimchii GJ2 were used as probiotics. These probiotics were isolated from kimchi, a traditional Korean fermented vegetable. The genera Lactobacillus and Leuconostoc, used as starter cultures in kimchi fermentation, are the dominant lactic acid bacteria of kimchi [7]. Previous studies have reported that Lactobacillus sakei OK67 may contribute to lowering epididymal fat accumulation and pro-inflammatory factors such as tumor necrosis factor-alhpa (TNF-α), interleukin-6 (IL-6) and interleukin-1 beta (IL-1β) [8]. Jo et al. reported that Leuconostoc kimchii GJ2 may improve cardiovascular diseases and hypercholesterolemia by reducing serum cholesterol, triglyceride and low-density lipoprotein cholesterol levels [9]. We tested the effects of d-allulose as a prebiotic. d-allulose, a low-calorie (0.2 kcal/g) functional sweetener, has been recently reported as being beneficial for the prevention of obesity due to its anti-hyperlipidemic effect, brought on by the altering of lipid-regulating enzyme activities and gene expressions levels [10,11]. Moreover, Kimoto-Nira et al. has reported that d-allulose may have a beneficial effect on the growth and activity of probiotics in vitro [12]. Thus, further investigative studies on the potential use of d-allulose as a prebiotic in preventing and ameliorating obesity and its complications are felt to be necessary.
This study was undertaken to evaluate whether allulose may act synergistically with two probiotic species such as Lactobacillus sakei LS03 and Leuconostoc kimchi GJ2 in reducing obesity. We measured body weight and white adipose tissue weight, and analyzed lipid profiles in the plasma, hepatic enzyme activities related to lipid metabolism, as well as gene expression in the liver and epididymal white adipose tissue.
2. Materials and Methods
2.1. Animals and Diets
Five weeks old male C57BL/6J mice (n = 70) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA), and bred in another institution (KPC, Gwangju, Gyeonggi Province, Republic of Korea). d-allulose and the probiotics were supplied by CJ CheilJedang Corp (Seoul, Korea). The mice were maintained in a room with controlled temperature (20–25 °C) under 12 h dark/light alternating conditions. The mice were fed commercial chow diet pellets during the 1 week adaptation period. Next, the mice were fed a normal diet (ND; 5% fat (corn oil), w/w) for 16 weeks and a high-fat diet (HFD; 20% fat, 1% cholesterol, w/w) for 4 weeks to induce obesity. HFD contained 40 kcal% fat, 17 kcal% protein, and 43 kcal% carbohydrate with the fat sources consisting of lard (85% (w/w) of total fat) and corn oil (15% (w/w) of total fat). After induction of obesity, the mice fed a HFD were randomly divided into seven groups and fed HFD (n = 10), HFD with Lactobacillus sakei LS03 at 109 cfu/day (LS; n = 10), HFD with Leuconostoc kimchi GJ2 at 109 cfu/day (GJ; n = 10), HFD with 3% allulose at 3% allulose substituted for sucrose in HFD, w/w (allulose (AL); n = 10), HFD with allulose and Lactobacillus sakei LS03 at 109 cfu/day (AL-LS; n = 10), HFD with allulose and Leuconostoc kimchi GJ2 109 cfu/day (AL-GJ; n = 10), and HFD with allulose, Lactobacillus sakei LS03 at 109 cfu/day and Leuconostoc kimchi GJ2 at 109 cfu/day (AL-LSGJ; n = 10) for 12 weeks (Table 1). The mice had free access to water and food, except probiotics, during the entire experimental period. Diets were supplied in pellet form and fresh probiotics were orally administered daily during the experimental period. Probiotics were dissolved in 250 μL of phosphate buffered saline (PBS) and the amounts of probiotics given were 109 cfu/day. Just PBS, without probiotics, was orally administered to the control, HFD and AL groups. After 12 weeks, all mice were anesthetized with diethyl ether and sacrificed for 16 h fast. Blood was taken from the inferior vena cava for plasma analysis. The liver and adipose tissue were removed, rinsed with physiological saline, weighed and immediately frozen in liquid nitrogen, and stored at −80 °C until analysis. The animal study protocols were approved by the Ethics Committee at KPC (approval no. P150067).
Table 1.
Composition of experimental diets with or without allulose supplement.
| ND | HFD | AL | |
|---|---|---|---|
| Casein | 20 | 20 | 20 |
| D, L-Methionine | 0.3 | 0.3 | 0.3 |
| Corn starch | 15 | 11.1 | 11.1 |
| Sucrose | 50 | 37 | 34 |
| Cellulose | 5 | 5 | 5 |
| Corn oil | 5 | 3 | 3 |
| Lard | 17 | 17 | |
| Mineral mix 1 | 3.5 | 4.2 | 4.2 |
| Vitamin mix 2 | 1 | 1.2 | 1.2 |
| Choline bitartrate | 0.2 | 0.2 | 0.2 |
| Cholesterol | 1 | 1 | |
| Tert-Butylhydroquinone | 0.001 | 0.004 | 0.004 |
| Allulose | 3 | ||
| Total (%) | 100 | 100 | 100 |
1 AIN-76 mineral mixture (Harlan Teklad Co., Madison, WI, USA). 2 AIN-76 vitamin mixture (Harlan Teklad Co., Madison, WI, USA). ND: normal diet; HFD: high-fat diet; AL: allulose.
2.2. Plasma Biomarkers
Plasma lipid concentration was determined using commercially available kits. Plasma triglyceride (TG), total-cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), glutamic oxaloacetic transaminase (GOT), and glutamic pyruvic transaminase (GPT) levels were determined using Asan enzymatic kits (Asan, Seoul, Korea). Plasma apolipoprotein AI (ApoA-I) and apolipoprotein B (ApoB) were measured using enzymatic kits (Eiken, Japan). Plasma free fatty acid (FFA) was measured using Nittobo enzymatic kit (Nittobo Medical Co., Tokyo, Japan). Plasma adipokines (leptin, resistin, and adiponectin) and cytokines (TNF-α, IL-6 and IL-1β) were determined via a multiplex detection kit from Bio-Rad (Hercules, CA, USA). Data analyses were performed using Bio-Plex Manager software version 4.1.1 (Bio-Rad, Hercules, CA, USA).
2.3. Hepatic Lipid Contents
Hepatic lipid was extracted [13], and dried lipid residues were dissolved in 1 mL of ethanol for triglyceride, cholesterol, and fatty acid assays. A solution of Triton X-100 and sodium cholate in distilled water was added to 200 μL of a dissolved lipid solution for the purpose of emulsification. Triglyceride, cholesterol, and fatty acid contents were analyzed using the same enzymatic kit used for the plasma analysis.
2.4. Hepatic Lipid-Regulating Enzymes Activities
Hepatic cytosolic and mitochondrial fractions were prepared and analyzed according to the method developed by Hulcher and Oleson [14]. Protein concentration in the enzyme sources was determined using the Bradford method. Glucose-6-phosphate dehydrogenase (G6PD) [15] and malic enzyme (ME) [16] activities were determined using the previously described method. Carnitine palmitoyl transferase (CPT) was determined using the method described by Markwell et al. [17]. Fatty acid β-oxidation was determined via Lazarow’s method [18].
2.5. Real-Time qPCR Analysis
Liver and epididymal white adipose tissue (WAT) was prepared from each group of mice. Total RNA, extracted using TRIzol reagent (Invitrogen, Grand Island, NY, USA), was used to synthesize cDNA via the QuantiTect Reverse Transcription kit (QIAGEN Gmbh, Hilden, Germany). RNA expression was quantified by way of a quantitative real-time polymerase chain reaction (PCR) using the QuantiTect SYBR Green PCR kit (QIAGEN Gmblh, Hilden, Germany). Primers were designed to detect fatty acid synthase (FAS), acetyl-CoA carboxylase 1 (ACC1), peroxisome proliferator-activated receptor alpha (PPARα), carnitine palmitoyltransferase 1-alpha and 2 (CPT1α and CPT2), peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α), sirtuin 1 (SIRT1), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Table 2). The amplification was performed as follows: 10 min at 90 °C, 15 s at 95 °C, and 60 s at 60 °C, for a total of 35 cycles. The Ct data were normalized using GAPDH, and the relative gene expression level was calculated with the 2 ∆∆Ct method.
Table 2.
Primer sequences used for RT-qPCR validation of the microarray data.
| Gene | Primer Direction | Primer Sequence |
|---|---|---|
| GAPDH | Forward | 5′-AGGTCGGTGTGAACGGATTTG-3′ |
| Reverse | 5′-TGTAGACCATGTAGTTGAGGTCA-3′ | |
| FAS | Forward | 5′-GCTGCGGAAACTTCAGGAAAT-3′ |
| Reverse | 5′-AGAGACGTGTCACTCCTGGACTT-3′ | |
| ACC1 | Forward | 5′-GCCTCTTCCTGACAAACGAG-3′ |
| Reverse | 5′-TGACTGCCGAAACATCTCTG-3′ | |
| CPT1α | Forward | 5′-ATCTGGATGGCTATGGTCAAGGTC-3′ |
| Reverse | 5′-GTGCTGTCATGCGTTGGAAGTC-3′ | |
| CPT2 | Forward | 5′-GCCTGCTGTTGCGTGACTG-3′ |
| Reverse | 5′-TGGTGGGTACGATGCTGTGC-3′ | |
| PPARα | Forward | 5′-GGCACCCTCACATCATCAAACTG-3′ |
| Reverse | 5′-TGGAACAGACGGCGGCTTTC-3′ | |
| SIRT1 | Forward | 5′-TGTGAAGTTACTGCAGGAGTGTAAA-3′ |
| Reverse | 5′-GCATAGATACCGTCTCTTGATCTGAA-3′ | |
| PGC1α | Forward | 5′-AAGTGTGGAACTCTCTGGAACTG-3′ |
| Reverse | 5′-GGGTTATCTTGGTTGGCTTTATG-3′ |
GAPDH: glyceraldehyde-3-phosphate dehydrogenase; FAS: fatty acid synthase; ACC1: acetyl-CoA; carboxylase 1; CPT1α and CPT2: carnitine palmitoyltransferase 1-alpha and 2; PPARα: peroxisome proliferator-activated receptor alpha; SIRT1: sirtuin 1; PGC1α: peroxisome proliferator-activated receptor gamma coactivator 1-alpha.
2.6. Histopathological Analysis
Liver samples from each mouse were removed and fixed in 10% (v/v) formalin/PBS buffer solution. Fixed samples were embedded in paraffin in preparation for staining with hematoxylin and eosin. Stained slices were viewed under an optical microscope (Nikon, Tokyo, Japan) set at 200 × magnification.
2.7. Statistical Analysis
All data were presented as mean ± standard error of the mean (SEM) and were analyzed using SPSS (SPSS Inc. Chicago, IL, USA). Statistical differences between the ND group and the HFD group were determined using Student’s t-test. One-way analysis of variance (one-way ANOVA) was used to evaluate statistical significance between groups fed the high-fat diet (HFD, LS, GJ, AL, AL-LS, AL-GJ, and AL-LSGJ). The statistical significance between group means was determined via Duncan’s multiple-range test, multiple comparison procedure at p < 0.05.
3. Results
3.1. Body Weights and Food Efficiency Ratio
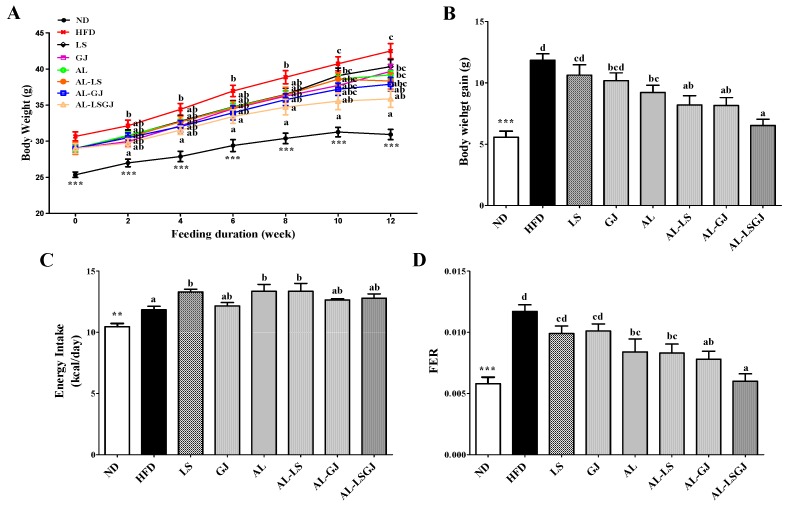
Supplementation of HFD significantly increased body weight (BW) and decreased food efficiency ratio (FER) throughout the experiment compared to the ND (Figure 1). Probiotic LS- and GJ-supplemented groups did not significantly affect BW gains and FER. AL significantly decreased the BW gain, despite the increase in energy intake. These led to a decreased FER in the AL group compared with the HFD group. Allulose with probiotic LS (AL-LS) significantly decreased the BW from 12th week, and allulose with probiotic GJ (AL-GJ) markedly decreased the BW from 6th week compared to HFD (Figure 1A). AL-LS and AL-GJ also exhibited a significant decrease in BW gain and FER when compared to the HFD group. Additionally, synergies were observed between allulose and probiotics in BW gain and FER. AL-LS tend to decrease the BW gain, and AL-GJ also tend to decrease both the BW gain and FER compared to the AL group. Furthermore, the AL-LSGJ group, that was fed allulose with two species of probiotics, had the lowest BW gain and FER among HFD-fed groups (Figure 1B,D). AL-LSGJ significantly decreased the BW from the second week when compared to the HFD group (Figure 1A). Similar to the BW results, the BW gain and FER were significantly lower in the AL-LSGJ group compared to that in the HFD, probiotics (LS, GJ), and AL groups (Figure 1B,D).
Figure 1.
Effect of synbiotic supplementation for 12 weeks on body weight, measured weekly. (A) body weight, (B) body weight gain, (C) energy intake and (D) food deficiency ratio (FER) in diet-induced obese mice. Data represented as mean ± standard error of the mean (S.E.); Significant differences ND versus HFD are indicated; ** p < 0.01, *** p < 0.001; abcd Means with different superscript letters are significantly different among the groups (p < 0.05); ND: normal diet (5% fat, w/w); HFD: high fat diet (20% fat, 1% cholesterol, w/w); LS: HFD + Lactobacillus sakei LS03 109 cfu/day; GJ: HFD + Leuconostoc kimchi GJ2 109 cfu/day; AL: HFD + 3% allulose; AL-LS: AL + Lactobacillus sakei LS03 109 cfu/day; AL-GJ: AL + Leuconostoc kimchi GJ2 109 cfu/day; AL-LSGJ: AL + Lactobacillus sakei LS03 + L. Leuconostoc kimchi GJ2 109 cfu/each/day; FER: food efficiency ratio = body weight gain/energy intake per day.
3.2. Adipose Tissue Weights
The weight of liver and all white adipose tissue depots (epididymal, perirenal, mesenteric, subcutaneous, retroperitoneal, and interscapular) were significantly increased, while kidney weight was markedly decreased by the HFD when compared to the ND (Table 3). Probiotic LS- and GJ-supplemented groups significantly decreased only interscapular WAT compared with the HFD group. AL group vs. HFD group exhibited significant decrease in the weights of perirenal, mesenteric, interscapular and visceral WAT (including epididymal, perirenal, retroperitoneal and mesenteric WAT depots). AL-LS showed a significant decrease in perirenal, mesenteric, interscapular, visceral and total WAT when compared to the HFD group. The AL-GJ and AL-LSGJ groups showed a dramatic decrease in all WAT depots compared to the HFD group. Additionally, a synergic effect between allulose and probiotics was observed. The AL-GJ markedly decreased the weights of subcutaneous interscapular and total WAT, and the AL-LSGJ significantly decreased the weights of all WAT depots, except retroperitoneal WAT, when compared to the AL group.
Table 3.
Effect of synbiotic supplementation for 12 weeks on organ and white adipose tissue weights.
| ND | HFD | LS | GJ | AL | AL-LS | AL-GJ | AL-LSGJ | |
|---|---|---|---|---|---|---|---|---|
| Liver | 3.27 ± 0.05 *** | 4.67 ± 0.20 | 4.18 ± 0.16 | 4.27 ± 0.25 | 4.56 ± 0.12 | 4.49 ± 0.10 | 4.31 ± 0.19 | 4.18 ± 0.11 |
| Kidney | 0.92 ± 0.02 *** | 0.72 ± 0.01 a | 0.76 ± 0.03 ab | 0.76 ± 0.03 ab | 0.83 ± 0.03 bc | 0.85 ± 0.02 c | 0.90 ± 0.03 c | 0.89 ± 0.05 c |
| Epididymal WAT | 3.11 ± 0.21 *** | 6.05 ± 0.11 c | 6.03 ± 0.27 c | 5.98 ± 0.12 bc | 5.89 ± 0.25 bc | 5.43 ± 0.18 abc | 5.18 ± 0.34 ab | 4.84 ± 0.25 a |
| Perirenal WAT | 0.40 ± 0.03 *** | 0.94 ± 0.04 d | 0.79 ± 0.07 bcd | 0.90 ± 0.11 cd | 0.71 ± 0.06 abc | 0.66 ± 0.05 ab | 0.57 ± 0.07 ab | 0.54 ± 0.04 a |
| Mesenteric WAT | 0.88 ± 0.07 *** | 1.97 ± 0.12 e | 1.77 ± 0.19 de | 1.60 ± 0.14 cd | 1.26 ± 0.13 bc | 1.19 ± 0.08 abc | 1.07 ± 0.10 ab | 0.86 ± 0.06 a |
| Subcutaneous WAT | 2.67 ± 0.24 *** | 6.10 ± 0.22 b | 5.59 ± 0.38 b | 5.65 ± 0.32 b | 5.68 ± 0.50 b | 5.17 ± 0.27ab | 4.37 ± 0.41 a | 4.35 ± 0.31 a |
| Retroperitoneal WAT | 0.75 ± 0.06 *** | 1.44 ± 0.03 b | 1.34 ± 0.05 ab | 1.28 ± 0.11 ab | 1.38 ± 0.07 ab | 1.30 ± 0.06 ab | 1.19 ± 0.09 a | 1.21 ± 0.08 a |
| Interscapular WAT | 1.53 ± 0.13 *** | 3.27 ± 0.08 c | 2.77 ± 0.20 b | 2.70 ± 0.13 b | 2.57 ± 0.24 b | 2.33 ± 0.14 ab | 2.02 ± 0.19 a | 2.03 ± 0.16 a |
| Total WAT | 9.34 ± 0.68 *** | 19.63 ± 0.40 d | 19.29 ± 0.65 d | 18.11 ± 0.64 cd | 17.49 ± 1.14 cd | 16.49 ± 0.44 bc | 14.69 ± 1.00 ab | 14.09 ± 0.63 a |
| Visceral WAT | 5.14 ± 0.35 *** | 10.39 ± 0.18 d | 9.93 ± 0.51 cd | 9.76 ± 0.22 cd | 9.23 ± 0.43 bc | 8.58 ± 0.32 ab | 8.01 ± 0.56 ab | 7.45 ± 0.36 a |
Data are mean ± standard error of the mean (S.E.); Significant differences ND versus HFD are indicated; *** p < 0.001; abcde Means with different superscript letters are significantly different among the groups (p < 0.05); ND: normal diet (5% fat, w/w); HFD: high fat diet (20% fat, 1% cholesterol, w/w); LS: HFD + Lactobacillus sakei LS03 109 cfu/day; GJ: HFD + Leuconostoc kimchi GJ2 109 cfu/day; AL: HFD + 3% allulose; AL-LS: AL + Lactobacillus sakei LS03 109 cfu/day; AL-GJ: AL + Leuconostoc kimchi GJ2 109 cfu/day; AL-LSGJ: AL + Lactobacillus sakei LS03 + L. Leuconostoc kimchi GJ2 109 cfu/each/day; FER, food efficiency ratio = body weight gain/energy intake per day; WAT: white adipose tissue.
Kidney weight of the AL group was significantly increased compared to that of the HFD group. Supplementation of AL-LS and AL-GJ significantly increased kidney weight compared to LS and GJ, respectively. However, the kidney weight in the allulose-based groups (AL, AL-LS, AL-GJ, and AL-LSGJ) was not increased than in the ND group (Table 3).
3.3. Plasma Lipid Profiles
Supplementation of HFD significantly increased the plasma TG, TC, FFA, HDL-C, nonHDL-C, Apo B, and Apo A-I:Apo B ratio compared to the ND group (Table 4). The supplementation of GJ, AL and AL-LS significantly decreased the levels of plasma TG, non HDL-C and AI compared to the HFD. Conversely, the HDL-C:TC ratio (HTR) in the GJ, AL, and AL-LS groups was significantly higher than that in the HFD group. LS also significantly decreased non-HDL-C and AI, while increased HTR compared to the HFD. AL-GJ supplementation led to a significantly lower concentration of plasma TG, TC, nonHDL-C and ApoB, and AI, whereas HTR and the Apo A-I:Apo B ratio were significantly increased by AL-GJ supplementation compared to the HFD group. Moreover, the AL-LSGJ group fed allulose-supplemented HFD with 2 species of probiotics significantly altered all plasma lipid parameters (TG, TC, FFA, HDL-C, nonHDL-C, HTR, AI, Apo A-I, Apo B, and the Apo A-I:Apo B ratio compared to the HFD group. Especially, TC level was significantly decreased by AL-LSGJ, while the Apo A-I level and Apo A-I:Apo B ratio were significantly higher in the AL-LSGJ group when compared with the LS, GJ, and AL groups. Accordingly, these markers were lowest in the AL-LSGJ group in entire HFD-fed groups (Table 4).
Table 4.
Effect of synbiotic supplementation for 12 weeks on plasma lipid profiles in diet-induced obese mice.
| ND | HFD | LS | GJ | AL | AL-LS | AL-GJ | AL-LSGJ | |
|---|---|---|---|---|---|---|---|---|
| TG (mmol/L) | 0.80 ± 0.05 ** | 0.98 ± 0.05 b | 0.87 ± 0.05 ab | 0.83 ± 0.05 a | 0.78 ± 0.05 a | 0.75 ± 0.06 a | 0.82 ± 0.03 a | 0.73 ± 0.03 a |
| Total-C (mmol/L) | 3.37 ± 0.14 *** | 5.29 ± 0.22 c | 4.62 ± 0.26 bc | 4.56 ± 0.18 bc | 4.81 ± 0.29 bc | 4.85 ± 0.26 bc | 4.32 ± 0.30 a | 4.32 ± 0.15 a |
| FFA (mmol/L) | 0.71 ± 0.01 * | 0.77 ± 0.02 b | 0.74 ± 0.02 ab | 0.72 ± 0.02 ab | 0.73 ± 0.02 ab | 0.71 ± 0.02 ab | 0.75 ± 0.02 ab | 0.69 ± 0.02 a |
| HDL-C (mmol/L) | 1.03 ± 0.06 *** | 1.62 ± 0.04 a | 1.64 ± 0.11 a | 1.76 ± 0.07 ab | 1.75 ± 0.09 ab | 1.71 ± 0.06 ab | 1.58 ± 0.08 a | 1.88 ± 0.06 b |
| nonHDL-C (mmol/L) | 2.45 ± 0.12 *** | 3.79 ± 0.19 b | 2.96 ± 0.17 a | 2.78 ± 0.16 a | 3.10 ± 0.21 a | 3.15 ± 0.14 a | 2.59 ± 0.20 a | 2.69 ± 0.13 a |
| HTR (%) | 30.63 ± 0.63 | 29.29 ± 0.73 a | 35.46 ± 0.64 b | 36.80 ± 1.17 b | 35.80 ± 1.15 b | 35.04 ± 1.51 b | 37.03 ± 1.03 b | 37.90 ± 1.07 b |
| AI | 2.24 ± 0.07 | 2.46 ± 0.10 b | 1.80 ± 0.04 a | 1.69 ± 0.10 a | 1.87 ± 0.09 a | 1.82 ± 0.11 a | 1.76 ± 0.08 a | 1.72 ± 0.07 a |
| ApoA-I (mg/dL) | 44.72 ± 0.43 | 44.87 ± 0.42 b | 43.39 ± 0.28 a | 44.51 ± 0.32 ab | 44.61 ± 0.75 ab | 43.91 ± 0.51 ab | 45.20 ± 0.42 b | 49.05 ± 0.60 c |
| ApoB 100 (mg/dL) | 2.74 ± 0.24 *** | 5.91 ± 0.61 b | 5.10 ± 0.38 ab | 4.62 ± 0.82 ab | 5.10 ± 0.25 ab | 5.07 ± 0.59 ab | 3.78 ± 0.35 a | 3.37 ± 0.37 a |
| ApoA-I/ApoB | 19.01 ± 0.97 *** | 8.56 ± 0.72 a | 8.85 ± 0.51 a | 11.97 ± 1.43 ab | 8.89 ± 0.39 a | 10.13 ± 1.43 ab | 13.15 ± 1.47 bc | 16.30 ± 2.09 c |
Data are mean ± S.E.; Significant differences ND versus HFD are indicated; * p < 0.05, ** p < 0.01, *** p < 0.001; abc Means with different superscript letters are significantly different among the groups (p < 0.05); ND: normal diet (5% fat, w/w); HFD: high fat diet (20% fat, 1% cholesterol, w/w); LS: HFD + Lactobacillus sakei LS03 109 cfu/day; GJ: HFD + Leuconostoc kimchi GJ2 109 cfu/day; AL: HFD + 3% allulose; AL-LS: AL + Lactobacillus sakei LS03 109 cfu/day; AL-GJ: AL + Leuconostoc kimchi GJ2 109 cfu/day; AL-LSGJ: AL + Lactobacillus sakei LS03 + L. Leuconostoc kimchi GJ2 109 cfu/each/day; HTR: (HDL-C /Total-C) ×100; AI: atherogenic index = (Total-C) − (HDL-C)/(HDL-C); ApoA-I: ApoB ratio; TG: triglyceride; FFA: free fatty acid; Total-C: total-cholesterol; HDL-C: high-density lipoprotein cholesterol.
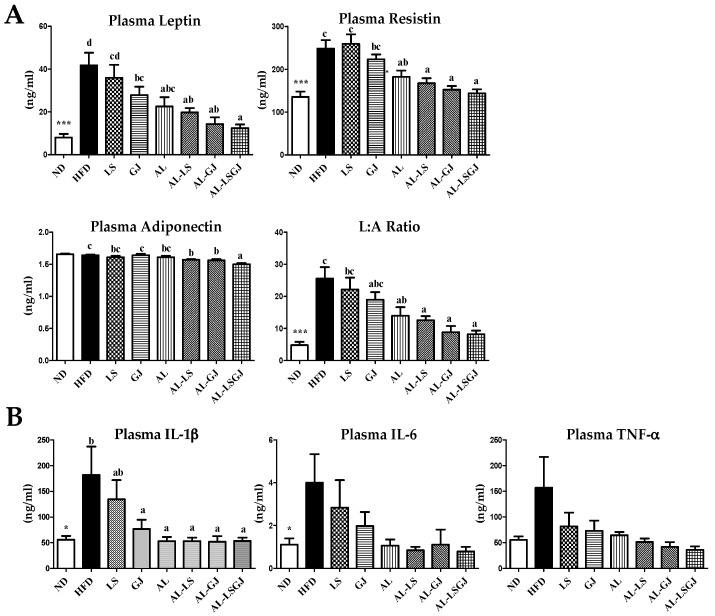
3.4. Plasma Adipokine and Cytokine Levels
The HFD group dramatically increased the level of plasma adipokines (leptin, resistin) and pro-inflammatory cytokines (IL-1β and IL-6) when compared to the ND group (Figure 2). LS did not significantly affect the plasma adipokine/cytokine profiles. GJ supplementation significantly decreased plasma leptin and IL-1β levels compard to the HFD group. The plasma leptin, resistin, leptin: adiponectin ratio (L:A ratio) and IL-1β were dramatically decreased in the AL, AL-LS, AL-GJ, and AL-LSGJ groups compared to those in the HFD group.
Figure 2.
Effect of synbiotic supplementation for 12 weeks on plasma adipokine levels. (A) adipokine levels and (B) cytokine levels in diet-induced obese mice. Data are mean ± S.E.; Significant differences of ND versus HFD are indicated; * p < 0.05, *** p < 0.001; abcd Means with different superscript letters are significantly different among the groups (p < 0.05); ND: normal diet (5% fat, w/w); HFD: high fat diet (20% fat, 1% cholesterol, w/w); LS: HFD + Lactobacillus sakei LS03 109 cfu/day; GJ: HFD + Leuconostoc kimchi GJ2 109 cfu/day; AL: HFD + 3% allulose; AL-LS: AL + Lactobacillus sakei LS03 109 cfu/day; AL-GJ: AL + Leuconostoc kimchi GJ2 109 cfu/day; AL-LSGJ: AL + Lactobacillus sakei LS03 + L. Leuconostoc kimchi GJ2 109 cfu/each/day; IL-6: interleukin-6; IL-1β: interleukin-1 beta; TNF-α: tumor necrosis factor-alhpa.
3.5. Hepatic Lipids Levels, Enzyme Activity, and mRNA Expression
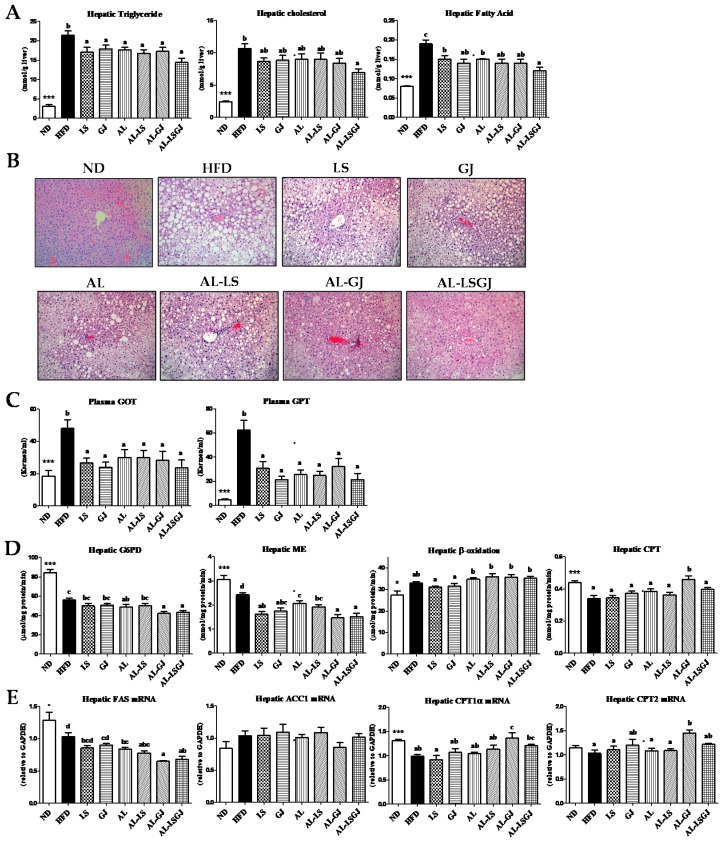
The HFD group dramatically increased the levels of hepatic lipids (TC, TG, and FFA) and hepatic lipotoxicity markers (plasma GOT and GPT) when compared to the ND group (Figure 3A). The levels of hepatic TG and FFA were significantly lower in the allulose- and probiotics-fed groups (LS, GJ, AL, AL-LS, AL-GJ, and AL-LSGJ) compared to those in the HFD group (Figure 3A). Especially, AL-LSGJ supplementation markedly decreased hepatic TC as well as TG and FFA compared to the HFD, and hepatic FA level in the AL-LSGJ group was markedly lower than those in the LS, GJ, and AL groups. Similar to the hepatic lipid levels, hematoxylin and eosin (H&E) staining results indicated that hepatic lipid droplets numbers and sizes were decreased in AL-LSGJ-treated mice compared to those in other groups. In addition, levels of plasma GOT and GPT were also reduced in the allulose- and probiotics-fed groups than in the HFD group (Figure 3B,C).
Figure 3.
Effect of synbiotic supplementation for 12 weeks on hepatic morphology. (A) hepatic lipid levels, (B) hepatic morphology (X200), (C) hepatic lipotoxicity markers, (D) hepatic lipid regulating enzyme activities and (E) hepatic gene expressions in diet-induced obese mice. Data are mean ± S.E.; Significant differences ND versus HFD are indicated; * p < 0.05, *** p < 0.001; abc Means with different superscript letters are significantly different among the groups (p < 0.05); ND: normal diet (5% fat, w/w); HFD: high fat diet (20% fat, 1% cholesterol, w/w); LS: HFD + Lactobacillus sakei LS03 109 cfu/day; GJ: HFD + Leuconostoc kimchi GJ2 109 cfu/day; AL: HFD + 3% allulose; AL-LS: AL + Lactobacillus sakei LS03 109 cfu/day; AL-GJ: AL + Leuconostoc kimchi GJ2 109 cfu/day; AL-LSGJ: AL + Lactobacillus sakei LS03 + L. Leuconostoc kimchi GJ2 109 cfu/each/day; GOT: glutamic oxaloacetic transaminase; GPT: glutamic pyruvic transaminase; G6PD: Glucose-6-phosphate dehydrogenase; ME: malic enzyme; FAS: fatty acid synthase; ACC1: acetyl-CoA carboxylase 1; CPT: carnitine palmitoyltransferase.
The activities of hepatic enzymes related to FA synthesis (G6PD and ME) were significantly decreased by AL supplementation compared to HFD (Figure 3D). Moreover, the activity of ME in LS- and GJ-groups was significantly reduced compared to that of the HFD group. Also, G6PD activity was significantly lowered in both AL-GJ- and AL-LSGJ-groups than in the LS and GJ groups, respectively, and ME activity was significantly lowered in both AL-GJ- and AL-LSGJ-groups than in the AL group. Activity of β-oxidation was tended to be higher in the allulose-fed groups (AL, AL-LS, AL-GJ, and AL-LSGJ) compared to that in the HFD group. Hepatic CPT activity was significantly increased in the AL-GJ group only compared to those in the HFD group. The mRNA expression of FAS was significantly lower in the allulose treated groups (AL, AL-LS, AL-GJ, and AL-LSGJ) compared to that in the HFD group (Figure 3E). The AL-GJ group markedly reduced hepatic FAS mRNA expression compared to LS, GJ and AL groups. By contrast, mRNA expression of CPT1α and CPT2 were significantly up-regulated by the AL-GJ treatment compared to the HFD group. Furthermore, mRNA expression of CPT1α was dramatically increased by AL-GJ treatment compared to the LS, GJ, and AL treatment.
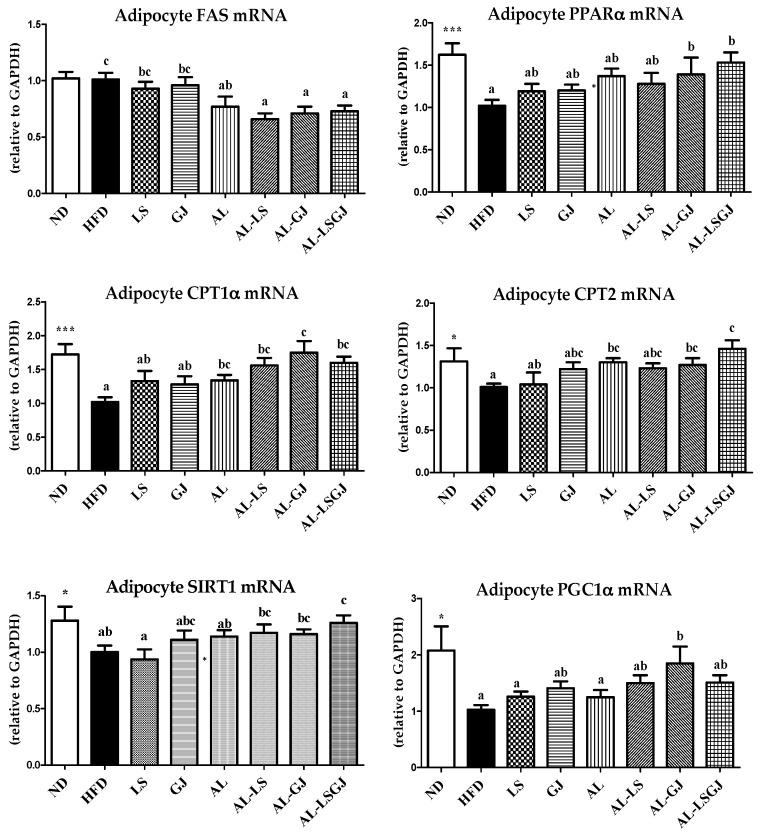
3.6. mRNA Expression in Epididymal White Adipose Tissue
The HFD group significantly decreased the expression of adipocyte PPARα, CPT1α, CPT2, SIRT1, and PGC1α genes involved in fatty acid oxidation (FAO) compared to the ND group (Figure 4). The supplementation of AL significantly decreased FAS gene expression involved in lipogenesis, while increased FAO-associated gene expressions (CPT1α and CPT2) compared to the HFD. In addition, supplementation of AL-LS and AL-GJ significantly down-regulated FAS gene expression compared to that of LS and GJ. Especially, AL-GJ significantly increased PPARα, CPT1α, CPT2, and PGC1α gene expressions compared to the HFD group. AL-LS also markedly increased CPT1α expression compared with the HFD group. Furthermore, supplementation of AL-LSGJ significantly increased the PPARα, CPT1α, CPT2, and SIRT1 gene expression compared to HFD, in addition to which the SIRT1 gene expression was also significantly increased in AL-LSGJ compared to that in LS and AL.
Figure 4.
Effect of synbiotic supplementation for 12 weeks on epididymal adipose tissue gene expressions in diet-induced obese mice. Data are mean ± S.E.; Significant differences ND versus HFD are indicated; * p < 0.05, *** p < 0.001; abc Means with different superscript letters are significantly different among the groups (p < 0.05); ND: normal diet (5% fat, w/w); HFD: high fat diet (20% fat, 1% cholesterol, w/w); LS: HFD + Lactobacillus sakei LS03 109 cfu/day; GJ: HFD + Leuconostoc kimchi GJ2 109 cfu/day; AL: HFD + 3% allulose; AL-LS: AL + Lactobacillus sakei LS03 109 cfu/day; AL-GJ: AL + Leuconostoc kimchi GJ2 109 cfu/day; AL-LSGJ: AL + Lactobacillus sakei LS03 + L. Leuconostoc kimchi GJ2 109 cfu/each/day; PPARα: peroxisome proliferator-activated receptor alpha; SIRT1: sirtuin 1; PGC1α: proliferator-activated receptor gamma coactivator 1-alpha.
4. Discussion
Probiotics and prebiotics have been consumed for centuries, either as natural components of food, or as fermented foods [19]. Combinations of probiotics and prebiotics are defined as synbiotics, which are able to enhance health and reduce disease related issues [20]. Synbiotics are being targeted more and more for their potential as functional food ingredients [21]. Previous studies have indicated that allulose may have beneficial effects on obesity related metabolic disturbances [11]. However, only a few studies have been conducted on the effects of allulose as prebiotics. Additionally, the previous studies have reported that Lactobacillus sakei LS03 and/or Leuconostoc kimchi GJ2 isolated from a traditional Korean fermented vegetable kimchi may improve adiposity, inflammation, and cardiovascular diseases [7,8,9]. Therefore, we investigated the levels of plasma, hepatic lipids, enzyme activities, and gene expressions to investigate the suitability of allulose as prebiotics for two probiotics Lactobacillus sakei LS303 and Leuconostoc kimchi GJ2. Results indicated that BW gain was significantly decreased in the AL group compared to the HFD group, it was tended to be lower in the AL-LS and AL-GJ groups than in the AL group, and the addition of allulose to two species of probioticss represented by AL-LSGJ group, led to a significant decrease in BW as well as BW gain compared to supplementation of AL alone. Similarly, the weight of total WAT was significantly lower in the AL-GJ group than in the GJ and AL groups. Supplementations of AL-GJ and AL-LSGJ significantly decreased the weight of total WAT, and AL-LSGJ markedly decreased not only total WAT but also visceral WAT weights compared to supplementation of AL alone. Adipose tissue, which is the major site of lipid storage, produces and releases a variety of pro-inflammatory and anti-inflammatory factors, including the adipokine leptin, adiponectin, resistin, as well as cytokines, such as IL-1β, IL-6, and TNF-α, among others [22,23]. In those with obesity, pro-inflammatory factors are released excessively and these excess amounts are considered to play an important role in the pathogenesis of obesity-related complications such as insulin resistance, type II diabetes, nonalcoholic fatty liver disease, and dyslipidemia [22]. During the development of obesity, plasma leptin levels are increased, while plasma adiponectin levels are decreased [24,25]. In this study, addition of allulose to HFD along with oral probiotics (AL-LS, AL-GJ, AL-LSGJ), as well as the allulose supplement alone, consistently resulted in the decrease of body weight, FER, body fat mass, as well as the concentration of plasma leptin, resistin and IL-1β. Leptin and IL-1β levels were significantly decreased by the addition of probiotic feeding of GJ to AL. Although the changes in plasma adiponectin levels were not dramatic, the ratio of leptin to adiponectin was lowered in the AL-, AL-LS-, AL-LS-, and AL-LSGJ groups. Among these 4 groups, the AL-LSGJ group which consisted of allulose and 2 species of probiotics were most effective to reduce some abnormalities of obesity. This observation suggests that allulose was effective in improving body weight and adiposity, revealed a synergy effect with probiotics, and synbiotic containing both allulose and 2 species of probiotics were most effective. Also, decreased adiposity by these synbiotics is partially linked to improvement of adipokines/cytokines dysregulation involved in inflammation.
Metabolic and endocrine functions of adipose tissues, which contribute to obesity related metabolic disorders, are partly associated with adipose tissue gene expression [26]. mRNA expression of FAS in epididymal white adipose tissue was related to adipogenesis. FAS, is one of the major genes related to fatty acid synthesis [27]. On the contrary, PPARα induces FAO by activating FAO-related genes, such as CPT1α, CPT2, and others [28,29]. PGC1α and SIRT1 are important genes that induce transcription of mitochondrial FAO [30]. Interestingly, the supplementation of AL significantly down-regulated adipocyte FAS gene expression, while up-regulated adipocyte CPT1α, and CPT2 expressions when compared to the HFD, thereby reducing adiposity. Moreover, the mRNA expression of PGC1α was significantly increased by the addition of allulose to the GJ treated mice (AL-GJ), and mRNA expression of SIRT1 in the allulose supplemented with two probiotic species group (AL-LSGJ) was significantly up-regulated compared to the allulose group. The results of the present work suggest that allulose was effective to reduce body weight and adiposity by decreasing adipocyte lipogenesis and increasing FAO, and allulose exerted synergic effect with probiotics in the prevention of adiposity by further increasing the adipocyte gene expressions of PGC1α by AL-GJ, and SIRT1 by AL-LSGJ.
Triglyceride, cholesterol, and other lipids are transported in the blood by lipoproteins, such as very low-density lipoprotein (VLDL), low-density lipoprotein (LDL) and HDL [31]. ApoA-1 is the principal apolipoprotein component of HDL-C, and ApoB-100 is a major apolipoprotein component of VLDL, intermediate-density lipoprotein (IDL), and LDL [32]. In general, obesity causes abnormalities in lipoprotein metabolism such as dyslipidemia and hyperlipidemia by increasing the synthesis of VLDL, LDL, ApoB, and total body cholesterol [33]. In the present study, supplementation of allulose and/or probiotics led to elevated HTR while significantly decreasing AI to levels when compared to HFD group. Supplementation of AL-GJ significantly decreased plasma TC, while significantly increased ApoA-I/ApoB ratio compared to that in the GJ and AL groups. Moreover, supplementation of AL-LSGJ dramatically decreased plasma TC and FFA levels, while markedly increasing the plasma Apo A-I level and ApoA-I/ApoB ratio compared to that in the LS, GJ and AL groups. It appears that when allulose was present as a prebiotic in the HFD with LS and GJ mixture, the overall effect was more beneficial than LS or GJ alone. In addition, supplementation of allulose to GJ-treated mice (AL-GJ) resulted in a decrease in plasma TC and ApoB, and an increase in ApoA-I and the ApoA-I/ApoB ratio, with the decreased total WAT weight, compared to the AL-LS group. Therefore, GJ probiotic appeared to be more responsive to the prebiotic allulose.
Liver plays an important role in lipid metabolism via pathways such as hepatic fatty acid synthesis and oxidation. However, obesity may lead to hepatic steatosis due to the accumulation of lipid droplets in the hepatocytes [34]. Both hepatic TG and FFA were significantly decreased by AL compared to the HFD. LS and GJ probiotics also significantly reduced hepatic TG and FFA compared to the HFD. In particular, hepatic TG, cholesterol, and FFA in the AL-LSGJ group were significantly decreased compared to the HFD group, and hepatic FFA in the AL-LSGJ group was also markedly decreased compared to the LS and AL groups. Such results could be partly due to alteration of the activities of hepatic lipid-regulating enzymes or the expression of hepatic genes. Based on G6PD and ME activities, fatty acid synthesis were significantly lowered by the AL supplement alone, while ME activity was significantly lowered by LS and GJ supplements, compared to the HFD group. In addition, combination of AL-GJ and AL-LSGJ led to a decrease in ME activity compared to that in AL and significantly recued G6PD activity compared to the LS and GJ groups. On the other hand, the hepatic enzyme activities, β-oxidation, related to fatty acid oxidation was tended to be higher in alluloase-supplemented groups (AL, AL-LS, AL-GJ, and AL-LSGJ) compared to HFD group. Also, supplementation of allulose to the GJ-treatment led to elevated CPT activity compared to that in mice fed HFD-based diet.
On the other hand, the hepatic enzyme activities, β-oxidation, and CPT, related to fatty acid oxidation were significantly increased by the of allulose supplementation to GJ-treated mice. Also, CPT activity was most significantly increased in the AL-GJ group and the mRNA expression of CPT1α and CPT2 were significantly increased by supplementation of allulose to GJ-treated mice. Consequently, our study indicates that the most beneficial effects reaped by mice given the allulose supplement with both GJ and LG probiotics were the improvements seen in dyslipidemia and hepatic steatosis due to the inhibition of fat accumulation in the liver. Accordingly, although treatment with the probiotics GJ or LG may improve some obesity related metabolic markers, supplementation of allulose may further improve obesity related markers, thus supporting its role as a beneficial prebiotic. Based on the overall result, a combination of both probiotics and allulose may, in the least, produce a synergistic regulatory effect on body fat metabolism.
5. Conclusions
Results of the current study indicate that allulose, known as a functional sugar substitute with 0.2 kcal/g energy, exhibited a beneficial effect by lowering the body weight and abdominal fat mass in DIO mice. Furthermore, the anti-obesity effect of allulose was superior to that of probiotics (LS, GJ), and allulose had a synergy effect with probiotics in improving the deleterious effect of diet-induced obesity and its complications as evidenced by further decreased adiposity, and the levels of plasma lipid and pro-inflammatory adipokines/cytokines, and hepatic lipids by AL-GJ and/or AL-LSGJ when compared to the probiotics or allulose alone. When compared to AL, the supplementation AL-GJ significantly decreasing the total WAT weight by increasing adipocyte PGC1α mRNA expression, which contributed to decreased plasma TC, and increased ApoA-I/ApoB ratio. Especially, synbiotic containing both allulose and two species of probiotics (AL-LSGJ) was the most effective in the prevention of hepatic steatosis, adiposity and dyslipidemia. AL-LSGJ improved adiposity by increasing adipocyte SIRT1 mRNA expression involved in FAO, which contributed to reduce the dyslipidemia and inflammation. Moreover, AL-LSGJ improved hepatic steatosis via decreased hepatic lipogenesis by suppressing the ME enzyme activity, which also contributed to improve dyslipidemia. These observations have suggested that allulose may act as a prebiotic for the two probiotics tested in the study. This new synbiotic mixture with allulose may help ameliorate the deleterious effects of diet-induced obesity and contribute to the growth of the food industry.
Acknowledgments
All the samples were provided by the CJ Cheil Jedang Corporation (Seoul, Korea).
Author Contributions
B.-R.C. performed the experiments, analyzed the data, and wrote the manuscript. E.-Y.K. performed the experiments and reviewed the manuscript. H.-J.K. contributed to the preparation of prebiotics and probiotics for this experiment, and participated in writing the discussions. M.-S.C. supervised this work and had full access to all the data and therefore takes full responsibility for the integrity of the results and accuracy of the data analysis.
Funding
This study was supported by the National Research Foundation of Korea (NRF) grant, funded by the Korea government (NRF-2016R1A2B4011329 and 2015R1C1A2A01051533).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Sáez-Lara M.J., Robles-Sanchez C., Ruiz-Ojeda F.J., Plaza-Diaz J., Gil A. Effects of Probiotics and Synbiotics on Obesity, Insulin Resistance Syndrome, Type 2 Diabetes and Non-Alcoholic Fatty Liver Disease: A Review of Human Clinical Trials. Int. J. Mol. Sci. 2016;17:928. doi: 10.3390/ijms17060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Upadhyay N., Moudgal V. Probiotics: A review. JCOM. 2012;19:76–84. [Google Scholar]
- 3.Yoo J.Y., Kim S.S. Probiotics and Prebiotics: Present Status and Future Perspectives on Metabolic Disorders. Nutrients. 2016;8:173. doi: 10.3390/nu8030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umeki M., Oue K., Mochizuki S., Shirai Y., Sakai K. Effect of Lactobacillus rhamnosus KY-3 and cellobiose as synbiotics on lipid metabolism in rats. J. Nutr. Sci. Vitaminol. 2004;50:330–334. doi: 10.3177/jnsv.50.330. [DOI] [PubMed] [Google Scholar]
- 5.Marteau P., Boutron-Ruault M.C. Nutritional advantages of probiotics and prebiotics. Br. J. Nutr. 2002;87:S153–S157. doi: 10.1079/BJN2002531. [DOI] [PubMed] [Google Scholar]
- 6.Sharma P., Singh A., Mathur N. Review Synbiotics “Combinationof Probiotics and Prebiotics”. IOSR-JBB. 2017;3:19–24. doi: 10.9790/264X-03041924. [DOI] [Google Scholar]
- 7.Jung J.Y., Lee S.H., Lee H.J., Seo H.Y., Park W.S., Jeon C.O. Effects of Leuconostoc mesenteroides starter cultures on microbial communities and metabolites during kimchi fermentation. Int. J. Food. Microbiol. 2012;153:378–387. doi: 10.1016/j.ijfoodmicro.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 8.Lim S.M., Jeong J.J., Woo K.H., Han M.J., Kim D.H. Lactobacillus sakei OK67 ameliorates high-fat diet-induced blood glucose intolerance and obesity in mice by inhibiting gut microbiota lipopolysaccharide production and inducing colon tight junction protein expression. Nutr. Res. 2016;36:337–348. doi: 10.1016/j.nutres.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Jo S.Y., Choi E.A., Lee J.J., Chang H.C. Characterization of starter kimchi fermented with Leuconostoc kimchii GJ2 and its cholesterol-lowering effects in rats fed a high-fat and high-cholesterol diet. J. Sci. Food Agric. 2015;95:2750–2756. doi: 10.1002/jsfa.7018. [DOI] [PubMed] [Google Scholar]
- 10.Hossain A., Yamaguchi F., Matsuo T., Tsukamoto I., Toyoda Y., Ogawa M., Nagata Y., Tokuda M. Rare sugar d-allulose: Potential role and therapeutic monitoring in maintaining obesity and type 2 diabetes mellitus. Pharmacol. Ther. 2015;155:49–59. doi: 10.1016/j.pharmthera.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Han Y., Han H.J., Kim A.H., Choi J.Y., Cho S.J., Park Y.B., Jung U.J., Choi M.S. d-Allulose supplementation normalized the body weight and fat-pad mass in diet-induced obese mice via the regulation of lipid metabolism under isocaloric fed condition. Mol. Nutr. Food Res. 2016;60:1695–1706. doi: 10.1002/mnfr.201500771. [DOI] [PubMed] [Google Scholar]
- 12.Kimoto-Nira H., Moriya N., Hayakawa S., Kuramasu K., Ohmori H., Yamasaki S., Ogawa M. Effects of rare sugar d-allulose on acid production and probiotic activities of dairy lactic acid bacteria. J. Dairy Sci. 2017;100:5936–5944. doi: 10.3168/jds.2016-12214. [DOI] [PubMed] [Google Scholar]
- 13.Folch J., Lees M., Sloane Stanley G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 14.Hulcher F.H., Oleson W.H. Simplified spectrophotometric assay for microsomal 3-hydroxy-3-methylglutaryl CoA reductase by measurement of coenzyme A. J. Lipid Res. 1973;14:625–631. [PubMed] [Google Scholar]
- 15.Rudack D., Chisholm E.M., Holten D. Rat liver glucose 6-phosphate dehydrogenase. Regulation by carbohydrate diet and insulin. J. Biol. Chem. 1971;246:1249–1254. [PubMed] [Google Scholar]
- 16.Ochoa S. Malic enzyme: Malic enzymes from pigeon and wheat germ. In: Colowick S.P., Kaplan N.O., editors. Methods in Enzymology. Academic Press; New York, NY, USA: 1955. pp. 323–326. [Google Scholar]
- 17.Markwell M.A., Mc Groarty E.J., Bieber L.L., Tolbert N.E. The subcellular distribution of carnitine acyltransferases in mammalian liver and kidney. A new peroxisomal enzyme. J. Biol. Chem. 1973;248:3426–3432. [PubMed] [Google Scholar]
- 18.Lazarow P.B. Assay of peroxisomal beta-oxidation of fatty acids. Method Enzymol. 1981;72:315–319. doi: 10.1016/s0076-6879(81)72021-4. [DOI] [PubMed] [Google Scholar]
- 19.Patterson J.A., Burkholder K.M. Application of prebiotics and probiotics in poultry production. Poult. Sci. 2003;82:627–631. doi: 10.1093/ps/82.4.627. [DOI] [PubMed] [Google Scholar]
- 20.Kolida S., Gibson G.R. Synbiotics in health and disease. Ann. Rev. Food Sci. Technol. 2011;2:373–393. doi: 10.1146/annurev-food-022510-133739. [DOI] [PubMed] [Google Scholar]
- 21.Rastall R.A., Maitin V. Prebiotics and synbiotics: Towards the next generation. Curr. Opin. Biotechnol. 2002;13:490–496. doi: 10.1016/S0958-1669(02)00365-8. [DOI] [PubMed] [Google Scholar]
- 22.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 23.Grundy S.M. Obesity, metabolic syndrome, and cardiovascular disease. J. Clin. Endocrinol. Metab. 2004;89:2595–2600. doi: 10.1210/jc.2004-0372. [DOI] [PubMed] [Google Scholar]
- 24.Satoh N., Naruse M., Usui T., Tagami T., Suganami T., Yamada K., Kuzuya H., Shimatsu A., Ogawa Y. Leptin-to-adiponectin ratio as a potential atherogenic index in obese type 2 diabetic patients. Diabetes Care. 2004;27:2488–2490. doi: 10.2337/diacare.27.10.2488. [DOI] [PubMed] [Google Scholar]
- 25.Vendrell J., Broch M., Vilarrasa N., Molina A., Gómez J.M., Gutiérrez C., Simón I., Soler J., Richart C. Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: Relationships in obesity. Obes. Res. 2004;12:962–971. doi: 10.1038/oby.2004.118. [DOI] [PubMed] [Google Scholar]
- 26.Kershaw E.E., Flier J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 27.Kersten S., Desvergne B., Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 28.Poulsen L.l., Siersbæk M., Mandrup S. PPARs: Fatty acid sensors controlling metabolism. Semin. Cell Dev. Biol. 2012;23:631–639. doi: 10.1016/j.semcdb.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Wahli W., Michalik L. PPARs at the crossroads of lipid signaling and inflammation. Trends Endocrinol. Metab. 2012;23:351–363. doi: 10.1016/j.tem.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Gerhart-Hines Z., Rodgers J.T., Bare O., Lerin C., Kim S.H., Mostoslavsky R., Alt F.W., Wu Z., Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rottiers V., Näär A.M. MicroRNAs in metabolism and metabolic disorders. Nat. Rev. Mol. Cell Biol. 2012;13:239–250. doi: 10.1038/nrm3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrisett J.D., Abdel-Fattah G., Hoogeveen R., Mitchell E., Ballantyne C.M., Pownall H.J., Opekun A.R., Jaffe J.S., Oppermann S., Kahan B.D. Effects of sirolimus on plasma lipids, lipoprotein levels, and fatty acid metabolism in renal transplant patients. J. Lipid Res. 2002;43:1170–1180. [PubMed] [Google Scholar]
- 33.Egusa G., Beltz W.F., Grundy S.M., Howard B.V. Influence of obesity on the metabolism of apolipoprotein B in humans. J. Clin. Investig. 1985;76:596–603. doi: 10.1172/JCI112011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen P., Leray V., Diez M., Serisier S., Le Bloc’h J., Siliart B., Dumon H. Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. 2008;92:272–283. doi: 10.1111/j.1439-0396.2007.00752.x. [DOI] [PubMed] [Google Scholar]