Abstract
Background and Aims
While there is increasing recognition of Batesian floral mimicry in plants, there are few confirmed cases where mimicry involves more than one model species. Here, we test for pollination by mimicry in Diuris (Orchidaceae), a genus hypothesized to attract pollinators via mimicry of a range of co-occurring pea plants (Faboideae).
Methods
Observations of pollinator behaviour were made for Diuris brumalis using arrays of orchid flowers. An analysis of floral traits in the co-flowering community and spectral reflectance measurements were undertaken to test if Di. brumalis and the pea plants showed strong similarity and were likely to be perceived as the same by bees. Pollen removal and fruit-set were recorded at 18 sites over two years to test if fitness of Di. brumalis increased with the abundance of the model species.
Key Results
Diuris brumalis shares the pollinator species Trichococolletes capillosus and T. leucogenys (Hymenoptera: Colletidae) with co-flowering Faboideae from the genus Daviesia. On Di. brumalis, Trichocolletes exhibited the same stereotyped food-foraging and mate-patrolling behaviour that they exhibit on Daviesia. Diuris and pea plants showed strong morphological similarity compared to the co-flowering plant community, while the spectral reflectance of Diuris was similar to that of Daviesia spp. Fruit-set and pollen removal of Di. brumalis was highest at sites with a greater number of Daviesia flowers.
Conclusions
Diuris brumalis is pollinated by mimicry of co-occurring congeneric Faboideae species. Evidence for mimicry of multiple models, all of which share pollinator species, suggests that this may represent a guild mimicry system. Interestingly, Di. brumalis belongs to a complex of species with similar floral traits, suggesting that this represents a useful system for investigating speciation in lineages that employ mimicry of food plants.
Keywords: Diuris brumalis, Daviesia, Faboideae, Colletidae, mimicry, food deception specialization, pollination, pollinator behaviour, plant fitness
INTRODUCTION
Batesian mimicry represents an interaction between model, mimic and operator (the signal receiver), in which the operator mistakes the mimic for the model leading to a fitness benefit for the mimic (Vane-Wright, 1980). Mimicry can be achieved through a diversity of cues, including visual, acoustic, chemical, tactile and possibly electrical (Stoddard, 1999; Norman et al., 2001; Barbero et al., 2009; Schiestl and Johnson, 2013; Bohman et al., 2018). Despite mimicry in animals being well supported in multiple systems, the phenomenon remained rarely confirmed and largely controversial in plants (Ruxton et al., 2004). Only in the last three decades has evidence been presented suggesting that floral mimicry may be widespread in some plant families (Johnson and Schiestl, 2016).
Orchids (Orchidaceae) are an unusual group among flowering plants in that approximately one-third of known species (6500–9000 species) are believed to attract their pollinators via deception (Dafni,1984; Ackerman, 1986). Non-rewarding orchids exhibit a range of mechanisms to attract pollinators, including sexual deception (Coleman, 1928; Schiestl et al., 1999, 2003), brood site mimicry (Van der Niet et al., 2011; Martos et al., 2015) and alarm pheromone imitation (Brodmann et al., 2009). However, the majority of deceptive orchids attract pollinators by falsely advertising floral rewards to pollinators (Ackerman, 1986), using traits such as inflorescence shape and architecture, flower colour and brightness, scent, nectar guides and pollen marks (Kunze and Gumbert, 2001; Galizia et al., 2005; Jersáková et al., 2012). The most common form of food deception is generalized food deception, where food-seeking animals are attracted by general floral signals rather than the traits of any particular rewarding species (Van der Cingel, 1995; Jersáková et al., 2006). Alternatively, deceptive orchids that exhibit similar floral traits to those of a particular rewarding flower are predicted to be using Batesian mimicry to attract pollinators (Jersáková et al., 2006), where the mimic receives a benefit from co-flowering plant species through increased reproductive success (Jersáková et al., 2006).
The most comprehensive evidence to date for Batesian mimicry in orchids comes from research undertaken on the South African flora. For example, Peter and Johnson (2008) employed UV-manipulation experiments to show that Eulophia zeyheriana mimics the floral colour of nectar-rewarding Wahlenbergia cuspidata (Campanulaceae) to attract Lipotriches (Halictidae) bees. Similarly, Jersáková et al. (2012) demonstrated that Disa pulchra attracts long-proboscid tabanid flies by mimicking the rewarding iris Watsonia lepida through closely matching the floral reflectance spectra of the species. In these cases, and most others where floral Batesian mimicry has been hypothesized, in any given population there is evidence for mimicry of a single model species (Dafni et al., 1981; Nilsson, 1983; Johnson, 2000; Benitez-Vieyra et al., 2007). However, in orchids there is some evidence for guild mimicry, where a rewardless species mimics a range of model species that have similar floral traits and share the same pollinator species (Brown and Brown, 1979; Dafni and Bernhardt, 1990; Johnson and Schiestl, 2016). For example, the European orchid Traunsteinera globosa attracts pollinators by mimicking the colour and inflorescence shape of representatives of three morphologically similar co-occurring genera in the Dipsacaceae and Caprifoliaceae (Juillet et al., 2007; Jersáková et al., 2016). This strategy may be advantageous over other more specialized forms of Batesian mimicry as the mimic may receive a fitness benefit from co-flowering with a wider range of model plants.
The Australian orchid genus Diuris has been long hypothesized to engage in guild mimicry (Dafni and Bernhardt, 1990). Some clades of Diuris are superficially similar in both colour and shape to those of a guild of yellow and brown pea plants (Faboideae). Floral mimicry of Faboideae was first tested in the eastern Australian species Diuris maculata (Beardsell et al., 1986; Indsto et al., 2006), where it was shown that Diuris and some Faboideae share pollinators and have similar floral coloration according to a bee visual model. While Diuris encompasses a range of floral shapes and colorations, this yellow and brown Faboideae-like flower type has evolved at least twice within the genus (Indsto et al., 2009), suggesting that these traits could be adaptations to the mimicry of Faboideae (see argument of Johnson et al., 2003). However, to determine if this is truly a pollination strategy based on mimicry, or convergent evolution of floral signals that are attractive to bee pollinators, requires comparison with the floral traits of the broader plant community (de Jager et al., 2016), observations of pollinator behaviour on model and mimic, and data on reproductive success of the orchid in relation to the abundance of the model (Roy and Widmer, 1999; Peter and Johnson, 2008). Furthermore, a compelling line of evidence for the existence of mimicry would be if the pollinator is deceived into engaging in the same specific behaviours with the putative mimic that it typically exhibits only with the model species. Such behavioural evidence confirms that the orchid is functioning as a mimic, regardless of whether some of its floral traits originally evolved through selection for mimicry, or independently to exploit the foraging behaviour of the bee.
We tested the mimicry hypothesis in Diuris brumalis, an orchid species that co-occurs with a range of Faboideae species that exhibit similar yellow–brown colour patterns. Having identified candidate model species based on the diet of the bee species involved in pollination of Di. brumalis, we tested the following predictions: (1) that the colour and morphology overlap between models and mimic, but not with the remainder of the floral community; (2) that the flowering phenology of the proposed mimic overlaps with the models; (3) that the pollinator exhibits with the mimic a deceived behaviour normally only associated with the model; (4) that the fitness of the mimic is greater in the presence of the models; and (5) that the fitness of the mimic increases with the number of flowers of the model species. Furthermore, to investigate if this mimicry system operates with more than one model species, observations of pollinator behaviour were undertaken in habitats that differed in the pea plant species present.
MATERIAL AND METHODS
Study species
Diuris is a primarily Australian genus comprising approx. 100 species, with centres of diversity in south-western and south-eastern Australia (Jones, 2006). Diuris are terrestrial geophytes, with a solitary scape produced per plant in any given year (Jones, 2006). Most species of Diuris appear to be capable of clonal reproduction through vegetative multiplication of daughter tubers (Dixon et al., 1989). Diuris brumalis produces yellow–brown nectarless flowers during July and August, with between three and 15 flowers per inflorescence (Brown et al., 2013). A vector is required for pollination, and the flowers are self-compatible (Supplementary Data Appendix S1). Pollination within a given flowering season is primarily pollen-limited, with most or all flowers on a scape forming fruit after pollination by hand (Elliott and Ladd, 2002; Appendix S1). Diuris brumalis occurs in a range of habitats, which differ in their community of winter-flowering Faboideae species. Unlike Di. brumalis, these Faboideae produce floral nectar (Appendix S1).
Study sites
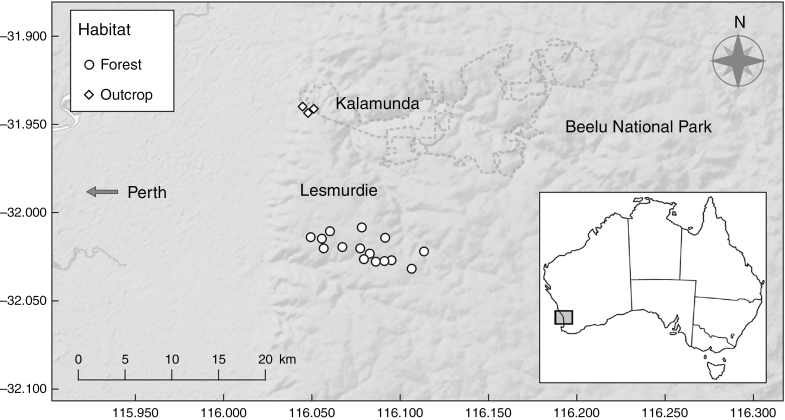
Data were collected from Di. brumalis populations in the Darling Range, near Perth, Western Australia, during 2016 and 2017 (Fig. 1). The populations were selected across two different habitat types (Fig. 1; Table S1): Jarrah forest (hereafter referred to as ‘forest’; 15 sites) and heathland surrounding granite outcrops (hereafter ‘outcrop’; three sites). No other species of Diuris was observed flowering at any site during the study period.
Fig. 1.
Distribution of the 18 Diuris brumalis study sites in the Darling Range, Western Australia. Fifteen sites were in Jarrah forest and three in granite outcrops.
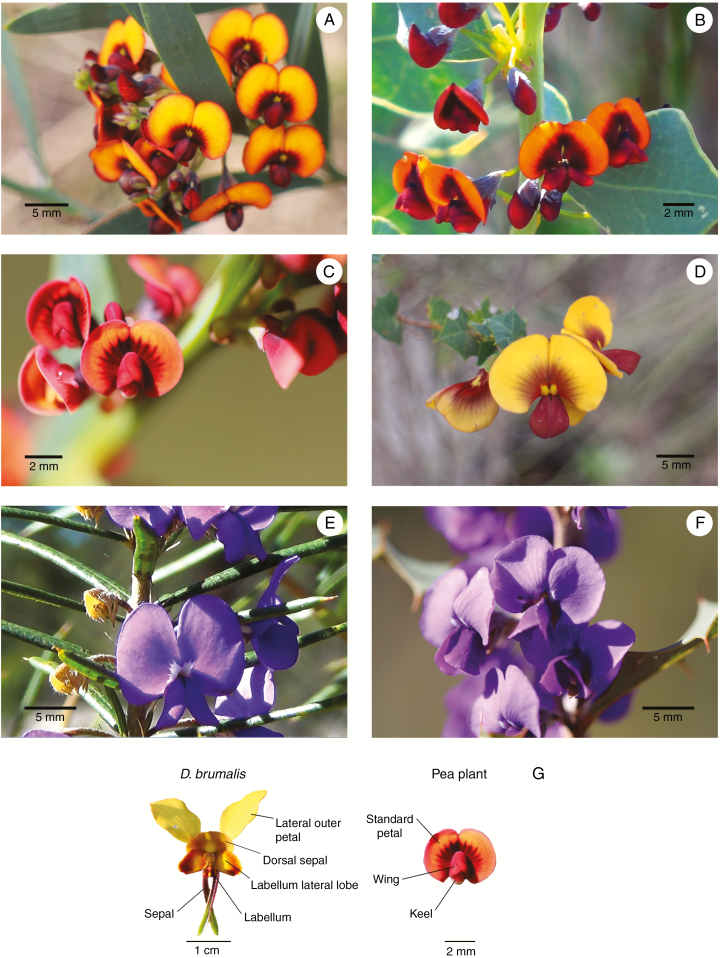
Diuris brumalis frequently co-occurs with several species of Faboideae (Fabaceae; Marshall, 1995). Six species of flowering Faboideae, commonly referred to as pea plants, were identified at the study sites (Fig. 2A–F), namely Daviesia decurrens, Da. horrida, Da. rhombifolia, Hovea chorizemifolia, H. pungens and Bossiaea aquifolium (Table S1). While Da. decurrens, Da. rhombifolia, H. chorizemifolia, H. pungens and B. aquifolium were present at forest sites and only Da. horrida and H. pungens were present at outcrop sites. Voucher specimens of all studied species were collected and accessioned at Herbarium of Western Australia in Perth (Table S2).
Fig. 2.
Faboideae co-occurring with Diuris brumalis: A, Daviesia horrida; B, Da. rhombifolia; C, Da. decurrens; D, Bossiaea aquifolium; E, Hovea pungens; F, Hovea chorizemifolia; G, pea-like floral morphology of Di. brumalis formed by two lateral petals, the dorsal sepal, labellum lateral lobe, labellum and two basal sepals (Hoffman and Brown, 2011).
Observation of pollinators on Diuris brumalis
To identify the pollinators of Di. brumalis and quantify their behaviour, observations of pollinator visitation to orchid flowers were undertaken at three sites (F1, F2 and O3) between 13 July to 15 August 2016 and 12 July to 13 August 2017. A total of 191, 15-min observation periods were conducted (for a total of 2865 min of observation), with insect behaviour recorded using an EOS M video camera (Canon, Tokyo, Japan) for subsequent viewing in slow motion. Observations were conducted between 1000 and 1530 h when temperatures were above 17 °C (temperature ranged between 8 and 19 °C, as measured with a Smartsensor AR827, set 20 cm above the ground). Arrays of orchid flowers were designed to replicate the colony-forming habit of Di. brumalis and comprised multiple inflorescences that had been cut and placed in glass vials (two inflorescences per vial, each with 4–6 flowers). For each observation period, three vials were spaced 10–20 cm apart to create a conspicuous floral display, with vials placed 1–2 m from flowering individuals of Da. decurrens, Da. rhombifolia or Da. horrida. While artificial arrays of flowers were used as the basis for pollinator observations, naturally occurring Di. brumalis were common at each of these three sites where observations were undertaken.
For each individual insect visiting a flower of Diuris and pea plants, the behaviour was recorded and categorized as follows: (1) the insect approached the flower, (2) the type of behaviour exhibited upon approaching the flower: zig-zag flight = moving side to side in flight as they approached the flowering plant; direct flight = flying in a straight line as they approached the flower; aligned = body of visitor aligned along the midpoint of the labellum/keel during attempts to forage; patrolling = appearing to inspect multiple flowers around the plant; searching = the bee approaches a flower closely (<5 cm) but then chooses to alight on a different flower, (3) the insect was carrying pollinia of Di. brumalis, (4) if the insect landed on the flower, (5) the length of time spent on the flower (if >1 s), (6) if the insect attempted to forage on the flower, either attempting to manipulate the labellum/keel by opening the wings, or attempting to feed on nectar (Fig. 2G), (7) if the insect removed or deposited pollen of Di. brumalis or of pea plants (based on filament contact with the insect) and (8) if the insect visited additional Di. brumalis or pea plant flowers (Tables S3, S4). Behaviour was only recorded for the first flower visited, as due to the very rapid movement of pollinators, it was often impossible to accurately quantify visits to subsequent flowers.
To determine whether pollinator behaviour differs in response to flowers of Diuris compared with Da. decurrens and Da. rhombifolia, we compared the proportion of floral visitors exhibiting food-foraging behaviours between pea plants and Diuris using a Generalized Linear Model with a Bernoulli distribution of the response variable. Plant species was the fixed effect and was treated as a categorical variable. Specifically, we tested (1) if between Diuris and pea plants (Da. decurrens and Da. rhombifolia in the forest habitat) there is a difference in the proportion of bees landing on the flower, and (2) if among the bees landing on the flower, there a difference in the proportion of bees that exhibit foraging behaviour, either by manipulating the labellum/keel or attempting to forage on nectar.
Observation of pollinators on co-flowering plants
To determine if Di. brumalis shares pollinators with co-flowering pea plants, pollinator observations were undertaken at two forest sites (F1, F2) and one outcrop site from 13 July to 6 September in 2016 and from 11 July to 9 September 2017. Observations were made between 1100 and 1500 h daily, with the same video camera set up as described above. A total of 32 observation periods were undertaken for B. aquifolium, Da. decurrens, Da. horrida, Da. rhombifolia, H. chorizemifolia and H. pungens individuals, each of 15 min, yielding a total of 480 min of observation for each plant species. The pollinator behaviours recorded corresponded to those used for visitors to the Diuris (see above), to enable a formal behavioural comparison. To test if bees that visited Di. brumalis also visited members of the plant community other than pea plants, additional 10-min observation periods were undertaken for other dominant co-flowering species: Acacia pulchella, Adenanthos barbiger, Calothamnus sanguineus, Hakea lissocarpha, Hibbertia hypericoides and Hypocalymna robustum (from four to five observation periods per species, for a total of 270 min).
Identification of pollinators
Pollinators observed on Di. brumalis and pea plant flowers (particularly individuals carrying the distinctive white pollinia of Di. brumalis) were collected for identification. All collected insect pollinators were sent to the Western Australian Museum as voucher specimens (Table S5). Native bee pollinators observed were sexed and identified according to Batley and Houston (2012) based on behavioural (patrolling – males; collecting pollen on the abdomen – females) and morphological features including differences in antennae length (generally longer in males), body size (larger in females), abdomen width (larger in females) and number of hairs on the head (more abundant on males).
Quantification of pollen loads of floral visitors
As a complementary approach to resolving the food plants of the floral visitors, pollen was identified from the bodies of bees caught (Table S5) visiting Di. brumalis and pea plants. Pollen observed on the tibiae or abdomen of pollinators during identification was removed by washing the insect with distilled water, acetolysed following the methods of Erdtman (1960) and mounted on glass microscope slides. All pollen samples were identified under high magnification (Olympus-BX 51 microscope with Olympus–DP71 camera; Olympus, Tokyo, Japan) by comparison with acetolysed mounted pollen samples from herbarium specimens of Di. brumalis, B. aquifolium, Da. decurrens, Da. horrida, Da. rhombifolia, H. chorizemifolia, H. pungens and other commonly co-flowering plant taxa.
Morphological evaluation of floral traits and spectral reflectance
To test if Di. brumalis shows greater overlap in floral morphology with the candidate model species than the remainder of the plant community, a morphological evaluation of the floral traits of the dominant co-flowering plant species, including functional pollinators traits, was conducted at three forest sites (F1, F2 and F3). Morphological evaluation was conducted on Di. brumalis and 20 co-flowering species from 11 families in accordance with the descriptions in Marchant et al. (1987). The traits included were corolla symmetry (zygomorphic, actinomorphic), corolla shape (rotate, papillionaceous, bilabiate, ligulate), flower width and length (in mm), flower orientation (pendant, upright, horizontal), plant height (in cm), petal projections as a platform for pollinators (presence or absence), anther position (exposed or not exposed) and inflorescence type (umbel, raceme, spike, panicle, solitary) (Table S6). Morphological similarity of floral traits was evaluated using non-metric multi-dimensional scaling (NMDS) following the methodology of Jolles (2015).
To test if the floral colour of Di. brumalis flowers is likely to be distinguishable by bees from the co-flowering pea plants species (Da. decurrens, Da. horrida, Da. rhombifolia, H. chorizemifolia, H. pungens and B. aquifolium), we took spectral reflectance measurements and analysed them using the Chittka (1992) model of bee vision. In addition, spectral reflectance was also measured for two yellow-flowered species that occurred at all sites, Hibbertia hypericoides (Dilleniaceae) and Acacia pulchella (Fabaceae), to test if other yellow-flowered species could also be part of the same guild as the pea plants. Spectral reflectance was measured on two flowers per plant for six randomly selected individuals of each species using a spectrometer (Jaz, DH-2000 UV-VIS-NIR Light source) with an integration time of 50 milliseconds. For Di. brumalis, measurements of spectral reflectance were taken from the outer lateral petals, the central dorsal sepal, the labellum and labellum lateral lobes; for pea plant species measurements were taken from the standard and wing petals (Fig. 2G) and for Hibbertia hypericoides and Acacia pulchella from the most conspicuous part of the floral display (the corolla and stamens, respectively). Spectral reflectance was analysed using the colour hexagon model, which is based on the sensitivities of photoreceptors of the bee Apis mellifera (Chittka, 1992; Chittka and Kevan, 2005). For quantifying similarity of spectral reflectance, the distance between colour loci coordinates was measured as the Euclidean distance.
Comparative flowering phenology of Diuris brumalis and candidate model species
To test the prediction that mimics overlap in flowering period with their proposed models, the extent of flowering across the study period was quantified for Di. brumalis and the co-occurring pea plants (Da. decurrens, Da. horrida, Da. rhombifolia, H. chorizemifolia, H. pungens and B. aquifolium). For each species, weekly counts of open flowers were undertaken in 30 × 30-m quadrats at intact forest sites (F1, F2 and F3) from 28 June to 11 October 2017. For pea plants, due to the high number of flowers, we scored the total number of flowers per quadrat as (binned category): (1) 1–100, (2) 101–200, (3) 301–400, (4) 401–500 and so on, up to 2000 for a total of 19 categories (1–19). However, in the case of H. chorizemifolia and Di. brumalis, due to the paucity of flowers per inflorescence, the exact number of flowers on each plant was counted. The average number of flowers (or binned category) was calculated as the measure of flowering during any given week.
Reproductive success of the mimic in relation to the abundance of the model
To test if the fitness of Di. brumalis (Table S7) increased with the number of flowers of the putative model species, the proportion of flowers with pollen removal and the proportion of flowers with fruit formation was quantified at 18 populations. In 2016 (15 sites) and 2017 (18 sites), we delimited a single 30 × 30-m quadrat per site, and at the peak flowering period for Di. brumalis we recorded: (1) the number of pea plants of each species within the quadrat; (2) the estimated number of flowers for each pea plant species; and (3) how many Di. brumalis plants and flowers were present (counted at the end of the flowering season in August). Variable (2) was estimated by counting the number of flowers on ten stems per pea plant to enable calculation of a mean, then multiplying this value by the total number of stems on the plant. Following evidence that the pollinators of Di. brumalis fed almost entirely on Daviesia, this variable was modified to be the estimated number of flowers of Daviesia. In both years, at the end of the flowering period of Di. brumalis we collected data on the number of flowers without pollinia and the number of fruits produced. The proportion of flowers with pollinia removal was used as a proxy for male fitness, while the proportion of flowers setting fruit was used as a proxy for female fitness.
Pollinia removed and fruit set were analysed by Generalized Linear Mixed Models (GLMMs) using the package glmmTMB in R Studio (Version 3.3.2). First, we tested if pollinia removal and fruit-set were greater at sites where Daviesia was present. Secondly, we tested if pollinia removal and fruit-set increased when there were more Daviesia flowers. In these latter models we included the abundance of Daviesia flowers, habitat and year all as fixed effects. In each model, site was treated as a random effect, as pollinia removal and fruit-set was quantified at the same sites in two field seasons. Because pollinia removal and fruit-set were analysed as proportions of the total number of flowers, they were assumed to be binomially distributed. However, when using a binomial distribution, the models for pollinia removed showed overdispersion (overdispersion parameters: 5.833 for the model of presence–absence and 3.897 for the model testing the effect of Daviesia flower abundance, habitat and year; see Zuur et al., 2013 for calculations), necessitating a switch to a betabinomial distribution. Evaluation of which model was most strongly supported by the data was undertaken using the corrected Akaike’s information criterion (AICc) index, which dropped 120 and 57 points respectively for the two models by switching to the betabinomial distribution. Models testing the effect of the covariates (see above) on fruit-set were not over-dispersed. Therefore, fruit-set was confirmed to be a binomially distributed response variable. The abundance of Daviesia flowers was log-transformed to improve the fit of the fruit-set model. The improvement of the model following a log transformation was confirmed by the AICc index, which dropped 6.5 points, and verified using the ‘anova’ R function (χ233,4 = 6.371, P < 0.001). For all models we undertook the checks suggested by Zuur et al. (2013) to confirm that the underlying assumptions of the model are not violated.
RESULTS
Pollinators of Diuris brumalis
During baiting experiments a total of 132 insects were observed visiting Di. brumalis. Of these, 102 visits were by Trichocolletes spp. (Colletidae) and 25 by Apis mellifera (Apidae). Other visitors included Syrphidae (three) and Leioproctus sp. (two; Colletidae). Only Trichocolletes spp. and Apis mellifera were observed with orchid pollinia attached, in each case to the frontal region of the head (Fig. 3). However, only in the case of Trichocolletes was deposition of pollinia observed, with parts of the pollinia deposited in visits to subsequent flowers.
Fig. 3.
Pollinators of Diuris brumalis and Daviesia spp.: A, inflorescence of Diuris brumalis (Orchidaceae); B, female of Trichocolletes leucogenys with pea plant pollen (orange in colour) on the abdomen and posterior legs, feeding on Daviesia rhombifolia by positioning the abdomen over the keel of the flower; C, male of Trichocolletes capillosus carrying Diuris brumalis pollinia on the head.
A total of 32 insects were caught for identification during baiting experiments and observations of pea plants (Table S5). In 2016 a total of 14 Trichococolletes capillosus, two T. leucogenys and one T. dives were caught, while seven T. leucogenys were caught in 2017. A total of 14 Trichocolletes were observed carrying pollen of Di. brumalis, eight while visiting Di. brumalis, and six while foraging on Daviesia spp. (see example in Supplementary video). Of the eight individuals observed carrying pollina while visiting Di. brumalis, two arrived at the plant already carrying pollinia, and six removed pollinia while being observed. As the six individuals removing pollinia were all captured for identification, the remaining bees must have all sourced pollinia from wild Di. brumalis, independent of our artificial arrays. The identification of captured visitors and/or pollinators shows that the Trichocolletes spp. individuals caught on Di. brumalis and on pea plants with orchid pollinia included both females (four) and males (six). On two occasions Apis mellifera were collected with attached Di. brumalis pollinia. Of the Trichocolletes collected during the study, ten carried on their hind legs pollen of the same colour as seen in pea plants (yellow–orange). Trichocolletes capillosus was recorded in 2016 in the habitat forest, whereas T. leucogenys was recorded in both 2016 and 2017 in the habitats forest and outcrop (Table S5).
Pollinators of co-occurring pea plants
Based on observations of contact with the reproductive structures, Da. decurrens and Da. rhombifolia (occurring at only the forest sites) were pollinated by both T. capillosus and T. leucogenys, while Da. horrida (occurring only at the outcrop sites) was pollinated only by T. leucogenys. Apis mellifera was also observed to pollinate all three species as well as H. pungens and B. aquifolium (Table S4). No Trichocolletes species were observed visiting other pea plants or other plant species in the community.
Quantification of pollinator behaviour
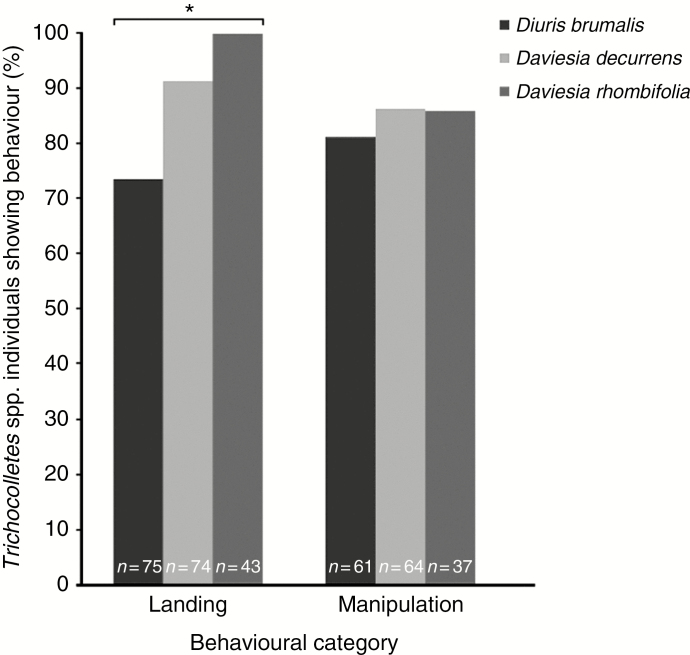
Of the 102 Trichocolletes spp. visiting Di. brumalis, 74.6 % alighted on the flower. In each case the insect aligned along the labellum with its head facing the column. Those individuals that flew around the flowers without landing (25.6 %) were mostly ‘patrolling’ and could be visually distinguished as males by longer antennas and smaller body size, suggesting mate-searching behaviour (Barrows, 1976). Occasionally, males behaving in this fashion were observed mating with females that had been located while foraging on Daviesia spp. Both male and female of Trichocolletes spp. landed on the flowers of Di. brumalis for 1–2 s. However, we were unable to record the behaviour of Trichocolletes that landed for less than 1 s due to the rapidity of visits. Of the bees alighting on the flower, 81.3 % attempted to manipulate the labellum by articulations of the anterior legs and/or pushing of the abdomen onto the labellum, as seen when foraging on nectar and pollen from Daviesia spp. (Fig. 4). On 50.8 % of occasions, attempting to manipulate the labellum resulted in pollinia removal, with 29.5 % attributable to females and 21.3 % to males. After the visit, 19.3 % of insects extracting orchid pollinia visited other orchid flowers within the clump, with the remaining 80.7 % of bees going on to forage on Daviesia spp. flowers.
Fig. 4.
Comparison of the foraging behaviour of Trichocolletes spp. (Trichocolletes capillosus, T. leucogenys) on Diuris brumalis and Daviesia spp. (Daviesia decurrens, Da. rhombifolia, Da. horrida). Bars represent the proportion of individuals engaging in: 1, Landing: flying directly to the flower and alighting on keel or labellum; 2, Manipulation: attempted to manipulate the flower as part of foraging behaviour for either nectar or pollen. * indicates a significant difference.
The behaviour exhibited by Trichocolletes spp. on Di. brumalis is characterized by similar behaviour as seen when foraging on the flowers of Daviesia spp. in the forest habitat (Da. decurrens and Da. rhombifolia). However, significantly more visitors landed on the Daviesia spp. than on Di. brumalis (Di. brumalis 74.2 %, n = 102 vs. Da. rhombifolia 100 %, n = 43, P = 0.009; Di. brumalis vs. Da. decurrens 91.3 %, n = 74, P = 0.004), Alternatively, among the bees that landed, there was no difference in how frequently the bees attempted to forage on the flower (Di. brumalis 81.3 %, n = 75 vs. Da. rhombifolia 86 %, n = 37, P = 0.513; Di. brumalis vs. Da. decurrens 86.4 %, n = 64; P = 0.394).
Floral similarity of mimics and models
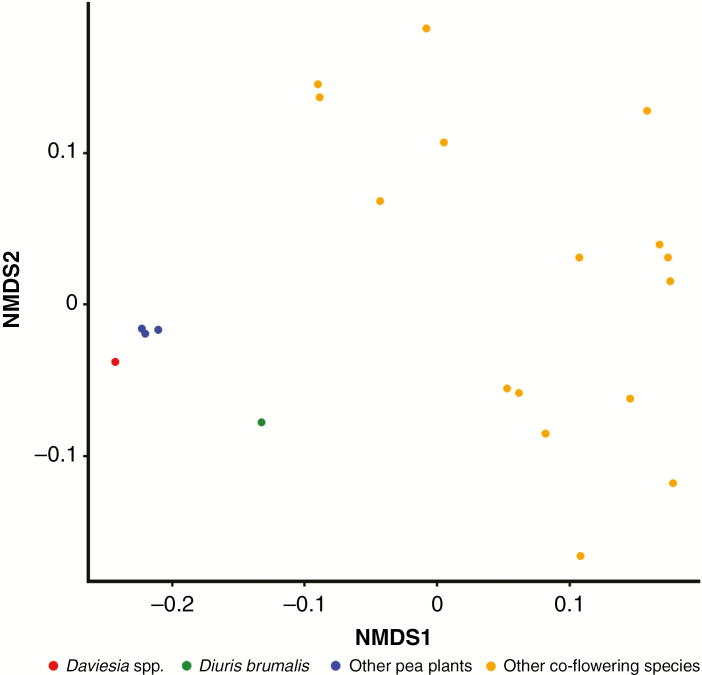
An NMDS plot shows that all pea plants are morphologically similar, and formed a tight cluster, with a pronounced similarity of Daviesia spp. that overlap in the plot (Fig. 5). Diuris brumalis is more similar to pea plants than the remainder of the co-flowering plant community, but does not overlap with the morphology of peas in the NMDS plot (Fig. 5). Investigation of the species by trait matrix reveals that Di. brumalis matches pea plants for all morphological traits except height of plant and flower size. In the case of flower size, Di. brumalis is much larger because of the prominently projecting lateral sepals (Fig. 2G).
Fig. 5.
Non-metric multi-dimensional scaling plot of floral traits for Diuris brumalis and co-flowering species in the forest habitat. Diuris brumalis and co-occurring pea plants (Faboideae) form a distinct cluster reflecting strong morphological similarity compared to the remainder of the plant community.
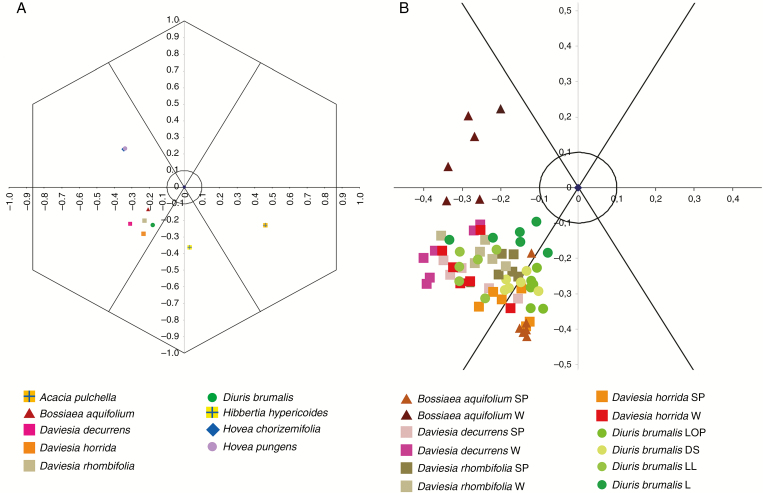
Analysis of spectral reflectance using the hexagon bee vision model (Chittka, 1992; Chittka and Kevan, 2005) showed that the average colour loci of Di. brumalis, all three Daviesia spp. and B. aquifolium corresponded to the UV region. The colour loci for Hovea spp. fell in the UV–blue region (Fig. 6A), and the colour loci for Acacia pulchella and Hibbertia hypericoides were located in the UV–green and green region, respectively. The mean distance of the colour loci measured on flower parts between Di. brumalis and Da. decurrens, Da. rhombifolia, Da. horrida and B. aquifolium was 0.12, 0.05, 0.06 and 0.1 respectively (Table S9). Colour loci for individual plants of Daviesia spp., distributed in the coordinates range y: [−0.39; −0.10] x: [−0.12; −0.40], overlap the visual space of Di. brumalis individuals ranging across the positions y: [−0.34; −0.09] x: [−0.33; −0.08] (Fig. 6B). In B. aquifolium the overlap of colour space of individual colour loci with Di. brumalis is limited to the dorsal petals, as the keel is in the UV–blue region (Fig. 6B).
Fig. 6.
(A) Mean values of colour loci calculated for floral parts of Daviesia decurrens, Da. horrida, Da. rhombifolia, Hovea chorizemifolia, H. pungens and Bossiaea aquifolium. In addition, colour loci are presented for two commonly occurring yellow-flowered species present at all sites, Hibbertia hypericoides (Dilleniaceae) and Acacia pulchella (Fabaceae), to test model similarity based on floral colour. (B) Distribution of colour loci most similar to the Di. brumalis colour signal. Measurements of spectral reflectance were taken for Di. brumalis: LOP = lateral outer petal; DS = dorsal sepal; LL = labellum lateral lobe; L = labellum; for pea plant species (Faboideae): SP = standard petal; W = wing petals. The calculations were made using the Hexagon colour model of bee vision (Chittka, 1992).
Quantification of the pollen load of floral visitors
Pollen counts (Table S8) showed that the pollen assemblage carried by four Trichocolletes specimens consisted of almost 100 % Daviesia pollen with traces (<10 pollen grains in the scanned slide) of Myrtaceae spp. and Grevillea. One specimen of T. leucogenys from the outcrop habitat (Table S8, no. 2), which was caught on Da. horrida, contained 97.5 % Daviesia pollen and traces of pollen of Myrtaceae (2 %) and Hovea (0.5 %). The amount of Daviesia pollen in samples from Apis mellifera specimens caught foraging on Daviesia plants was variable (80–98 %), and also they contained pollen of Banksia, Acacia and Myrtaceae (1–20 %). On the Apis mellifera specimen caught foraging on B. aquifolium (Table S8, no. 10), Bossiaea pollen comprised 97.5 % of the assemblage with 1.5 % Banksia pollen, 1 % Myrtaceae pollen and traces of Acacia pollen.
Flowering phenology of target species
There was pronounced variation in the overlap of the flowering periods of Di. brumalis and the co-occurring pea plants (Fig. S1). Among the species that are frequently visited by Trichocolletes spp., flowering of Da. decurrens and Da. rhombifolia peaked 2 and 5 weeks respectively after the peak of Di. brumalis. Flowering of H. chorizemifolia peaks 2 weeks before Di. brumalis, while the peak of H. pungens corresponded to the peak of Di. brumalis. Peak flowering for B. aquifolium occurred near the end of the Di. brumalis flowering period.
Reproductive success of the mimic in relation to the abundance of the model
Pollinia removed did not show any significant difference between sites where Daviesia spp. were present (marginal mean of 0.119 ± <0.001 s.e.) or absent (0.1 ± <0.001, P = 0.592). However, fruit-set was higher in the presence of Daviesia ssp. (0.027 ± 0.003) than in their absence (0.008 ± 0.001, P = 0.049).
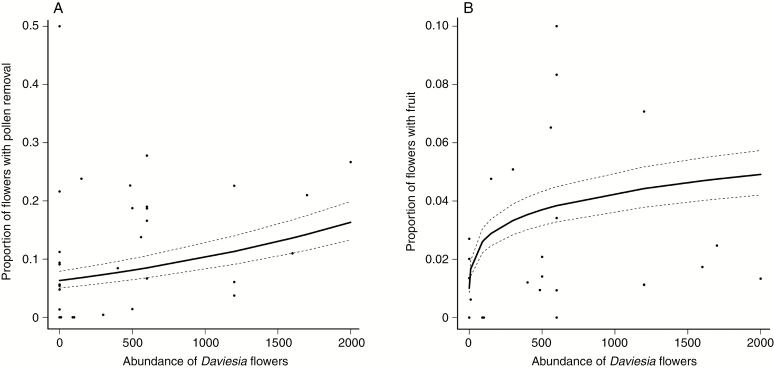
The proportion of pollinia removed exhibited a positive relationship (mean = 0.2982 ± 0.1237, P = 0.016) with the abundance of Daviesia flowers (Fig. 7A). Year also had a significant effect on the proportion of pollinia removed from Di. brumalis flowers (2017 = 0.146 ± 0.013; 2016 = 0.069 ± 0.006, P = 0.019). The proportion of pollinia removed was marginally higher in the jarrah forest (0.123 ± 0.011) compared to the outcrop habitat (0.057 ± 0.015, P = 0.068), although the difference was non-significant. Fruit-set showed a positive relationship with the number of Daviesia flowers (log-transformed mean = 0.21398 ± 0.08328, P = 0.01) (Fig. 7B). Fruit-set was significantly different between years (2016 = 0.031 ± 0.006; 2017 = 0.01 ± 0.002, P < 0.001), but did not differ between the forest (0.021 ± 0.004) and outcrop habitats (0.017 ± 0.006, P = 0.692). The relationship between the number of Daviesia flowers and both pollen removal and fruit-set was influenced by several sites where Daviesia spp. did not occur, and there was very little or no reproductive success in Di. brumalis.
Fig. 7.
The proportion of flowers with pollen removal (A) and fruit-set (B) of Diuris brumalis as a function of the number of flowers of Daviesia spp.
DISCUSSION
Pollinator sharing between models and mimic
One of the most fundamental criteria to assess the occurrence of floral mimicry is to establish whether the proposed model and mimic species share the same pollinators (Roy and Widmer, 1999; Johnson and Schiestl, 2016). Data from this study indicate that Di. brumalis shares the same pollinators (the bees Trichocolletes capillosus and T. leucogenys) with Da. decurrens and Da. rhombifolia in jarrah forest, and Da. horrida in heathland with granite outcrops. Additionally, observations of pollinator foraging, and analysis of pollen collected from the bodies of pollinators, revealed that in the study areas both T. capillosus and T. leucogenys feed primarily on Da. decurrens, Da. rhombifolia and Da. horrida (Table S8). Trichocolletes capillosus individuals were observed and caught only in 2016 in forest sites, while T. leucogenys were observed and caught in both 2016 and 2017 in forest and outcrop sites. Previous observations suggest that depending on seasonal conditions, the numbers of Trichocolletes that emerge at particular sites can vary from year to year, and in some years none may emerge (T. Houston, Western Australian Museum, pers. comm.). Apis mellifera and T. dives are potential pollinators as they have been observed to extract orchid pollinia, but they were not observed to deposit pollen on Di. brumalis. However, given their ability to remove and carry pollen, they may be responsible for occasional pollination events.
Behavioural evidence for mimicry
Exhibiting specific behaviours typically associated with the model species provides strong evidence that the proposed mimic has deceived the operator. In the present study, T. capillosus and T. leucogenys exhibit very similar foraging behaviour on Di. brumalis and Daviesia spp. (Fig. 4) [Supplementary data, Video], suggesting that the orchid is sufficiently similar to the model to deceive pollinators. For all three Daviesia species observed, Trichocolletes spp. show abdomen bending around the keel when attempting to collect nectar/pollen on Daviesia. Furthermore, female bees part the keel using their hind legs to collect pollen from the anthers. Our observations of T. capillosus and T. leucogenys individuals on Di. brumalis flowers suggested that they attempt to use the same stereotyped foraging behaviour. Both species landed along the midline of the labellum and push their abdomen upon it, unsuccessfully attempting to open it using their anterior legs in a similar fashion to the pollen-collecting behaviour they exhibit on Daviesia [Supplementary data, Video]. Crucially, Trichocolletes have only been recorded exhibiting this keel-parting behaviour on pea plants, meaning that this behaviour indicates mimicry of Faboideae, not other plant groups. Interestingly, other insects observed visiting Daviesia (particularly Apis mellifera and Leioproctus spp.) that have broader foraging preferences beyond the Faboideae were seen to land on the flowers and probe the keel with the body orientated in different directions, not necessarily along the keel.
When visiting Di. brumalis flowers, some male T. capillosus and T. leucogenys individuals appeared to exhibit patrolling behaviour, where the male searches for females in specific landmarks or rendezvous places that can be resourced-based (Haas, 1960; Barrows, 1976; Paxton, 2005). In Trichocolletes landmarks are represented by flowering Daviesia bushes, where males often approach closely without landing, probably searching for females engaged in foraging behaviour. Males exhibiting this same apparent patrolling behaviour were observed occasionally to mate with females foraging on Daviesia plants. Exhibiting this ‘patrolling’ behaviour provides evidence that male Trichocolletes confuse Di. brumalis with Daviesia food sources, even though courtship or patrolling behaviour is not directly involved in pollination.
Physical similarity between the mimic and the models
A multivariate analysis of floral morphological traits indicated that Di. brumalis is more similar to species of Faboideae than any other co-flowering species in the studied communities (Fig. 5). While Di. brumalis did not overlap with the Fabaceae in the NMDS plot, all of the characters scored were matching between pea plants and Di. brumalis except for plant height and overall flower size. Among the pea plants, the spectral reflectance of Di. brumalis was most similar to that of the Daviesia species on which Trichocolletes feed (Fig. 6A, B). The similarity of colour loci between Di. brumalis and Daviesia appeared particularly pronounced between the standard (model) and dorsal petal (mimic), and between the wing (model) and labellum lateral lobe (mimic), suggesting a level of colour matching between morphologically corresponding floral parts (Figs 2G and 6B). Between Daviesia species and Di. brumalis the distances between mean colour loci (averaged across floral parts) ranged between 0.05 and 0.10. In some individuals, the coloration of Da. rhombifolia, Da. decurrens and Da. horrida overlapped in colour space with Di. brumalis, and was less than the 0.06 units whereby bumble bees and honey bees cannot distinguish colours (Dyer and Chittka, 2004a, b; Giurfa, 2004). However, due to only partial overlap of colour loci of individual plants in Daviesia and Di. brumalis, colours of model species and mimic are likely to often be distinguishable by pollinators. Furthermore, it is likely that precise colour patterns differ between Di. brumalis and the Daviesia species. Nonetheless, mimics do not have to be identical, as long as they are perceived as similar by the pollinator (Dalziell and Welbergen, 2016). Indeed, Diuris may benefit from an unspecific mimicry of a range of pea plants, rather than appearing identical to a single species, as this may enable them to function effectively with multiple model species.
While the labellum, dorsal sepal and labellum lateral lobes of Di. brumalis appear to replicate the keel, dorsal and wing petals of Daviesia, the prominently projecting external petals in Di. brumalis are a component of floral architecture that is absent in Daviesia spp. (Fig 2G). However, it may be possible that some floral parts are involved in the mimicry of pea plants while others are not essential for mimicry and are free to vary. For example, in some genera of sexual deceptive orchids, where the role of floral traits in pollinator attraction is well studied, mimicry of the sex pheromone of the pollinator is often precise (Peakall et al., 2010; Bohman et al., 2018), while colour is not a close match to the female (Gaskett et al., 2016). Similarly, parts of the flower involved in positioning of the pollinator may be under stronger selection than morphologically inactive parts (Rakosy et al., 2017; de Jager and Peakall, 2018). In Diuris flowers, selection may operate through a dual mechanism, where floral traits involved in mimicry, such as colour and shape of the labellum and column wings, have evolved to resemble pea plants whereas the projecting outer petals may have evolved exaggerated size to increase long-distance attraction of pollinators. Indeed, there is a large body of supporting evidence suggesting that a greater floral display increases pollinator visitation rates (e.g. Peter and de Jong, 1990; Karron et al., 2004).
Overlap between mimics and models in flowering phenology
An overlap in flowering phenology between mimic and model is another key requirement of floral mimicry (Roy and Widmer, 1999; Johnson and Schiestl, 2016). Here, we have shown that the flowering periods of Di. brumalis and Daviesia spp. overlap, but that the flowering peak of Di. brumalis precedes the peak of the model species (2 weeks before Da. decurrens and 5 weeks before Da. rhombifolia) (Fig. S1). In Trichocolletes, males often emerge several days prior to females (observations by T. Houston, Western Australian Museum, unpubl. res.), meaning that Diuris may take advantage of early emerging males that are searching for females and nectar on pea plants. This interpretation was supported in the present study, where most observations at the start of the flowering period were of males, but the number of females increased as the Daviesia came into flower. The exploitation of naïve pollinators appears to be a common characteristic of food-deception systems. Species that use generalized food deception often flower when naïve pollinators emerge (Pellissier et al., 2010) and are yet to learn that the orchid flowers are rewardless (Internicola and Harder, 2012). Alternatively, pollinators can exhibit an innate sensory bias to certain colours and shapes and, following emergence, automatically searching for food sources with these traits (Çakmak and Wells, 1995; Lunau and Maier, 1995). In the case of Di. brumalis, pollinators may attempt to forage on the mimetic Diuris through either naivety or an innate preference for pea-like flowers, even though flowering individuals of the model pea plants may be scarce at the time of emergence.
Does fitness of the mimic increase in the presence of the model?
Adaptive resemblance between mimic and model species is achieved when pollinators are not able to distinguish between them, and this ‘misclassification’ behaviour enhances the fitness of the mimic (Endler, 1981; Skelhorn and Ruxton, 2010). As such, it is expected that in mimicry systems the fitness of the mimic should be greater when the model is more abundant (Anderson and Johnson, 2006). However, in practice it is difficult to separate the effects on fitness of reduced pollinator learning in the presence of the more model flowers, and of greater pollinator abundance in the presence of more model flowers. This challenge applies to Di. brumalis, as the Trichocolletes species foraged primarily on the model flowers, making pollinator abundance likely to be highly correlated with abundance of the model. For example, fruit-set was lowest at sites where Trichocolletes were not observed and Daviesia were almost absent. Furthermore, fruit-set increased with the number of Daviesia flowers, with this relationship likely to be influenced by sites where there were few or no Daviesia, and thus very low reproductive success of Di. brumalis. As expected under pollinator learning, rates of pollinia removal increased when there were greater numbers of Daviesia flowers, although this could also potentially be attributable to greater numbers of pollinator at these sites. To resolve this issue, it would be of interest to compare the response of Trichocolletes to experimentally presented Diuris flowers in areas with and without natural populations of Diuris.
Interestingly, even at sites where Trichocolletes were not observed and Daviesia were largely absent, occasional cases of pollen removal and deposition occurred. These events may be partly attributable to the introduced honey bee Apis mellifera, which was frequently observed foraging on co-occurring flowering plants in both habitats, including sites where Trichocolletes was not observed. However, forest sites without Daviesia exhibited a level of fruit-set approaching zero, despite some level of pollen removal, suggesting that honey bees may fail to complete pollination through pollen deposition. At present, there is very little information on the potential negative or positive effects of Apis mellifera on pollination of Australian orchids (e.g. Adams and Lawson, 1993; Phillips et al., 2009), although given the occasional visitation witnessed here, Diuris may represent an interesting study genus to tackle this issue.
Is there evidence for guild mimicry in Diuris brumalis?
While pollination via mimicry of flowering plants usually involves a particular model species, there is evidence that some plants mimic a guild of plant species rather than a specific model (Jersáková et al., 2016). Plant guilds are recognized by both sharing a particular pollinator (or group of related pollinators) and having very similar floral traits (Manning and Goldblatt, 1996), which are likely to represent adaptations to the particular pollinator(s) (Johnson, 2010). Based on some sharing of pollinators and their striking resemblance, Diuris have been hypothesized to mimic a guild of pea plants (Beardsell et al., 1986; Dafni and Bernhardt, 1990; Indsto et al., 2006). The present study shows that while Daviesia spp. share pollinators and may form the basis of a guild, this does not extend to all pea plant species in the community. However, based on behavioural observations and floral traits, we provide evidence that mimicry functions with different Daviesia species in different habitats (Da. decurrens, Da. rhombifolia in jarrah forest; Da. horrida in the outcrop heath). As such, through the use of more than one model species the Diuris–Daviesia mimicry system may meet some of the conditions for guild mimicry.
While the guild mimicry hypothesis has received support from observational studies in orchids (e.g. Jersakova et al., 2016 and the present study), at present experimental tests are lacking. A complementary approach to conducting field observations in different habitats would be to move experimental arrays of orchids between pea plant communities, thereby testing if any given population of Di. brumalis can attract pollinators in the presence of other pea plant species. In addition, it would be of interest to investigate the breadth of phenotypes that can achieve mimicry through the use of models or manipulated Diuris flowers. Alternatively, experiments with bees conditioned on different species of pea plant could be used understand the full range of models that Di. brumalis can mimic. However, the outcomes of such experiments would also be partly affected by whether the bees learn to associate rewards with particular pea plants, or if the attraction is innate. If the attraction is innate it is possible that Di. brumalis may be attractive to pollinators regardless of the pollinators’ prior experience with food plants.
CONCLUSIONS
Here we present evidence that Di. brumalis achieves pollination by mimicking the flowers of multiple co-flowering species of Daviesia. In addition to meeting the criteria for sharing pollinators and flowering times, pollinators exhibited pea plant-specific foraging behaviour on the Diuris, providing strong evidence that the mimic had successfully deceived the pollinator. This evidence was further supported by data on morphology and colour, showing that not only are Diuris and Daviesia spp. very similar compared to the remainder of the co-flowering community, but that based on bee vision models, in many cases the colour of Diuris and the proposed model species will not be readily distinguishable to pollinators. Fruit-set and pollen removal of Di. brumalis was more frequent in the presence of Daviesia, although evidence suggests that this is probably through some combination of both learning and greater pollinator abundance at sites where the model is present. The diversity of species related to Di. brumalis with pea-like floral traits (Diuris corymbosa complex) suggests that this may be an effective system for understanding diversification in lineages that use floral Batesian mimicry.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Fig. S1: phenology of Diuris brumalis and co-occurring Faboideae. Table S1: habitat assigned with description. Table S2: plant species vouchered at the WA Herbarium. Table S3: observations of floral visitors to Diuris brumalis. Table S4: observations of floral visitors to Faboideae. Table S5: insects caught on Diuris brumalis and co-occurring Faboideae. Table S6: Floral traits of Diuris brumalis and the 20 most abundant co-flowering species. Table S7: populations and reproductive data of Diuris brumalis. Table S8: composition of pollen loads. Table S9: means and standard deviation of colour loci of Diuris brumalis and pea plants. Appendix S1: floral biology of Diuris brumalis and co-occurring Faboideae. Video: Trichocolletes behaviour on Daviesia decurrens (model) and Diuris brumalis (mimic). Key behaviours illustrated: ‘patrolling’, courtship behaviour by males looking for females, keel (model) or labellum (mimic) ‘manipulation’, ‘foraging’ behaviour by females, including searching for sources without landing. Video is presented in slow motion.
ACKNOWLEDGEMENTS
We thank Andrea Aromatisi for extensive assistance in the field and video making; Terry Houston at the Western Australian Museum for identification of insects and advice on the ecology of pollinator species; Adam Cross for assistance with editing; Andrew Brown for suggestions for field sites; Nicola Tommasi for assistance with artwork; Massimo Labra for facilitating the link with University of Milano-Bicocca research staff; Karl Duffy and Myles Menz for advice on statistical analysis; and two anonymous reviewers for suggestions that improved the final manuscript. This work was supported by Endeavour Fellowship Program [ID 5117_2016], Australian Orchid Foundation [308.16], Curtin University [CIPRS, CSIRS_2017] and Università degli Studi di Napoli Federico II [Short mobility program D.M. 976_2017].
LITERATURE CITED
- Ackerman JD. 1986. Coping with the epiphytic existence: pollination strategies. Selbyana 9: 52–60. [Google Scholar]
- Adams PB, Lawson SD. 1993. Pollination in australian orchids - a critical-assessment of the literature 1882–1992. Australian Journal of Botany 41: 553–575. [Google Scholar]
- Anderson B, Johnson SD. 2006. The effects of floral mimics and models on each others’ fitness. Proceedings of the Royal Society B: Biological Sciences 273: 969–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbero F, Bonelli S, Thomas JA, Balletto E, Schönrogge K. 2009. Acoustical mimicry in a predatory social parasite of ants. The Journal of Experimental Biology 212: 4084–4090. [DOI] [PubMed] [Google Scholar]
- Barrows EM. 1976. Mating behavior in halictine bees (Hymenoptera: Halictidae): patrolling and age-specific behavior in males. Journal of the Kansas Entomological Society 49: 105–119. [Google Scholar]
- Batley M, Houston TF. 2012. Revision of the Australian bee genus Trichocolletes Cockerell (Hymenoptera: Colletidae: Paracolletini). Records of the Australian Museum 64: 1–50. [Google Scholar]
- Beardsell DV, Clements MA, Hutchinson JF, Williams EG. 1986. Pollination of Diuris maculata R. Br. (Orchidaceae) by floral mimicry of the native legumes Daviesia spp. and Pultenaea scabra R. Br. Australian Journal of Botany 34: 165–173. [Google Scholar]
- Benitez-Vieyra S, de Ibarra NH, Wertlen AM, Cocucci AA. 2007. How to look like a mallow: evidence of floral mimicry between Turneraceae and Malvaceae. Proceedings of the Royal Society B: Biological Sciences 274: 2239–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohman B, Karton A, Flematti GR, Scaffidi A, Peakall R. 2018. Structure-activity studies of semiochemicals from the spider orchid Caladenia plicata for sexual deception. Journal of Chemical Ecology 44: 1–8. [DOI] [PubMed] [Google Scholar]
- Brodmann J, Twele R, Francke W, Yi-bo L, Xi-qiang S, Ayasse M. 2009. Orchid mimics honey bee alarm pheromone in order to attract hornets for pollination. Current Biology 19: 1368–1372. [DOI] [PubMed] [Google Scholar]
- Brown A, Dixon K, French C, Brockman G. 2013. Field Guide to the Orchids of Western Australia. Freemantle: Simon Nevill Publications. [Google Scholar]
- Brown JH, Brown AK. 1979. Convergence, competition, and mimicry in a temperate community of hummingbird-pollinated flowers. Ecology 60: 1022–1035. [Google Scholar]
- Çakmak I, Wells H. 1995. Honey bee forager indiviudual constancy: innate or learned?Bee Science 3: 165–173. [Google Scholar]
- Chittka L. 1992. The colour hexagon: a chromaticity diagram based on photoreceptor excitations as a generalized representation of colour opponency. Journal of Comparative Physiology A 170: 533–543. [Google Scholar]
- Chittka L, Kevan PG. 2005. Flower colour as advertisement. In: Dafni A, Kevan PG, Husband BC, eds. Pratical Pollination Biology. Cambridge: Enviroquest Ltd, 157–196. [Google Scholar]
- Coleman E. 1928. Pollination of Cryptostylis leptochila. Victorian Naturalist 44: 333–340. [Google Scholar]
- Dafni A. 1984. Mimicry and deception in pollination. Annual Review of Ecology and Systematics 15: 259–278. [Google Scholar]
- Dafni A, Bernhardt P. 1990. Pollination of terrestrial orchids of southern Australia and the Mediterranean region. Evolutionary Biology 24: 193–252. [Google Scholar]
- Dafni A, Ivri Y, Brantjes NBM. 1981. Pollination of Serapias vomeracea Briq. (Orchidaceae) by imitation of holes for sleeping solitary male bees (Hymenoptera). Acta Botanica Neerlandica 30: 69–73. [Google Scholar]
- Dalziell AH, Welbergen JA. 2016. Mimicry for all modalities. Ecology Letters 19: 609–619. [DOI] [PubMed] [Google Scholar]
- de Jager ML, Peakall R. 2018. Experimental examination of pollinator-mediated selection in a sexually deceptive orchid. Annals of Botany in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jager M, Newman E, Theron G, Botha P, Barton M, Anderson B. 2016. Pollinators can prefer rewarding models to mimics: consequences for the assumptions of Batesian floral mimicry. Plant Systematics and Evolution 302: 409–418. [Google Scholar]
- Dixon KW, Buirchell BJ, Collins MT. 1989. Orchids of Western Australia: Cultivation and Natural History, 2nd edn. Victoria Park: Western Australian Native Orchid Study and Conservation Group. [Google Scholar]
- Dyer AG, Chittka L. 2004a Biological significance of distinguishing between similar colours in spectrally variable illumination: bumblebees (Bombus terrestris) as a case study. Journal of Comparative Physiology A 190: 105–114. [DOI] [PubMed] [Google Scholar]
- Dyer AG, Chittka L. 2004b Bumblebees (Bombus terrestris) sacrifice foraging speed to solve difficult colour discrimination tasks. Journal of Comparative Physiology A 190: 759–763. [DOI] [PubMed] [Google Scholar]
- Elliott CP, Ladd PG. 2002. Pollen limitation of fruit set in Western Australian terrestrial orchids. Journal of the Royal Society of Western Australia 85: 165–168. [Google Scholar]
- Endler JA. 1981. An overview of the relationships between mimicry and crypsis. Biological Journal of the Linnean Society 16: 25–31. [Google Scholar]
- Erdtman G. 1960. The acetholysis method: a revised description. Svensk Botanisk Tidskrift 54: 341–350. [Google Scholar]
- Galizia CG, Kunze J, Gumbert A, et al. 2005. Relationship of visual and olfactory signal parameters in a food-deceptive flower mimicry system. Behavioral Ecology 16: 159–168. [Google Scholar]
- Gaskett AC, Endler JA, Phillips RD. 2016. Convergent evolution of sexual deception via chromatic and achromatic contrast rather than colour mimicry. Evolutionary Ecology 31: 205–227. [Google Scholar]
- Giurfa M. 2004. Conditioning procedure and color discrimination in the honeybee Apis mellifera. Naturwissenschaften 91: 228–231. [DOI] [PubMed] [Google Scholar]
- Haas A. 1960. Vergleichende Verhaltensstudien zum Paarungsschwarm solitärer Apiden. Zeitschrift für Tierpsychologie 17: 402–416. [PubMed] [Google Scholar]
- Hoffman N, Brown A. 2011. Orchids of south-west Australia, 3rd edn. Gooseberry Hill: Noel Hoffman. [Google Scholar]
- Indsto JO, Weston PH, Clements MA, Dyer AG, Batley M, Whelan RJ. 2006. Pollination of Diuris maculata (Orchidaceae) by male Trichocolletes venustus bees. Australian Journal of Botany 54: 669–679. [Google Scholar]
- Indsto JO, Weston PH, Clements MA. 2009. A molecular phylogenetic analysis of Diuris (Orchidaceae) based on AFLP and ITS reveals three major clades and a basal species. Australian Systematic Botany 22: 1–15. [Google Scholar]
- Internicola AI, Harder LD. 2012. Bumble-bee learning selects for both early and long flowering in food-deceptive plants. Proceedings of the Royal Society of London B: Biological Sciences 279: 1538–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jersáková J, Johnson SD, Kindlmann P. 2006. Mechanisms and evolution of deceptive pollination in orchids. Biological Reviews 81: 219–235. [DOI] [PubMed] [Google Scholar]
- Jersáková J, Jürgens A, Šmilauer P, Johnson SD, Johnson M. 2012. The evolution of floral mimicry: identifying traits that visually attract pollinators. Functional Ecology 26: 1381–1389. [Google Scholar]
- Jersáková J, Spaethe J, Streinzer M, Neumayer J, Paulus H, Dotter S, Johnson SD. 2016. Does Traunsteinera globosa (the globe orchid) dupe its pollinators through generalized food deception or mimicry? Botanical Journal of the Linnean Society 180: 269–294.
- Johnson SD. 2000. Batesian mimicry in the non-rewarding orchid Disa pulchra, and its consequences for pollinator behaviour. Biological Journal of the Linnean Society 71: 119–132. [Google Scholar]
- Johnson SD. 2010. The pollination niche and its role in the diversification and maintenance of the Southern African Flora. Philosophical Transactions: Biological Sciences 365: 499–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SD, Schiestl FP. 2016. Floral Mimicry, 1st edn. Oxford: Oxford University Press. [Google Scholar]
- Johnson SD, Alexandersson R, Linder HP. 2003. Experimental and phylogenetic evidence for floral mimicry in a guild of fly-pollinated plants. Biological Journal of the Linnean Society 80: 289–304. [Google Scholar]
- Jolles DJ. 2015. Morphometric analysis of floral variation in the Pyrola picta species complex (Ericaceae): interpretation and implications for ecological and phylogenetic differentiation. Botanical Journal of the Linnean Society 177: 462–480. [Google Scholar]
- Jones DL. 2006. A Complete Guide to Native Orchids of Australia, Including the Island Territories, 2nd edn. Frenchs Forest: Reed New Holland. [Google Scholar]
- Juillet N, Gonzalez MA, Page PA, Gigord LDB. 2007. Pollination of the European food-deceptive Traunsteinera globosa (Orchidaceae): the importance of nectar-producing neighbouring plants. Plant Systematics and Evolution 265: 123–129. [Google Scholar]
- Karron JD, Mitchell RJ, Holmquist KG, Bell JM, Funk B. 2004. The influence of floral display size on selfing rates in Mimulus ringens. Heredity 92: 242–248. [DOI] [PubMed] [Google Scholar]
- Kunze J, Gumbert A. 2001. The combined effect of color and odor on flower choice behavior of bumble bees in flower mimicry systems. Behavioral Ecology 12: 447–456. [Google Scholar]
- Lunau K, Maier EJ. 1995. Innate colour preferences of flower visitors. Journal of Comparative Physiology A 177: 1–19. [Google Scholar]
- Manning JC, Goldblatt P. 1996. The Prosoeca peringueyi (Diptera: Nemestrinidae) pollination guild in Southern Africa: long-tongued flies and their tubular flowers. Annals of the Missouri Botanical Garden 83: 67–86. [Google Scholar]
- Marchant NG, Wheeler JR, Rye BL, Bennett EM, Lander NS, Macfarlane TD. 1987. Flora of the Perth region. Perth: Western Australian Herbarium Department of Agriculture. [Google Scholar]
- Marshall J. 1995. Wildflowers of the West Coast Hills Region. North Perth: Wildflower Society of Western Australia. [Google Scholar]
- Martos F, Cariou ML, Pailler T, Fournel J, Bytebier B, Johnson SD. 2015. Chemical and morphological filters in a specialized floral mimicry system. New Phytologist 207: 225–234. [DOI] [PubMed] [Google Scholar]
- Nilsson LA. 1983. Anthecology of Orchis mascula (Orchidaceae). Nordic Journal of Botany 3: 157–179. [Google Scholar]
- Norman MD, Finn J, Tregenza T. 2001. Dynamic mimicry in an Indo-Malayan octopus. Proceedings of the Royal Society of London B: Biological Sciences 268: 1755–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton RJ. 2005. Male mating behaviour and mating systems of bees: an overview. Apidologie 36: 145–156. [Google Scholar]
- Peakall R, Ebert D, Poldy J, et al. 2010. Pollinator specificity, floral odour chemistry and the phylogeny of Australian sexually deceptive Chiloglottis orchids: implications for pollinator‐driven speciation. New Phytologist 188: 437–450. [DOI] [PubMed] [Google Scholar]
- Pellissier L, Vittoz P, Internicola AI, Gigord LDB. 2010. Generalized food-deceptive orchid species flower earlier and occur at lower altitudes than rewarding ones. Journal of Plant Ecology 3: 243–250. [Google Scholar]
- Peter CI, Johnson SD. 2008. Mimics and magnets: the importance of color and ecological facilitation in floral deception. Ecology 89: 1583–1595. [DOI] [PubMed] [Google Scholar]
- Peter GLK, de Jong TJ. 1990. Effects of plant size, plant density and sex differential nectar reward on pollinator visitation in the protandrous Echium vulgare (Boraginaceae). Oikos 57: 399–405. [Google Scholar]
- Phillips RD, Faast R, Bower CC, Brown GR, Peakall R. 2009. Implications of pollination by food and sexual deception for pollinator specificity, fruit set, population genetics and conservation of Caladenia (Orchidaceae). Australian Journal of Botany 57: 287–306. [Google Scholar]
- Rakosy D, Cuervo M, Paulus HF, Ayasse M. 2017. Looks matter: changes in flower form affect pollination effectiveness in a sexually deceptive orchid. Journal of Evolutionary Biology 30: 1978–1993. [DOI] [PubMed] [Google Scholar]
- Roy BA, Widmer A. 1999. Floral mimicry: a fascinating yet poorly understood phenomenon. Trends in Plant Science 4: 325–330. [DOI] [PubMed] [Google Scholar]
- Ruxton GD, Sherratt TN, Speed MP. 2004. Avoiding Attack: The Evolutionary Ecology of Crypsis, Warning Signals and Mimicry, 1st edn. Oxford: Oxford University Press. [Google Scholar]
- Schiestl FP, Johnson SD. 2013. Pollinator-mediated evolution of floral signals. Trends in Ecology and Evolution 28: 307–315. [DOI] [PubMed] [Google Scholar]
- Schiestl FP, Ayasse M, Paulus HF, et al. 1999. Orchid pollination by sexual swindle. Nature 399: 421. [Google Scholar]
- Schiestl FP, Peakall R, Mant JG, et al. 2003. The chemistry of sexual deception in an orchid-wasp pollination system. Science 302: 437–438. [DOI] [PubMed] [Google Scholar]
- Skelhorn J, Ruxton GD. 2010. Predators are less likely to misclassify masquerading prey when their models are present. Biology Letters 6: 597–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard PK. 1999. Predation enhances complexity in the evolution of electric fish signals. Nature 400: 254. [DOI] [PubMed] [Google Scholar]
- Van der Cingel NA. 1995. An Atlas of Orchid Pollination: European Orchids. Rotterdam: A. A. Balkema. [Google Scholar]
- Van der Niet T, Hansen DM, Johnson SD. 2011. Carrion mimicry in a South African orchid: flowers attract a narrow subset of the fly assemblage on animal carcasses. Annals of Botany 107: 981–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane-Wright RI. 1980. On the definition of mimicry. Biological Journal of the Linnean Society 13: 1–6. [Google Scholar]
- Zuur AF, Hilbe JM, Ieno EN. 2013. Beginner Guide to GLM and GLMM with R: a Frequentist and Bayesian Perspective for Ecologists. Newburgh: Highland Statistics Limited. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.