Abstract
This study aims to investigate the prospective associations between fruit and vegetable (FV) intakes and their polyphenol content with subsequent sleep duration in UK women. In this study, 13,958 women with ~4 years of follow-up in the UK Women’s Cohort Study were included in the analyses. FV intakes were assessed at baseline using a food frequency questionnaire (FFQ), and average hours of sleep per day were self-reported in follow-up. Polyphenol intake was calculated by matching FV items from the FFQ with the Phenol-Explorer database. Linear regression models, adjusting for confounders, were used for the analyses. Consuming an additional portion of apples, kiwi, oranges, pineapple, and 100% pure juice were associated with shorter sleep. Similarly, an additional portion of cabbage, celery, aubergine, olives, and peppers were inversely associated with sleep duration. An additional gram of total polyphenols was associated with shorter sleep by 18 min (99% CI −31 to −4, p < 0.001). FV consumption and total polyphenol content were inversely associated with sleep duration; however, effect sizes were small, and polyphenol classes from FV intakes were not associated with sleep duration. Future intervention studies considering the time of FV consumption in relation to sleep are needed to clarify the underlying mechanisms.
Keywords: sleep, fruits and vegetables, polyphenols
1. Introduction
Epidemiological studies have shown that short sleep duration is associated with hypertension [1], type 2 diabetes [2], cardiovascular disease [3], all-cause mortality [4,5], and a 45% increased risk of obesity compared to normal sleep duration [6,7]. These associations, in part, may be mediated through changes in dietary intake including fruit and vegetables (FV) that influence bodyweight and chronic disease risk [8,9]. However, the directionality of these associations are not well established; a bi-directional relationship has been suggested [10,11]. Recently, St-onge et al. suggested that plant-based diets could have potential benefits for reducing cardiovascular risk by improving sleep [12]. These benefits may be facilitated by dietary polyphenols that have been shown to modulate the circadian rhythms [13] and sleep-wake cycles [14] in rodents. In humans, consuming 2 kiwi fruits an hour before bedtime improved sleep onset, duration, and efficiency in 24 healthy adults during a 4-week open clinical trial [15]. Furthermore, a double-blinded pilot study showed that fresh tart cherry juice reduced insomnia in 15 elderly subjects [16]. The effects of cherries were also observed to increase sleep duration and reduce the number of awakenings, as measured by actigraphy in 12 Spanish participants [17]. These clinical evidence studies suggest sleep-promoting effects of certain fruits; however, they were conducted on small study groups. The limited number of longitudinal epidemiological studies in this area suggests the need for cohort studies with validated dietary intake measures to clarify this association. Therefore, the aim of this study was to explore the prospective associations between specific FV intakes and polyphenol content of FV and sleep duration in a large cohort of UK women. To our knowledge, we are the first to report on prospective associations between specific FV intakes and their polyphenol content with sleep duration using a large cohort. We hypothesized that FV intakes and their polyphenol contents are associated with longer sleep durations.
2. Materials and Methods
2.1. Participants
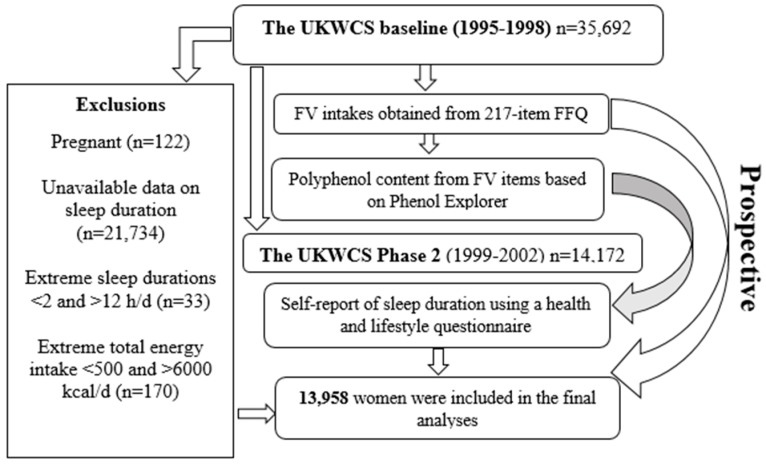
The UK Women’s Cohort study (UKWCS) is a large prospective cohort that was established to explore links between diet and chronic diseases. Participants were taken from responders to the World Cancer Research Fund’s direct mail survey including those living in England, Wales, Scotland, and Northern Ireland. Ethical approval was granted at its initiation in 1993 (Research Ethics Committee reference number is 15/YH/0027). The National Research Ethics Committee for Yorkshire and the Humber, Leeds East has now taken on responsibility for the ongoing cohort. [18]. Baseline data collection was between 1995 and 1998 (Figure 1) using a postal food frequency questionnaire (FFQ). Follow-up data (Phase 2) was collected between 1999 and 2002 around 4 years later, and 14,172 women (40% of baseline) completed a follow-up health and life style questionnaire including sleep, and 12,453 women also completed a 4-day food diary and a 1-day activity diary.
2.2. Baseline Characteristics
Age, height, weight, medical history, and smoking habits were self-reported. Physical activity was recorded using a binary question in the FFQ which questioned if participants spent time on activities vigorous enough to cause sweating or a faster heartbeat, which indicated moderate physical activity (MET-hours/day). Supplement usage was identified by asking whether participants took any vitamins, minerals, fish oils, fibre, or other food supplements. Participants also self-reported their status regarding vegetarian and vegan diets. Classification of socio-economic status (SES) was undertaken based on occupation, according to the United Kingdom National Statistics-Socio-Economic Classification (NS-SEC), where women are divided into three categories (managerial/professional, intermediate, or routine/manual) [19]. Socio-demographic information such as marital status was determined by self-report questions asking for marital status (married or living as married, divorced, single, widowed, or separated).
2.3. Fruit and Vegetable Intakes
Diet was assessed at baseline using a detailed 217-item FFQ developed from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Oxford cohort [20]. The FFQ was validated on a subsample of 303 cohort subjects against a 4-day food diary, as well as fasting blood measures of specific nutrients [18,21,22]. The FFQ were sent to 61,000 participants who had previously responded to a direct mail survey from the World Cancer Research Fund, and a total of 35,692 women completed the FFQ. Participants were asked to choose their frequency of consumption for each food listed in the FFQ by answering the question “how often have you eaten these foods in the last 12 months?” using one of ten response categories; never, less than once a month, 1–3 per month, once a week, 2–4 per week, 5–6 per week, once per day, 2–3 per day, 4–5 per day, and 6+ per day. These were consequently converted to weight of each food consumed per day based on the Food Standards Agency portion sizes book [23]. For the current study, FV food items were used individually, and also their polyphenol contents were calculated from Phenol Explorer [24]. FV consumption was expressed as grams per day (g/day); however, in order to have a better estimate, results were presented per portion size unless stated otherwise, and non-response to FV questions were taken as missing data.
2.4. Estimation of Polyphenol Intake from Fruits and Vegetables
The polyphenol intake was calculated by matching FV intakes from the FFQ and the recently developed Phenol Explorer database (www.phenol-explorer.eu) [24] that contains data on the content of 500 polyphenols in over 400 foods. The individual polyphenol intake from each item of FV was calculated by multiplying the content of each polyphenol by the daily consumption of each FV item. Polyphenol classes included flavonoids, phenolic acids, stilbenes, lignans, and other polyphenols (see Appendix A, Table A1, for polyphenol sub-classes). The total polyphenol intake was calculated as the sum of all polyphenol classes intake from all FV reported by the FFQ. This estimation method has been previously used in other studies [25,26,27]. In the Phenol-Explorer database, polyphenol intake was calculated using High performance liquid chromatography (HPLC); however, in the case of lignans and phenolic acids in some FV items (e.g., olives), polyphenol content was calculated using HPLC after hydrolysis, because these treatments are needed to release phenolic compounds that otherwise cannot be analysed [25]. Polyphenol contents from FV items that were analysed using HPLC after hydrolysis are indicated in the tables.
2.5. Sleep Duration
Participants were asked about sleep duration using the lifestyle questionnaire in phase 2 in two separate questions in the following form (Figure S1);
“On an average weekday, how is your day spent?”
“On an average weekend, how is your day spent?”
Participants were asked to record number of hours and/or minutes that were spent sleeping in an average weekday and weekend. Two separate variables were generated for sleep duration based on weekdays and weekends for all women. Average sleep duration was calculated using the following equation [9]
| ((minutes slept during the week × 5) + (minutes slept during weekends × 2))/7 | (1) |
2.6. Statistical Analyses
Descriptive statistics, such as means and proportions, described women from the UKWCS according to FV quintiles. We have previously reported from cross-sectional data that sleep duration and FV intakes are non-linearly associated [9]. In order to use linear regression models, we assessed the relationship between FV intakes and sleep duration using locally weighted scatterplot smoothing (LOWESS) [28], which is a tool that creates a smooth line through a scatterplot to help see the trend between variables. The association between total FV intakes (grams/day) and sleep duration (hours/day) showed a linear association (Figure S2). Normal distribution of the outcome variable (sleep duration) was checked using histogram plot (Figure S3). Linear regression models were used to assess the prospective association between FV intakes (continuous exposure in grams/day) or their polyphenols (continuous exposure) and sleep duration (continuous outcome in minutes/day). Unadjusted and adjusted models were used where potential confounders were identified based on previous studies [9]. Confounders were age, SES (professional and managerial, intermediate, routine and manual), smoking (yes, no), ethnicity (white, Bangladeshi, Indian, Chinese, Pakistani, black-Caribbean, black-other, other) and total energy intake. Models that investigated polyphenol intake from FV and sleep duration were further adjusted for other polyphenol content; for example, flavonoids were adjusted for phenolic acids, stilbenes, lignans, and other polyphenols. Total polyphenol intake in association with sleep duration was adjusted for the confounders mentioned above without further adjustment of other polyphenol content. There is not sufficient experimental evidence that body mass index (BMI) and physical activity independently influence fruit and vegetable consumption and their polyphenols to include as an adjustment in the main analyses; however, there is evidence of BMI influencing sleep duration. We adjusted for BMI and physical activity but it did not meaningfully change the results; therefore, the results are shown without adjusting for these covariates. The final number (n column in Tables 2–4 and in Appendix A) of women included in the analyses indicates complete data of all covariates included in the model, and difference in (n) is due to missing data of any of the included covariates in the model.
Sensitivity analyses were conducted on polyphenol content from FV intakes since they are unstable compounds and can be affected by alcohol intake, supplement use, and medications [29]. Sensitivity analyses included stratifications of certain variables selected prior to analyses between polyphenol content from FV and sleep duration. Variables explored were alcohol consumption frequency (more than once a week, once a week or less, never), BMI (obese vs. non-obese), and dietary habits (vegetarian/vegan vs. non-vegetarian/vegan). Separate analyses were conducted after excluding (1) supplement users, (2) those who self-reported currently having a longstanding illness (see Appendix A Table A5 for excluded illnesses), and (3) those who self-reported taking prescribed medicines. Further sensitivity analyses included considering weekdays and weekends sleep duration separately, since sleep and dietary habits differ between weekdays and weekends (controlled for confounders stated above in the adjusted model). Further adjustment for BMI and physical activity (MET/hours) in the adjusted model stated above was conducted. To take account of multiple testing, significance level was determined by a p value of <0.01 to reflect 99% confidence intervals in main and sensitivity analyses. All analyses were conducted using Stata V. 15.1 statistical software (StataCorp LLC, College Station, TX, USA) [30].
3. Results
3.1. Socio-Demographic Characteristics
Cohort participants who had unavailable data on sleep duration, extreme sleep duration <2 and >12 h/day, pregnant women, and those with extreme total energy intake <500 and >6000 were excluded, and a total of 13,958 women were included in the analyses (Figure 1). Baseline characteristics of the participants based on FV quintiles are shown in Table 1. The mean age was 52 years (95% CI 52 to 53), and mean BMI was 24.5 (95% CI 24.4 to 24.5). Women had on average 7.5 h/day of sleep (95% CI 7.5 to 7.6) and 24 min/day (95% CI 23 to 25) of physical activity. In total, 32% (95% CI 31 to 32) of women were vegetarian or vegan, 98% (95% CI 98 to 99) were white, and 60% (95% CI 59 to 61) reported supplement usage.
Figure 1.
Participants’ flow chart. d (day), FFQ (food frequency questionnaire), FV (fruits and vegetables), h (hours), UKWCS (The UK Women’s Cohort Study).
Table 1.
Baseline characteristics by quintile of total FV intakes from the UKWCS.
| Characteristics | 0–348 | 348–486 | 486–624 | 624–817 | 817–1597 | Total |
|---|---|---|---|---|---|---|
| Number of Women (n) | 2364 | 2710 | 2873 | 2996 | 3015 | 13,958 |
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | |
| Age (years) | 51 (50, 51) | 52 (51, 52) | 52 (52, 52) | 52 (52, 52) | 53 (52, 53) | 52 (52, 53) |
| BMI (kg/m2) | 24.4 (24.2, 24.5) | 24.1(24.0, 24.3) | 24.2 (24.0, 24.3) | 24.0 (23.9, 24.2) | 24.1 (23.9, 24.2) | 24.5(24.4, 24.5) |
| Total energy intake (kcal/day) | 2036 (2004, 2069) | 2115 (2092, 2138) | 2272 (2249, 2295) | 2422 (2398, 2446) | 2698 (2672, 2724) | 2307 (2299, 2314) |
| Sleep duration * | 7.5 (7.5, 7.6) | 7.5 (7.5, 7.6) | 7.5 (7.5, 7.5) | 7.5 (7.5, 7.6) | 7.4 (7.4, 7.5) | 7.5 (7.5, 7.6) |
| Weekday sleep duration * | 7.5 (7.4, 7.5) | 7.5 (7.5, 7.5) | 7.5 (7.4, 7.5) | 7.5 (7.4, 7.5) | 7.4 (7.4, 7.4) | 7.5 (7.4, 7.5) |
| Weekend sleep duration * | 7.9 (7.9, 8.0) | 7.9 (7.9, 7.9) | 7.9 (7.8, 7.9) | 7.9 (7.8, 7.9) | 7.7 (7.7, 7.8) | 7.9 (7.8, 7.9) |
| Physical activity (minutes/day) | 27 (24, 29) | 21 (20, 22) | 23 (21, 24) | 23 (22, 24) | 28 (26, 29) | 24 (23, 25) |
| % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | |
| Has longstanding illness (yes) | 25 (23, 27) | 24 (23, 26) | 26 (24, 28) | 27 (25, 28) | 28 (26, 29) | 26 (25, 27) |
| Prescribed medicine (yes) | 31.2 (29, 33) | 32.1 (30, 33) | 30.6 (28, 32) | 31.8 (30, 33) | 31.8 (30, 33) | 31.5 (30, 32) |
| Smoking (yes) | 13 (12, 14) | 9 (8, 10) | 8 (7, 9) | 6 (5, 7) | 6 (5, 7) | 8 (8, 9) |
| Supplement use (yes) | 55 (53, 57) | 56 (54, 57) | 59 (56, 60) | 62 (60, 63) | 67 (65, 69) | 60 (59, 61) |
| Vegetarian or vegan (yes) | 24 (22, 26) | 27 (25, 28) | 30 (28, 31) | 34 (33, 36) | 42 (40, 43) | 32 (31, 32) |
| Ethnicity (white) | 98 (98, 99) | 99 (98, 99) | 99 (98, 99) | 98 (98, 99) | 98 (97, 98) | 98 (98, 99) |
| Employer (employed) | 62 (60, 63) | 60 (58, 62) | 59 (57, 61) | 58 (56, 60) | 56 (54, 57) | 59 (58, 60) |
| SES (professional) | 61 (59, 63) | 63 (61, 65) | 67 (65, 69) | 67 (65, 68) | 70 (68, 71) | 66 (65, 66) |
| Marital status (married) | 73 (71, 75) | 75 (74, 73) | 77 (75, 78) | 77(75, 78) | 77 (75, 78) | 76 (75, 77) |
| Number of children (2 children) | 51 (49, 54) | 52 (50, 54) | 51 (49, 53) | 48 (46, 51) | 49 (47, 51) | 50 (49, 51) |
* Hours/day, BMI (body mass index), CI (confidence interval), FV (fruit and vegetables), mg (milligram), µg (microgram), m (metre), SES (socio-economic status), UKWCS (The UK Women’s Cohort Study).
3.2. Prospective Associations between Individual FV Items and Sleep Duration
In adjusted models (Table 2), consuming an additional portion of the following fruits were associated with shorter sleep: apples 2 min/day (99% CI −4 to −1, p < 0.001), kiwi 10 min/day (99% CI −19 to −2, p = 0.001), oranges 3 min/day (99% CI −5 to 0.8, p < 0.001), pineapple 17 min/day (99% CI −33 to −1, p = 0.006), and 100% pure juice 3 min/day (99% CI −3 to −0.07, p = 0.001). Similarly, consuming an additional portion of the following vegetables was associated with shorter sleep: cabbage 6 min/day (99% CI −12 to −0.4, p = 0.006), celery 12 min/day (99% CI −23 to −0.5, p = 0.007), aubergine 21 min/day (99%CI −41 to −1, p = 0.007), olives 37 min/day (99% CI −71 to −3, p = 0.004), and peppers 13 min/day (99% CI −23 to −3, p = 0.001) (Table 2).
Table 2.
The prospective associations between FV intakes and sleep duration of women from the UKWCS.
| Sleep Duration (minutes/day) | ||||||
|---|---|---|---|---|---|---|
| Models | Unadjusted | Adjusted * | ||||
| Fruit | Coefficient per Additional 80 g/day (99% CI) | p Value | n | Coefficient per Additional 80 g/day (99% CI) | p Value | n |
| Apples | −2 (−4, −1) | <0.001 | 13,530 | −2 (−4, −1) | <0.001 | 12,862 |
| Avocados | −8 (−22, 4) | 0.1 | 8468 | −8 (−23, 5) | 0.1 | 8033 |
| Bananas | −0.4 (−2, 1) | 0.5 | 13,092 | −0.6 (−2, 1) | 0.4 | 12,437 |
| Grapes | −1 (−4, 0.5) | 0.05 | 13,312 | −1 (−3, 1) | 0.2 | 12,664 |
| Kiwi | −10 (−19, −2) | 0.001 | 11,709 | −10 (−19, −2) | 0.001 | 11,147 |
| Mangoes | −6 (−16, 4) | 0.1 | 7293 | −6 (−17, 4) | 0.1 | 6930 |
| Oranges, satsumas, grapefruit | −2 (−4, 0.4) | 0.002 | 12,967 | −3 (−5, −0.8) | <0.001 | 12,330 |
| Papaya | −4 (−18, 9) | 0.4 | 3646 | −5 (−20, 9) | 0.3 | 3445 |
| Pears | −0.3 (−3, 2) | 0.7 | 12,177 | −0.1 (−3, 2) | 0.8 | 11,576 |
| Pineapple | −20 (−35, −4) | 0.001 | 11,810 | −17 (−33, −1) | 0.006 | 11,243 |
| Apricots | −44 (−89, 1) | 0.01 | 11,010 | −40 (−88, 6) | 0.02 | 10,456 |
| Melon | −1 (−4, 0.8) | 0.07 | 13,110 | −1 (−4, 1) | 0.2 | 12,478 |
| Nectarines | −3 (−26, 19) | 0.6 | 12,678 | −8 (−32, 14) | 0.3 | 12,062 |
| Peaches | −32 (−71, 6) | 0.03 | 12,945 | −32 (−73, 8) | 0.04 | 12,315 |
| Plums | −29 (−74, 16) | 0.09 | 12,839 | −31 (−77, 15) | 0.08 | 12,217 |
| Raspberries | −4 (−14, 6) | 0.2 | 12,643 | −2 (−13, 7) | 0.4 | 12,040 |
| Redcurrants, blackcurrants | −4 (−12, 2) | 0.09 | 10,426 | −4 (−11, 3) | 0.1 | 9910 |
| Rhubarb | 1 (−2, 4) | 0.3 | 11,359 | 1 (−2, 4) | 0.3 | 10,829 |
| Strawberries | −12 (−31, 6) | 0.09 | 13,321 | −11 (−31, 8) | 0.1 | 12,673 |
| Orange juice-pure fruit per 125 g/day | −1 (−3, 0.5) | 0.06 | 12,224 | −1 (−3, 0.4) | 0.04 | 11,642 |
| 100% pure fruit juice per 125 g/day | −3 (−6, −0.08) | 0.001 | 10,345 | −3 (−6, −0.07) | 0.001 | 9834 |
| Dried fruit per 25 g/day | ||||||
| Dates | −2 (−8, 3) | 0.2 | 9577 | −3 (−9, 3) | 0.1 | 9117 |
| Figs | −0.008 (−3, 3) | 0.9 | 7231 | −0.2 (−3, 3) | 0.8 | 6860 |
| Prunes | −4 (−11, 2) | 0.09 | 9205 | −4 (−11, 2) | 0.1 | 8739 |
| Mixed dried fruit | −0.9 (−6, 4) | 0.6 | 8972 | −0.8 (−6, 4) | 0.6 | 8531 |
| Currants, raisins, sultanas | 0.4 (−6, 7) | 0.8 | 12,605 | 0.8 (−6, 8) | 0.7 | 12,003 |
| Vegetables | ||||||
| Beetroot | −16 (−35, 1) | 0.01 | 11,516 | −12 (−31, 7) | 0.1 | 10,951 |
| Broccoli, spring greens, kale | −2 (−7, 1) | 0.1 | 13,488 | −1 (−6, 3) | 0.5 | 12,822 |
| Brussels | −3 (−9, 2) | 0.1 | 12,783 | −0.6 (−7, 6) | 0.8 | 12,153 |
| Cabbage | −8 (−13, −3) | <0.001 | 13,051 | −6 (−12, −0.4) | 0.006 | 12,425 |
| Carrots | −2 (−7, 2) | 0.1 | 13,713 | −1 (−6, 3) | 0.5 | 13,037 |
| Cauliflower | −0.04 (−0.1, 0.01) | 0.06 | 13,503 | −0.03 (−0.1, 0.03) | 0.2 | 12,842 |
| Celery | −14 (−25, −3) | 0.001 | 12,483 | −12 (−23, −0.5) | 0.007 | 11,865 |
| Coleslaw | −6 (−27, 15) | 0.4 | 10,018 | −4 (−27, 17) | 0.5 | 9546 |
| Courgettes, marrow, squash | −6 (−14, 2) | 0.05 | 11,697 | −7 (−16, 1) | 0.03 | 11,134 |
| Cucumber | −17 (−32, −1) | 0.005 | 12,911 | −15 (−32, 0.8) | 0.01 | 12,293 |
| Green and runner beans | −6 (−11, −1) | 0.001 | 13,344 | −5 (−10, 0.6) | 0.02 | 12,688 |
| Lettuce | −15 (−31, −0.1) | 0.009 | 13,550 | −14 (−30, 2) | 0.02 | 12,885 |
| Aubergine, okra | −25 (−44, −6) | <0.001 | 8258 | −21 (−41, −1) | 0.007 | 7853 |
| Olives | −36 (−69, −3) | 0.005 | 6461 | −37 (−71, −3) | 0.004 | 6129 |
| Parsnips | −0.7 (−12, 10) | 0.8 | 11,704 | 1 (−19, 13)) | 0.6 | 11,137 |
| Peas, mushy peas, mange tout | −5 (−15, 3) | 0.1 | 13,132 | −5 (−15, 4) | 0.1 | 12,510 |
| Peppers | −10 (−20, −1) | 0.004 | 12,371 | −13 (−23, −3) | 0.001 | 11,780 |
| Swedes | −0.07 (−11, 11) | 0.9 | 11,052 | 1 (−10, 13) | 0.7 | 10,512 |
| Tomatoes-raw, canned, sauce | −2 (−5, 0.1) | 0.01 | 13,526 | −2 (−5, 0.1) | 0.01 | 12,872 |
| Turnip | −5 (−20, 9) | 0.3 | 8998 | −5 (−21, 10) | 0.3 | 8568 |
| Mustard, cress, watercress | −21 (−73, 31) | 0.3 | 11,283 | −21 (−75, 32) | 0.3 | 10,736 |
| Boiled/mashed potatoes | 0.3 (−1, 1) | 0.5 | 13,330 | 0.9 (−0.6, 2) | 0.1 | 12,692 |
* Adjusted for age, socio-economic status, smoking, ethnicity, and total energy intake.
3.3. Prospective Associations between Total Polyphenol Content from FV and Sleep Duration
Table 3 shows the prospective associations between total polyphenol intake from FV and sleep duration. In unadjusted analyses (Table 3), an additional gram of flavonoids, lignans, other polyphenols and total polyphenols were inversely associated with sleep duration (p < 0.01). In adjusted analyses (Table 3), an additional gram of total polyphenols was associated with shorter sleep by 18 min (99% CI −31 to −4, p < 0.001).
Table 3.
The prospective associations between total polyphenols from FV consumption and sleep duration in the UKWCS.
| Sleep Duration (minutes/day) | ||||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted * | |||||
| Polyphenol Class | Coefficient per Additional Gram (99% CI) | p Value | n | Coefficient per Additional Gram (99% CI) | p Value | n |
| Total flavonoids | −30 (−54, −6) | 0.001 | 13,636 | −30 (−61, 1) | 0.01 | 12,816 |
| Total phenolic acids | −22 (−61, 17) | 0.1 | 13,635 | 22 (−30, 74) | 0.2 | 12,816 |
| Total other polyphenols | −180 (−330, −30) | 0.002 | 13,805 | −136 (−299, 27) | 0.03 | 12,816 |
| Total stilbenes | −4011 (−8731, 708) | 0.03 | 13,670 | −538 (−6418, 5341) | 0.08 | 12,816 |
| Total lignans | −28 (−54, −2) | 0.005 | 13,880 | −14 (−43, 15) | 0.2 | 12,816 |
| Total polyphenols from FV ** | −16 (−28, −5) | <0.001 | 13,636 | −18 (−31, −4) | 0.001 | 12,971 |
* Adjusted for age, socio-economic status, smoking, ethnicity, total energy intake, and other polyphenol components. ** Total polyphenols (not adjusted for other polyphenol components) = the sum of total flavonoids, total phenolic acids, total other polyphenols, total stilbenes, and total lignans.
3.4. Prospective Associations between Polyphenol Classes from FV and Sleep Duration
Table 4 shows the prospective associations between polyphenol classes from FV and sleep duration. In unadjusted analyses (Table 4), flavonoids from apples, kiwi, oranges, cabbage, cucumber, green beans, lettuce, olives, and peppers were negatively associated with sleep duration. Phenolic acids from lettuce, aubergine, olives, and peppers were also negatively associated with sleep duration. Other polyphenols from celery and olives were negatively associated with sleep duration. Lignans from oranges, cabbage, and cucumber were negatively associated with sleep duration.
Table 4.
The prospective associations between polyphenol classes from FV consumption and sleep duration in the UKWCS.
| Sleep Duration (minutes/day) | ||||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted * | |||||
| Polyphenol Classes | Coefficient per Additional mg (99% CI) | p Value | n | Coefficient per Additional mg (99% CI) | p Value | n |
| Flavonoids | ||||||
| Apple | −0.5 (−0.8, −0.2) | <0.001 | 13,530 | −0.5 (−0.8, −0.1) | <0.001 | 12,536 |
| Avocados | −19 (−50, 11) | 0.1 | 8468 | −7 (−41, 26) | 0.5 | 7900 |
| Banana | −0.1 (−0.6, 0.4) | 0.5 | 13,092 | −0.1 (−0.7, 0.4) | 0.5 | 12,115 |
| Grape | −0.3 (−0.8, 0.1) | 0.05 | 13,312 | −0.001 (−0.5, 0.5) | 0.9 | 12,413 |
| Kiwi | −19 (−34, −5) | 0.001 | 11,709 | −15 (−31, 0.8) | 0.02 | 10,939 |
| Mangoes | −4 (−11, 2) | 0.1 | 7293 | −1 (−9, 7) | 0.6 | 6831 |
| Oranges ** | −0.07 (−0.13, 0.01) | 0.002 | 12,967 | −0.08 (−0.1, −0.01) | 0.002 | 12,054 |
| Pears | −0.09 (−0.81, 0.62) | 0.7 | 12,177 | 0.1 (−0.6, 0.9) | 0.5 | 11,352 |
| Apricots | −7 (−14, 0.3) | 0.01 | 11,010 | −4 (−12, 4) | 0.2 | 10,304 |
| Nectarines | −0.3 (−2, 1) | 0.6 | 12,678 | −0.3 (−2, 1) | 0.6 | 11,824 |
| Peaches | −17 (−38, 3) | 0.03 | 12,945 | −13 (−39, 11) | 0.1 | 12,063 |
| Plums | −0.3 (−0.9, 0.2) | 0.09 | 12,839 | −0.3 (−1, 0.2) | 0.1 | 11,981 |
| Raspberries | −0.05 (−0.1, 0.08) | 0.2 | 12,643 | −0.03 (−0.2, 0.1) | 0.6 | 11,815 |
| Redcurrants | −0.1 (−0.3, 0.08) | 0.09 | 10,426 | −0.02 (−0.6, 0.5) | 0.9 | 9784 |
| Rhubarb | 0.4 (−0.81, 1) | 0.3 | 11,359 | 0.9 (−0.4, 2) | 0.09 | 10,620 |
| Strawberries | −0.1 (−0.4, 0.1) | 0.09 | 13,321 | −0.1 (−0.4, 0.2) | 0.4 | 12,433 |
| Orange juice (pure fruit) | −0.02 (−0.06, 0.01) | 0.06 | 12,224 | −0.01 (−0.05, 0.02) | 0.3 | 12,054 |
| Figs | −0.2 (−121, 120) | 0.9 | 7231 | −6 (−141, 127) | 0.8 | 6739 |
| Prunes | −7 (−17, 3) | 0.09 | 9205 | −8 (−21, 4) | 0.08 | 8565 |
| Raisins | 3 (−49, 56) | 0.8 | 12,605 | 6 (−52, 64) | 0.7 | 11,726 |
| Beetroot ** | −42 (−87, 3) | 0.02 | 11,516 | −27 (−77, 22) | 0.1 | 10,681 |
| Broccoli | −0.1 (−0.3, 0.08) | 0.1 | 13,488 | −0.01 (−0.2, 0.2) | 0.8 | 12,490 |
| Brussels ** | −3 (−8, 2) | 0.1 | 12,783 | 0.8 (−5, 7) | 0.7 | 11,846 |
| Cabbage ** | −262 (−431, −93) | <0.001 | 13,051 | −180 (−373, 11) | 0.02 | 12,107 |
| Cucumber ** | −209 (−403, −15) | 0.005 | 12,911 | −165 (−372, 41) | 0.04 | 12,002 |
| Green beans | −1 (−1, −0.2) | 0.001 | 13,344 | −0.4 (−1, 0.4) | 0.1 | 12,363 |
| Lettuce | −4 (−9, −0.03) | 0.009 | 13,550 | −3 (−8, 1) | 0.07 | 12,555 |
| Olives | −0.2 (−0.5, −0.02) | 0.005 | 6461 | 1 (−0.8, 2) | 0.1 | 6049 |
| Parsnips ** | −0.9 (−15, 13) | 0.8 | 11,705 | 4 (−11, 19) | 0.4 | 10,885 |
| Peas | −356 (−939, 226) | 0.1 | 13,132 | −272 (−908, 364) | 0.2 | 12,195 |
| Peppers | −3 (−5, −0.3) | 0.004 | 12,371 | −3 (−6, −0.4) | 0.003 | 11,527 |
| Swedes ** | −0.01 (-2, 2) | 0.9 | 11,052 | 0.4 (−1, 2) | 0.6 | 10,268 |
| Tomatoes | −10 (−22, 0.5) | 0.01 | 13,526 | −8 (−20, 4) | 0.08 | 12,534 |
| Watercress ** | −1 (−6, 2) | 0.3 | 11,283 | −0.6 (−5, 4) | 0.7 | 10,531 |
| Phenolic acids | ||||||
| Bananas | −0.5 (−3, 2) | 0.5 | 13,092 | −0.2 (−3, 2) | 0.8 | 12,115 |
| Grapes (green) | −0.2 (−0.4, 0.06) | 0.05 | 13,312 | −0.008 (−0.3, 0.3) | 0.9 | 12,413 |
| Pears | −0.03 (−0.3, 0.2) | 0.7 | 12,177 | 0.1 (−0.1, 0.4) | 0.3 | 11,352 |
| Apricots | −5 (−11, 0.2) | 0.01 | 11,010 | −2 (−9, 3) | 0.2 | 10,304 |
| Nectarines | −0.4 (−3, 2) | 0.6 | 12,678 | 0.2 (−3, 3) | 0.8 | 11,824 |
| Peaches | −1 (−3, 0.3) | 0.03 | 12,945 | −0.7 (−3, 1) | 0.4 | 12,063 |
| Plums | −0.4 (−1, 0.2) | 0.09 | 12,839 | −0.2 (−0.9, 0.5) | 0.4 | 11,981 |
| Raspberries | −0.04 (−0.1, 0.06) | 0.2 | 12,643 | 0.03 (−0.1, 0.1) | 0.5 | 11,815 |
| Redcurrants | −2 (−5, 1) | 0.09 | 10,426 | −0.2 (−7, 7) | 0.9 | 9784 |
| Strawberries | −1 (−3, 0.6) | 0.09 | 13,321 | −0.1 (−2, 2) | 0.9 | 12,433 |
| Dates | −0.4 (−1, 0.5) | 0.2 | 9577 | −0.3 (−1, 0.7) | 0.4 | 8942 |
| Prunes | −0.09 (−0.2, 0.04) | 0.09 | 9205 | −0.07 (−0.2, 0.07) | 0.2 | 8565 |
| Raisins | 0.1 (−1, 2) | 0.8 | 12,605 | 0.3 (−1, 2) | 0.6 | 11,726 |
| Broccoli | −0.1 (−0.5, 0.1) | 0.1 | 13,488 | 0.05 (−0.3, 0.4) | 0.6 | 12,490 |
| Carrots | −0.1 (−0.4, 0.1) | 0.1 | 13,713 | −0.03 (−0.3, 0.3) | 0.7 | 12,679 |
| Cauliflower | −0.6 (−1, 0.2) | 0.06 | 13,503 | −0.2 (−1, 0.8) | 0.5 | 12,509 |
| Lettuce | −5 (−10, −0.03) | 0.009 | 13,550 | −3 (−9, 2) | 0.1 | 12,555 |
| Aubergine | −21 (−36, −5) | <0.001 | 8258 | −14 (−31, 2) | 0.03 | 7735 |
| Olives | −0.3 (−0.6, −0.02) | 0.005 | 6461 | −0.5 (−3, 2) | 0.6 | 6049 |
| Peppers | −29 (−55, −3) | 0.004 | 12,371 | −31 (−60, −2) | 0.005 | 11,527 |
| Tomatoes | −0.8 (−1, 0.04) | 0.01 | 13,526 | −0.4 (−1, 0.4) | 0.2 | 12,534 |
| Boiled/mashed potatoes | 0.01 (−0.04, 0.07) | 0.5 | 13,330 | 0.04 (−0.02, 0.1) | 0.1 | 12,346 |
| Other polyphenols | ||||||
| Pears | −9 (−78, 60) | 0.7 | 12,177 | 28 (−47, 103) | 0.3 | 11,352 |
| Orange juice (pure fruit) | −0.5 (−1, 0.2) | 0.06 | 12,224 | 1 (−0.02, 2) | 0.01 | 11,418 |
| Celery | −6 (−12, −1) | 0.001 | 12,483 | −4 (−10, 0.8) | 0.02 | 11,623 |
| Olives | −0.1 (−0.3, −0.01) | 0.005 | 6461 | −0.1 (−0.3, 0.02) | 0.03 | 6049 |
| Stilbenes | ||||||
| Grapes | −7 (−17, 2) | 0.05 | 13,312 | −1 (−13, 10) | 0.7 | 12,413 |
| Redcurrants | −3 (−9, 2) | 0.09 | 10,426 | −0.1 (−7, 6) | 0.9 | 9784 |
| Strawberries | −44 (−114, 24) | 0.09 | 13,321 | −15 (−98, 67) | 0.6 | 12,433 |
| Lignans | ||||||
| Oranges ** | −0.2 (−0.4, −0.03) | 0.002 | 12,967 | −0.2 (−0.4, 0.05) | 0.03 | 12,054 |
| Pineapple ** | −0.1 (−0.2, −0.02) | 0.001 | 11,810 | −0.09 (−0.2, 0.004) | 0.01 | 11,027 |
| Melon * | −0.02 (−0.05, 0.009) | 0.07 | 13,110 | −0.005 (−0.04, 0.02) | 0.6 | 12,195 |
| Brussels ** | −0.06 (−0.1, 0.04) | 0.1 | 12,783 | 0.006 (−0.1, 0.1) | 0.9 | 11,846 |
| Cabbage ** | −132 (−218, −47) | <0.001 | 13,051 | −89 (−185, 6.8) | 0.01 | 12,107 |
| Squash ** | −862 (−2011, 287) | 0.05 | 11,697 | −874 (−2124, 375) | 0.07 | 10,914 |
| Cucumber ** | −5 (−10, −0.4) | 0.005 | 12,911 | −4 (−9, 0.9) | 0.03 | 12,002 |
| Parsnips ** | −19 (−303, 264) | 0.8 | 11,705 | 70 (−233, 374) | 0.5 | 10,885 |
| Swedes ** | −19 (−2822, 2783) | 0.9 | 11,052 | 434 (−2671, 3539) | 0.7 | 10,268 |
| Turnip ** | −45 (−173, 83) | 0.3 | 8998 | −57 (−194, 80) | 0.2 | 8381 |
* Adjusted for age, socio-economic status, smoking, ethnicity, total energy intake and other polyphenol components. ** Analysed using chromatography after hydrolysis.
In adjusted analyses (Table 4), an additional mg of flavonoids from apples was associated with 0.5 min/day shorter sleep (99% CI −0.8 to −0.1, p < 0.001), an additional mg of flavonoids from oranges was associated with 0.08 min/day shorter sleep (99% CI −0.1, −0.01, p = 0.002), an additional mg of flavonoids from peppers was associated with 3 min/day shorter sleep (99% CI −0.6, −0.4, p = 0.003), and an additional mg of phenolic acids from peppers was associated with 31 min/day shorter sleep (99% CI −60 to −2, p = 0.005).
3.5. Sensitivity Analyses
Sensitivity analyses showed broadly similar results (available Appendix A, Table A2, Table A3, Table A4, Table A5, Table A6 and Table A7). Analyses between polyphenol content from FV and sleep duration stratified by BMI (<25 kg/m2 vs. ≥25 kg/m2) showed that an additional gram of total polyphenols were associated with shorter sleep by 20 min/day (99% CI −36 to −4, p = 0.001) in women with a BMI <25 kg/m2, whereas no association was observed in women with a BMI ≥ 25 kg/m2 (Appendix A) (Table A2). Stratification of analyses by vegetarian/vegan status showed that an additional gram of total flavonoids were associated with shorter sleep by 52 min/day (99% CI −92 to −13, p = 0.001) and total polyphenols by 18 min/day (99% CI −35 to −1, p = 0.006) in non-vegan/vegetarian women (Appendix A) (Table A3). When considering weekday and weekend sleep duration separately (Appendix A) (Table A4), total polyphenol intakes were negatively associated with sleep duration on both weekdays and weekends. Analyses between FV intakes and sleep duration stratified by frequency of alcohol consumption showed that total flavonoids from FV were associated with shorter sleep by 44 min/day (99% CI −89 to −0.7, p = 0.009) in women consuming alcohol more than once a week (Appendix A) (Table A5). Similarly, total polyphenols from FV were associated with shorter sleep by 23 min/day (99% CI −45 to −2, p = 0.005) in women consuming alcohol once a week or less. After excluding women who reported supplement intake, results were attenuated and no association was found between polyphenol intakes and sleep duration (Appendix A) (Table A6), although not significant, polyphenol intakes tended to be negatively associated with sleep duration. After excluding women who reported having a long-term illness, an additional gram of total polyphenols from FV were associated with 18 min/day shorter (99% CI −34 to −3, p = 0.001) (Appendix A) (Table A6), and after excluding women who reported medication use, other polyphenols were negatively associated with sleep duration (Appendix A) (Table A6). Results were attenuated between polyphenol intakes and sleep duration after further adjustment of BMI and physical activity (Appendix A) (Table A7); however, the associations tended to be negative.
4. Discussion
To our knowledge, this is the first study to report prospective associations between specific FV items and their polyphenol content with sleep duration in a large cohort of UK women. A total of 13,958 women were followed up for approximately 4 years and were included in this study. Our results showed that intakes of apples, kiwi, oranges, pineapple, 100% pure juice, cabbage, celery, cucumber, aubergine, olives, peppers, tomato, and total FV were associated with shorter sleep. In terms of polyphenol content, total polyphenols from FV were negatively associated with sleep duration. Flavonoids from apples, oranges, and peppers were associated with shorter sleep, and phenolic acids from peppers were associated with shorter sleep. Collectively, these findings suggest that among UK women, specific FV items, and total polyphenol intake from FV were associated with shorter sleep. This was not what we had anticipated from previous, smaller scale studies [15,16,17].
Our results are in contrast to some human studies; for example, tart cherry juice has been shown to reduce insomnia severity in older adults in a pilot study [16]. However, no improvement was observed in sleep latency, duration, or efficiency; this may be due to the small sample size (n = 15) and the short period of intervention (2 weeks). In addition, the consumption of cherry increased sleep duration and 6-sulfatoxymelatonin (a metabolite that is considered to reflect the nocturnal melatonin concentration) concentrations in the urine in middle aged and elderly participants [17]. However, this study also had a small sample size (n = 12) and short period of intervention (3 days). Similarly, the consumption of 2 kiwifruits 1 h before bedtime for 4 weeks increased sleep duration by 13% in adults reporting sleep disturbances [15]. In our results, an additional portion of kiwi intake was associated with 10 min/day shorter sleep (99% CI −19 to −2, p = 0.001). Furthermore, in regard to polyphenol intake and sleep, a recent study investigated the effects of chronic supplementation of Holisfiit®, a polyphenol-rich extract-based food supplement developed from FV, on both body composition and subjective perception of mental and physical health in 33 overweight and obese participants [31]. After 16-week of supplementation, awakening during the night, total sleep duration, and sleep quality improved by 38% (p = 0.04), 50% (p = 0.02) and 43% (p = 0.03), respectively. In this study, total polyphenol intake was associated with shorter sleep. However, it is important to note that Holisfiit® provided bioactive compounds of polyphenols from flavonoids, delivering flavanones, anthocyanins, and flavanols, and natural components of the methylxanthine family from both an extract of green tea and an extract of yerba mate leaves as well as vitamin B3. Polyphenol effects from supplements differ in bioavailability [32] and concentration to polyphenols from foods [33], which may be one explanation of our different results. Furthermore, a considerable amount of evidence is supporting the hypothesis that high-dose polyphenols from supplements can cause adverse effects through pro-oxidative action, whereas the risk of toxicity from food is low due to poor absorption [33]. Since polyphenols are unstable compounds, some factors need to be considered in comparing our results with previous studies, such as food processing, storage, and ripening stage that impact dietary polyphenol composition [29]. Furthermore, polyphenol content between foods is highly variable, and even within a specific food item the polyphenol content may vary [33]. These conflicting results may be due to the different study designs and participants. Experimental trials on participants with sleep problems differ from healthy free-living individuals; therefore, it is required to consider the potential for non-representative samples taking part in experimental studies.
Our findings are in line with one animal study by Pifferi et al. [14]. They tested the effects of resveratrol dietary supplement, a dietary polyphenolic compound present in FV, on non-human primate grey mouse lemur sleep-wake cycle. After three weeks of resveratrol supplementation, the animals exhibited a significantly-increased proportion of active-wake time occurring during the resting phase (light) of the sleep-wake cycle. Negligible changes in active-wake time during the active phase (dark) of the sleep wake cycle suggesting that resveratrol activity depends largely on the time of administration. Dosing-time dependency of polyphenols were shown previously on melatonin levels [34], the expression pattern of clock genes in the hypothalamus of rats [34], rat tissue lipoperoxidation [35], and adjustment of the clock system in rat liver [36]. Resveratrol administration on male rats behaved as an antioxidant during the night and as a pro-oxidant during day-time [35]. Grape seed proanthocyanidin extract treatment maintained nocturnal melatonin levels and modulated the circadian rhythms when it was administered at the start of the day, rather than at night, in rats [34]. Similarly, grape seed proanthocyanidin modulated the molecular clock by repressing nicotinamide phosphoribosyltransferase (Nampt)—a gene that undergoes transcription by the enhancement of circadian locomotor output cycles (CLOCK)—when the lights were turned on, and overexpressed when the lights were turned off, in rat livers [36]. The effectiveness of polyphenols during periods of the day could be due to the discrepant functionality of the hypothalamus suprachiasmatic nucleus (SCN). It has been shown that SCN cells are extensively coupled during the day, when the cells exhibit synchronous neural activity, but minimally coupled during the night, when the cells are electrically silent [34,37]. Although the timing of FV intakes was not assessed in this study, this could be one explanation of the negative associations we found between FV intakes and sleep duration. FV intake during the day may have a different effect on sleep to when consumed at night. However, as rats are nocturnal animals, in contrast to humans who are diurnal, assessing the zeitgeber time when polyphenol rich foods can entertain circadian rhythms and sleep measures in humans is necessary by conducting interventional trials to determine if there is a time-dependency effect of FV intakes on sleep duration.
Several potential pathways and mechanisms explain the associations between FV intake and their polyphenol content with sleep duration. One of the functions of sleep is to protect the body against the effects of free radicals produced by a high metabolic rate during waking hours [14]. A study suggested that during sleep, metabolic rate and brain temperature are lower than during awake time; this may provide an opportunity to renew the enzymes affected by free radicals. Thus, foods with antioxidant effects are expected to affect the regulation of sleep measures [14]. The action of FV intakes and their polyphenols on sleep measures could be by their improvement on mitochondrial function and energy metabolism by decreasing fat mass, which may lead to changes in sleep [14]. The negative associations between FV intakes and sleep duration in this study could be due to the high antioxidant content in FV that contributes to a decrease in the production of free radicals, and may lead to lower requirements of sleep [14]. It is important to note that the UKWCS contains a higher proportion of vegetarians and well-educated participants, who tend to eat more healthily than the general population; thus, results need to be carefully interpreted. Since the metabolic state of a cell is coupled to the molecular clock, diet may modify rhythmic cellular activities [38]. In light of this, another proposed pathway of how antioxidants may affect sleep is through the protective activation of hormetic, involving proteins such as ion channels, kinases, deacetylases, and transcription factors which regulate the expression of genes that encode cytoprotective enzymes [39]-pathways that promote sirtuin 1(SIRT1) protein expression [40]. SIRT1 has a central role for reactive oxygen species mainly produced as a consequence of mitochondrial functions [41]. It has been identified that several polyphenols, such as resveratrol, act as dietary activators of SIRT1 [40]. In turn, SIRT1 modulates transcription factors including period (PER) 2 [42], which are circadian clock genes that regulate the daily rhythms of locomotor activity, metabolism, and behaviour. Additionally, SIRT1 modulates the ventromedial hypothalamic clock, a brain region that contains neuronal food-synchronized clocks that contribute to regulation of the circadian rhythm in feeding behaviour [43].
Alternatively, polyphenols could adjust the central clock at intestinal levels through the gut-brain axis [34]. Researchers have related the bi-directional interactions between the central nervous system, the enteric nervous system, and the gastrointestinal tract to the prominent role of the gut microbiota in these gut-brain interactions [44]. The bi-directional relationship between microbiota and the circadian system have been shown in germ-free mice that have altered clock gene expression [45,46]. In light of the bi-directional relationship of the gut-brain axis, the polyphenols in FV may affect sleep through nocturnal secretion of melatonin by the pineal gland which is directly controlled in the brain by the SCN. It has been shown that grape seed proanthocyanidin extract administration increased plasma melatonin levels in the middle of the light period, maintaining similar levels at dusk in Male Wistar rats [34]. The beneficial effects of polyphenols may be controlled by the specific microbiota composition of each individual, and there is a strong inter-individual variability in polyphenol bioconversion by the gut microbiota [47]. Some studies suggest that polyphenols have the capacity to alter the gut microbiota composition by increasing the population of beneficial microflora in the gut [48]. It has been previously reported that the bioavailability of polyphenols and the presence of bioactive metabolites in rat plasma depend on the rat sex and the amount ingested [49]. Recently, diverse effects of resveratrol in induced colitis in mice depended on the sex of the animal [50]. Our studies included only women, and further human studies are needed to confirm if the effects of FV intakes and their polyphenols differ by sex.
Although several mechanisms have been proposed on how FV intakes and their polyphenols may affect sleep and the circadian system, more studies are needed to define the mechanisms by which polyphenols could adjust sleep measures and the central clock. Future studies that can demonstrate that the effects mediated by polyphenol supplements mimic the effects of whole foods are essential. Furthermore, dose-response and time of administration-response studies allowing for identification of the most effective doses and times are needed. Studies demonstrating that some polyphenols are transported to the brain or are present in the circulation at the times of the beneficial effects on sleep measures are important to clarify the underlying mechanisms between FV intakes and sleep.
Strengths and Limitations
In interpreting the results of our analyses, certain limitations of the study should be considered. Dietary intakes were collected at one time point only, which means any changes in dietary pattern over time were not taken into account. Self-report of FV intakes in the UKWCS are above the national average [51], possibly due to over-reporting through the FFQ [52], which was observed in other cohort studies using this dietary assessment method [53]. The method to assess sleep duration has not been validated, and self-reported sleep duration may cause over-reporting [54], and any change in sleep patterns were not taken into account. In addition, it was difficult to exclude the effects of other dietary polyphenols from other sources such as caffeine, red wine, and tea [55]. Whilst inverse associations between FV intakes and sleep duration have been observed, effect size was small, and may not be clinically significant. Interpretation of the extent of causality should be undertaken with caution, since observational studies have substantial potential for biases caused by incomplete adjustments for confounders, measurement error in the exposure estimate, and biases in participation selection. On the other hand, our analyses have several strengths. The UKWCS is a large prospective cohort which includes health-conscious women with wide diversity in dietary intakes, which facilitates clarifying the associations between FV intakes and sleep duration. Furthermore, to our knowledge, this is the first study that has extensively investigated the associations between FV items and their polyphenol content on sleep duration in UK women using a validated dietary assessment tool. The use of Phenol Explorer as a reference database for polyphenol content has several advantages due to the high quality literature articles on polyphenol composition, the impacts of food processing on the polyphenols, and metabolite composition in the body. These advantages ensure that the polyphenol content of FV applied here were sensible with regard to the variety of polyphenols in each FV item.
5. Conclusions
FV intakes and their polyphenol content were associated with shorter sleep in a sub-group of UK women. Further investigations are required to assess the relationship between FV intakes and sleep measures in the general population using objective methods of sleep. Overall, the findings of this study do not provide strong evidence to suggest that polyphenol classes from FV intakes are important in relation to sleep duration. Until further knowledge is obtained from intervention studies, consumption of five or more servings/day of FV and sleeping 7–9 h/day for adults is recommended.
Acknowledgments
We thank participants of the UKWCS for their time and cooperation and Darren Greenwood for his advice on the statistical analyses.
Supplementary Materials
The following are available online at http://www.mdpi.com/2072-6643/10/11/1803/s1, Figure S1: Sleep duration assessment method, Figure S2: The relationship between fruit and vegetable intakes and sleep duration using locally weighted scatterplot smoothing (LOWESS), Figure S3: Normal distribution of sleep duration (outcome) using histogram plot.
Appendix A
Table A1.
Polyphenol classes and sub-classes based on Phenol Explorer database.
| Flavonoids |
| Anthocyanins |
| Chalcones |
| Dihydrochalcones |
| Dihydroflavonols |
| Flavanols |
| Flavanones |
| Flavones |
| Flavonols |
| Isoflavonoids |
| Phenolic acids |
| Hydroxybenzoic acids |
| Hydroxycinnamic acids |
| Hydroxyphenylacetic acids |
| Hydroxyphenylpropanoic acids |
| Hydroxyphenylpentanoic acids |
| Stilbenes |
| Stilbenes |
| Lignans |
| Lignans |
| Other polyphenols |
| Alkylmethoxyphenols |
| Alkylphenols |
| Curcuminoids |
| Furanocoumarins |
| Hydroxybenzaldehydes |
| Hydroxybenzoketones Hydroxycinnamaldehydes |
| Hydroxycoumarins |
| Hydroxyphenylpropenes |
| Methoxyphenols |
| Naphtoquinones |
| Phenolic terpenes |
| Tyrosols |
| Other polyphenols |
Table A2.
Prospective associations between polyphenol content from fruit and vegetable intakes and sleep duration for women from the UKWCS stratified by BMI.
| Sleep Duration (minutes/day) | ||||||
|---|---|---|---|---|---|---|
| Body Mass Index < 25 kg/m2 (n = 9017) | Body Mass Index ≥ 25 kg/m2 (n = 4908) | |||||
| Polyphenol Class | Coefficient per Additional Gram (99% CI) * | p Value | n | Coefficient per Additional Gram (99% CI) * | p Value | n |
| Total flavonoids | −32 (−70, 4) | 0.02 | 8336 | −23 (−82, 35) | 0.2 | 4480 |
| Total phenolic acids | 30 (−430, 92) | 0.1 | 8336 | 6 (−91, 104) | 0.8 | 4480 |
| Total other polyphenols | −88 (−264, 86) | 0.1 | 8336 | −294 (−681, 93) | 0.05 | 4480 |
| Total stilbenes | −1619 (−8596, 5357) | 0.5 | 8336 | 1435 (−9291, 12,162) | 0.7 | 4480 |
| Total lignans | −21 (−56, 13) | 0.1 | 8336 | 0.3 (−54, 55) | 0.9 | 4480 |
| Total polyphenols ** | −20 (−36, −4) | 0.001 | 8433 | −12 (−38, 12) | 0.1 | 4538 |
* Adjusted for age, socio-economic status, smoking, ethnicity, total energy intake and other polyphenol components. ** Total polyphenols = the sum of total flavonoids, total phenolic acids, total other polyphenols, total stilbenes and total lignans. 122 pregnant women were excluded; Extreme values of body mass index (<18 and >50 kg/m2) were excluded (n = 183); FV, fruits and vegetables.
Table A3.
Prospective associations between polyphenol content from fruit and vegetable intakes and sleep duration for women from the UKWCS stratified by vegan/vegetarian status.
| Sleep Duration (minutes/day) | ||||||
|---|---|---|---|---|---|---|
| Vegan or Vegetarian (n = 4469) | Non-Vegan or Non-Vegetarian (n = 9455) | |||||
| Polyphenol Class | Coefficient per Additional Gram (99% CI) * | p Value | n | Coefficient per Additional Gram (99% CI) * | p Value | n |
| Total flavonoids | 9 (−43, 62) | 0.6 | 4148 | −52 (−92, −13) | 0.001 | 8668 |
| Total phenolic acids | −6 (−96, 84) | 0.8 | 4148 | 34 (−30, 99) | 0.1 | 8668 |
| Total other polyphenols | −94 (−315, 125) | 0.2 | 4148 | −138 (−389, 112) | 0.1 | 8668 |
| Total stilbenes | −4589 (−14,484, 5305) | 0.2 | 4148 | 2380 (−4955, 9717) | 0.4 | 8668 |
| Total lignans | −22 (−69, 24) | 0.2 | 4148 | −4 (−43, 33) | 0.7 | 8668 |
| Total polyphenols ** | −14 (−35, 7) | 0.08 | 4200 | −18 (−35, −1) | 0.006 | 8771 |
* Adjusted for age, socio-economic status, smoking, ethnicity, total energy intake and other polyphenol components. ** Total polyphenols= the sum of total flavonoids, total phenolic acids, total other polyphenols, total stilbenes and total lignans. 122 pregnant women were excluded; FV, fruits and vegetables.
Table A4.
Prospective associations between polyphenol content from fruit and vegetable intakes and sleep duration for women from the UKWCS based on weekdays and weekends.
| Sleep Duration (minutes/day) | ||||||
|---|---|---|---|---|---|---|
| Weekdays | Weekends | |||||
| Polyphenol Class | Coefficient per Additional Gram (99% CI) * | p Value | n | Coefficient per Additional Gram (99% CI) * | p Value | n |
| Total flavonoids | −28 (−58, 1) | 0.02 | 12,769 | −23 (−56, 9) | 0.07 | 12,605 |
| Total phenolic acids | 43 (−6, 93) | 0.02 | 12,769 | −4 (−59, 50) | 0.8 | 12,605 |
| Total other polyphenols | −90 (−247, 66) | 0.1 | 12,769 | −61 (−230, 107) | 0.3 | 12,605 |
| Total stilbenes | −581 (−6164, 5001) | 0.7 | 12,769 | −3688 (−9789, 2412) | 0.1 | 12,605 |
| Total lignans | −19 (−47, 8) | 0.07 | 12,769 | −23 (−54, 6) | 0.04 | 12,605 |
| Total polyphenols ** | −14 (−27, −1) | 0.004 | 12,922 | −25 (−39, −11) | <0.001 | 12,755 |
* Adjusted for age, socio-economic status, smoking, ethnicity, total energy intake and other polyphenol components. ** Total polyphenols = the sum of total flavonoids, total phenolic acids, total other polyphenols, total stilbenes and total lignans. 122 pregnant women were excluded; FV, fruits and vegetables.
Table A5.
Prospective associations between polyphenol content from fruit and vegetable intakes and sleep duration for women from the UKWCS based on alcohol consumption frequency.
| Sleep Duration (minutes/day) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| More Than Once a Week | Once a Week or Less | Never | |||||||
| Polyphenol Class | Coefficient per Additional Gram (99% CI) * |
p Value | n | Coefficient per Additional 80 g/day (99% CI) * |
p Value | n | Coefficient per Additional Gram (99% CI) * |
p Value | n |
| Total flavonoids | −44 (−89, −0.7) | 0.009 | 6633 | −8 (−59, 42) | 0.6 | 4662 | −27 (−133, 78) | 0.5 | 1271 |
| Total phenolic acids | 23 (−49, 96) | 0.4 | 6633 | 43 (−44, 132) | 0.2 | 4662 | −110 (−274, 53) | 0.08 | 1271 |
| Total other polyphenols | −152 (−357, 53) | 0.05 | 6633 | −287 (−648, 73) | 0.04 | 4662 | 140 (−302, 582) | 0.4 | 1271 |
| Total stilbenes | 5000 (−3428, 13,428) | 0.1 | 6633 | −7235 (−16,632, 2161) | 0.04 | 4662 | 4286 (−13,504, 22,076) | 0.5 | 1271 |
| Total lignans | 4 (−40, 49) | 0.7 | 6633 | −33 (−79, 13) | 0.06 | 4662 | −26 (−111, 59) | 0.4 | 1271 |
| Total polyphenols ** | −11 (−31, 8) | 0.1 | 6677 | −23 (−45, −2) | 0.005 | 4712 | −29 (−67, 9) | 0.05 | 1327 |
* Adjusted for age, socio-economic status, smoking, ethnicity, total energy intake and other polyphenol components. ** Total polyphenols = the sum of total flavonoids, total phenolic acids, total other polyphenols, total stilbenes and total lignans. 122 pregnant women were excluded; FV, fruits and vegetables.
Table A6.
Prospective associations between polyphenol content from fruit and vegetable intakes and sleep duration for women from the UKWCS after excluding participants who self-reported supplement intake, long-term illness and medication use.
| Sleep Duration (minutes/day) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Excluding Supplement Users (n = 7634) | Excluding Those with Long-Term Illness (n = 3673) | Excluding Those Who Reported Medication Use (n = 4165) | |||||||
| Polyphenol Class | Coefficient per Additional Gram (99% CI) * |
p Value | n | Coefficient per Additional Gram (99% CI) * |
p Value | n | Coefficient per Additional Gram (99% CI)* |
p Value | n |
| Total flavonoids | −36 (−83, 11) | 0.05 | 5786 | −27 (−62, 8) | 0.05 | 9584 | −18 (−54, 17) | 0.1 | 8969 |
| Total phenolic acids | 56 (−23, 136) | 0.07 | 5786 | 41 (−20, 103) | 0.08 | 9473 | 37 (−23, 97) | 0.1 | 8969 |
| Total other polyphenols | −214 (−449, 20) | 0.02 | 5786 | −141 (−324, 42) | 0.04 | 9473 | −239 (−421, −56) | 0.001 | 8969 |
| Total stilbenes | 662 (−7887, 9211) | 0.8 | 5786 | −3358 (−9941, 3223) | 0.1 | 9473 | −1080 (−7762, 5600) | 0.6 | 8969 |
| Total lignans | −14 (−59, 31) | 0.4 | 5786 | −18 (−52, 14) | 0.1 | 9473 | −9 (−43, 25) | 0.4 | 8969 |
| Total polyphenols ** | −15 (−36, 4) | 0.04 | 5845 | −18 (−34, −3) | 0.001 | 9593 | −11 (−27, 3) | 0.04 | 9058 |
* Adjusted for age, socio-economic status, smoking, ethnicity, total energy intake and other polyphenol components. ** Total polyphenols = the sum of total flavonoids, total phenolic acids, total other polyphenols, total stilbenes and total lignans. 122 pregnant women were excluded; FV, fruits and vegetables; Illness that were excluded in Table A5: Heart attack, coronary thrombosis, myocardial infarction; Angina; Stroke; High bold pressure (Hypertension); High blood cholesterol (hyperlipidaemia); Diabetes; Gallstones; Polyps in the large intestine; Cancer.
Table A7.
Prospective associations between polyphenol content from fruit and vegetable intakes and sleep duration for women from the UKWCS after further adjusting for BMI and physical activity.
| Sleep Duration (minutes/day) | |||
|---|---|---|---|
| Adjusted * (Including BMI and Physical Activity) | |||
| Polyphenol Class | Coefficient per Additional Gram (99% CI) | p Value | n |
| Total flavonoids | −22 (−60, 15) | 0.1 | 7360 |
| Total phenolic acids | 27 (−40, 94) | 0.2 | 7360 |
| Total other polyphenols | −40 (−248, 166) | 0.6 | 7360 |
| Total stilbenes | −1053 (−8105, 5999) | 0.7 | 7360 |
| Total lignans | −13 (−47, 21) | 0.3 | 7360 |
| Total polyphenols ** | −11 (−27, 4) | 0.06 | 7424 |
* Adjusted for age, socio-economic status, smoking, ethnicity, total energy intake and other polyphenol components. ** Total polyphenols = the sum of total flavonoids, total phenolic acids, total other polyphenols, total stilbenes and total lignans. 122 pregnant women were excluded and extreme values of body mass index (<18 and >50 kg/m2) were excluded (n = 183); BMI, body mass index, FV, fruits and vegetables. Number of participants (n) differ from Table 3 due to available data of physical activity and sleep duration of only 8177 women and difference in (n) is due to missing data of any of the included covariates in the model.
Author Contributions
Conceptualization, E.N., L.H. and J.C.; Methodology, E.N., L.H. and J.C.; Software, E.N.; Validation, E.N., L.H. and J.C.; Formal Analysis, E.N.; Investigation, E.N.; Resources, E.N.; Data Curation, E.N. and J.C.; Writing-Original Draft Preparation, E.N.; Writing-Review and Editing, E.N., L.H. and J.C.; Visualization, E.N.; Supervision, L.H. and J.C.; Project Administration, L.H. and J.C.; Funding Acquisition, E.N.
Funding
E.N. is in receipt of a scholarship from Umm Al-Qura University, Makkah, Saudi Arabia. J.C. was funded by (The UK Medical Research Council) grant number (MR/L02019X/1).
Conflicts of Interest
J.C. is a director of the University of Leeds spin out company Dietary Assessment. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- 1.Stranges S., Dorn J.M., Cappuccio F.P., Donahue R.P., Rafalson L.B., Hovey K.M., Freudenheim J.L., Kandala N.B., Miller M.A., Trevisan M. A population-based study of reduced sleep duration and hypertension: The strongest association may be in premenopausal women. J. Hypertens. 2010;28:896–902. doi: 10.1097/HJH.0b013e328335d076. [DOI] [PubMed] [Google Scholar]
- 2.Chaput J.P., Després J.P., Bouchard C., Tremblay A. Association of sleep duration with type 2 diabetes and impaired glucose tolerance. Diabetologia. 2007;50:2298–2304. doi: 10.1007/s00125-007-0786-x. [DOI] [PubMed] [Google Scholar]
- 3.Cappuccio F.P., Cooper D., D’Elia L., Strazzullo P., Miller M.A. Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. Eur. Heart J. 2011;32:1484–1492. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 4.Cappuccio F.P., D’Elia L., Strazzullo P., Miller M.A. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–592. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin J., Jin X., Shan Z., Li S., Huang H., Li P., Peng Z., Yu K., Bao W., Yang W., et al. Relationship of sleep duration with all-cause mortality and cardiovascular events: A systematic review and dose-response meta-analysis of prospective cohort studies. J. Am. Heart Assoc. 2017;6:e005947. doi: 10.1161/JAHA.117.005947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.St-Onge M.P. Sleep–obesity relation: Underlying mechanisms and consequences for treatment. Obes. Rev. 2017 doi: 10.1111/obr.12499. [DOI] [PubMed] [Google Scholar]
- 7.Wu Y., Zhai L., Zhang D. Sleep duration and obesity among adults: A meta-analysis of prospective studies. Sleep Med. 2014;15:1456–1462. doi: 10.1016/j.sleep.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Aune D., Giovannucci E., Boffetta P., Fadnes L.T., Keum N., Norat T., Greenwood D.C., Riboli E., Vatten L.J., Tonstad S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017;46:1029–1056. doi: 10.1093/ije/dyw319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noorwali E.A., Cade J.E., Burley V.J., Hardie L.J. The relationship between sleep duration and fruit/vegetable intakes in UK adults: A cross-sectional study from the National Diet and Nutrition Survey. BMJ Open. 2018;8:e020810. doi: 10.1136/bmjopen-2017-020810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peuhkuri K., Sihvola N., Korpela R. Diet promotes sleep duration and quality. Nutr. Res. 2012;32:309–319. doi: 10.1016/j.nutres.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Frank S., Gonzalez K., Lee-Ang L., Young M.C., Tamez M., Mattei J. Diet and sleep physiology: Public health and clinical implications. Front. Neurol. 2017;8:393. doi: 10.3389/fneur.2017.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St-Onge M.P., Crawford A., Aggarwal B. Plant-based diets: Reducing cardiovascular risk by improving sleep quality? Curr. Sleep Med. Rep. 2018;4:74–78. doi: 10.1007/s40675-018-0103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pifferi F., Dal-Pan A., Menaker M., Aujard F. Resveratrol dietary supplementation shortens the free-running circadian period and decreases body temperature in a prosimian primate. J. Biol. Rhythms. 2011;26:271–275. doi: 10.1177/0748730411401788. [DOI] [PubMed] [Google Scholar]
- 14.Pifferi F., Rahman A., Languille S., Auffret A., Babiloni C., Blin O., Lamberty Y., Richardson J.C., Aujard F. Effects of dietary resveratrol on the sleep-wake cycle in the non-human primate gray mouse lemur (Microcebus murinus) Chronobiol. Int. 2012;29:261–270. doi: 10.3109/07420528.2011.654019. [DOI] [PubMed] [Google Scholar]
- 15.Lin H.H., Tsai P.S., Fang S.C., Liu J.F. Effect of kiwifruit consumption on sleep quality in adults with sleep problems. Asia Pac. J. Clin. Nutr. 2011;20:169–174. [PubMed] [Google Scholar]
- 16.Pigeon W.R., Carr M., Gorman C., Perlis M.L. Effects of a tart cherry juice beverage on the sleep of older adults with insomnia: A pilot study. J. Med. Food. 2010;13:579–583. doi: 10.1089/jmf.2009.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrido M., Paredes S.D., Cubero J., Lozano M., Toribio-Delgado A.F., Muñoz J.L., Reiter R.J., Barriga C., Rodríguez A.B. Jerte Valley cherry-enriched diets improve nocturnal rest and increase 6-sulfatoxymelatonin and total antioxidant capacity in the urine of middle-aged and elderly humans. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65:909–914. doi: 10.1093/gerona/glq099. [DOI] [PubMed] [Google Scholar]
- 18.Cade J.E., Burley V.J., Alwan N.A., Hutchinson J., Hancock N., Morris M.A., Threapleton D.E., Greenwood D.C. Cohort profile: The UK Women’s Cohort Study (UKWCS) Int. J. Epidemiol. 2017;46:e11. doi: 10.1093/ije/dyv173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The National Statistics Socio-Economic Classification User Manual Office for National Statistics. [(accessed on 5 February 2018)];2005 Available online: https://www.ons.gov.uk/ons/guide-method/classifications/archived-standard-classifications/soc-and-sec-archive/the-national-statistics-socio-economic-classification--user-manual.pdf.
- 20.Riboli E., Kaaks R. The EPIC Project: Rationale and study design. European Prospective Investigation into Cancer and Nutrition. Int. J. Epidemiol. 1997;26:S6–S14. doi: 10.1093/ije/26.suppl_1.S6. [DOI] [PubMed] [Google Scholar]
- 21.Cade J.E., Burley V.J., Greenwood D.C. UK Women’s Cohort Study Steering Group. The UK Women’s Cohort Study: Comparison of vegetarians, fish-eaters and meat-eaters. Public Health Nutr. 2004;7:871–878. doi: 10.1079/PHN2004620. [DOI] [PubMed] [Google Scholar]
- 22.Cade J.E., Burley V.J., Greenwood D.C. UK Women’s Cohort Study Steering Group Dietary fibre and risk of breast cancer in the UK Women’s Cohort Study. Int. J. Epidemiol. 2007;36:431–438. doi: 10.1093/ije/dyl295. [DOI] [PubMed] [Google Scholar]
- 23.Agency F.S. Food Portion Sizes. The Stationary Office; London, UK: 2002. [Google Scholar]
- 24.Neveu V., Perez-Jiménez J., Vos F., Crespy V., du Chaffaut L., Mennen L., Knox C., Eisner R., Cruz J., Wishart D., et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database. 2010 doi: 10.1093/database/bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miranda A.M., ASteluti J., Fisberg R.M., Marchioni D.M. Dietary intake and food contributors of polyphenolsin adults and elderly adults of Sao Paulo: A population-based study. Br. J. Nutr. 2016;115:1061–1070. doi: 10.1017/S0007114515005061. [DOI] [PubMed] [Google Scholar]
- 26.Miranda A.M., Steluti J., Fisberg R.M., Marchioni D.M. Association between Polyphenol Intake and Hypertension in Adults and Older Adults: A Population-Based Study in Brazil. PLoS ONE. 2016;11:e0165791. doi: 10.1371/journal.pone.0165791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lefèvre-Arbogast S., Gaudout D., Bensalem J., Letenneur L., Dartigues J.F., Hejblum B.P., Féart C., Delcourt C., Samieri C. Pattern of polyphenol intake and the long-term risk of dementia in older persons. Neurology. 2018;90:e1979–e1988. doi: 10.1212/WNL.0000000000005607. [DOI] [PubMed] [Google Scholar]
- 28.Cleveland W.S. Robust locally weighted regression and smoothing scatterplots. J. Am. Stat. Assoc. 2012;74:829–836. doi: 10.1080/01621459.1979.10481038. [DOI] [Google Scholar]
- 29.Cheynier V. Polyphenols in foods are more complex than often thought. Am. J. Clin. Nutr. 2005 doi: 10.1093/ajcn/81.1.223S. [DOI] [PubMed] [Google Scholar]
- 30.Stata Statistical Software: Release 15. StataCorp LLC; College Station, TX, USA: 2017. [Google Scholar]
- 31.Romain C., Alcaraz P.E., Chung L.H., Cases J. Regular consumption of HolisFiit, a polyphenol-rich extract-based food supplement, improves mind and body well-being of overweight and slightly obese volunteers: A randomized, double-blind, parallel trial. Int. J. Food Sci. Nutr. 2017;68:840–848. doi: 10.1080/09637486.2017.1292221. [DOI] [PubMed] [Google Scholar]
- 32.Manach C., Williamson G., Morand C., Scalbert A., Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005;81:S230–S242. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 33.Martin K.R., Appel C.L. Polyphenols as dietary supplements: A double-edged sword. Nutr. Diet. 2009;2:1–12. doi: 10.2147/NDS.S6422. [DOI] [Google Scholar]
- 34.Ribas-Latre A., Del Bas J.M., Baselga-Escudero L., Casanova E., Arola-Arnal A., Salvadó M.J., Arola L., Bladé C. Dietary proanthocyanidins modulate melatonin levels in plasma and the expression pattern of clock genes in the hypothalamus of rats. Mol. Nutr. Food Res. 2015;59:865–878. doi: 10.1002/mnfr.201400571. [DOI] [PubMed] [Google Scholar]
- 35.Gadacha W., Ben-Attia M., Bonnefont-Rousselot D., Aouani E., Ghanem-Boughanmi N., Touitou Y. Resveratrol opposite effects on rat tissue lipoperoxidation: Pro-oxidant during day-time and antioxidant at night. Redox Rep. 2009;14:154–158. doi: 10.1179/135100009X466131. [DOI] [PubMed] [Google Scholar]
- 36.Ribas-Latre A., Baselga-Escudero L., Casanova E., Arola-Arnal A., Salvadó M.J., Bladé C., Arola L. Dietary proanthocyanidins modulate BMAL1 acetylation, Nampt expression and NAD levels in rat liver. Sci. Rep. 2015;5:10954. doi: 10.1038/srep10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colwell C.S. Rhythmic coupling among cells in the suprachiasmatic nucleus. J. Neurobiol. 2000;43:379–388. doi: 10.1002/1097-4695(20000615)43:4<379::AID-NEU6>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Potter G.D., Cade J.E., Grant P.J., Hardie L.J. Nutrition and the circadian system. Br. J. Nutr. 2016;116:434–442. doi: 10.1017/S0007114516002117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattson M.P. Hormesis defined. Ageing Res. Rev. 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramis M.R., Esteban S., Miralles A., Tan D.X., Reiter R.J. Caloric restriction, resveratrol and melatonin: Role of SIRT1 and implications for aging and related-diseases. Mech. Ageing Dev. 2015;146–148:28–41. doi: 10.1016/j.mad.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 42.Asher G., Gatfield D., Stratmann M., Reinke H., Dibner C., Kreppel F., Mostoslavsky R., Alt F.W., Schibler U., Stratmann M., et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 43.Orozco-Solis R., Ramadori G., Coppari R., Sassone-Corsi P. SIRT1 relays nutritional inputs to the circadian clock through the sf1 neurons of the ventromedial hypothalamus. Endocrinology. 2015;156:2174–2184. doi: 10.1210/en.2014-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayer E.A., Tillisch K., Gupta A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015;125:926–938. doi: 10.1172/JCI76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leone V., Gibbons S.M., Martinez K., Hutchison A.L., Huang E.Y., Cham C.M., Pierre J.F., Heneghan A.F., Nadimpalli A., Hubert N., et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe. 2015;17:681–689. doi: 10.1016/j.chom.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang X., Bushman F.D., FitzGerald G.A. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc. Natl. Acad. Sci. USA. 2015;112:10479–10484. doi: 10.1073/pnas.1501305112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bladé C., Aragonès G., Arola-Arnal A., Muguerza B., Bravo F.I., Salvadó M.J., Arola L., Suárez M. Proanthocyanidins in health and disease. Biofactors. 2016;42:5–12. doi: 10.1002/biof.1249. [DOI] [PubMed] [Google Scholar]
- 48.Selma M.V., Espín J.C., Tomás-Barberán F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009;57:6485–6501. doi: 10.1021/jf902107d. [DOI] [PubMed] [Google Scholar]
- 49.Margalef M., Guerrero L., Pons Z., Bravo F.I., Arola L., Muguerza B., Arola-Arnal A. A dose–response study of the bioavailability of grape seed proanthocyanidin in rat and lipid-lowering effects of generated metabolites in HepG2 cells. Food Res. Int. 2014;64:500–507. doi: 10.1016/j.foodres.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 50.Wagnerova A., Babickova J., Liptak R., Vlkova B., Celec P., Gardlik R. Sex Differences in the Effect of Resveratrol on DSS-Induced Colitis in Mice. Gastroenterol. Res. Pract. 2017;2017:8051870. doi: 10.1155/2017/8051870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitton C., Nicholson S.K., Roberts C., Prynne C.J., Pot G.K., Olson A., Fitt E., Cole D., Teucher B., Bates B., et al. National diet and nutrition survey: UK food consumption and nutrient intakes from the first year of the rolling programme and comparisons with previous surveys. Br. J. Nutr. 2011;106:1899–1914. doi: 10.1017/S0007114511002340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cade J.E., Burley V.J., Warm D.L., Thompson R.L., Margetts B.M. Food-frequency questionnaires: A review of their design, validation and utilisation. Nutr. Res. Rev. 2004;17:5–22. doi: 10.1079/NRR200370. [DOI] [PubMed] [Google Scholar]
- 53.Brunner E., Stallone D., Juneja M., Bingham S., Marmot M. Dietary assessment in Whitehall II: Comparison of 7 day diet diary and food-frequency questionnaire and validity against biomarkers. Br. J. Nutr. 2001;86:405–414. doi: 10.1079/BJN2001414. [DOI] [PubMed] [Google Scholar]
- 54.Lauderdale D.S., Knutson K.L., Yan L.L., Liu K., Rathouz P.J. Self-reported and measured sleep duration: How similar are they? Epidemiology. 2008;19:838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pérez-Jiménez J., Neveu V., Vos F., Scalbert A. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010 doi: 10.1038/ejcn.2010.221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.