Abstract
Astaxanthin and lutein, antioxidants used in nutraceutics and cosmetics, can be extracted from several microalgal species. In this work, investigations on astaxanthin and lutein extraction from Haematococcus pluvialis (H. pluvialis) in the red phase were carried out by means of the supercritical fluid extraction (SFE) technique, in which CO2 supercritical fluid was used as the extracting solvent with ethanol as the co-solvent. The experimental activity was performed using a bench-scale reactor in semi-batch configuration with varying extraction times (20, 40, 60, and 80 min), temperatures (50, 65, and 80 °C) and pressures (100, 400, and 550 bar). Moreover, the performance of CO2 SFE with ethanol was compared to that without ethanol. The results show that the highest astaxanthin and lutein recoveries were found at 65 °C and 550 bar, with ~18.5 mg/g dry weight (~92%) astaxanthin and ~7.15 mg/g dry weight (~93%) lutein. The highest astaxanthin purity and the highest lutein purity were found at 80 °C and 400 bar, and at 65 °C and 550 bar, respectively.
Keywords: CO2 supercritical fluid extraction, Haematococcus pluvialis, lutein, astaxanthin, ethanol, co-solvent, antioxidants, purity, recovery, extraction
1. Introduction
Currently, the interest in microalgae is on the rise, as the knowledge of their benefits in several aspects of human life increases. Astaxanthin and lutein, high-value molecules for many industrial sectors, e.g., pharmaceuticals, nutraceuticals, additive/functional foods, natural medicine, and cosmetics, can be extracted from microalgal species [1,2]. Microalgae can live both in freshwater and seawater environments, using their photosynthesis activity to convert atmospheric carbon dioxide into oxygen and sugars in the presence of sunlight [3]. During their life cycle, they can be used for carbon dioxide sequestration; in fact, for each kilogram of dry biomass, about 1.8–2 kg of CO2 can be captured.
Astaxanthin and lutein are two very valuable carotenoids on the market. Their properties make them useful as food additives. Thanks to its antioxidant and antiaging properties, astaxanthin is also used in the cosmetic sector [4], while lutein is also used as a dietary supplement for its beneficial effects on eye health [5].
A number of factors, such as the type of molecule, the end use of the extract (e.g., pharmaceutical, cosmetics, and food), and its thermolability, affect the selection of a proper extraction process [6]. Squeezing, maceration, infusion, percolation, steam distillation, and solvent extraction are traditional techniques for extracting principal components from vegetables. The main drawbacks of these techniques are the thermal degradation of molecules, due to the high temperatures of extraction, and the presence of solvent residues in the extracts, which can compromise their end use. In order to solve these issues and, at the same time, minimize energy costs and environmental impact, innovative extraction methods, such as ultrasonic extraction, microwave extraction, accelerated solvent extraction, and extraction with supercritical fluid, were proposed by the research community in the past few decades [7,8,9,10,11,12].
Extraction with supercritical fluid (SFE) is an advanced technology with great potential for the extraction of molecules that require high standards in terms of yield without any traces of solvents, which is especially important when the extracts are intended for nutraceutics [13,14,15,16,17]. Extraction with supercritical fluid using CO2 in supercritical condition as the extracting solvent (CO2-SFE) is generally applied [18] as an alternative to traditional extraction technologies [19,20,21,22,23], to extract natural compounds. While the use of CO2 is the most common approach, many substances can be used in supercritical conditions (hexane, methanol, pentane, butane, nitrous oxide, sulfur hexafluoride, and fluorinated hydrocarbons). Thanks to its critical conditions (Pc = 7.38 MPa, Tc = 31.1 °C), particularly in terms of low critical temperature, it is possible to extract thermally unstable substances with CO2-SFE while reducing thermal degradation effects [24]. Moreover, CO2 is non-flammable and less toxic than conventional solvents. After extraction, CO2 can easily be recovered for successive cyclic extractions, since it is in a gaseous state at room temperature and atmospheric pressure [25,26]. Unlike traditional solvents with larger molecules, carbon dioxide (CO2) in supercritical conditions spreads faster through cellular walls thanks to its high permeability and diffusivity. Because of these advantages, CO2-SFE appears to be the preferable extraction process when high-quality standards are required to extract high-value compounds [27,28,29,30,31].
The main drawback of this technique is the chemical behavior of CO2, similar to lipophilic solvents, i.e., better able to extract non-polar molecules. In order to overcome this obstacle, substances with high polarity such as water, methanol, or ethanol and other polar compounds (called co-solvents) are often used [32,33]. Their choice depends on aspects like polarity, toxicity, and environmental impact [34,35,36]. Among co-solvents, ethanol, a generally recognized as safe (GRAS) solvent, according to the Food and Drug Administration classification [37], is often used. Actually, ethanol is normally employed in the pharmaceutical and food industry, since 50 mg of residual ethanol per day is acceptable for human health.
In the last twenty years, CO2-SFE was used to extract bio-products from over 300 vegetables [21,23], as highlighted in the many of papers and patents reported in the literature [38,39,40]. Currently, CO2-SFE is also used for the extraction of several active principal components from algae that can be used in the food industry [41,42,43], such as high-value lipids [44].
Haematococcus pluvialis is a microalgal species that can grow both in freshwater and in aquatic environments with considerable concentrations of NaCl [45,46,47], and it is characterized by its ability to accumulate significant concentrations of astaxanthin and lutein. Several experiments focusing on the extraction of astaxanthin from H. pluvialis via CO2-SFE were carried out without [48,49,50,51] and with ethanol as a co-solvent [50,52,53]. For example, Kwan and co-workers [51] developed a method of selectively purifying astaxanthin and other by-products (triacylglycerides) from H. pluvialis using supercritical CO2. Cheng et al. [50] investigated the low-pressure supercritical CO2 extraction of astaxanthin from H. pluvialis using a microfluidic reactor with and without co-solvent (ethanol and olive oil), observing the increase in astaxanthin recovery and the decrease in extraction time in the presence of a co-solvent (astaxanthin recovery = 92%, at 55 °C, 8 MPa, and 15 min). Lutein extraction via CO2-SFE in the presence of co-solvents from other microalgal species, such as Chlorella [54,55] and Scenedesmus [56], was also investigated. CO2-SFE extraction from microalgae is the starting point for the production of natural compounds with a sustainable approach and a low environmental impact.
In this paper, astaxanthin and lutein extraction from H. pluvialis in the red phase (HPR) was investigated using CO2-SFE technology with ethanol as the co-solvent. The effects of temperature (50–80 °C) and pressure (100–550 bar) on recovery and purity over extraction time (20–80 min), i.e., four extraction cycles of 20 min each, were tested by keeping flow rates of carbon dioxide and ethanol constant at 3.62 g/min and 1 mL/min, respectively, using a bench-scale experimental apparatus. Moreover, at the same operative conditions, a comparison between the experimental findings from CO2-SFE with ethanol and the experimental findings from CO2-SFE without ethanol was carried out. Experimental findings from CO2-SFE without ethanol were published in a previous study by Di Sanzo et al [57].
2. Results and Discussion
2.1. Extraction Yield
Total extraction yields for each operative condition are summarized in Table 1, in which the total yields extracted without co-solvent are also included. The results are expressed as mg of extract per gram of dry weight of HPR, and values were obtained at the end of the extraction procedure. In the presence of a co-solvent, the total extraction yield ranged from 207.67 mg/g (T = 80 °C, P = 100 bar) to 292.70 mg/g (T = 65 °C, P = 400 bar); without co-solvent, the total extraction yield varied in the range of 0.1 mg/g (T = 50 °C, P = 100 bar) to 277.1 mg/g (T = 65 °C, P = 400 bar). As shown, with and without co-solvent, the highest total extraction yield was found at the same operative conditions (T = 65 °C, P = 400 bar); however, CO2-SFE extraction with a co-solvent increased the highest total extraction yield from 277.1 mg/g to 292.7 mg/g.
Table 1.
Extraction yield during CO2 superfluid extraction (CO2-SFE) with and without co-solvent [57].
| Operative Conditions | Total Extraction Yield (mg/g) (Ethanol Flow Rate = 1 mL/min) |
Total Extraction Yield (mg/g) (Without Co-Solvent) |
|
|---|---|---|---|
| Extraction Time = 80 min | |||
| CO2 Flow Rate = 3.62 g/min | |||
| T (°C) | P (bar) | ||
| 50 | 100 | 209.99 | 0.1 |
| 50 | 400 | 235.47 | 132.4 |
| 50 | 550 | 241.64 | 234.8 |
| 65 | 100 | 241.62 | 4.8 |
| 65 | 400 | 292.70 | 277.1 |
| 65 | 550 | 280.78 | 184.6 |
| 80 | 100 | 207.67 | 10.6 |
| 80 | 400 | 253.03 | 158.7 |
| 80 | 550 | 222.57 | 59.4 |
Data in Table 1 show that the total extraction yield increases as temperature and pressure increase, until a maximum is reached, before a subsequent decrease. This result is due to counteracting phenomena: increasing the pressure increases the CO2 density (the solvation power of the fluids [56]), but a pressure too high may obstruct the diffusion of supercritical fluid into the matrix; increasing the temperature decreases the viscosity of the solvent improving the mass transfer, but a temperature too high may degrade the extracted compounds [48,58,59].
2.2. Astaxanthin Recovery
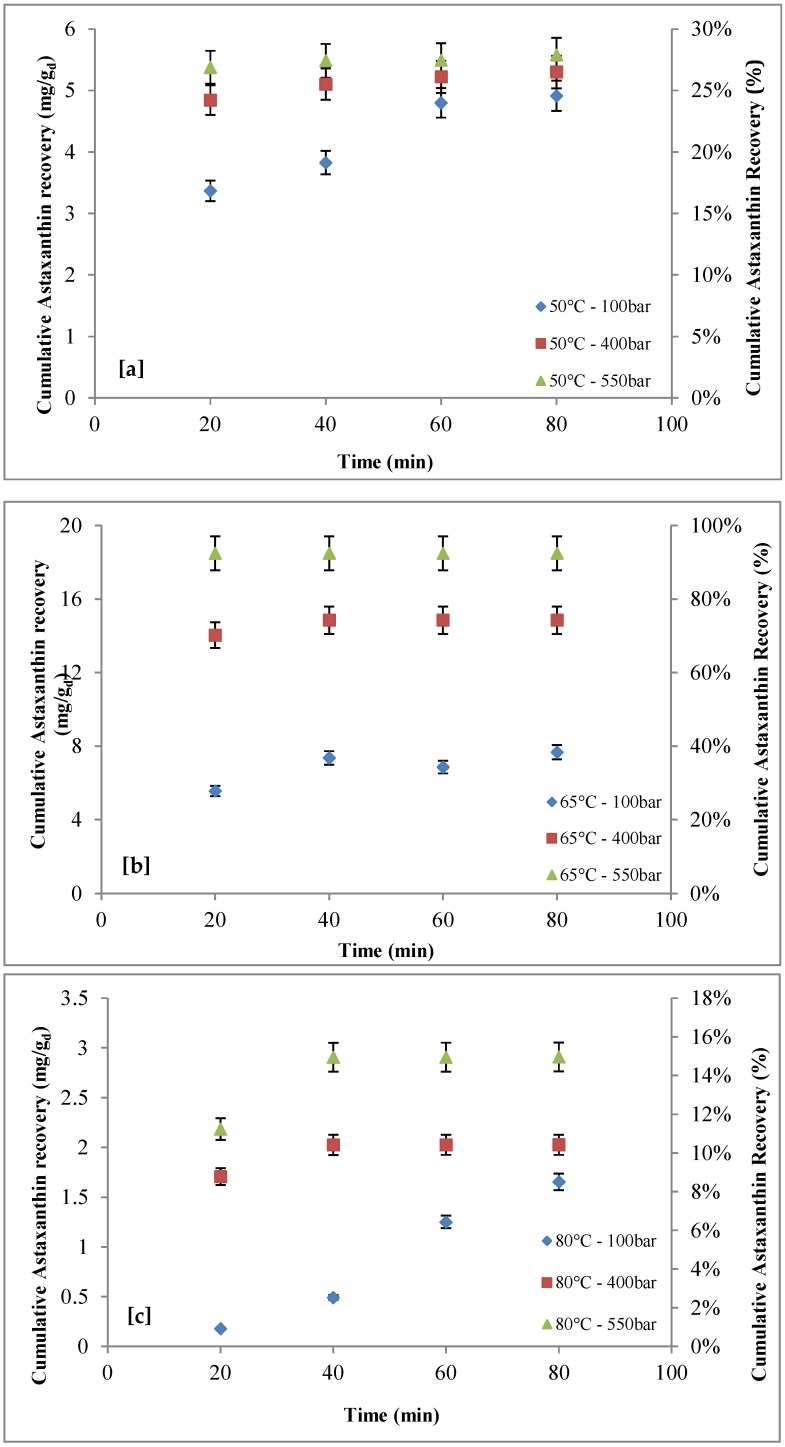
The effects of pressure (100–550 bar), temperature (50–80 °C), and extraction time (extraction cycle number; 20–80 min) on the cumulative recovery of astaxanthin are reported in Figure 1 and Figure 2. Figure 1a–c shows that, while keeping extraction temperature constant, astaxanthin recovery increased with extraction time and pressure.
Figure 1.
Effect of pressure (100–550 bar) on astaxanthin recovery as function of extraction time: (a) T = 50 °C; (b) T = 65 °C; (c) T = 80 °C.
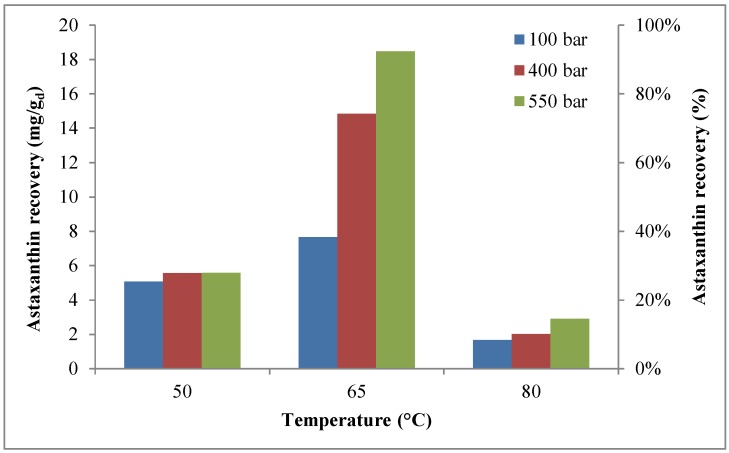
Figure 2.
Effects of temperature (50–80 °C) and pressure (100–550 bar) on cumulative astaxanthin recovery (extraction time = 80 min).
At 50 °C (Figure 1a), with an extraction time of 20 min (first extraction cycle), increasing pressure from 100 to 550 bar resulted in astaxanthin recovery growing from ~3.4 mg/gd (~17%) to ~5.4 mg/gd (~27%). With an extraction time of 80 min (four extraction cycles), astaxanthin extraction yield increased from ~4.9 mg/gd (~25%) to ~5.6 mg/gd (~28%). Increasing extraction time from 20 min to 80 min, at 100 bar, astaxanthin recovery increased from ~3.4 mg/gd to ~4.9 mg/gd; at 400 bar, astaxanthin recovery slightly increased from ~4.8 mg/gd to ~5.3 mg/gd; at 550 bar, astaxanthin recovery slightly increased from ~5.4 mg/gd to ~5.6 mg/gd. At 65°C (Figure 1b) and at 80 °C (Figure 1c), for the first extraction cycle, astaxanthin recovery reached a maximum at ~18.4 mg/gd (~92%) at 65 °C and 550 bar, while this value was only ~0.2 mg/gd (~1%) at 80 °C and 100 bar. However, it is worth highlighting that, at 65 °C and 550 bar, astaxanthin recovery was relatively unaffected by the extraction time.
For each operative condition, the astaxanthin yield at the end of a cycle of extraction is shown in Figure 2. This figure clearly shows that, whatever the pressure, astaxanthin recovery increased with extraction temperature, reaching a maximum at 65 °C, corresponding to ~18.5 mg/gd (~93%), before decreasing, probably due to thermal degradation [60,61,62]. Results show that the maximum astaxanthin recovery was obtained at the highest pressure (550 bar) and at an intermediate temperature (65 °C). It is worth pointing out that, under the best extraction conditions, a negligible influence of extraction time was basically observed, as, at 20 min, an astaxanthin extraction yield of about 18.4 mg/gd was achieved, with respect to an astaxanthin extraction yield of about 18.5 mg/gd reached at 80 min.
Pan et al. [63] investigated the effects of H. pluvialis loading, CO2 flow rate, time, pressure, and temperature of extraction, as well as ethanol loading, on astaxanthin extraction, observing a yield of about 71% at the following operating conditions: temperature = 50 °C, pressure = 310 bar, CO2 flow rate = 6 L/min, time = 160 min, and H. pluvialis loading = 6.5 g. Machmudah et al. [53] reported an extraction yield of about 80% at a pressure of 400 bar and a temperature of 343 K, with a CO2 flow rate of 3 mL/min and an ethanol concentration of 5%; a comparable finding was found by the same research group at a pressure of 550 bar and a temperature of 343 K, with CO2 flow rate of 3 mL/min without ethanol. It is worth highlighting that, in this study, an astaxanthin yield of about 93% at 550 bar and 65 °C, with a CO2 flow rate of 3.62 g/min and an ethanol flow rate of 1 mL/min was observed.
The comparison between cumulative recovery of astaxanthin with and without co-solvent [57] is reported in Table 2. As shown, the maximum recovery of astaxanthin (~94%) was found without co-solvent at 550 bar and 50 °C. However, a comparable result was achieved with co-solvent (~92%) at 550 bar and 65 °C.
Table 2.
Comparison between astaxanthin cumulative recovery with and without co-solvent [57].
| CO2 Flow Rate = 3.62 g/min; Extraction Time = 80 min | ||||||
|---|---|---|---|---|---|---|
| T (°C) | P (bar) | |||||
| 100 | 400 | 550 | 100 | 400 | 550 | |
| Cumulative Recovery with Co-Solvent (%) (Ethanol flow rate = 1 mL/min) |
Cumulative Recovery without Co-Solvent (%) | |||||
| 50 | 24.5 | 27.8 | 27.9 | <0.01 | 91.6 | 94.5 |
| 65 | 38.3 | 74.2 | 92.4 | 0.05 | 79.8 | 35.2 |
| 80 | 8.4 | 10.1 | 14.5 | 5.7 | 72.7 | 13.5 |
The purity of astaxanthin for each stage of extraction, under different operative conditions, with and without co-solvent is reported in Table 3. With co-solvent, the highest purity (~18%) was achieved with an extraction time of 40 min at 80 °C and 400 bar. Comparable findings were observed by Reyes et al. [64], who investigated the effect of ethanol content in the CO2 flow on astaxanthin extraction from H. pluvialis by CO2-SFE, observing a maximum purity of about 23% at 70 °C and 275 bar with an ethanol content in the CO2 flow of 13% (w/w). Without co-solvent, the highest purity (~34%) was found with an extraction time of 80 min at the same pressure and temperature.
Table 3.
Purity of astaxanthin for each stage under different operative conditions with and without co-solvent [57].
| CO2 Flow Rate = 3.62 g/min | ||||||||
|---|---|---|---|---|---|---|---|---|
| T and P | Astaxanthin Purity (%) with Co-Solvent | Astaxanthin Purity (%) without Co-Solvent | ||||||
| 20 min | 40 min | 60 min | 80 min | 20 min | 40 min | 60 min | 80 min | |
| 50 °C 100 bar |
0.86 | 10.33 | 5.13 | 4.85 | 0 | 0 | 0 | 0 |
| 50 °C 400 bar |
2.71 | 1.34 | 1.55 | 1.84 | 11.81 | 29.41 | 32.96 | 32.48 |
| 50 °C 550 bar |
2.33 | 1.21 | 4.82 | 8.60 | 7.30 | 17.31 | 22.57 | 26.67 |
| 65 °C 100 bar |
3.28 | 2.01 | 0.53 | 1.18 | 0 | 0 | 0 | 0 |
| 65 °C 400 bar |
5.24 | 4.39 | 0.01 | 0.01 | 4.96 | 20.86 | 28.53 | 29.46 |
| 65 °C 550 bar |
6.67 | 0.00 | 0.40 | 0.08 | 2.42 | 15.21 | 17.75 | 27.14 |
| 80 °C 100 bar |
0.18 | 0.89 | 2.86 | 1.96 | 0 | 0 | 0 | 0 |
| 80 °C 400 bar |
0.72 | 18.11 | 0.06 | 0.08 | 7.70 | 13.32 | 29.11 | 34.23 |
| 80 °C 550 bar |
1.02 | 14.03 | 0.03 | 0.19 | 2.21 | 8.03 | 19.63 | 22.15 |
As shown in Table 3, an effective improvement of the extraction of astaxanthin in the presence of co-solvent was not observed, despite CO2-SFE with ethanol showing a valuable level of astaxanthin recovery. Moreover, a reduction in purity was found; this result may be explained by considering that, in the presence of ethanol, the total extraction yield increased, reducing the purity of astaxanthin.
2.3. Lutein Recovery
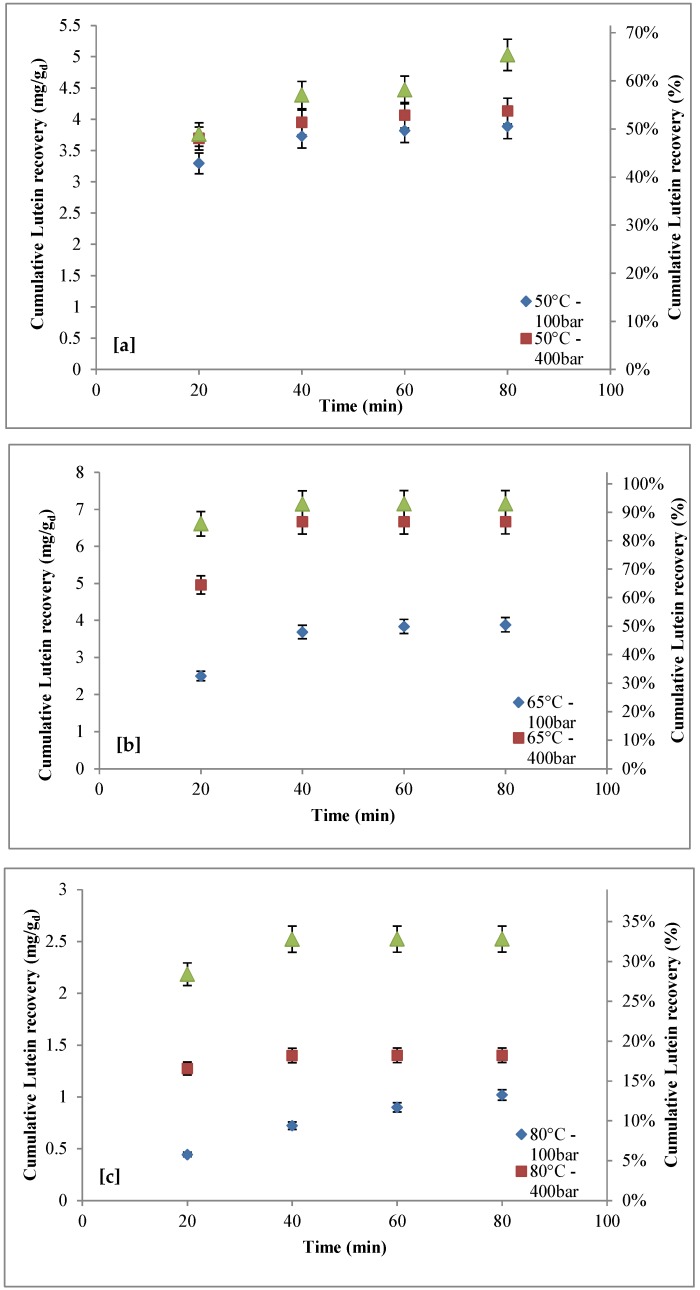
The effects of pressure, temperature, and extraction time (extraction cycle number) on total lutein recovery are reported in Figure 3. Figure 3a–c shows that, keeping extraction temperature constant, lutein recovery increased with extraction time and pressure.
Figure 3.
Effect of pressure (100–550 bar) on lutein recovery as function of the extraction time: (a) T = 50 °C; (b) T = 65 °C; (c) T = 80 °C.
At 50 °C (Figure 3a) with an extraction time of 20 min (first extraction cycle), increasing pressure from 100 to 550 bar resulted in lutein recovery growing from ~3.3 mg/gd (~43%) to ~3.7 mg/gd (~49%). With an extraction time of 80 min (four extraction cycles), lutein extraction yield increased from ~3.9 mg/gd (~50%) to ~5.0 mg/gd (~65%). Increasing extraction time from 20 min to 80 min, at 100 bar, lutein recovery slightly increased from ~3.3 mg/gd (~43%) to ~3.9 mg/gd (~50%); at 400 bar, lutein recovery slightly increased from ~3.7 mg/gd (~48%) to ~4.1 mg/gd (~54%); at 550 bar, lutein recovery increased from ~3.7 mg/gd (~48%) to ~5.0 mg/gd (~65%). At 65 °C (Figure 3b) and at 80 °C (Figure 3c), the influence of extraction time on lutein recovery was quite similar to that observed at 50 °C (Figure 3a); however, the maximum lutein recovery, close to 7.15 mg/gd (~93%) was reached in the second extraction cycle at 65 °C and 550 bar, before decreasing to ~0.4 mg/gd (~6%) at 80 °C and 100 bar. Moreover, it is worth highlighting that, at the best extraction conditions (65 °C and 550 bar), starting from 40 min, the effect of extraction time was negligible (Figure 3b); therefore, a 40 min extraction was sufficient to achieve the maximum lutein extraction yield.
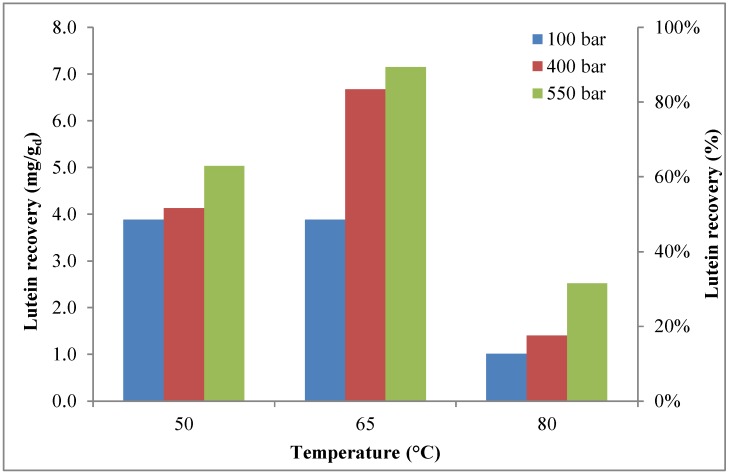
For each operative condition, the lutein yield at the end of a complete cycle of extraction is shown in Figure 4. This figure clearly shows that, at the pressure of 100 bar, almost the same recovery value was obtained at 50 °C and 65 °C, while, at higher extraction pressures, temperature played a crucial role, as lutein recovery increased with temperature, reaching a maximum at 65 °C before decreasing, probably due to the thermal instability of carotenoids, as reported by several authors [63,65]. At the best extraction conditions, it is possible to assume that about 93% of lutein was extracted.
Figure 4.
Effects of temperature (50–80 °C) and pressure (100–550 bar) on cumulative lutein recovery (extraction time = 80 min).
To the best of the authors’ knowledge, few papers focusing on lutein extraction from H. pluvialis via supercritical fluid extraction were published. Nobre et al. [52] reported lutein recovery from H. pluvialis close to 92% at a pressure of 300 bar and a temperature of 60 °C, using CO2-SFE with ethanol as a co-solvent (ethanol 10%/CO2 90%, (v/v)). As shown, our experimental findings are relatively in line with literature data.
The comparison between the cumulative recovery of lutein with and without co-solvent [57] is reported in Table 4. As shown, the presence of ethanol strongly improved the extraction of lutein, as the highest recovery increased from about 51% without co-solvent (P = 550 bar, T = 50 °C) to about 93% with co-solvent (P = 550 bar, T = 65 °C).
Table 4.
Comparison between lutein cumulative recovery with and without co-solvent [57].
| CO2 Flow Rate = 3.62 g/min; Extraction Time = 80 min | ||||||
|---|---|---|---|---|---|---|
| T (°C) | P (bar) | |||||
| 100 | 400 | 550 | 100 | 400 | 550 | |
| Cumulative Recovery with Co-Solvent (%) (Ethanol Flow Rate = 1 mL/min) |
Cumulative Recovery without Co-Solvent (%) | |||||
| 50 | 50.5 | 53.7 | 57.5 | 0.1 | 44.5 | 50.9 |
| 65 | 50.4 | 86.6 | 92.9 | 0 | 33.9 | 37.7 |
| 80 | 13.2 | 18.2 | 32.8 | 1.6 | 17.3 | 1.2 |
The purity of lutein for each stage of extraction, under different operative conditions, with and without co-solvent is reported in Table 5. With co-solvent, the highest purity (~16%) was achieved with an extraction time of 40 min at 65 °C and 550 bar, which are the same conditions that resulted in the highest lutein recovery, as shown in Figure 3. Without co-solvent, the highest purity (~7.5%) was found with an extraction time of 80 min at 400 bar and 50 °C. As shown, ethanol was effective for the enhancement of lutein purity, as the maximum purity increased from about 7.5% to about 16%.
Table 5.
Purity of lutein for each stage under different operative conditions with and without co-solvent [57].
| CO2 Flow Rate = 3.62 g/min | ||||||||
|---|---|---|---|---|---|---|---|---|
| T and P | Lutein Purity (%) with Co-Solvent | Lutein Purity (%) without Co-Solvent | ||||||
| 20 min | 40 min | 60 min | 80 min | 20 min | 40 min | 60 min | 80 min | |
| 50 °C 100 bar |
0.61 | 2.88 | 1.94 | 1.90 | 0 | 0 | 0 | 0 |
| 50 °C 400 bar |
1.44 | 0.93 | 1.04 | 1.09 | 2.27 | 3.89 | 6.36 | 7.54 |
| 50 °C 550 bar |
1.18 | 5.93 | 3.51 | 0.33 | 1.58 | 2.83 | 2.04 | 4.63 |
| 65 °C 100 bar |
1.08 | 0.98 | 0.29 | 0.44 | 0 | 0 | 0 | 0 |
| 65 °C 400 bar |
1.36 | 6.78 | 0.06 | 0.03 | 0.93 | 0.96 | 1.52 | 3.62 |
| 65 °C 550 bar |
1.73 | 16.57 | 0.35 | 0.28 | 1.64 | 0.68 | 2.31 | 2.80 |
| 80 °C 100 bar |
0.33 | 0.58 | 0.50 | 0.43 | 2.33 | 0.92 | 0.63 | 0.67 |
| 80 °C 400 bar |
0.39 | 5.17 | 0.16 | 0.28 | 0.83 | 0.59 | 1.25 | 1.44 |
| 80 °C 550 bar |
0.76 | 4.89 | 0.08 | 0.08 | 0 | 0.32 | 1.62 | 1.66 |
2.4. Comparison with Literature for Recovery of Astaxanthin and Lutein Using CO2-SFE with Co-Solvent
The comparison between the operative conditions allowing the maximum recovery of astaxanthin and lutein defined in the present work and data available in literature is reported in Table 6. As shown, the supercritical extraction process is an effective technology for the extraction of astaxanthin and lutein, with recoveries higher than 90% for both compounds. In particular, the operative conditions identified in our study, including the type of pre-treatment, produced the highest recovery of astaxanthin and lutein. However, as shown in Figure 1 and Figure 3, in terms of the effect of extraction time on recovery, it is worth highlighting that, at the operative conditions with which the maximum recoveries were achieved (65 °C; 550 bar), the recoveries were relatively unaffected by extraction time. Therefore, the maximum recoveries of astaxanthin and lutein were found during the first extraction cycle (20 min) and the second extraction cycle (40 min), respectively, and they did not vary along with the increase in extraction time (Figure 1 and Figure 3).
Table 6.
Comparison of operative conditions for astaxanthin and lutein recovery from Haematococcus pluvialis.
| Optimum Extraction Conditions | Carotenoid Recovery e | Reference | ||||||
|---|---|---|---|---|---|---|---|---|
| Biomass Loading (g) | CO2 Flow Rate (g/min) | Co-Solvent a | Pre-Treatment | Pb (bar) | Tc (°C) | td (h) | ||
| n.a. § | 100 µL·min−1 | 20% (v/v) ethanol | Hydrotermal | 80 | 55 | 15 min | Astaxanthin 98.3% | [50] |
| n.a. § | 100 µL·min−1 | 20% (v/v) olive oil | Hydrotermal | 80 | 55 | 15 min | Astaxanthin 98.6% | |
| 7 | 1.41 g/min | 5% (v/v) ethanol | Drying | 400 | 70 | 4 | Astaxanthin 77.9% | [53] |
| 2 | 1.41 g/min | 10% (v/v) ethanol | Freeze drying and ball milling | 300 | 60 | - | Astaxanthin >90%; Lutein >90% |
[52] |
| 6 | 1.41 g/min | 10% (v/v) olive oil | Drying | 400 | 70 | 5 | Asthaxanthin 36% | [61] |
| 240 | 7.8 g/min | 2.3 mL/g sample ethanol | Freeze drying (powder form) | 435 | 65 | 3.5 | Astaxanthin 87.42% | [66] |
| 1.38 | 3.62 g/min | 12.5% (v/v) ethanol | Ball milling | 550 | 65 | 1.33 (20 min for Astaxanthin; 40 min for Lutein) # | Astaxanthin 92.4%; Lutein 92.9% |
This study |
a Ethanol/vegetable oils mentioned in the column served as a co-solvent in the extraction; b operating pressure; c operating temperature; d total extraction time; e recovery at optimum conditions; § n.a. = not available; # at the operative conditions with which the maximum recoveries were achieved (65 °C; 550 bar), the recoveries were not affected by extraction time.
3. Materials and Methods
3.1. Samples and Chemicals
Experimental activity was carried out using lyophilized H. pluvialis microalgae in the red phase (HPR) provided by Micoperi Blue Growth (Ravenna, Italy). HPR has a mesh particle sieve size lower than 50 µm, with a total content of astaxanthin of 20.0 mg/g dry weight, corresponding to 2% (w/w) dry weight, which was measured using the method proposed by Li et al. [67]. Since no standard method to measure lutein content in HPR is reported in the literature, lutein content was measured using the same method proposed by Li et al. [67], except that lutein measurement was carried out using ultra (u)HPLC analysis. A value of about 7.7 mg/g dry weight, equal to 38.5% (w/w) astaxanthin content was found, in agreement with Vidhyavathi et al. [68], who found that, for the HPR cystic phase, lutein was about 30–40% (w/w) astaxanthin content. HPR was stored at −20 °C in a vacuum-sealed plastic bag to avoid degradation, and was brought to atmospheric conditions before extraction. All chemicals and standards (astaxanthin and lutein) were of analytical grade and purchased from Sigma Aldrich (St. Louis, MO, USA). All other reagents were of uHPLC grade unless otherwise stated. Carbon dioxide (industrial grade) with a purity of 99.999% was purchased from Rivoira (Bari, Italy).
3.2. Experimental Set-Up
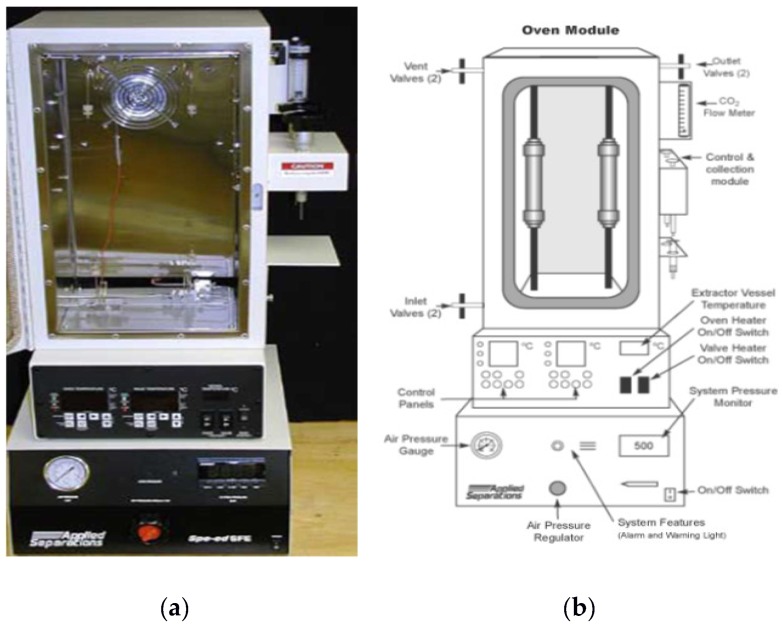
The experimental set-up, shown in Figure 5, was a laboratory-scale equipment with a reactor volume of 30 mL [64]. The bench-scale experimental apparatus for CO2-SFE was equipped with a heater in order to achieve temperatures up to 250 °C, and a pumping system for the compression of CO2 up to 680 bar. Two vessels were located inside the module: the first was used as a CO2 pre-heater and the second was used for extraction. In the extraction vessel, two pressure control systems—inlet and outlet valves (Wika Transmitter, Klingenberg, Germany), with precision of 0.6 mbar—were installed, whereas the CO2 flow rate was controlled using a flow meter LPN/S80 AL G 2.5 (SACOFGAS, Milano, Italy). The inlet flow rate was adjustable up to 25 mL/min and the flow control was carried out on the expanded gas. Temperature was monitored using thermocouples, while the inlet and outlet flow streams were controlled using micrometric valves. The experimental apparatus was also equipped with a specific line for supplying co-solvent, using a syringe pump (Speed SFE Modifier Pump Module-PN 7170, Allentown, PA, USA) to compress the co-solvent up to 680 bar and to regulate the flow rate up to 10 mL/min.
Figure 5.
(a) Picture of the CO2 superfluid extractor (CO2-SFE) 2; (b) schema of bench-scale CO2-SFE.
The experimental apparatus was also equipped with acoustic and visual high-pressure alerts and, as the primary security system, a rupture disc was installed. All parameters of the process were controlled using a distributed control system (DCS). Before each extraction stage, HPR was mechanically pre-treated using the Mixer Mill (Retsch MM400, Haan, Germany) with three steel spheres of 1 cm for 5 min at 400 rpm, in which 1.4 g of HPR was mixed with 0.8 g of diatom land [69].
For each experimental test, four extraction cycles of 20 min each (extraction time = 20–80 min) were carried out. Carbon dioxide and ethanol flow rates were kept constant at 3.62 g/min and 1 mL/min, respectively. Temperature (T) and pressure (P) were varied in the ranges of 50–80 °C and 100–550 bar, respectively. The experimental plan, in which HPR biomass loading was included, is reported in the Table 7.
Table 7.
CO2-SFE extraction conditions. HPR—H. pluvialis in the red phase.
| Operative Conditions | ||
|---|---|---|
| T (°C) | P (bar) | HPR Biomass Loading (g) |
| 50 | 100 | 1.43 |
| 50 | 400 | 1.43 |
| 50 | 550 | 1.37 |
| 65 | 100 | 1.36 |
| 65 | 400 | 1.36 |
| 65 | 550 | 1.38 |
| 80 | 100 | 1.35 |
| 80 | 400 | 1.38 |
| 80 | 550 | 1.34 |
The effects of pressure and temperature on astaxanthin and lutein extraction were reported in terms of cumulative recovery and purity [57]. Cumulative recovery was expressed both as mg of the compound extracted for g of dry HRP biomass loading (Equation (1)) and as percentage of the total content (Equation (2)). Purity was calculated as the ratio between the recovery (mg/g) and the total extraction yield (Equation (3)).
| (1) |
| (2) |
| (3) |
where WC,i (mg) is the weight of the compound extracted for each extraction cycle, WB (g) is the weight of HPR biomass loading (Table 7), WT (mg/g) is the total content of the compound (Section 3.1; 20.0 mg/g dry weight for astaxanthin; 7.7 mg/g dry weight for lutein), and WE (mg/g) is the total extraction yield (Table 1).
Each experimental condition was investigated in triplicate.
3.3. Analytical Methods
After each extraction cycle, the sample was collected in an amber vial and subjected to basic hydrolysis in the presence of NaOH (saponification), in order to remove lipids and chlorophylls from the sample, avoiding the overlap of the spectra with the species present in the carotenoid family [56]. Specifically, saponification was carried out by adding 1 mL of NaOH solution in methanol (0.05 M) to 5 mL of extract. This solution was left in the dark in an inert atmosphere for 7 h. Once this step was completed, the sample was neutralized with 3 mL of an NH4Cl solution in methanol (0.05 M). After saponification, astaxanthin and lutein were measured using a uHPLC Agilent 1290 Infinity II with a Zorbax reverse-phase C18 column with methanol/water (95:5, v/v) as a mobile phase solvent, while the sample was dissolved in a mixture of methanol/chloroform (90:10 containing 0.1% butylated hydroxytoluene (BHT) as an antioxidant). The flow rate and column temperature were kept constant at 0.4 mL/min and 28 °C, respectively [69]. The extraction procedure was detailed previously in Di Sanzo et al. [57].
4. Conclusions
In the present work, the effects of time (20–80 min), temperature (50–80 °C), and pressure (100–550 bar) on astaxanthin and lutein extraction from H. pluvialis in the red phase via CO2-SFE with ethanol (GRAS solvent) as a co-solvent were investigated. Moreover, a comparison of CO2-SFE with without ethanol was also carried out.
The results highlight that, for both astaxanthin and lutein, temperature played a crucial role in both extraction yield and carotenoid degradation. At 65 °C and 550 bar, astaxanthin and lutein extraction yields were maximized, avoiding carotenoid thermal degradation. Moreover, at the best extraction conditions (65 °C and 550 bar), a negligible effect of extraction time was observed; therefore, to basically achieve the maximum recovery, an extraction time of 20 min was sufficient for astaxanthin, while an extraction time of 40 min was required for lutein. These conditions led to an astaxanthin extraction yield of about 92%, and a lutein extraction yield of about 93%. From the comparison between experimental findings and literature data, it is possible to see that the operative conditions identified in our study, including the type of pre-treatment, produced the highest recovery of astaxanthin and lutein. In terms of purity of astaxanthin, similar results were achieved.
By comparing the performance of CO2-SFE with and without ethanol, it is worth highlighting that a real effectiveness of ethanol as a co-solvent for astaxanthin extraction was not observed, with the drawback of a reduction in astaxanthin purity. On the other hand, the presence of ethanol was found to be effective for the extraction of lutein, as an enhancement was observed both in terms of recovery and purity.
Acknowledgments
Authors wish to thank prof. Evangelos Hristoforou and dr. Nikos Stefanakis for the helpful suggestions regarding discussion about results.
Author Contributions
Methodology, A.M.; investigation, G.D.S., V.L. and M.M.; writing—original draft preparation, S.M., A.I. and P.C.; writing—review and editing, S.C.; supervision, A.M. and D.M.
Funding
This paper received funding from the Bio Based Industries Joint Undertaking under the European Union’s Horizon 2020 research and innovation program under grant agreement No 745695 (VALUEMAG).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Pulz O., Gross W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004;65:635–648. doi: 10.1007/s00253-004-1647-x. [DOI] [PubMed] [Google Scholar]
- 2.Spolaore P., Joannis-Cassan C., Duran E., Isambert A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006;101:87–96. doi: 10.1263/jbb.101.87. [DOI] [PubMed] [Google Scholar]
- 3.Mendes R.L., Coelho J.P., Fernandes H.L., Marrucho I.J., Cabral J.M.S., Novais J.M., Palavra A.F. Applications of supercritical CO2 extraction to microalgae and plants. J. Chem. Technol. Biotechnol. 1995;62:53–59. doi: 10.1002/jctb.280620108. [DOI] [Google Scholar]
- 4.Ambati R.R., Moi P.S., Ravi S., Aswathanarayana R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs. 2014;12:128–152. doi: 10.3390/md12010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaswir I., Noviendri1 D., Hasrini R.F., Octavianti F. Carotenoids: Sources, medicinal properties and their application in food and nutraceutical industry. J. Med. Plants Res. 2011;5 doi: 10.5897/JMPRX11.011. [DOI] [Google Scholar]
- 6.De Melo M.M.R., Silvestre A.J.D., Silva C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids. 2014;92:115–176. doi: 10.1016/j.supflu.2014.04.007. [DOI] [Google Scholar]
- 7.Ameer K., Shahbaz H.M., Kwon J.H. Green extraction methods for polyphenols from plant matrices and their byproducts: A review. Compr. Rev. Food Sci. Food Saf. 2017;16:295–315. doi: 10.1111/1541-4337.12253. [DOI] [PubMed] [Google Scholar]
- 8.Crampon C., Boutin O., Badens E. Supercritical carbon dioxide extraction of molecules of interest from microalgae and seaweeds. Ind. Eng. Chem. Res. 2011;50:8941–8953. doi: 10.1021/ie102297d. [DOI] [Google Scholar]
- 9.Mendes R.L., Nobre B.P., Cardoso M.T., Pereira A.P., Palavra A.F. Supercritical carbon dioxide extraction of compounds with pharmaceutical importance from microalgae. Inorg. Chim. Acta. 2003;356:328–334. doi: 10.1016/S0020-1693(03)00363-3. [DOI] [Google Scholar]
- 10.Palavra A.M.F., Coelho J.P., Barroso J.G., Rauter A.P., Fareleira J.M.N.A., Mainar A., Urieta J.S., Nobre B.P., Gouveia L., Mendes R.L., et al. Supercritical carbon dioxide extraction of bioactive compounds from microalgae and volatile oils from aromatic plants. J. Supercrit. Fluids. 2011;60:21–27. doi: 10.1016/j.supflu.2011.04.017. [DOI] [Google Scholar]
- 11.Sovová H. Rate of the vegetable oil extraction with supercritical CO2—I. Modeling of extraction curves. Chem. Eng. Sci. 1994;49:409–414. doi: 10.1016/0009-2509(94)87012-8. [DOI] [Google Scholar]
- 12.Wood J.A., Bernards M.A., Wan W.-K., Charpentier P.A. Extraction of ginsenosides from North American ginseng using modified supercritical carbon dioxide. J. Supercrit. Fluids. 2006;39:40–47. doi: 10.1016/j.supflu.2006.01.016. [DOI] [Google Scholar]
- 13.Herrero M., Mendiola J.A., Cifuentes A., Ibáñez E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A. 2010;1217:2495–2511. doi: 10.1016/j.chroma.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Mattea F., Martín Á., Cocero M.J. Carotenoid processing with supercritical fluids. J. Food Eng. 2009;93:255–265. doi: 10.1016/j.jfoodeng.2009.01.030. [DOI] [Google Scholar]
- 15.Smith R.M. Supercritical fluids in separation science—The dreams, the reality and the future. J. Chromatogr. A. 1999;856:83–115. doi: 10.1016/S0021-9673(99)00617-2. [DOI] [PubMed] [Google Scholar]
- 16.Temelli F. Perspectives on supercritical fluid processing of fats and oils. J. Supercrit. Fluids. 2009;47:583–590. doi: 10.1016/j.supflu.2008.10.014. [DOI] [Google Scholar]
- 17.Uddin M.S., Sarker M.Z.I., Ferdosh S., Akanda M.J.H., Easmin M.S., Bt Shamsudin S.H., Yunus K. Bin Phytosterols and their extraction from various plant matrices using supercritical carbon dioxide: A review. J. Sci. Food Agric. 2015;95:1385–1394. doi: 10.1002/jsfa.6833. [DOI] [PubMed] [Google Scholar]
- 18.Huang Z., Shi X., Jiang W. Theoretical models for supercritical fluid extraction. J. Chromatogr. A. 2012;1250:2–26. doi: 10.1016/j.chroma.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 19.Brunner G. Supercritical fluids: Technology and application to food processing. J. Food Eng. 2005;67:21–33. doi: 10.1016/j.jfoodeng.2004.05.060. [DOI] [Google Scholar]
- 20.Crampon C., Nikitine C., Zaier M., Lépine O., Tanzi C.D., Vian M.A., Chemat F., Badens E. Oil extraction from enriched Spirulina platensis microalgae using supercritical carbon dioxide. J. Supercrit. Fluids. 2017;119:289–296. doi: 10.1016/j.supflu.2016.10.006. [DOI] [Google Scholar]
- 21.Phelps C.L., Smart N.G., Wai C.M. Past, present, and possible future applications of supercritical fluid extraction technology. J. Chem. Educ. 1996;73:1163. doi: 10.1021/ed073p1163. [DOI] [Google Scholar]
- 22.Raventós M., Duarte S., Alarcón R. Application and possibilities of supercritical CO2 extraction in food processing industry: An overview. Food Sci. Technol. Int. 2002;8:269–284. doi: 10.1106/108201302029451. [DOI] [Google Scholar]
- 23.Span R., Wagner W. A new equation of state for carbon dioxide covering the fluid region from the triple-point temperature to 1100 K at pressures up to 800 MPa. J. Phys. Chem. Ref. Data. 1996;25:1509–1596. doi: 10.1063/1.555991. [DOI] [Google Scholar]
- 24.Fernández-Ronco M.P., De Lucas A., Rodríguez J.F., García M.T., Gracia I. New considerations in the economic evaluation of supercritical processes: Separation of bioactive compounds from multicomponent mixtures. J. Supercrit. Fluids. 2013;79:345–355. doi: 10.1016/j.supflu.2013.01.018. [DOI] [Google Scholar]
- 25.Shilpi A., Shivhare U.S., Basu S. Supercritical CO2 extraction of compounds with antioxidant activity from fruits and vegetables waste—A review. Focus. Mod. Food Ind. 2013;2:43–62. [Google Scholar]
- 26.Halim R., Danquah M.K., Webley P.A. Extraction of oil from microalgae for biodiesel production: A review. Biotechnol. Adv. 2012;30:709–732. doi: 10.1016/j.biotechadv.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Brennecke J.F., Eckert C.A. Phase equilibria for supercritical fluid process design. AIChE J. 1989;35:1409–1427. doi: 10.1002/aic.690350902. [DOI] [Google Scholar]
- 28.Han X., Poliakoff M. Continuous reactions in supercritical carbon dioxide: problems, solutions and possible ways forward. Chem. Soc. Rev. 2012;41:1428. doi: 10.1039/c2cs15314a. [DOI] [PubMed] [Google Scholar]
- 29.Mohamed R.S., Debenedetti P.G., Prud’homme R.K. Effects of process conditions on crystals obtained from supercritical mixtures. AIChE J. 1989;35:325–328. doi: 10.1002/aic.690350220. [DOI] [Google Scholar]
- 30.Randolph T.W. Supercritical fluid extractions in biotechnology. Trends Biotechnol. 1990;8:78–82. doi: 10.1016/0167-7799(90)90140-S. [DOI] [PubMed] [Google Scholar]
- 31.Rozzi N.L., Singh R.K. Supercritical fluids and the food industry. Compr. Rev. Food Sci. Food Saf. 2002;1:33–44. doi: 10.1111/j.1541-4337.2002.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 32.Francis A.W. Ternary systems of liquid carbon dioxide. J. Phys. Chem. 1954;58:1099–1114. doi: 10.1021/j150522a014. [DOI] [Google Scholar]
- 33.Reverchon E., De Marco I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids. 2006;38:146–166. doi: 10.1016/j.supflu.2006.03.020. [DOI] [Google Scholar]
- 34.Kitzberger C.S.G., Smânia A., Pedrosa R.C., Ferreira S.R.S. Antioxidant and antimicrobial activities of shiitake (Lentinula edodes) extracts obtained by organic solvents and supercritical fluids. J. Food Eng. 2007;80:631–638. doi: 10.1016/j.jfoodeng.2006.06.013. [DOI] [Google Scholar]
- 35.Mendiola J.A., Herrero M., Cifuentes A., Ibañez E. Use of compressed fluids for sample preparation: Food applications. J. Chromatogr. A. 2007;1152:234–246. doi: 10.1016/j.chroma.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 36.Zancan K.C., Marques M.O.M., Petenate A.J., Meireles M.A.A. Extraction of ginger (Zingiber officinale roscoe) oleoresin with CO2 and co-solvents: A study of the antioxidant action of the extracts. J. Supercrit. Fluids. 2001;24:57–76. doi: 10.1016/S0896-8446(02)00013-X. [DOI] [Google Scholar]
- 37.Food and Drug Administration Substances Generally Recognized as Safe; Final Rule. Fed. Regist. 2016;81:54959–55055. [Google Scholar]
- 38.Lang Q., Wai C.M. Supercritical fluid extraction in herbal and natural product studies—A practical review. Talanta. 2001;53:771–782. doi: 10.1016/S0039-9140(00)00557-9. [DOI] [PubMed] [Google Scholar]
- 39.Reverchon E. Supercritical fluid extraction and fractionation of essential oils and related products. J. Supercrit. Fluids. 1997;10:1–37. doi: 10.1016/S0896-8446(97)00014-4. [DOI] [Google Scholar]
- 40.Sahena F., Zaidul I.S.M., Jinap S., Karim A.A., Abbas K.A., Norulaini N.A.N., Omar A.K.M. Application of supercritical CO2 in lipid extraction—A review. J. Food Eng. 2009;95:240–253. doi: 10.1016/j.jfoodeng.2009.06.026. [DOI] [Google Scholar]
- 41.Herrero M., del PilarSánchez-Camargo A., Cifuentes A., Ibáñez E. Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. TrAC Trends Anal. Chem. 2015;71:26–38. doi: 10.1016/j.trac.2015.01.018. [DOI] [Google Scholar]
- 42.Mäki-Arvela P., Hachemi I., Murzin D.Y. Comparative study of the extraction methods for recovery of carotenoids from algae: Extraction kinetics and effect of different extraction parameters. J. Chem. Technol. Biotechnol. 2014;89:1607–1626. doi: 10.1002/jctb.4461. [DOI] [Google Scholar]
- 43.Yen H.W., Yang S.C., Chen C.H., Chang J.S. Supercritical fluid extraction of valuable compounds from microalgal biomass. Bioresour. Technol. 2015;184:291–296. doi: 10.1016/j.biortech.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 44.Catchpole O., Moreno T., Montañes F., Tallon S. Perspectives on processing of high value lipids using supercritical fluids. J. Supercrit. Fluids. 2018;134:260–268. doi: 10.1016/j.supflu.2017.12.001. [DOI] [Google Scholar]
- 45.Sarada R., Tripathi U., Ravishankar G.A. Influence of stress on astaxanthin production in Haematococcus pluvialis grown under different culture conditions. Process Biochem. 2002;37:623–627. doi: 10.1016/S0032-9592(01)00246-1. [DOI] [Google Scholar]
- 46.Chekanov K., Lobakova E., Selyakh I., Semenova L., Sidorov R., Solovchenko A. Accumulation of astaxanthin by a new Haematococcus pluvialis strain BM1 from the white sea coastal rocks (Russia) Mar. Drugs. 2014;12:4504–4520. doi: 10.3390/md12084504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heimann K., Huerlimann R. Microalgal Classification: Major Classes and Genera of Commercial Microalgal Species. Handb. Mar. Sci. 2015;578:25–41. doi: 10.1016/B978-0-12-800776-1.00003-0. [DOI] [Google Scholar]
- 48.Thana P., Machmudah S., Goto M., Sasaki M., Pavasant P., Shotipruk A. Response surface methodology to supercritical carbon dioxide extraction of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2008;99:3110–3115. doi: 10.1016/j.biortech.2007.05.062. [DOI] [PubMed] [Google Scholar]
- 49.Valderrama J.O., Perrut M., Majewski W. Extraction of Astaxantine and phycocyanine from microalgae with supercritical carbon dioxide. J. Chem. Eng. Data. 2003;48:827–830. doi: 10.1021/je020128r. [DOI] [Google Scholar]
- 50.Cheng X., Qi Z.B., Burdyny T., Kong T., Sinton D. Low pressure supercritical CO2 extraction of astaxanthin from Haematococcus pluvialis demonstrated on a microfluidic chip. Bioresour. Technol. 2018;250:481–485. doi: 10.1016/j.biortech.2017.11.070. [DOI] [PubMed] [Google Scholar]
- 51.Kwan T.A., Kwan S.E., Peccia J., Zimmerman J.B. Selectively biorefining astaxanthin and triacylglycerol co-products from microalgae with supercritical carbon dioxide extraction. Bioresour. Technol. 2018;269:81–88. doi: 10.1016/j.biortech.2018.08.081. [DOI] [PubMed] [Google Scholar]
- 52.Nobre B., Marcelo F., Passos R., Beirão L., Palavra A., Gouveia L., Mendes R. Supercritical carbon dioxide extraction of astaxanthin and other carotenoids from the microalga Haematococcus pluvialis. Eur. Food Res. Technol. 2006;223:787–790. doi: 10.1007/s00217-006-0270-8. [DOI] [Google Scholar]
- 53.Machmudah S., Shotipruk A., Goto M., Sasaki M., Hirose T. Extraction of astaxanthin from Haematococcus pluvialis using supercritical CO2 and ethanol as entrainer. Ind. Eng. Chem. Res. 2006;45:3652–3657. doi: 10.1021/ie051357k. [DOI] [Google Scholar]
- 54.Kitada K., Machmudah S., Sasaki M., Goto M., Nakashima Y., Kumamoto S., Hasegawa T. Supercritical CO2 extraction of pigment components with pharmaceutical importance from Chlorella vulgaris. J. Chem. Technol. Biotechnol. 2009;84:657–661. doi: 10.1002/jctb.2096. [DOI] [Google Scholar]
- 55.Ruen-Ngam D., Shotipruk A., Pavasant P., Machmudah S., Goto M. Selective extraction of lutein from alcohol treated Chlorella vulgaris by supercritical CO2. Chem. Eng. Technol. 2012;35:255–260. doi: 10.1002/ceat.201100251. [DOI] [Google Scholar]
- 56.Yen H.W., Chiang W.C., Sun C.H. Supercritical fluid extraction of lutein from Scenedesmus cultured in an autotrophical photobioreactor. J. Taiwan Inst. Chem. Eng. 2012;43:53–57. doi: 10.1016/j.jtice.2011.07.010. [DOI] [Google Scholar]
- 57.Sanzo G., Mehariya S., Martino M., Larocca V., Casella P., Chianese S., Musmarra D., Balducchi R., Molino A. Supercritical carbon dioxide extraction of astaxanthin, lutein, and fatty acids from Haematococcus pluvialis microalgae. Mar. Drugs. 2018;16:334. doi: 10.3390/md16090334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poojary M.M., Barba F.J., Aliakbarian B., Donsì F., Pataro G., Dias D.A., Juliano P. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar. Drugs. 2016;14:214. doi: 10.3390/md14110214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fahmy T.M., Johnson D.M., McNally M.P., Fahmy T.M., Paulaitis M.E. Modifier effects in the supercritical fluid extraction of solutes from clay, soil, and plant materials. Anal. Chem. 1993;65:1462–1469. doi: 10.1021/ac00058a026. [DOI] [Google Scholar]
- 60.Chafer A., Fornari T., Berna A., Stateva R.P. Solubility of quercetin in supercritical CO2+ ethanol as a modifier: Measurements and thermodynamic modelling. J. Supercrit. Fluids. 2004;32:89–96. doi: 10.1016/j.supflu.2004.02.005. [DOI] [Google Scholar]
- 61.Krichnavaruk S., Shotipruk A., Goto M., Pavasant P. Supercritical carbon dioxide extraction of astaxanthin from Haematococcus pluvialis with vegetable oils as co-solvent. Bioresour. Technol. 2007;99:5556–5560. doi: 10.1016/j.biortech.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 62.Bustamante A., Roberts P., Aravena R., Valle J. Supercritical extraction of astaxanthin from H. pluvialis using ethanol-modified CO2. Experiments and modeling; Proceedings of the 11th International Conference of Eng Food; Athens, Greece. 22–26 May 2011. [Google Scholar]
- 63.Pan J.L., Wang H.M., Chen C.Y., Chang J.S. Extraction of astaxanthin from Haematococcus pluvialis by supercritical carbon dioxide fluid with ethanol modifier. Eng. Life Sci. 2012;12:638–647. doi: 10.1002/elsc.201100157. [DOI] [Google Scholar]
- 64.Reyes F.A., Mendiola J.A., Ibañez E., Del Valle J.M. Astaxanthin extraction from Haematococcus pluvialis using CO2-expanded ethanol. J. Supercrit. Fluids. 2014;92:75–83. doi: 10.1016/j.supflu.2014.05.013. [DOI] [Google Scholar]
- 65.Ruen-ngam D., Shotipruk A., Pavasant P. Comparison of extraction methods for recovery of astaxanthin from Haematococcus pluvialis. Sep. Sci. Technol. 2011;46:64–70. doi: 10.1080/01496395.2010.493546. [DOI] [Google Scholar]
- 66.Vasapollo G., Longo L., Rescio L., Ciurlia L. Innovative supercritical CO2 extraction of lycopene from tomato in the presence of vegetable oil as co-solvent. J. Supercrit. Fluids. 2004;29:87–96. doi: 10.1016/S0896-8446(03)00039-1. [DOI] [Google Scholar]
- 67.Li Y., Miao F., Geng Y., Lu D., Zhang C., Zeng M. Accurate quantification of astaxanthin from Haematococcus crude extract spectrophotometrically. Chin. J. Oceanol. Limnol. 2012;30:627–637. doi: 10.1007/s00343-012-1217-5. [DOI] [Google Scholar]
- 68.Vidhyavathi R., Venkatachalam L., Kamath B.S., Sarada R., Ravishankar G.A. Differential expression of carotenogenic genes and associated changes in pigment profile during regeneration of Haematococcus pluvialis cysts. Appl. Microbiol. Biotechnol. 2007;75:879–887. doi: 10.1007/s00253-007-0876-1. [DOI] [PubMed] [Google Scholar]
- 69.Molino A., Rimauro J., Casella P., Cerbone A., Larocca V., Chianese S., Karatza D., Mehariya S., Ferraro A., Hristoforou E., et al. Extraction of astaxanthin from microalga Haematococcus pluvialis in red phase by using generally recognized as safe solvents and accelerated extraction. J. Biotechnol. 2018;283:51–61. doi: 10.1016/j.jbiotec.2018.07.010. [DOI] [PubMed] [Google Scholar]