Abstract
Invasive pancreatic ductal adenocarcinoma (PDAC) can infiltrate back into and spread along preexisting pancreatic ducts and ductules in a process known as cancerization of ducts (COD). Histologically COD can mimic high-grade pancreatic intraepithelial neoplasia (HG-PanIN). We reviewed pancreatic resections from 100 patients with PDAC for the presence or absence of ducts with histologic features of COD. Features supporting COD included adjacent histologically similar invasive PDAC and an abrupt transition between markedly atypical intraductal epithelium and normal duct epithelium or circumferential involvement of a duct. As the TP53 and SMAD4 genes are frequently targeted in invasive PDAC but not HG-PanIN, paired PDAC and histologically suspected COD lesions were immunolabeled with antibodies to the p53 and Smad4 proteins. Suspected COD was identified on hematoxylin and eosin sections in 89 (89%) of the cases. Immunolabeling for p53 and Smad4 was performed in 68 (76%) of 89 cases. p53 was interpretable in 55 cases and all 55 (100%) cases showed concordant labeling between COD and invasive PDAC. There was matched aberrant p53 immunolabeling in 37 (67%) cases including overexpression in 30 (55%) cases and lack of expression in 7 (13%) cases. Smad4 immunolabeling was interpretable in 61 cases and 59 (97%) cases showed concordant labeling between COD and invasive PDAC. Matched loss of Smad4 was seen in 28 (46%) cases. The immunolabeling of invasive PDAC and COD for p53 and Smad4 supports the high prevalence of COD observed on hematoxylin and eosin and highlights the utility of p53 and Smad4 immunolabeling in differentiating COD and HG-PanIN.
Key Words: cancerization of ducts, pancreatic ductal adenocarcinoma, pancreatic intraepithelial neoplasia, p53, Smad4
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive and deadly form of cancer. It is the third leading cause of death in the United States and has a 5-year survival rate of only 8%.1 The recognition and molecular characterization of precursor lesions, particularly pancreatic intraepithelial neoplasia (PanIN), has improved our understanding of the pathogenesis of invasive PDAC and provided a target for early detection.2–4 PanIN is classified into low-grade (LG-PanIN) or high-grade (HG-PanIN) depending on the degree of dysplasia.5 HG-PanIN is characterized by severe dysplasia and is considered a direct precursor to invasive PDAC.2,6
PDACs are characteristically highly infiltrative neoplasms, and often infiltrate into blood vessels and when they do they can grow for some distance within these vessels.7 Similarly, PDACs can grow from the stroma into and extend along preexisting ducts, a pattern of growth known as “cancerization of ducts” (COD).5,8–10 Both COD and HG-PanIN are intraductal epithelial lesions showing marked dysplasia and may appear very similar on histology. COD is suspected when there is immediately adjacent invasive carcinoma, which is histologically similar to the duct lesion. COD will frequently show an abrupt transition between the marked dysplasia of the neoplastic cells and the complete absence of dysplasia in the normal duct epithelium, although circumferential duct involvement can be seen. Importantly, invasive PDAC and HG-PanIN have distinct molecular alterations and immunolabeling patterns, which can aid in the distinction of COD versus HG-PanIN. Specifically, mutations in TP53 and SMAD4 genes are late events in the genetic progression of PDAC and are prevalent in invasive PDAC but less so in isolated HG-PanIN lesions.3 Likewise, immunohistochemistry shows aberrant expression of p53 in 60% to 70% and loss of Smad4 in 55% of invasive PDAC, while aberrant immunolabeling for these proteins is rarely seen in isolated HG-PanIN.3,11
In this study, we describe the histologic features and prevalence of COD in a series of surgically resected pancreata and validate our findings using immunolabeling for p53 and Smad4.
MATERIALS AND METHODS
Case Selection
After obtaining institutional review board approval, our pathology database was searched to identify pancreatic resections from patients with PDAC from 2009 to 2010. Patients treated with neoadjuvant chemotherapy or preoperative radiation therapy were excluded. One hundred pancreatic resections from 100 patients were included and electronic medical records for each patient were reviewed for recurrence and survival.
Identification of Duct Lesions
Available hematoxylin and eosin (H&E)–stained slides from each case were reviewed by 2 of the authors (D.H. and R.H.H.) for the presence or absence of ducts with histologic features of COD. Duct lesions were considered suspicious for COD when the lesion was immediately adjacent to an invasive carcinoma, the cytology of the lesion was similar to that of the invasive PDAC (eg, both with clear cytoplasm) and when there was an abrupt transition between lesional and normal ductal epithelium or circumferential involvement of a duct. Unstained sections containing suspected COD and invasive PDAC were cut from formalin-fixed paraffin-embedded blocks for immunohistochemical analysis.
Immunolabeling
Immunohistochemistry was performed with anti-p53 mouse monoclonal antibody (Clone BP-53-11; Ventana Medical Systems Inc., Oro Valley, AZ) and anti-Smad4 mouse monoclonal antibody (clone B8; Santa Cruz Biotechnology Inc., Dallas, TX) and interpreted by 2 authors (D.H. and R.H.H.) as previously described.3,12,13 Briefly, p53 immunolabeling was considered aberrant when there was diffuse nuclear labeling in >60% of cells (overexpression) or complete absence of nuclear labeling (lack of expression). Scattered nuclear labeling (wild-type) of nonneoplastic stromal cells was used as a positive internal control. Smad4 immunolabeling was considered lost when there was complete absence of nuclear and cytoplasmic labeling and intact when there was diffuse or partial nuclear and/or cytoplasmic labeling. Nuclear and cytoplasmic labeling of nonneoplastic pancreatic ductal cells and islet cells were used as a positive internal control.
Statistical Analysis
Statistical analyses were performed using R statistical programming language (R Foundation, Vienna, Austria). Median values for age, tumor size and number of positive lymph nodes between cases with and without COD were compared using Mann-Whitney U test. All remaining clinicopathologic characteristics were compared using Pearson χ2 test. A Cox proportional hazards model adjusting for age, race, tumor size and lymph node status was used to test for differences in survival between cases with and without COD.
RESULTS
Case Characteristics
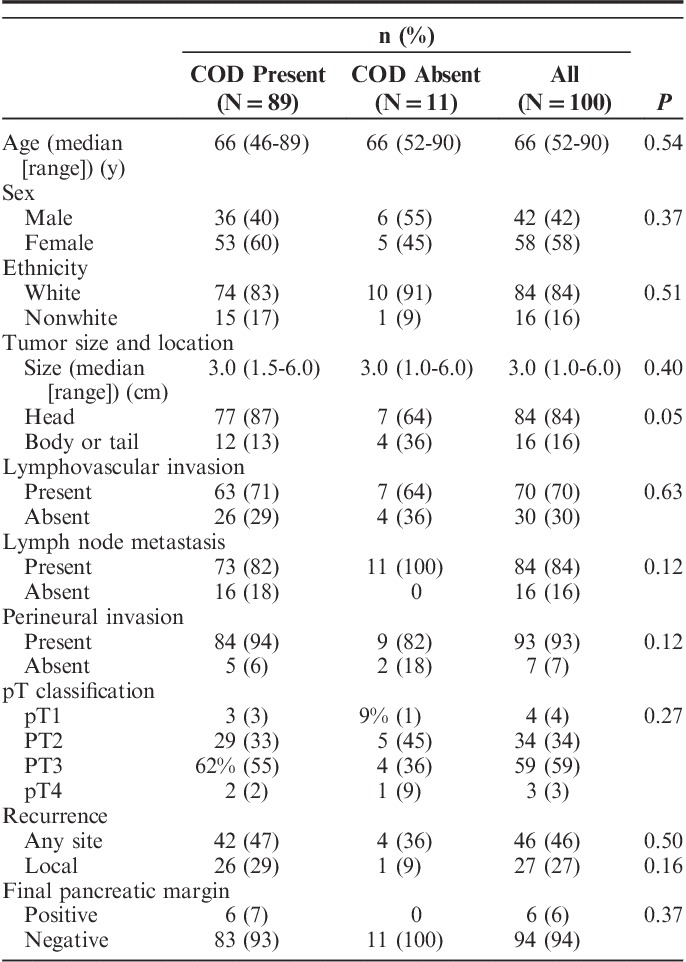
The case characteristics are summarized in Table 1. Of the 100 patients, 58 (58%) were female, 84 (84%) were of white ethnicity and the median age was 66 years (range: 46 to 90 y). The median tumor size was 3.0 cm (range: 1.0 to 6.0 cm) and 84 (84%) of the cancers were located in the head of the pancreas. Lymphovascular and perineural invasion were reported in 70 (70%) cases and 93 (93%) cases, respectively. Lymph node metastasis were present in 84 (84%) of cases and the median number of positive lymph nodes per case was 3 (range: 1 to 15). The final pancreatic parenchymal margin was negative in 94 (94%) cases. The follow-up period ranged from 0.1 to 96 months (median: 15.8 mo) and 64 (64%) patients were followed until death. The median survival was 16.1 months. There was recurrence of PDAC in 46 cases including local recurrence in 27 cases and/or metastasis to liver, lung, lymph nodes, vertebra or omentum in 19 cases.
TABLE 1.
Clinicopathologic Characteristics of Pancreatic Ductal Adenocarcinoma With and Without Cancerization of Ducts
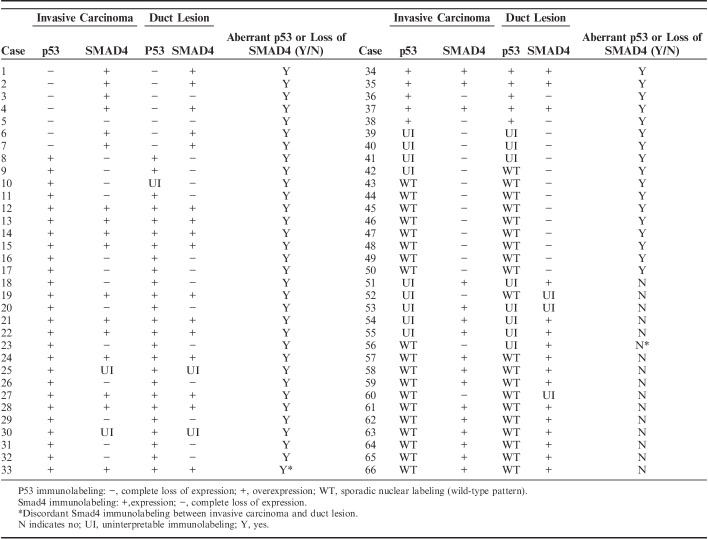
Immunolabeling for p53 and Smad4
Duct lesions suspicious for COD were observed on routine H&E stained sections in 89 (89%) of 100 cases. Immunolabeling for p53 and Smad4 was performed in 68 (76%) of these 89 cases. Tissue blocks were not available or the duct lesion of interest was not present on deeper sections in 21 cases. In 2 cases, p53 and Smad4 immunolabeling was not interpretable in both the invasive carcinoma and duct lesion. The results for 66 cases with interpretable immunolabeling are presented in Table 2.
TABLE 2.
Immunolabeling of Paired Duct Lesions and Invasive Carcinoma With p53 and SMAD4
Overall, 50 (76%) of 66 cases had matched aberrant p53 and/or loss of Smad4 in the invasive PDAC and duct lesion. p53 immunolabeling was interpretable in both the duct lesion and invasive PDAC in 55 cases. All 55 (100%) cases showed concordant p53 labeling between the duct lesion and invasive PDAC. There was aberrant p53 immunolabeling in 37 (67%) cases including overexpression in 30 (55%) cases and complete lack of expression in 7 (13%) cases (Fig. 1). Eighteen (33%) cases showed sporadic nuclear p53 labeling (wild-type pattern). p53 was not interpretable in the duct lesion and/or invasive PDAC in 11 cases due to lack of positive internal control, tissue edge effect or heterogenous labeling of the lesional cells.
FIGURE 1.
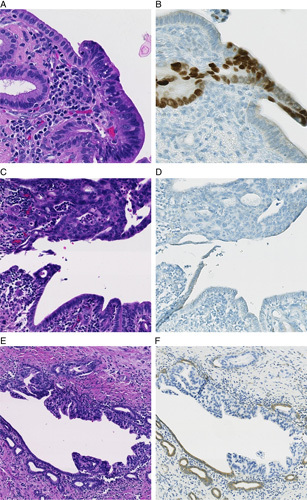
Cancerization of ducts, or intraductal spread of invasive ductal adenocarcinoma, showing overexpression of p53 (B), lack of expression of p53 (D) and loss of Smad4 (F). H&E stain (A, C, E). p53 immunostain (B, D). Smad4 immunostain (E).
Immunolabeling for Smad4 was interpretable in both the duct lesion and invasive PDAC in 61 cases. The duct lesion and invasive PDAC had concordant labeling in 59 (97%) of these 61 cases. There was matched loss of Smad4 expression in the duct lesion and invasive PDAC in 28 (46%) cases (Fig. 1). Intact Smad4 labeling in both components was seen in 31 (51%) cases. Two (3%) cases showed discordant labeling. In one case, Smad4 was lost in the invasive PDAC and retained in the duct lesion (Table 2, case 56). In the second case, Smad4 was intact in the invasive PDAC and lost in duct lesion (Table 2, case 33). Smad4 was not interpretable in 5 cases due to lack of positive internal control or tissue edge effect.
Cancerization of Ducts
Of the 89 suspicious duct lesions, the p53 and/or Smad4 immunolabeling pattern confirmed the presence of COD in 50 (56%) cases. All 39 cases with wild-type p53 and intact Smad4 or uninterpretable or absent immunolabeling were considered most consistent with COD based on histologic features. All 11 cases without COD also lacked HG-PanIN. For the statistical analyses we combined the 50 definitive COD cases (supported by both H&E histology and immunolabeling) together with the 39 suspected COD cases (consistent by H&E but inconclusive by immunolabeling). The clinicopathologic characteristics of cases with and without COD were compared and are summarized in Table 1. There were no statistically significant associations between COD and age, sex, ethnicity, tumor size, tumor location, lymphovascular invasion, presence or absence of lymph node metastasis, perineural invasion, pT classification, recurrence or pancreatic margin status. Among the 84 total cases with lymph node metastasis, COD was associated with more positive lymph nodes per case (median: 4; n=73) versus those without COD (median: 1; n=11; P=0.004). Survival analysis did not show a statistically significant difference between cases with COD versus those without (hazard ratio, 1.90; P=0.15).
DISCUSSION
Our results demonstrate that COD, or intraductal spread of PDAC, is common in pancreatic cancer. A similar pattern of intraductal growth has been reported in acinar cell carcinoma of the pancreas and colon cancer metastatic to the liver.14–18 We identified COD histologically in 89% of cases suggesting that it is even more prevalent than previously reported.8–10 Interestingly, all 11 cases without histologic evidence of COD also lacked HG-PanIN suggesting that COD is much more common than HG-PanIN.
We validated our histologic impression of COD using immunolabeling of paired duct lesions and invasive PDAC for p53 and Smad4. Hosoda et al3 recently whole exome sequenced a series of well-characterized, isolated PanIN lesions and demonstrated that isolated HG-PanIN lesions without associated invasive PDAC rarely have mutations in TP53 and SMAD4 and rarely show aberrant p53 and Smad4 immunolabeling. This is in contrast to prior studies of lesions interpreted to be HG-PanIN that were adjacent to pancreatic cancer, which were likely confounded by the presence of COD.6,19–21 In this present study, the duct lesions consistent with COD histologically had a high prevalence of aberrant immunolabeling for p53 and for Smad4, and there was a high concordance of immunolabeling between invasive PDAC and these duct lesions for p53 (100%) and Smad4 (96%). We saw matched aberrant p53 in 67% of cases and loss of Smad4 in 46% similar to the reported prevalence of these alterations in invasive PDAC.11
Taken together, our histologic findings and immunolabeling results indicate that the duct lesions that we studied most likely represent COD. However, we acknowledge that in some situations it may be difficult and even impossible to distinguish COD from HG-PanIN. For example, the histologic features of HG-PanIN may overlap with those of COD (eg, an abrupt transition from markedly dysplastic epithelium to normal epithelium). Immunolabeling for p53 and Smad4 is useful but can be unrevealing in carcinomas which lack TP53 and SMAD4 alterations. Finally, although it would be extremely rare to actually catch the event, aberrant p53 and/or loss of Smad4 cannot exclude HG-PanIN lesions which have acquired p53 and/or Smad4 alterations in the process of transformation into an invasive carcinoma.
In clinical practice, the utilization of Smad4 and p53 immunolabeling to distinguish COD from HG-PanIN may of particular value in evaluating surgical margins. Matthaei and colleagues showed that the presence of HG-PanIN at a surgical margin has no impact on survival, while the presence of invasive carcinoma at a resection margin has been associated with shortened survival.22,23 As COD is an extension of invasive carcinoma, the presence of COD at a surgical margin may have prognostic significance. Additional studies are needed to definitively address this.
In our analysis of COD and clinicopathologic characteristics, we found that COD was associated with more positive lymph nodes per case (median: 4) which is an adverse prognostic factor.22,24–26 We saw no difference in survival or any other clinicopathologic parameters between cases with and without COD, however, our statistical power was limited due to the relatively small number of cases without COD (n=11).
In addition to clinical significance, recognition of the high prevalence of COD in pancreata with PDAC has important implications for researchers. Studies that include PanIN lesions harvested from pancreata with a PDAC may include COD lesions misclassified as PanIN lesions. As PDAC can grow along ducts for some distance, the only way to avoid this blunder is to study PanIN lesions isolated from pancreata without an invasive cancer. Moreover, the parallels between COD and growth of PDAC into veins suggests an avenue for future research—to determine if and why pancreatic cancer has a predilection to grow into and along preexisting tubular structures.7
In summary, we reviewed a large series of resected pancreata and found that COD, the intraductal spread of invasive PDAC, is extraordinarily common. We immunolabeled paired duct lesions and invasive PDAC for p53 and Smad4 and found there was high concordance in immunolabeling pattern thereby validating the high prevalence of COD observed on H&E and highlighting the utility of p53 and Smad4 immunolabeling in distinguishing COD and HG-PanIN.
Footnotes
Conflicts of Interest and Source of Funding: Supported by the NIH SPORE grant P50 CA62924; NIH/NIDDK K08 DK107781; Sol Goldman Pancreatic Cancer Research Center; Buffone Family Gastrointestinal Cancer Research Fund; Kaya Tuncer Career Development Award in Gastrointestinal Cancer Prevention; AGA-Bernard Lee Schwartz Foundation Research Scholar Award in Pancreatic Cancer; Sidney Kimmel Foundation for Cancer Research Kimmel Scholar Award; AACR-Incyte Corporation Career Development Award for Pancreatic Cancer Research; Rolfe Pancreatic Cancer Foundation; Joseph C Monastra Foundation; The Gerald O Mann Charitable Foundation (Harriet and Allan Wulfstat, Trustees). The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
REFERENCES
- 1.Siegel R, Miller K, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Andea A, Sarkar F, Adsay VN. Clinicopathological correlates of pancreatic intraepithelial neoplasia: a comparative analysis of 82 cases with and 152 cases without pancreatic ductal adenocarcinoma. Mod Pathol. 2003;16:996–1006. [DOI] [PubMed] [Google Scholar]
- 3.Hosoda W, Chianchiano P, Griffin JF, et al. Genetic analyses of isolated high‐grade pancreatic intraepithelial neoplasia (HG‐PanIN) reveal paucity of alterations in TP53 and SMAD4. J Pathol. 2017;242:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lennon AM, Wolfgang CL, Canto MI, et al. The early detection of pancreatic cancer: what will it take to diagnose and treat curable pancreatic neoplasia? Cancer Res. 2014;74:3381–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basturk O, Hong S-M, Wood LD, et al. A revised classification system and recommendations from the Baltimore consensus meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol. 2015;39:1730–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brat DJ, Lillemoe KD, Yeo CJ, et al. Progression of pancreatic intraductal neoplasias to infiltrating adenocarcinoma of the pancreas. Am J Surg Pathol. 1998;22:163–169. [DOI] [PubMed] [Google Scholar]
- 7.Hong S-M, Goggins M, Wolfgang CL, et al. Vascular invasion in infiltrating ductal adenocarcinoma of the pancreas can mimic pancreatic intraepithelial neoplasia: a histopathologic study of 209 cases. Am J Surg Pathol. 2012;36:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamasaki S, Suda K, Nobukawa B, et al. Intraductal spread of pancreatic cancer. Pancreatology. 2002;2:407–412. [DOI] [PubMed] [Google Scholar]
- 9.Ishii M, Kimura Y, Sugita S, et al. Surgical and oncological impact of main pancreatic duct spread in invasive ductal adenocarcinoma: a clinicopathological study of 184 resected cases. Pancreatology. 2015;15:681–687. [DOI] [PubMed] [Google Scholar]
- 10.Klöppel G, Bommer G, Rückert K, et al. Intraductal proliferation in the pancreas and its relationship to human and experimental carcinogenesis. Virchows Archiv A Pathol Anat Histol. 1980;387:221–233. [DOI] [PubMed] [Google Scholar]
- 11.Hruban RH, Fukushima N. Pancreatic adenocarcinoma: update on the surgical pathology of carcinomas of ductal origin and PanINs. Mod Pathol. 2007;20:S61–S70. [DOI] [PubMed] [Google Scholar]
- 12.Yemelyanova A, Vang R, Kshirsagar M, et al. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol. 2011;24:1248–1253. [DOI] [PubMed] [Google Scholar]
- 13.Wilentz RE, Iacobuzio-Donahue CA, Argani P, et al. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res. 2000;60:2002–2006. [PubMed] [Google Scholar]
- 14.Fabre A, Sauvanet A, Flejou J-F, et al. Intraductal acinar cell carcinoma of the pancreas. Virchows Arch. 2001;438:312–315. [DOI] [PubMed] [Google Scholar]
- 15.Imamura M, Kimura Y, Ito H, et al. Acinar cell carcinoma of the pancreas with intraductal growth: report of a case. Surg Today. 2009;39:1006–1009. [DOI] [PubMed] [Google Scholar]
- 16.Basturk O, Zamboni G, Klimstra DS, et al. Intraductal and papillary variants of acinar cell carcinomas: a new addition to the challenging differential diagnosis of intraductal neoplasms. Am J Surg Pathol. 2007;31:363–370. [DOI] [PubMed] [Google Scholar]
- 17.Toll AD, Mitchell D, Yeo CJ, et al. Acinar cell carcinoma with prominent intraductal growth pattern: case report and review of the literature. Int J Surg Pathol. 2011;19:795–799. [DOI] [PubMed] [Google Scholar]
- 18.Riopel MA, Klimstra DS, Godellas C, et al. Intrabiliary growth of metastatic colonic adenocarcinoma: a pattern of intrahepatic spread easily confused with primary neoplasia of the biliary tract. Am J Surg Pathol. 1997;21:1030–1036. [DOI] [PubMed] [Google Scholar]
- 19.Boschman CR, Stryker S, Reddy JK, et al. Expression of p53 protein in precursor lesions and adenocarcinoma of human pancreas. Am J Pathol. 1994;145:1291–1295. [PMC free article] [PubMed] [Google Scholar]
- 20.Maitra A, Adsay NV, Argani P, et al. Multicomponent analysis of the pancreatic adenocarcinoma progression model using a pancreatic intraepithelial neoplasia tissue microarray. Mod Pathol. 2003;16:902–912. [DOI] [PubMed] [Google Scholar]
- 21.Murphy SJ, Hart SN, Lima JF, et al. Genetic alterations associated with progression from pancreatic intraepithelial neoplasia to invasive pancreatic tumor. Gastroenterology. 2013;145:1098–1109. e1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcea G, Dennison A, Ong S, et al. Tumour characteristics predictive of survival following resection for ductal adenocarcinoma of the head of pancreas. Eur J Surg Oncol. 2007;33:892–897. [DOI] [PubMed] [Google Scholar]
- 23.Matthaei H, Hong S-M, Mayo SC, et al. Presence of pancreatic intraepithelial neoplasia in the pancreatic transection margin does not influence outcome in patients with R0 resected pancreatic cancer. Ann Surg Oncol. 2011;18:3493–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen JW, Bhandari M, Astill DS, et al. Predicting patient survival after pancreaticoduodenectomy for malignancy: histopathological criteria based on perineural infiltration and lymphovascular invasion. HPB. 2010;12:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strobel O, Hinz U, Gluth A, et al. Pancreatic adenocarcinoma: number of positive nodes allows to distinguish several N categories. Ann Surg. 2015;261:961–969. [DOI] [PubMed] [Google Scholar]
- 26.Basturk O, Saka B, Balci S, et al. Substaging of lymph node status in resected pancreatic ductal adenocarcinoma has strong prognostic correlations: proposal for a revised N classification for TNM staging. Ann Surg Oncol. 2015;22:1187–1195. [DOI] [PubMed] [Google Scholar]