Abstract
Background and aims
Dysphagia can be associated with significant morbidity in cancer patients. We aimed to develop and evaluate dysphagia screener tools for use in observational studies (phase 1) and for routine symptom monitoring in clinical care (phase 2).
Methods
Various dysphagia or odynophagia screening questions, selected after an expert panel reviewed the content, criterion, and construct validity, were compared with either functional assessment of cancer therapy ‐ esophageal cancer (FACT‐E) Swallowing Index Cut‐Off Values or to questions adapted from the Patient Reported Outcomes for Common Terminology Criteria for Adverse Events. Sensitivity, specificity, and patient acceptability were assessed.
Results
In Phase 1 (n = 178 esophageal cancer patients), the screening question “How are you currently eating?” had the highest sensitivities and specificities against various Swallowing Index Cut‐Off Value cut‐offs, with the best optimal cutoff associated with weight loss (80% sensitivity and 75% specificity). In phase 2 (255 head and neck, gastro‐esophageal, and thoracic cancer patients), a single question screener (“Do you experience any difficulty or pain upon swallowing?”) versus a Patient Reported Outcomes for Common Terminology Criteria for Adverse Events–like gold standard generated sensitivities between 86% and 94% and specificities between 93% and 100%. This screening question (+/− follow‐up questions) had a median completion time of under 2 minutes, and >90% of patients were willing to complete the survey electronically, did not feel that survey made clinic visit more difficult, and did not find the questions upsetting or distressful.
Conclusion
Our results demonstrate that these screener tools (“How are you currently eating?”, “Do you experience any difficulty or pain upon swallowing?”) can effectively screen dysphagia symptoms without increasing cancer outpatient clinic burden, both in observational studies and for routine clinical monitoring.
Keywords: dysphagia, esophageal cancer, gastric cancer, head and neck cancer, lung cancer, patient‐reported outcomes, symptom screening
Short abstract
Background and aims: Dysphagia can be associated with significant morbidity in cancer patients. We aimed to develop and evaluate dysphagia screener tools for use in observational studies (phase 1) and for routine symptom monitoring in clinical care (phase 2).
Methods: Various dysphagia or odynophagia screening questions, selected after an expert panel reviewed the content, criterion, and construct validity, were compared with either functional assessment of cancer therapy ‐ esophageal cancer (FACT‐E) Swallowing Index Cut‐Off Values or to questions adapted from the Patient Reported Outcomes for Common Terminology Criteria for Adverse Events. Sensitivity, specificity, and patient acceptability were assessed.
Results: In Phase 1 (n = 178 esophageal cancer patients), the screening question “How are you currently eating?” had the highest sensitivities and specificities against various Swallowing Index Cut‐Off Value cut‐offs, with the best optimal cutoff associated with weight loss (80% sensitivity and 75% specificity). In phase 2 (255 head and neck, gastro‐esophageal, and thoracic cancer patients), a single question screener (“Do you experience any difficulty or pain upon swallowing?”) versus a Patient Reported Outcomes for Common Terminology Criteria for Adverse Events–like gold standard generated sensitivities between 86% and 94% and specificities between 93% and 100%. This screening question (+/− follow‐up questions) had a median completion time of under 2 minutes, and >90% of patients were willing to complete the survey electronically, did not feel that survey made clinic visit more difficult, and did not find the questions upsetting or distressful.
Conclusion: Our results demonstrate that these screener tools (“How are you currently eating?”, “Do you experience any difficulty or pain upon swallowing?”) can effectively screen dysphagia symptoms without increasing cancer outpatient clinic burden, both in observational studies and for routine clinical monitoring.
1. INTRODUCTION
A patient‐reported outcome (PRO) is any health status report that is provided directly by the patient, without interpretation of the patient's response by a clinician or anyone else.1 Patient‐reported outcomes can take on a variety of forms, including measurements of symptom severity and/or health‐related quality of life (HRQoL).2 Patient‐reported outcomes with a symptomatic focus are of the utmost importance in cancer care. Many cancer‐related symptoms are associated with the disease, but some symptoms such as fatigue, neuropathy, and affective symptoms can also be caused by cancer therapy.3 Regardless of cause, when symptoms are not managed effectively, they can impact patients' functional status, affect, and HRQoL.4, 5, 6, 7, 8, 9 Further, unrelieved symptoms can cause patients to interrupt or abandon treatment10, 11 or decrease adherence to treatment regimens.12, 13, 14, 15, 16, 17, 18
As essential a role that symptom PROs play in multidisciplinary cancer care, barriers exist that may result in suboptimal symptom assessment. Outpatient clinics must balance the need for comprehensive symptom assessments, while minimizing patient burden. One method is to implement short symptom screener tools, followed by more comprehensive questions if the patient elicits a positive screen result; this may maximize symptom reporting while reducing clinic burden on both patients and clinicians.19, 20, 21
Symptom screening may also have merits in the context of prospective observational studies. Unlike clinical trial patients, who are aware that they are potentially benefiting from specialized treatment regimens, patients enrolled in observational studies often perceive no direct benefits. These patients may lack motivation to complete long questionnaires—a burden that can be avoided with effective screening tools.
Although dysphagia (difficulty swallowing) is not one of the most prevalent symptoms in the general cancer population, it has high morbidity. Decreased HRQoL accompanies difficulty in eating or drinking.22, 23 In the extreme setting, severe dysphagia managed by nasogastric feeding can lead to sustained symptoms due to muscle atrophy.24, 25 Dysphagia is associated with malnutrition and a subsequent increased risk of infection.22 When dehydration occurs, renal failure and other adverse effects such as constipation are common, which can associate with impaired mental status and delirium.22, 26, 27, 28, 29 Mechanical obstructive tumors can lead to weight loss and shorter survival times.30, 31, 32
Within certain cancer populations, dysphagia is highly prevalent: Dysphagia is observed in approximately 50% of head and neck patients33, 34, 35; progressive dysphagia and/or odynophagia (pain upon swallowing) are the most common presenting symptoms in gastroesophageal cancer patients.36 Radiation‐induced dysphagia and odynophagia occur in patients receiving radiation to the chest or mediastinum (the central compartment of the thoracic cavity), often up to 2 months posttreatment.37
The main goals of this study were (1) to develop a dysphagia screening tool for use in both observational study screening and routine symptom monitoring, (2) to compare the tool's sensitivity and specificity with gold‐standard tools in capturing dysphagia symptoms, and (3) to assess the feasibility and acceptability of implementing our dysphagia screener tool on an electronic tablet (Apple's iPad) in an outpatient clinic. Because we were devising screening tools for research purposes (observational study) and separately for routine symptom monitoring, we have discussed them separately in Sections 2 and 3, as phase 1 (research/observational study) and phase 2 (routine use).
2. METHODS
2.1. Study design and population
This study was approved by the Research Ethics Board of the University Health Network in Toronto, Ontario, Canada; informed consent was obtained from all participants. The purpose of the study was to develop dysphagia screening tools for prospective observational studies (phase 1) and for routine clinical care (phase 2). In both phases, an expert panel using a consensus approach38 reviewed potential screening and follow‐up questions for criterion, content, and construct validity, as well as feasibility, where possible, validated gold standards were chosen. The phases were performed at separate times, with phase 2 occurring after the end of phase 1 recruitment.
2.2. Stakeholder and expert panel for phases 1 and 2
Two panels were created because of the number of individuals required; individuals were randomly assigned to each panel (13 per panel), with one panel focused on phase 1 and the other panel on phase 2. Decisions of one panel were then introduced to the other panel for commentary and discussion. Individuals for the 2 panels included 3 gastrointestinal cancer clinic nurses from 2 Toronto institutions; 2 outpatient clinic nurses from institutions in Ottawa and Edmonton; 2 speech, swallowing, and language clinicians from Toronto and Houston; 2 PhD researchers focusing on esophageal and head and neck cancer translational research from Toronto and Vancouver; 1 PhD researcher focused on knowledge translational science (Calgary); 3 gastrointestinal cancer medical oncology fellows originally from 3 continents but currently training in Canada; 2 radiation oncology fellows from North America; 2 staff surgeons and 2 radiation oncologist staff; 3 medical oncology staff from 3 centers in North America; and 4 patient representatives, including one from the Canadian Cancer Society. There was 1 face‐to‐face initial meeting of a subset of both of these panels at the Canadian Clinical Trials Group Annual Meeting. Stakeholders then met in 2 formal 2‐hour virtual meetings, with further communications performed online through eletronic mail.
2.3. Statistical analyses
Descriptive statistics were performed separately for phases 1 and 2, including median, minimum, and maximums for continuous variables, and frequency tabulations by categorial variables. All statistical analyses were completed using SAS 9.4.
2.3.1. Phase 1 screeners for research purposes
In phase 1, when developing a screening tool useful for prospective observational studies, the primary objective was to identify a highly sensitive screening question for dysphagia to reduce the burden of completing the full validated FACT‐E swallowing index (45 total questions and 8 swallowing‐specific questions).39 The panel selected 2 potential single‐screening questions: Screener 1, “How are you currently eating?”, was derived from O'Rourke et al,40 with response choices of “normal,” “eats soft food only,” “eats pureed food only,” “drinks liquid only,” and “no swallowing at all” (Figure 2), and for screener 2, one of the FACT‐E questions itself was chosen as a putative screening question: “I can swallow easily and naturally,” with response choices of “very much,” “quite a bit,” “somewhat,” “a little bit,” and “not at all.” Given a gold standard based on FACT‐E that is focused on esophageal cancer patients, all phase 1 patients were restricted to those with a diagnosis of gastroesophageal cancer.
Figure 2.
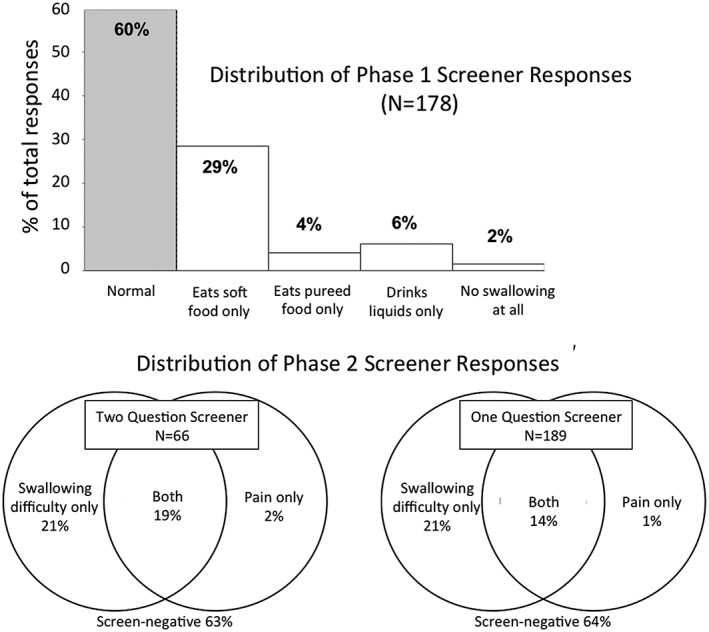
Prevalence of phases 1 and 2 screener questions. Graphs illustrating screener response distribution for phase 1 and 2, separately. Nearly 40% of all patients in each phase were screen‐positive for dysphagia. For phase 1, screener 1 is reported. For phase 2, the 2‐question screeners used screeners 3 and 4, while the 1‐question screener used screener 5
2.4. Recruitment and data collection procedures
At outpatient clinics, patients were prescreened for study eligibility by the clinical team. Patients over the age of 18 years with a gastroesophageal or esophageal cancer, who were capable of communcating in English and were deemed to be competent to understanding the study by their physicians, were deemed eligible. A life expectancy by their physicians of at least 12 weeks was also an eligibility criterion, as the institutional review board felt that approaching actively dying patients was unethical. Eligible patients were approached in clinic waiting areas and examination rooms prior to their appointments. In phase 1, patients completed screeners 1 and 2 along with the 5‐question FACT‐E Swallowing Index subset.39 The full questionnaire included clinico‐demographic questions and the EQ5D‐3L, a 5‐question tool that can be converted into a health utility score.41 All patients included in phase 1 completed the questionnaire on paper. Cancer clinical data for patients was abstracted from the electronic medical records. Clinicians were not given the dysphagia data but were asked to manage their patients in a usual fashion.
2.5. Statistical analysis
For phase 1, patients were considered screen‐positive if they responded anything other than “normal” to Screener 1 or “very much” to Screener 2 (see Figure 2 for full response set). Sensitivities and specificities were calculated for each screener against various Swallowing Index Cut‐off Values (SICVs), a summation of scores from 5 FACT‐E questions (E1, E2, E3, E5, and HN7), with possible SICV values of 0 to 20; higher scores represented lower severity of symptoms. Negatively worded questions were reverse scored. Swallowing Index Cut‐off Values were determined from clinical factors previously described by Darling et al39: scores of 5.5 (2 standard deviations below mean of surgical cancer patients at 3‐4 months follow‐up), 7.1 (1 standard deviation below mean of cancer patients who experienced weight loss), 12.16 (mean of cancer patients who experienced weight loss), 13.3 (mean of surgical cancer patients), 14.5 (mean of surgical cancer patients at 3‐4 months follow‐up), and 16.0 (mean of cancer patients without pain). Receiver operating characteristic curves were generated using the same screener and these various SICV criteria.
2.5.1. Phase 2 screeners for routine clinical monitoring
In phase 2, the objective was to develop and evaluate practical, routine screening question(s) for detecting swallowing disturbances in routine cancer patient management. Similar to our previous evaluations,42 we used more comprehensive and focused symptom questions in the style of Patient Reported Outcomes for Common Terminology Criteria for Adverse Events (PRO‐CTCAE). However, while PRO‐CTCAE has a single question about dysphagia, our expert panel determined that for clinical management purposes, severity of symptoms and functional interference were both of clinical relevance; symptom frequency, however, was too confounded by a patient's frequency of attempts at eating. The panel also identified that difficulty swallowing (dysphagia) and pain upon swallowing (odynophagia) of solids, and independently, of liquids, were important separate symptoms requiring different management and should be assessed separately. In the first part of phase 2, the evaluation was, therefore, of 2 separate screening questions, 1 for dysphagia (screener 3) and 1 for odynophagia (screener 4), and initially tested on patients in this manner. Upon a suggestion at a prespecified mid–phase 2 stakeholder and expert panel meeting, the 2 screening questions were later combined into a single screening question (screener 5): “Do you experience any difficulty or pain upon swallowing?”, with response choices of “no,” “difficulty swallowing only,” “pain swallowing only,” and “both difficulty and pain swallowing”; the last half of the phase 2 section then utilized the 1 question screener (screener 5). With the purpose of screening for routine cancer care, dual assessments of feasibility/patient acceptability and sensitivity/specificity of the screener were performed. Given this same objective, patients with head and neck and gastroesophageal cancers, and those receiving mediastinal radiation, were chosen as test subjects. All PRO‐CTCAE data were dichotomized into no symptoms versus symptoms of any grade, in order to ensure that the screener questions could be sensitive enough to capture even subtle symptoms that could be addressed in follow‐up questions.
2.6. Recruitment and data collection procedures
At outpatient clinics, patients were prescreened for study eligibility with the clinical team. Eligibility criteria were the same as phase 1, except inclusion was expanded to include individuals with a head and neck cancer, a gastroesophageal or esophageal cancer, or a lung cancer that was being treated primarily with radiation or chemoradiation; these diagnoses are associated with high prevalences of dysphagia and odynophagia.43, 44 Eligible patients were approached in clinic waiting areas and examination rooms prior to their appointments. The phase 2 electronic tablet–based questionnaire included either the 1‐question or 2‐question screener and the PRO‐CTCAE–like comprehensive panel of follow‐up questions (see Table 2, phase 2), a set of 9 questions designed to assess the feasibility and acceptability of implementing the survey in a clinical setting, clinico‐demographic questions, and the EQ5D‐3L. The total number of questions was 23 to 24, depending on whether the patient completed the 1‐question or 2‐question screener. Some phase 2 patients answered all the questions regardless of their answers to the screening questions, which allowed their data to be assessed for screener sensitivity/specificity. The remaining phase 2 patients only answered the follow‐up questions when they were screen‐positive; it was this latter set of patients that was administered the feasibility/acceptability survey. Cancer clinical data for patients was abstracted from the electronic medical records. Clinicians were not given the dysphagia data but were asked to manage their patients in a usual fashion.
Table 2.
Distribution of answers to comprehensive gold standard questions on dysphagia and odynophagia
| Phase 1 (N = 178) | |||||
|---|---|---|---|---|---|
| Very much | Quite a bit | Somewhat | A little bit | Not at all | |
| “I have difficulty swallowing solid foods” | 26 (15%) | 25 (14%) | 28 (16%) | 31 (17%) | 68 (38%) |
| “I have difficulty swallowing soft or mashed foods” | 6 (3%) | 12 (7%) | 25 (14%) | 25 (14%) | 110 (62%) |
| “I have difficulty swallowing liquids” | 4 (2%) | 10 (6%) | 13 (7%) | 24 (13%) | 127 (71%) |
| “I choke when I swallow” | 2 (1%) | 7 (4%) | 16 (9%) | 26 (15%) | 127 (71%) |
| “I can swallow naturally and easily” | 61 (34%) | 40 (22%) | 33 (19%) | 25 (14%) | 19 (11%) |
| Phase 2 (N > 90)b | |||||
|---|---|---|---|---|---|
| Very much | Quite a bit | Somewhat | A little bit | Not at all | |
| Severity of difficulty swallowinga | 3 (2%) | 8 (6%) | 45 (34%) | 36 (27%) | 42 (31%) |
| Difficulty upon swallowing – Solid food interference | 5 (4%) | 18 (15%) | 22 (18%) | 29 (24%) | 45 (38%) |
| Difficulty upon swallowing – Fluids interference | 2 (2%) | 5 (5%) | 5 (5%) | 29 (31%) | 53 (56%) |
| Severity of pain swallowinga | 1 (1%) | 8 (8%) | 14 (15%) | 16 (17%) | 57 (50%) |
| Pain upon swallowing – Solid food interference | 1 (1%) | 8 (9%) | 10 (11%) | 14 (15%) | 58 (64%) |
| Pain upon swallowing – Fluids interference | 0 (0%) | 6 (6%) | 3 (3%) | 16 (17%) | 69 (73%) |
For these questions on severity, the category levels (from left to right) are severe, very severe, moderate, mild, none.
Because not all patients were required to complete every component of phase 2, the sample size for each specific question ranged from 91 through 134 patients. Shaded boxes refer to the answer associated with lack of symptoms.
2.7. Statistical analysis
For phase 2, sensitivities and specificities were calculated for the 1‐question screener against the PRO‐CTCAE–like follow‐up questions. Using a strict definition, all follow‐up questions had to be answered in the negative (“none” or “not at all”) for the patient to be considered to have either no dysphagia or no odynophagia (these 2 symptoms were assessed independently).
3. RESULTS
3.1. Phases 1 and 2 description
Demographic and clinical characteristics are presented in Table 1. Figure 1 describes the recruitment and analysis details of the population, captured in a CONSORT diagram. Prevalence of dysphagia (Figure 2) based on screener questions was approximately 36% to 40%, regardless of whether it was assessed as the ability to eat (phase 1) or the presence of difficulty or pain upon swallowing (phase 2), and regardless of whether it was a 2‐question or 1‐question screener.
Table 1.
Clinico‐demographic characteristics of the patients
| Variables | Phase 1 | Phase 2 |
|---|---|---|
| N | 178 | 255 |
| Age in years: median (range) | 68.7 (33.1‐84.0) | 63.2 (21.5‐89.8) |
| Sex: female | 43 (24%) | 64 (25%) |
| Married or living with partner | 115 (68%) | 173 (68%) |
| Ethnicity | ||
| White | 145 (81%) | 190 (75%) |
| Asian | 17 (10%) | 27 (11%) |
| Other | 7 (4%) | 16 (6%) |
| Prefer not to answer | 9 (5%) | 22 (9%) |
| Highest level of education | ||
| Secondary or less | 63 (35%) | 82 (32%) |
| Postsecondary | 113 (63%) | 142 (56%) |
| Prefer not to answer | 2 (1%) | 31 (12%) |
| Disease site | ||
| Gastroesophageal | 178 (100%) | 84 (33%) |
| Head and neck | 0 (0%) | 155 (61%) |
| Thoracic | 0 (0%) | 16 (6%) |
Figure 1.
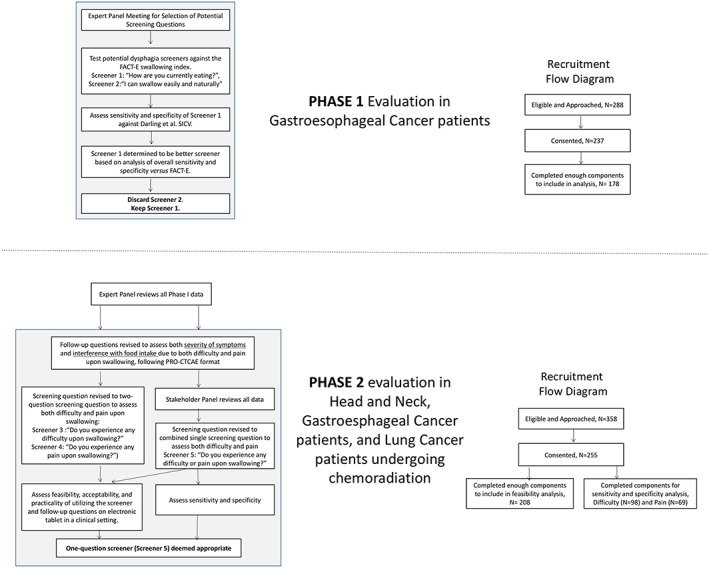
CONSORT and recruitment flow diagrams for swallowing difficulties assessment. For phase 1, the sensitivity and specificity were assessed for 2 potential screeners versus the FACT‐E swallowing index in gastroesophageal cancer patients. For phase 2, dual assessments of feasibility/patient acceptability (1‐question and 2‐question screeners) and sensitivity/specificity of the 1‐question screener versus a Patient Reported Outcomes for Common Terminology Criteria for Adverse Events (PRO‐CTCAE) gold standard. SICV, Swallowing Index Cut‐off Value
Proper evaluation of a screening tool requires a broad distribution of patients with and without swallowing issues. As expected, both phases 1 and 2 patients reported difficulty swallowing solid foods more often than soft/mashed foods or liquids. The median summative SICV score was 17 (range: 2‐20); one‐quarter of patients fell below the 12.16 SICV, the mean value of patients who had suffered associated weight loss (Table 2).
3.2. Sensitivity and specificity of phase 1 screening questions
In phase 1, initial analysis found that screener 1, “How are you currently eating?”, with anchoring responses of “Able to eat normally” and “Unable to swallow anything,” had significantly better test characteristics (sensitivity/specificity) than screener 2, “I can swallow easily and naturally.” Sensitivities and specificities were evaluated against various gold‐standard SICVs (Table 3). In screener 1, sensitivities ranged from 66% to 86%, and specificities ranged from 63% to 83%. Areas under the curve ranged from 0.752 to 0.885 (Figure 3). The sum of sensitivity and specificity was greatest when SICV of 12.16 was used as a gold standard cut‐off (80% sensitivity and 75% specificity).
Table 3.
Assessment of sensitivities and specificities of screening questions in phases 1 and 2a
| Phase 1: screener 1, “How are you currently eating” | |||||||
|---|---|---|---|---|---|---|---|
| Cut‐off values for gold standard | True positive | True negative | False positive | False negative | Sensitivity | Specificity | |
| SICV | Prevalence of having swallowing difficulty using this cut‐off | ||||||
| 5.5 | 3.9% | 6 | 108 | 63 | 1 | 86% | 63% |
| 7.1 | 10.1% | 15 | 106 | 54 | 3 | 83% | 66% |
| 12.16 | 25.3% | 36 | 100 | 33 | 9 | 80% | 75% |
| 13.3 | 32.0% | 44 | 96 | 25 | 13 | 77% | 79% |
| 14.5 | 37.6% | 48 | 90 | 21 | 19 | 72% | 81% |
| 16 | 44.4% | 52 | 82 | 17 | 27 | 66% | 83% |
| Phase 1: screener 2, “I can swallow naturally and easily” | |||||||
|---|---|---|---|---|---|---|---|
| Cut‐off values for gold standard | True positive | True negative | False Positive | False negative | Sensitivity | Specificity | |
| SICV | Prevalence of having swallowing difficulty using this cut‐off | ||||||
| 5.5 | 3.9% | 3 | 98 | 73 | 2 | 71% | 57% |
| 7.1 | 10.1% | 12 | 96 | 64 | 6 | 67% | 60% |
| 12.16 | 25.3% | 30 | 85 | 48 | 15 | 67% | 64% |
| 13.3 | 32.0% | 35 | 80 | 41 | 22 | 61% | 66% |
| 14.5 | 37.6% | 40 | 78 | 33 | 27 | 60% | 70% |
| 16 | 44.4% | 45 | 75 | 24 | 34 | 57% | 76% |
| Phase 2 single question screener: difficulty swallowing question | ||||||
|---|---|---|---|---|---|---|
| None/not at all only in all subsequent questions vs all others | 61 | 33 | 0 | 4 | 94% | 100% |
| Phase 2 single question screener: pain swallowing question | ||||||
|---|---|---|---|---|---|---|
| None/not at all in all subsequent questions only vs all others | 24 | 38 | 3 | 4 | 86% | 93% |
Abbreviation: SICV, Swallowing Index Cut‐off Value.
For phase 1, screener 1, response choices were “normal,” “eats soft food only,” “eats pureed food only,” “drinks liquid only,” and “no swallowing at all.”
Figure 3.
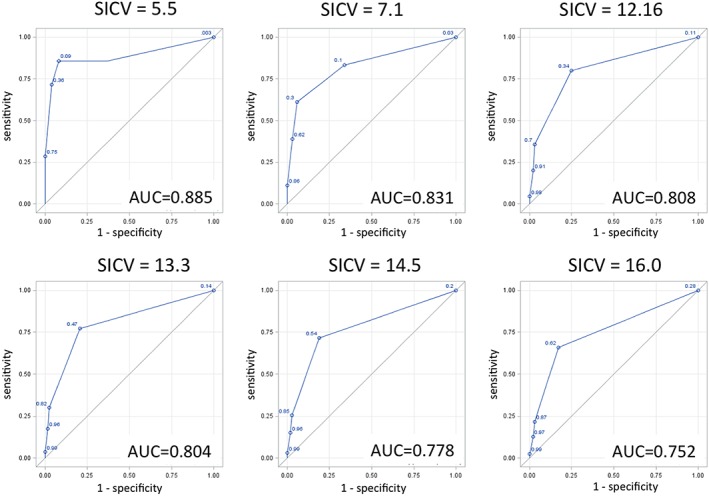
Receiver operator curves for various cutoffs of the FACT‐E swallowing index (Swallowing Index Cut‐off Value [SICV]). Area under the curve (AUC) ranges from 0.752 to 0.885
3.3. Sensitivity and specificity, feasibility, and acceptaibility of phase 2 screening questions
In phase 2, sensitivity and specificity were evaluated only on the 1‐question screener (screener 5). When compared with the gold standard PRO‐CTCAE questions on severity and functional interference for both dysphagia and odynophagia, the sensitivities ranged from 86% to 94% for each of the follow‐up questions, while the specificities ranged from 93% to 100% (Table 3). The screener was slightly more sensitive and specific to difficulty swallowing than to pain swallowing (Table 3).
Feasibility and acceptability, and practicality of dysphagia symptom screening via electronic tablet, were assessed for both the 2‐question (screeners 3 and 4) and 1‐question (screener 5) dysphagia screeners based on responses of 208 of 255 total patients included in phase 2; missing results were because we selected a random sample for completion of this section. Not every patient was approached because of the added coordinator time to track and time patients, and of those who were asked to complete this section, 97% had complete data. Median completion time for the 2‐question screener and follow‐up questions was 127 seconds; this time was reduced to 107 seconds for the 1‐question screener. Over 90% of patients completing both versions of the screener were willing to complete the surveys on a touchscreen tablet, did not feel like completing the surveys made their clinic visit more difficult, and did not find the questions to be upsetting or distressful. Over 80% felt that completing the surveys was not time consuming and that the completion of the surveys was a useful method to communicate with their clinician. However, less than 20% of patients wished to see or keep a printout of their dysphagia assessments (Figure S1).
4. DISCUSSION
This study successfully identified several dysphagia screener questions that have potential use in screening for both observational studies and routine symptom assessment. The phase 1 screener (screener 1) provided a solid base for further investigation into dysphagia screener tools. It yielded sensitivity greater than 80% versus FACT‐E at 3 SICVs (equal to 5.5, 7.1, and 12.16), suggesting that it is useful for screening for dysphagia in prospective observational studies. The 1‐question screener (screener 5) developed and analyzed during phase 2 of the study was not only highly sensitive and specific when compared with our gold standard (PRO‐CTCAE–like follow‐up questions) but was also feasible and acceptable for use in routine multidisciplinary care. When symptoms were in the mild through very severe range that is consistent with a need for clinical intervention, the sensitivity and specificity of the screener were high (>85% all values). Further, the screener and follow‐up questions, on average, took less than 2 minutes to complete, suggesting that the tool is practical for implementation in busy outpatient cancer clinics. Our analysis of the feasibility/acceptability responses by patients provides strong evidence that the tool minimizes patient burden, is easy for patients to use and understand, and improves communication of dysphagia symptoms between patient and clinician. The phase 2 screener (screener 5) may be an effective way to accurately detect the presence and assess the extent of patients' dysphagia symptoms without significantly increasing clinic burden.
Currently, PRO‐CTCAE v1.0 is becoming more prevalent for toxicity evaluation.45 However, this tool only contains a single swallowing item, and while this may be sufficient for gathering toxicity information, it may not be enough for clinical management of symptoms. Our 6 focused assessment questions balance between extensive toxicity assessment and symptom control. These questions distinguish differences in difficulty and pain upon swallowing and describe the extent of severity and functional interference due to symptoms. Further, our follow‐up is formatted in a PRO‐CTCAE style, which allows for easy integration and comparison with other PRO‐CTCAE items.
This study supports the findings of prior studies19, 20, 21 that suggest that short screener tools can effectively assess cancer‐related symptoms and reduce outpatient clinic burden. This study, however, is the only one, to our knowledge, to develop a highly sensitive and specific screening tool explicitly for dysphagia/odynophagia. While more than one‐third of patients included in this study responded positively to screeners for dysphagia, swallowing disturbances are often not assessed in an outpatient clinic setting. For example, outpatient cancer clinics in Ontario routinely assess symptoms with the Edmonton Symptom Assessment System46; this tool collects data on 9 common cancer symptoms, but dysphagia and odynophagia are not included. Our results suggest that the chosen phase 2 single screener question will be a highly effective clinical monitoring tool.
A recent randomized clinical trial (RCT) by Basch et al47 reported increased overall survival in a randomized trial where the experimental arm had received rapid symptom screening (in the form of PRO‐CTCAE) and subsequent management, when compared with a control arm of patients receiving “usual” care. Thus, implementation of our screener routinely may have far‐reaching effects beyond improving HRQoL.
This study was not without limitations. Consenting patients were fluent in English, so this study does not suggest that our tool, as it currently exists, is generalizable to patients who speak other languages. Future validation in multiple languages would be important. In phase 1, we identified a screener question that would allow our patients to be dichotomized into those with and without dysphagia when using FACT‐E; we should caution that researchers who want to use the full scale of FACT‐E or analyze the data in a continuous manner should continue to use FACT‐E in its current state. Our study made no direct comparisons of the screener's effectiveness between the 3 groups included (head and neck, gastroesophageal cancers, and thoracic cancers undergoing radiation); however, the 1‐question screener was extremely straightforward and direct, so it is highly unlikely that the characteristics of our test would noticeably change across disease sites. We also did not assess clinician perceptions directly, as we tried to avoid clinician bias in influencing patient perception; instead, we avoided providing the clinicians with the dysphagia results completely and asked them to manage their patients in a usual fashion. Finally, there is still controversy as to whether the gold standard reporting of symptoms or toxicities should be “filtered” through a clinician's viewpoint (ie, physician interpretation of an open discussion of toxicities) or be directly ascertained from the patient using PROs; our study used the patient‐reported symptoms and toxicity as the gold standard.
Our study determined 2 highly sensitive (>85% sensitivity phase 1 and >90% sensitivity phase 2) screener questions for swallowing issues. One screener was designed for use in research settings, as it was designed for use instead of FACT‐E. The second screener was designed with a different purpose in mind: for routine clinical use. The screeners were neither burdensome on patients nor time consuming. Future studies should look to assess the practical implication of our screener in populations of non‐English–speaking patients in outpatient clinics. Comparison to other gold standard research tools for dysphagia should also be considered. In the meantime, we have established, in principle, the utility of implementing single‐question screeners in both the observational study setting and for routine clinical practice. We expect to reduce survey burden in patients by reducing the number of patients who need to complete the more extensive follow‐up questions that will refine the degree, frequency, and severity of symptoms required to manage these symptoms properly in the clinical setting.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Conceptualization: Geoffrey Liu, Mark Doherty, Catherine Brown, Wei Xu, Doris Howell
Data curation: Michael Borean, Kishan Shani, Catherine Brown, Geoffrey Liu, Gail Darling, Rebecca K. Wong, Raymond Jang, Elena Elimova, Grainne O'Kane
Formal analysis: Michael Borean, Kishan Shani, Catherine Brown, Wei Xu, Geoffrey Liu
Funding acquisition: Geoffrey Liu, Doris Howell
Investigation: Michael Borean, Kishan Shani, Judy Chen, Mindy Liang, Joel Karkada
Methodology: Michael Borean, Geoffrey Liu
Project administration: Geoffrey Liu
Resources: Geoffrey Liu, Doris Howell
Supervision: Geoffrey Liu, Wei Xu, M. Catherine Brown, Doris Howell.
Visualization: Michael Borean, Kishan Shani, M. Catherine Brown, Geoffrey Liu
Writing – Original Draft Preparation: Michael Borean
Writing – Review and Editing: Michael Borean, Kishan Shani, M. Catherine Brown, Judy Chen, Mindy Liang, Joel Karkada, Simranjit Kooner, Mark Doherty, Grainne M O'Kane, Dr. Raymond Jang, Elena Elimova, Rebecca K Wong, Gail Darling, Wei Xu, Doris Howell, Geoffrey Liu
Supporting information
SUPPLEMENTAL FIGURE 1. Feasibility and acceptability responses for Phase 2 Screener. DY = definitely yes, PY = probably yes, NS = not sure, PN = probably not, DN = definitely not.
ACKNOWLEDGMENTS
This research was funded by Cancer Care Ontario through the Ontario Patient Reported Outcomes of Symptoms and Toxicity (ON‐PROST) and the Alan B. Brown Chair, Princess Margaret Cancer Foundation.
Borean M, Shani K, Brown MC, et al. Development and evaluation of screening dysphagia tools for observational studies and routine care in cancer patients. Health Sci Rep. 2018;1:e48 10.1002/hsr2.48
Contributor Information
Doris Howell, Email: doris.howell@uhn.ca.
Geoffrey Liu, Email: geoffrey.liu@uhn.ca.
REFERENCES
- 1. Guidance for industry patient‐reported outcome measures: use in medical product development to support labeling claims. In: Services HaH, ed2009. [DOI] [PMC free article] [PubMed]
- 2. Cleeland CS. Symptom burden: multiple symptoms and their impact as patient‐reported outcomes. JNCI Monographs. 2007;2007(37):16‐21. [DOI] [PubMed] [Google Scholar]
- 3. Cleeland CS, Bennett GJ, Dantzer R, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine‐immunologic model of cancer symptoms. Cancer. 2003;97(11):2919‐2925. [DOI] [PubMed] [Google Scholar]
- 4. Glover J, Dibble SL, Dodd MJ, Miaskowski C. Mood states of oncology outpatients: Does pain make a difference? J Pain Symptom Manage. 1995;10(2):120‐128. [DOI] [PubMed] [Google Scholar]
- 5. Burrows M, Dibble SL, Miaskowski C. Differences in outcomes among patients experiencing different types of cancer‐related pain. Oncol Nurs Forum. 1998;25(4):735‐741. [PubMed] [Google Scholar]
- 6. Miaskowski C, Lee KA. Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: a pilot study. J Pain Symptom Manage. 1999;17(5):320‐332. [DOI] [PubMed] [Google Scholar]
- 7. Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum. 2001;28(3):465‐470. [PubMed] [Google Scholar]
- 8. Kurzrock R. The role of cytokines in cancer‐related fatigue. Cancer. 2001;92(6 Suppl):1684‐1688. [DOI] [PubMed] [Google Scholar]
- 9. Valentine AD, Meyers CA. Cognitive and mood disturbance as causes and symptoms of fatigue in cancer patients. Cancer. 2001;92(6 Suppl):1694‐1698. [DOI] [PubMed] [Google Scholar]
- 10. Borden EC, Parkinson D. A perspective on the clinical effectiveness and tolerance of interferon‐alpha. Semin Oncol. 1998;25(1 Suppl 1):3‐8. [PubMed] [Google Scholar]
- 11. Cleeland CS. Cancer‐related symptoms. Semin Radiat Oncol. 2000;10(3):175‐190. [DOI] [PubMed] [Google Scholar]
- 12. Ahlberg K, Ekman T, Gaston‐Johansson F, Mock V. Assessment and management of cancer‐related fatigue in adults. Lancet (London, England). 2003;362(9384):640‐650. [DOI] [PubMed] [Google Scholar]
- 13. Berger A. Treating fatigue in cancer patients. Oncologist. 2003;8(Suppl 1):10‐14. [DOI] [PubMed] [Google Scholar]
- 14. Bernard S, Gill P, Rosen P, et al. A phase I trial of alpha‐interferon in combination with pentostatin in hematologic malignancies. Med Pediatr Oncol. 1991;19(4):276‐282. [DOI] [PubMed] [Google Scholar]
- 15. Manzullo E, Liu W, Escalante C. Treatment for cancer‐related fatigue: an update. Expert Rev Anticancer Ther. 2003;3(1):99‐106. [DOI] [PubMed] [Google Scholar]
- 16. Jacobsen PB, Donovan KA, Weitzner MA. Distinguishing fatigue and depression in patients with cancer. Semin Clin Neuropsychiatry. 2003;8(4):229‐240. [PubMed] [Google Scholar]
- 17. Stasi R, Abriani L, Beccaglia P, Terzoli E, Amadori S. Cancer‐related fatigue: evolving concepts in evaluation and treatment. Cancer. 2003;98(9):1786‐1801. [DOI] [PubMed] [Google Scholar]
- 18. ten Tije AJ, Smorenburg CH, Seynaeve C, et al. Weekly paclitaxel as first‐line chemotherapy for elderly patients with metastatic breast cancer. A multicentre phase II trial. Eur J Cancer (Oxford, England: 1990). 2004;40(3):352‐357. [DOI] [PubMed] [Google Scholar]
- 19. Bagha SM, Macedo A, Jacks LM, et al. The utility of the Edmonton symptom assessment system in screening for anxiety and depression. Eur J Cancer Care. 2013;22(1):60‐69. [DOI] [PubMed] [Google Scholar]
- 20. Vodermaier A, Linden W, Siu C. Screening for emotional distress in cancer patients: a systematic review of assessment instruments. J Natl Cancer Inst. 2009;101(21):1464‐1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ripamonti CI, Bandieri E, Pessi MA, Maruelli A, Buonaccorso L, Miccinesi G. The Edmonton symptom assessment system (ESAS) as a screening tool for depression and anxiety in non‐advanced patients with solid or haematological malignancies on cure or follow‐up. Support Care Cancer. 2014;22(3):783‐793. [DOI] [PubMed] [Google Scholar]
- 22. Raber‐Durlacher JE, Brennan MT, Verdonck‐de Leeuw IM, et al. Swallowing dysfunction in cancer patients. Support Care Cancer. 2012;20(3):433‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jensen K, Lambertsen K, Grau C. Late swallowing dysfunction and dysphagia after radiotherapy for pharynx cancer: frequency, intensity and correlation with dose and volume parameters. Radiother Oncol: J Eur Soc Ther Radiol Oncol. 2007;85(1):74‐82. [DOI] [PubMed] [Google Scholar]
- 24. Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol. 2006;24(17):2636‐2643. [DOI] [PubMed] [Google Scholar]
- 25. Mekhail TM, Adelstein DJ, Rybicki LA, Larto MA, Saxton JP, Lavertu P. Enteral nutrition during the treatment of head and neck carcinoma: Is a percutaneous endoscopic gastrostomy tube preferable to a nasogastric tube? Cancer. 2001;91(9):1785‐1790. [PubMed] [Google Scholar]
- 26. Santarpia L, Contaldo F, Pasanisi F. Nutritional screening and early treatment of malnutrition in cancer patients. J Cachexia Sarcopenia Muscle. 2011;2(1):27‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Finestone HM, Foley NC, Woodbury MG, Greene‐Finestone L. Quantifying fluid intake in dysphagic stroke patients: a preliminary comparison of oral and nonoral strategies. Arch Phys Med Rehabil. 2001;82(12):1744‐1746. [DOI] [PubMed] [Google Scholar]
- 28. Whelan K. Inadequate fluid intakes in dysphagic acute stroke. Clin Nutr (Edinburgh, Scotland). 2001;20(5):423‐428. [DOI] [PubMed] [Google Scholar]
- 29. Wotton K, Crannitch K, Munt R. Prevalence, risk factors and strategies to prevent dehydration in older adults. Contemp Nurse. 2008;31(1):44‐56. [DOI] [PubMed] [Google Scholar]
- 30. Dhanapal R, Saraswathi T, Govind RN. Cancer cachexia. J Oral Maxillofac Pathol: JOMFP. 2011;15(3):257‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Donohoe CL, Ryan AM, Reynolds JV. Cancer cachexia: mechanisms and clinical implications. Gastroenterol Res Pract. 2011;2011:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim CH, Weaver AL, Hsu JJ, Rainwater L, Zinsmeister AR. Discriminate value of esophageal symptoms: a study of the initial clinical findings in 499 patients with dysphagia of various causes. Mayo Clin Proc. 1993;68(10):948‐954. [DOI] [PubMed] [Google Scholar]
- 33. García‐Peris P, Parón L, Velasco C, et al. Long‐term prevalence of oropharyngeal dysphagia in head and neck cancer patients: impact on quality of life. Clin Nutr. 2007;26(6):710‐717. [DOI] [PubMed] [Google Scholar]
- 34. Nguyen NP, Moltz CC, Frank C, et al. Severity and duration of chronic dysphagia following treatment for head and neck cancer. Anticancer Res. 2005;25(4):2929‐2934. [PubMed] [Google Scholar]
- 35. Larsson M, Hedelin B, Johansson I, Athlin E. Eating problems and weight loss for patients with head and neck cancer: a chart review from diagnosis until one year after treatment. Cancer Nurs. 2005;28(6):425‐435. [DOI] [PubMed] [Google Scholar]
- 36. Layke JC, Lopez PP. Esophageal cancer: a review and update. Am Fam Physician. 2006;73(12):2187‐2194. [PubMed] [Google Scholar]
- 37. Mascarenhas F, Silvestre ME, Sa da Costa M, Grima N, Campos C, Chaves P. Acute secondary effects in the esophagus in patients undergoing radiotherapy for carcinoma of the lung. Am J Clin Oncol. 1989;12(1):34‐40. [DOI] [PubMed] [Google Scholar]
- 38. Obbarius A, van Maasakkers L, Baer L, et al. Standardization of health outcomes assessment for depression and anxiety: recommendations from the ICHOM depression and anxiety working group. Qual Life Res Int J Qual Life Asp Treat Care Rehab. 2017;26(12):3211‐3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Darling G, Eton DT, Sulman J, Casson AG, Celia D. Validation of the functional assessment of cancer therapy esophageal cancer subscale. Cancer. 2006;107(4):854‐863. [DOI] [PubMed] [Google Scholar]
- 40. O'Rourke I, Tiver K, Bull C, Gebski V, Langlands A. Swallowing performance after radiation therapy for carcinoma of the esophagus. Cancer. 1988;61(10):2022‐2026. [DOI] [PubMed] [Google Scholar]
- 41. Brooks R. EuroQol: the current state of play. Health Policy (Amsterdam, Netherlands). 1996;37(1):53‐72. [DOI] [PubMed] [Google Scholar]
- 42. Wang T. Active Surveillance of skin toxicities through web‐based patient‐reported (PRO) toxicity reporting tool in outpatient cancer patients on EGFR tyrosine kinase inhibitors (TKI): a feasibility study. Poster presented at Conference on Pharmacoepidemiology & Therapeutic Risk Management; August, 2015; Boston, USA
- 43. Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non‐small‐cell lung cancer: a phase 3 randomised trial. Lancet (London, England). 2015;386(9998):1049‐1056. [DOI] [PubMed] [Google Scholar]
- 44. Krekeler BN, Broadfoot CK, Johnson S, Connor NP, Rogus‐Pulia N. Patient adherence to dysphagia recommendations: a systematic review. Dysphagia. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Di Maio M, Basch E, Bryce J, Perrone F. Patient‐reported outcomes in the evaluation of toxicity of anticancer treatments. Nat Rev Clin Oncol. 2016;13(5):319‐325. [DOI] [PubMed] [Google Scholar]
- 46. Dudgeon D, King S, Howell D, et al. Cancer Care Ontario's experience with implementation of routine physical and psychological symptom distress screening. Psychooncology. 2012;21(4):357‐364. [DOI] [PubMed] [Google Scholar]
- 47. Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient‐reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL FIGURE 1. Feasibility and acceptability responses for Phase 2 Screener. DY = definitely yes, PY = probably yes, NS = not sure, PN = probably not, DN = definitely not.


