Abstract
Staphylococcus aureus is a nosocomial pathogen that can cause chronic to persistent infections. Among different mediators of pathogenesis, toxin-antitoxin (TA) systems are emerging as the most prominent. These systems are frequently studied in Escherichia coli and Mycobacterial species but rarely explored in S. aureus. In the present study, we thoroughly analyzed the S. aureus genome and screened all possible TA systems using the Rasta bacteria and toxin-antitoxin database. We further searched E. coli and Mycobacterial TA homologs and selected 67 TA loci as putative TA systems in S. aureus. The host inhibition of growth (HigBA) TA family was predominantly detected in S. aureus. In addition, we detected seven pathogenicity islands in the S. aureus genome that are enriched with virulence genes and contain 26 out of 67 TA systems. We ectopically expressed multiple TA genes in E. coli and S. aureus that exhibited bacteriostatic and bactericidal effects on cell growth. The type I Fst toxin created holes in the cell wall while the TxpA toxin reduced cell size and induced cell wall septation. Besides, we identified a new TA system whose antitoxin functions as a transcriptional autoregulator while the toxin functions as an inhibitor of autoregulation. Altogether, this study provides a plethora of new as well as previously known TA systems that will revitalize the research on S. aureus TA systems.
Keywords: Staphylococcus aureus, toxin-antitoxin systems, HigBA, pathogenicity islands
1. Introduction
Staphylococcus aureus is a commensal pathogen that can cause a diverse array of infections and syndromes such as toxic shock syndrome, bacteremia, endocarditis, osteomyelitis, pneumonia, soft-tissue infection and many more [1]. S. aureus can detect and respond to diverse environmental stimuli such as nutrient starvation and stress to increase its fitness by altering the expression of numerous genes such as the toxin-antitoxin (TA) system and two-component system [2,3]. A typical TA system consists of two components: a toxin that can disrupt a cellular process and an antitoxin that functions as an antidote for the toxin. TA systems have been involved in virulence and persistent infections in Escherchia coli [4], Mycobacterium [5], and Salmonella [6]. They are classified into six types based on the mechanism of interaction between toxin and antitoxin [7]. The type I TA system has been characterized by an RNA-RNA interaction of the antitoxin antisense RNA with the toxin mRNA, while the type II TA system exhibits protein-protein interaction between toxin and antitoxin. In the type III TA system, the activity of the toxic protein is inhibited by the interaction with the antitoxin RNA, while toxin and antitoxin proteins in the type IV TA system compete for the same target [4,7,8]. The antitoxin protein in the type V TA system cleaves the toxin mRNA [9]. The newly identified type VI TA system differs from others in a way that the antitoxin functions as an adaptor to facilitate toxin proteolysis [10,11]. The abundance of TA loci has been considered to have evolved through horizontal gene transfer, by which TA systems successfully integrate into the bacterial genome and are involved in genetic stability as well as pathogenic potency [12]. Under unfavorable conditions such as starvation, bacteriophage attack, heat shock, and antibiotic stresses, the unstable antitoxin is degraded, and as a result, the toxin is freed to target vital cellular processes such as translation, transcription, and replication [7,10]. TA systems have been extensively studied in E. coli [4] and Mycobacterium [5], while the study on TA systems in S. aureus is less advanced. S. aureus possesses a number of TA genes in its genome, however just three type II and two type I TA systems have been studied [3]. The rest of TA systems still await discovery and analysis. Here, we have performed an extensive screening and assessment of TA loci in the genome of methicillin-resistant S. aureus MW2 utilizing available software and databases. We screened TA systems with Rasta bacteria [13], the toxin-antitoxin database (TADB) [14], and position-specific iterative basic local alignment search tool (PSI-BLAST) searches supported by the “guilt by association” principle and classified them into different families and types. HigBA was the most abundant TA family with 15 systems, followed by six GCN5-related acetyltransferases (GNATs) and five RelBE TA systems. Other TA families include MazEF, VapBC, HicBA, CcdBA, ParDE, HipBA, transporter TA systems, abortive infection (Abi) TA systems, and some with a domain of unknown function. Further, we ectopically expressed some toxin and antitoxin genes to assess their predicted functions. Most of the type II toxins did inhibit cell growth while their antitoxins did not show any inhibitory effects. We overexpressed RelE, MazF, and HigB toxins that exhibited growth inhibition. We detected seven type I TA systems including previously characterized type I toxins Fst and TxpA [15]. Transmission electron microscopy (TEM) analysis of the bacterial cell wall revealed that the Fst toxin broke the cell wall while TxpA and holin toxins increased cell wall septation. We also detected a new TA system and found that its toxin and antitoxin did not show any growth inhibition upon overexpression. In this new TA system, toxin and antitoxin can be co-transcribed, and the antitoxin can bind to the promoter and autoregulate its operon. The toxin protein interfered with the antitoxin function and inhibited binding of the antitoxin to the promoter. In summary, our present study has greatly expanded the plethora of TA systems in S. aureus and revealed a variety of genes involved in bacterial cellular processes.
2. Results and Discussion
2.1. Comprehensive Screening of Toxin-Antitoxin Systems in S. aureus
The typical toxin-antitoxin (TA) system consists of a stable toxin and an unstable antitoxin and is widely distributed in bacterial genomes. The toxin and antitoxin form a conjugative pair of genes that are co-transcribed, and are involved in various cellular processes, and importantly counterbalance each other. The fraction of the TA system encoded by a bacterial genome varies from one pathogen to another. Mycobacterium contains 88 [5], E. coli contains 40 [4], Salmonella typhimurium carries 27 [6,16], and Pseudomonas aeruginosa has 26 TA systems [17]. To date, three type II TA systems have been well characterized in S. aureus, namely Axe1-Txe1, Axe2-Txe2, and MazEF. These type II TA systems show similarity to E. coli Phd-Doc, YefM-YoeB, and MazEF systems, respectively [3].
In the present work, we combined multiple approaches and explored the S. aureus genome for putative TA loci. We screened for TA genes and predicted a total of 67 putative TA systems with Rasta bacteria, TADB, and PSI-BLAST. To make the classification more accurate, we used the TA systems of E. coli [4], Klebsiella pneumoniae [18], and Mycobacterium [5,19] as modal and manually curated each TA system and classified them into different families. We found type I, II, III, IV, and type V TA systems in S. aureus on the basis of software prediction, conserved domain analysis, and orthologues searches from Kyoto Encyclopedia of Genes and Genomes (KEGG). Details of the screening method and TA systems is given (Figure 1A,B), and the selected TA families are provided in Table 1.
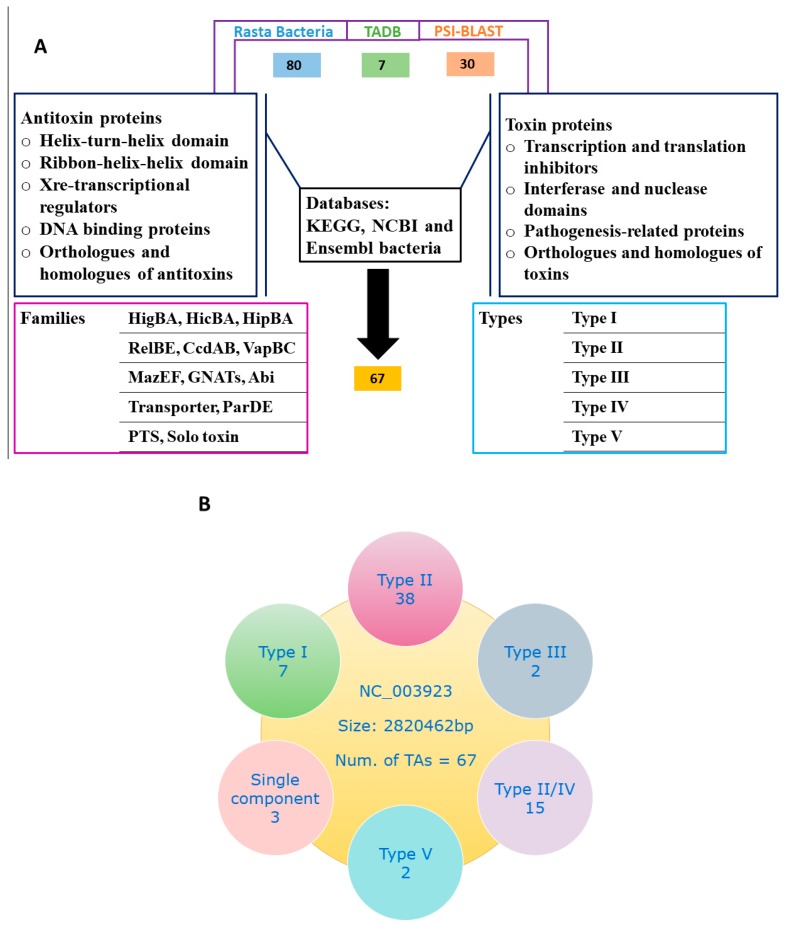
Figure 1.
Screening of TA systems. (A) Preliminary screening of TA loci in S. aureus genome was performed with Rasta bacteria and toxin-antitoxin database (TADB) and followed by position-specific iterative basic local alignment search tool (PSI-BLAST) searches. In total, 80 putative TA systems are predicted by Rasta bacteria, seven by TADB, and 30 by PSI-BLAST. TA systems were manually curated by conserved domains such as helix-turn-helix (HTH), ribbon-helix-helix (RHH) and transcriptional regulators for antitoxin, and conserved domains such as interferases, nucleases, transcription and translation inhibitors and pathogenesis-related proteins for toxins. All selected TA system orthologues and homologs were analyzed, and finally, 67 putative TA systems were selected. (B) The selected 67 TA systems were classified into different families and types. Type II TA systems were abundantly detected followed by putative type II/IV TA systems.
Table 1.
List of all selected TA systems and their predicted families. The TA systems were arranged in order of their proximity to the TA family. The length of toxin and antitoxin proteins is given in amino acids (aa).
| Family | Toxin Genes | Length (aa) |
| TxpA/Fst/Holin (type I toxins) | MW1888-TxpA | 44 |
| MW2354-Fst | 84 | |
| MW0911-TxpA | 35 | |
| MW1440-TxpA | 34 | |
| MW0405 | 64 | |
| MW1434 | 81 | |
| MW1381-Holin | 100 | |
| Family | Toxin/Antitoxin Genes | Length (aa) Toxin/Anti-toxin |
| HigBA (type II) | MW1419/MW1418 | 84/57 |
| MW1725/MW1724 | 59/117 | |
| MW2494/MW2493 | 76/106 | |
| MW1228/MW1227 | 79/77 | |
| MW1413/MW1412 | 62/66 | |
| MW0749/MW0748 | 74/48 | |
| MW1338/MW1337 | 59/116 | |
| MW1928/MW1927 | 106/53 | |
| MW1397/MW1396 | 92/110 | |
| MW1822/MW1821 | 91/154 | |
| MW0789/MW0788 | 98/128 | |
| MW1919/MW1918 | 72/134 | |
| MW2464/MW2463 | 72/275 | |
| MW0907/MW0906 | 72/189 | |
| MW1056/MW1055 | 44/62 | |
| RelBE (type II) | MW2329/MW2330 | 88/83 |
| MW2380/MW2381 | 88/85 | |
| MW1230/MW1231 | 80/155 | |
| MW0324/MW0325 | 129/67 | |
| MW1394/MW1395 | 131/133 | |
| GNATs (type II) | MW2441/MW2440 | 94/144 |
| MW1089/MW1088 | 70/203 | |
| MW0641/MW0640 | 180/99 | |
| MW0924/MW0923 | 160/71 | |
| MW2449/MW2450 | 163/56 | |
| MW2497/MW2498 | 99/185 | |
| Nucleases (type II) (CcdAB/ParDE) | MW1311/MW1312 | 73/166 |
| MW0461/MW0462 | 87/130 | |
| MW1403/MW1402 | 104/101 | |
| MW1733/MW1732 | 85/159 | |
| VapBC (type II) | MW1070/MW1071 | 263/224 |
| MW0302/MW0303 | 266/315 | |
| MW0572/MW0573 | 211/295 | |
| MazEF (type II) | MW1992/MW1993 | 120/56 |
| MW1492/MW1493 | 114/144 | |
| HicBA (type II) | MW0075/MW0076 | 109/83 |
| MW0771/MW0772 | 72/94 | |
| Transporters (type II/IV) | MW1042/MW1043 | 61/77 |
| MW1045/MW1046 | 48/65 | |
| MW2414/MW2413 | 77/102 | |
| MW1722/MW1723 | 100/147 | |
| MW2025/MW2026 | 77/134 | |
| MW1779/MW1780 | 121/68 | |
| MW0350/MW0349 | 69/83 | |
| Transporters (type V/II) | MW2507/MW2506 | 91/122 |
| MW0268/MW0269 | 617/164 | |
| Abi (type III/IV) | MW2234/MW2233 | 245/104 |
| MW0420/MW0421 | 89/131 | |
| HipBA (type II) | MW0980/MW0981 | 91/179 |
| PTS (type II/IV) | MW1437/MW1436 | 133/107 |
| MW1004/MW1005 | 84/129 | |
| MW0777/MW0776 | 78/67 | |
| MW1150/MW1149 | 94/391 | |
| MW1196/MW1195 | 83/34 | |
| MW1292/MW1291 | 89/102 | |
| MW1432/MW1431 | 71/87 | |
| MW1164/MW1165 | 275/130 | |
| MW0101 | 50 | |
| MW0036 | 103 | |
| MW1827 | 128 |
2.2. Distribution of TA Genes in Different Families
The toxin gene codes for a poison protein and antitoxin is the cognate antidote. The toxin and antitoxin sequence domains are the determinants for families and TA types. The antitoxin mostly contains a DNA-binding domain such as helix-turn-helix (HTH) or ribbon-helix-helix (RHH) of the transcriptional regulator. For example, the RelBE family in E. coli is the most diverse family and most of the RelB antitoxins bind to the promoter region and autoregulate the TA operon [20]. In Mycobacterium, VapBC is the most abundant TA family, and the VapBC protein complex can autoregulate the TA operon [5]. When we screened S. aureus genome, we observed the enriched profusion of the host inhibition of growth (HigBA) TA family, which is a sub-family of RelBE super-family [14]. The HigBA TA system has the unique genetic annotation in type II TA systems, i.e., the toxin gene is upstream of the antitoxin, which makes it different from the rest of type II TA families [21,22]. HigB is a ribonuclease in function followed by HigA antitoxin, and they can form a heterotetrameric complex. The antitoxin contains an HTH-Xre domain and can bind to DNA, while the toxin targets 50S ribosomal subunit [22]. To date, no HigBA TA system has been reported in S. aureus. In this study, we identified 15 HigBA TA systems in the S. aureus genome and classified them into three groups by pairwise alignment to its close members (Figure 2A–C).
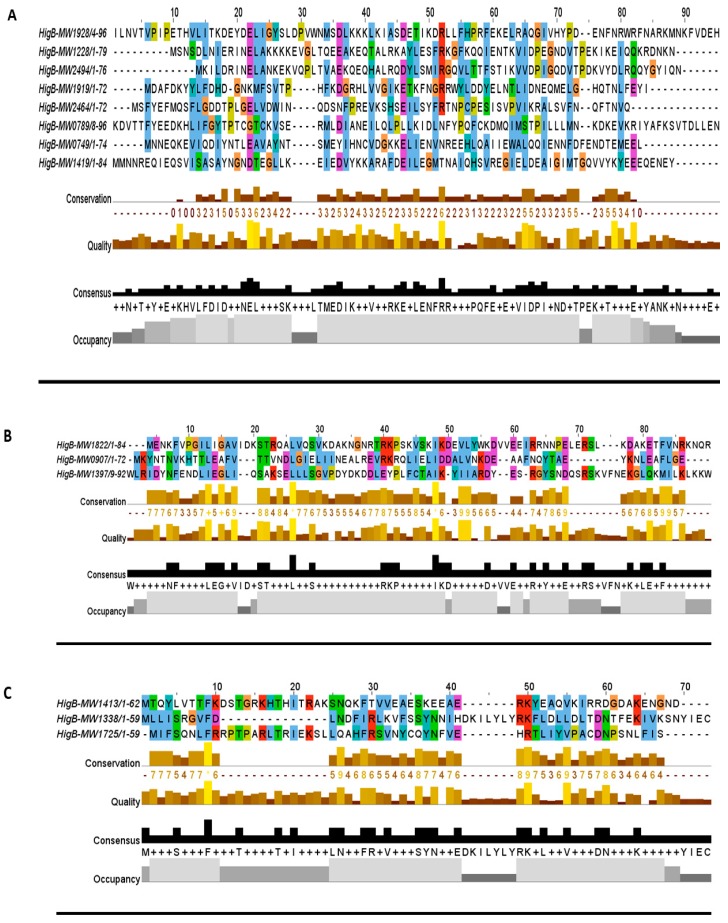
Figure 2.
Multiple sequence alignment of HigB toxins. (A–C) The HigB sequences were aligned by MUSCLE software across the S. aureus genome. HigB toxins that were short in length and showed divergence in their family were aligned together in a sub-family as shown in (B,C).
In all three groups, the HigB toxin is followed by the HigA antitoxin, which satisfies the first requirement in predicating the HigBA TA system (Table 1). Such enriched abundance of HigBA TA systems has not been reported in other bacteria, but possible homologs can be detected. For example, homologues of HigB toxins (MW1228, MW1928, and MW1413) are prevalent in Lactobacillus ceti, Enterococcus faecium, Streptococcus pneumoniae, E. coli, P. aeruginosa, Bacillus cereus, and Staphylococcus species (Figure 3A–C). We aligned HigB toxin MW1228 with homologues from L. ceti, E. faecium, S. pneumoniae, E. coli, P. aeruginosa, and B. cereus, and detected a highly conserved domain of 35 residues (13–48 aa) at the N-terminus (Figure 3A). We did not detect a similar domain in other HigB toxins such as MW1928 and MW1413, but they also shared a unique domain with their homologues. When we aligned the homologues of MW1928 and MW1413, we detected a highly conserved domain of 17 residues (10–27 aa) in MW1928 and 11 residues (20–31 aa) in MW1413 homologues (Figure 3B,C). Although the homologues of the HigBA TA family are widely detected in many bacteria, mostly remain uncharacterized except in Proteus vulgaris, Mycobacterium [19], Vibrio cholera [23], and P. aeruginosa. In P. aeruginosa, the HigB toxin functions as RNase and reduces biofilm formation, pyocyanin and pyochelin production, and swarming motility [22]. The endonuclease toxin HigB cleaved AAA rich sequences as well as single monomer (A) in the coding region of P. vulgaris [24]. Interestingly, the HigB target coincided with amino acid lysine (AAA), one of the most frequent amino acid in the GC-less and AT-rich Staphylococcus species. Therefore, the enriched profusion of the HigBA system highlights the target specificity and functional sites in S. aureus.
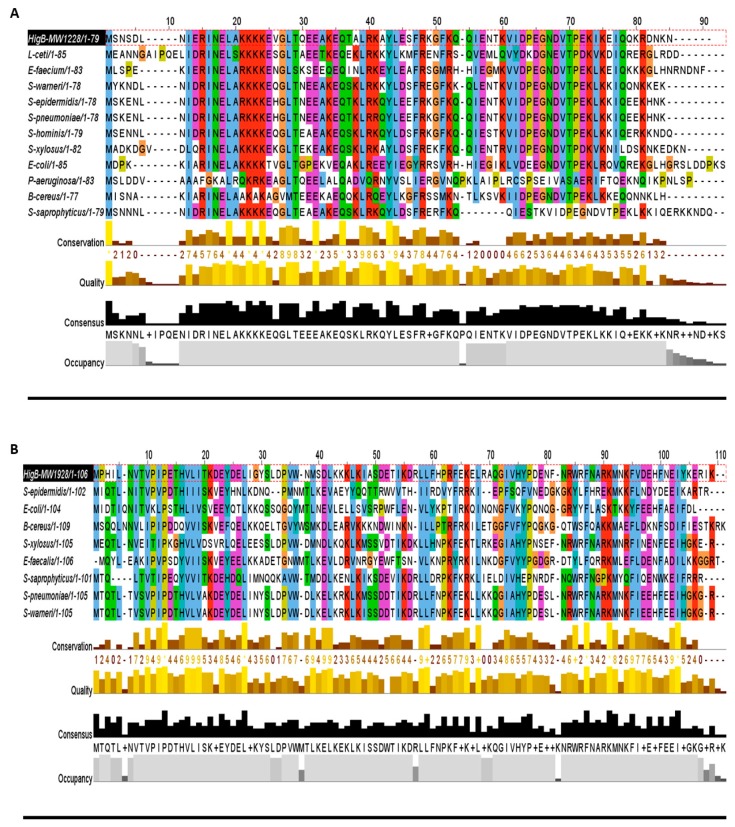
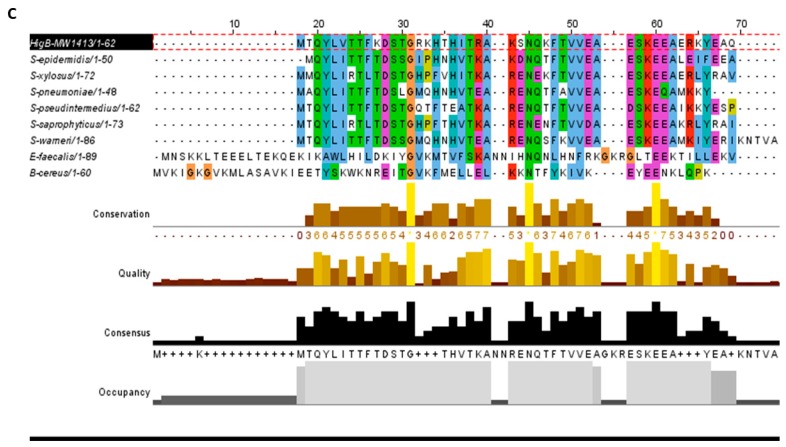
Figure 3.
Homologs of the HigB toxins. (A) The HigB toxin MW1228 showed high conservation in L. ceti, E. faecium, S. pneumoniae, E. coli, P. aeruginosa, and B. cereus. The N-terminal 35 residues (13–48 aa) was the highly conserved region in all of the selected homologues. (B) The toxin MW1928 was the largest protein (106 aa) in the HigBA family and displayed a highly conserved region at N-terminal (10–27 aa) and a less conserved region at C-terminal (95–109 aa). (C) The toxin MW1413 is a small protein (62 aa) in the HigBA family and highly conserved in Staphylococcus species such as S. epidermidius, S. xylosus, S. pseudointermedius, S. saprophyticus, S. warneri, and S. pneumoniae. MW1413 was less conserved in E. faecalis and B. cereus.
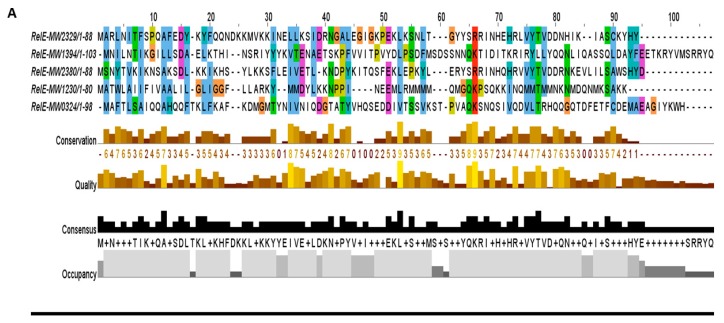
The RelBE super-family has sub-families, namely YefM-YoeB, MqsAR, PrlF-YhaV, and RelBE in E. coli [14]. The RelBE sub-family antitoxin RelB is degraded upon a stringent response that leads to the activation of the RelE toxin and inhibition of growth via targeting translation. Upon stringent response, RelB is degraded and subsequently leads to the activation of RelE, which targets translation and results in growth inhibition. The YefM-YoeB sub-family has been well characterized in S. aureus [3], S. quorum [25], S. suis [26], Streptomyces [27], E. coli, and S. pneumoniae [28]. The YoeB toxin can form a complex with the YefM antitoxin and target translation. In this study, we detected five RelBE TA systems (Figure 4A) that can be further classified into sub-families depending on their functions. For example, we characterized a new TA system of the RelBE super-family that showed resemblance with YefM-YoeB sub-family, and was involved in virulence control in S. aureus [20].
Figure 4.
The predicted toxins were aligned by MUSCLE and sorted by pairwise alignment to close matching protein. (A) The RelE toxins MW2329, MW2380, and MW1230 were shorter in length and showed homology towards YefM-YoeB sub-family, while the MW0324 and MW1394 were longer in length and displayed less conservation, possibly making a new sub-family. (B) The GNATs toxins showed a functional site of 28 residues (41–68 aa) that contained the most conserved region in this family. (C) A moderate consensus site of 20 residues (30–49 aa) was detected in the nuclease family. The nuclease toxins MW1311, MW1403, and MW1733 were closely related in sequence alignment, while MW0461 was less conserved.
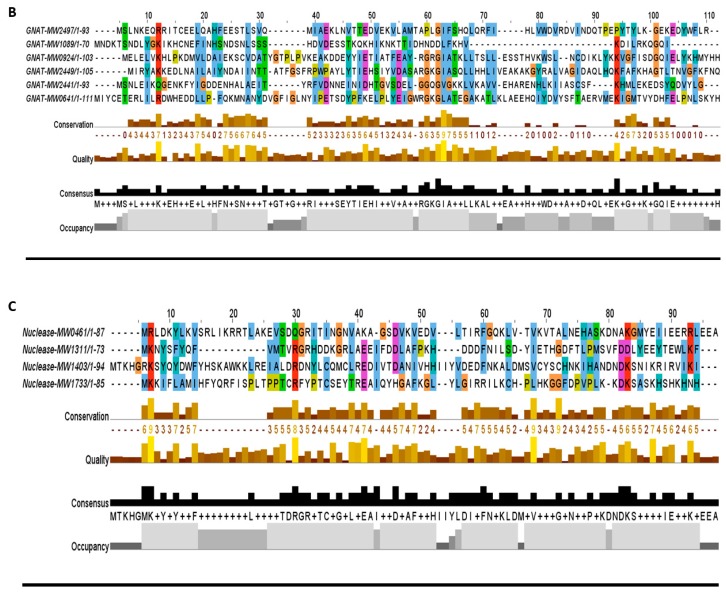
The GNATs-like TA system is the new addition of the TA family that can prevent peptide bond formation by acetylating tRNA and inhibiting translation in Salmonella and Klebsilla [18,29]. The TacT toxin inhibits translation via acetylating the initiator tRNA, and induces persister cell formation [29]. Six loci were detected in S. aureus that showed resemblance with GNATs TA systems (Figure 4B), and their toxins showed acetyltransferase-like domains.
Four TA loci showed resemblance with DNA gyrase inhibitors (nucleases) and were predicted to be either the ParDE or the CcdBA TA system (Figure 4C). The par toxin targets DNA gyrase and inhibits DNA replication in E. coli and M. tuberculosis [4,5]. The ParE-ParD components have been reported to form a heterotetrameric complex via the ParD N-terminal RHH domain that can increase the dimerization of ParD, while the C-terminal domain has a specific ParE toxin-binding site [30]. The CcdB toxin can bind to DNA gyrase, form a gyrase-DNA complex, and create a double-strand break into DNA. The CcdA antitoxin can resolve the complex by binding to the toxin protein and dislodge CcdB from gyrase [31]. So far, these loci have not been studied in detail in S. aureus and characterization of these systems will reveal the mechanism of toxin action.
The first persistence-associated gene hipA was first detected in 1983 [32] and later studied in E. coli as the HipBA TA system [33]. We found one HipBA-like system in S. aureus that is different in sequence and length, but its orthologue search revealed its relatedness with the HipBA family. Here we named it as the first HipBA TA system in S. aureus. In the hip operon, HipA is a toxin that phosphorylates glutamyl-tRNA synthetase, while HipB is the antitoxin that counteracts the HipA toxicity [33].
The VapBC TA system is the most abundant TA system in prokaryotes and is found copiously in E. coli, Mycobacterium, Shigella, Sulfolobus, and Leptospira [2,4,5,34], while there is no report of vapBC loci in S. aureus. The toxin VapC targets translation by cleaving mRNA or tRNA. The VapB antitoxin is usually a transcriptional regulator that has a DNA binding domain and forms a complex with VapC. VapBC can respond to different stress conditions such as hypoxia, antibiotics stress, and latent infections [34,35]. Collectively, we detected 3 VapBC TA systems in S. aureus (Table 1), which have not been characterized so far.
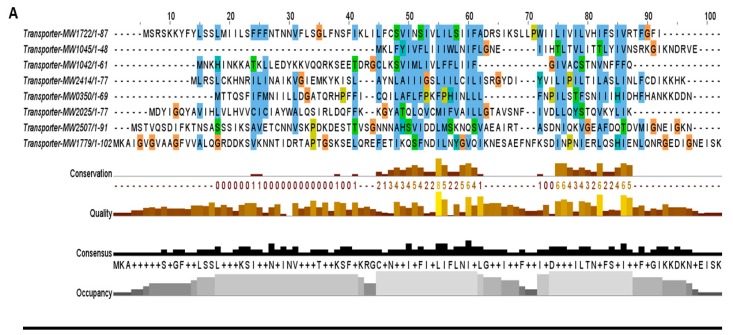
The second most abundant family that consists of nine TA systems belongs to the transporter TA family in S. aureus. These TA systems are either located in secretion systems or contain conserved domains associated with transport pathways. For example, toxin MW0268 and antitoxin MW0269 are located in the type VII secretion system while toxin MW2507 is an effector protein that showed proximity towards the type VII secretion system secretory proteins EsxA and EsxB. Similarly, toxins MW1042 and MW1722 are associated with ATP-binding cassette transporter permeases, while toxin MW2414 has orthologue homology with TerC protein that has been implicated in the efflux of tellurium ions in E. coli [36]. The toxins MW1779 and MW0350 are related to sodium and hydrogen antiporter systems. These putative TA systems were grouped into the transporter TA family, and their toxin proteins were aligned together that were less conserved and more divergent (Figure 5A). We believed that these systems might be involved in inhibition and regulation of secretion systems and transport pathways.
Figure 5.
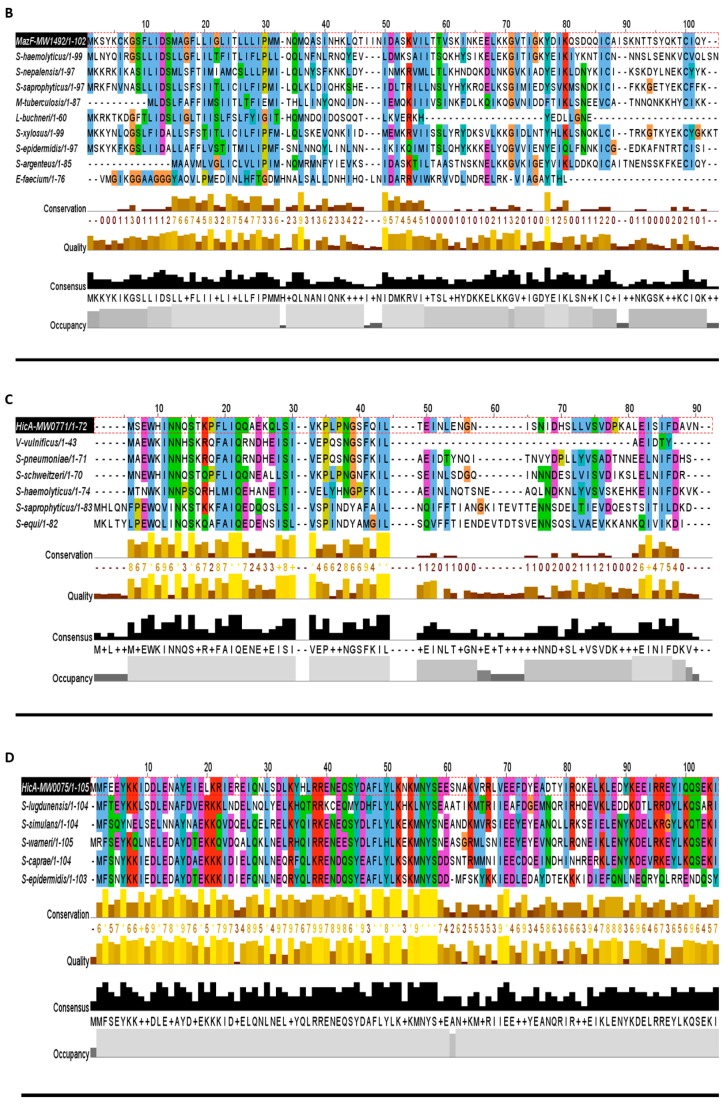
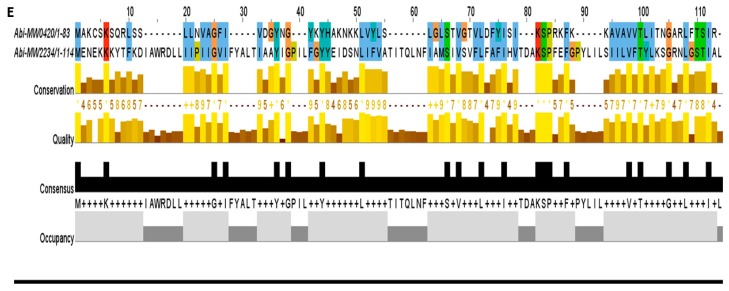
The toxins were aligned by MUSCLE and sorted by pairwise alignment to close matching protein. (A) The transporter family toxins displayed less conservation among all TA families because each of the protein is associated with different secretion systems and transport pathways. The 15 residues (48–62 aa) are the highly consensus sites in this family. (B) Homologs of MazF toxin MW1492 were aligned that showed a high degree of conservation in M. tuberculosis, L. buchneri, E. faecium, and Staphylococcus species. The highly conserved region contains 25 residues (8–32 aa) at N-terminus. (C) The HicA toxin MW0771 homologs were detected in Vibrio vulnificus, S. pneumoniae, and Staphylococcus species. The 20 residues (11–30 aa) are the highly consensus sites in the homologs of MW00771 protein. (D) The homologs of MW0075 could not be detected in M. tuberculosis, L. buchneri, E. faecium, V. vulnificus, S. pneumoniae and B. cereus. The MW0075 toxin is a highly conserved protein in Staphylococcus species. (E) The two Abi toxins were aligned by pairwise alignment that showed 16% sequence similarity. Both Abi toxin homologs could be detected in different bacterial species.
The MazEF family has been characterized by the toxin MazF that acts as an endoribonuclease targeting mRNA, 16S and 23S ribosomal RNAs in Mycobacterium and E. coli [5,10]. Components of the MazEF TA system are commonly called global translation inhibitors [37], and are activated upon nutrient or oxidative stress, DNA damaging response, and heat shock [5]. The MazEF TA systems have been studied in E. coli, M. tuberculosis, Clostridium difficile, and S. aureus [2,3,4,5]. In the present study, we detected one new and one previously studied MazEF systems. The new MazF toxin showed a high degree of conservation in other bacteria including E. faecium, M. tuberculosis, and lactobacillus buchneri (Figure 5B).
When we mined the S. aureus genome, we discovered two HicBA TA systems (Figure 5C,D). In the HicBA TA system, hicA codes for the toxin that cleaves mRNA and reduces the rate of translation, while hicB rescues the bacteriostatic effects induced by hicA [38]. The transcription of hicBA is induced upon carbon and amino acid starvation and depends on lon protease in E. coli [39].
Apart from the type II TA system, two loci were predicted as the abortive infection system (Abi) TA system that can probably constitute the type III or IV TA system (Figure 5E). The TA system ToxIN in Pectobacterium atrosepticum was the first TA system described that also acted as the abortive infection system. This system can lead to altruistic cell death and viral replication inhibition [40,41]. The Abi TA system has not been discovered in S. aureus so far and awaits further investigation
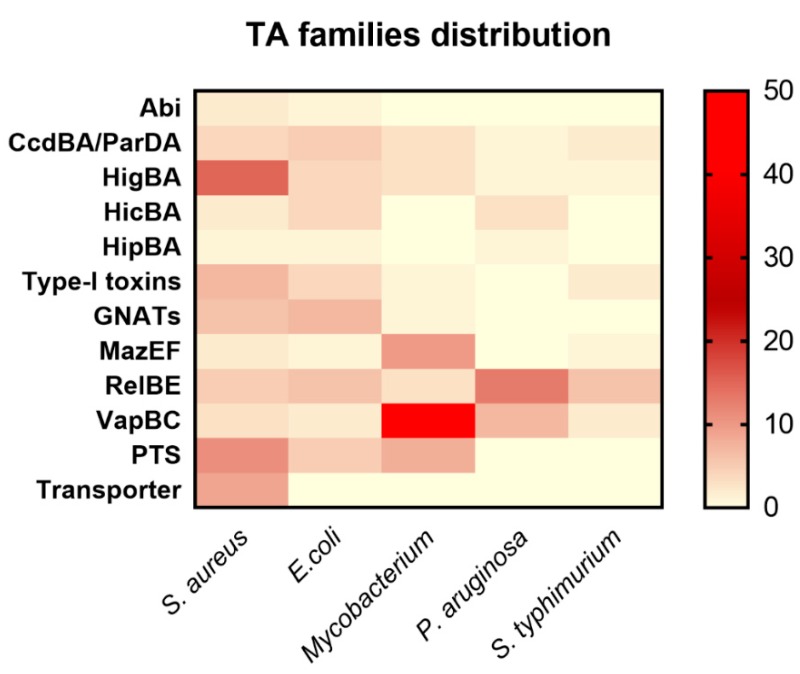
The S. aureus genome contains 11 TA systems that neither show any similarity with the reported TA families nor contain conserved domain. Therefore, we kept them as putative TA systems (PTS). Among these PTS, 3 TA systems can be three components (MW1430-1431-1432, MW1149-1150-1151, and MW0776-0777-0778) or two components including toxin and antitoxin as shown in Table 1. The third component can be either a second antitoxin or transcriptional regulator that probably alters the expression of TA genes. Three of the PTS were classified as a solo toxin (MW0101, MW0036, and MW1827) that can probably constitute different types of TA systems or might function differently (Table 1). In general, three-component TA systems and some solo toxins have been studied in detail in cyanobacteria [42]. Further research on these PTS systems may reveal their classification and function in S. aureus. In conclusion, we compared S. aureus TA families with the reported TA families of E. coli, Mycobacterium, P. aeruginosa, and S. typhimurium, and our results could advance our understanding of the distribution of TA families in bacteria (Figure 6).
Figure 6.
Distribution of TA families in S. aureus, E. coli, Mycobacterium, P. aruginosa, and S. typhimurium. The selected TA families were compared with the reported TA families of other bacteria. The highest number of TA systems in Mycobacterium is 50 VapBC, 10 MazEF, 8 PTS, and 3 HigBA TA systems. P. aruginosa has 13 RelBE, 7 VapBC, and 3 HicBA systems. E. coli has 7 GNATs, 6 RelBE, 5 nuclease toxins, and 4 each HigBA and HicBA systems. S. typhimurium has 6 RelBE, 2 VapBC, and 2 nuclease toxins. Seven type I toxins were found in S. aureus, 4 in E. coli, 2 in S. typhimurium, and 1 in Mycobacterium. The HipBA TA system was the rarest family that has not been discovered in any bacteria except E. coli, P. aeruginosa, and S. aureus.
2.3. Pathogenicity Islands TA Systems
Pathogenicity islands (PIs) are unique regions of DNA and consist of virulence genes and mobile genetic elements. We detected 7 PIs in S. aureus genomes by using PIs database [12]. These islands are enriched with TA-like genes. The roles of TA systems in PIs include but are not limited to successful integration, maintenance and stability of islands, and defense against phages [12,43]. The PI phi-Sa-2 and VSa-gamma have 11 and 4 TA systems (Table 2). We predicted that PIs-TA systems are the most functional TA systems in S. aureus. This prediction is supported by previous reports that mechanistically related TA systems can display different functions depending on their localization in regions such as PIs [43]. In total, we detected 26 TA systems out of 67 that are located within pathogenicity island (PI) regions with an abundance of virulence genes in the neighborhood.
Table 2.
The predicted seven PIs in the genome of S. aureus. Each PI has different number of TA systems and unique virulence genes.
| PIs | Size (bp) | Number of TA Systems | Representative Toxins from TA Systems | Virulence Genes in PIs |
|---|---|---|---|---|
| Phi-Sa-2 | 45,550 | 11 | MW1381, MW1394, MW1397, MW1403, MW1413, MW1419, MW1432, MW1434, MW1437, MW1440, MW1492 | Panton–Valentine leukocidin |
| VSa-gamma | 20,881 | 4 | MW1042, MW1045, MW1056, MW1070 | Alpha hemolysin, phenol soluble modulin |
| Phi-Sa-3 | 43,598 | 3 | MW1888, MW1919, MW1928 | Staphylokinase |
| VSa-beta | 31,977 | 3 | MW1725, MW1733, MW1779 | Proteases |
| VSa-alpha | 34,481 | 3 | MW0302, MW0324, MW0405 | -- |
| VSa-3 | 14,380 | 2 | MW0749, MW0771 | Enterotoxins, exotoxin |
| VSa-4 | 2710 | 0 | -- |
2.4. Functional Assessment of the Type I TA System
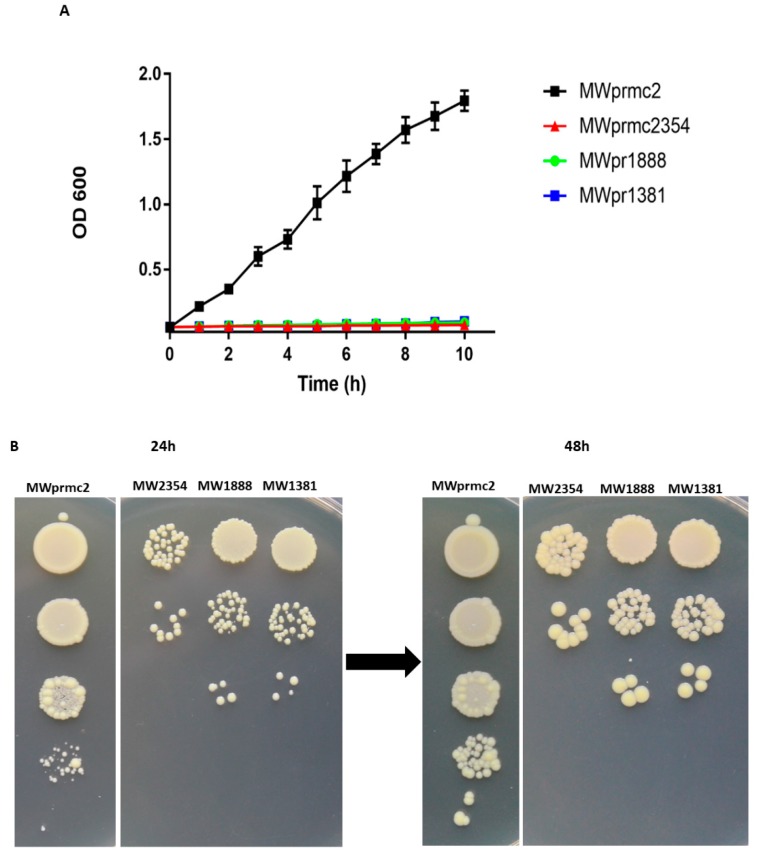
The type I TA system toxins are called bacterial time bombs because these toxins target the bacterial cell wall and induce cell death. Importantly, type I toxins are considered to be the most critical peptides to eradicate S. aureus cells [3,44]. Here we have detected seven type I TA systems that have toxin domains such as TxpA, Fst, and holin (Table 1). We have also revealed two type I TA systems Fst and TxpA that were previously reported [3,15]. To assess the activity of three type I toxins that target cell wall, we constructed different recombinant vectors as shown in Materials and Methods. The toxin was ectopically overexpressed in S. aureus and the OD600 value was determined. The expression of toxins inhibited cell growth, confirming the deadly nature of type I toxins (Figure 7A). Further, the cellular toxicity assay was performed on anhydrotetracycline (ATC) inducible plates. The results indicated that cell growth was inhibited and cannot be restored (Figure 7B), demonstrating that type I toxins are bactericidal and can induce cell death in S. aureus.
Figure 7.
Overexpression of type I toxins. (A) Normal growth was observed in MWprmc2 that contains an empty pRMC2 vector while complete growth inhibition was observed in MWprmc2354, MWprmc1888 and MWprmc1381 that contain Fst, TxpA, and holin toxins, respectively. (B) The bactericidal nature of three type I toxins was determined by ATC inducible plates, and the results showed that cells expressing Fst, TxpA, and holin toxins were unable to restore growth as compared to control MWprmc2.
2.5. Transmission Eelectron Microscopy Analysis of the Bacterial Cell Wall
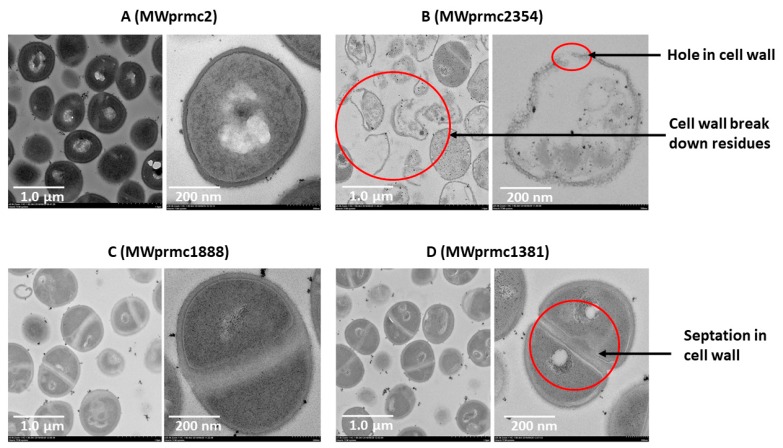
Type I toxins are short peptides and apoptotic in nature and target cell wall [45]. We ectopically expressed three type I toxins for 1 h, harvested the cells, and analyzed the cell wall by transmission electron microscope (TEM). As a control, the S. aureus with empty pRMC2 vector was used (Figure 8A). The type I toxins targeted the cell wall differently. The MWprmc2354 (Fst toxin) created holes and broke the cell wall, and its lysis activity was intense. Apart from this, empty cells with no cellular entities were observed, and cells unable to withstand the toxin effects and ultimately cell wall remaining remnants were detected with ectopic expression of Fst (Figure 8B). The ectopic expression of MWprmc1888 (TxpA toxin) and MWprmc1381 (holin toxin) led to neither holes nor breakage, and instead increased wall septation. Two cells intersecting their cell walls and septum formation were ostensible with the TxpA (Figure 8C) and holin (Figure 8D) toxins as shown by TEM micrographs (Figure 8A–D).
Figure 8.
The bacterial cell wall was analyzed by TEM. Single cell micrographs were captured at magnification ×25.0 K and scale bar 200 nm while the group of cells micrographs were captured at magnification ×8.0 K and scale bar 1.0 µm. (A) Mwprmc2 was an empty vector and used as control. (B) The Fst toxin (MWprmc2354) broke down the cell wall as well as created holes. (C,D) The TxpA (MWprmc1888) and holin (MWprmc1381) toxins increased cell wall septation and reduced cell size compared to the control MWprmc2 with the empty vector.
2.6. Response and Function of Cellular Proteases in S. aureus
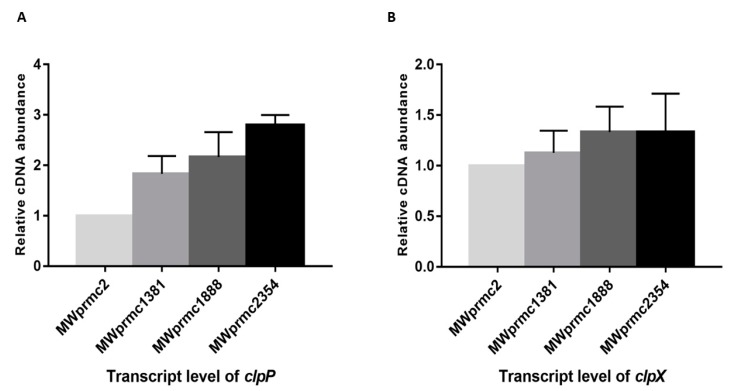
Most of TA systems contain a liable antitoxin that is either degraded by cellular proteases or suppressed to enhance toxin expression under stress conditions, suggesting that the antitoxin can be a positive or a negative regulator of the toxin. We detected six different types of Clp proteases; ClpA, ClpB, ClpC, ClpP, ClpQ, and ClpX in S. aureus. Among them, ClpC and ClpX form complexes with ClpP and are involved in the protein homeostasis by either conditional degradation or disposing of the unwanted proteins [46]. In the present study, we observed the upregulation of clpP towards type I toxins, while clpX remained steady (Figure 9A,B). Our data indicated that clpP was activated when the toxin was ectopically expressed. This result suggests that the proteases play an essential role in keeping the toxin level in the balanced state in order not to severely damage the bacterial cells. The response and function of proteases towards TA systems are an exciting topic and need further study.
Figure 9.
The transcript levels of clpP and clpX were determined by quantitative real-time PCR (qRT-PCR). The transcript levels were compared with MWprmc2 containing empty pRMC2 vector. (A) The ectopic expression of MWprmc2354 toxin activated clpP (p ≤ 0.06) when compared to other type I toxins MWprmc1888 and MWprmc1381 (p ≤ 0.08). (B) The ectopic expression of type I toxins did not alter the transcript level of clpX.
2.7. Functional Assessment of Type II TA Systems
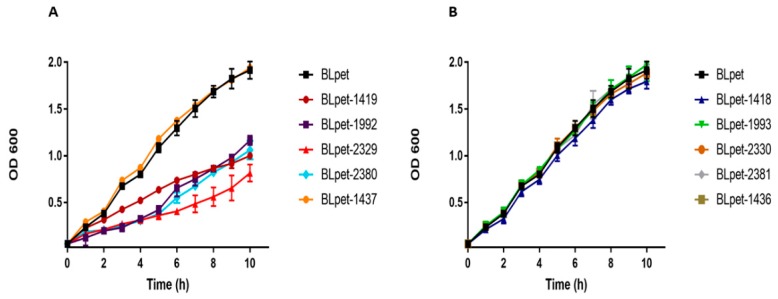
The type II TA system is well studied in E. coli, Mycobacterium, Pseudomonas, and Staphylococcus. After screening the TA loci in S. aureus, we randomly selected five type II TA systems from different families, i.e., MW2329-MW2330 and MW2380-MW2381 TA systems from the RelBE family, MW1992-MW1993 TA system from the MazEF family, MW1419-MW1418 TA system from the HigBA family, and MW1436-MW1437 TA system from the PTS family. We aimed to confirm whether the predicted toxin or antitoxin can inhibit cell growth or not. We ectopically expressed toxins and antitoxins in E. coli BL21 and monitored the bacterial growth. All the toxins inhibited cell growth except MW1437, while all the antitoxins were similar to control BLpet with empty pET28a plasmid (Figure 10A,B). We conclude that most of the type II TA system toxins target metabolic pathways and induce bacteriostatic conditions, contrasting to type I toxins with bactericidal activities.
Figure 10.
Expression of type II toxins and antitoxins in E. coli BL21. (A) The ectopic expression of RelE toxins (BLpet-2329 and BLpet-2380), HigB toxin (BLpet-1419), and MazF toxin (BLpet-1992) inhibited cell growth while BLpet-1437 has no inhibitory effects on cell growth. (B) RelB antitoxins (BLpet-2330 and BLpet-2381), HigA antitoxin (BLpet-1418), MazE antitoxin (BLpet-1993), and BLpet-1436 did not inhibit cell growth.
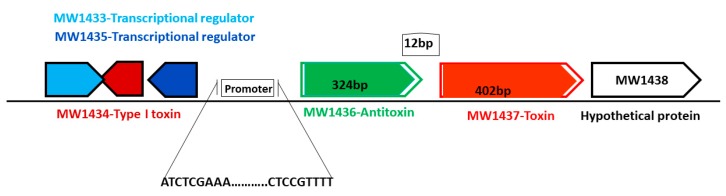
The MW1436-MW1437 TA locus was predicted to be either a type II or a type IV TA system and classified as PTS (Table 1). Locus MW1437 codes for a toxin while MW1436 codes for an antitoxin. The two genes are separated by a 12 bp intergenic region and are located in the phi-Sa-2 PI region within the neighborhood of one of the type I TA system (MW1434) (Figure 11). The toxin protein has a Zn-dependent protease-like domain, while the antitoxin is an HTH domain-containing transcriptional regulator. The ectopic expression of both toxin BLpet-1437 and antitoxin BLpet-1436 were growth-supportive, and no cellular toxicity was observed (Figure 10A,B).
Figure 11.
Schematic representation of a new TA system from phi-Sa-2 pathogenicity island. The toxin-coding gene is 402 bp, the antitoxin-coding gene is 324 bp in length, and they are located close to the type I TA system and transcriptional regulator.
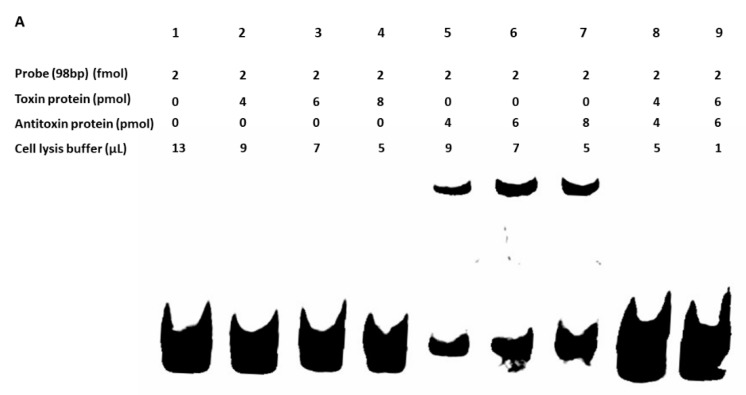
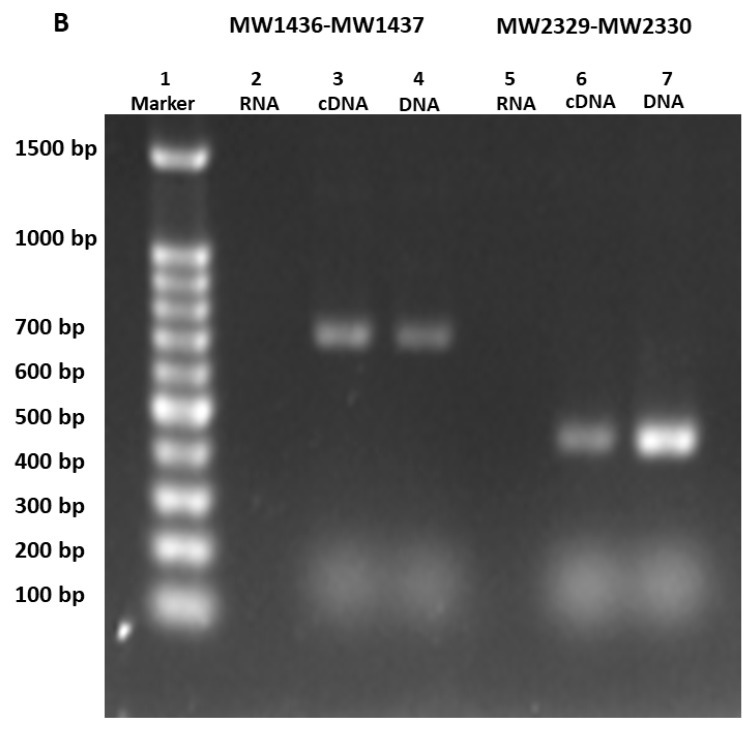
We did not detect the in vivo protein–protein interaction between toxin (BLpet-1437) and antitoxin (BLpet-1436) proteins (data not shown). Further, we evaluated the DNA-binding ability of antitoxin and toxin proteins. Firstly, we determined the 98 bp promoter region of this operon using SoftBerry software. We incubated the toxin and antitoxin separately as well as together with the 98 bp biotin-labeled promoter probe. Our gel-shift assay results indicated that the antitoxin can bind to the probe and probably autoregulate this operon (Figure 12A, lanes 5–7), while the toxin could not bind to the probe (Figure 12A, lanes 2–4). Interestingly, the addition of the toxin protein to antitoxin and the probe mixture inhibited antitoxin–probe complex formation, suggesting that the toxin protein can inhibit the binding of antitoxin to the promoter region and prevent autoregulation (Figure 12A, lanes 8 and 9). To further determine whether the two genes can be co-transcribed, we performed RT-PCR using RNA, cDNA, and DNA as templates and detected the 700 bp co-transcript of MW1436-MW1437 (Figure 12B, lanes 3 and 4). Our data also showed that relBE genes MW2329-MW2330 were co-transcribed (Figure 12B, lanes 6 and 7). In summary, although the toxin of this TA system did not inhibit cell growth, it may be involved in some other functions such as autoregulation of the neighbor genes or stability of the phi-Sa-2 island.
Figure 12.
(A) Gel-shift assay was performed to detect the binding of toxin and antitoxin to the promoter. The up-shift represents the binding of the antitoxin protein to the 98 bp probe (lanes 5–7), while the toxin protein was unable to bind (lanes 2–4). No shift was observed when antitoxin and toxin proteins were mixed together with the 98 bp probe (lanes 8 and 9). (B) The TA genes MW1436-MW1437, and MW2329-M2330 were co-transcribed. 1 = marker, 2, 5 = RNA, 3, 6 = cDNA, and 4, 7 = DNA. The co-transcript product (lanes 3 and 4) represents the 700 bp size of MW1436-MW1437, and lanes 6 and 7 represent the 400 bp size of MW2329-MW2330.
3. Conclusions
We comprehensively screened all the possible TA loci in S. aureus genome and predicted previously unidentified 67 TA systems, and classified them into different families and types. Our data substantially expanded the collection of TA systems in S. aureus and revealed the nature of type I and type II toxins. We detected a new TA system whose toxin did not inhibit cell growth but prevented cognate antitoxin binding to the promoter. The presence of these chromosomally encoded TA systems suggests that these loci may be involved in S. aureus physiology and genome stability. More importantly, our prediction may open a new window for further study of these uncharacterized TA systems, especially, their association with S. aureus pathogenicity and virulence.
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
The bacterial strains used in this study are listed in Table S1 of the Supplementary Material. S. aureus was grown in tryptic soy broth (TSB) medium and E. coli was grown in lysogeny broth (LB). pRMC-2, an ATC inducible shuttle vector, and pET28a, an isopropyl β-D-1-thiogalactopyranoside (IPTG) inducible plasmid, were used in S. aureus and E. coli BL21, respectively. For plasmid maintenance, each medium was supplemented with appropriate antibiotic (ampicillin 150 µg/mL, chloramphenicol 15 µg/mL, and kanamycin 50 µg/mL). Cells were washed with normal saline. Normal growth conditions for S. aureus and E. coli were 37 °C and 220 rpm. Growth curves were determined by measuring optical density at 600 nm using a BioTek microplate reader.
4.2. Screening of TA Systems in S. aureus
Initially, we identified all putative TA genes in S. aureus by Rasta bacteria and TADB. Rasta bacteria cutoff score >53 was set and 80 TA systems were selected, and seven TA systems were predicted by TADB. Further, we performed PSI-BLAST searches using Mycobacterium and E. coli known TA systems as modal and selected 30 more TA systems in S. aureus. To improve the screening strategy, we analyze the predicted TA domains in databases such as KEGG, NCBI, and Ensembl bacteria, and analyzed the orthologues and homologs. Then the selected TA systems were classified into different families and types. The approach is summarized in Figure 1A.
4.3. Construction of Vectors
TA genes were amplified by PCR and inserted into pRMC2 vectors via KpnI-SacI-EcoRI and pET28a via NdeI-BamHI-EcoRI-XhoI restriction enzymes, resulting in the recombinant vectors MWprmc2354, MWprmc1888, MWprmc1381, BLpet2329, BLpet2330, BLpet2380, BLpet2381, BLpet1992, BLpet1993, BLpet1418, and BLpet1419. MWprmc2 and BLpet were empty vectors and used as controls in S. aureus and E. coli, respectively. All the constructed vectors were confirmed by DNA sequencing. The primers used for the construction of vectors are provided in Table S1 of the Supplementary Material.
4.4. Cellular Toxicity Assay
The constructed vectors were transformed into E. coli Trans T1 and BL21 by heat shock at 42 °C for 1 min. The pRMC2-vectors were initially transformed into E. coli Trans T1 by heat shock and then to S. aureus RN4220 and S. aureus MW2 by electroporation. Further, the 24 h culture was diluted 200 times and grown at 37 °C for 2 h. When the culture reached OD600 = 0.45–0.48, ATC (200 ng/mL) or IPTG (0.1 mM) was added, and the growth pattern was monitored for 10 h at 1 h intervals. The bactericidal nature of type I toxins was assessed on ATC plates. S. aureus was grown as described above to OD600 = 0.85–0.90. Cells (normally 5 × 108 CFU/mL) were serially diluted in water, and 10 µL from each dilution was spotted on ATC inducible plates. The plates were incubated at 37 °C and observed for the restoration of growth after 24 h and 48 h intervals.
4.5. RNA Extraction and qRT-PCR
S. aureus was grown in TSB medium under specified conditions such as ATC induction for 1 h. Then, 1 mL of culture was centrifuged at 12,000 rpm for 2 min and the cells were washed and suspended in RNAiso Plus. Cells were treated with 0.1 mm silica beads in RNA isolation tube by the FastPrep-24 Automated system for 40 s twice. Trichloromethane was added in the RNA isolation tube and kept at 4 °C for 5 min, and then centrifuged at 12,000 rpm at 4 °C for 20 min. The up-layer was taken and mixed with an equal volume of isopropanol and kept at −20 °C for 2 h. Then the tubes were centrifuged at 12,000 rpm for 30 min, washed with 75% ethanol, air dried and dissolved in water (50 µL). RNase-free DNase I was used for residual DNA removal, and then RNAiso Plus and trichloromethane were added and centrifuged at 12,000 rpm at 4 °C for 20 min. The up-layer supernatant was transferred to a new tube and mixed with an equal volume of isopropanol and kept at −20 °C for 2 h. The tubes were centrifuged at 12,000 rpm at 4 °C for 30 min, and RNA was collected and washed with 75% ethanol and air dried. RNA concentration and quality were determined by Beckman Coulter and NanoDrop. The PrimeScript First Strand cDNA synthesis kit and SYBR Premix Ex Taq kit (TaKaRa) were used for RT and real-time PCR, respectively. The gene signal was normalized with gyrA or hu cDNA abundance [47]. All the qRT-PCR primers were designed by Beacon designer 7 and synthesized by General Biosystems China and listed in Table S1 of the Supplementary Material.
4.6. Preparation of Samples for Transmission Electron Microscopy
The type I toxins were ectopically expressed for 1 h in S. aureus, and cells were harvested and washed with phosphate buffered saline (PBS). The samples were then fixed with 2% paraformaldehyde and 2.5% glutaraldehyde in PBS buffer at 4 °C overnight, and washed three times in PBS buffer, then fixed with 2% Osmium tetroxide in PBS buffer at 4 °C overnight, and washed three times in PBS buffer. After fixation, the samples were dehydrated by a graded series of ethanol (30%, 50%, 70%, 80%, 90%, 95%, and 100%) for 20 min at each step, and transferred to absolute acetone for 20 min. The samples were placed in 1:1 mixture of Spurr resin and absolute acetone at room temperature for 1 h, and then transferred to 3:1 mixture of Spurr resin and absolute acetone at room temperature for 3 h, and to Spurr resin mixture overnight. Then the samples were placed in capsules contained embedding medium and heated at 70 °C overnight. The specimen sections were stained by uranyl acetate and alkaline lead citrate for 5–10 min, respectively, and images were captured with a Hitachi HT-7700 transmission electron microscope.
4.7. Gel-Shift Assay
Toxin and antitoxin expression vectors BLpet1437 and BLpet1436 were transferred into E. coli BL21 cells, and the transformants were grown in LB medium at 37 °C to OD600 = 0.45–0.48, and induced with IPTG (0.1 mM) at 30 °C for 5 h. Kanamycin 50 µg/mL was added into LB medium to maintain the plasmid. Cells were harvested, washed with normal saline, and lysed by sonication for 15 min in lysis buffer (50 mM Tris-HCl, pH 8.0, 300 mM NaCl). The proteins were purified by Ni2+-NTA histidine affinity column and concentrated by Millipore protein concentration column. Then, the biotin label primer was synthesized and the 98 bp promoter region was amplified by PCR and incubated with toxin and antitoxin proteins at 37 °C for 1 h. The 15µL reaction mixture contains probe (2 fmol), each toxin and antitoxin protein (4–8 pmol), and lysis buffer. The reaction was stopped by adding gel-shift loading dye and heated at 95 °C for 10 min, and then loaded on 4% native polyacrylamide gel and run in 0.5× Tris-borate-EDTA buffer at 400 mA for 30 min as previously described [20]. DNA bands were transferred onto nylon membrane and detection was performed with chemiluminescent detection kit. Image Quant LAS 4000 mini imaging system was used to detect the membrane.
4.8. Statistical Analysis
The nucleotides and protein sequences were obtained from the NCBI database and analyzed by Vector NTI software. For protein sequence alignment, MUSCLE [48] software was used and visualized by Jalview. Graph pad prism and Origin pro 9 was used for data analysis. Primers were designed by Beacon designer 7. Welch t-test was used for statistical significance.
Acknowledgments
We thank Zeyu Jin and Jiade Zhu for technical assistance. We are grateful to the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) for providing the staphylococcal strains.
Supplementary Material
The following is available online at http://www.mdpi.com/2072-6651/10/11/473/s1, Table S1: List of all bacterial strains, vectors, and primers used in this study. The lower case letters represent restriction enzyme sites.
Author Contributions
B.S. and G.H. designed the project; G.H. and Q.Z. performed the experiments; G.H. analyzed the data and wrote the manuscript; All authors proofread the manuscript.
Funding
Gul Habib was supported by the World Academy of Sciences Ph.D. Presidential Fellowship Program. This study was funded by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB29020000).
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
This study reveals 67 toxin-antitoxin systems, 13 TA families classified in five TA types, and seven pathogenicity islands in Staphylococcus aureus.
References
- 1.Baba T., Takeuchi F., Kuroda M., Yuzawa H., Aoki K., Oguchi A., Nagai Y., Iwama N., Asano K., Naimi T., et al. Genome and virulence determinants of high virulence community-acquired mrsa. Lancet. 2002;359:1819–1827. doi: 10.1016/S0140-6736(02)08713-5. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-Garcia L., Blasco L., Lopez M., Bou G., Garcia-Contreras R., Wood T., Tomas M. Toxin-antitoxin systems in clinical pathogens. Toxins. 2016;8:227. doi: 10.3390/toxins8070227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuster C.F., Bertram R. Toxin-antitoxin systems of staphylococcus aureus. Toxins. 2016;8:140. doi: 10.3390/toxins8050140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamaguchi Y., Park J.H., Inouye M. Toxin-antitoxin systems in bacteria and archaea. Annu. Rev. Genet. 2011;45:61–79. doi: 10.1146/annurev-genet-110410-132412. [DOI] [PubMed] [Google Scholar]
- 5.Sala A., Bordes P., Genevaux P. Multiple toxin-antitoxin systems in mycobacterium tuberculosis. Toxins. 2014;6:1002–1020. doi: 10.3390/toxins6031002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De la Cruz M.A., Zhao W., Farenc C., Gimenez G., Raoult D., Cambillau C., Gorvel J.P., Meresse S. A toxin-antitoxin module of salmonella promotes virulence in mice. PLoS Pathog. 2013;9:e1003827. doi: 10.1371/journal.ppat.1003827. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Page R., Peti W. Toxin-antitoxin systems in bacterial growth arrest and persistence. Nat. Chem. Biol. 2016;12:208–214. doi: 10.1038/nchembio.2044. [DOI] [PubMed] [Google Scholar]
- 8.Wen Z., Wang P., Sun C., Guo Y., Wang X. Interaction of type iv toxin/antitoxin systems in cryptic prophages of escherichia coli k-12. Toxins. 2017;9:77. doi: 10.3390/toxins9030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X., Lord D.M., Cheng H.Y., Osbourne D.O., Hong S.H., Sanchez-Torres V., Quiroga C., Zheng K., Herrmann T., Peti W., et al. A new type v toxin-antitoxin system where mrna for toxin ghot is cleaved by antitoxin ghos. Nat. Chem. Biol. 2012;8:855–861. doi: 10.1038/nchembio.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kedzierska B., Hayes F. Emerging roles of toxin-antitoxin modules in bacterial pathogenesis. Molecules. 2016;21:790. doi: 10.3390/molecules21060790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aakre C.D., Phung T.N., Huang D., Laub M.T. A bacterial toxin inhibits DNA replication elongation through a direct interaction with the beta sliding clamp. Mol. Cell. 2013;52:617–628. doi: 10.1016/j.molcel.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon S.H., Park Y.K., Kim J.F. Paidb v2.0: Exploration and analysis of pathogenicity and resistance islands. Nucleic Acids Res. 2015;43:D624–D630. doi: 10.1093/nar/gku985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sevin E.W., Barloy-Hubler F. Rasta-bacteria: A web-based tool for identifying toxin-antitoxin loci in prokaryotes. Genome Biol. 2007;8:R155. doi: 10.1186/gb-2007-8-8-r155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie Y., Wei Y., Shen Y., Li X., Zhou H., Tai C., Deng Z., Ou H.Y. Tadb 2.0: An updated database of bacterial type ii toxin-antitoxin loci. Nucleic Acids Res. 2018;46:D749–D753. doi: 10.1093/nar/gkx1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fozo E.M., Makarova K.S., Shabalina S.A., Yutin N., Koonin E.V., Storz G. Abundance of type i toxin-antitoxin systems in bacteria: Searches for new candidates and discovery of novel families. Nucleic Acids Res. 2010;38:3743–3759. doi: 10.1093/nar/gkq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lobato-Marquez D., Moreno-Cordoba I., Figueroa V., Diaz-Orejas R., Garcia-del Portillo F. Distinct type i and type ii toxin-antitoxin modules control salmonella lifestyle inside eukaryotic cells. Sci. Rep. 2015;5:9374. doi: 10.1038/srep09374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen S.B., Ghoul M., Griffin A.S., Petersen B., Johansen H.K., Molin S. Diversity, prevalence, and longitudinal occurrence of type ii toxin-antitoxin systems of pseudomonas aeruginosa infecting cystic fibrosis lungs. Front. Microbiol. 2017;8:1180. doi: 10.3389/fmicb.2017.01180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeo C.C. Gnat toxins of bacterial toxin-antitoxin systems: Acetylation of charged trnas to inhibit translation. Mol. Microbiol. 2018;108:331–335. doi: 10.1111/mmi.13958. [DOI] [PubMed] [Google Scholar]
- 19.Schuessler D.L., Cortes T., Fivian-Hughes A.S., Lougheed K.E., Harvey E., Buxton R.S., Davis E.O., Young D.B. Induced ectopic expression of higb toxin in mycobacterium tuberculosis results in growth inhibition, reduced abundance of a subset of mrnas and cleavage of tmrna. Mol. Microbiol. 2013;90:195–207. doi: 10.1111/mmi.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen W., Liu B., Xue L., Zhu Z., Niu L., Sun B. Autoregulation and virulence control by the toxin-antitoxin system savrs in staphylococcus aureus. Infect. Immun. 2018;86:IAI-00032. doi: 10.1128/IAI.00032-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armalyte J., Jurenas D., Krasauskas R., Cepauskas A., Suziedeliene E. The higba toxin-antitoxin module from the opportunistic pathogen acinetobacter baumannii—Regulation, activity, and evolution. Front. Microbiol. 2018;9:732. doi: 10.3389/fmicb.2018.00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood T.L., Wood T.K. The higb/higa toxin/antitoxin system of pseudomonas aeruginosa influences the virulence factors pyochelin, pyocyanin, and biofilm formation. Microbiologyopen. 2016;5:499–511. doi: 10.1002/mbo3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Budde P.P., Davis B.M., Yuan J., Waldor M.K. Characterization of a higba toxin-antitoxin locus in vibrio cholerae. J. Bacteriol. 2007;189:491–500. doi: 10.1128/JB.00909-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurley J.M., Woychik N.A. Bacterial toxin higb associates with ribosomes and mediates translation-dependent mrna cleavage at a-rich sites. J. Biol. Chem. 2009;284:18605–18613. doi: 10.1074/jbc.M109.008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nolle N., Schuster C.F., Bertram R. Two paralogous yefm-yoeb loci from staphylococcus equorum encode functional toxin-antitoxin systems. Microbiology. 2013;159:1575–1585. doi: 10.1099/mic.0.068049-0. [DOI] [PubMed] [Google Scholar]
- 26.Zheng C., Xu J., Ren S., Li J., Xia M., Chen H., Bei W. Identification and characterization of the chromosomal yefm-yoeb toxin-antitoxin system of streptococcus suis. Sci. Rep. 2015;5:13125. doi: 10.1038/srep13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sevillano L., Diaz M., Yamaguchi Y., Inouye M., Santamaria R.I. Identification of the first functional toxin-antitoxin system in streptomyces. PLoS ONE. 2012;7:e32977. doi: 10.1371/journal.pone.0032977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nieto C., Cherny I., Khoo S.K., de Lacoba M.G., Chan W.T., Yeo C.C., Gazit E., Espinosa M. The yefm-yoeb toxin-antitoxin systems of escherichia coli and streptococcus pneumoniae: Functional and structural correlation. J. Bacteriol. 2007;189:1266–1278. doi: 10.1128/JB.01130-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheverton A.M., Gollan B., Przydacz M., Wong C.T., Mylona A., Hare S.A., Helaine S. A salmonella toxin promotes persister formation through acetylation of trna. Mol. Cell. 2016;63:86–96. doi: 10.1016/j.molcel.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalton K.M., Crosson S. A conserved mode of protein recognition and binding in a pard-pare toxin-antitoxin complex. Biochemistry. 2010;49:2205–2215. doi: 10.1021/bi902133s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Jonge N., Garcia-Pino A., Buts L., Haesaerts S., Charlier D., Zangger K., Wyns L., De Greve H., Loris R. Rejuvenation of ccdb-poisoned gyrase by an intrinsically disordered protein domain. Mol. Cell. 2009;35:154–163. doi: 10.1016/j.molcel.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 32.Moyed H.S., Bertrand K.P. Hipa, a newly recognized gene of escherichia coli k-12 that affects frequency of persistence after inhibition of murein synthesis. J. Bacteriol. 1983;155:768–775. doi: 10.1128/jb.155.2.768-775.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Germain E., Castro-Roa D., Zenkin N., Gerdes K. Molecular mechanism of bacterial persistence by hipa. Mol. Cell. 2013;52:248–254. doi: 10.1016/j.molcel.2013.08.045. [DOI] [PubMed] [Google Scholar]
- 34.Cooper C.R., Daugherty A.J., Tachdjian S., Blum P.H., Kelly R.M. Role of vapbc toxin-antitoxin loci in the thermal stress response of sulfolobus solfataricus. Biochem. Soc. Trans. 2009;37:123–126. doi: 10.1042/BST0370123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramage H.R., Connolly L.E., Cox J.S. Comprehensive functional analysis of mycobacterium tuberculosis toxin-antitoxin systems: Implications for pathogenesis, stress responses, and evolution. PLoS Genet. 2009;5:e1000767. doi: 10.1371/journal.pgen.1000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orth D., Grif K., Dierich M.P., Wurzner R. Variability in tellurite resistance and the ter gene cluster among shiga toxin-producing escherichia coli isolated from humans, animals and food. Res. Microbiol. 2007;158:105–111. doi: 10.1016/j.resmic.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Pandey D.P., Gerdes K. Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res. 2005;33:966–976. doi: 10.1093/nar/gki201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li G., Shen M., Lu S., Le S., Tan Y., Wang J., Zhao X., Shen W., Guo K., Yang Y., et al. Identification and characterization of the hicab toxin-antitoxin system in the opportunistic pathogen pseudomonas aeruginosa. Toxins. 2016;8:113. doi: 10.3390/toxins8040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jorgensen M.G., Pandey D.P., Jaskolska M., Gerdes K. Hica of escherichia coli defines a novel family of translation-independent mrna interferases in bacteria and archaea. J. Bacteriol. 2009;191:1191–1199. doi: 10.1128/JB.01013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dy R.L., Przybilski R., Semeijn K., Salmond G.P., Fineran P.C. A widespread bacteriophage abortive infection system functions through a type iv toxin-antitoxin mechanism. Nucleic Acids Res. 2014;42:4590–4605. doi: 10.1093/nar/gkt1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Short F.L., Akusobi C., Broadhurst W.R., Salmond G.P.C. The bacterial type iii toxin-antitoxin system, toxin, is a dynamic protein-rna complex with stability-dependent antiviral abortive infection activity. Sci. Rep. 2018;8:1013. doi: 10.1038/s41598-017-18696-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kopfmann S., Roesch S.K., Hess W.R. Type ii toxin-antitoxin systems in the unicellular cyanobacterium synechocystis sp. Pcc 6803. Toxins. 2016;8:228. doi: 10.3390/toxins8070228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng Y., Chen C.J., Su L.H., Hu S., Yu J., Chiu C.H. Evolution and pathogenesis of staphylococcus aureus: Lessons learned from genotyping and comparative genomics. FEMS Microbiol. Rev. 2008;32:23–37. doi: 10.1111/j.1574-6976.2007.00086.x. [DOI] [PubMed] [Google Scholar]
- 44.Pinel-Marie M.L., Brielle R., Felden B. Dual toxic-peptide-coding staphylococcus aureus rna under antisense regulation targets host cells and bacterial rivals unequally. Cell Rep. 2014;7:424–435. doi: 10.1016/j.celrep.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 45.Sayed N., Nonin-Lecomte S., Rety S., Felden B. Functional and structural insights of a staphylococcus aureus apoptotic-like membrane peptide from a toxin-antitoxin module. J. Biol. Chem. 2012;287:43454–43463. doi: 10.1074/jbc.M112.402693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donegan N.P., Thompson E.T., Fu Z., Cheung A.L. Proteolytic regulation of toxin-antitoxin systems by clppc in staphylococcus aureus. J. Bacteriol. 2010;192:1416–1422. doi: 10.1128/JB.00233-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valihrach L., Demnerova K. Impact of normalization method on experimental outcome using rt-qpcr in staphylococcus aureus. J. Microbiol. Methods. 2012;90:214–216. doi: 10.1016/j.mimet.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 48.Edgar R.C. Muscle: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.