Abstract
Patients with lung adenocarcinoma harboring common epidermal growth factor receptor (EGFR) mutations usually have a good response rate (RR) and longer progression-free survival (PFS) to EGFR tyrosine kinase inhibitors (TKIs). However, the treatment efficacy to uncommon EGFR mutations remains controversial. We, therefore, performed a retrospective study, screening 2958 patients. A total of 67 patients with lung adenocarcinoma harboring uncommon EGFR mutations were enrolled and 57 patients with stage IV diseases receiving a first-line EGFR TKI were included for further analyses. The patients were classified into 27 (47%) “a single sensitizing uncommon mutation”, 7 (12%) “multiple sensitizing mutations”, 5 (9%) “a sensitizing mutation and a resistant uncommon mutation”, and 18 (32%) “other resistant uncommon mutations”. No significant difference was noted in PFS or overall survival (OS) between groups. Patients receiving different first-line EGFR TKIs had similar PFS and OS. The elder patients had a significantly poorer performance status than the younger patients but a significantly longer PFS than the younger patients (median PFS: 10.5 vs. 5.5 months, p = 0.0320). In conclusion, this is the first study to identify that elderly patients with stage IV lung adenocarcinoma harboring uncommon EGFR mutation might have a longer PFS. Large-scale prospective studies are mandatory to prove our findings.
Keywords: lung cancer, adenocarcinoma, gefitinib, erlotinib, afatinib, elderly, epidermal growth factor receptor, tyrosine kinase inhibitor
1. Introduction
Lung cancer is a leading cause of death in the world. Platinum-based chemotherapy had been a standard therapy for metastatic lung cancer, but the treatment result is still limited and disappointing. In the past decade, non-small cell lung cancer (NSCLC) patients had been proved to have longer progression-free survival (PFS) and better response rate to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) if they a had sensitizing EGFR mutation in phase 3 clinical trials [1,2,3,4,5]. Therefore, EGFR TKIs had been the standard therapy in NSCLC patients with EGFR mutation. Of all EGFR mutations in lung cancer, approximately 90% are common mutations, including in-frame deletions in exon 19 and an L858R point mutation in exon 21 [5,6,7]. However, many uncommon mutations, also called rare or non-classical mutations, were detected but the response to EGFR TKIs was inconsistent due to a limited number of cases enrolled in the trials [8,9,10,11,12,13,14]. For example, Chiu et al. claimed that both gefitinib and erlotinib are active in lung adenocarcinoma patients with G719X/L861Q/S768I mutations but they had short PFS (a median of 7.7 months) than in those with common EGFR mutations (a median of 11.4 months) (p < 0.01) [13]. A post-hoc analysis of the LUX-Lung 2, 3 and 6 trials demonstrated that patients harboring G719X, L861G, or S768I mutation responded to afatinib [15]. In a retrospective study by Shen et al., afatinib provided significantly better PFS than gefitinib/erlotinib in 51 patients with stage IIIB-IV lung adenocarcinoma with non-classical mutations (median PFS: 11.0 vs. 3.6 months, p = 0.03) [9]. Tu et al. indicated that non-classical mutations were more common in smokers (30.7% vs. 24.3%, p = 0.039) and males (54.1% vs. 44.4%, p = 0.007), and favorable efficacy was observed in patients harboring L858R mutation (median PFS: 15.2 months) or G719X mutation (median PFS: 11.6 months) [8].
Identifying the predicting factors for the clinical efficacy of EGFR TKIs in these patients with lung adenocarcinoma harboring uncommon EGFR mutation is urgent. We, therefore, conducted a retrospective study in two university-affiliated hospitals. We made a comprehensive analysis of the clinical efficacy of three different EGFR TKIs in these patients.
2. Patients and Methods
2.1. Patient Identification
In this retrospective study, all patients of lung adenocarcinoma diagnosed and received EGFR mutation exam in two university-affiliated hospitals in Taiwan between January 2009 and March 2018 were screened. All patients had uncommon EGFR mutation and received an EGFR TKI were enrolled and followed until March 2018. The diagnosis of lung cancer was confirmed pathologically, according to World Health Organization pathology classification. The tumor staging was designated according to the seventh American Joint Committee on Cancer staging system by a special committee constituted of clinical pulmonologists, medical oncologists, chest surgeons, radiologists, pathologists and radiation oncologists. Patients were included if: (1) they had adequate tumor specimens for EGFR mutation examination, (2) the exam revealed an uncommon EGFR gene mutation, and (3) they received an EGFR TKI treatment with gefitinib, erlotinib, or afatinib. To clearly identify the effects of EGFR TKI as the first-line treatment for stage IV lung adenocarcinoma harboring uncommon mutation, patients with stage I-III cancer and those received an EGFR TKI after other treatment modalities were excluded from the further analyses.
As in our previous reports [16,17,18,19,20,21], mutations in EGFR gene were analyzed using protocol developed and validated by the Division of Molecular Diagnostics, Department of Laboratory Medicine, Kaohsiung Medical University Hospital (KMUH), which utilized amplification refractory mutation specific (ARMS) polymerase chain reaction (PCR) and Scorpion technologies for the detection, and direct sequencing was performed when a negative result was found in ARMS PCR.
Although a few studies have discussed rare EGFR mutation (Table A1), the most appropriate method for classifying rare EGFR mutation remained inconclusive because of the rare entity. Therefore, in addition to classifying the mutation patterns by exons, we also classified the mutations by drug sensitivity, including “single sensitizing uncommon mutation” (exon18 G719X and exon21 L861Q), “multiple sensitizing mutations” (exon18 G719X + exon20 S768I, exon18 G719X + exon21 L861Q, and exon21 L858R + exon20 S768I), “a sensitizing mutation and a resistant uncommon mutation” (exon18 G719X + exon18 other and exon21 L858R + exon20 Q812*), and “other resistant uncommon mutations” (exon 18 other mutation, exon 20 insertion, and exon 20 point mutation).
Baseline clinical characteristics were collected by retrospective chart review, including age at diagnosis, sex, initial Eastern Cooperative Oncology Group (ECOG) performance status, smoking history. Smoking status was categorized as current smoker or never smoker (<100 lifetime cigarettes).
Based on serial imaging studies, the Initial treatment response was classified as a progressive disease (PD), stable disease (SD), partial response (PR), or complete response (CR), using the RECIST 1.1 criteria. The primary outcome of this study was PFS on an EGFR TKI, defined by the duration between the start of an EGFR TKI and the onset of progression under the treatment. The secondary outcome was overall survival (OS), defined by the duration between the date of confirmed diagnosis and the date of death. The study protocol was approved by the KMUH Institutional Review Board (KMUHIRB-E(II)-20150162).
2.2. Statistical Analyses
Continuous variables and categorical variables were compared using the Kruskal-Wallis test and Fisher’s exact test, respectively. Survival times, including PFS and OS, were estimated using the Kaplan-Meier method, and differences between the groups were compared using the log-rank test. Cox proportional hazards regression analyses were used to identify the effect of different variables on PFS or OS. After univariate analyses, all variables were included to obtain a maximal model of multivariable analysis to assess the independent effect of different variables. We also used a backward variable selection method, keeping only variables with a p value less than 0.15, to develop reduced multivariable models. All statistical analyses were performed using the SAS system (version 9.4 for Windows, SAS Institute Inc., Cary, NC, USA). The statistical significance level was set at a two-sided p value of <0.05.
3. Results
3.1. Patients Characteristics
In 2958 patients with lung adenocarcinoma having their specimens tested for EGFR mutation, a total of 67 patients with lung adenocarcinoma harboring uncommon EGFR gene mutations, who had received an EGFR TKI treatment, were enrolled. Five patients with stage I to III disease (Table A2) were excluded from further analyses to control the confounding effects from the cancer stage. In the 62 patients with stage IV lung cancer (Table 1), 36 (58%) were female and 26 (42%) were male, and the mean age was 65 years. Most of them were never smokers (71%) and had a performance status of ECOG ≤ 1 (71%). All patients had their tumor positive for thyroid transcription factor 1 (TTF-1), except for a patient whose tumor was not tested. The overall disease control rate on an EGFR TKI was 73%, and the median PFS on an EGFR TKI was 7.5 months (Table 1).
Table 1.
Characteristics of the patients with stage IV lung cancer harboring uncommon mutation.
| All Stage IV Patients | Patients Receiving First-Line TKI | |
|---|---|---|
| n | 62 | 57 |
| Age (year), mean ± SD | 65 ± 12 | 66 ± 12 |
| Age (year), median (IQR) | 64 (56–74) | 65 (56–75) |
| Sex, n (%) | ||
| Female | 36 (58%) | 32 (56%) |
| Male | 26 (42%) | 25 (44%) |
| Smoking history, n (%) | ||
| Never | 44 (71%) | 40 (70%) |
| Current | 18 (29%) | 17 (30%) |
| Performance status, n (%) | ||
| ECOG 0–1 | 44 (71%) | 39 (68%) |
| ECOG 2–4 | 18 (29%) | 18 (32%) |
| TKI medication, n (%) | ||
| Afatinib | 19 (31%) | 17 (30%) |
| Gefitinib | 32 (52%) | 31 (54%) |
| Erlotinib | 11 (18%) | 9 (16%) |
| TKI use, n (%) | ||
| 1st-line treatment | 57 (92%) | 57 (100%) |
| 2nd-line treatment | 3 (5%) | |
| after 2nd-line treatment | 2 (3%) | |
| Number of metastatic sites, n (%) | ||
| =1 | 29 (47%) | 27 (47%) |
| ≥2 | 33 (53%) | 30 (53%) |
| Metastatic site, n (%) | ||
| Brain | 16 (26%) | 14 (25%) |
| Leptomeningeal | 2 (3%) | 2 (4%) |
| Lung | 23 (37%) | 20 (35%) |
| Bone | 32 (52%) | 30 (53%) |
| Pleural | 29 (47%) | 29 (51%) |
| Pericardial | 5 (8%) | 5 (9%) |
| Liver | 10 (16%) | 9 (16%) |
| Adrenal | 5 (8%) | 5 (9%) |
| Renal | 1 (2%) | 0 (0%) |
| Mutation site, n (%) | ||
| Exon 18 G719X | 14 (23%) | 14 (25%) |
| Exon 18 other mutation | 2 (3%) | 2 (4%) |
| Exon 18 G719X + exon 18 other mutation | 5 (8%) | 5 (9%) |
| Exon 20 insertion | 16 (26%) | 13 (23%) |
| Exon 20 point mutation | 3 (5%) | 3 (5%) |
| Exon 21 L861Q | 13 (21%) | 13 (23%) |
| Exon 18 G719X + exon 20 S768I | 3 (5%) | 3 (5%) |
| Exon 18 G719X + exon 21 L861Q | 2 (3%) | 2 (4%) |
| Exon 21 L858R + exon 20 S768I | 3 (5%) | 2 (4%) |
| Exon 21 L858R + exon 20 Q812* | 1 (2%) | 0 (0%) |
| Mutation site classified by exon, n (%) | ||
| Mutation only in exon 18 | 21 (34%) | 21 (37%) |
| Mutation only in exon 20 | 19 (31%) | 16 (28%) |
| Mutation only in exon 21 | 13 (21%) | 13 (23%) |
| Mutations in multiple exons | 9 (15%) | 7 (12%) |
| Mutation site classified by susceptibility †, n (%) | ||
| Single sensitizing uncommon mutation | 27 (44%) | 27 (47%) |
| Multiple sensitizing mutations | 8 (13%) | 7 (12%) |
| A sensitizing mutation and a resistant uncommon mutation | 6 (10%) | 5 (9%) |
| Other resistant uncommon mutations | 21 (34%) | 18 (32%) |
| Initial response to TKI, n (%) | ||
| Partial response | 21 (34%) | 19 (33%) |
| Stable disease | 24 (39%) | 22 (39%) |
| Progressive disease | 17 (27%) | 16 (28%) |
| Response rate to TKI | 21 (34%) | 19 (33%) |
| Disease control rate to TKI | 45 (73%) | 41 (72%) |
| Median follow-up time (months) | 13.0 | 11.5 |
| Median PFS on TKI (months) | 7.5 | 7.4 |
| Median OS (month) | 17.0 | 16.1 |
Abbreviations: ECOG = Eastern Cooperative Oncology Group; TTF-1 = thyroid transcription factor 1. † Mutation site classified by susceptibility: “Single sensitizing uncommon mutation” included exon18 G719X and exon21 L861Q; “multiple sensitizing mutations” included exon18 G719X + exon20 S768I, exon18 G719X + exon21 L861Q, and exon21 L858R + exon20 S768I; “a sensitizing mutation and a resistant uncommon mutation” included exon18 G719X + exon18 other and exon21 L858R + exon20 Q812*; “other resistant uncommon mutations” included exon 18 other mutation, exon 20 insertion, and exon 20 point mutation.
In the patients with stage IV diseases, 57 (92%) patients received an EGFR TKI as their first-line treatment. In these patients, similarly, 32 (56%) were female and 25 (44%) were male, and the mean age was 66 years. Most of them were never smokers (70%) and had a performance status of ECOG ≤ 1 (68%). To avoid bias from various preceding lines of treatment, only patients receiving an EGFR TKI as the first-line treatment were included for further survival analyses.
3.2. Various Outcomes Related to Different EGFR Mutation Patterns
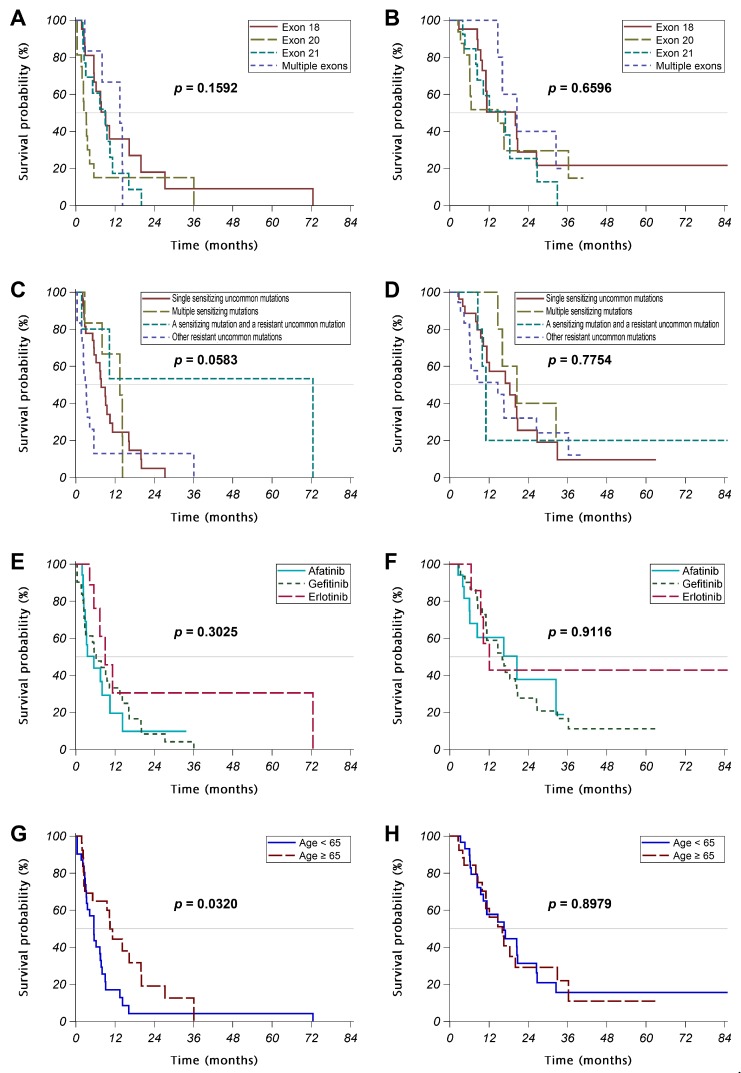
In the stage IV patients receiving a first-line EGFR TKI, 21 (37%), 16 (28%), 13 (23%), and 7 (12%) had mutation in exons 18, 20, 21, and multiple exons, respectively (Table 1 and Table 2). Patients with their tumors harboring mutations in different exons of EGFR were similar in age, sex, smoking history, and performance status. The response rate and disease control rate were significantly higher in those having mutations in multiple exons (Table 2). However, no significant difference was noted in the PFS or OS between groups (Table 2, Figure 1A,B).
Table 2.
Characteristics of the patients with stage IV lung cancer harboring uncommon mutation receiving a first-line tyrosine kinase inhibitor (TKI), classified by the exon of epidermal growth factor receptor (EGFR) mutation.
| Mutation Only in exon 18 | Mutation Only in exon 20 | Mutation Only in exon 21 | Mutations in Multiple Exons | p Value * | |
|---|---|---|---|---|---|
| n | 21 (37%) | 16 (28%) | 13 (23%) | 7 (12%) | |
| Age (year), mean ± SD | 66 ± 13 | 63 ± 10 | 69 ± 13 | 64 ± 11 | 0.6964 |
| Age (year), median (IQR) | 64 (55–79) | 65 (55–70) | 67 (56–78) | 63 (60–73) | 0.6964 |
| Age (year), n (%) | 0.7749 | ||||
| <65 | 11 (52%) | 9 (56%) | 6 (46%) | 5 (71%) | |
| ≥65 | 10 (48%) | 7 (44%) | 7 (54%) | 2 (29%) | |
| Sex, n (%) | 1.0000 | ||||
| Female | 12 (57%) | 9 (56%) | 7 (54%) | 4 (57%) | |
| Male | 9 (43%) | 7 (44%) | 6 (46%) | 3 (43%) | |
| Smoking history, n (%) | 0.3188 | ||||
| Never | 14 (67%) | 11 (69%) | 8 (62%) | 7 (100%) | |
| Current | 7 (33%) | 5 (31%) | 5 (38%) | 0 (0%) | |
| Performance status, n (%) | 0.0799 | ||||
| ECOG 0–1 | 17 (81%) | 7 (44%) | 9 (69%) | 6 (86%) | |
| ECOG 2–4 | 4 (19%) | 9 (56%) | 4 (31%) | 1 (14%) | |
| No. of metastatic sites, n (%) | 0.3715 | ||||
| =1 | 11 (52%) | 7 (44%) | 4 (31%) | 5 (71%) | |
| ≥2 | 10 (48%) | 9 (56%) | 9 (69%) | 2 (29%) | |
| Metastatic site, n (%) | |||||
| Brain | 5 (24%) | 5 (31%) | 3 (23%) | 1 (14%) | 0.8976 |
| Leptomeningeal | 0 (0%) | 2 (13%) | 0 (0%) | 0 (0%) | 0.2644 |
| Lung | 4 (19%) | 5 (31%) | 9 (69%) | 2 (29%) | 0.0288 |
| Bone | 11 (52%) | 8 (50%) | 9 (69%) | 2 (29%) | 0.3946 |
| Pleural | 11 (52%) | 8 (50%) | 7 (54%) | 3 (43%) | 1.0000 |
| Pericardial | 3 (14%) | 0 (0%) | 1 (8%) | 1 (14%) | 0.4222 |
| Liver | 1 (5%) | 2 (13%) | 6 (46%) | 0 (0%) | 0.0109 |
| Adrenal | 3 (14%) | 2 (13%) | 0 (0%) | 0 (0%) | 0.5324 |
| TKI medication, n (%) | 0.2374 | ||||
| Afatinib | 4 (19%) | 8 (50%) | 2 (15%) | 3 (43%) | |
| Gefitinib | 14 (67%) | 7 (44%) | 7 (54%) | 3 (43%) | |
| Erlotinib | 3 (14%) | 1 (6%) | 4 (31%) | 1 (14%) | |
| Initial response to TKI, n (%) | 0.0212 | ||||
| Partial response | 8 (38%) | 1 (6%) | 5 (38%) | 5 (71%) | |
| Stable disease | 10 (48%) | 6 (38%) | 5 (38%) | 1 (14%) | |
| Progressive disease | 3 (14%) | 9 (56%) | 3 (23%) | 1 (14%) | |
| Response rate to TKI | 8 (38%) | 1 (6%) | 5 (38%) | 5 (71%) | 0.0118 |
| Disease control rate with TKI | 18 (86%) | 7 (44%) | 10 (77%) | 6 (86%) | 0.0357 |
| Median PFS on TKI (month) | 9.2 | 2.8 | 9.0 | 13.5 | 0.1592 |
| Median OS (month) | 20.0 | 14.7 | 17.0 | 20.5 | 0.6596 |
* Kruskal-Wallis test or Fisher’s exact test or Log-rank test.
Figure 1.
Kaplan-Meier curves of the progression-free survival (PFS) (A,C,E,G) and overall survival (OS) (B,D,F,H) of the patients receiving a first-line EGFR TKI, while classified the patients by (A,B) the exon of EGFR mutation, (C,D) the drug sensitivity of EGFR mutation, (E,F) the EGFR TKI used, and (G,H) the age group. Abbreviation: PFS = progression-free survival; OS = overall survival.
While classifying the EGFR mutation pattern by drug sensitivity, the patients were reclassified into 27 (47%) “single sensitizing uncommon mutation”, 7 (12%) “multiple sensitizing mutations”, 5 (9%) “a sensitizing mutation and a resistant uncommon mutation” (all of them were exon 18 G719X with another exon 18 point mutation), and 18 (32%) “other resistant uncommon mutations” (Table 1 and Table 3). Patients with their tumors harboring different EGFR mutation patterns showed significantly different treatment responses to EGFR TKIs, while those with “multiple sensitizing mutations” had the best response rate to TKIs (Table 3). However, no significant difference was noted in the PFS or OS between groups (Table 3, Figure 1C,D).
Table 3.
Characteristics of the patients with stage IV lung cancer harboring uncommon mutation receiving a first-line tyrosine kinase inhibitor (TKI), classified by the drug susceptibility of EGFR mutation †.
| Single Sensitizing Uncommon Mutation | Multiple Sensitizing Uncommon Mutations | A Sensitizing Mutation and a Resistant Uncommon Mutation | Other Resistant Uncommon Mutations | p Value * | |
|---|---|---|---|---|---|
| n | 27 (47%) | 7 (12%) | 5 (9%) | 18 (32%) | |
| Age (year), mean ± SD | 67 ± 12 | 64 ± 11 | 77 ± 13 | 61 ± 10 | 0.0971 |
| Age (year), median (IQR) | 64 (56–78) | 63 (60–73) | 84 (75–85) | 64 (49–70) | 0.0971 |
| Age (year), n (%) | 0.3426 | ||||
| <65 | 14 (52%) | 5 (71%) | 1 (20%) | 11 (61%) | |
| ≥65 | 13 (48%) | 2 (29%) | 4 (80%) | 7 (39%) | |
| Sex, n (%) | 0.4195 | ||||
| Female | 17 (63%) | 4 (57%) | 1 (20%) | 10 (56%) | |
| Male | 10 (37%) | 3 (43%) | 4 (80%) | 8 (44%) | |
| Smoking history, n (%) | 0.0286 | ||||
| Never | 20 (74%) | 7 (100%) | 1 (20%) | 12 (67%) | |
| Current | 7 (26%) | 0 (0%) | 4 (80%) | 6 (33%) | |
| Performance status, n (%) | 0.1784 | ||||
| ECOG 0-1 | 21 (78%) | 6 (86%) | 3 (60%) | 9 (50%) | |
| ECOG 2-4 | 6 (22%) | 1 (14%) | 2 (40%) | 9 (50%) | |
| Number of metastatic sites, n (%) | 0.1168 | ||||
| =1 | 9 (33%) | 5 (71%) | 4 (80%) | 9 (50%) | |
| ≥2 | 18 (67%) | 2 (29%) | 1 (20%) | 9 (50%) | |
| Metastatic site, n (%) | |||||
| Brain | 7 (26%) | 1 (14%) | 1 (20%) | 5 (28%) | 0.9629 |
| Leptomeningeal | 0 (0%) | 0 (0%) | 0 (0%) | 2 (11%) | 0.3571 |
| Lung | 13 (48%) | 2 (29%) | 0 (0%) | 5 (28%) | 0.1805 |
| Bone | 18 (67%) | 2 (29%) | 1 (20%) | 9 (50%) | 0.1168 |
| Pleural | 15 (56%) | 3 (43%) | 3 (60%) | 8 (44%) | 0.8305 |
| Pericardial | 3 (11%) | 1 (14%) | 0 (0%) | 1 (6%) | 0.8033 |
| Liver | 7 (26%) | 0 (0%) | 0 (0%) | 2 (11%) | 0.3156 |
| Adrenal | 2 (7%) | 0 (0%) | 1 (20%) | 2 (11%) | 0.5715 |
| TKI medication, n (%) | 0.1599 | ||||
| Afatinib | 5 (19%) | 3 (43%) | 0 (0%) | 9 (50%) | |
| Gefitinib | 16 (59%) | 3 (43%) | 4 (80%) | 8 (44%) | |
| Erlotinib | 6 (22%) | 1 (14%) | 1 (20%) | 1 (6%) | |
| Initial response to TKI, n (%) | 0.0062 | ||||
| Partial response | 11 (41%) | 5 (71%) | 2 (40%) | 1 (6%) | |
| Stable disease | 12 (44%) | 1 (14%) | 2 (40%) | 7 (39%) | |
| Progressive disease | 4 (15%) | 1 (14%) | 1 (20%) | 10 (56%) | |
| Response rate to TKI | 11 (41%) | 5 (71%) | 2 (40%) | 1 (6%) | 0.0037 |
| Disease control rate with TKI | 23 (85%) | 6 (86%) | 4 (80%) | 8 (44%) | 0.0188 |
| Median PFS on TKI (month) | 7.7 | 13.5 | 72.5 | 3.1 | 0.0583 |
| Median OS (month) | 18.4 | 20.5 | 11.0 | 14.7 | 0.7754 |
* Kruskal-Wallis test or Fisher’s exact test or Log-rank test. † Mutation site classified by susceptibility: “Single sensitizing uncommon mutation” included exon18 G719X and exon21 L861Q; “multiple sensitizing mutations” included exon18 G719X + exon20 S768I, exon18 G719X + exon21 L861Q, and exon21 L858R + exon20 S768I; “a sensitizing mutation and a resistant uncommon mutation” included exon18 G719X + exon18 other and exon21 L858R + exon20 Q812*; “other resistant uncommon mutations” included exon 18 other mutation, exon 20 insertion, and exon 20 point mutation.
3.3. Different TKIs Showed Similar Treatment Response
In the 57 patients with stage IV disease receiving an EGFR TKI as their first-line treatment, 17 (30%), 31 (54%), and 9 (16%) patients used afatinib, gefitinib, and erlotinib, respectively (Table 4). The response rate to the TKI was similar in three groups, while patients receiving erlotinib seemed to have a trend for better disease control rate (p = 0.0914). No significant difference was noted in the PFS or OS between patients receiving different first-line EGFR TKIs (Table 4, Figure 1E,F).
Table 4.
Characteristics of the patients with stage IV lung cancer harboring uncommon mutation receiving a first-line tyrosine kinase inhibitor (TKI), classified by the drug used.
| Afatinib | Gefitinib | Erlotinib | p Value * | |
|---|---|---|---|---|
| n | 17 (30%) | 31 (54%) | 9 (16%) | |
| Age (year), mean ± SD | 64 ± 9 | 67 ± 14 | 62 ± 11 | 0.4356 |
| Age (year), median (IQR) | 65 (61–70) | 67 (56–80) | 61 (55–67) | 0.4356 |
| Age (year), n (%) | 0.5820 | |||
| <65 | 11 (65%) | 15 (48%) | 5 (56%) | |
| ≥65 | 6 (35%) | 16 (52%) | 4 (44%) | |
| Sex, n (%) | 0.1084 | |||
| Female | 8 (47%) | 16 (52%) | 8 (89%) | 0.2477 |
| Male | 9 (53%) | 15 (48%) | 1 (11%) | |
| Smoking history, n (%) | 0.2477 | |||
| Never | 13 (76%) | 19 (61%) | 8 (89%) | |
| Current | 4 (24%) | 12 (39%) | 1 (11%) | |
| Performance status, n (%) | 0.3383 | |||
| ECOG 0–1 | 10 (59%) | 21 (68%) | 8 (89%) | |
| ECOG 2–4 | 7 (41%) | 10 (32%) | 1 (11%) | |
| Number of metastatic sites, n (%) | 0.3891 | |||
| =1 | 6 (35%) | 17 (55%) | 4 (44%) | |
| ≥2 | 11 (65%) | 14 (45%) | 5 (56%) | |
| Metastatic site, n (%) | ||||
| Brain | 6 (35%) | 5 (16%) | 3 (33%) | 0.2500 |
| Leptomeningeal | 2 (12%) | 0 (0%) | 0 (0%) | 0.1078 |
| Lung | 9 (53%) | 8 (26%) | 3 (33%) | 0.1661 |
| Bone | 8 (47%) | 17 (55%) | 5 (56%) | 0.9344 |
| Pleural | 7 (41%) | 19 (61%) | 3 (33%) | 0.2398 |
| Pericardial | 4 (24%) | 1 (3%) | 0 (0%) | 0.0542 |
| Liver | 3 (18%) | 5 (16%) | 1 (11%) | 1.0000 |
| Adrenal | 2 (12%) | 2 (6%) | 1 (11%) | 0.6897 |
| Mutation site classified by exon, n (%) | 0.2374 | |||
| Mutation only in exon 18 | 4 (24%) | 14 (45%) | 3 (33%) | |
| Mutation only in exon 20 | 8 (47%) | 7 (23%) | 1 (11%) | |
| Mutation only in exon 21 | 2 (12%) | 7 (23%) | 4 (44%) | |
| Mutations in multiple exons | 3 (18%) | 3 (10%) | 1 (11%) | |
| Mutation site classified by susceptibility †, n (%) | 0.1599 | |||
| Single sensitizing uncommon mutation | 5 (29%) | 16 (52%) | 6 (67%) | |
| Multiple sensitizing mutations | 3 (18%) | 3 (10%) | 1 (11%) | |
| A sensitizing mutation and a resistant uncommon mutation | 0 (0%) | 4 (13%) | 1 (11%) | |
| Other resistant uncommon mutations | 9 (53%) | 8 (26%) | 1 (11%) | |
| Initial response to TKI, n (%) | 0.1943 | |||
| Partial response | 7 (41%) | 9 (29%) | 3 (33%) | |
| Stable disease | 5 (29%) | 11 (35%) | 6 (67%) | |
| Progressive disease | 5 (29%) | 11 (35%) | 0 (0%) | |
| Response rate to TKI | 7 (41%) | 9 (29%) | 3 (33%) | 0.7450 |
| Disease control rate with TKI | 12 (71%) | 20 (65%) | 9 (100%) | 0.0914 |
| Median PFS on TKI (month) | 5.5 | 6.2 | 9.0 | 0.3025 |
| Median OS (month) | 20.5 | 16.1 | 12.1 | 0.9116 |
* Kruskal-Wallis test or Fisher’s exact test or Log-rank test. † Mutation site classified by susceptibility: “Single sensitizing uncommon mutation” included exon18 G719X and exon21 L861Q; “multiple sensitizing mutations” included exon18 G719X + exon20 S768I, exon18 G719X + exon21 L861Q, and exon21 L858R + exon20 S768I; “a sensitizing mutation and a resistant uncommon mutation” included exon18 G719X + exon18 other and exon21 L858R + exon20 Q812*; “other resistant uncommon mutations” included exon 18 other mutation, exon 20 insertion, and exon 20 point mutation.
3.4. Elder Patients Had Better PFS but Similar OS
In the patients with stage IV disease receiving first-line EGFR TKI, 26 (46%) were elder (age ≥ 65 years old) patients (Table 5). There were significantly more male patients in the elder group than in the younger group (62% vs. 29%, p = 0.0176). The elder patients had significantly poorer performance status than the younger patients (percentage of patients with EGOG 2-4: 46% vs. 19%, p = 0.0453). The response rate to TKI and disease control rate with TKI was similar between the elder and younger patients. The elder patients had a significantly longer PFS than the younger patients (median PFS: 10.5 vs. 5.5 months, p = 0.0320), whereas the OS was similar between groups (p = 0.8979) (Table 5, Figure 1G,H).
Table 5.
Characteristics of the patients with stage IV lung cancer harboring uncommon mutation receiving a first-line tyrosine kinase inhibitor (TKI), classified by the age group.
| Age < 65 | Age ≥ 65 | p Value * | |
|---|---|---|---|
| n | 31 (54%) | 26 (46%) | |
| Age (year), mean ± SD | 57 ± 7 | 76 ± 7 | <0.0001 |
| Age (year), median (IQR) | 59 (50–62) | 77 (71–81) | <0.0001 |
| Sex, n (%) | 0.0176 | ||
| Female | 22 (71%) | 10 (38%) | |
| Male | 9 (29%) | 16 (62%) | |
| Smoking history, n (%) | 0.2494 | ||
| Never | 24 (77%) | 16 (62%) | |
| Current | 7 (23%) | 10 (38%) | |
| Performance status, n (%) | 0.0453 | ||
| ECOG 0–1 | 25 (81%) | 14 (54%) | |
| ECOG 2–4 | 6 (19%) | 12 (46%) | |
| Number of metastatic sites, n (%) | 0.4311 | ||
| =1 | 13 (42%) | 14 (54%) | |
| ≥2 | 18 (58%) | 12 (46%) | |
| Metastatic site, n (%) | |||
| Brain | 9 (29%) | 5 (19%) | 0.5392 |
| Leptomeningeal | 1 (3%) | 1 (4%) | 1.0000 |
| Lung | 11 (35%) | 9 (35%) | 1.0000 |
| Bone | 17 (55%) | 13 (50%) | 0.7931 |
| Pleural | 14 (45%) | 15 (58%) | 0.4287 |
| Pericardial | 4 (13%) | 1 (4%) | 0.3624 |
| Liver | 6 (19%) | 3 (12%) | 0.4876 |
| Adrenal | 3 (10%) | 2 (8%) | 1.0000 |
| Mutation site classified by exon, n (%) | 0.7749 | ||
| Mutation only in exon 18 | 11 (35%) | 10 (38%) | |
| Mutation only in exon 20 | 9 (29%) | 7 (27%) | |
| Mutation only in exon 21 | 6 (19%) | 7 (27%) | |
| Mutations in multiple exons | 5 (16%) | 2 (8%) | |
| Mutation site classified by susceptibility †, n (%) | 0.3426 | ||
| Single sensitizing uncommon mutation | 14 (45%) | 13 (50%) | |
| Multiple sensitizing mutations | 5 (16%) | 2 (8%) | |
| A sensitizing mutation and a resistant uncommon mutation | 1 (3%) | 4 (15%) | |
| Other resistant uncommon mutations | 11 (35%) | 7 (27%) | |
| TKI medication, n (%) | 0.5820 | ||
| Afatinib | 11 (35%) | 6 (23%) | |
| Gefitinib | 15 (48%) | 16 (62%) | |
| Erlotinib | 5 (16%) | 4 (15%) | |
| Initial response to TKI, n (%) | 0.7086 | ||
| Partial response | 9 (29%) | 10 (38%) | |
| Stable disease | 12 (39%) | 10 (38%) | |
| Progressive disease | 10 (32%) | 6 (23%) | |
| Response rate to TKI | 9 (29%) | 10 (38%) | 0.5748 |
| Disease control rate with TKI | 21 (68%) | 20 (77%) | 0.5581 |
| Median PFS on TKI (month) | 5.5 | 10.5 | 0.0320 |
| Median OS (month) | 16.6 | 16.1 | 0.8979 |
* Kruskal-Wallis test or Fisher’s exact test or Log-rank test. † Mutation site classified by susceptibility: “Single sensitizing uncommon mutation” included exon18 G719X and exon21 L861Q; “multiple sensitizing mutations” included exon18 G719X + exon20 S768I, exon18 G719X + exon21 L861Q, and exon21 L858R + exon20 S768I; “a sensitizing mutation and a resistant uncommon mutation” included exon18 G719X + exon18 other and exon21 L858R + exon20 Q812*; “other resistant uncommon mutations” included exon 18 other mutation, exon 20 insertion, and exon 20 point mutation.
3.5. Factors Related to PFS and OS
Cox regression analyses were used to identify prognostic factors related to the PFS and OS in patients with stage IV lung adenocarcinoma harboring uncommon mutation treated with a first-line EGFR TKI. Univariate analyses identified significantly good prognostic factors for PFS included elder age (HR = 0.52 [95% CI: 0.28–0.95], p = 0.0349) and having a sensitizing mutation and a resistant uncommon mutation (HR = 0.19 [95% CI: 0.04–0.85], p = 0.0296) (Table 6). As for the metastatic site, leptomeningeal metastasis was the only one associated with a trend for poorer PFS (Table A3). On multivariable analyses, including maximal models and reduced models developed with backward variable selection method, elder age and female sex were independent prognostic factors for better PFS, while the mutation patterns also had significant effects on PFS. Smoking history, performance status, or the types of EGFR TKIs did not significantly affect the PFS.
Table 6.
Cox proportional hazards regression analyses for identifying the relationship between clinical features and progression-free survival in patients with stage IV lung cancer harboring uncommon mutation receiving first-line TKI.
| Univariate Analysis | Multivariable Analyses | ||||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | ||
| Sex | |||||
| Female | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Male | 1.50 [0.83–2.72] | 2.83 [1.04–7.72] | 2.90 [1.07–7.88] | 2.13 [1.11–4.09] | 2.10 [1.11–3.97] |
| Age (year) | |||||
| <65 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| ≥65 | 0.52 [0.28–0.95] | 0.27 [0.12–0.58] | 0.28 [0.13–0.60] | 0.35 [0.17–0.72] | 0.35 [0.17–0.73] |
| Smoking history | |||||
| Never | 1.00 | 1.00 | 1.00 | ||
| Current | 1.33 [0.70–2.52] | 0.44 [0.14–1.42] | 0.46 [0.15–1.46] | ||
| Performance status | |||||
| ECOG 0–1 | 1.00 | 1.00 | 1.00 | ||
| ECOG 2–4 | 1.60 [0.85–3.02] | 1.51 [0.60–3.84] | 1.52 [0.62–3.74] | ||
| Number of metastatic sites | |||||
| =1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| ≥2 | 1.60 [0.85–3.02] | 1.65 [0.78–3.51] | 1.87 [0.89–3.94] | 1.75 [0.87–3.54] | 1.73 [0.84–3.58] |
| Mutation site classified by exon | |||||
| Mutation only in exon 18 | 1.02 [0.36–2.84] | 1.31 [0.42–4.09] | 1.11 [0.38–3.20] | ||
| Mutation only in exon 20 | 2.25 [0.78–6.47] | 3.70 [0.93–14.83] | 2.84 [0.94–8.60] | ||
| Mutation only in exon 21 | 1.53 [0.54–4.38] | 1.84 [0.53–6.43] | 1.59 [0.51–5.01] | ||
| Mutations in multiple exons | 1.00 | 1.00 | 1.00 | ||
| Mutation site classified by susceptibility † | |||||
| Single sensitizing uncommon mutation | 0.57 [0.29–1.10] | 0.39 [0.16–0.96] | 0.48 [0.23–1.02] | ||
| Multiple sensitizing mutations | 0.43 [0.15–1.21] | 0.27 [0.07–1.01] | 0.34 [0.12–1.01] | ||
| A sensitizing mutation and a resistant uncommon mutation | 0.19 [0.04–0.85] | 0.20 [0.04–1.08] | 0.18 [0.04–0.83] | ||
| Other resistant uncommon mutations | 1.00 | 1.00 | 1.00 | ||
| TKI medication | |||||
| Afatinib | 1.00 | 1.00 | 1.00 | ||
| Gefitinib | 0.90 [0.46–1.76] | 1.85 [0.76–4.46] | 1.90 [0.82–4.43] | ||
| Erlotinib | 0.46 [0.16–1.30] | 0.91 [0.27–3.05] | 1.24 [0.37–4.19] | ||
Model 1 and Model 2 are maximal models. Model 3 and Model 4 are reduced multivariable models developed with backward variable selection method, keeping only variables with p value less than 0.15, from Model 1 and Model 2, respectively. † Mutation site classified by susceptibility: “Single sensitizing uncommon mutation” included exon18 G719X and exon21 L861Q; “multiple sensitizing mutations” included exon18 G719X + exon20 S768I, exon18 G719X + exon21 L861Q, and exon21 L858R + exon20 S768I; “a sensitizing mutation and a resistant uncommon mutation” included exon18 G719X + exon18 other and exon21 L858R + exon20 Q812*; “other resistant uncommon mutations” included exon 18 other mutation, exon 20 insertion, and exon 20 point mutation.
Female sex and never smoker were significant factors for better OS on univariate analyses but became insignificant in the multivariable models (Table 7). As for metastatic site, brain and leptomeningeal metastases were associated with poorer OS (Table A3), while this finding might be biased by small sample size. Better initial performance status remained a significant factor for better OS in the univariate model and multivariable models, including maximal models and the reduced model. The mutation patterns or the types of EGFR TKIs did not significantly affect the OS. Elder age was not associated with a better OS on univariate analysis, whereas multivariable models showed that elder age had a trend for better OS.
Table 7.
Cox proportional hazards regression analyses for identifying the relationship between clinical features and overall survival in patients with stage IV lung cancer harboring uncommon mutation receiving first-line TKI.
| Univariate Analysis | Multivariable Analyses | |||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||
| Sex | ||||
| Female | 1.00 | 1.00 | 1.00 | 1.00 |
| Male | 2.31 [1.19–4.47] | 1.81 [0.65–4.98] | 1.86 [0.67–5.16] | 1.83 [0.88–3.78] |
| Age (year) | ||||
| <65 | 1.00 | 1.00 | 1.00 | 1.00 |
| ≥65 | 1.04 [0.55–1.98] | 0.45 [0.18–1.12] | 0.46 [0.19–1.15] | 0.47 [0.20–1.14] |
| Smoking history | ||||
| Never | 1.00 | 1.00 | 1.00 | |
| Current | 2.40 [1.21–4.74] | 1.11 [0.37–3.3] | 1.1 [0.36–3.33] | |
| Performance status | ||||
| ECOG 0–1 | 1.00 | 1.00 | 1.00 | 1.00 |
| ECOG 2–4 | 2.77 [1.42–5.39] | 3.43 [1.21–9.75] | 3.32 [1.19–9.28] | 3.40 [1.32–8.72] |
| Number of metastatic sites | ||||
| =1 | 1.00 | 1.00 | 1.00 | |
| ≥2 | 2.77 [1.42–5.39] | 1.10 [0.49–2.46] | 1.20 [0.55–2.60] | |
| Mutation site classified by exon | ||||
| Mutation only in exon 18 | 1.39 [0.45–4.30] | 1.56 [0.43–5.62] | ||
| Mutation only in exon 20 | 1.73 [0.55–5.48] | 1.26 [0.34–4.73] | ||
| Mutation only in exon 21 | 1.95 [0.61–6.24] | 1.90 [0.43–8.48] | ||
| Mutations in multiple exons | 1.00 | 1.00 | ||
| Mutation site classified by susceptibility | ||||
| Single sensitizing uncommon mutation | 0.86 [0.41–1.78] | 1.15 [0.49–2.7] | ||
| Multiple sensitizing mutations | 0.56 [0.18–1.74] | 0.75 [0.21–2.67] | ||
| A sensitizing mutation and a resistant uncommon mutation | 1.01 [0.33–3.12] | 1.23 [0.34–4.44] | ||
| Other resistant uncommon mutations | 1.00 | 1.00 | ||
| TKI medication | ||||
| Afatinib | 1.00 | 1.00 | 1.00 | |
| Gefitinib | 1.04 [0.48–2.24] | 0.85 [0.33–2.21] | 0.91 [0.35–2.36] | |
| Erlotinib | 0.83 [0.25–2.71] | 0.93 [0.22–3.95] | 1.01 [0.22–4.49] | |
* Model 1 and Model 2 are maximal models. Model 3 is a reduced multivariable models developed with backward variable selection method, keeping only variables with p value less than 0.15, from both Model 1 and Model 2. † Mutation site classified by susceptibility: “Single sensitizing uncommon mutation” included exon18 G719X and exon21 L861Q; “multiple sensitizing mutations” included exon18 G719X + exon20 S768I, exon18 G719X + exon21 L861Q, and exon21 L858R + exon20 S768I; “a sensitizing mutation and a resistant uncommon mutation” included exon18 G719X + exon18 other and exon21 L858R + exon20 Q812*; “other resistant uncommon mutations” included exon 18 other mutation, exon 20 insertion, and exon 20 point mutation.
4. Discussion
In the past decade, EGFR TKIs had replaced platinum-based chemotherapy to be a standard therapy in patients of NSCLCs harboring EGFR mutation, because many phase 3 randomized controlled trials demonstrated that patients receiving EGFR TKIs had better response rate and longer PFS. However, most of these studies enrolled common mutations, including exon 19 deletion mutation and exon 21 L858R point mutation. To date, the effect of EGFR TKIs in NSCLCs with uncommon mutations are not completely understood. This study is probably one of the largest-scale comprehensive studies about the effect of a first-line EGFR TKI in patients having lung adenocarcinoma harboring uncommon EGFR mutations. We found that the elder patients, although having significantly poorer initial performance status, had a significantly longer PFS than the younger patients.
Approximately 90% EGFR mutations are exon 19 deletion or exon 21 L858R point mutation, and both of them are sensitizing to EGFR TKIs. However, uncommon mutations, also known as non-classic or rare mutations, have been rarely discussed, because they are rarely seen and only parts of them receive first- or second-generation EGFR TKIs. Uncommon or non-classical mutation is a heterogeneous group of molecular alterations with variable responses to EGFR-targeted drugs. Only several retrospective small-scale studies to date tried to identify the clinical efficacy in these patients but the results were inconclusive [8,9,10,13,14,22,23,24]. Exon 18 G719X, exon 20 S768I, and exon 21 L861Q are generally considered as sensitizing uncommon mutations. In contrast, the de novo exon 20 T790M mutation and exon 20 insertion predict primary resistance to clinically achievable levels of EGFR TKIs [8,9,14].
Patients with lung cancer harboring a “single sensitizing uncommon mutation”, including G719X, S768I, and L861Q, usually had a good treatment response to EGFR TKI, although the response was generally still inferior to those harboring common sensitizing mutations. Chen et al. reported that the patients with NSCLC harboring a single uncommon sensitizing mutation (either G719X, L861Q, or S768I) had a response rate of 32.4% and disease control rate of 83.8% [14]. Yun et al. showed G719S mutation had a 14-fold higher affinity to adenosine triphosphate than the wild-type but a weaker affinity to gefitinib than L858R mutation, suggesting that gefitinib could inhibit cancers harboring G719S mutation with less effectiveness [6]. Both studies by Chen et al. [7] and Kancha et al. [25] indicated S768I mutation had resistance to EGFR TKI in vitro, but some case reports indicated S768I mutation was still responsive to EGFR TKI clinically. In our current study, the patients with adenocarcinoma harboring a single sensitizing uncommon mutation had a fine initial treatment response to first-line EGFR TKIs (disease control rate of 85%), PFS (median, 7.7 months), and OS (median, 18.4 months).
Some uncommon mutations are sporadic, and most of them are resistant to an EGFR TKI. Shen et al. showed that G779F, L747P, and M825L with R831C were sensitizing mutations, whereas V717G, I715V, K716E, and complex non-classic mutation, such as V742F, A743V, and H773R, were resistant mutations [9]. Ibrahim et al. reported a patient with stage IV lung adenocarcinoma harboring delE709 T710insD in exon 18 who had a good response to afatinib [26].
In patients with lung cancer harboring uncommon mutation, a few of them had compound mutations. “Multiple sensitizing mutations” is a complex group, mostly having co-existing sensitizing mutations in different exons. Chiu et al. reported that co-existence of G719X and L861Q had a high objective response rate of 88.9% but co-existence of G719X and S768I had an objective response rate of only 50%. They further indicated that patients with compound uncommon EGFR mutations (G719X + L861Q or G719X + S768I) had a significantly longer PFS than the patients with a single mutation did (median PFS: 11.9 vs. 6.5 months; p = 0.010) [13]. In a report by Kobayashi et al., two cases with tumor harboring G719X + S768I had partial responses to erlotinib but the PFSs were only 5 and 7 months [11]. Tu et al. reported that patients with lung cancer harboring compound L858R mutations and G719X mutations, which comprised the majority of uncommon EGFR mutations, had objective response rates of 75% and 50% and median PFSs of 15.2 and 11.6 months, respectively [8]. However, Chen et al. presented several cases with cancer harboring G719X + L861Q or G719X + S768I, who had no response to EGFR TKI [14]. Our present study indicated that the “multiple sensitizing mutations” group had a significantly better initial treatment response to first-line EGFR TKI (response rate of 71% and disease control rate of 86%), and a fine PFS (median, 13.5 months), and OS (median, 20.5 months). Nevertheless, these cases were very rare and heterogeneous, and a simple conclusion is hard to be made.
As another type of compound mutation, an even smaller group of patients had their cancer harboring “a sensitizing (common or uncommon) mutation and a resistant uncommon mutation.” In the study by Peng et al., five patients had adenocarcinoma harboring L858R compound mutations, including one case of L858R + S768I. The four cases with L858R and a resistant uncommon mutation had a stable disease as the initial treatment response to gefitinib treatment and had a median PFS of 10 months [10]. In the report by Kobayashi et al., a case with adenocarcinoma harboring G719X and a resistant uncommon mutation and two cases with adenocarcinoma harboring L858R and a resistant uncommon mutation had a partial response as the initial treatment response to first-line erlotinib treatment [11]. In another report, a patient with co-existence of I706T and G719A had a good response and a PFS of at least 22 months, but a patient with co-existence of E709K and G719A had a very poor response to EGFR TKI [27]. Kauffmann-Guerrero et al. reported a patient with co-existence of G857E and R836R in exon 21, who had a very poor response to EGFR TKI [12]. In our current study, interestingly, all of five patients with stage IV adenocarcinoma harboring a sensitizing mutation and a resistant uncommon mutation involved two point mutations in a single exon (exon 18 G719X with another exon 18 point mutation). These patients, treated with a first-line EGFR TKI, had a fine initial treatment response rate (40%), disease control rate (80%) and a very long PFS (median PFS: 72.5 months).
Patients with lung cancer harboring a “single sensitizing uncommon mutation”, including G719X, S768I, and L861Q, usually had a good treatment response to EGFR TKI, although the response was generally still inferior to those harboring common sensitizing mutations as the report of Chiu et al. [13].
Traditionally, EGFR TKIs are less effective to uncommon mutations than to common sensitizing mutations. Some studies indicated that the irreversible EGFR TKI such as afatinib might be more effective for the patients with lung cancer harboring an uncommon mutation [9,15]. Yang et al. summarized Lux-Lung series and indicated afatinib was active in NSCLC harboring certain types of uncommon EGFR mutations, especially G719X, S768I, and L861Q but was less active in those harboring other uncommon mutation types [15]. In the study by Shen et al., the analysis of 51 patients having lung adenocarcinoma harboring uncommon EGFR mutations except for exon 20 insertion showed that afatinib provided significantly longer PFS than gefitinib/erlotinib did (median PFS: 11.0 vs. 3.6 months, p = 0.03) [9]. However, the inclusion of patients with stage IIIB disease and those receiving an EGFR TKI as the second or third line of treatment, as well as the exclusion of five cases with exon 20 insertion, might confound the analysis. Our current study clearly focused on a clear group with patients receiving an EGFR TKI as the first-line treatment for their stage IV lung adenocarcinoma harboring uncommon mutations and found no significant difference in the initial treatment response, PFS, and OS between patients receiving different EGFR TKIs.
Several studies have tried to demonstrate the prognostic factors of lung cancer patients. In a study of patients receiving EGFR TKI (as any line of treatment) for advanced lung adenocarcinoma harboring (either common or uncommon) EGFR mutation by Chiu et al. [13], female sex, elderly (age > 70 years), and common EGFR mutation were independent factors suggesting better PFS. However, the analysis might be confounded by the enrollment of patients with stage IIIB disease and those receiving EGFR TKI as the second- or later-line treatment and by the absence of patients receiving afatinib. In addition, these studies did not report the prognostic factors specifically in the uncommon mutation group. In the current study, we first identified that the elder was an independent prognostic factor for better PFS in the patients treated with a first-line EGFR TKI for their stage IV lung adenocarcinoma harboring uncommon mutation.
Furthermore, large-scale prospective studies are warranted to elucidate the efficacy and prognostic factors of different EGFR TKIs in patients with different rare or compound mutations. Osimertinib, a 3rd generation EGFR TKI, has been used to treat the de novo T790M mutation [28], and several case reports have demonstrated that it may be effective in some patients harboring uncommon mutations [29,30]. However, more evidence is still needed.
Several limitations of this study should be addressed. First of all, this study was retrospective, and selection bias cannot be completely avoided. Many patients with uncommon mutation did not receive EGFR TKI because previous studies had shown poor clinical efficacy of EGFR TKIs in this group of patients. Second, some patients may not have undergone follow-up imaging on schedule. Although regular imaging follow-up is required for the patients to receive reimbursements for EGFR TKIs every three months, missing a follow-up exam still occurs. This and the unavoidable bias from a retrospective study design may be overcome through future well-designed prospective studies. Third, due to the uncommon entity, we might be unable to collect sufficient cases to do the analysis. However, this study might be one of the largest-scale comprehensive studies about this topic to date.
5. Conclusions
In conclusion, the elder patients with stage IV lung adenocarcinoma harboring uncommon EGFR mutation, although having significantly poor performance status, may have a longer PFS than the younger patients, while treated with a first-line EGFR TKI, but the OS was similar in the elder and younger patients. No significant difference in PFS or OS was observed in patients receiving either gefitinib, erlotinib, or afatinib as the first-line treatment. A further large-scale study is necessary to validate our findings, as well as to determine the best treatment modality for patients with lung adenocarcinoma harboring uncommon EGFR mutation.
Acknowledgments
The authors thank the help from the Statistical Analysis Laboratory, Department of Medical Research, Kaohsiung Medical University Hospital.
Appendix A
Table A1.
Studies about lung cancer harboring rare EGFR mutations.
| Author, Year | N † | EGFR TKIs | Categories of Mutation Types | Response Rate (%) | Median PFS (months) | Median OS (months) |
|---|---|---|---|---|---|---|
| Kobayasi, et al., 2013 | 8 | Erlotinib | Compound mutations (sensitive mutation with other mutation): | |||
| G719X with others (n = 3) | 100% | |||||
| L858R with others (n = 3) | 66.7% | |||||
| L861Q + E709A (n = 1) | 100% | |||||
| delL747_T751+R776S (n = 1) | 100% | |||||
| Chiu, et al., 2015 | 151 | Erlotinib, Gefitinib | Uncommon mutation (G719X, L861Q, S768I, G719X + L861Q, and G719X + S768I) |
41.6% | 7.7 | 24.0 |
| Common mutation | 66.5% | 11.4 | 29.7 | |||
| Peng, et al., 2015 | 6 | Gefitinib | L858R compound mutations | 16.7% | 12.2 | 28.6 |
| Yang, et al., 2015 | 75 | Afatinib | Group 1: point mutations and duplications in exons 18-21(L861G, G719X, S768I, and other point mutations alone or in combination with each other) | 71.1% | 10.7 | 19.4 |
| Group 2: de-novo T790M mutation in exon 20 (alone or in combination with other mutations) | 14.3% | 2.9 | 14.9 | |||
| Group 3: exon 20 insertions | 8.7% | 2.7 | 9.2 | |||
| Chen, et al., 2017 | 66 | Icotinib, Gefitinib, Erlotinib, Afatinib | Group 1: sensitizing rare mutations (G719X, L861Q, S768I) | 32.4% | 4.1 | |
| Group 2: resistant mutation (exon 20 insertion) | 11.1% | 3.1 | ||||
| Group 3: complex mutation (L858R + T790M) | 30% | 8.6 | ||||
| Kauffmann-Guerrero, et al., 2017 | 12 | Erlotinib, Afatinib | Rare mutation | 28.5 | ||
| Complex mutation | 20% | |||||
| Tu, et al., 2018 | 62 | EGFR TKIs | G719X | 50% | 11.6 | 25.2 |
| Exon 20 insertion | 0% | 3 | 12.5 | |||
| T790M | 0% | 1 | 2.4 | |||
| Complex L858R | 75% | 15.2 | 27.7 | |||
| Others | 10% | 2.9 | 11.7 | |||
| Shen, et al., 2018 | 56 | Gefitinib, Erlotinib, Afatinib | Group 1: exon 20 insertions (except A763_Y764 insFQEA) | 20% | ||
| Group 2: non-classical mutations with Del19 or L858R | 58.8% | |||||
| Group 3: non-classical mutations without a combination of Del19 or L858R | 58.8% |
Abbreviation: EGFR = epidermal growth factor receptor; TKI = tyrosine kinase inhibitor; PR = partial response. † Case number of the patients receiving an EGFR TKI.
Table A2.
Patients with stage I to III lung cancer harboring rare EGFR mutation.
| Patient No. | Age | Sex | Smoking | Initial Stage | TTF-1 | EGFR Mutation | TKI Treatment | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Exon | Mutation | Medication | Line of Treatment | Initial Response | ||||||
| 1 | 68 | Female | never | IA | + | exon 21 | L861Q | Gefitinib | 1st line | PR |
| 2 | 60 | Female | never | IA | + | exon 21 | L861Q | Afatinib | 1st line | SD |
| 3 | 42 | Male | 20 pack-year | IIA | + | exon 18 | G719X | Afatinib | 1st line | PD |
| 4 | 72 | Male | never | IIIA | + | exon 20 | S768I | Erlotinib | 2nd line | SD |
| 5 | 53 | Female | never | IIIB | + | exon 18 | G724S | Gefitinib | 2nd line | PR |
Abbreviation: ECOG = Eastern Cooperative Oncology Group; TTF-1 = thyroid transcription factor 1, TTF-1 was often used to confirmed the diagnosis of lung adenocarcinoma.
Table A3.
Univariate Cox proportional hazards regression analysis to identify the relationship between metastatic sites and survival in patients with stage IV lung cancer harboring rare mutations receiving first-line TKIs.
| Metastatic Site | Progression-Free Survival | Overall Survival | ||
|---|---|---|---|---|
| HR [95% CI] | p Value | HR [95% CI] | p Value | |
| Brain | 1.68 [0.77–3.67] | 0.1902 | 3.82 [1.57–9.26] | 0.0030 |
| Leptomeningeal | 4.23 [0.97–18.38] | 0.0545 | 15.09 [3.02–75.30] | 0.0009 |
| Lung | 1.03 [0.56–1.9] | 0.9202 | 0.63 [0.31–1.30] | 0.2145 |
| Bone | 1.78 [0.97–3.29] | 0.0638 | 1.29 [0.67–2.47] | 0.4440 |
| Pleural | 1.35 [0.75–2.44] | 0.3112 | 1.26 [0.66–2.39] | 0.4816 |
| Pericardial | 0.94 [0.33–2.64] | 0.9021 | 1.01 [0.31–3.30] | 0.9931 |
| Liver | 1.73 [0.75–3.97] | 0.1956 | 1.53 [0.58–4.04] | 0.3885 |
| Adrenal | 0.5 [0.15–1.66] | 0.2565 | 0.36 [0.08–1.51] | 0.1621 |
Abbreviation: HR = hazard ratio; CI = confidence interval.
Author Contributions
M.-J.T. and C.-J.Y.: design the study and wrote the manuscript, J.-Y.H. and M.-H.L.: data collection and statistical review. C.-Y.K., Y.-C.T., T.-C.L.: data collection and critical history review and statistical analysis. M.-S.H. and I.-W.C.: manuscript review and as consultants. All authors have read and approved the final version of the manuscript.
Funding
The entire study was funded by grants from kmtth-106-006 in Kaohsiung Municipal Ta-Tung Hospital.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Yang C.H., Yu C.J., Shih J.Y., Chang Y.C., Hu F.C., Tsai M.C., Chen K.Y., Lin Z.Z., Huang C.J., Shun C.T., et al. Specific EGFR mutations predict treatment outcome of stage IIIb/IV patients with chemotherapy-naive non-small-cell lung cancer receiving first-line gefitinib monotherapy. J. Clin. Oncol. 2008;26:2745–2753. doi: 10.1200/JCO.2007.15.6695. [DOI] [PubMed] [Google Scholar]
- 2.Mok T.S., Wu Y.L., Thongprasert S., Yang C.H., Chu D.T., Saijo N., Sunpaweravong P., Han B., Margono B., Ichinose Y., et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 3.Mitsudomi T., Morita S., Yatabe Y., Negoro S., Okamoto I., Tsurutani J., Seto T., Satouchi M., Tada H., Hirashima T., et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 4.Maemondo M., Inoue A., Kobayashi K., Sugawara S., Oizumi S., Isobe H., Gemma A., Harada M., Yoshizawa H., Kinoshita I., et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 5.Lynch T.J., Bell D.W., Sordella R., Gurubhagavatula S., Okimoto R.A., Brannigan B.W., Harris P.L., Haserlat S.M., Supko J.G., Haluska F.G., et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 6.Yun C.H., Boggon T.J., Li Y., Woo M.S., Greulich H., Meyerson M., Eck M.J. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: Mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell. 2007;11:217–227. doi: 10.1016/j.ccr.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y.R., Fu Y.N., Lin C.H., Yang S.T., Hu S.F., Chen Y.T., Tsai S.F., Huang S.F. Distinctive activation patterns in constitutively active and gefitinib-sensitive EGFR mutants. Oncogene. 2006;25:1205–1215. doi: 10.1038/sj.onc.1209159. [DOI] [PubMed] [Google Scholar]
- 8.Tu H.Y., Ke E.E., Yang J.J., Sun Y.L., Yan H.H., Zheng M.Y., Bai X.Y., Wang Z., Su J., Chen Z.H., et al. A comprehensive review of uncommon EGFR mutations in patients with non-small cell lung cancer. Lung Cancer. 2017;114:96–102. doi: 10.1016/j.lungcan.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Shen Y.C., Tseng G.C., Tu C.Y., Chen W.C., Liao W.C., Chen W.C., Li C.H., Chen H.J., Hsia T.C. Comparing the effects of afatinib with gefitinib or erlotinib in patients with advanced-stage lung adenocarcinoma harboring non-classical epidermal growth factor receptor mutations. Lung Cancer. 2017;110:56–62. doi: 10.1016/j.lungcan.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Peng L., Song Z., Jiao S. Comparison of uncommon EGFR exon 21 l858R compound mutations with single mutation. Onco Targets Ther. 2015;8:905–910. doi: 10.2147/OTT.S78984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi S., Canepa H.M., Bailey A.S., Nakayama S., Yamaguchi N., Goldstein M.A., Huberman M.S., Costa D.B. Compound EGFR mutations and response to EGFR tyrosine kinase inhibitors. J. Thorac. Oncol. 2013;8:45–51. doi: 10.1097/JTO.0b013e3182781e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kauffmann-Guerrero D., Huber R.M., Reu S., Tufman A., Mertsch P., Syunyaeva Z., Jung A., Kahnert K. Nsclc patients harbouring rare or complex EGFR mutations are more often smokers and might not benefit from first-line tyrosine kinase inhibitor therapy. Respiration. 2018;95:169–176. doi: 10.1159/000484175. [DOI] [PubMed] [Google Scholar]
- 13.Chiu C.H., Yang C.T., Shih J.Y., Huang M.S., Su W.C., Lai R.S., Wang C.C., Hsiao S.H., Lin Y.C., Ho C.L., et al. Epidermal growth factor receptor tyrosine kinase inhibitor treatment response in advanced lung adenocarcinomas with G719X/l861Q/S768I mutations. J. Thorac. Oncol. 2015;10:793–799. doi: 10.1097/JTO.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 14.Chen K., Yu X., Wang H., Huang Z., Xu Y., Gong L., Fan Y. Uncommon mutation types of epidermal growth factor receptor and response to EGFR tyrosine kinase inhibitors in chinese non-small cell lung cancer patients. Cancer Chemother. Pharmacol. 2017;80:1179–1187. doi: 10.1007/s00280-017-3464-9. [DOI] [PubMed] [Google Scholar]
- 15.Yang J.C., Sequist L.V., Geater S.L., Tsai C.M., Mok T.S., Schuler M., Yamamoto N., Yu C.J., Ou S.H., Zhou C., et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: A combined post-hoc analysis of lux-lung 2, lux-lung 3, and lux-lung 6. Lancet Oncol. 2015;16:830–838. doi: 10.1016/S1470-2045(15)00026-1. [DOI] [PubMed] [Google Scholar]
- 16.Yang C.J., Tsai M.J., Hung J.Y., Tsai Y.M., Lee J.Y., Chou S.H., Liu T.C., Shen M.C., Huang M.S., Chong I.W. Poorer prognosis in taiwanese female ever smokers with stage iv lung adenocarcinoma who were readministered a tyrosine kinase inhibitor. Onco Targets Ther. 2016;9:1511–1518. doi: 10.2147/OTT.S100169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang C.J., Tsai M.J., Hung J.Y., Liu T.C., Chou S.H., Lee J.Y., Hsu J.S., Tsai Y.M., Huang M.S., Chong I.W. Pemetrexed had significantly better clinical efficacy in patients with stage IV lung adenocarcinoma with susceptible EGFR mutations receiving platinum-based chemotherapy after developing resistance to the first-line gefitinib treatment. Onco Targets Ther. 2016;9:1579–1587. doi: 10.2147/OTT.S100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang C.J., Tsai M.J., Hung J.Y., Lee M.H., Tsai Y.M., Tsai Y.C., Hsu J.F., Liu T.C., Huang M.S., Chong I.W. The clinical efficacy of afatinib 30 mg daily as starting dose may not be inferior to afatinib 40 mg daily in patients with stage IV lung adenocarcinoma harboring exon 19 or exon 21 mutations. BMC Pharmacol. Toxicol. 2017;18:82. doi: 10.1186/s40360-017-0190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang C.J., Hung J.Y., Tsai M.J., Wu K.L., Liu T.C., Chou S.H., Lee J.Y., Hsu J.S., Huang M.S., Chong I.W. The salvage therapy in lung adenocarcinoma initially harbored susceptible EGFR mutation and acquired resistance occurred to the first-line gefitinib and second-line cytotoxic chemotherapy. BMC Pharmacol. Toxicol. 2017;18:21. doi: 10.1186/s40360-017-0130-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu K.L., Tsai M.J., Yang C.J., Chang W.A., Hung J.Y., Yen C.J., Shen C.H., Kuo T.Y., Lee J.Y., Chou S.H., et al. Liver metastasis predicts poorer prognosis in stage IV lung adenocarcinoma patients receiving first-line gefitinib. Lung Cancer. 2015;88:187–194. doi: 10.1016/j.lungcan.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Hsu J.S., Huang M.S., Chen C.Y., Liu G.C., Liu T.C., Chong I.W., Chou S.H., Yang C.J. Correlation between EGFR mutation status and computed tomography features in patients with advanced pulmonary adenocarcinoma. J. Thorac. Imaging. 2014;29:357–363. doi: 10.1097/RTI.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 22.Schwentner I., Witsch-Baumgartner M., Sprinzl G.M., Krugmann J., Tzankov A., Jank S., Zwierzina H., Loeffler-Ragg J. Identification of the rare EGFR mutation p.G796S as somatic and germline mutation in white patients with squamous cell carcinoma of the head and neck. Head Neck. 2008;30:1040–1044. doi: 10.1002/hed.20831. [DOI] [PubMed] [Google Scholar]
- 23.Pallan L., Taniere P., Koh P. Rare EGFR exon 20 S768I mutation predicts resistance to targeted therapy: A report of two cases. J. Thorac. Oncol. 2014;9:e75. doi: 10.1097/JTO.0000000000000308. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi Y., Togashi Y., Yatabe Y., Mizuuchi H., Jangchul P., Kondo C., Shimoji M., Sato K., Suda K., Tomizawa K., et al. EGFR exon 18 mutations in lung cancer: Molecular predictors of augmented sensitivity to afatinib or neratinib as compared with first-or third-generation tkis. Clin. Cancer Res. 2015;21:5305–5313. doi: 10.1158/1078-0432.CCR-15-1046. [DOI] [PubMed] [Google Scholar]
- 25.Kancha R.K., von Bubnoff N., Peschel C., Duyster J. Functional analysis of epidermal growth factor receptor (EGFR) mutations and potential implications for EGFR targeted therapy. Clin. Cancer Res. 2009;15:460–467. doi: 10.1158/1078-0432.CCR-08-1757. [DOI] [PubMed] [Google Scholar]
- 26.Ibrahim U., Saqib A., Atallah J.P. EGFR exon 18 dele709_t710insd mutated stage IV lung adenocarcinoma with response to afatinib. Lung Cancer. 2017;108:45–47. doi: 10.1016/j.lungcan.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 27.Gauthier H., Douchet G., Lehmann-Che J., Meignin V., Raynaud C., Sabatier P., de Cremoux H., Poirot B., Culine S., Pouessel D., et al. Two cases of non-small-cell lung cancer with rare complex mutation of EGFR exon 18 but different response to targeted therapy. J. Thorac. Oncol. 2014;9:e78–e79. doi: 10.1097/JTO.0000000000000328. [DOI] [PubMed] [Google Scholar]
- 28.Tian P., Wang Y., Wang W., Li Y., Wang K., Cheng X., Tang Y., Han-Zhang H., Ye J., Chuai S., et al. High-throughput sequencing reveals distinct genetic features and clinical implications of NSCLC with de novo and acquired EGFR T790M mutation. Lung Cancer. 2018;124:205–210. doi: 10.1016/j.lungcan.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 29.Zeng L., Zhang Y., Yang N. EGFR exon 18 dele709_t710insd as an acquired resistance mechanism to afatinib in an advanced egfr exon 18 E709H lung adenocarcinoma. J. Thorac. Oncol. 2018;13:e93–e95. doi: 10.1016/j.jtho.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Yang M., Tong X., Xu X., Zheng E., Ni J., Li J., Yan J., Shao Y.W., Zhao G. Case report: Osimertinib achieved remarkable and sustained disease control in an advanced non-small-cell lung cancer harboring EGFR H773L/V774M mutation complex. Lung Cancer. 2018;121:1–4. doi: 10.1016/j.lungcan.2018.04.006. [DOI] [PubMed] [Google Scholar]