Abstract
Background
Anti‐Mϋllerian hormone (AMH) plays an important role regulating ovarian sensitivity to follicle‐stimulating hormone and luteinizing hormone in folliculogenesis. Anti‐Mϋllerian hormone is well established as a biomarker of ovarian reserve but may also have utility in predicting pregnancy outcomes. Few studies have described AMH levels in pregnancy and, among those that have, most have used cross‐sectional study designs and are limited to participants seeking fertility treatment. Our aim was to analyze AMH longitudinally in low‐risk pregnancies.
Methods
We conducted a prospective cohort study at Baystate Medical Center, a large tertiary care hospital in Springfield, MA, USA. We recruited women (n = 30) with low risk, singleton pregnancies, aged 18 to 35 years, with BMI between 18 and 40 kg/m2, and without preexisting disease. Anti‐Mϋllerian hormone (pmol/L) was measured in plasma samples collected at 5 prenatal care visits throughout gestation.
Results
Anti‐Mϋllerian hormone levels varied significantly over gestation (Friedman's analysis of variance, P value < .0001). At gestational weeks 7 to 10, average AMH was 36.7 pmol/L (standard error = 8.1) and at weeks 34 to 37 was 9.5 pmol/L (standard error = 1.9). Initial AMH varied between women, and an overall significant log‐linear decline was observed.
Conclusions
Anti‐Mϋllerian hormone varies between women and declines exponentially during pregnancy. The biological mechanism of the heterogeneity of AMH decline over gestation is unclear. Future studies evaluating AMH throughout pregnancy that also assess gravid health and pregnancy outcomes are warranted.
Keywords: anti‐Mϋllerian hormone, folliculogenesis, gestation, longitudinal, pregnancy
1. INTRODUCTION
Anti‐Müllerian hormone (AMH) is a glycoprotein hormone from the transforming growth factor‐beta family1, 2, 3 and is expressed by granulosa cells of antral and preantral follicles.4 In fetal development, AMH prevents development of the Müllerian ducts during male sex differentiation.5 In addition, AMH plays an important role in ovarian function and folliculogenesis, modulating ovarian sensitivity to gonadotropins,6 and in gene expression, leading to production of ovarian steroids.6, 7 Anti‐Müllerian hormone is a well‐described biomarker of ovarian reserve and has been used to predict age‐at‐menopause8, 9 and primary ovarian insufficiency.10, 11 Studies suggest that AMH levels have minimal variability across the menstrual cycle.12, 13, 14 Influences on AMH, other than age,12, 15 include use of oral contraceptives16, 17 and smoking status.13
Although well established as a biomarker of fecundability, few studies have described AMH levels during pregnancy, and there is some uncertainty regarding how levels vary across gestation in healthy pregnancies. The limited studies of AMH in pregnancy include cross‐sectional comparisons,18 assessment in women seeking fertility treatment,19 assessment in women with gestational diabetes,20 and evaluation in healthy women by using biospecimens collected once each trimester.21 To further evaluate variation in AMH levels over gestation and between women in pregnancy, we used data from a prospective pregnancy study with longitudinal biospecimen collection from women with singleton normal pregnancies. Characterization of the variability of AMH levels during uncomplicated pregnancy is an important step toward assessment of the association of AMH with adverse pregnancy outcomes.
2. METHODS
2.1. Study sample
Women in our sample (n = 30) were recruited as part of the Patterns of Inflammation in Normal Gestation study to assess the variability of inflammatory biomarkers across gestation in low‐risk pregnancies and, secondarily, to evaluate feasibility of recruitment and retention of women in longitudinal pregnancy studies. Women with a singleton pregnancy between the ages of 18 and 35 years, with BMI between 18 and 40 kg/m2, without preexisting proteinuria, and without current or history of autoimmune conditions, hypertension, diabetes, cardiovascular disease, cancer, chronic renal disease, or chronic inflammatory conditions were eligible for participation. The study protocol entailed collection of 5 blood draws at normal prenatal care visits starting in early pregnancy (at gestational week 8‐14) and completed proximal to parturition (gestational week 35‐38). Recruitment took place over 3 months, between November 4 2013 and February 5 2014. Follow‐up was completed on August 19, 2014. Participant characteristics were recorded from medical record abstractions. The study was approved by the Institutional Review Boards of the University of Massachusetts Amherst and Baystate Health, and all participating women provided written informed consent.
2.2. AMH measurement
Blood samples were collected at prenatal care visits via venipuncture. Purple top BD Vacutainer tubes with K2EDTA additives were used to collect 10‐mL aliquots of whole blood and put in ice until processing. Blood samples were cold centrifuged, and plasma was aliquoted into 0.5‐mL samples and stored at −80°C. Samples were processed within 2 hours of collection. Anti‐Müllerian hormone was measured by using an ultra‐sensitive ELISA from ANSH Labs (picoAMH kit, catalog number AL‐124, Webster, TX) at the Boston Children's Hospital, completed in accordance with the manufacturer's instructions. Percent variation for repeated ELISA samples was between 3.2% and 5.8%.
2.3. Statistical analyses
Plasma concentrations of AMH (pmol/L) were used to estimate mean AMH by visit and by gestational age at sample collection. Due to variation in timing of clinic visits and, accordingly, gestational age at biospecimen collection, and the limited sample size, gestational age at sample collection was assigned to groups defined by mainly 4‐week intervals. A plot of mean AMH by gestational age of pregnancy was generated to visualize AMH trends. Means were compared by using repeated measures analysis of variance, with Tukey post hoc test for pairwise comparisons. To address potential violations of distributional assumptions, we used the nonparametric Friedman's test, which utilizes ranks to compare AMH by gestational age. Pearson correlation coefficients were estimated to assess the linear correlation of overall change in AMH levels with initial levels. To assess variability in AMH between women relative to temporal variation over gestation, we estimated intraclass correlation coefficients. In addition, we used mixed models of natural log transformed AMH to evaluate log‐linear trends over gestation. For these models, a random intercept was tested to evaluate individual variability in baseline AMH. All statistical analyses were carried out by using SAS software v.9.4 (SAS Institute Inc., Cary, NC).
3. RESULTS
3.1. Participant characteristics
Of the 30 study participants, 29 women completed all 5 scheduled visits (97%), and the remaining participant completed 4 visits. Thus, overall compliance with the study protocol was high, with a total of 149 biospecimens collected of a possible 150. An additional 31st participant dropped out of the study shortly after informed consent due to relocation and is not included in the analyses. Participant characteristics and demographics are described in Table 1. The participants were young, with average age of 23.4 years (SD = 3.8). Just over half the participants self‐identified as Hispanic (53%), and over half of the participants were nulliparous (52%). The mean gestational ages in weeks (range) for each visit were as follows: visit one, 11.0 (7‐15); visit 2, 16.8 (range 15–20); visit 3, 24.7 (23‐27); visit 4, 30.8 (29‐34); and visit 5, 35.6 (35‐37). Women gave birth at an average of 39.6 weeks' gestation; 1 woman delivered at 36 weeks 2 days, with the remainder delivering between 37 weeks 3 days and 41 weeks 3 days. One woman was diagnosed with preeclampsia and delivered at 41 weeks; otherwise, no women experienced complications in pregnancy.
Table 1.
Characteristics of participants in the Patterns of Inflammation in Normal Gestation study (n = 30)
| n | % | |
|---|---|---|
| Hispanic ethnicity | 16 | 53% |
| Racea | ||
| White | 9 | 30% |
| Black | 8 | 27% |
| Other | 7 | 23% |
| Unknown/missing | 6 | 20% |
| Marital status | ||
| Single, living alone | 11 | 37% |
| Single, living with partner | 15 | 50% |
| Married | 4 | 13% |
| Education | ||
| 1+ years college | 10 | 33% |
| Completed high school/GED | 17 | 57% |
| Some high school | 3 | 10% |
| Parity | ||
| 0 | 16 | 52% |
| 1 | 9 | 29% |
| >1 | 6 | 19% |
| Mean | SD | |
| Age (years) | 23.4 | 3.8 |
| Gestational age at delivery (weeks) | 39.6 | 1.2 |
| BMI (kg/m2) | 26.7 | 5.0 |
By self‐report.
3.2. AMH throughout pregnancy
Differences in AMH by timing of gestation were evaluated with nonparametric, Friedman analysis of variance test making comparisons by gestational age. Anti‐Müllerian hormone was observed to vary significantly by gestational age (P < .0001). Figure 1 depicts AMH levels (mean and 95% confidence intervals [CIs]) by GA to illustrate the pattern of AMH across pregnancy. Mean AMH decreased across gestation. Mean and standard error (SE) as well as median and first and third quartiles of AMH (pmol/L) by 4‐week gestational age intervals are given in Table 2. In samples collected at 7 to 10 weeks' GA, AMH was 36.7 pmol/L (SE = 8.1); at GA weeks 19 to 24, average AMH was 13.9 (SE = 2.0) pmol/L; and at weeks 34 to 37 was 9.5 (SE = 1.9) pmol/L. There was a statistically significant mean difference in AMH between samples collected at GA weeks 7 to 10 and those collected at weeks 11 to 14 by using Tukey post hoc tests (P < .05). Substantial intraindividual and interindividual variabilities were observed, as reflected by the estimated coefficient of within‐subject variance of 0.52 (95%CI: 0.38, 0.71) and the intraclass correlation coefficient estimate (0.67, 95%CI: 0.52, 0.79).
Figure 1.
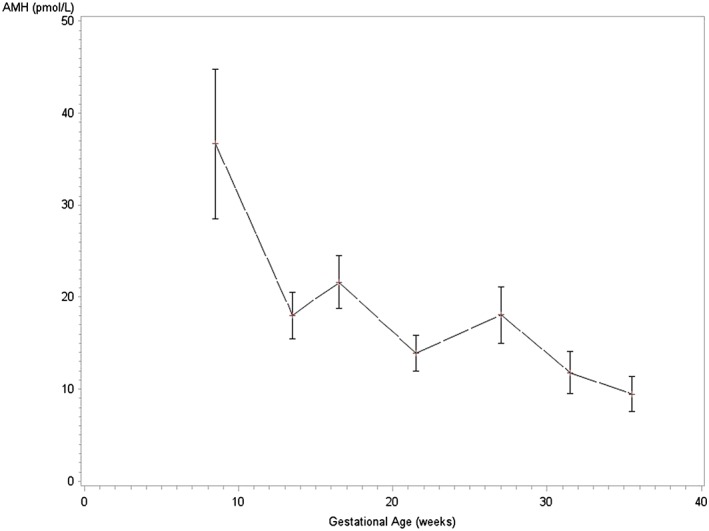
Anti‐Mϋllerian hormone (AMH; pmol/L) throughout pregnancy (mean and 95% CI). Anti‐Müllerian hormone (pmol/L) means and 95% confidence intervals are shown by gestational age in weeks at blood sample collection. Means were significantly different by gestational age (P < .0001) in nonparametric comparisons of AMH by gestational age using Friedman test
Table 2.
Average and median AMH by gestational age at sample collection among participants in the Patterns of Inflammation in Normal Gestation study (n = 30)
| GA at Sample Collection* (Weeks) | N | AMH (pmol/L) | ||||
|---|---|---|---|---|---|---|
| Mean | SE | Median | 25th Percentile | 75th Percentile | ||
| 7‐10 | 12 | 36.7 | 8.1 | 22.3 | 13.4 | 63.6 |
| 11‐14 | 17 | 18.0 | 2.5 | 13.0 | 10.4 | 25.0 |
| 15‐18 | 27 | 21.6 | 2.9 | 16.3 | 11.1 | 31.6 |
| 19‐24 | 19 | 13.9 | 2.0 | 13.5 | 4.5 | 17.8 |
| 25‐29 | 16 | 18.0 | 3.1 | 14.3 | 9.5 | 22.7 |
| 30‐33 | 27 | 11.8 | 2.3 | 7.9 | 5.0 | 12.7 |
| 34‐37 | 31 | 9.5 | 1.9 | 5.8 | 3.9 | 9.7 |
Abbreviations: AMH, anti‐Mϋllerian hormone; GA, gestational age; SE, standard error.
The trajectory of AMH during pregnancy was further explored by using linear mixed models with a natural log transformation of AMH and specifying a random intercept, which was used to address the variability of initial levels of AMH among participants. Based on correlation analysis, initial AMH levels were related to the amount of decline over pregnancy (r = −0.92, P < .0001). A significant log‐linear trend was observed that corresponded to a 0.045 unit decrease in the natural log of AMH per gestational week (95%CI: −0.050, −0.040: P < .0001). A likelihood ratio test indicated that the random intercept significantly improved model fit (P < .0001). The regression line from these models, along with individual trajectories of natural log transformed AMH, is shown in Figure 2.
Figure 2.
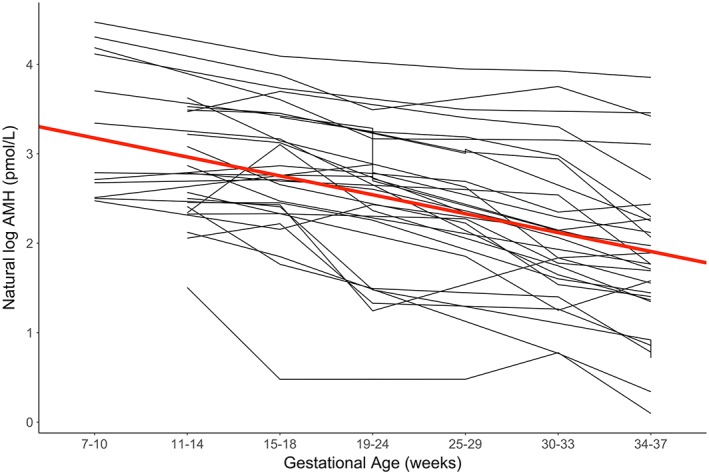
Trajectories of natural log transformed anti‐Mϋllerian hormone (AMH) by gestational age. Natural log transformed AMH (pmol/L) is shown plotted by gestational age at sample collection for each cohort member. Mixed models of log AMH regressed on gestational age yielded the estimates shown in the plot along with the bold red regression line
4. DISCUSSION
In this study, we evaluated AMH in plasma samples collected at 5 time points between 8 and 36 weeks of gestation. Our results suggest significant variation in AMH over gestation. Our study included women with low‐risk pregnancies and not seeking fertility treatment, which provides information regarding the variability of AMH between women and across gestation in pregnancy. Although 1 of the 30 participants experienced preeclampsia, no other women experienced complications in pregnancy, and results were unaltered after repeating analysis excluding this 1 participant.
We observed decreasing AMH throughout pregnancy; as seen in plots of AMH over pregnancy, and supported by the Tukey post hoc tests, a large decline occurred in the first trimester, between 7 and 14 weeks' gestation. In addition to this gestational variability, we observed AMH levels to vary significantly between women. A random intercept model of natural log transformed AMH provided a good fit to the data, indicating the log‐linear trend of declining AMH over pregnancy as well as the interindividual variation. Further, initial AMH levels were strongly correlated with the amount of decline in AMH over gestation, consistent with what has been reported previously.21 This result is consistent with a conserved rate of AMH decline that is dependent on initial concentration of AMH.
Some women had initially low concentrations of AMH that stayed at low levels throughout gestation. A recent review by McCredie et al examined 8 studies of AMH in pregnancy and noted a similar rate of decline during the first trimester.22 Different mechanisms have been associated with heterogeneity in AMH decline during pregnancy including fetal sex and maternal BMI. Stojsin‐Carter et al observed in cattle that female cattle pregnant with male fetuses had higher AMH in early pregnancy (days 35‐175) compared with those with female fetuses.23 Evidence on BMI‐specific effects on AMH decline have been inconclusive with reports from studies suggesting both positive and negative associations between maternal BMI and AMH concentration during gestation.22 Other mechanisms may explain this observed trend, such as pregnancy‐induced hemodilution with concurrent plasma‐protein binding increase and estrogen‐induced AMH suppression during pregnancy.24, 25 Given the observation of the most dramatic decreases in AMH very early in pregnancy, some women may have had a somewhat later initial visit than other women and provided samples too late in gestation to capture this very early gestational variation.
Prior studies of AMH in pregnancy have considered a mix of study samples and used different approaches.17, 18, 19, 20, 21, 26 Using a cross‐sectional approach among 84 women, no significant differences were observed in AMH levels compared among those in the first trimester (n = 27), in second trimester (n = 21), in the third trimester (n = 13), and a nonpregnant (n = 15) group.18 In contrast, cross‐sectional comparison of AMH levels among 554 women by trimester and postpartum suggested that levels decline over pregnancy and rapidly rise in the postpartum period.26 Decreasing AMH has been observed in the first few weeks of gestation among women seeking fertility care (n = 85),19 and declines in AMH have been observed late in pregnancy in a prospective study of women with and without GDM during the third trimester, with significant decreases between 28 to 32 and 34 to 36 weeks of gestation.20
Our results are similar to those of Nelson et al, evaluating AMH in a pregnancy cohort (n = 60) of women recruited at their first prenatal visit (GA range: 8‐14 weeks) who had a normal pregnancy and no history of pregnancy complications.21 Based on plasma samples collected approximately at 3 time points per woman at 12, 26, and 35 weeks' gestation, AMH levels were observed to decline during the second and third trimesters of pregnancy.21 Similarly, we observed a declining AMH throughout pregnancy independent of the age of the participant; through collection of 5 time points for biospecimen collection across pregnancy, we have described AMH trajectories in fine detail, both individually and as an overall trend. We observed somewhat larger changes in AMH in early pregnancy; however, declining mean AMH levels were observed throughout gestation.
Longitudinal assessment of AMH in gestation may provide insight into its role in folliculogenesis. Early pregnancy concentrations of AMH are thought to be similar to those before pregnancy.19, 21 We observed high levels continuing to 10 weeks' gestation but rapid declines shortly thereafter, which may reflect the timing of ovarian function and follicular recruitment in pregnancy.21 As AMH decreases in latter gestational ages, follicular recruitment declines, but the observation of continuing detectable levels suggests that the process is not halted completely in pregnancy. Likely, gonadotropin suppression during early pregnancy causes decreased AMH concentration and explains low concentrations of AMH from early pregnancy through postpartum.27 Although our study characterized AMH in uncomplicated pregnancies, AMH has been suggested by some to be related to increased risk of adverse pregnancy outcomes, such as maternal hypertension or preeclampsia.28, 29 Studies that assess AMH concentration prepregnancy, during gestation, and postpartum may help to better understand the mechanism of follicular recruitment and role of gonadotropin in AMH concentration in women who have completed pregnancy. Prospective pregnancy studies that evaluate maternal and pregnancy outcomes in addition to other biomarkers in pregnancy are important to better understand how AMH in pregnancy is related to these maternal and fetal characteristics.
Despite the small sample size, a strength of our study was the high level of compliance with the study protocol, which resulted in a large number of biospecimens across a wide range of gestation. Participants in the study provided all but 1 of the 5 scheduled biospecimen collections per participant, which helped us characterize within and between‐person AMH concentration variability. In addition, this supports the feasibility of longitudinal studies of biomarkers during pregnancy. Finally, eligibility for our study was relatively broad, contributing to the generalizability of our findings. We evaluated AMH with regard to race/ethnicity and other covariates and observed no associations, but because of the sample size (n = 30), the statistical power was low and these might have been missed.
5. CONCLUSIONS
Our data suggest that, on average, AMH levels during gestation decrease but do not completely disappear and that patterns vary among women, particularly by baseline AMH levels. Additional studies may help improve understanding of the significance of AMH trajectories in gestation for pregnancy outcomes.
CONFLICT OF INTEREST
The authors report no conflicts of interest or financial interests to disclose in this work.
AUTHOR CONTRIBUTIONS
Conceptualization: Andrew J. Healy, Brian W. Whitcomb
Formal analysis: Joshua R. Freeman, Nicholas G. Reich, Brian W. Whitcomb
Funding acquisition: Andrew J. Healy, Brian W. Whitcomb
Supervision: Andrew J. Healy, Brian W. Whitcomb
Writing—original draft: Joshua R. Freeman, Brian W. Whitcomb
Writing—review and editing: Elizabeth R. Bertone‐Johnson, Andrew J. Healy, Amrita Roy, Nicholas G. Reich
ACKNOWLEDGEMENTS
This work was supported by a grant from the Collaborative Biomedical Research Program (BWW and AJH) jointly funded by Baystate Health and the University of Massachusetts Amherst.
Freeman JR, Whitcomb BW, Roy A, Bertone‐Johnson ER, Reich NG, Healy AJ. A pilot longitudinal study of anti‐Müllerian hormone levels throughout gestation in low risk pregnancy. Health Sci Rep. 2018;1:e53 10.1002/hsr2.53
REFERENCES
- 1. Grootegoed JA, Baarends WM, Themmen AP. Welcome to the family: the anti‐Mϋllerian hormone receptor. Mol Cell Endocrinol. 1994;100(1‐2):29‐34. [DOI] [PubMed] [Google Scholar]
- 2. Baarends WM, Uilenbroek JT, Kramer P, et al. Anti‐Mϋllerian hormone and anti‐Mϋllerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin‐induced follicle growth. Endocrinology. 1995;136(11):4951‐4962. [DOI] [PubMed] [Google Scholar]
- 3. Weenen C, Laven JS, Von Bergh AR, et al. Anti‐Mϋllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10(2):77‐83. [DOI] [PubMed] [Google Scholar]
- 4. Fleming R, Seifer DB, Frattarelli JL, Ruman J. Assessing ovarian response: antral follicle count versus anti‐Mϋllerian hormone. Reprod Biomed Online. 2015;31(4):486‐496. [DOI] [PubMed] [Google Scholar]
- 5. Jarminska‐Jackowiak T, Warenik‐Szymankiewicz A, Trzeciak WH. Anti‐Mϋllerian hormone. Structure and role in sexual differentiation. Ginekol Pol. 1995;66(1):51‐58. [PubMed] [Google Scholar]
- 6. Sacchi S, D'Ippolito G, Sena P, et al. The anti‐Mϋllerian hormone (AMH) acts as a gatekeeper of ovarian steroidogenesis inhibiting the granulosa cell response to both FSH and LH. J Assist Reprod Genet. 2016;33(1):95‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kevenaar ME, Themmen AP, Laven JS, et al. Anti‐Mϋllerian hormone and anti‐Mϋllerian hormone type II receptor polymorphisms are associated with follicular phase estradiol levels in normo‐ovulatory women. Hum Reprod. 2007;22(6):1547‐1554. [DOI] [PubMed] [Google Scholar]
- 8. Daan NM, Fauser BC. Menopause prediction and potential implications. Maturitas. 2015;82(3):257‐265. [DOI] [PubMed] [Google Scholar]
- 9. Tehrani FR, Solaymani‐Dodaran M, Tohidi M, Gohari MR, Azizi F. Modeling age at menopause using serum concentration of anti‐Mϋllerian hormone. J Clin Endocrinol Metabol. 2013;98(2):729‐735. [DOI] [PubMed] [Google Scholar]
- 10. Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti‐Mϋllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20(5):688‐701. [DOI] [PubMed] [Google Scholar]
- 11. Visser JA, Schipper I, Laven JS, Themmen AP. Anti‐Mϋllerian hormone: an ovarian reserve marker in primary ovarian insufficiency. Nat Rev Endocrinol. 2012;8(6):331‐341. [DOI] [PubMed] [Google Scholar]
- 12. Lambert‐Messerlian G, Plante B, Eklund EE, Raker C, Moore RG. Levels of anti‐Mϋllerian hormone in serum during the normal menstrual cycle. Fertil Steril. 2016;105(1):208, e1‐213. [DOI] [PubMed] [Google Scholar]
- 13. Kissell KA, Danaher MR, Schisterman EF, et al. Biological variability in serum anti‐Mϋllerian hormone throughout the menstrual cycle in ovulatory and sporadic anovulatory cycles in eumenorrheic women. Hum Reprod. 2014;29(8):1764‐1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cook CL, Siow Y, Taylor S, Fallat ME. Serum Mϋllerian‐inhibiting substance levels during normal menstrual cycles. Fertil Steril. 2000;73(4):859‐861. [DOI] [PubMed] [Google Scholar]
- 15. Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WH. A validated model of serum anti‐Mϋllerian hormone from conception to menopause. PloS one. 2011;6(7):e22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Birch Petersen K, Hvidman HW, Forman JL, et al. Ovarian reserve assessment in users of oral contraception seeking fertility advice on their reproductive lifespan. Hum Reprod. 2015;30(10):2364‐2375. [DOI] [PubMed] [Google Scholar]
- 17. Dólleman M, Verschuren WM, Eijkemans MJ, et al. Reproductive and lifestyle determinants of anti‐Mϋllerian hormone in a large population‐based study. J Clin Endocrinol Metabol. 2013;98(5):2106‐2115. [DOI] [PubMed] [Google Scholar]
- 18. La Marca A, Giulini S, Orvieto R, De Leo V, Volpe A. Anti‐Mϋllerian hormone concentrations in maternal serum during pregnancy. Hum Reprod. 2005;20(6):1569‐1572. [DOI] [PubMed] [Google Scholar]
- 19. Hamilton K, Hadlow N, Roberts P, et al. Longitudinal changes in maternal serum concentrations of anti‐Mϋllerian hormone in individual women during conception cycles and early pregnancy. Fertil Steril. 2016;106(6):1407‐1413.e2. [DOI] [PubMed] [Google Scholar]
- 20. Gerli S, Favilli A, Brozzetti A, et al. Anti‐Mϋllerian hormone concentration during the third trimester of pregnancy and puerperium: a longitudinal case‐control study in normal and diabetic pregnancy. Endocrine. 2015;50(1):250‐255. [DOI] [PubMed] [Google Scholar]
- 21. Nelson SM, Stewart F, Fleming R, Freeman DJ. Longitudinal assessment of anti‐Mϋllerian hormone during pregnancy‐relationship with maternal adiposity, insulin, and adiponectin. Fertil Steril. 2010;93(4):1356‐1358. [DOI] [PubMed] [Google Scholar]
- 22. McCredie S, Ledger W, Venetis CA. Anti‐Müllerian hormone kinetics in pregnancy and post‐partum: a systematic review. Reprod Biomed Online. 2017;34(5):522‐533. [DOI] [PubMed] [Google Scholar]
- 23. Stojsin‐Carter A, Costa NN, De Morais R, et al. Fetal sex alters maternal anti‐Müllerian hormone during pregnancy in cattle. Anim Reprod Sci. 2017;186:85‐92. [DOI] [PubMed] [Google Scholar]
- 24. La Marca A, Grisendi V, Griesinger G. How much does AMH really vary in normal women? Int J Endocrinol. 2013;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grynberg M, Pierre A, Rey R, et al. Differential regulation of ovarian anti‐Mϋllerian hormone (AMH) by estradiol through alpha‐ and beta‐estrogen receptors. J Clin Endocrinol Metab. 2012;97(9):E1649‐E1657. [DOI] [PubMed] [Google Scholar]
- 26. Köninger A, Kauth A, Schmidt B, et al. Anti‐Mϋllerian‐hormone levels during pregnancy and postpartum. Reprod Biol Endocrinol. 2013;11(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hagen CP, Sorensen K, Anderson RA, Juul A. Serum levels of anti‐Mϋllerian hormone in early maturing girls before, during, and after suppression with GnRH agonist. Fertil Steril. 2012;98(5):1326‐1330. [DOI] [PubMed] [Google Scholar]
- 28. Tokmak A, Güney G, Aksoy RT, et al. May maternal anti‐Mϋllerian hormone levels predict adverse maternal and perinatal outcomes in preeclampsia? J Matern Fetal Neonatal Med. 2015;28:1451‐1456. [DOI] [PubMed] [Google Scholar]
- 29. Shand AW, Whitton K, Pasfield A, et al. Evaluation of anti‐Mϋllerian hormone in the first trimester as a predictor for hypertensive disorders of pregnancy and other adverse pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2014;54(3):244‐249. [DOI] [PubMed] [Google Scholar]


