Abstract
Background and aims
Wound healing requires appropriate oxygen and pH levels. Oxygen therapy and pH‐modulating treatments have shown positive effects on wound healing. Thus, a dressing, which combines high levels of dissolved oxygen (DO) with the pH of intact skin, may improve wound healing. Our aims were to (1) formulate an in situ gelling dressing with high levels of DO and with the pH level of intact skin, (2) evaluate physical and chemical properties of the dressing, and (3) elucidate basic effects of elevated levels of DO on human skin cells in vitro.
Methods
A dressing was formulated with 15 to 16 wt% poloxamer 407, acetate buffer, and oxygenated water. Stability of pH and DO, rheology, and shelf life were analysed. Furthermore, in vitro studies of the effect of increased levels of DO were performed.
Results
An in situ gelling wound dressing, with a DO concentration ranging between 25 and 35 mg/L and a pH of 5.5, was formulated. The DO concentration was stable above 22 mg/L for at least 30 hours when applied on a surface at 35°C and covered for directed diffusion into the intended wound area. At storage, the dressing had stable pH for 3 months and stable DO concentration over 30 mg/L for 7 weeks. Increasing DO significantly enhanced intracellular ATP in human skin cells, without changing reactive oxygen species production, proliferation rate, or viability.
Conclusion
The developed dressing may facilitate wound healing by delivering controlled and stable oxygen levels, providing adjustable pH for optimized healing, and increasing intracellular ATP availability.
Keywords: hyperoxia, intracellular ATP, thermo sensitive, topical dressing, wound healing
Short abstract
Background and aims: Wound healing requires appropriate oxygen and pH levels. Oxygen therapy and pH‐modulating treatments have shown positive effects on wound healing. Thus, a dressing, which combines high levels of dissolved oxygen (DO) with the pH of intact skin, may improve wound healing. Our aims were to (1) formulate an in situ gelling dressing with high levels of DO and with the pH level of intact skin, (2) evaluate physical and chemical properties of the dressing, and (3) elucidate basic effects of elevated levels of DO on human skin cells in vitro.
Methods: A dressing was formulated with 15 to 16 wt% poloxamer 407, acetate buffer, and oxygenated water. Stability of pH and DO, rheology, and shelf life were analysed. Furthermore, in vitro studies of the effect of increased levels of DO were performed.
Results: An in situ gelling wound dressing, with a DO concentration ranging between 25 and 35 mg/L and a pH of 5.5, was formulated. The DO concentration was stable above 22 mg/L for at least 30 hours when applied on a surface at 35°C and covered for directed diffusion into the intended wound area. At storage, the dressing had stable pH for 3 months and stable DO concentration over 30 mg/L for 7 weeks. Increasing DO significantly enhanced intracellular ATP in human skin cells, without changing reactive oxygen species production, proliferation rate, or viability.
Conclusion: The developed dressing may facilitate wound healing by delivering controlled and stable oxygen levels, providing adjustable pH for optimized healing, and increasing intracellular ATP availability.
1. INTRODUCTION
Oxygen is a key player in wound healing, as elevated metabolism in the healing skin cells demands increased oxygen supply.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 Blood perfusion, capillary density, partial oxygen pressure (pO2), the oxyhemoglobin dissociation condition, and cell consumption of oxygen are parameters influencing oxygen availability.1 Vascular complications are primarily responsible for wound ischemia in chronic wounds, limiting the delivery of oxygen rich blood and ultimately leading to hypoxia.4 Although it has been shown that acute hypoxia is important in the early stages of wound healing, prolonged hypoxia impairs the healing process.11 Sustained hypoxia diminishes the availability of intracellular ATP and, thus, reduces the synthesis, repair, and turnover of enzymes, DNA, RNA, and cell membrane components, which may result in cell death.1 Sufficient level of oxygen is also crucial for vital cell processes including proliferation,12 collagen synthesis,13, 14, 15, 16, 17 and antibacterial defence.18, 19, 20, 21, 22
Administration of oxygen, both systemically1, 2, 4, 5, 6, 7, 8, 9, 23 and locally,24, 25, 26 has been shown to accelerate the healing process of chronic wounds. These oxygen therapies are cost and personnel demanding; thus, oxygenated dressings have been developed to offer a more economic and easy‐to‐use alternative. Use of oxygenated dressings has shown encouraging results such as accelerated wound healing, lower pain score, and improved histological profiles.27, 28, 29, 30, 31, 32 Documentation of the oxygen concentration and stability of these products is, however, limited.
Chronic wounds have been reported to have a pH ranging between 7.2 and 8.9,33 impeding wound healing.34, 35 It has further been shown that wounds become acidic as they progress towards healing.36 Several studies33, 34, 35, 36 have concluded that an acidic environment at the wound bed supports the healing process. Lowering the pH of wounds has been shown to increase the available pO2 in the wound bed, due to increased release of oxygen from haemoglobin.35 Acidification has also been proven to promote angiogenesis and increase macrophage and fibroblast activity.34 Hydrogels are dressings formed by a network of polymers filled with liquid that can maintain a moist wound environment and protect wounds from secondary infection.37 The block copolymer poloxamers are important in the development of temperature sensitive in situ forming hydrogels. Poloxamers offer advantageous properties for dermal drug delivery, facilitating application and allowing the formulation to act as a depot and, thereby, promoting delivery of active substances for an extended period after application.38, 39 Hydrogels can be applied and removed with minimal pain or trauma to the wound bed and the patient.37
In this study, poloxamer 407 was used to formulate Oxy Dressing, an oxygenated in situ gelling application with pH 5.5. The developed dressing was characterized for physical and chemical properties, and in vitro effects of elevated dissolved oxygen (DO) levels on human skin cells were investigated.
2. METHODS
2.1. Production of oxygenated water, Oxy Water
Water with elevated levels of DO was produced as described in patent WO 2016/071691 (Oxy Solutions, Oslo, Norway). In brief, reverse osmosis water and oxygen was fed into the mixing chamber of the oxygenation device. Water and oxygen were mixed in a chamber and subsequently passed through a piping system including a venturi. The system ran continuously at 2.9 BAR and was fed with 98% O2. At the outlet, oxygenated water containing up to 100 mg/L DO was collected, tapped on glass bottles, and stored at room temperature until use.
2.2. Winkler titration for determination of DO concentrations
All reagents were supplied from Sigma‐Aldrich (St. Louis, MO, USA). Samples were mixed with manganous sulphate (2.2 M, prepared from catalogue number M7634) and alkaline iodide azide (12 M NaOH, prepared from catalogue number 221465, 0.86 M KI, prepared from catalogue numbers 30315 and 71290). The precipitation was allowed to settle halfway twice before 98% sulphuric acid (catalogue number 258105) was added, giving a dark amber colour. The solution was then titrated with sodium thiosulfate (0.0375 M, prepared from catalogue numbers 217247 and 221465). Starch indicator solution (prepared from catalogue numbers S9765 and 247588) was added when the solution reached a light yellow colour, and further titrated until the solution was transparent. Each 1.0 mL of sodium thiosulfate used was equivalent to 3 mg/L DO.
2.3. Formulation of oxygenated in situ gelling dressing, Oxy Dressing
Oxy Dressing was formulated with Oxy Water (~65 mg/L) and poloxamer 407 (Lutrol F127, BASF, Ludwigshafen, Germany, catalogue numbers 50424592 and 50259528). In a mixing vial, concentrated (40‐50 wt% (weight percentage)) Lutrol F127 stock solution was prepared by dissolving the poloxamer powder in Oxy Water (2°C‐4°C) under gentle agitation. When the poloxamer was dissolved, the stock solution was diluted with Oxy Water (0°C) to a final concentration of 15 to 16 wt%.
When formulating the Oxy Dressing in 20 mM acetate buffer for pH control, 30 wt% of poloxamer 407 was dissolved in a 38 mM acetate buffer by gentle mixing on an ice bath (0°C). After dissolution of poloxamer 407 in the stock solution, the 30 wt% poloxamer solution was mixed with Oxy Water cooled to 0°C, to a final concentration of 15 to 16 wt% poloxamer 407.
2.4. Stability testing of pH
The Oxy Dressing formulated in 20 mM acetate buffer was stored in capped glass vials at room temperature (23°C) and at 5°C for up to 3 months; pH was assessed at 0 and 3 months.
2.5. Oxygen stability at 35°C (skin surface temperature)
An oxygen optode developed by RISE (Borås, Sweden) was used to evaluate the oxygen concentration and stability of the Oxy Dressing over time. The optode was assembled on to the tip of a fiber optic probe (NeoFox, Ocean Optics; Dunedin, Florida, USA) and mounted inverted (facing upwards) on a heating block controlling the surface temperature. Oxy Water or calibrating solutions were poured into a small brass pipe (15 ml) assembled onto a seal surrounding the optode film prior to measurements. Calibration of the heated (35°C) optode was made by exposing the assembled sensor to argon and oxygen saturated ultra‐purified water, respectively, at 35°C. The oxygen contents of the calibrating solutions were also confirmed by Winkler titration. During measurements, a smaller black seal was used as container during gel application, facilitating an exact volume (1.5 mL) and surface area (3.5 cm2). The area of the gel measured corresponded to an uncured thickness of 4 mm. To avoid the gel from drying and restricting the diffusion of oxygen into the atmosphere, measurements were performed when covering the gel with a glass lid. Temperature and oxygen levels were simultaneously and continuously measured for up to 40 hours. Humidity and temperature in the room were determined before and after the measurements (29.7% relative humidity and 21.5°C). Control dressing with 15 to 16 wt% poloxamer 407 prepared with MilliQ water was used as reference.
2.6. Gelling properties
Gelling properties were assessed either by visual inspection, by lowering a vial containing the formulation into a water bath at 35°C to 37°C, by direct application of the formulation onto the skin, or by using a Kinexus Pro Rheometer Malvern Instruments, Worcestershire, UK, with a CP4/40 (cone plate 4°‐40‐mm diameter). For the rheology measurements, 1.2 mL of formulation was applied on a cool (18°C‐20°C) plate, and the temperature was increased 1°C/min at a frequency of 0.3 Hz and a shear stress of 1 Pa.
2.7. Shelf life testing
For shelf life testing, the Oxy Dressing was gently filled in capped glass vials (Agilent HS, crimp, RB 20 mL with Hdspc Al crmp cap, PTFE/SI, 5301 Stevens Creek Blvd Santa Clara, CA, USA) with minimum head spacing, and stored at 20°C or at 4°C for up to 7 weeks. The DO concentration was measured weekly by Winkler titration.
2.8. Cell cultures
All reagents were from Sigma Aldrich (St. Louis, MO, USA). Human skin fibroblasts (HSF) (ATCC, Manassas, VA, USA, catalogue number CRL‐7449) were grown in complete cell medium (DMEM, catalogue number D6429) with 10% fetal bovine serum (FBS, catalogue number F7524) and 1% penicillin/streptomycin (catalogue number P4333). At confluence, cells were detached using 5% trypsin in 1 mM ethylene diamine tetra acetic acid (catalogue number T3924) and reseeded according to the experimental plans. Cells were cultured at 37°С in an incubator with humidified atmosphere and 5% СО2. Cell authentication was performed by PCR‐single‐locus‐technology at Eurofins Genomics (Ebersberg, Germany).
2.9. Preparation of oxygenated cell medium
Oxygenated medium was prepared using powder DMEM (Sigma Aldrich, St. Louis, MO, USA with or without phenol red, catalogue numbers D5648 and D9785) and Oxy Water. The mixing ratio depended on the desired concentration of DO in the final cell medium. DMEM was supplied with additives and FBS as described under the sections for individual cell experiments. Phenol red free DMEM was used for the reactive oxygen species (ROS) experiment to avoid disturbance of the fluorescent readings.
As oxygen evaporates faster from the liquid at high temperatures, plated cells were left at room temperature for 30 minutes after stimulation to maintain the elevated DO concentration for a longer period of time. Control cells were treated the same way.
2.10. Intracellular ATP concentration
HSF were seeded at a concentration of 2.5 × 105 cells/9.5 cm2 and grown to confluence for 48 hours. The cells were treated with complete cell medium (10% FBS) with DO concentrations of 8 or 33 mg/L. Cells were re‐stimulated with the respective treatments after 1, 2, and 3 hours. After 4 hours, the cells were rinsed with PBS and lysed on ice with lysis buffer (200 mM Tris, pH 7.5, 2 M NaCl, 20 mM EDTA 0.2% Triton X‐100). The lysate was centrifuged at 10 000 ×g for 5 minutes and added to a standard reaction solution (ATP Determination Kit, Thermo Fisher, Waltham, MA, USA, catalogue number A22066). Luminescence was read with a plate reader (FLUOStar Omega, BMG Labtech), and signal was compared with an ATP standard curve. Cells in corresponding wells were subsequently harvested, and trypan blue (Sigma Aldrich, St. Louis, MO, USA, catalogue number T8154) added, and the number of cells was determined by manually counting live and dead cells using a hemocytometer. The data were corrected for blank readings and presented as nM intracellular ATP/105 live cells.
2.11. ROS production
HSFs were seeded at a density of 2.5 × 104 cells/0.32 cm2 and grown to confluence overnight. Cells were stained with 20‐μM DCFDA solution (2′,7′‐dichlorofluorescin diacetate; DCFDA Cellular ROS Detection Assay Kit, Abcam, Cambridge, UK, catalogue number ab113851) and incubated for 45 minutes at 37°C, protected from light exposure. ROS kinetics were studied by stimulating HSF with complete cell medium (10% FBS) with DO concentrations of 9 or 32 mg/L, or 500‐μM H2O2 (positive control). Fluorescence was read after 15 minutes, 30 minutes, 1 hour, and 4 hours, with an FLx800 plate reader (BioTek, Winooski, USA), with excitation wavelength at 485 nm and emission wavelength 520 nm. An additional experiment was performed, stimulating the HSF with complete cell medium (10% FBS) with DO concentrations of 9 or 31 mg/L, or 500‐μM H2O2 (positive control). The cells were re‐stimulated hourly with the respective treatments, and fluorescence was read after 4 hours. All values were corrected for blank readings and presented as relative fluorescence (RFU ×103).
2.12. Proliferation and cell viability
HSFs were seeded at a density of 5 × 104 cells/25 cm2 and incubated overnight. Cells were treated with 5‐mL control cell medium (1% FBS, DO: 11 mg/L), oxygenated cell medium (1% FBS, DO: 23, 31, 41, 50 mg/L), or cell medium with 10% FBS (positive control, DO: 10 mg/L). The stimulation was repeated every 24 hours up to 4 days. On days 2, 3, and 4, cells were harvested, and live cells were manually counted using a hemocytometer. Dead cells were counted using trypan blue and a TC20 Automated cell counter (BioRad, USA). A 1‐minute‐long treatment of the cell suspension with Triton X (final concentration 1%) was used as a positive control for cell death.
2.13. Statistics
Determination of DO concentration was performed on 5 samples for each condition. Stability of pH was measured in 3 to 9 different samples for each group. Oxygen stability at 35°C was performed on 3 samples. Rheology was measured in triplicate on 2 individual batches per group. Shelf life study was performed on 2 samples per group for each condition. All in vitro experiments were performed in triplicates and repeated at least 3 times.
Results were analysed in SPSS version 22 using Mann‐Whitney U test, Independent 2‐tailed t‐test or ANOVA with Bonferroni post hoc test comparing the average values of each experiment. Values of P < 0.05 were considered statistically significant.
3. RESULTS
The new technology enabled preparation of Oxy Water that contained a DO concentration significantly higher than control water (67 ± 3.6 mg/L for Oxy Water versus 15 ± 1.1 mg/L for control water, P = 0.014, Mann‐Whitney U test, Figure 1A). The oxygenated water was further used to develop Oxy Dressing, an in situ gelling formulation containing 15 to 16 wt % poloxamer 407 and a final DO concentration ranging between 25 and 35 mg/L. The Oxy Dressing was also formulated to a pH of 5.5 by using acetate buffer. The pH of the dressing formulated in acetate buffer was stable at approximately 5.5 for 3 months when stored at room temperature and under refrigerated conditions (5°C, Figure 1B). An oxygen optode was developed in order to measure the oxygen concentration and stability of the Oxy Dressing over time (Figure 2A). The average DO level of 2 of the measured Oxy Dressings remained stably above 22 mg/L for more than 30 hours when applied on a surface with controlled temperature at 35°C and covered with a glass lid (Figure 2B). One of the replicates had a lower initial DO concentration and dropped to 10 mg/L DO after approximately 7 hours. This was due to drying of the dressing, thus loss of oxygen. Oxy Dressing showed significantly lower gelling temperature in the range of 24.4°C to 24.8°C when compared with the Control dressing (P = 0.04) (Mann‐Whitney U test, Figure 2C). Gelling was induced in both the Oxy Dressing and the control dressing in the temperature range 24°C to 25°C, indicating that the dressing will form a gel after administration to the skin, having a temperature of >32°C. Further, the Oxy Dressing maintained stable DO levels above 30 mg/L when stored at 4°C, and above 20 mg/L when stored at room temperature (20°C) capped glass vials for 7 weeks (Figure 2D).
Figure 1.
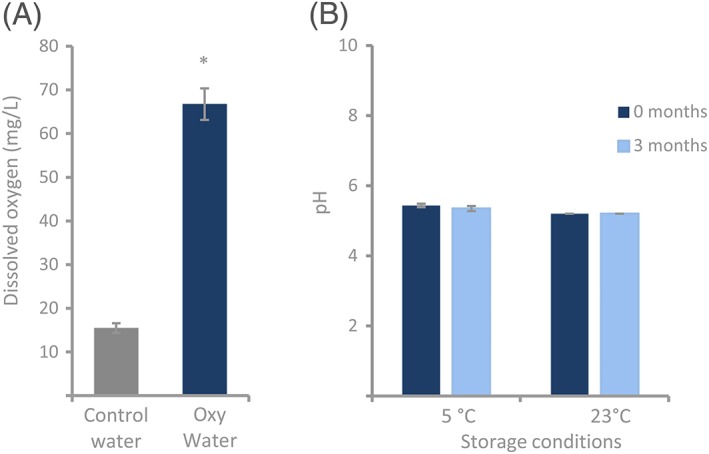
Development of Oxy Dressing. A, The new oxygenation technology enabled production of Oxy Water with a significantly higher level of DO compared with control water. Data are presented as average ± SD (n = 5, * P < 0.05, Mann‐Whitney U test). B, The pH value of poloxamer 407 formulated in 20 mM acetate buffer, stored at 5°C and 23°C for 3 months, was measured. The pH of 5.43 ± 0.05 was stable for 3 months in the Oxy Dressing formulated with 20 mM acetate buffer. Data are presented as average ± SD (n = 3 to 9)
Figure 2.
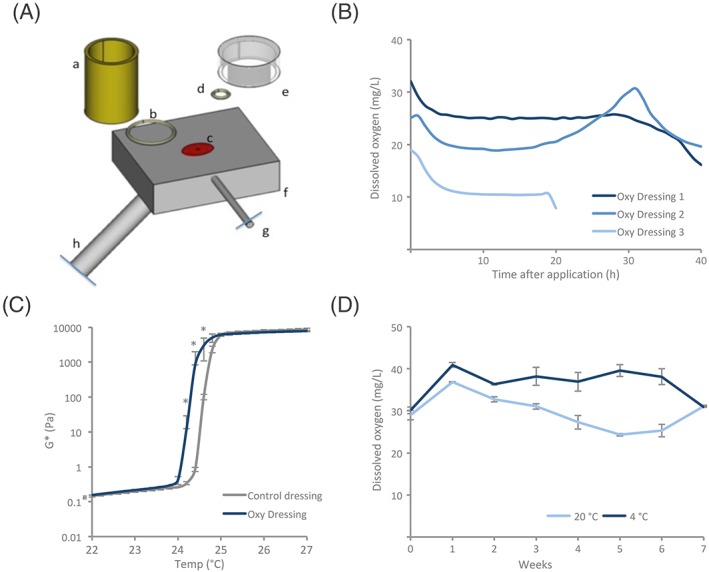
Characterisation of Oxy Dressing. A, Schematic figure of the oxygen optode setup for continuous determination of DO. The sample reservoir (a), seal (b), optode (c), seal for gels (d), lid for restricted diffusion (e), heating block (f), temperature probe (g), and fiber optic probe (h). B, Stability of DO after application to a 35°C surface was measured with the setup displayed in A (n = 3). Each replicate is shown separately. The average DO concentration in Oxy Dressing replicate 1 and 2 were kept above 22 mg/L for more than 30 hours at 35°C when covered. The third Oxy Dressing replicate had a lower initial DO concentration and dropped to 10 mg/L after approximately 7 hours. C, The influence of viscosity (G*: Shear modulus complex component) on increased temperature for poloxamer 407 formulated in 20 mM acetate buffer with either MilliQ (control dressing) or Oxy Water (Oxy Dressing). Significant gelling was induced at 24°C to 25°C, in both groups. The gelling temperature was significantly lower in the Oxy Dressing in the temperature interval 24.4°C to 24.8°C. Data are presented as average ± SD per 0.2°C (n = 2, * P < 0.05, Mann‐Whitney U test). D, DO was measured in Oxy Dressings after storage at room temperature (20°C) or in fridge (4°C) for 7 weeks. The Oxy Dressing maintained stable DO levels above 30 mg/L when stored at 4°C, and above 20 mg/L when stored at room temperature in capped glass vials for 7 weeks. Data are presented as average ± SD (n = 2 per time point and storage condition)
Investigation of basic effects of elevated levels of DO on human skin cells in vitro was also performed. As shown in Figure 3A, intracellular ATP levels were significantly higher in HSFs after 4 hours of hourly treatment with 33 mg/L DO (113.1 ± 10.1 nM ATP/105 live cells) compared with control (8 mg/L DO, 70.9 ± 20.5 nM ATP/105 live cells, P = 0.03, Independent 2‐tailed t‐test). ROS kinetics were studied at 15 minutes, 30 minutes, 1 hour, and 4 hours after a single stimulation of 32 mg/L DO, control (9 mg/L DO), or the positive control, H2O2 (Figure 3B). There were no significant differences between 32 mg/L DO and control at any of the time points measured. The ROS levels gradually decreased over time for both groups. The positive control had significantly higher and increasing ROS levels over the time period of 4 hours. ROS production was also studied after 4 hours with hourly re‐stimulation with 31 mg/L DO, control (8 mg/L DO), or the positive control, H2O2 (Figure 3C). The ROS level was higher in the HSF treated with 31 mg/L DO (14.3 ± 10.2 RFU ×103) when compared with control (7.3 ± 0.04 RFU ×103); however, the difference was not significant. The positive control (72.6 ± 14.5 RFU ×103) had significantly higher ROS levels than control. Increasing the DO concentration up to 50 mg/L for up to 4 days did not change cell proliferation of HSF (Figure 4A; 50 mg/L DO Day 4: 100 ± 16.1 cell number (× 103) or control (11 mg/L DO): 112 ± 11.3 cell number (× 103), P = 0.17, ANOVA with Bonferroni post hoc test). Similarly, it did not alter the level of cell viability (Figure 4B; 50 mg/L DO Day 4: 1 ± 2% dead cells of total cell number, control (11 mg/L DO): 2 ± 3.3% dead cells of total cell number (P = 0.65, ANOVA with Bonferroni post hoc test).
Figure 3.
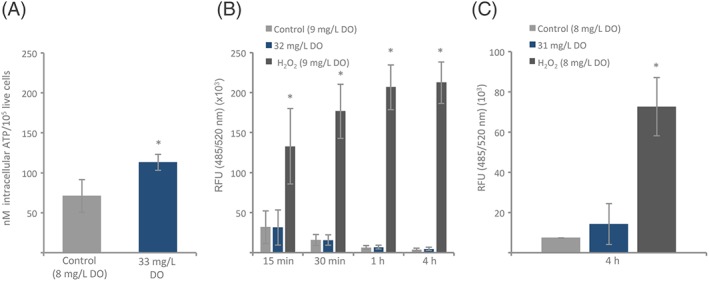
In vitro effects of dissolved oxygen (DO) on intracellular ATP and reactive oxygen species (ROS). A, Intracellular ATP levels were measured in HSFs incubated for 4 hours in DMEM with (33 mg/L) or without (8 mg/L) elevated levels of DO. The cells were restimulated every hour with the respective treatments. Cells treated with 33 mg/L DO showed significantly higher intracellular ATP levels than control (n = 3, *P < 0.05, independent 2‐tailed t‐test). B, ROS kinetics were measured in HSFs at 15 minutes, 30 minutes, 1 hour, and 4 hours, after incubation in 32 mg/L DO (DMEM w/10% FBS), control (DMEM w/10% FBS, 9 mg/L DO), or positive control (DMEM w/10% FBS, 9 mg/L DO, and 500‐μM H2O2). ROS levels are presented as relative fluorescence (RFU, 485/520 nm) × 103. There were no significant differences between the ROS production in the cells treated with 32 mg/L DO when compared with control. The positive control had significantly higher levels of ROS compared with control at all time points (n = 3, *P < 0.05, ANOVA with Bonferroni post hoc test). C, ROS was measured in HSF incubated for 4 hours in DMEM with 10% FBS with 31 mg/L or 8 mg/L DO. The cells were restimulated every hour with the respective treatments. Cells treated with 31 mg/L had increased ROS levels after 4 hours when compared with control, but the change was not significant. The positive control (DMEM w/10% FBS, 8 mg/L DO, and 500 μM H2O2) had significantly higher levels of ROS compared with control (n = 3, *P < 0.05, ANOVA with Bonferroni post hoc test)
Figure 4.
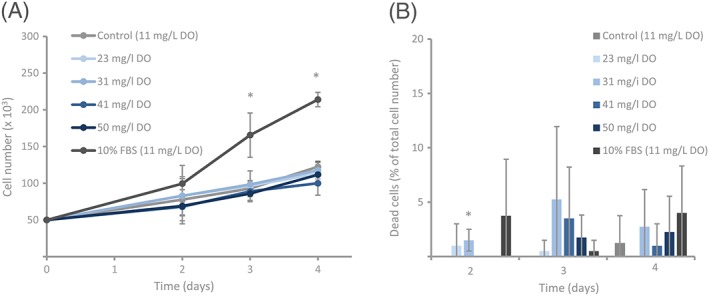
In vitro effect of dissolved oxygen (DO) on proliferation and cell death. Human skin fibroblasts (HSFs) were grown in DMEM with 1% FBS with (23‐50 mg/L) or without (11 mg/L) elevated levels of DO. DMEM with 10% FBS (11 mg/L) served as positive control. A) Treating HSFs with DO up to 50 mg/L (50 mg/L DO) did not significantly change the proliferation rate when compared with control. The positive control had significantly higher proliferation rate on day 3 and day 4 compared with control. Data are expressed as average ± SD (n = 4, *P < 0.05, ANOVA with Bonferroni post hoc test). B, Treating HSFs with DO up to 50 mg/L did not significantly alter the number of dead cells compared with control over the course of 4 days, with the exception of 31 mg/L at day 2. Triton X (not shown in figure) significantly increased the number of dead cells compared with control. Data are expressed as the percentage of dead cells relative to total cell number and represent the average ± SD (n = 4, *P < 0.05, ANOVA with Bonferroni post hoc test)
4. DISCUSSION
Wound oxygenation and pH modification are closely tied to successful wound healing.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 33, 34, 35, 36 Due to the importance of oxygen and pH in wound healing, the main objective of the current project was to develop and characterize a highly oxygenated topical dressing with the pH of intact skin and evaluate the effects of DO on human skin cells in vitro.
4.1. Topical wound healing dressing
The new oxygenation technology offers a new possibility of formulating a wound healing dressing that can topically introduce elevated, controlled, and stable levels of DO to the wound bed. Currently available oxygen dressings primarily contain oxygen in the form of oxygen gas or release oxygen via chemical or biochemical reactions.10, 30 For it to be available for the cells in the wounded tissue, the gas first needs to be dissolved in the surrounding liquid. For this reason, topical applied DO has been reported to penetrate skin more efficient than gaseous oxygen.29 Roe et al demonstrated in 2010 that topically applied DO penetrates through at least 700 μm of human skin.29 It had previously been shown that atmospheric oxygen gas supplies the outer 250 to 400 μm of human skin in vivo.40 By combining the oxygenated water with the thermo sensitive poloxamer 407, forming the Oxy Dressing, oxygen may be supplied more efficiently to the wound site compared with formulations administrating oxygen gas.29 Poloxamer 407 alone has, in addition, been shown to display wound healing properties, by significantly increasing wound closure, vascular density, collagen formation, and leukocyte infiltration, in an experimental model.41 Thus, the possible wound healing effects of the poloxamer may add to the advantages of increased DO concentration. Importantly, the concept of an in situ gelling formulation is advantageous in order to be able to administer the formulation to a wounded skin area without causing pain during application. Stability and shelf life studies show that the in situ gelling dressing can supply significantly elevated DO concentration for more than 30 hours when covered, and the high DO concentration is maintained also when stored.
Formulating the Oxy Dressing by using buffer may enable pH control at the wound site over time. While normal skin has a pH of approximately 5.5, chronic wounds have been shown to have a pH range between 7.2 and 8.9.33, 42 An acidic environment at the wound bed has been shown to suppress wound infection, increase antimicrobial activity, and enhance re‐epithelization and angiogenesis.33 A shift towards a more acidic milieu will also lead to an increased release of oxygen from hemoglobin.33 Thus, in light of the importance of pH to wound healing, lowering the pH can itself be favourable for the healing process,43 and represent an additional advantage to the wound‐healing properties of the Oxy Dressing.
Wound healing involves a dynamic series of events, and the different steps of healing require different oxygen concentrations and pH.42, 44 The technology presented in this study enables production of a dressing with various DO concentrations and pH values; thus, the formulation can be customized for different steps of wound healing and to each patient's needs.
4.2. In vitro effect of elevated DO levels
The effects of oxygen on wound healing are well established.1, 5, 8, 45, 46, 47, 48, 49, 50 Knowledge about the basic mechanisms, however, is still limited and requires further evaluation. Therefore, in vitro studies aiming to elucidate effects of DO on human skin cells were performed in this study.
Adequate oxygen supply is essential for proper cell function. A partial oxygen deficiency can result in impaired energy synthesis and ATP depletion.1, 5 Sufficient amount of oxygen is important for tissue healing, as reparative processes including synthesis, repair, and turnover of essential macromolecules, lead to increased energy demand.1, 5, 13 In vivo studies have shown enhanced healing, development of granulation tissue, and re‐epithelisation, when applying intracellular ATP delivery techniques.51, 52 In the current project, a significant increase in intracellular ATP levels in HSFs was observed when stimulating the cells with 33 mg/L DO. This indicates an advantageous effect of DO concentration on the intracellular energy level of human skin cells, possibly facilitating wound healing.
Traditionally, ROS have been viewed as damaging to cells, with a negative influence on wound healing.53 Research has shown, however, that ROS can also serve as cellular messengers to support healing processes.53, 54, 55 In the current project, no significant change in ROS kinetics was found in HSFs stimulated with elevated levels of DO up to 4 hours when compared with control (Figure 3B). When replicating the protocol used in the intracellular ATP experiment and stimulating the cells every hour up to 4 hours, the results showed a slight increase in ROS production in the elevated DO group when compared with control, but the change was not significant (Figure 3C). One may speculate if this minor increase in ROS can lead to the observed increased intracellular ATP levels.
Changes in the accessibility of oxygen, both hypoxia and hyperoxia, have been shown to influence cell proliferation. While hypoxia (< 1% O2 in the cell incubator) decreases cell proliferation in vitro,56, 57 increased oxygen availability has been reported to either increase or decrease cell proliferation, depending on the oxygen concentration, length of exposure, and number of treatments.58, 59, 60 Prolonged exposure to elevated levels of oxygen is known to be toxic.61, 62 In the current project, no significant change in cell proliferation or viability after treating HFSs with DO concentrations of up to 50 mg/L for 4 days was observed. These results indicate that treatment with DO concentrations up to 50 mg/L does not induce changes in cell proliferation or cell death.
5. CONCLUSION
The developed in situ gelling topical dressing has elevated and stable levels of DO and a pH of 5.5, both after application at the temperature of skin and during storage. Elevated accessibility of DO increased intracellular ATP levels without affecting the production of ROS, cell proliferation, or cell viability in vitro. The developed dressing is a new wound healing application that may facilitate healing by increasing DO delivery, modulating and controlling pH levels, and increasing energy availability. The technology enables production of dressings with various DO concentrations and pH, thus, the wound treatment can be optimized for each patient's needs. Further studies are, however, needed in order to elucidate the effect of the dressing on wound healing both in vitro and in vivo.
CONFLICT OF INTEREST
I.M., H.U., M.A.M., and C.H. are employees of Oxy Solutions AS. N.S., A.K., E.S., L.R., and H.B. are employees of RISE.
AUTHOR CONTRIBUTION
Conceptualization: CH, MAM.
Methodology and investigation: IM, HU, NS, AK, ES
Formal analysis: IM, HU, NS, ES, AK, HB, MAM, CH
Writing—original draft: IM, NS, LR, HB, MAM, CH
Writing—Review and editing: IM, NS, LR, MAM, CH
Funding acquisition: CH, HB
Project administration: CH
All authors have read and approved the manuscript.
ACKNOWLEDGEMENTS
Funding sources: Eurostars project E!10397 Innovative wound care, Norwegian Research Council, and Vinnova.
Moen I, Ugland H, Strömberg N, et al. Development of a novel in situ gelling skin dressing: Delivering high levels of dissolved oxygen at pH 5.5 Health Sci Rep. 2018;1:e57 10.1002/hsr2.57
REFERENCES
- 1. Howard MA, Asmis R, Evans KK, Mustoe TA. Oxygen and wound care: a review of current therapeutic modalities and future direction. Wound Repair Regen. 2013;21(4):503‐511. [DOI] [PubMed] [Google Scholar]
- 2. Rodriguez PG, Felix FN, Woodley DT, Shim EK. The role of oxygen in wound healing: a review of the literature. Dermatol Surg. 2008;34(9):1159‐1169. [DOI] [PubMed] [Google Scholar]
- 3. Gordillo GM, Hunt TK, Sen CK. Significance of oxygen therapeutics. Wound Repair Regen. 2003;11(5):393; author reply. [DOI] [PubMed] [Google Scholar]
- 4. Sen CK. Wound healing essentials: let there be oxygen. Wound Repair Regen. 2009;17(1):1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Babilas P. Oxygen in acute and chronic wound healing. Br J Dermatol. 2010;163(2):257‐268. [DOI] [PubMed] [Google Scholar]
- 6. Hunt TK, Ellison EC, Sen CK. Oxygen: at the foundation of wound healing—introduction. World J Surg. 2004;28(3):291‐293. [DOI] [PubMed] [Google Scholar]
- 7. Gottrup F. Oxygen in wound healing and infection. World J Surg. 2004;28(3):312‐315. [DOI] [PubMed] [Google Scholar]
- 8. Eisenbud DE. Oxygen in wound healing: nutrient, antibiotic, signaling molecule, and therapeutic agent. Clin Plast Surg. 2012;39(3):293‐310. [DOI] [PubMed] [Google Scholar]
- 9. Ladizinsky D, Roe D. New insights into oxygen therapy for wound healing. Wounds. 2010;22(12):294‐300. [PubMed] [Google Scholar]
- 10. Dissemond J, Kroger K, Storck M, Risse A, Engels P. Topical oxygen wound therapies for chronic wounds: a review. J Wound Care. 2015;24(2):53‐54, 6–60, 2–3. [DOI] [PubMed] [Google Scholar]
- 11. Knighton DR, Silver IA, Hunt TK. Regulation of wound‐healing angiogenesis‐effect of oxygen gradients and inspired oxygen concentration. Surgery. 1981;90(2):262‐270. [PubMed] [Google Scholar]
- 12. Hunt TK, Niinikoski J, Zederfeldt B. Role of oxygen in repair processes. Acta Chir Scand. 1972;138(2):109‐110. [PubMed] [Google Scholar]
- 13. Stephens FO, Hunt TK. Effect of changes in inspired oxygen and carbon dioxide tensions on wound tensile strength: an experimental study. Ann Surg. 1971;173(4):515‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jonsson K, Jensen JA, Goodson WH 3rd, et al. Tissue oxygenation, anemia, and perfusion in relation to wound healing in surgical patients. Ann Surg. 1991;214(5):605‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ishii Y, Miyanaga Y, Shimojo H, Ushida T, Tateishi T. Effects of hyperbaric oxygen on procollagen messenger RNA levels and collagen synthesis in the healing of rat tendon laceration. Tissue Eng. 1999;5(3):279‐286. [DOI] [PubMed] [Google Scholar]
- 16. Lo JF, Brennan M, Merchant Z, et al. Microfluidic wound bandage: localized oxygen modulation of collagen maturation. Wound Repair Regen. 2013;21(2):226‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davis SD, Cazzaniga AL, Ricotti C, et al. Topical oxygen emulsion. Arch Dermatol. 2007;143(10):1252‐1256. [DOI] [PubMed] [Google Scholar]
- 18. Allen DB, Maguire JJ, Mahdavian M, et al. Wound hypoxia and acidosis limit neutrophil bacterial killing mechanisms. Arch Surg. 1997;132(9):991‐996. [DOI] [PubMed] [Google Scholar]
- 19. Babior BM. Oxygen‐dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978;298(12):659‐668. [DOI] [PubMed] [Google Scholar]
- 20. Hopf HW, Hunt TK, West JM, et al. Wound tissue oxygen tension predicts the risk of wound infection in surgical patients. Arch Surg. 1997;132(9):997‐1004; discussion 5. [DOI] [PubMed] [Google Scholar]
- 21. Knighton DR, Halliday B, Hunt TK. Oxygen as an antibiotic. The effect of inspired oxygen on infection. Arch Surg. 1984;119(2):199‐204. [DOI] [PubMed] [Google Scholar]
- 22. Knighton DR, Halliday B, Hunt TK. Oxygen as an antibiotic. A comparison of the effects of inspired oxygen concentration and antibiotic administration on in vivo bacterial clearance. Arch Surg. 1986;121(2):191‐195. [DOI] [PubMed] [Google Scholar]
- 23. Gordillo GM, Sen CK. Revisiting the essential role of oxygen in wound healing. Am J Surg. 2003;186(3):259‐263. [DOI] [PubMed] [Google Scholar]
- 24. Gordillo GM, Roy S, Khanna S, et al. Topical oxygen therapy induces vascular endothelial growth factor expression and improves closure of clinically presented chronic wounds. Clin Exp Pharmacol Physiol. 2008;35(8):957‐964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blackman E, Moore C, Hyatt J, Railton R, Frye C. Topical wound oxygen therapy in the treatment of severe diabetic foot ulcers: a prospective controlled study. Ostomy Wound Manage. 2010;56(6):24‐31. [PubMed] [Google Scholar]
- 26. Driver VR, Yao M, Kantarci A, Gu G, Park N, Hasturk H. A prospective, randomized clinical study evaluating the effect of transdermal continuous oxygen therapy on biological processes and foot ulcer healing in persons with diabetes mellitus. Ostomy Wound Manage. 2013;59(11):19‐26. [PubMed] [Google Scholar]
- 27. Lairet KF, Baer D, Leas ML, Renz EM, Cancio LC. Evaluation of an oxygen‐diffusion dressing for accelerated healing of donor‐site wounds. J Burn Care Res. 2014;35(3):214‐218. [DOI] [PubMed] [Google Scholar]
- 28. Ivins N, Simmonds W, Turner A, Harding K. The use of an oxygenating hydrogel dressing in VLU. Wounds (UK). 2007;3(1):1‐5. [Google Scholar]
- 29. Roe DF, Gibbins BL, Ladizinsky DA. Topical dissolved oxygen penetrates skin: model and method. J Surg Res. 2010;159(1):e29‐e36. [DOI] [PubMed] [Google Scholar]
- 30. Zellner S, Manabat R, Roe DF. A dissolved oxygen dressing: a pilot study in an ischemic skin flap model. J Int Med Res. 2015;43(1):93‐103. [DOI] [PubMed] [Google Scholar]
- 31. Lafferty B, Wood L, Davis P. Improved care and reduced costs with advanced wound dressings. Wounds (UK). 2011;7(1):14‐23. [Google Scholar]
- 32. Kellar RS, Audet RG, Roe DF, Rheins LA, Draelos ZD. Topically delivered dissolved oxygen reduces inflammation and positively influences structural proteins in healthy intact human skin. J Cosmet Dermatol. 2013;12(2):86‐95. [DOI] [PubMed] [Google Scholar]
- 33. Percival SL, McCarty S, Hunt JA, Woods EJ. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair Regen. 2014;22(2):174‐186. [DOI] [PubMed] [Google Scholar]
- 34. Gethin G. The significance of surface pH in chronic wounds. Wounds UK. 2007;3(3):52‐55. [Google Scholar]
- 35. Leveen HH, Falk G, Borek B, et al. Chemical acidification of wounds. An adjuvant to healing and the unfavorable action of alkalinity and ammonia. Ann Surg. 1973;178(6):745‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kaufman T, Eichenlaub EH, Angel MF, Levin M, Futrell JW. Topical acidification promotes healing of experimental deep partial thickness skin burns: a randomized double‐blind preliminary study. Burns Incl Therm Inj. 1985;12(2):84‐90. [DOI] [PubMed] [Google Scholar]
- 37. Fonder MA, Lazarus GS, Cowan DA, Aronson‐Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol. 2008;58(2):185‐206. [DOI] [PubMed] [Google Scholar]
- 38. Escobar‐Chavez JJ, Lopez‐Cervantes M, Naik A, Kalia YN, Quintanar‐Guerrero D, Ganem‐Quintanar A. Applications of thermo‐reversible pluronic F‐127 gels in pharmaceutical formulations. J Pharm Pharm Sci. 2006;9(3):339‐358. [PubMed] [Google Scholar]
- 39. Devi DR, Sandhya P, Vedha Hari BN. Poloxamer: a novel functional molecule for drug delivery and gene therapy. J Pharm Sci & Res. 2013;5(8):159‐165. [Google Scholar]
- 40. Stucker M, Struk A, Altmeyer P, Herde M, Baumgartl H, Lubbers DW. The cutaneous uptake of atmospheric oxygen contributes significantly to the oxygen supply of human dermis and epidermis. J Physiol. 2002;538(Pt 3):985‐994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kant V, Gopal A, Kumar D, et al. Topical pluronic F‐127 gel application enhances cutaneous wound healing in rats. Acta Histochem. 2014;116(1):5‐13. [DOI] [PubMed] [Google Scholar]
- 42. Schneider LA, Korber A, Grabbe S, Dissemond J. Influence of pH on wound‐healing: a new perspective for wound‐therapy? Arch Dermatol Res. 2007;298(9):413‐420. [DOI] [PubMed] [Google Scholar]
- 43. Schneider LA, Wlaschek M, Dissemond J, Scharffetter‐Kochanek K. Examples for the importance of radiophysical measurements in clinical phototherapy. J Dtsch Dermatol Ges. 2007;5(5):384‐389. [DOI] [PubMed] [Google Scholar]
- 44. Sinno H, Prakash S. Complements and the wound healing cascade: an updated review. Plast Surg Int. 2013;2013(146764). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kranke P, Bennett M, Roeckl‐Wiedmann I, Debus S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2004;(2):CD004123. [DOI] [PubMed] [Google Scholar]
- 46. Hammarlund C, Sundberg T. Hyperbaric oxygen reduced size of chronic leg ulcers: a randomized double‐blind study. Plast Reconstr Surg. 1994;93(4):829‐833; discussion 34. [PubMed] [Google Scholar]
- 47. Kessler L, Bilbault P, Ortega F, et al. Hyperbaric oxygenation accelerates the healing rate of nonischemic chronic diabetic foot ulcers: a prospective randomized study. Diabetes Care. 2003;26(8):2378‐2382. [DOI] [PubMed] [Google Scholar]
- 48. Abidia A, Laden G, Kuhan G, et al. The role of hyperbaric oxygen therapy in ischaemic diabetic lower extremity ulcers: a double‐blind randomised‐controlled trial. Eur J Vasc Endovasc Surg. 2003;25(6):513‐518. [DOI] [PubMed] [Google Scholar]
- 49. Fries RB, Wallace WA, Roy S, et al. Dermal excisional wound healing in pigs following treatment with topically applied pure oxygen. Mutat Res. 2005;579(1–2):172‐181. [DOI] [PubMed] [Google Scholar]
- 50. Kalliainen LK, Gordillo GM, Schlanger R, Sen CK. Topical oxygen as an adjunct to wound healing: a clinical case series. Pathophysiology. 2003;9(2):81‐87. [DOI] [PubMed] [Google Scholar]
- 51. Chiang B, Essick E, Ehringer W, et al. Enhancing skin wound healing by direct delivery of intracellular adenosine triphosphate. Am J Surg. 2007;193(2):213‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang J, Wan R, Mo Y, Li M, Zhang Q, Chien S. Intracellular delivery of adenosine triphosphate enhanced healing process in full‐thickness skin wounds in diabetic rabbits. Am J Surg. 2010;199(6):823‐832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sen CK. The general case for redox control of wound repair. Wound Repair Regen. 2003;11(6):431‐438. [DOI] [PubMed] [Google Scholar]
- 54. Paty PB, Graeff RW, Mathes SJ, Hunt TK. Superoxide production by wound neutrophils. Evidence for increased activity of the NADPH oxidase. Arch Surg. 1990;125(1):65‐69. [DOI] [PubMed] [Google Scholar]
- 55. Shaked G, Alkan M, Nagauker O, Charuzi I, Levy R. Superoxide production by neutrophils from trauma patients: regulation of NADPH oxidase activity. J Trauma. 1994;37(1):22‐29. [DOI] [PubMed] [Google Scholar]
- 56. Oberringer M, Meins C, Bubel M, Pohlemann T. A new in vitro wound model based on the co‐culture of human dermal microvascular endothelial cells and human dermal fibroblasts. Biol Cell. 2007;99(4):197‐207. [DOI] [PubMed] [Google Scholar]
- 57. Vogler M, Vogel S, Krull S, et al. Hypoxia modulates fibroblastic architecture, adhesion and migration: a role for HIF‐1alpha in cofilin regulation and cytoplasmic actin distribution. PLoS one. 2013;8(7):e69128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dimitrijevich SD, Paranjape S, Wilson JR, Gracy RW, Mills JG. Effect of hyperbaric oxygen on human skin cells in culture and in human dermal and skin equivalents. Wound Repair Regen. 1999;7(1):53‐64. [DOI] [PubMed] [Google Scholar]
- 59. Saretzki G, Feng J, von Zglinicki T, Villeponteau B. Similar gene expression pattern in senescent and hyperoxic‐treated fibroblasts. J Gerontol a Biol Sci Med Sci. 1998;53(6):B438‐B442. [DOI] [PubMed] [Google Scholar]
- 60. Lin HI, Chu SJ, Perng WC, Wu CP, Lin ZY, Huang KL. Hyperbaric oxygen attenuates cell growth in skin fibroblasts cultured in a high‐glucose medium. Wound Repair Regen. 2008;16(4):513‐519. [DOI] [PubMed] [Google Scholar]
- 61. Smith JL. The pathological effects due to increase of oxygen tension in the air breathed. J Physiol. 1899;24(1):19‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bert P, Hitchcock MA, Hitchcock FA. Barometric Pressure. Columbus, Ohio: College Book Company; 1943. [Google Scholar]


