Abstract
The combination of increased referral for genetic testing and the current shortage of genetic counselors has necessitated the development and implementation of alternative models of genetic counseling and testing for hereditary cancer assessment. The purpose of this scoping review is to provide an overview of the patient outcomes that are associated with alternative models of genetic testing and genetic counseling for hereditary cancer, including germline-only and tumor testing models. Seven databases were searched, selecting studies that were: (1) full-text articles published ≥2007 or conference abstracts published ≥2015, and (2) assessing patient outcomes of an alternative model of genetic counseling or testing. A total of 79 publications were included for review and synthesis. Data-charting was completed using a data-charting form that was developed by the study team for this review. Seven alternative models were identified, including four models that involved a genetic counselor: telephone, telegenic, group, and embedded genetic counseling models; and three models that did not: mainstreaming, direct, and tumor-first genetic testing models. Overall, these models may be an acceptable alternative to traditional models on knowledge, patient satisfaction, psychosocial measures, and the uptake of genetic testing; however, particular populations may be better served by traditional in-person genetic counseling. As precision medicine initiatives continue to advance, institutions should consider the implementation of new models of genetic service delivery, utilizing a model that will best serve the needs of their unique patient populations.
Keywords: BRCA, genetic counseling, genetic testing, hereditary cancer, genetic service delivery, patient outcomes
1. Introduction
In just over five years, multiple events have increased the demand for genetic counseling (GC) and genetic testing (GT) for hereditary breast and ovarian cancer. In 2013, Angelina Jolie’s opinion editorial in the New York Times prompted an influx of patients requesting GT of the BRCA1 and BRCA2 genes that was so significant that it was dubbed the “Angelina Jolie effect” [1,2]. In 2014 and 2018, the FDA approved poly (ADP-ribose) polymerase (PARP) inhibitors for BRCA-associated breast and ovarian cancers, respectively, which again prompted an increase in GC referrals [3], only this time with some urgency, given that results are needed to inform treatment decisions. At the same time, there has been increased awareness of the high BRCA mutation rates [4,5,6], and historically low genetic GC/GT rates [7,8,9,10] among women with ovarian cancer. Multi-disciplinary meetings have been convened in the United States (USA) [11] and Canada [12] to define a list of priorities to ensure that BRCA GT is routinely performed for all women with high-grade ovarian cancer. Additionally, the National Cancer Institute developed a “Traceback” framework to identify and provide GT to previously unreferred ovarian cancer patients and their unaffected family members [13], and the National Institute of Health recently announced a funding opportunity to support pilot research projects using this approach to identify individuals with an elevated cancer risk [14]. Ultimately, continued increases in awareness and requests for GT will have positive outcomes for hereditary cancer patients and their family members; however, there is currently a shortage of genetic counselors working in direct patient care to provide the necessary GC services [15]. The growing issue of supply and demand for GT in the field of hereditary breast and ovarian cancer requires a rapid development and implementation of alternative models of genetic service delivery.
In the traditional GC model, a health-care provider refers a patient for GC, the patient is scheduled a separate in-person appointment with a genetic counselor for pre-test GC, and results are disclosed by a genetic counselor in person or through other means [16]. While comprehensive in nature, the traditional model is time-intensive [17,18], and cancer genetic counselors using this model see fewer patients and have longer wait times compared to those who employ other models of care [18]. Alternative models of GC and GT have been developed and implemented to improve patient access; however, there remains concern regarding the quality of GC, and few genetic counselors routinely employ these models [17,18]. It is evident that models of GT where cancer patients begin with tumor testing, rather than blood testing, will soon be commonplace. Similar to the universal tumor screening model that is used in Lynch syndrome, where immunohistochemistry/microsatellite instability testing is completed automatically as part of the pathology protocol, these models would help identify patients who would benefit from GC and GT while simultaneously increasing the efficiency of genetic services. While previous review articles have described various alternative models of GC, none have considered tumor testing as an alternative model [19,20,21,22,23,24,25,26]. The purpose of this review is to provide a single comprehensive overview of patient-reported outcomes for all alternative models of GC and GT, including tumor testing, which are currently utilized for hereditary cancer to serve as a resource for clinicians to identify and implement alternative models that are relevant to their clinical resources and patient population.
2. Methods
A scoping review was completed to capture a wide breath of studies. Scoping reviews are considered exploratory projects that systematically map the literature that is available on a topic, and summarize a range of evidence [27,28]. They are uniquely suited to disciplines such as GC, which have emerging evidence and where there is a paucity of randomized controlled trials [27]. The methodology that was used for this review was based on the framework outlined by Arksey and O’Malley [29] and recommendations made by Levac et al. [27]. The review included five key steps: (1) identifying the research question, (2) identifying relevant studies, (3) study selection, (4) charting the data, and (5) collating, summarizing, and reporting the results. The review did not complete the optional sixth step of completing a consultation exercise.
2.1. Identifying the Research Question
The review was anchored in the following question: “What are the alternative models of pre-test GC and GT that are used for hereditary cancer, and what is the patient impact of employing these alternative models of care?” For the purposes of this review, any model that did not involve a separately scheduled appointment for an in-person pre-test GC from a genetic counselor or medical geneticist was considered an alternative model of care.
2.2. Identifying Relevant Studies
To conduct a wide review, relevant studies were identified from seven databases: Medline, Epub Ahead of Print, Embase, PyschINFO, CINAHL, the Cochrane Database of Systematic Reviews, and the Cochrane Central Register of Controlled Trials. A librarian at the University Health Network was consulted to develop a search strategy (Appendix A). MeSH headings and keywords were used to combine cancer terms (including “Neoplasms”, “cancer”, “tumor”, “HBOC” (hereditary breast/ovarian cancer), “HNPCC”, “Lynch”) with genetic terms (including “Genetic Services”, “Genetic Counseling”, “Genetic Testing”, “germline test”, “molecular screen”, “universal screen”, “somatic test”) and patient outcomes terms (including “Patient Outcome Assessment”, “Patient Reported Outcome Measures”, “Patient Satisfaction”, Stress Psychological”, “access”). Results were limited to English articles published ≥2007 and conference abstracts published in ≥2015. An initial search conducted on 22 November 2017, and an updated search was completed on 6 June 2018. The search strategy identified a total of 14,993 articles for review. Citations were imported into EndNote citation software (Clarivate Analytics; Boston, MA, USA) for manual de-duplication. The resulting 10,270 articles were imported into a web-based citation screening platform, Covidence (Veritas Health Innovation Ltd.; Melbourne, AUS), where an additional 538 duplicates were removed, leaving a total of 9732 articles for review.
2.3. Study Selection
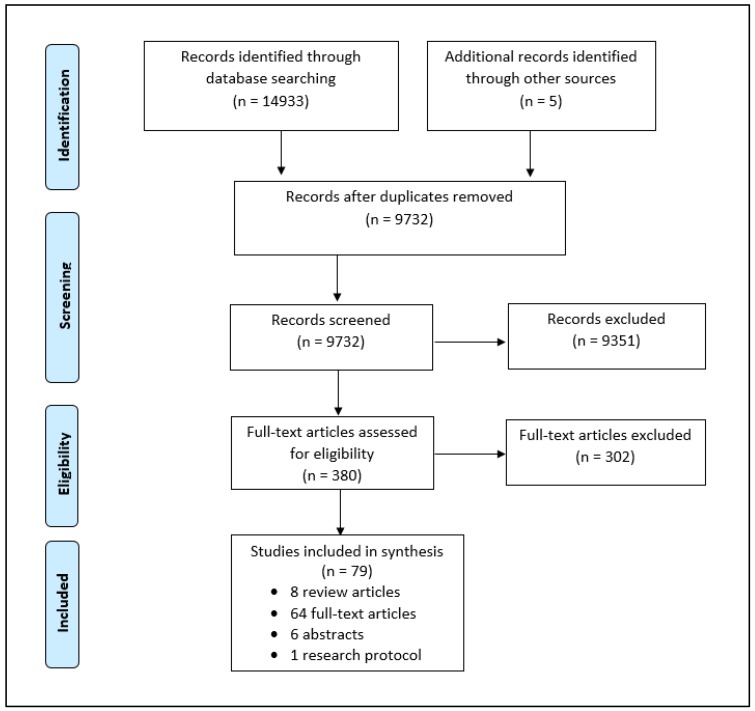
Inclusion and exclusion criteria were developed by the study team (Appendix B). Articles were included if they assessed the patient outcomes of adults receiving GT or GC for hereditary cancer using alternative models of care. Published protocols for ongoing studies and conference abstracts without full-text publications were also included. Due to limited resources, articles were excluded if they were not available in English. To ensure the relevancy of information, articles published <2007 and conference abstracts published <2015 were excluded. Additional exclusion criteria included studies involving individuals at a 50% risk of a hereditary cancer syndrome, direct to consumer GT, tumor GT for somatic targets only, GT for non-cancer indications, and articles that did not provide a direct assessment of patients’ experiences or outcomes. All of the articles were screened by the first author (J.M.M.) and one of three secondary reviewers (S.R.A., A.V., M.C.). All of the reviewers have at least five years of GC experience. Conflicts were resolved through discussion amongst reviewers. When a resolution could not be achieved, an independent third reviewer (K.A.M.) was consulted. The search strategy results identified 74 relevant publications. Screening the reference lists from eight selected review articles [19,20,21,22,23,30,31,32] identified an additional five articles, resulting in a total of 79 publications (Figure 1).
Figure 1.
Prisma Flow Diagram.
2.4. Charting the Data and Reporting Results
Data charting and extraction was completed by J.M.M. and recorded using a data extraction form. Extracted data included article title, authors, country, publication year, study population, details of GC/GT intervention, study design, and patient outcomes measured. Articles were grouped by invention type, and the data extraction form was used to summarize and report the results.
3. Results
A total of 79 publications (8 review articles, 6 conference abstracts, 1 protocol, and 64 full-text articles) describing 69 studies were included in this review. Five articles were qualitative in nature. Studies were grouped into seven categories based on the GC intervention (Table 1, Table 2, Table 3, Table 4, Table 5, Table 6 and Table 7), which can be dichotomized into two groups: those that involved a genetic counselor prior to GT (alternative GC models: telephone, telegenetic, group, and embedded GC models), and those that did not (alternative GT models: mainstreaming, direct GT, and tumor-first GT models). Among publications of alternative GC models, the most common outcomes that were examined were patient satisfaction (n = 16), psychosocial outcomes (n = 13), knowledge (n = 13), and uptake of GT (n = 13). Of those reporting on alternative GT models, the most common outcomes discussed were the uptake of GT (n = 25), psychosocial outcomes (n = 19), attendance at GC (n = 17), and referral for GC (n = 16). While not a specific outcome of interest for this review, a cost reduction was noted in the telephone (n = 1) telegenetic (n = 1), group (n = 2), mainstreaming (n = 1), and direct GT (n = 2) models. Detailed information about each model is provided in the following sub-sections.
Table 1.
Telephone genetic counseling models.
| Study | Country | Population | Selected Outcomes: GC Referral Rates [A], GC Attendance [B], GT Uptake [C], Wait Time [D], GC Time [E], Satisfaction [F], Knowledge [G], Psychosocial [H], Health Behaviors [I], Patient Preferences [J] |
|---|---|---|---|
| Pal et al. (2010) [33] | USA | African American women with BC ≤ 50 receiving telephone GC with tailored counseling aid (n = 37) |
|
| Sutphen et al. (2010) [34] | USA | Health insurance company employees receiving telephone GC after screening positive for HBOC risk (n = 39) |
|
| Kinney et al. (2014) [35] * | USA | Randomized trial of rural and urban women aged 25–74 years with a personal/family history suggestive of HBOC who had telephone (n = 437) or traditional (n = 464) GC |
|
| Schwartz et al. (2014) [36] ** | USA | Randomized trial of women aged 21–85 years with ≥10% risk of a BRCA mutation who had telephone (n = 298) or traditional (n = 302) GC |
|
| Butrick et al. (2015) [37] ** | USA | Randomized trial of women aged 21–85 years with ≥10% risk of a BRCA mutation who had telephone (n = 298) or traditional (n = 302) GC |
|
| Peshkin et al. (2015) [38] ** | USA | Randomized trial of women aged 21–85 years with ≥10% risk of a BRCA mutation who had telephone (n = 272) or traditional (n = 282) GC |
|
| Kinney et al. (2016) [39] * | USA | Randomized trial of rural and urban women aged 25–74 years with a personal or family history suggestive of HBOC who had telephone (n = 409) or traditional (n = 383) GC |
|
| Steffen et al. (2017) [40] * | USA | Randomized trial of rural and urban women aged 25–74 years with known mutation status who had telephone (n = 402) or traditional (n = 379) GC for HBOC |
|
GC = genetic counseling; GT = genetic testing; OC = ovarian cancer; BC = breast cancer; HBOC = hereditary breast/ovarian cancer * publications from the same study; ** publications from the same study.
Table 2.
Telegenetic genetic counseling models.
| Study | Country | Population | Selected Outcomes: GC Referral Rates [A], GC Attendance [B], GT Uptake [C], Wait Time [D], GC Time [E], Satisfaction [F], Knowledge [G], Psychosocial [H], Health Behaviors [I], Patient Preferences [J] |
|---|---|---|---|
| d’Agincourt-Canning et al. (2008) [41] | CAN | Patients (n = 43) and family members (n = 5) having telegenetic GC for hereditary cancer |
|
| Zilliacus et al. (2010) [42] * | AUS | Qualitative study of women who received GC for HBOC, where a genetic physician provided telegenic GC with a local genetic counselor present in-person (n = 12) |
|
| Zilliacus et al. (2011) [43] * | AUS | Patients receiving traditional (n = 89) or telegenetic (n = 106) GC for HBOC, where a genetic physician provided telegenic GC with a local genetic counselor present in-person |
|
| Meropol et al. (2011) [44] | USA | Patients and families receiving telegenetic GC for hereditary CRC or HBOC (n = 31) |
|
| Buchanan et al. (2015) [45] | USA | Randomized trial of individuals receiving telegenetic (n = 59) or traditional (n = 71) GC for hereditary cancer risk. |
|
| Bradbury et al. (2016) [46] | USA | Patients >20 years eligible for GT (HBOC or CRC) receiving telegenic GC (n = 61) |
|
| Mette et al. (2016) [47] | USA | Patients receiving telegenetic (n = 56) or traditional (n = 63) GC for hereditary cancer risk |
|
| Solomons et al. (2018) [48] | USA | New patients seen by genetics for personal/family history of cancer receiving telegenetic (n = 90) or traditional (n = 68) GC |
|
GC = genetic counseling; GT = genetic testing; OC = ovarian cancer; BC = breast cancer; CRC = colorectal cancer; HBOC = hereditary breast/ovarian cancer. *publications from the same study.
Table 3.
Group genetic counseling models.
| Study | Country | Population | Selected Outcomes: GC Referral Rates [A], GC Attendance [B], GT Uptake [C], Wait Time [D], GC Time [E], Satisfaction [F], Knowledge [G], Psychosocial [H], Health Behaviors [I], Patient Preferences [J] |
|---|---|---|---|
| Mangerich et al. (2008) [49] | USA | Individuals interested in BRCA GT (n = 15; 6 with BC) attending a group education class |
|
| Ridge et al. (2009) [50] | CAN | Women offered appointments for GC, including: group GC (n = 42), traditional GC (n = 37), OC patients receiving group GC (n = 10) |
|
| Rothwell et al. (2012) [51] | USA | Women with or at high risk of BC/OC receiving group (n = 17) or traditional (n = 32) GC |
|
| Manchanda et al. (2016) [52] | UK | Randomized trial of Ashkenazi Jewish men/women without previous BRCA testing receiving group DVD (n = 409) or traditional (n = 527) GC |
|
| Benusiglio et al. (2017) [53] | FRA | BC and OC patients eligible for BRCA GT receiving group (n = 210) or traditional (n = 47) GC |
|
| Wiesman et al. (2017) [54] | USA | Ashkenazi Jewish men/women at low risk of a BRCA mutation receiving group GC (n = 88) |
|
GC = genetic counseling; GT = genetic testing; OC = ovarian cancer; BC = breast cancer.
Table 4.
Genetics embedded models.
| Study | Country | Population | Selected Outcomes: GC Referral Rates [A], GC Attendance [B], GT Uptake [C], Wait Time [D], GC Time [E], Satisfaction [F], Knowledge [G], Psychosocial [H], Health Behaviors [I], Patient Preferences [J] |
|---|---|---|---|
| Kentwell et al. (2017) [55] | AUS | Non-mucinous OC patients diagnosed <70 years before (n = 134) and after (n = 99) the implementation of embedded GC |
|
| Senter et al. (2017) [56] | USA | Newly diagnosed OC patients before (n = 401) and after (n = 336) the implementation of embedded GC |
|
| Bednar et al. (2017) [57] * | USA | OC patients (n = 1636) seen after implementation of embedded GC, mainstreaming GT, and GC-assisted referral processes |
|
| Pederson et al. (2018) [58] | USA | Newly diagnosed BC patients before (n = 471) and after (n = 440) implementation of embedded GC |
|
GC = genetic counseling; GT = genetic testing; OC = ovarian cancer; * outcomes are the result of multiple methods including embedded GC.
Table 5.
Mainstreaming genetic testing models.
| Study | Country | Population | Selected Outcomes: GC Referral Rates [A], GC Attendance [B], GT Uptake [C], Wait Time [D], GC Time [E], Satisfaction [F], Knowledge [G], Psychosocial [H], Health Behaviors [I], Patient Preferences [J] |
|---|---|---|---|
| George et al. (2016) [59] | UK | OC patients (n = 207) receiving BRCA GT via their oncology team |
|
| Bednar et al. (2017) [57] | USA | OC patients (n = 197) seen at regional oncology clinic |
|
| Yoon et al. (2017) [60] | MAL | Conference abstract: OC patients (n = 208) receiving BRCA GT via their oncology team |
|
| Colombo et al. (2018) [61] | USAITAESP | OC patients (n = 634) receiving BRCA GT via their oncology team |
|
| Rahman et al. (2018) [62] | UK | OC patients (n = 122) receiving BRCA GT via oncology team |
|
GC = genetic counseling; GT = genetic testing; OC = ovarian cancer; VUS = variant of uncertain significance.
Table 6.
Direct genetic testing models.
| Study | Country | Population | Selected Outcomes: GC Referral Rates [A], GC Attendance [B], GT Uptake [C], Wait Time [D], GC Time [E], Satisfaction [F], Knowledge [G], Psychosocial [H], Health Behaviors [I], Patient Preferences [J] |
|---|---|---|---|
| Brierley et al. (2010) [63] | USA | Series of cases without pre-test GC (n = 21) |
|
| Metcalfe et al. (2010) [64] * | CAN | Ashkenazi Jewish women aged 25–80 years (n = 1516) pursuing BRCA GT using the direct GT model with written information |
|
| Metcalfe et al. (2012) [65] * | CAN | Ashkenazi Jewish women aged 25–80 years identified to have a BRCA mutation via direct GT with written information (n = 19) |
|
| Pal et al. (2014) [66] | USA | Women in the Inherited Cancer Registry database with BRCA mutation (n = 438) |
|
| Armstrong et al. (2015) [67] | USA | Women who had BRCA GT with (n = 1334) or without (n = 2247) pre-test GC |
|
| Sie et al. (2014) [68] # | NED | Women with BC referred for BRCA GT electing to have direct GT (n = 95) or pre-test GC (n = 66) |
|
| Sie et al. (2016) [69] # | NED | Women with BC referred for BRCA GT electing to have direct GT (n = 59, incl 5 BRCA+) or pre-test GC (n = 49, incl 1 BRCA+) |
|
| Plaskocinska et al. (2016) [70] ## | UK | Women with a recent (<12 months) diagnosis of OC (n = 173) who had direct BRCA GT |
|
| Shipman et al. (2017) [71] ## | UK | Qualitative Study of women diagnosed with OC in last 12 months with positive (n = 4), negative (n = 5) and inconclusive (n = 3) GT results from direct BRCA GT |
|
| Meiser et al. (2016) [72] ^ | AUS | Qualitative Study of women aged 18–49 years with BC who received BRCA GT with pre-test GC or direct GT with written information who were BRCA+/fhx+ (n = 5) BRCA+/fhx− (n = 5), BRCA−/fhx+ (n = 5), or BRCA−/fhx− (n = 5) |
|
| Quinn et al. (2017) [73] ^ | AUS | Women aged 18–49 years with BC who received treatment focused BRCA GT with pre-test GC (n = 70) or direct GT with written information (n = 65) |
|
| Høberg-Vetti et al. (2016) [74] ^^ | NOR | Women offered direct BRCA GT with written information after a new diagnosis of BC (n = 893) or OC (n = 122) |
|
| Augestad et al. (2017) [75] ^^ | NOR | Qualitative study of women newly diagnosed with BC (n = 13) or OC (n = 4) who received direct BRCA GT with written information |
|
| Lieberman et al. (2017a) [76] ** | ISR | Ashkenazi Jewish individuals aged ≥25 years who were self- (n = 744) or recruiter (n = 1027) enrolled for direct BRCA GT with written information |
|
| Lieberman et al. (2017b) [77] ** | ISR | Qualitative Study of Ashkenazi Jewish individuals ≥25 who were BRCA+ (n = 26) or BRCA− (n = 10) after direct GT with written information |
|
GC = genetic counseling; GT = genetic testing; BC = breast cancer; OC = ovarian cancer; fhx = family history; * publications from the same study, ** publications from the same study; ^ publications from the same study, ^^ publications from the same study; # publications from the same study, ## publications from the same study.
Table 7.
Tumor-first genetic testing models.
| Study | Country | Population | Selected Outcomes: GC Referral Rates [A], GC Attendance [B], GT Uptake [C], Wait Time [D], GC Time [E], Satisfaction [F], Knowledge [G], Psychosocial [H], Health Behaviors [I], Patient Preferences [J] |
|---|---|---|---|
| Studies of tumor screening for Lynch Syndrome: | |||
| Landsbergen et al. (2012) [78] | NED | Recently diagnosed CRC < 50 OR second CRC < 70 years old (n = 400) who had tumor screening. |
|
| Heald et al. (2013) [79] | USA | CRC where universal screening results went only to the surgeon (n = 237), to the surgeon and genetics (n = 87), and to the surgeon and genetics with a genetic counselor contacting the patient (n = 784) |
|
| Marquez et al. (2013) [80] | USA | Universal tumor screening of CRC ≤ 70 years old (n = 129) |
|
| Moline et al. (2013) [81] | USA | Universal tumor screening of EC patients (n = 245) |
|
| Ward et al. (2013) [82] | AUS | CRC patients with mismatch repair deficient tumors (n = 245) |
|
| Batte et al. (2014) [83] | USA | Universal screening of unselected EC (retrospective = 408; prospective = 206) |
|
| Hall et al. (2014) [84] | USA | Consecutive CRC and EC patients who had reliable internet access (n = 66) whose tumor screening results were disclosed directly via their electronic medical record |
|
| Frolova et al. (2015) [85] | USA | EC before (n = 395) and after (n = 242) the implementation of universal tumor screening |
|
| Kidambi et al. (2015) [86] | USA | CRC in selected (<50 or <60 with features of Lynch syndrome; n = 107) and universal screening groups (n = 285) |
|
| Hunter et al. (2015) [87] | USA | CRC patients undergoing universal tumor screening (n = 145) |
|
| Goverde et al. (2016) [88] | NED | Consecutive series of EC patients ≤70 years (n = 179) having tumor screening |
|
| Brennan et al. (2017) [89] | AUS | Consecutive series of CRC patients (n = 1612) having tumor screening |
|
| Holter et al. (2017) [90] | CAN | Conference abstract: CRC cancer patients <60 years (n = 502) undergoing tumor screening |
|
| Hunter et al. (2017) [91] | USA | Newly diagnosed CRC patients (n = 189) undergoing tumor screening |
|
| Kupfer et al. (2017) [92] | USA | Conference abstract: CRC in White (n = 266) African American (n = 174), and Hispanic (n = 125) patients having tumor screening |
|
| Livi et al. (2017) [93] | ITA | Conference abstract: Consecutive EC patients (n = 166) having tumor screening |
|
| Najdawi et al. (2017) [94] | AUS | Patients with EC (any histology) and endometroid or clear cell gynecological cancer (n = 124) having tumor screening |
|
| O’Kane et al. (2017) [95] | IRL | CRC patients having tumor screening at one of three centers (n = 3906) |
|
| Patel et al. (2017) [96] | USA | Conference abstract: Consecutive CRC patients (n = 1597) having tumor screening |
|
| Watkins et al. (2017) [97] | USA | EC patients (n = 242) having tumor screening |
|
| Martin et al. (2018) [98] | USA | Newly diagnosed CRC patients (n = 78) having tumor screening |
|
| Metcalfe et al. (2018) [99] | USA | Consecutive upper tract urothelial cancer patients (n = 115) having tumor screening |
|
| Miesfeldt et al. (2018) [100] | USA | CRC (n = 175) or EC (n = 276) patients where results were sent to a surgeon, patient navigator, or both |
|
| Studies of tumor genetic testing | |||
| Gray et al. (2016) [101] | USA | Patients with stage IV lung or CRC (n = 167) enrolled in a tumor testing study |
|
| Pinheiro et al. (2017) [102] | USA | Cancer patients being offered or receiving tumor results (n = 66) |
|
| Best et al. (2018) [103] | AUS | Patients with advanced solid tumors participating in a molecular tumor screening study (n > 369) |
|
GC = genetic counseling; GT = genetic testing; CRC = colorectal cancer; EC = endometrial cancer.
3.1. Telephone Genetic Counseling Models
Telephone GC models involve the provision of GC completely by telephone, and may involve mailed resources provided to patients in advance of their appointments. Eight articles were identified, representing four separate studies conducted in the USA (Table 1) [33,34,35,36,37,38,39,40]. All eight studies involved GC for hereditary breast/ovarian cancer. Two articles described patient experience with telephone GC without comparison to traditional GC, and the remaining six articles reported results from two randomized non-inferiority trials comparing telephone and traditional GC models. All of the studies demonstrated improved patient knowledge following telephone GC. Both non-inferiority trials demonstrated telephone GC to be non-inferior to traditional GC on knowledge, psychosocial, and counseling measures [35,36,39]. The Schwartz trial also noted a cost benefit of telephone GC by comparing estimates of patient, clinician, testing, and overhead costs [36]. In assessments of patient satisfaction, those receiving telephone GC were more likely to report that GC was convenient; however, they were less likely to report having no difficulty in maintaining attention or perceiving a high level of emotional support/recognition [38]. Most women were very satisfied with telephone GC; however, these results may be biased, as one-third of patients declined participation in the trial, citing a preference for in-person GC [36]. Unfortunately, both non-inferiority trials reported lower, non-equivalent rates of GT in the telephone group. Follow-up studies suggest that ethnicity, cancer-specific distress, and perceived mutation risk may moderate patient decisions to pursue GT, where ethnic minorities and patients with higher levels of distress or perceived risk were less likely to pursue GT after telephone, rather than traditional GC [37,40].
3.2. Telegenetic Genetic Counseling Models
In telegenetic GC models, GC is provided remotely via videoconference. Eight articles were identified, representing one Australian study with separate publications for quantitative and qualitative analyses, and six North American studies (Table 2) [41,42,43,44,45,46,47,48]. All eight studies involved GC for hereditary cancer, two of which focused on breast/ovarian cancer. In contrast to other studies where genetic counselors and/or geneticists are located remotely, the Australian telegenetic GC model involved a telegenetic appointment with a remote geneticist and a local genetic counselor [42,43]. Qualitative analyses suggest that patients are satisfied with telegenetic GC, but this model may be best suited for patients seeking information rather than emotional support [42]. Quantitative analyses comparing traditional and telegenetic GC models observed no significant differences in knowledge, satisfaction, or measures of anxiety and depression; however, telegenetic GC was significantly better at meeting patients’ expectations and promoting perceived personal control [43]. Only 7% of patients receiving telegenetic GC would have preferred a face-to-face appointment [43]. Among North American studies, three assessed telegenetic GC alone [41,44,46] and three compared telegenetic GC to traditional GC, including one randomized trial [45,47,48]. Knowledge significantly increased after telegenic GC [46,48]. In three studies assessing psychosocial outcomes, levels of anxiety, depression, and worry about developing cancer, all decreased after telegenetic GC [46,47,48]. All six North American studies reported high levels of patient satisfaction with telegenetic GC; however, a third of patients would have preferred in-person GC [44,45,48], and almost a quarter of patients in one study declined a telegenetic appointment, preferring to schedule an in-person appointment [41]. Buchanan et al. also reported lower attendance rates for telegenic compared to in-person GC appointments [45]. One study demonstrated telegenetic counseling to be a cost-effective alternative to outreach GC by comparing the total cost of telegenic GC (GC service + telegenetic set-up and support) to the total cost of outreach GC (GC service + travel time + mileage) [45]. In other centers, the travel costs may be borne by patients who are required to travel to the genetics clinic.
3.3. Group Genetic Counseling Models
Group GC models involve an informative group session that may or may not be followed by brief one-on-one GC. Six articles were identified, including four North American studies and two European studies (Table 3) [49,50,51,52,53,54]. All six used group GC models for hereditary breast and ovarian cancer, two of which were limited to Ashkenazi Jewish individuals [52,54]. Four articles compared group GC to traditional GC [50,51,52,53]. Importantly, a non-inferiority trial assessing the use of a group DVD GC session followed by individualized GC demonstrated group GC to be non-inferior with respect to knowledge, risk perception, and GC satisfaction measures [52]. Three additional studies assessed knowledge scores [49,51,53], and only Rothwell et al. did not observe significantly improved knowledge scores; however, this was attributed to high baseline knowledge scores [51]. Rothwell et al. also assessed psychosocial outcomes, and found that both traditional and group GC increased perceived personal control, decreased cancer-specific distress, and improved depression, with no significant differences between the two groups [51]. All six studies reported high levels of patient satisfaction with group GC. Two studies found that many women prefer individual to group GC [50,51]; however, in a retrospective study of low-risk Ashkenazi Jewish individuals, only 8% reported a preference for traditional GC [54]. Time was assessed in five studies [49,50,51,52,53], and cost was assessed in two [49,52]. In studies providing direct comparisons to a traditional GC model, notable time-saving was reported for the group GC model [51,52,53]. Two studies extrapolated the GC time to demonstrate a cost benefit to the group GC [49,52]. Two studies examining the uptake of GT reported discrepant results, with Rothwell et al. reporting a significantly lower uptake after group GC [51], and Manchanda et al. reporting equivalent GT rates [52].
3.4. Embedded Genetic Counseling Models
In the embedded GC model, genetic counselors are integrated into oncology clinics, where genetic counselors provide genetic counseling and facilitate genetic testing during oncology visits. Additional roles may also include genetic risk assessment, the identification of appropriate genetic referral, referral triage, and clinician education. One Australian and three American studies were identified (Table 4) [55,56,57,58]. All four studies reported improvements in GC referral rates for breast or ovarian cancer patients. While Senter et al. reported an overall increase in GC referral and patient follow-through, the highest rates were recorded when genetic counselors and oncologists were in the same oncology clinic on the same day [56]. In addition to improved referral rates, all of the studies noted improved efficiencies, such as reduced patient wait times for GC, and shorter GC sessions. Importantly, Pederson reported that the reduced wait times increased the likelihood for patients to have GT prior to breast surgery, resulting in a decrease in the time to treat breast cancer patients [58].
3.5. “Mainstreaming” Genetic Testing Models
Popularized by the Mainstreaming Cancer Genetics Program in the United Kingdom (UK) [59], mainstreaming GT models engage non-genetics clinicians to order GT, typically with support from genetic clinicians. Referrals to clinical genetic services are processed in the event of positive or inconclusive GT results. This model has also been referred to as a Triage GC model [16]. Five publications were identified: two from the UK, one from the USA, one from an international study, and one abstract from a Malaysian study (Table 5) [57,59,60,61,62]. All five studies assessed GT of the BRCA1 and BRCA2 genes. Patient satisfaction with the mainstreaming model was high, and patients were glad to have had GT organized during their oncology visits [59,61]. The uptake of GT with this model was high, potentially improving access to GT. In their study, Bednar et al. reported that over half of ovarian cancer patients at a regional clinic in the USA received GT via their physician, many of whom may not have otherwise had access to GT [57]. Following positive results, the rates of GC referral [59,62] and patient attendance for GC [62] were high; however, Rahman et al. reported that only 22% for women with a variant of uncertain significance were referred for GC [62]. Considering the reduced number of GC appointments required in the mainstreamed GC model, one study estimated that this model provides a fourfold reduction in patient wait times and 13-fold reduction in health-care costs [59]. Yoon et al. was the only study using a validated tool to assess the psychosocial impact of mainstreamed GT. While distress levels decreased after post-test GC, 17% of patients still required additional support. Distress may be attributed to their cancer diagnosis, rather than GT results, as most had distress related to living with cancer, and about half frequently worried about getting cancer again [60].
3.6. Direct Genetic Testing Models
In direct GT models, patients are offered GT with limited to no pre-test discussion. Written documents or other resources may be provided in lieu of GC. Fifteen articles, representing nine studies from six countries, were identified (Table 6) [63,64,65,66,67,68,69,70,71,72,73,74,75,76,77]. Although not in the context of a research study, Brierly et al. explored a series of cases where pre-test GC was not provided by genetics professionals, and cautioned of the negative outcomes of this model, including unnecessary prophylactic surgeries, unnecessary testing, psychosocial distress, and false reassurance resulting in inappropriate medical management [63]. These concerns were not evident in the results of the 14 remaining articles. All 14 articles involved GT of the BRCA1 and BRCA2 genes.
Two studies (four articles) focused on providing direct GT to individuals of Ashkenazi Jewish descent, including one qualitative study [64,65,76,77]. Satisfaction with the direct GT model was high among BRCA mutation carriers and non-carriers, and the majority would recommend this model of GT to others [64,76]. All of the women who were identified to have a BRCA mutation attended an in-person post-test GC [64,76], and compliance with high-risk screening and risk-reducing surgeries was high [65]. With respect to psychosocial measures, levels of cancer-specific distress following GT were significantly higher among BRCA mutation carriers [64,76]; however, in a two-year follow-up study of BRCA mutation carriers, Metcalfe et al. found that levels of distress decreased significantly, especially among women who pursued risk-reducing surgeries [65]. With respect to knowledge, cancer genetics knowledge levels increased only among those who received in-person post-test GC (BRCA carriers and non-carriers with a family history) [76]. Two additional studies, which were not limited to the Ashkenazi Jewish population, included women with and without a personal diagnosis of cancer [66,67]. BRCA mutation carriers reported longer GC sessions and a higher uptake of risk-reducing mastectomy or breast MRI when counseling was provided by a genetic professional [66]. Reported levels of knowledge and satisfaction were higher among those who reported having pre-test GC with a genetic counselor [67].
The remaining four studies (eight publications) included women diagnosed with ovarian cancer [70,71], breast cancer [68,69,72,73], or both [74,75]; qualitative and quantitative analyses were published for each patient group. The qualitative data suggested that the impact of GT was minimal in comparison to a cancer diagnosis [71,72,75]; however, some women still reported a preference for discussing GT with a health-care provider prior to GT [75]. The decision to pursue GT was often motivated by altruistic factors [71,75] and to inform treatment [72]. Among five quantitative publications, two involved patients receiving direct GT [70,74], and three compared direct GT to the traditional GC model, including a randomized non-inferiority trial [68,69,72,73]. Congruent with the qualitative data, the quantitative data demonstrated that levels of distress, depression, and anxiety were lower in response to GT than in response to an ovarian cancer diagnosis [70]. The average level of depression and anxiety among newly diagnosed breast/ovarian cancer patients pursuing GT was comparable to that of breast and gynecologic cancer patients in general [74]. The results of the non-inferiority trial demonstrated that the direct GT model was non-inferior to the traditional GC model on measures of decisional conflict about GT, knowledge, and psychosocial measures [73]. Patient satisfaction and acceptability of the direct GT model was high. In a patient preference study, no differences in the quality of life, breast cancer worry, or risk perceptions were noted in women who chose direct GT or traditional GC; however, levels of decisional conflict were higher, and the uptake of GT was lower, among women who chose traditional GC [68,69]. Considering the costs associated with GC services, two studies reported a cost benefit of direct GT models, as compared to traditional GC [70,73].
3.7. Tumor-First Genetic Testing Models
In tumor-first GC models, genetic screening is first performed on a sample of tumor tissue, often as part of a pathology workflow, with GC offered based on the tumor results. A total of 26 publications from six countries that reviewed patient outcomes of tumor testing were identified (Table 7) [78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103]. In total, 23 publications [78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100], including four abstracts [90,92,93,96], assessed patient outcomes of immunohistochemistry and/or the microsatellite instability testing of tumor tissue as an initial screening test for Lynch syndrome. Ten out of 15 studies reporting the rate of GC referral following abnormal tumor screening for Lynch syndrome reported a 100% GC referral rate [79,80,85,86,88,90,94,96,99,100]; however, one study reported an 18% referral rate [82]. Referral rates increased when a genetic counselor or navigator was directly involved in the screening protocol, and when patients had additional features suggestive of Lynch syndrome [79,82,86,100]. In a study assessing the role of ethnicity, Kupfer et al. reported high rates of GC referral and GT in Caucasian patients [92]. A total of 14 studies reported the uptake of GC among patients who screened positive for Lynch syndrome [79,80,81,82,83,85,86,88,89,90,95,96,99,100]. The majority of patients who were referred for GC attended an appointment. Three studies reported low GC rates (30–42%); however, it is unclear whether patients had been contacted [83] or referred [89,100]. Of patients who attended GC, >70% pursued GT [79,80,81,82,83,85,88,90,93,96,97,98,100]. Four studies reported on the psychosocial impacts of tumor GT [78,84,87,91]. In a model where tumor results were disclosed directly to patients via a patient portal, satisfaction was high, anxiety levels did not change immediately after tumor results, and 80% of patients with abnormal tumor results pursued GC/GT [84]. Patient attitudes about tumor testing were also generally positive [87,91]. When surveyed prior to tumor results, patients reported low levels of distress [78,87]; however, one study found that 40% of patients with abnormal tumor results had high levels of cancer-related distress, which subsequently decreased over time [78].
The remaining three publications assessed the use of DNA sequencing of tumor tissue for patients with advanced disease to identify potential druggable targets. Two studies examining patient preferences reported positive patient attitudes toward tumor testing; however, neither study directly reported data on the patient impact of receiving tumor genetic test results [101,102]. The published protocol of a mixed-methods longitudinal study outlined ongoing research to evaluate patient knowledge and preferences, as well as the behavioral, decisional, and psychological outcomes of tumor testing in individuals with advanced cancer diagnoses [103].
4. Discussion
The purpose of this scoping review was to first determine the various alternative models of pre-test GC and GT used for hereditary cancer, and second to determine the patient impact of employing these models of care. Seven alternative models of care were identified and dichotomized into alternative GC models (telephone, telegenetic, group, embedded GC models) and alternative GT models (mainstreaming, direct, and tumor-first GT models). The results of this review provide insight into rates of GC/GT, patient preferences, and psychosocial outcomes associated with alternative models of genetic service delivery, which can be carefully evaluated by the clinicians who are considering utilizing these models. While not an outcome of interest for this review, the cost–benefit reported in several studies may be an important consideration for policy makers.
4.1. Alternative GC Models
The results of this scoping review indicate that all four alternative models of GC can improve patient access to genetic services; however, this may not necessarily translate into an increased uptake of GC and GT. Both telephone and telegenetic models extend the reach of genetic counselors by eliminating or reducing the travel required to access genetic services. Despite this advantage, a lower uptake of GC and GT has been identified in telegenetic and telephone models, respectively. Data suggests that low rates of GT among telephone GC may be related to ethnicity [37] and high cancer-specific distress or perceived hereditary cancer risk [40]. Group GC can also improve patient access to genetic services by increasing the efficiency of genetics clinics and allowing genetic counselors to see higher volumes of patients; however, patient uptake of GC with the model may be low [50], and there is conflicting data regarding patient uptake of GT following group GC [51,52]. Finally, the embedded GC model may improve patient access to GC and GT by improving GC referral rates and facilitating GC/GT during oncology appointments. Based on the small number of studies included in this review, the embedded GC model appears to improve the patient uptake of both GC and GT.
While it is important to improve patient access to genetic services, it is paramount that patient experience is considered when implementing alternative GC models. The studies in this review, including four non-inferiority studies, suggest that telephone, telegenetic, and group GC models improve patient knowledge and psychosocial functioning. While patients appear to be satisfied with these alternative models, some patients may still prefer the traditional GC model. It is important to consider these results in the context of the potential bias created by patients who declined study participation in order to receive traditional GC, as reported in studies of telephone [36], telegenetic [41], and group [50] GC models. Studies evaluating the embedded GC model did not examine its psychosocial impact on cancer patients, demonstrating the need for future research regarding patient psychosocial outcomes associated with this model of care.
Overall, the four alternative GC models may be useful in increasing patient access to genetic services; however, they may not be appropriate for all patients. The combination of patient preference data and reports of lower GT rates among specific patient groups indicate that traditional GC should remain available as an option to patients, particularly for those seeking emotional support during the GT process.
4.2. Alternative GT Models
By eliminating pre-test GC with a genetic counselor and facilitating GT outside of the genetics clinic, alternative GT models undoubtably improve patient access to genetic information. Indeed, the small number of included studies evaluating mainstreaming GT models reported a high patient uptake of GT. It is concerning that one study that reported patients with positive or inconclusive results may not be referred for GC in a timely manner, or at all [62]. While many studies of direct GT models evaluated the psychosocial impact of direct GT models, few reported on the patient uptake of GT and post-test GC. The data from one study suggested that patient uptake may be higher with a direct GT model as compared to the traditional GT model; however, participants were not randomized to a specific GT model, and this discrepancy may be attributed to patient characteristics rather than the model itself [68]. Additionally, patients receiving negative GT results may be less likely to attend post-test GC recommended based on family history [76]. Most studies assessing GC referral, GC attendance, and the uptake of germline GT after tumor testing to screen for Lynch syndrome reported high uptake of these services; however, low rates have also been reported [82,89,92,95,99]. The data from this review demonstrates that facilitation by a genetic counselor or navigator results in a higher uptake of GC and germline GT, suggesting that some central oversight may be required when implementing tumor-first GT models.
The results of this review indicate that patient satisfaction with alternative GT models is high; however, additional data may be required to further evaluate patient psychosocial outcomes. Only one study involving mainstreaming GT employed validated tools to assess the psychosocial impact of this model, suggesting that further research is needed. In contrast, multiple quantitative and qualitative studies of the direct GT model evaluated its psychosocial impact. While this model does not appear to cause increased distress or anxiety among cancer patients, some studies suggest that a subset of women may benefit from having GC prior to GT, including those with higher levels of decisional conflict [68], baseline distress levels [68], unaffected individuals [64], and those requiring additional emotional support throughout the GT process [75]. While only four studies evaluating tumor screening for Lynch syndrome reported on psychosocial outcomes, this model does not appear to have a negative impact on patients. Based on this review, it seems that cancer patients are interested in tumor GT; however, included studies did not assess the patient impact of tumor GT outside of screening for Lynch syndrome. As with the mainstreaming GT model, additional research regarding the psychosocial outcomes of tumor-first GT models is required.
The three alternative GT models included in this review have the potential to improve patient access to GT. Yet, some patients may prefer to receive GC prior to GT. As such, further research is required to evaluate the psychosocial outcomes associated with mainstreaming and tumor-first GT models. In addition to those with positive GT results, patients with negative or inconclusive results may also benefit from post-test GC, as they can experience increased distress or misinterpret the clinical implications of their results [104,105]. As cautioned by Brierley et al., care must also be taken to ensure that patients receiving negative test results from mainstreaming, direct GT, or tumor-first models are not falsely reassured [63]. Thus, patients with negative GT results and a relevant history of cancer should be referred for further genetic evaluation. The implementation of alternative GT models will likely require “buy-in” from all of the stakeholders, and should involve educational training for ordering clinicians.
4.3. Future Directions
As targeted therapies continue to develop, more emphasis may be placed on tumor-first models to streamline GT processes. By reducing the reliance on clinicians to refer patients for GC, or order GT themselves, tumor-first models may ensure equal and rapid access to genetic information for all patients. Since DNA sequencing of tumor tissue can identify both germline and acquired pathogenic variants, this model can both inform treatment and identify the subset of patients who may have a hereditary cancer risk and warrant a referral for GC and targeted GT. With the rare exception of gene reversions, where pathogenic variants revert to wild-type in response to treatment, negative genetic tumor tests correlate with negative germline results. Therefore, only those patients with positive tumor results, negative tumor results with a suggestive family history, or negative tumor results in pre-treated tumor tissue, need to be seen for GC. Based on studies of alternative GT models, individuals with abnormal results have high rates of follow-through for post-test GC, which allows genetic counselors to provide information and support as well as discuss the familial implications of their result, creating opportunities for cancer prevention in at-risk relatives. As evidenced by this review, there is currently no data on the patient impact of automatic DNA sequencing of tumor tissue at the time of cancer diagnosis. While data from a limited number of studies involving tumor screening for Lynch syndrome suggests that automated tumor testing does not have a negative effect on patients, the immunohistochemistry and microsatellite instability testing conducted for Lynch syndrome may be perceived differently by patients and clinicians than DNA sequencing of tumor tissue. Thus, the reported patient outcomes associated with tumor testing in Lynch syndrome may not be indicative of patient outcomes associated with other tumor GT models. Likewise, the available data on the DNA sequencing of tumor tissue is comprised of patients with advanced stage disease, whose motivations and perceptions of GT may be vastly different from individuals with early-stage disease. As tumor GT models are implemented earlier in a patient’s treatment cycle, it will be critical to assess the impact of these models with respect to the timing and stage of the disease.
4.4. Study Limitations
The results of this review should be considered in the context of several limitations. First, it is not a systematic review, which is often considered the gold standard for reviewing available literature. Many of the included articles were qualitative in nature (n = 5), non-randomized studies comparing alternative and traditional strategies (n = 13), or did not provide a comparison to traditional strategies (n = 44). Despite their potential lack of rigor, the articles provide valuable insights for genetic counselors considering changes to their practice, and would have been missed in a systematic review. Second, as is the norm for scoping reviews, a quality assessment was not completed for the included articles. Third, scoping reviews have been criticized for their oversimplification and potential to mask variation between studies [106]. By grouping studies by model of service delivery, this review does not consider the nuances in study design and patient populations. The educational and emotional needs of individuals with and without cancer, as well as those diagnosed with breast, ovarian, or colon cancer, are vastly different, and were not assessed separately during this review. Fourth, the majority of the published literature regarding the tumor-first GT model has been conducted in the context of Lynch syndrome, and the patient perspective of immunohistochemistry and microsatellite instability testing may not translate to patient perspectives of the DNA sequencing of tumor tissue. Likewise, the few perspectives obtained from patients with advanced disease may not be applicable to those with a new cancer diagnosis. As tumor GT becomes part of routine care for cancer patients, further research is necessary to determine its impact on patients and families.
5. Conclusions
The field of hereditary cancer has changed significantly since the discovery of the BRCA1 and BRCA2 genes in the mid-1990s, and it is important that models of genetic service delivery evolve as well. Alternative models of GT and GC are unlikely to ever be as comprehensive as the traditional GC model; however, in the current era of precision medicine, many patients may be better served by models that increase their access to GT or GC, either by minimizing the number of in-person appointments or reducing clinical wait times. Yet, it remains important for providers to recognize that alternative models of GT and GC are not appropriate for, or acceptable to, all patients or clinical situations. The traditional GC model is likely to continue to have a role in GT for hereditary cancer, particularly for individuals without a personal diagnosis of cancer, or those requiring greater emotional support. It is evident from this review that there is no “one size fits all” approach that will suit every patient, clinician, or institution. The benefits and limitations of each model should be evaluated from the lens of each stakeholder when deciding which models of care to utilize. As GT continues to evolve, moving from the germline to tumor GT, new and exciting opportunities will emerge, allowing genetic counselors to expand their current roles, integrate GC into the field of oncology, and explore new models of genetic service delivery.
Appendix A. Medline Search Strategy
| 1 | exp Neoplasms/ |
| 2 | neoplas*.mp,kw. |
| 3 | paraneoplas*.mp,kw |
| 4 | cancer*.mp,kw. |
| 5 | tumo?r*.mp,kw. |
| 6 | onco*.mp,kw. |
| 7 | metast*.mp,kw. |
| 8 | malignan*.mp,kw. |
| 9 | adenocarcin*.mp,kw. |
| 10 | carcin*.mp,kw. |
| 11 | HBOC.mp,kw. |
| 12 | HNPCC.mp,kw. |
| 13 | lynch*.mp,kw. |
| 14 | or/1-13 |
| 15 | exp Genetic Services/ |
| 16 | Genetic Counseling/ |
| 17 | Genetic Testing/ |
| 18 | Genes, Neoplasm/ |
| 19 | Germ-Line Mutation/ |
| 20 | (genetic? adj2 service?).mp,kw. |
| 21 | (genetic? adj2 counsel*).mp,kw. |
| 22 | (genetic? adj2 screen*).mp,kw. |
| 23 | (genetic? adj2 test*).mp,kw. |
| 24 | (somatic adj2 screen*).mp,kw. |
| 25 | (somatic adj2 test*).mp,kw. |
| 26 | (germline? adj2 screen*).mp,kw. |
| 27 | (germline? adj2 test*).mp,kw. |
| 28 | (profiling* adj2 screen*).mp,kw. |
| 29 | (molecular* adj2 screen*).mp,kw. |
| 30 | or/15-29 |
| 31 | (universal* adj3 screen*).mp,kw. |
| 32 | 14 and 31 |
| 33 | “Outcome and Process Assessment (Health Care)”/ |
| 34 | “Outcome Assessment (Health Care)”/ |
| 35 | exp Patient Outcome Assessment/ |
| 36 | Patient Reported Outcome Measures/ |
| 37 | “Early Detection of Cancer”/ |
| 38 | Incidental Findings/ |
| 39 | Psychological Trauma/ |
| 40 | exp Stress Psychological/ |
| 41 | Stress Disorders, Traumatic/ |
| 42 | Stress Disorders, Post-Traumatic/ |
| 43 | Stress Disorders, Traumatic, Acute/ |
| 44 | exp Patient Satisfaction/ |
| 45 | Patient Preference/ |
| 46 | Patient Access to Records/ |
| 47 | (patient? adj3 outcome?).mp,kw. |
| 48 | (clinc* adj3 outcome?).mp,kw. |
| 49 | (counsel* adj3 outcome?).mp,kw. |
| 50 | (earl* adj3 detect*).mp,kw. |
| 51 | (profiling* adj2 detect*).mp,kw. |
| 52 | (incidental* adj2 finding?).mp,kw. |
| 53 | (incidental* adj2 germline?).mp,kw. |
| 54 | (psycho* adj3 trauma*).mp,kw. |
| 55 | (psycho* adj3 measur*).mp,kw. |
| 56 | (psycho* adj3 outcome?).mp,kw. |
| 57 | (psycho* adj3 function*).mp,kw. |
| 58 | (psycho* adj3 impact*).mp,kw. |
| 59 | (psycho* adj3 effect?).mp,kw. |
| 60 | (psycho* adj3 distress*).mp,kw. |
| 61 | (patient? adj2 satisfact*).mp,kw. |
| 62 | (patient? adj2 prefer*).mp,kw. |
| 63 | access*.mp,kw. |
| 64 | mainstream*.mp,kw. |
| 65 | main-stream*.mp,kw. |
| 66 | universal*.mp,kw. |
| 67 | model?.mp,kw. |
| 68 | or/33-67 |
| 69 | 14 and 30 and 68 |
| 70 | 32 or 69 |
| 71 | exp animals/not (exp animals/and exp humans/) |
| 72 | 70 not 71 |
| 73 | limit 72 to “all child (0 to 18 years)” |
| 74 | limit 72 to “all adult (19 plus years)” |
| 75 | 73 not 74 |
| 76 | 72 not 75 |
| 77 | limit 76 to yr = “2007-Current” |
| 78 | limit 77 to English language |
| 79 | remove duplicates from 78 |
Appendix B. Study Inclusion and Exclusion Criteria
| Inclusion | Exclusion |
|
|
Author Contributions
All authors provided substantial contribution to this manuscript, including conceptualization and methodology by J.M.M., R.H.K. and K.A.M.; abstract and full-text screening by J.M.M., S.R.A, M.C., A.V. and K.A.M.; data extraction by J.M.M.; original draft preparation by J.M.M.; review and editing by J.M.M., S.R.A., M.C., R.H.K. and K.A.M.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Evans D.G., Barwell J., Eccles D.M., Collins A., Izatt L., Jacobs C., Donaldson A., Brady A.F., Cuthbert A., Harrison R., et al. The Angelina Jolie effect: How high celebrity profile can have a major impact on provision of cancer related services. Breast Cancer Res. 2014;16:442. doi: 10.1186/s13058-014-0442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raphael J., Verma S., Hewitt P., Eisen A. The Impact of Angelina Jolie (AJ)’s Story on Genetic Referral and Testing at an Academic Cancer Centre in Canada. J. Genet. Couns. 2016;25:1309–1316. doi: 10.1007/s10897-016-9973-6. [DOI] [PubMed] [Google Scholar]
- 3.Buchtel K.M., Vogel Postula K.J., Weiss S., Williams C., Pineda M., Weissman S.M. FDA Approval of PARP Inhibitors and the Impact on Genetic Counseling and Genetic Testing Practices. J. Genet. Couns. 2018;27:131–139. doi: 10.1007/s10897-017-0130-7. [DOI] [PubMed] [Google Scholar]
- 4.Zhang S., Royer R., Li S., McLaughlin J.R., Rosen B., Risch H.A., Fan I., Bradley L., Shaw P.A., Narod S.A. Frequencies of BRCA1 and BRCA2 mutations among 1,342 unselected patients with invasive ovarian cancer. Gynecol. Oncol. 2011;121:353–357. doi: 10.1016/j.ygyno.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Harter P., Hauke J., Heitz F., Reuss A., Kommoss S., Marmé F., Heimbach A., Prieske K., Richters L., Burges A., et al. Prevalence of deleterious germline variants in risk genes including BRCA1/2 in consecutive ovarian cancer patients (AGO-TR-1) PLoS ONE. 2017;12:e0186043. doi: 10.1371/journal.pone.0186043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norquist B.M., Harrell M.I., Brady M.F., Walsh T., Lee M.K., Gulsuner S., Bernards S.S., Casadei S., Yi Q., Burger R.A., et al. Inherited Mutations in Women with Ovarian Carcinoma. JAMA Oncol. 2016;2:482–490. doi: 10.1001/jamaoncol.2015.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demsky R., McCuaig J., Maganti M., Murphy K.J., Rosen B., Armel S.R. Keeping it simple: Genetics referrals for all invasive serous ovarian cancers. Gynecol. Oncol. 2013;130:329–333. doi: 10.1016/j.ygyno.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 8.McGee J., Panabaker K., Leonard S., Ainsworth P., Elit L., Shariff S.Z. Genetics Consultation Rates Following a Diagnosis of High-Grade Serous Ovarian Carcinoma in the Canadian Province of Ontario. Int. J. Gynecol. Cancer. 2017;27:437–443. doi: 10.1097/IGC.0000000000000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Childers C.P., Childers K.K., Maggard-Gibbons M., Macinko J. National Estimates of Genetic Testing in Women With a History of Breast or Ovarian Cancer. J. Clin. Oncol. 2017;35:3800–3806. doi: 10.1200/JCO.2017.73.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoskins P.J., Gotlieb W.H. Missed therapeutic and prevention opportunities in women with BRCA-mutated epithelial ovarian cancer and their families due to low referral rates for genetic counseling and BRCA testing: A review of the literature. CA Cancer J. Clin. 2017;67:493–506. doi: 10.3322/caac.21408. [DOI] [PubMed] [Google Scholar]
- 11.Randall L.M., Pothuri B., Swisher E.M., Diaz J.P., Buchanan A., Witkop C.T., Bethan Powell C., Smith E.B., Robson M.E., Boyd J., et al. Multi-disciplinary summit on genetics services for women with gynecologic cancers: A Society of Gynecologic Oncology White Paper. Gynecol. Oncol. 2017;146:217–224. doi: 10.1016/j.ygyno.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 12.McCuaig J.M., Stockley T.L., Shaw P., Fung-Kee-Fung M., Altman A.D., Bentley J., Bernardini M.Q., Cormier B., Hirte H., Kieser K., et al. Evolution of genetic assessment for BRCA-associated gynaecologic malignancies: A Canadian multisociety roadmap. J. Med. Genet. 2018;55:571–577. doi: 10.1136/jmedgenet-2018-105472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samimi G., Bernardini M.Q., Brody L.C., Caga-Anan C.F., Campbell I.G., Chenevix-Trench G., Couch F.J., Dean M., de Hullu J.A., Domchek S.M., et al. Traceback: A Proposed Framework to Increase Identification and Genetic Counseling of BRCA1 and BRCA2 Mutation Carriers Through Family-Based Outreach. J. Clin. Oncol. 2017;35:2329–2337. doi: 10.1200/JCO.2016.70.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institutes of Health Traceback Testing: Increasing Identification and Genetic Counseling of Mutation Carriers through Family-based Outreach (U01 Clinical Trial Optional) [(accessed on 24 September 2018)]; Available online: https://grants.nih.gov/grants/guide/pa-files/PAR-18-616.html.
- 15.Hoskovec J.M., Bennett R.L., Carey M.E., DaVanzo J.E., Dougherty M., Hahn S.E., LeRoy B.S., O’Neal S., Richardson J.G., Wicklund C.A. Projecting the Supply and Demand for Certified Genetic Counselors: A Workforce Study. J. Genet. Couns. 2018;27:16–20. doi: 10.1007/s10897-017-0158-8. [DOI] [PubMed] [Google Scholar]
- 16.Cohen S.A., Gustafson S.L., Marvin M.L., Riley B.D., Uhlmann W.R., Liebers S.B., Rousseau J.A. Report from the National Society of Genetic Counselors service delivery model task force: A proposal to define models, components, and modes of referral. J. Genet. Couns. 2012;21:645–651. doi: 10.1007/s10897-012-9505-y. [DOI] [PubMed] [Google Scholar]
- 17.Cohen S.A., Marvin M.L., Riley B.D., Vig H.S., Rousseau J.A., Gustafson S.L. Identification of genetic counseling service delivery models in practice: A report from the NSGC Service Delivery Model Task Force. J. Genet. Couns. 2013;22:411–421. doi: 10.1007/s10897-013-9588-0. [DOI] [PubMed] [Google Scholar]
- 18.Trepanier A.M., Allain D.C. Models of service delivery for cancer genetic risk assessment and counseling. J. Genet. Couns. 2014;23:239–253. doi: 10.1007/s10897-013-9655-6. [DOI] [PubMed] [Google Scholar]
- 19.Buchanan A.H., Rahm A.K., Williams J.L. Alternate Service Delivery Models in Cancer Genetic Counseling: A Mini-Review. Front. Oncol. 2016;6:120. doi: 10.3389/fonc.2016.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fournier D.M., Bazzell A.F., Dains J.E. Comparing Outcomes of Genetic Counseling Options in Breast and Ovarian Cancer: An Integrative Review. Oncol. Nurs. Forum. 2018;45:96–105. doi: 10.1188/18.ONF.96-105. [DOI] [PubMed] [Google Scholar]
- 21.George A., Kaye S., Banerjee S. Delivering widespread BRCA testing and PARP inhibition to patients with ovarian cancer. Nat. Rev. Clin. Oncol. 2017;14:284–296. doi: 10.1038/nrclinonc.2016.191. [DOI] [PubMed] [Google Scholar]
- 22.Hoogerbrugge N., Jongmans M.C. Finding all BRCA pathogenic mutation carriers: Best practice models. Eur. J. Hum. Genet. 2016;24(Suppl. 1):S19–S26. doi: 10.1038/ejhg.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madlensky L., Trepanier A.M., Cragun D., Lerner B., Shannon K.M., Zierhut H. A Rapid Systematic Review of Outcomes Studies in Genetic Counseling. J. Genet. Couns. 2017;26:361–378. doi: 10.1007/s10897-017-0067-x. [DOI] [PubMed] [Google Scholar]
- 24.Trepanier A.M., Cohen S.A., Allain D.C. Thinking differently about genetic counseling service delivery. Curr. Genet. Med. Rep. 2015;3:49–56. doi: 10.1007/s40142-015-0069-7. [DOI] [Google Scholar]
- 25.Weitzel J.N., Blazer K.R., MacDonald D.J., Culver J.O., Offit K. Genetics, genomics, and cancer risk assessment: State of the Art and Future Directions in the Era of Personalized Medicine. CA Cancer J. Clin. 2011;61:327–359. doi: 10.3322/caac.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoll K., Kubendran S., Cohen S.A. The past, present and future of service delivery in genetic counseling: Keeping up in the era of precision medicine. Am. J. Med. Genet. C Semin. Med. Genet. 2018;178:24–37. doi: 10.1002/ajmg.c.31602. [DOI] [PubMed] [Google Scholar]
- 27.Levac D., Colquhoun H., O’Brien K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010;5:69. doi: 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grimshaw J. A Knowledge Synthesis Chapter. Canadian Institutes of Health Research; Ottawa, ON, Canada: 2010. pp. 1–56. [Google Scholar]
- 29.Arksey H., O’Malley L. Scoping Studies: Towards a Methodological Framework. Int. J. Soc. Res. 2005;8:19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 30.Foulkes W.D., Knoppers B.M., Turnbull C. Population genetic testing for cancer susceptibility: Founder mutations to genomes. Nat. Rev. Clin. Oncol. 2016;13:41–54. doi: 10.1038/nrclinonc.2015.173. [DOI] [PubMed] [Google Scholar]
- 31.Athens B.A., Caldwell S.L., Umstead K.L., Connors P.D., Brenna E., Biesecker B.B. A Systematic Review of Randomized Controlled Trials to Assess Outcomes of Genetic Counseling. J. Genet. Couns. 2017;26:902–933. doi: 10.1007/s10897-017-0082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meilleur K.G., Littleton-Kearney M.T. Interventions to improve patient education regarding multifactorial genetic conditions: A systematic review. Am. J. Med. Genet. A. 2009;149A:819–830. doi: 10.1002/ajmg.a.32723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pal T., Stowe C., Cole A., Lee J.H., Zhao X., Vadaparampil S. Evaluation of phone-based genetic counselling in African American women using culturally tailored visual aids. Clin. Genet. 2010;78:124–131. doi: 10.1111/j.1399-0004.2010.01466.x. [DOI] [PubMed] [Google Scholar]
- 34.Sutphen R., Davila B., Shappell H., Holtje T., Vadaparampil S., Friedman S., Toscano M., Armstrong J. Real world experience with cancer genetic counseling via telephone. Fam. Cancer. 2010;9:681–689. doi: 10.1007/s10689-010-9369-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinney A.Y., Butler K.M., Schwartz M.D., Mandelblatt J.S., Boucher K.M., Pappas L.M., Gammon A., Kohlmann W., Edwards S.L., Stroup A.M., et al. Expanding access to BRCA1/2 genetic counseling with telephone delivery: A cluster randomized trial. J. Natl. Cancer Inst. 2014;106 doi: 10.1093/jnci/dju328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartz M.D., Valdimarsdottir H.B., Peshkin B.N., Mandelblatt J., Nusbaum R., Huang A.T., Chang Y., Graves K., Isaacs C., Wood M., et al. Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. J. Clin. Oncol. 2014;32:618–626. doi: 10.1200/JCO.2013.51.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butrick M., Kelly S., Peshkin B.N., Luta G., Nusbaum R., Hooker G.W., Graves K., Feeley L., Isaacs C., Valdimarsdottir H.B., et al. Disparities in uptake of BRCA1/2 genetic testing in a randomized trial of telephone counseling. Genet. Med. 2015;17:467–475. doi: 10.1038/gim.2014.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peshkin B.N., Kelly S., Nusbaum R.H., Similuk M., DeMarco T.A., Hooker G.W., Valdimarsdottir H.B., Forman A.D., Joines J.R., Davis C., et al. Patient Perceptions of Telephone vs. In-Person BRCA1/BRCA2 Genetic Counseling. J. Genet. Couns. 2016;25:472–482. doi: 10.1007/s10897-015-9897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinney A.Y., Steffen L.E., Brumbach B.H., Kohlmann W., Du R., Lee J.H., Gammon A., Butler K., Buys S.S., Stroup A.M., et al. Randomized Noninferiority Trial of Telephone Delivery of BRCA1/2 Genetic Counseling Compared With In-Person Counseling: 1-Year Follow-Up. J. Clin. Oncol. 2016;34:2914–2924. doi: 10.1200/JCO.2015.65.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steffen L.E., Du R., Gammon A., Mandelblatt J.S., Kohlmann W.K., Lee J.H., Buys S.S., Stroup A.M., Campo R.A., Flores K.G., et al. Genetic Testing in a Population-Based Sample of Breast and Ovarian Cancer Survivors from the REACH Randomized Trial: Cost Barriers and Moderators of Counseling Mode. Cancer Epidemiol. Biomark. Prev. 2017;26:1772–1780. doi: 10.1158/1055-9965.EPI-17-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.d’Agincourt-Canning L., McGillivray B., Panabaker K., Scott J., Pearn A., Ridge Y., Portigal-Todd C. Evaluation of genetic counseling for hereditary cancer by videoconference in British Columbia. BC Med. J. 2008;50:554–559. [Google Scholar]
- 42.Zilliacus E.M., Meiser B., Lobb E.A., Kirk J., Warwick L., Tucker K. Women’s experience of telehealth cancer genetic counseling. J. Genet. Couns. 2010;19:463–472. doi: 10.1007/s10897-010-9301-5. [DOI] [PubMed] [Google Scholar]
- 43.Zilliacus E.M., Meiser B., Lobb E.A., Kelly P.J., Barlow-Stewart K., Kirk J.A., Spigelman A.D., Warwick L.J., Tucker K.M. Are videoconferenced consultations as effective as face-to-face consultations for hereditary breast and ovarian cancer genetic counseling? Genet. Med. 2011;13:933–941. doi: 10.1097/GIM.0b013e3182217a19. [DOI] [PubMed] [Google Scholar]
- 44.Meropol N.J., Daly M.B., Vig H.S., Manion F.J., Manne S.L., Mazar C., Murphy C., Solarino N., Zubarev V. Delivery of Internet-based cancer genetic counselling services to patients’ homes: A feasibility study. J. Telemed. Telecare. 2011;17:36–40. doi: 10.1258/jtt.2010.100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buchanan A.H., Datta S.K., Skinner C.S., Hollowell G.P., Beresford H.F., Freeland T., Rogers B., Boling J., Marcom P.K., Adams M.B. Randomized Trial of Telegenetics vs. In-Person Cancer Genetic Counseling: Cost, Patient Satisfaction and Attendance. J. Genet. Couns. 2015;24:961–970. doi: 10.1007/s10897-015-9836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bradbury A., Patrick-Miller L., Harris D., Stevens E., Egleston B., Smith K., Mueller R., Brandt A., Stopfer J., Rauch S., et al. Utilizing Remote Real-Time Videoconferencing to Expand Access to Cancer Genetic Services in Community Practices: A Multicenter Feasibility Study. J. Med. Internet Res. 2016;18:e23. doi: 10.2196/jmir.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mette L.A., Saldívar A.M., Poullard N.E., Torres I.C., Seth S.G., Pollock B.H., Tomlinson G.E. Reaching high-risk underserved individuals for cancer genetic counseling by video-teleconferencing. J. Commun. Support. Oncol. 2016;14:162–168. doi: 10.12788/jcso.0247. [DOI] [PubMed] [Google Scholar]
- 48.Solomons N.M., Lamb A.E., Lucas F.L., McDonald E.F., Miesfeldt S. Examination of the Patient-Focused Impact of Cancer Telegenetics Among a Rural Population: Comparison with Traditional In-Person Services. Telemed. J. E Health. 2018;24:130–138. doi: 10.1089/tmj.2017.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mangerich B., Stichler J.F. Breast and ovarian cancer: A new model for educating women. Nurs. Womens Health. 2008;12:490–499. doi: 10.1111/j.1751-486X.2008.00383.x. [DOI] [PubMed] [Google Scholar]
- 50.Ridge Y., Panabaker K., McCullum M., Portigal-Todd C., Scott J., McGillivray B. Evaluation of group genetic counseling for hereditary breast and ovarian cancer. J. Genet. Couns. 2009;18:87–100. doi: 10.1007/s10897-008-9189-5. [DOI] [PubMed] [Google Scholar]
- 51.Rothwell E., Kohlmann W., Jasperson K., Gammon A., Wong B., Kinney A. Patient outcomes associated with group and individual genetic counseling formats. Fam. Cancer. 2012;11:97–106. doi: 10.1007/s10689-011-9486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manchanda R., Burnell M., Loggenberg K., Desai R., Wardle J., Sanderson S.C., Gessler S., Side L., Balogun N., Kumar A., et al. Cluster-randomised non-inferiority trial comparing DVD-assisted and traditional genetic counselling in systematic population testing for BRCA1/2 mutations. J. Med. Genet. 2016;53:472–480. doi: 10.1136/jmedgenet-2015-103740. [DOI] [PubMed] [Google Scholar]
- 53.Benusiglio P.R., Di Maria M., Dorling L., Jouinot A., Poli A., Villebasse S., Le Mentec M., Claret B., Boinon D., Caron O. Hereditary breast and ovarian cancer: Successful systematic implementation of a group approach to genetic counselling. Fam. Cancer. 2017;16:51–56. doi: 10.1007/s10689-016-9929-x. [DOI] [PubMed] [Google Scholar]
- 54.Wiesman C., Rose E., Grant A., Zimilover A., Klugman S., Schreiber-Agus N. Experiences from a pilot program bringing BRCA1/2 genetic screening to theUS Ashkenazi Jewish population. Genet. Med. 2017;19:529–536. doi: 10.1038/gim.2016.154. [DOI] [PubMed] [Google Scholar]
- 55.Kentwell M., Dow E., Antill Y., Wrede C.D., McNally O., Higgs E., Hamilton A., Ananda S., Lindeman G.J., Scott C.L. Mainstreaming cancer genetics: A model integrating germline BRCA testing into routine ovarian cancer clinics. Gynecol. Oncol. 2017;145:130–136. doi: 10.1016/j.ygyno.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 56.Senter L., O’Malley D.M., Backes F.J., Copeland L.J., Fowler J.M., Salani R., Cohn D.E. Genetic consultation embedded in a gynecologic oncology clinic improves compliance with guideline-based care. Gynecol. Oncol. 2017;147:110–114. doi: 10.1016/j.ygyno.2017.07.141. [DOI] [PubMed] [Google Scholar]
- 57.Bednar E.M., Oakley H.D., Sun C.C., Burke C.C., Munsell M.F., Westin S.N., Lu K.H. A universal genetic testing initiative for patients with high-grade, non-mucinous epithelial ovarian cancer and the implications for cancer treatment. Gynecol. Oncol. 2017;146:399–404. doi: 10.1016/j.ygyno.2017.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pederson H.J., Hussain N., Noss R., Yanda C., O’Rourke C., Eng C., Grobmyer S.R. Impact of an embedded genetic counselor on breast cancer treatment. Breast Cancer Res. Treat. 2018;169:43–46. doi: 10.1007/s10549-017-4643-4. [DOI] [PubMed] [Google Scholar]
- 59.George A., Riddell D., Seal S., Talukdar S., Mahamdallie S., Ruark E., Cloke V., Slade I., Kemp Z., Gore M., et al. Implementing rapid, robust, cost-effective, patient-centred, routine genetic testing in ovarian cancer patients. Sci. Rep. 2016;6:29506. doi: 10.1038/srep29506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoon S.Y., Ahmad Bashah N.S., Wong S.W., Mariapun S., Hassan T., Padmanabhan H., Lim J., Lau S.Y., Rahman N., Thong M.K., et al. Mainstreaming Genetic Counselling for Genetic Testing of BRCA1 and BRCA2 in Ovarian Cancer Patients in Malaysia (MaGiC Study) Ann. Oncol. 2017;28(Suppl. 10):mdx729.004. doi: 10.1093/annonc/mdx729.004. [DOI] [Google Scholar]
- 61.Colombo N., Huang G., Scambia G., Chalas E., Pignata S., Fiorica J., Van Le L., Ghamande S., González-Santiago S., Bover I., et al. Evaluation of a Streamlined Oncologist-Led BRCA Mutation Testing and Counseling Model for Patients with Ovarian Cancer. J. Clin. Oncol. 2018;36:1300–1307. doi: 10.1200/JCO.2017.76.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rahman B., Lanceley A., Kristeleit R.S., Ledermann J.A., Lockley M., McCormack M., Mould T., Side L. Mainstreamed genetic testing for women with ovarian cancer: First-year experience. J. Med. Genet. 2018 doi: 10.1136/jmedgenet-2017-105140. [DOI] [PubMed] [Google Scholar]
- 63.Brierley K.L., Campfield D., Ducaine W., Dohany L., Donenberg T., Shannon K., Schwartz R.C., Matloff E.T. Errors in delivery of cancer genetics services: Implications for practice. Conn. Med. 2010;74:413–423. [PubMed] [Google Scholar]
- 64.Metcalfe K.A., Poll A., Llacuachaqui M., Nanda S., Tulman A., Mian N., Sun P., Narod S.A. Patient satisfaction and cancer-related distress among unselected Jewish women undergoing genetic testing for BRCA1 and BRCA2. Clin. Gen. 2010;78:411–417. doi: 10.1111/j.1399-0004.2010.01499.x. [DOI] [PubMed] [Google Scholar]
- 65.Metcalfe K.A., Mian N., Enmore M., Poll A., Llacuachaqui M., Nanda S., Sun P., Hughes K.S., Narod S.A. Long-term follow-up of Jewish women with a BRCA1 and BRCA2 mutation who underwent population genetic screening. Breast Cancer Res. Treat. 2012;133:735–740. doi: 10.1007/s10549-011-1941-0. [DOI] [PubMed] [Google Scholar]
- 66.Pal T., Lee J.H., Besharat A., Thompson Z., Monteiro A.N., Phelan C., Lancaster J.M., Metcalfe K., Sellers T.A., Vadaparampil S., et al. Modes of delivery of genetic testing services and the uptake of cancer risk management strategies in BRCA1 and BRCA2 carriers. Clin. Genet. 2014;85:49–53. doi: 10.1111/cge.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Armstrong J., Toscano M., Kotchko N., Friedman S., Schwartz M.D., Virgo K.S., Lynch K., Andrews J.E., Aguado Loi C.X., Bauer J.E., et al. Utilization and Outcomes of BRCA Genetic Testing and Counseling in a National Commercially Insured Population: The ABOUT Study. JAMA Oncol. 2015;1:1251–1260. doi: 10.1001/jamaoncol.2015.3048. [DOI] [PubMed] [Google Scholar]
- 68.Sie A.S., van Zelst-Stams W.A., Spruijt L., Mensenkamp A.R., Ligtenberg M.J., Brunner H.G., Prins J.B., Hoogerbrugge N. More breast cancer patients prefer BRCA-mutation testing without prior face-to-face genetic counseling. Fam. Cancer. 2014;13:143–151. doi: 10.1007/s10689-013-9686-z. [DOI] [PubMed] [Google Scholar]
- 69.Sie A.S., Spruijt L., van Zelst-Stams W.A., Mensenkamp A.R., Ligtenberg M.J., Brunner H.G., Prins J.B., Hoogerbrugge N. High Satisfaction and Low Distress in Breast Cancer Patients One Year after BRCA-Mutation Testing without Prior Face-to-Face Genetic Counseling. J. Genet. Couns. 2016;25:504–514. doi: 10.1007/s10897-015-9899-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Plaskocinska I., Shipman H., Drummond J., Thompson E., Buchanan V., Newcombe B., Hodgkin C., Barter E., Ridley P., Ng R., et al. New paradigms for BRCA1/BRCA2 testing in women with ovarian cancer: Results of the Genetic Testing in Epithelial Ovarian Cancer (GTEOC) study. J. Med. Gen. 2016;53:655–661. doi: 10.1136/jmedgenet-2016-103902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shipman H., Flynn S., MacDonald-Smith C.F., Brenton J., Crawford R., Tischkowitz M., Hulbert-Williams N.J., Group G.S. Universal BRCA1/BRCA2 Testing for Ovarian Cancer Patients is Welcomed, but with Care: How Women and Staff Contextualize Experiences of Expanded Access. J. Genet. Couns. 2017;26:1280–1291. doi: 10.1007/s10897-017-0108-5. [DOI] [PubMed] [Google Scholar]
- 72.Meiser B., Quinn V.F., Gleeson M., Kirk J., Tucker K.M., Rahman B., Saunders C., Watts K.J., Peate M., Geelhoed E., et al. When knowledge of a heritable gene mutation comes out of the blue: Treatment-focused genetic testing in women newly diagnosed with breast cancer. Eur. J. Hum. Genet. 2016;24:1517–1523. doi: 10.1038/ejhg.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quinn V.F., Meiser B., Kirk J., Tucker K.M., Watts K.J., Rahman B., Peate M., Saunders C., Geelhoed E., Gleeson M., et al. Streamlined genetic education is effective in preparing women newly diagnosed with breast cancer for decision making about treatment-focused genetic testing: A randomized controlled noninferiority trial. Genet. Med. 2017;19:448–456. doi: 10.1038/gim.2016.130. [DOI] [PubMed] [Google Scholar]
- 74.Høberg-Vetti H., Bjorvatn C., Fiane B.E., Aas T., Woie K., Espelid H., Rusken T., Eikesdal H.P., Listøl W., Haavind M.T., et al. BRCA1/2 testing in newly diagnosed breast and ovarian cancer patients without prior genetic counselling: The DNA-BONus study. Eur. J. Hum. Genet. 2016;24:881–888. doi: 10.1038/ejhg.2015.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Augestad M.T., Hoberg-Vetti H., Bjorvatn C., Sekse R.J. Identifying Needs: A Qualitative Study of women’s Experiences Regarding Rapid Genetic Testing for Hereditary Breast and Ovarian Cancer in the DNA BONus Study. J. Genet. Couns. 2017;26:182–189. doi: 10.1007/s10897-016-9996-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lieberman S., Tomer A., Ben-Chetrit A., Olsha O., Strano S., Beeri R., Koka S., Fridman H., Djemal K., Glick I., et al. Population screening for BRCA1/BRCA2 founder mutations in Ashkenazi Jews: Proactive recruitment compared with self-referral. Genet. Med. 2017;19:754–762. doi: 10.1038/gim.2016.182. [DOI] [PubMed] [Google Scholar]
- 77.Lieberman S., Lahad A., Tomer A., Cohen C., Levy-Lahad E., Raz A. Population screening for BRCA1/BRCA2 mutations: Lessons from qualitative analysis of the screening experience. Genet. Med. 2017;19:628–634. doi: 10.1038/gim.2016.175. [DOI] [PubMed] [Google Scholar]
- 78.Landsbergen K.M., Prins J.B., Brunner H.G., van Duijvendijk P., Nagengast F.M., van Krieken J.H., Ligtenberg M., Hoogerbrugge N. Psychological distress in newly diagnosed colorectal cancer patients following microsatellite instability testing for Lynch syndrome on the pathologist’s initiative. Fam. Cancer. 2012;11:259–267. doi: 10.1007/s10689-012-9510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heald B., Plesec T., Liu X., Pai R., Patil D., Moline J., Sharp R.R., Burke C.A., Kalady M.F., Church J., et al. Implementation of universal microsatellite instability and immunohistochemistry screening for diagnosing lynch syndrome in a large academic medical center. J. Clin. Oncol. 2013;31:1336–1340. doi: 10.1200/JCO.2012.45.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marquez E., Geng Z., Pass S., Summerour P., Robinson L., Sarode V., Gupta S. Implementation of routine screening for Lynch syndrome in university and safety-net health system settings: Successes and challenges. Genet. Med. 2013;15:925–932. doi: 10.1038/gim.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moline J., Mahdi H., Yang B., Biscotti C., Roma A.A., Heald B., Rose P.G., Michener C., Eng C. Implementation of tumor testing for lynch syndrome in endometrial cancers at a large academic medical center. Gynecol. Oncol. 2013;130:121–126. doi: 10.1016/j.ygyno.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 82.Ward R.L., Hicks S., Hawkins N.J. Population-based molecular screening for Lynch syndrome: Implications for personalized medicine. J. Clin. Oncol. 2013;31:2554–2562. doi: 10.1200/JCO.2012.46.8454. [DOI] [PubMed] [Google Scholar]
- 83.Batte B.A., Bruegl A.S., Daniels M.S., Ring K.L., Dempsey K.M., Djordjevic B., Luthra R., Fellman B.M., Lu K.H., Broaddus R.R. Consequences of universal MSI/IHC in screening ENDOMETRIAL cancer patients for Lynch syndrome. Gynecol. Oncol. 2014;134:319–325. doi: 10.1016/j.ygyno.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hall M.J., Herda M.M., Handorf E.A., Rybak C.C., Keleher C.A., Siemon M., Daly M.B. Direct-to-patient disclosure of results of mismatch repair screening for Lynch syndrome via electronic personal health record: A feasibility study. Genet. Med. 2014;16:854–861. doi: 10.1038/gim.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Frolova A.I., Babb S.A., Zantow E., Hagemann A.R., Powell M.A., Thaker P.H., Gao F., Mutch D.G. Impact of an immunohistochemistry-based universal screening protocol for Lynch syndrome in endometrial cancer on genetic counseling and testing. Gynecol. Oncol. 2015;137:7–13. doi: 10.1016/j.ygyno.2015.01.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kidambi T.D., Blanco A., Myers M., Conrad P., Loranger K., Terdiman J.P. Selective Versus Universal Screening for Lynch Syndrome: A Six-Year Clinical Experience. Dig. Dis. Sci. 2015;60:2463–2469. doi: 10.1007/s10620-014-3234-z. [DOI] [PubMed] [Google Scholar]
- 87.Hunter J.E., Zepp J.M., Gilmore M.J., Davis J.V., Esterberg E.J., Muessig K.R., Peterson S.K., Syngal S., Acheson L.S., Wiesner G.L., et al. Universal tumor screening for Lynch syndrome: Assessment of the perspectives of patients with colorectal cancer regarding benefits and barriers. Cancer. 2015;121:3281–3289. doi: 10.1002/cncr.29470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goverde A., Spaander M.C., van Doorn H.C., Dubbink H.J., van den Ouweland A.M., Tops C.M., Kooi S.G., de Waard J., Hoedemaeker R.F., Bruno M.J., et al. Cost-effectiveness of routine screening for Lynch syndrome in endometrial cancer patients up to 70 years of age. Gynecol. Oncol. 2016;143:453–459. doi: 10.1016/j.ygyno.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 89.Brennan B., Hemmings C.T., Clark I., Yip D., Fadia M., Taupin D.R. Universal molecular screening does not effectively detect Lynch syndrome in clinical practice. Ther. Adv. Gastroenterol. 2017;10:361–371. doi: 10.1177/1756283X17690990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Holter S.P.A., Brown C., Schaeffer D., Wang H., Deschenes J., Foulkes W., Karanicolas P., Hsieh E., Hart T., Streutker C., et al. Screening for Lynch syndrome through the Canadian colorectal cancer consortium. Fam. Cancer. 2017;16:S32–S33. [Google Scholar]
- 91.Hunter J.E., Arnold K.A., Cook J.E., Zepp J., Gilmore M.J., Rope A.F., Davis J.V., Bergen K.M., Esterberg E., Muessig K.R., et al. Universal screening for Lynch syndrome among patients with colorectal cancer: Patient perspectives on screening and sharing results with at-risk relatives. Fam. Cancer. 2017;16:377–387. doi: 10.1007/s10689-017-9972-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kupfer S.M.C., Barge W., Chang J., Peterson M., Stoll J., Berera S., Wideroff G., Sussman D., Melson J. Is universal tumor testing for lynch syndrome truly universal? Fam. Cancer. 2017;16:S80–S81. [Google Scholar]
- 93.Livi A., Turchetti D., Miccoli S., Godino L., Ferrari S., Zuntini R., Procaccini M., Della Gatta A.N., De Leo A., Ceccarelli C., et al. Routine mismatch repair immunohistochemistry analysis as a valuable method to improve lynch syndrome’s diagnosis among women with endometrial cancer. Fam. Cancer. 2017;16:S100–S101. [Google Scholar]
- 94.Najdawi F., Crook A., Maidens J., McEvoy C., Fellowes A., Pickett J., Ho M., Nevell D., McIlroy K., Sheen A., et al. Lessons learnt from implementation of a Lynch syndrome screening program for patients with gynaecological malignancy. Pathology. 2017;49:457–464. doi: 10.1016/j.pathol.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 95.O’Kane G.M., Ryan É., McVeigh T.P., Creavin B., Hyland J.M., O’Donoghue D.P., Keegan D., Geraghty R., Flannery D., Nolan C., et al. Screening for mismatch repair deficiency in colorectal cancer: Data from three academic medical centers. Cancer Med. 2017;6:1465–1472. doi: 10.1002/cam4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Patel S.R.-B.M., Mork M., Bannon S., Cuddy A., Vilar E., Pande M., Lynch P., You YN. Decision for non-completion of follow up among patients with abnormal screening test for hereditary colorectal cancer syndrome. Fam. Cancer. 2017;16:1–134. doi: 10.1007/s10689-017-0026-6. [DOI] [Google Scholar]
- 97.Watkins J.C., Yang E.J., Muto M.G., Feltmate C.M., Berkowitz R.S., Horowitz N.S., Syngal S., Yurgelun M.B., Chittenden A., Hornick J.L., et al. Universal Screening for Mismatch-Repair Deficiency in Endometrial Cancers to Identify Patients with Lynch Syndrome and Lynch-like Syndrome. Int. J. Gynecol. Pathol. 2017;36:115–127. doi: 10.1097/PGP.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 98.Martin B.B.J., Kim A., Batts K., Burgart L., Baldinger S., Jensen C. Treatment implications of universal mismatch repair gene screening in colorectal cancer patients. Dis. Colon. Rectum. 2018;61:e144. [Google Scholar]
- 99.Metcalfe M.J., Petros F.G., Rao P., Mork M.E., Xiao L., Broaddus R.R., Matin S.F. Universal Point of Care Testing for Lynch Syndrome in Patients with Upper Tract Urothelial Carcinoma. J. Urol. 2018;199:60–65. doi: 10.1016/j.juro.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 100.Miesfeldt S., Feero W.G., Lucas F.L., Rasmussen K. Association of patient navigation with care coordination in a Lynch syndrome screening program. Transl. Behav. Med. 2018;8:450–455. doi: 10.1093/tbm/ibx078. [DOI] [PubMed] [Google Scholar]
- 101.Gray S.W., Park E.R., Najita J., Martins Y., Traeger L., Bair E., Gagne J., Garber J., Jänne P.A., Lindeman N., et al. Oncologists’ and cancer patients’ views on whole-exome sequencing and incidental findings: Results from the CanSeq study. Genet. Med. 2016;18:1011–1019. doi: 10.1038/gim.2015.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pinheiro A.P.M., Pocock R.H., Switchenko J.M., Dixon M.D., Shaib W.L., Ramalingam S.S., Pentz R.D. Discussing molecular testing in oncology care: Comparing patient and physician information preferences. Cancer. 2017;123:1610–1616. doi: 10.1002/cncr.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Best M., Newson A.J., Meiser B., Juraskova I., Goldstein D., Tucker K., Ballinger M.L., Hess D., Schlub T.E., Biesecker B., et al. The PiGeOn project: Protocol of a longitudinal study examining psychosocial and ethical issues and outcomes in germline genomic sequencing for cancer. BMC Cancer. 2018;18:454. doi: 10.1186/s12885-018-4366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lumish H.S., Steinfeld H., Koval C., Russo D., Levinson E., Wynn J., Duong J., Chung W.K. Impact of Panel Gene Testing for Hereditary Breast and Ovarian Cancer on Patients. J. Genet. Couns. 2017;26:1116–1129. doi: 10.1007/s10897-017-0090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.O’Neill S.C., Rini C., Goldsmith R.E., Valdimarsdottir H., Cohen L.H., Schwartz M.D. Distress among women receiving uninformative BRCA1/2 results: 12-month outcomes. Psycho-Oncology. 2009;18:1088–1096. doi: 10.1002/pon.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grant M.J., Booth A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Info. Libr. J. 2009;26:91–108. doi: 10.1111/j.1471-1842.2009.00848.x. [DOI] [PubMed] [Google Scholar]