Abstract
Background
Evidence for the effects of Parkinson disease on energy expenditure is incomplete and contradictory. A number of studies showed increased resting energy expenditure among patients with Parkinson disease whereas others did not. It was hypothesized that energy expenditure increases during exercise, based on findings in patients with a variable regime of anti‐parkinsonian therapies and at different stages of the disease. However, energy expenditure during posture maintenance has been neglected. To better understand these issues, we studied energy expenditure in a homogenous population of Parkinson patients in an early stage of the disease and different states of activity.
Methods
Oxygen consumption was assessed in a group of 10 males with early Parkinson disease without dopaminergic treatment and controls matched for age and body composition. Oxygen consumption was measured at rest, during trunk unsupported sitting, and during exercise at different intensities (unloaded and loaded cycling).
Results
Resting energy expenditure was similar between groups. Higher energy consumption was observed during maintenance of trunk posture at rest and during light intensity aerobic exercise (P < .05 for all conditions). The increment in energy expenditure associated with increased physical demand tended to be steeper in Parkinson disease.
Conclusion
Resting energy expenditure is normal in Parkinson disease. However, energy expenditure increases during physical activity and even during the maintenance of unsupported posture among patients with Parkinson disease.
Keywords: energy expenditure, exercise, indirect calorimetry, Parkinson disease, weight loss
Short abstract
It has been hypothesized that weight loss in Parkinson disease (PD) is the result of increased resting energy expenditure, yet conclusive evidence for this hypothesis has been lacking. This study demonstrated that increased energy expenditure in PD patients occurs only during posture maintenance and physical activity, not during rest. Evidence for abnormally increased energy expenditure emerged as of the recruitment of axial muscles to support the body trunk in a sitting posture.
1. INTRODUCTION
Unexplained loss of weight in the early phases of Parkinson disease (PD) is common.1 In cases of normal (or even increased) caloric intake in early PD,1, 2 the cause of weight loss in this phase of the disease is often assumed to be increased energy expenditure. This hypothesis has been supported by certain studies that have reported increased energy expenditure in PD,3, 4 but disconfirmed by others.2, 5 These conflicting findings may be associated with differences in the use of dopaminergic agents, the severity of the disease, body composition, and/or unmatched gender between PD and control groups. Some studies have not made a distinction between resting energy expenditure and activity‐induced energy expenditure, and the experimental procedures were not designed to address these putative differences.
To better understand energy expenditure in PD, we measured oxygen consumption (V̇O2) at rest and during moderate aerobic effort under optimally controlled conditions, in a homogeneous group of PD male patients, naive to dopaminergic therapy, in the early phase of the disease.
2. METHODS
2.1. Participants
Ten male PD patients, aged 42 to 74 years, were recruited through the Hadassah Medical Center's neurology outpatient Clinic for Movement Disorders. Ten non‐parkinsonian and non–blood‐related male individuals served as age‐matched controls. The exclusion criteria included significant cognitive impairment, clinically significant cardiovascular conditions, drug‐induced parkinsonism, suspected Parkinson‐plus syndrome, and the use of medication that is known to modulate metabolic rate such as beta‐blockers or thyroid replacement therapy. The study was approved by the Institutional Review Board committee of Hadassah Medical Center (HMO‐0078‐15) and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All participants signed an informed consent before enrollment. A physician was present during all exercises.
To reduce the variability among participants (percentage of fat tissue, muscle mass, and hormonal state), we only enrolled males in this study. This choice was guided by the higher percentage of muscle mass in males, which enabled us to detect subtle differences in a relatively small sample in energy consumption related to fat‐free mass (FFM), which consists largely of muscle tissue. The patients with PD were not on dopaminergic therapy (levodopa or agonists). Two of the PD patients were on symptomatic therapy (amantadine), and 6 were taking MAO‐B inhibitors.
The participants were assessed on the Motor Unified Parkinson's Disease Rating Scale (UPDRS) on the day of the test. The level of regular physical activity was evaluated using the Physical Activity Scale for the Elderly6 and was similar between groups (t test P > .11 for all questions; see Table S1). To exclude unrelated factors that can alter baseline metabolism, participants were instructed to avoid eating (4 h), drinking caffeinated beverages (2 h), and smoking (2 h) before the procedure.7
2.2. Measurement of FFM and oxygen consumption
The FFM measurement was calculated for each patient by bioelectrical impedance analysis using a body composition monitor (Tanita model BC‐545, Tanita Corporation, Tokyo, Japan). The body composition monitor was validated against dual‐energy X‐ray absorptiometry8 prior to the test. Indirect calorimetry was performed by measuring V̇O2 (oxygen consumption) using a metabolic cart with a built‐in spiroergometry (Zan 600, nSpire Health GmbH, Oberthulba, Germany), which measures breath‐by‐breath oxygen consumption and carbon dioxide output.
2.3. Spirometry procedures
To assess resting energy expenditure, participants sat in a comfortable armchair, with their torso leaning back at 50 to 70 degrees, for 10 minutes. All respired air was collected by a well‐fitted face mask. Calculations were based on the measurements obtained at minutes 8 to 10 (steady state). Average values of V̇O2 in liters per minute were multiplied by 4.9 to calculate caloric consumption in kilocalorie per minute and again by 1440 (number of min per 24 h) to calculate daily resting energy expenditure. We then measured V̇O2 in 4 sequential conditions—3 minutes of relaxed sitting on the bike, 4 minutes of unloaded cycling (no resistance, constant cycling speed 60 rpm), and 6 minutes of 40‐W loaded cycling (constant cycling speed 60 rpm). This was followed by a 4‐minute recovery period in which participants stopped cycling but continued to sit relaxed on the bike. The working load was well below the anaerobic threshold of all participants and ensured steady‐state conditions. The respiratory exchange ratio was similar between groups and did not exceed 0.9. During exercise, the patients' blood pressure, oxygen saturation, and electrocardiography were monitored. Visual inspection of the V̇O2 graphs revealed that during these time intervals, oxygen consumption was stable.
2.4. Statistics
Our experiment (10 individuals in each arm) was designed to detect at least a 20% increment of energy expenditure in the PD group (1‐sided test with P < .1), assuming intra‐group standard deviation of 20% of the group's mean with a power of 80%.
3. RESULTS
We measured the energy expenditure of 10 male patients with early PD and compared their results against 10 matched individuals without PD (participant characteristics are summarized in Table 1). Patients with PD reported a relative recent onset of symptoms (within 1‐5 y). The UPDRS motor scores ranged from 14 to 33, indicating mild to moderate severity of disease. None of the patients used levodopa or dopamine agonists.
Table 1.
Participant characteristics
| Characteristic | PD (Mean ± SD) | Control (Mean ± SD) | P‐value |
|---|---|---|---|
| Age, y | 60.5 ± 11.1 | 60.8 ± 10.4 | .95 |
| Weight, kg | 77.4 ± 14.7 | 79.7 ± 12.0 | .69 |
| Height, cm | 173.0 ± 6.4 | 173.9 ± 7.3 | .81 |
| Body fat, % | 22.4 ± 6.3 | 24.7 ± 6.6 | .46 |
| Fat‐free mass, kg | 56.8 ± 9.6 | 56.6 ± 6.5 | .96 |
| BMI | 25.9 ± 4.9 | 26.5 ± 4.7 | .80 |
| Duration of symptoms, y | 2.60 ± 1.4 | NA | NA |
| UPDRS part III scoring | 20.6 ± 6.48 | NA | NA |
|
Use of PD medications (#): MAO‐B inhibitor Amantadine |
6/10 2/10 |
NA | NA |
The resting energy expenditure, calculated during supported sitting from measured V̇O2, was similar between groups (Figure 1 and Table 2). Energy expenditure was calculated as 1930 ± 390 kcal/day in PD and 1869 ± 310 kcal/day in controls (P = .68, t test). In contrast to the resting state with a supported body trunk posture, V̇O2 values in all the other conditions were significantly higher in the PD than in the control group (Figure 1 and Table 2).
Figure 1.
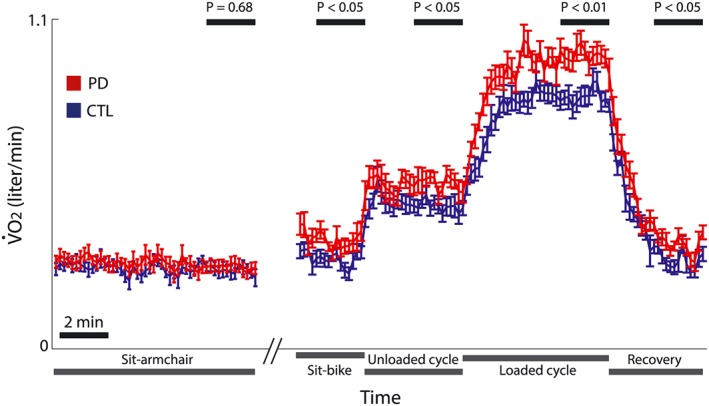
Breath‐by‐breath measure of oxygen consumption at rest and during exercise. Average rate of oxygen consumption (V̇O2) was calculated in patients with Parkinson disease (PD, red) and in healthy controls (CTL, blue) at a 10‐s sampling interval. Vertical bars represent standard error of the mean. The conditions are marked below the x‐axis, and the time intervals used for inter‐group comparison are marked above by black bars (average of the last 2 min for each condition). A time scale of 2 min is depicted by the black bar
Table 2.
Oxygen consumption (V̇O2) in the different conditions
| V̇O2 (Mean ± SD in L/min) | |||
|---|---|---|---|
| Condition of measurement | PD | Control | P‐value |
| Sitting in armchair | 0.27 ± 0.06 | 0.27 ± 0.04 | .68 |
| Sitting on bicycle | 0.36 ± 0.06 | 0.29 ± 0.05 | .02 |
| Unloaded cycling | 0.56 ± 0.07 | 0.48 ± 0.07 | .03 |
| Loaded cycling | 0.98 ± 0.09 | 0.84 ± 0.09 | .003 |
| Recovery | 0.35 ± 0.06 | 0.29 ± 0.05 | .048 |
To rule out the possibility of a confounding contribution of differences in muscle mass between individuals, we adjusted the V̇O2 to measure FFM (in liters per min per kilogram). This analysis yielded the same results, namely, a similar rest V̇O2 per kilogram in the PD and control groups (P‐value = .53) and a significantly higher V̇O2 per kilogram in the PD than in the controls in all 4 other conditions (P‐values = .005, .03, .04, and .045, respectively).
The physical tasks were increasingly demanding. When sitting in the armchair, both the axial and limb muscles were largely inactivated. Unsupported bike sitting required the activation of axial muscles, and for patients within each group, V̇O2 values were significantly higher while sitting on bicycle seat than when sitting in the armchair (PD: P = .0001, CRL: P = .01, paired t test). Unloaded cycling also required the activation of lower limb muscles. This activation further increased with loaded cycling. This served to test the hypothesis that the addition of muscle effort at different intensity levels would increase the abnormally high V̇O2 in PD. To calculate the additional oxygen consumption related to each of the exercises, for each individual, we subtracted his mean V̇O2 value at rest (calculated over the last 2 min of armchair rest) from the measured mean V̇O2 in each of the conditions. The increment in V̇O2 consumption in each phase was steeper in PD than in controls but was not statistically significant: The addition of unloaded cycling led to a V̇O2 increment of 0.20 ± 0.03 L/min in PD and 0.19 ± 0.06 in the controls (P = .53). The addition of a constant work load further increased V̇O2 0.42 ± 0.08 L/min in PD vs only 0.36 ± 0.08 in controls (P = .09).
There were no correlations between the motor severity of the disease (total UPDRS III) and the increment of V̇O2 relative to rest in any of the conditions (for bike rest, unloaded, and loaded cycling, P = .98, .46, and .86, respectively). Correlating the different subcomponents of the UPDRS III (rigidity, tremor, bradykinesia, and axial) with the increment of V̇O2 relative to rest did not yield any significant results (all P‐values > .08), except for a significant correlation between tremor and increased energy expenditure during unsupported bike sit (R = .69, P = .03). This single significant correlation, among multiple statistical tests, is of uncertain clinical significance.
4. DISCUSSION
By measuring oxygen consumption, we showed that in a homogenous population of males with early PD who were not on dopaminergic therapy, the energy expenditure at rest did not increase. Physical activity, on the other hand, led to an increment in energy expenditure that was higher than in the controls. Evidence for abnormally increased energy expenditure emerged from the recruitment of axial muscles to support the body trunk in a sitting posture.
Our observation of normal resting energy expenditure in PD is consistent with previous studies2, 5 but conflicts with others.3, 4 These contradictory results may have to do with unmatched PD and control group gender,3 a lack of adjustment for FFM,4 or the recruitment of patients on medication that modulates metabolic rate such as beta‐blockers or thyroid hormone replacement therapy or individuals who had undergone deep brain stimulation.9 We avoided these issues by only testing male patients (for their higher proportion of FFM), matching for anthropomorphic parameters, and excluding patients on potentially confounding medication or post‐PD surgeries.
Daily energy expenditure, which sums both resting energy expenditure and the energy consumption induced by physical activity, was found to be lower in PD than in the non‐affected controls when was measured over several days by a doubly labeled water technique.5 In light of the normal resting energy expenditure, the differences between the 2 groups thus resulted from differences in daily activity. Activity is prone to change with disease progression, motivation to exercise, etc. We did not measure daily energy expenditure but rather showed that similar physical activity is more energy consuming in PD compared to unaffected participants. We cannot rule out the possibility that impaired movement coordination in PD patients during cycling contributed to increased energy consumption in this group. However, our observation that the maintenance of trunk posture also requires increased energetic cost in PD rules out this possibility as a sole explanation. This last observation has not been documented in previous studies.
The energy consumed by an active muscle for a given work load reflects the muscle's energetic efficiency. We showed that in PD, muscles may have lower energetic efficiency. Muscle energy consumption is governed to a great extent by the number, size, and concentration of mitochondria in muscle tissue. It remains unclear whether there is impaired mitochondrial function in the muscles of individuals with PD.10, 11, 12 Our work lends weight to the possibility that mitochondrial respiratory chain enzymes have decreased activity in PD.12 Another possibility is that the energetic efficacy in PD is normal but that muscle rigidity creates an additional load for the active muscle. In this study, V̇O2 was not correlated with the severity of PD symptoms (except for a positive correlation with tremor severity during unsupported bike sit, which is of uncertain clinical significance). This absence of correlation, however, might stem from the small sample size and the relatively narrow range of disease severity.
Weight loss in PD is multifactorial.13 Caloric disequilibrium is not only due to increased energy expenditure but also due to reduced caloric intake secondary to depression, hyposmia, and dysphagia. Hormones such as leptin may also play a role in weight loss in PD.14 We did not asses caloric intake in this study or follow the weight of participants longitudinally. We can, therefore, only speculate that the increased energy expenditure, which is limited to the active state, could be considered a contributing factor to weight loss observed in PD patients in the early phases of the disease.
5. PERSPECTIVES
It has been hypothesized that weight loss, a major contributor to impaired quality of life in PD,15 is the result of increased resting energy expenditure, yet conclusive evidence for this hypothesis has been lacking. This study demonstrated that increased energy expenditure in PD patients occurs only during posture maintenance and physical activity, not during rest. Recent research showed that training with Nordic walking poles improved motor function and locomotion in PD patients.16 Nordic walking poles increase stability of gait during walking and may off‐load posture‐maintaining accessory muscles that, as we shown, are overworked in these patients. Based on our results, we predict improvement in exercise energetics for PD patients in conjunction with muscular function improvement following this type of training.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Table S1. Physical Activity Scale for the Elderly (PASE)
ACKNOWLEDGMENTS
We thank the 2 anonymous reviewers for their insightful comments and suggestions. This work was supported by the Prusiner‐Abramsky Research Award to D.A. H.B. is supported by research grants from the MAGNET program of the Office of the Chief Scientist (OCS) at the Israeli Ministry of Economy, the Israel Science Foundation (ISF), the German Israel Science Foundation (GIF), the Adelis, Rosetrees, and Vorst foundations, and the Simone and Bernard Guttman Chair in Brain Research.
Margaliot Kalifa T, Ziv N, Bergman H, Nusair S, Arkadir D. Increased energy expenditure during posture maintenance and exercise in early Parkinson disease. Health Sci Rep. 2018;1:e14 10.1002/hsr2.14
REFERENCES
- 1. Chen H, Zhang SM, Hernán MA, Willett WC, Ascherio A. Weight loss in Parkinson's disease: weight loss in PD. Ann Neurol. 2003;53:676‐679. [DOI] [PubMed] [Google Scholar]
- 2. Lorefält B, Ganowiak W, Pålhagen S, Toss G, Unosson M, Granérus A‐K. Factors of importance for weight loss in elderly patients with Parkinson's disease. Acta Neurol Scand. 2004;110:180‐187. [DOI] [PubMed] [Google Scholar]
- 3. Levi S, Cox M, Lugon M, Hodkinson M, Tomkins A. Increased energy expenditure in Parkinson's disease. BMJ. 1990;301:1256‐1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Markus HS, Cox M, Tomkins AM. Raised resting energy expenditure in Parkinson's disease and its relationship to muscle rigidity. Clin Sci. 1992;83:199‐204. [DOI] [PubMed] [Google Scholar]
- 5. Toth MJ, Fishman PS, Poehlman ET. Free‐living daily energy expenditure in patients with Parkinson's disease. Neurology. 1997;48:88‐91. [DOI] [PubMed] [Google Scholar]
- 6. Washburn RA, Smith KW, Jette AM, Janney CA. The physical activity scale for the elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153‐162. [DOI] [PubMed] [Google Scholar]
- 7. Compher C, Frankenfield D, Keim N, Roth‐Yousey L. Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc. 2006;106:881‐903. [DOI] [PubMed] [Google Scholar]
- 8. Rom O, Reznick AZ, Keidar Z, Karkabi K, Aizenbud D. Body composition in heavy smokers: comparison of segmental bioelectrical impedance analysis and dual‐energy X‐ray absorptiometry. Adv Exp Med Biol. 2015;840:1–11. [DOI] [PubMed] [Google Scholar]
- 9. Jorgensen HU, Werdelin L, Lokkegaard A, Westerterp KR, Simonsen L. Free‐living energy expenditure reduced after deep brain stimulation surgery for Parkinson's disease: decreased energy expenditure after STN‐DBS surgery. Clin Physiol Funct Imaging. 2012;32:214‐220. [DOI] [PubMed] [Google Scholar]
- 10. Blin O, Desnuelle C, Rascol O, et al. Mitochondrial respiratory failure in skeletal muscle from patients with Parkinson's disease and multiple system atrophy. J Neurol Sci. 1994;125:95‐101. [DOI] [PubMed] [Google Scholar]
- 11. Mann VM, Cooper JM, Krige D, Daniel SE, Schapira AHV, Marsden CD. Brain, skeletal muscle and platelet homogenate mitochondrial function in Parkinson's disease. Brain. 1992;115:333‐342. [DOI] [PubMed] [Google Scholar]
- 12. Winkler‐Stuck K, Kirches E, Mawrin C, et al. Re‐evaluation of the dysfunction of mitochondrial respiratory chain in skeletal muscle of patients with Parkinson's disease. J Neural Transm Vienna Austria 1996. 2005;112:499‐518. [DOI] [PubMed] [Google Scholar]
- 13. Bachmann CG, Trenkwalder C. Body weight in patients with Parkinson's disease. Mov Disord. 2006;21:1824‐1830. [DOI] [PubMed] [Google Scholar]
- 14. Fiszer U, Michałowska M, Baranowska B, et al. Leptin and ghrelin concentrations and weight loss in Parkinson's disease. Acta Neurol Scand. 2010;121:230‐236. [DOI] [PubMed] [Google Scholar]
- 15. Akbar U, He Y, Dai Y, et al. Weight loss and impact on quality of life in Parkinson's disease. PLOS ONE. 2015;10: e0124541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Monteiro EP, Franzoni LT, Cubillos DM, et al. Effects of Nordic walking training on functional parameters in Parkinson's disease: a randomized controlled clinical trial. Scand J Med Sci Sports. 2017;27:351‐358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Physical Activity Scale for the Elderly (PASE)


